Abstract
Corticotropin-releasing factor (CRF) is a neuropeptide that mediates the stress response. Long known to contribute to regulation of the adrenal stress response initiated in the hypothalamic-pituitary axis (HPA), a complex pattern of extrahypothalamic CRF expression is also described in rodents and primates. Cross-talk between the CRF and midbrain dopamine (DA) systems links the stress response to DA regulation. Classically CRF+ cells in the extended amygdala and paraventricular nucleus (PVN) are considered the main source of this input, principally targeting the ventral tegmental area (VTA). However, the anatomic complexity of both the DA and CRF system has been increasingly elaborated in the last decade. The DA neurons are now recognized as having diverse molecular, connectional and physiologic properties, predicted by their anatomic location. At the same time, the broad distribution of CRF cells in the brain has been increasingly delineated using different species and techniques. Here, we review updated information on both CRF localization and newer conceptualizations of the DA system to reconsider the CRF-DA interface.
Keywords: primate, rat, mouse, VTA, substantia nigra, retrorubral field, stress, extended amygdala
Graphical Abstract
1. 36 years later: ongoing challenges in localizing CRF in the CNS
Corticotropin-releasing factor (CRF) (also known as corticotropin releasing hormone, CRH) is a 41-amino acid peptide that was first isolated in 1981 (Swanson et al 1983, Vale et al 1981) in the rat hypothalamus. CRF, along with a family of CRF-related peptides, plays a role in acute and prolonged stress responses by integrating endocrine, autonomic and neural systems to promote adaptation in mammals (Burchfield 1979, Herman & Cullinan 1997, Ronan & Summers 2011, Sawchenko et al 1993). As the seat of the hypothalamic-pituitary (HPA) axis, CRF containing neurons localized in the hypothalamic paraventricular nucleus (PVN) send projections into median eminence. The median eminence is enriched with many small capillary loops from the superior hypophyseal artery, and CRF diffuses into this capillary bed, which in turn drains into sinusoids, and the venous system of the anterior pituitary. Here, CRF stimulates ‘corticotropes ‘, which release adrenocorticotropin hormone (ACTH) into the general circulation, stimulating the synthesis and release of glucocorticoids from the adrenal cortex (Antoni et al 1983, Rivier & Plotsky 1986). Glucocorticoid increases promote several adaptive responses including gluconeogenesis and temporary suppression of the immune system to support ‘fight or flight’ energy demands. Rising glucocorticoids eventually exert negative feedback inhibition on CRF-expressing cells in the PVN, downregulating CRF mRNA, and closing the HPA functional loop.
CRF is also synthesized and released at extrahypothalamic sites, exerting a direct neuroregulatory effect over brain structures involved in stress-sensitive function. Non-HPA axis CRF was initially identified as a putative neurotransmitter, but is now considered a ‘neuroregulator’, and is always expressed in concert with a primary transmitter (e.g. glutamate or GABA) (Gallagher et al 2008, Joels & Baram 2009, Orozco-Cabal et al 2006). While neurotransmitter activity elicits fast acting changes in membrane potential, the conventional view has been that physiological concentrations of CRF do not induce a membrane potential change (Gallagher et al 2008). Recent work indicates that endogenous CRF binds post-synaptic membrane receptors to influence the excitatory/inhibitory function of neuronal networks and the resultant effects depend upon stress duration (Gunn & Baram 2017, Gunn et al 2017, Radulovic et al 1999).
The monoamine system, which includes the serotonin, norepinephrine and dopamine (DA) systems, is a key target of CRF innervation, providing a link between stress and monoamine function (Joels & Baram 2009, Mejias-Aponte et al 2009, Reyes et al 2005, Rodaros et al 2007). Because the monoamine transmitters have all been therapeutic targets for psychiatric illnesses (Chiodo & Bunney 1983, Creese et al 1976, Leonard 1997, Van Praag 1977), the ways in which CRF influences them has long been a focus of pharmacologic research (Tilders & Berkenbosch 1986). While extensive research has focused on CRF-norepinephrine interactions during stress (Dunn & Swiergiel 2008, Koob 1999, Valentino & Van Bockstaele 2008), the midbrain DA neurons also play a central role in acute and chronic stress responses (Holly & Miczek 2016, Mantsch et al 2016, Spanagel & Weiss 1999). Stress-induced DA dysfunction is an important mechanism associated with drug-seeking (Koob & Volkow 2010), loss of motivational tone in mood disorders, and psychotic symptoms in both mood disorders and schizophrenia (Weinstein et al 2017, Yadid & Friedman 2008).
The midbrain DA system is more diverse than previously recognized, with neurochemical, connectional, and physiologic diversity not originally imagined (Barker et al 2016, Haber et al 2000, Lammel et al 2011, Lammel et al 2014, Lerner et al 2015, Margolis et al 2008). Specific DA subpopulations, rather than a homogenous cluster of DA cells, are regulated by specific combinations of afferent systems to influence distinct output paths (Geisler & Zahm 2005, Watabe-Uchida et al 2012). DA neurons were initially discovered to code prediction errors that support appetitive approach behaviors (Kobayashi & Schultz 2008, Schultz et al 1993). However, it is now known that some DA neurons signal the biologic relevance (salience) of both reward and non-reward predicting stimuli, emitting a general ‘salience’ signal that may be more involved in orienting or preparatory strategies to confront new or uncertain cues (Brischoux et al 2009, Bromberg-Martin et al 2010, Horvitz 2000, Matsumoto & Hikosaka 2009, Matsumoto & Takada 2013, Pignatelli & Bonci 2015, Volman et al 2013). These divergent physiologic responses of the DA cells tend to map topographically such that more rostromedially located DA cells (in the region of the ventral tegmental area (VTA) code reward prediction error, and more dorsolaterally and caudally located DA cells are involved in ‘salience coding’. CRF influences across the ventral midbrain DA neurons may therefore have different consequences for behavior depending on the specific DA subpopulations involved.
In this review, we first briefly revisit the well-known physiologic interface between the CRF and DA system, which has been a long-standing focus of research on addiction and other stress-mediated disorders (Deutch et al 1987, Deutch & Roth 1990, Kalivas 1985, Koob & Volkow 2010, Mantsch et al 2016, Meloni et al 2006). We then review and update information on localization of CRF neurons across species, including primates. Finally, we discuss new information on the organization and circuit heterogeneity of DA subpopulations and their possible differential regulation by specific CRF paths.
2. What is the functional role of CRF in the ventral midbrain?
In the ventral midbrain, pharmacologic and physiologic studies have focused almost exclusively on CRF actions in the VTA subregion because of its established connections with the ‘limbic’ forebrain, i.e. the ventral striatum and medial prefrontal cortex (Bardo et al 1996). The VTA constitutes a complex group of DA neurons that are primarily regulated through glutamatergic innervation, resulting in both short- and long-term changes in dopaminergic activity (Bonci & Malenka 1999). CRF is thought to have an excitatory role, inducing a potentiation of NMDAR (N-methyl-D-aspartate-receptor)-mediated synaptic transmission in DA neurons (Ungless et al 2003) resulting in glutamate release and dopaminergic activation (Korotkova et al 2006, Wise & Morales 2010). Both pharmacologic (Wang et al 2007) and electrophysiologic (Ungless et al 2003) data suggest that an association between CRF and the CRF binding protein (CRF-BP) is necessary to elicit this response. CRF may signal through the CRF type-2 receptor (CRFR2) as CRF activity is blunted in the presence of CRFR2 but not CRF typ-1 receptor (CRFR1) antagonists (Ungless et al 2003, Wang & Morales 2008). However, CRF has a higher affinity for CRFR1 than CRFR2 (Lovenberg et al 1995) and CRFR2 expression in VTA has been difficult to resolve (Van Pett et al 2000, Wise & Morales 2010). CRFR1 mRNA, however, has been found in VTA and localized in dopaminergic neurons (Tagliaferro et al., unpublished observations). Interestingly, following repeated cocaine exposure, CRF enhancement of NMDAR EPSCs was shown to involve both CRFR1 and CRFR2 that appeared to work in concert to amplify specific responses in the VTA (Hahn et al 2009). Therefore, precise CRF-glutamate-dopamine interactions in the VTA remain unresolved and may suggest multiple signaling mechanisms involving either CRFR1 or CRFR2 or both (reviewed in, Wise & Morales 2010).
From an anatomic perspective, CRF-positive fibers form both excitatory and inhibitory synapses in the VTA. DA cells receive CRF-positive synapses that are mostly asymmetric (glutamatergic) (Tagliaferro & Morales 2008, Wang et al 2005), while non-dopaminergic VTA neurons receive both asymmetric and symmetric (inhibitory) contacts from CRF-labeled terminals.
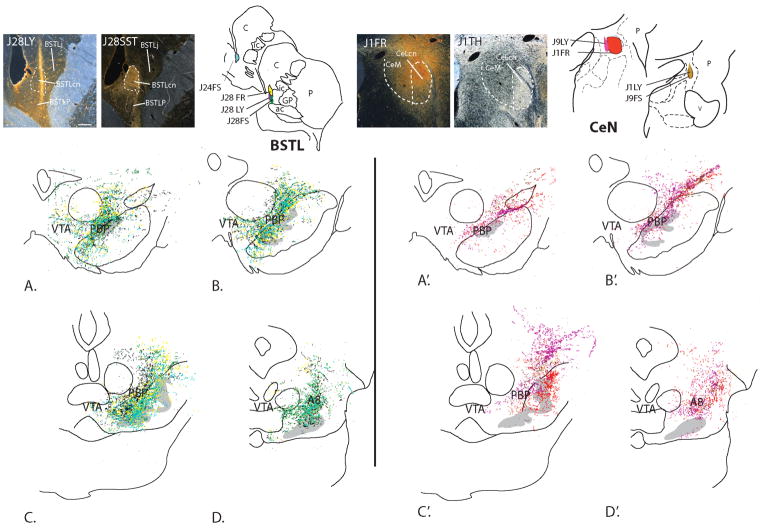
In rodents, the VTA receives CRF-containing afferents from lateral bed nucleus of stria terminalis (BSTL), the central nucleus of the amygdala (CeN), and the paraventricular nucleus of the hypothalamus (PVN) (Dabrowska et al 2016, Rodaros et al 2007). CRF innervation of other DA subregions has generally been ignored. However, recently, Dabrowska and colleagues (Dabrowska et al 2016) used genetic approaches in mice and rats to show that CRF synthesizing cells in the BSTL send relatively more intense fiber labeling over the substantia nigra pars compacta compared to the VTA. To examine the situation in nonhuman primates, we recently investigated inputs from the BSTL and CeN to the ventral midbrain in the monkey, and also found a relatively dense input to the dorsolateral substantia nigra and retrorubral field, but a relatively light input from these structures to the classic VTA. This topography is similar to previous findings in rats (Gonzales & Chesselet 1990, Wallace et al 1992, Zahm et al 2011) and monkeys (Fig. 1; Price & Amaral 1981). When examined with double-labeling experiments, retrograde tracer injections placed in these more lateral and caudal DA subpopulations resulted in many labeled cells in the BSTL and CeN that co-contained CRF (Fudge et al 2017). This prompted two questions: 1) what might be the role of CRF in DA cells outside the VTA? and, 2) Are more diverse sources of CRF responsible for DA modulation (Yadid & Friedman 2008)—particularly in the VTA--and if so, which ones? In other words, is the diversity of CRF inputs to the midbrain DA neurons greater than previously recognized? We revisited literature from mice, rats, monkeys, and human to ascertain the locations of CRF-containing neural populations throughout the brain, consider potential species differences, and uncover possible unexplored sources of CRF to specific DA subpopulations.
Figure 1.
Anterogradely labeled fibers resulting from all injections into the primate BSTL (A–D) and CeN (A′–D′) terminate mainly over the parabrachial pigmented nucleus (PBP), the lateral-most wing of the A10 neurons, and A8 (RRF), with some encroachment on the A9 neurons (gray). The VTA is receives fewer inputs. Photomicrographs show representative tracer injection sites the BSTL (J28LY) and CeN (J1FR) with adjacent sections labeled for either somatostatin (J28SST) or TH (J1TH) to localize relative injection site placement with nuclear subdivisions. Abbreviations: FR, fluororuby conjugated dextran amine; FS, fluorescein conjugated to dextran amine; LY, Lucifer yellow conjugated to dextran amine. Scale bar, 500 μm. *Figure reproduced with permission from (Fudge et al 2017)
3. Sources of CRF-positive cells in mice, rats, monkeys, and humans
3.1 Technical issues
Since its isolation in 1981 (Vale et al 1981), and the generation of specific antiserum to synthetic ovine CRF, numerous immunocytochemical studies have been conducted to document the distribution of CRF-immunoreactive (CRF-IR) neurons and fibers in the brain (Cummings et al 1983, Fellmann et al 1984, Joseph & Knigge 1983, Merchenthaler et al 1982, Merchenthaler et al 1984, Olschowka et al 1982, Sakanaka et al 1986, Swanson et al 1983, Wang et al 2011). However, detection of CRF-containing neurons has been, and remains, challenging.
3.1.1. Protein detection with immunocytochemistry
One obstacle in CRF detection is cross-reactivity of antibodies for CRF with urocortin, a structurally related protein (Donaldson et al 1996) and the melanin-concentrating hormone precursor (Nahon et al 1989). Another issue is that CRF-binding protein (CRF-BP), which is not uniformly present in all CRF-positive cells, may mute CRF immunoreactivity (Sawchenko et al 1993). Finally, there can be variable content of CRF protein in cell bodies due to unanticipated and/or unrecognized stress effects in experimental animals (Ceccatelli et al 1991). CRF levels in cells bodies drop rapidly following a stressor (Imaki et al 1991b, Kiss 1988), making detection of the CRF-positive cells difficult and unstable across conditions. One solution to visualizing CRF protein in cell bodies has been the use of colchicine, which inhibits microtubule polymerization, effectively arresting the movement of neurotransmitter containing vesicles down the axon shaft. This reduces cellular depletion, and also reduces the density of neuropil staining. In rodent studies, colchicine has the advantage of enhancing the intensity of CRF cellular labeling, resolving/and or enhancing immunoreactivity of soma labeling so that it can be visualized in neuropil-dense regions that would otherwise obscure it (Merchenthaler 1984, Sawchenko & Swanson 1983, Wang et al 2011). Comparing colchicine versus non-colchicine treated animals resulted in a significantly lower cell counts in one classic study (Swanson et al 1983), but not another (Sakanaka et al 1987b).
3.1.2. Other methods of detection
In situ hybridization detection of CRF mRNA is a highly specific method for detecting cell bodies prepared to generate CRF protein, although CRF-mRNA levels vary widely and do not necessarily correlate with the pattern of CRF protein expression (Imaki et al 1991b, Imaki et al 1989). Therefore, while this method is an important adjunct to immunocytochemical studies, it provides different information. To our knowledge there is not a study directly comparing the distribution of whole brain CRF protein (using immunocytochemistry) and in situ hybridization methods to assess CRF mRNA. Recent transgenic approaches detail similar, but not identical, findings compared to traditional immunocytochemical and in situ hybridization approaches (Alon et al 2009, Chen et al 2015, Kono et al 2017, Martin et al 2010, Peng et al 2017, Pomrenze et al 2015, Taniguchi et al 2011). These studies involve a variety of molecular techniques some of which reveal mismatches between virus-driven fluorescent protein expression and directly assessed CRF message and/or protein. Mismatch rates may vary between brain regions, with some regions having similar levels of expression and other regions having low rates of transgenic expression compared to native mRNA, or vice versa. These approaches require cross- validation with other assays and complementary techniques (see excellent discussions in Chen et al 2015, Kono et al 2017).
3.1.3. Species issues
In the non-human primate brain, analysis of CRF-IR distribution still relies on standard immunocytochemistry (Bassett & Foote 1992, Foote & Cha 1988, Fudge et al 2017, Lewis et al 1989). The active moiety of the CRF molecule is the same in human CRF and rat (Rivier et al 1983, Shibahara et al 1983, Vale et al 1981) and antibodies against this sequence are optimal in primate (although antibodies to ovine CRF result in similar but lower intensity staining). Primate studies do not use colchicine. The often-lethal side effects of colchicine, an inability to administer a standardized dose of the drug, and the suspected introduction of stress with administration are reasons against its use in larger species (Bassett & Foote 1992, Foote & Cha 1988, Lewis et al 1989). Regardless of this technical difference, CRF immunoreactivity is documented throughout the non-human primate brain in many areas coinciding with those found in rodent research.
3.1.4. Sex differences
Important to note, are recent findings of differential CRF regulation in males vs. female rats (Bangasser & Valentino 2012, Iwasaki-Sekino et al 2009). CRF mRNA appears to be regulated in gender-specific ways in several discrete brain regions following specific stressors. There has yet to be analysis of basal CRF expression in male versus female animals across specific brain regions, so this source of variability remains unknown.
4. Regional distribution of CRF protein and/or mRNA across species (Table 1)
4.1. Cortex
In rats and mice, CRF-IR cell bodies are scattered throughout the neocortex and are frequently bipolar in appearance with vertically oriented cell bodies. CRF-IR neurons appear to be more numerous in prefrontal, insular, and cingulate areas of rat cortex (Merchenthaler 1984, Merchenthaler et al 1982, Swanson et al 1983). Colchicine use may reveal a more homogeneous distribution across multiple cortical areas (Sakanaka et al 1987b). CRF is co-expressed with GABA and other neuropeptides in a lamina-specific fashion, indicating interneuron expression (Kubota et al 2011). Transgenic mouse studies indicate that CRF-producing cells are broadly expressed throughout the cortex, mainly in layers 2/3 with some cells in layer 6 (Kono et al 2017, Peng et al 2017, Taniguchi et al 2011). As in the rat, CRF-expressing cortical cells in mice are GABAergic, although one study indicates that CRF-expressing neurons in the piriform cortex and olfactory bulb co-express glutamate (Kono et al 2017).
In monkey, the overall density of CRF-IR cells and fibers, as well as their laminar distribution, differs substantially across cortical subregions (Lewis et al 1989). Similar to rodents, a high density of labeled cells and fibers are also found in the anterior cingulate cortex, but many CRF-positive cells are found in the association regions of the prefrontal, parietal, and temporal cortices. Within these regions, the posterior cingulate and medial and orbital frontal regions contain high densities of CRF labeled neurons, with the lowest density in the primary visual and primary motor regions. Because of their morphology and the fact that the distribution of labeled soma and fibers track one another across cortical regions and lamina, CRF-IR cells are presumed to be interneurons, similar to the situation in rodents.
4.2. Hippocampus
In rats and mice, CRF-IR cells in the hippocampus are distributed uniformly throughout all CA fields and scattered in the dentate gyrus (Chen et al 2001, Kono et al 2017, Merchenthaler 1984, Sakanaka et al 1987a, Swanson et al 1983, Williams & Milner 2011, Yan et al 1998) with the greatest number of CRF-IR cells localized in the most caudal part of CA4. The subiculum, presubiculum, and parasubiculum also contain significant numbers of CRF-IR cells (Sakanaka et al 1987b).
There are no published studies of CRF-IR distribution in macaque hippocampus, however light to moderate distribution of the CRF-Type 1 receptor (CRFR1) has been reported in the CA1-CA3 fields, subiculum, prosubiculum, and entorhinal area (Kostich et al 2004). In our material, we find a sparse distribution of CRF-IR cells in the CA fields with the highest concentration in the hilar region (unpublished observations) and scattered labeled cells in the subiculum and presubiculum (Fig. 2A–B).
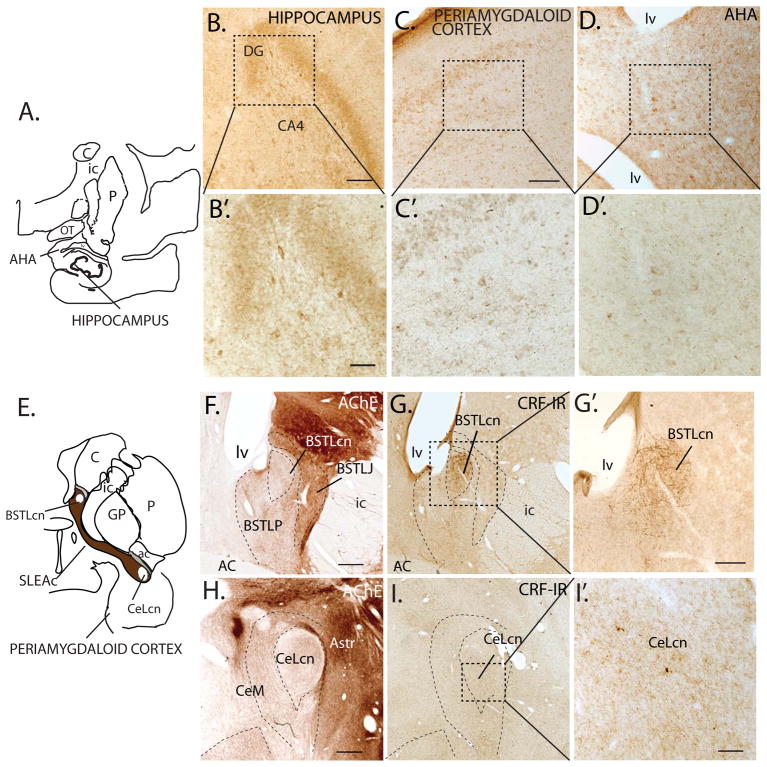
Figure 2.
A–D, B′–D′: CRF-IR cells in regions of monkey hippocampus and surrounding amygdala transition areas. B–B′. CRF-IR cells CA4 field of the hippocampus at low (B) and higher power (B′). C–C′. CRF-IR cells in the periamygdaloid cortex at low (C) and high (C′) power, and amygdalohippocampal area (AHA) at low (D) and high power (D′). E. Schematic showing the central extended amygdala. F. Subdivisions of the BSTL based on AChE staining. G–G′: CRF-IR cells and fibers stand out in the BSTLcn both at low (G) and high (G′) power. H. Subdivisions of the CeN in AChE. I–I′: Relatively low concentrations of CRF-IR cells and fibers in the CeLcn at low (I) and high magnification (I′) compared to the BSTLcn (G–G′).
Abbreviations: AC; anterior commissure; AChE, acetylcholinesterase; AHA, amygdalohippocampal area; Astr, amygdalostriatal area; BSTLcn, bed nucleus of the stria terminalis, lateral central division; BSTLJ, bed nucleus of the stria terminalis, lateral juxtacapsular division; BSTLP, bed nucleus of the stria terminalis, lateral posterior division; C, caudate nucleus; CeLcn, central nucleus, lateral central division; CeM, central nucleus, medial subdivision; DG; dentate gyrus; GP, globus pallidus; ic, internal capsule; lv, lateral ventricle; OT, optic tract; P, putamen; PAC, periamygdaloid cortex; SLEAc, sublenticular extended amygdala, central subdivision. Scale bars: = C, F, G, H, I = 500 μm; G′=250 μm; B, D and I′ = 100 μm; 40X magnification in B′, C′, D′ =50 μm.
4.3. Extended amygdala
The ‘central extended amygdala’, conceptualized as a forebrain macrostructure that is a key ‘receiving structure’ for amygdala output, includes the BSTL and the CeN. The BSTL and CeN are composed of medium-sized spiny neurons, and contain some of the densest concentrations of CRF-positive cells in the brain (Asan et al 2005, Cassell & Gray 1989, Cassell et al 1986, Cummings et al 1983, Ju et al 1989, Kono et al 2017, Olschowka et al 1982, Pomrenze et al 2015, Sakanaka et al 1986, Swanson et al 1983). Columns of CRF-IR cell bodies and fibers also extend from the BSTL and CeN into the basal forebrain region known as the ‘sublenticular extended amygdala’ (SLEAc; labeled ‘substantia innominata’ in early studies; Fig. 2E). The BSTL and CeN contain discrete sub compartments, which are described using various nomenclatures across the literature. We use the naming system of deOlmos and McDonald, as it is most readily translatable across species (De Olmos 2004, Martin et al 1991, McDonald 2003). In rodents, the lateral central subnuclei of the CeN (CeLcn) and BSTL (BSTLcn) are notable for having very high concentration of CRF-labeled cells and fibers (Cummings et al 1983, Ju et al 1989, Kono et al 2017, Merchenthaler 1984, Olschowka et al 1982, Pomrenze et al 2015, Sakanaka et al 1986, Swanson et al 1983). However, the medial CeN (CeM) and the posterolateral subdivision of the BSTL (BSTLP), and the contiguous SLEAc, also have many CRF-positive cells and fibers, as does the contiguous SLEAc (Alon et al 2009, Asan et al 2005, Cassell & Gray 1989, Paull et al 1984). The CRF-positive cells in the CeM-SLEAc-BSTLP continuum are frequently overlooked because they are more diffuse than the easily recognizable, high density of CRF cells in the CeLcn and BSTLcn. The CeM-SLEAc-BSTLP continuum is important because it covers a very large expanse of the forebrain and is the main output to the ventral midbrain (Zahm et al 2011). CRF-positive cells also occupy structures surrounding the extended amygdala: the ventral striatum, interstitial nucleus of the posterior limb of the anterior commissure (IPAC), amygdalostriatal area, and ventral septum (e.g. Asan et al 2005, Wang et al 2011).
In the monkey, the familiar high density of CRF-IR cells seen in rodent CeLcn is surprisingly reduced (Cha & Foote 1988)(Fig. 2H–I, I′). However, the BSTLcn contains denser concentration of CRF positive cells and fibers (unpublished observations; Fig. 2F–G, G′). CRF-IR cells and fibers are also found throughout the CeM, BSTLP, and contiguous SLEAc, where they are diffusely distributed (Fudge et al 2017).
4.4. Amygdala
The amygdala ‘proper’ differs significantly from the extended amygdala not only in cell types and ontogeny, but also in its connectivity and functional organization (McDonald 2003, Price et al 1987). In contrast to the central extended amygdala, the amygdala, comprised of basal, accessory basal and lateral nuclei, and the superficial regions, is ‘cortical-like’. It contains glutamatergic pyramidal neurons, heterogeneous populations of interneurons, and has connectivity patterns typical of the cortex, including participation in basal ganglia loop systems (Carlsen & Heimer 1988).
In rats, moderate densities of CRF-IR cell bodies are found in the main amygdala nuclei (especially the basal and accessory basal nuclei) after colchicine injections (Sakanaka et al 1986); but many studies find only scant or no CRF-IR cells in these ‘cortical-like’ nuclei (Merchenthaler 1984, Olschowka et al 1982, Swanson et al 1983). However, traditional CRF mRNA labeling and transgenic approaches reliably detect CRF mRNA in the basal nucleus in mouse (Alon et al 2009, Chen et al 2015, Kono et al 2017, Peng et al 2017).
In the monkey, immunocytochemical processing without colchicine is sufficient to detect many CRF-positive neurons in the basal and lateral nuclei (Bassett & Foote 1992). The periamygdaloid cortex and the amygdalohippocampal area contain occasional CRF-IR cells. CRF-IR cells in all these nuclei are small diameter cells, with morphology consistent with interneurons (Fig. 2C, D).
4.5. Hypothalamus
In rats and mice, CRF-IR and/or CRF mRNA positive cells define the lateral and medial parvocellular divisions of the PVN, where they are intensely stained and densely packed (Bloom et al 1982, Burlet et al 1983, Chen et al 2015, Cummings et al 1983, Kono et al 2017, Merchenthaler et al 1982, Olschowka et al 1982, Peng et al 2017, Piekut & Joseph 1986, Sawchenko et al 1984, Shioda et al 1985, Simmons & Swanson 2009, Swanson et al 1983). These cells are both ‘neuroendocrine’ (releasing CRF at the median eminence) and ‘non-neuroendocrine’ (also known as the ‘descending’ CRF system that project to the thalamus, brainstem and spinal cord) based on rat work (Rodaros et al 2007, Swanson 1991). The ‘descending’ neurons comprise a much smaller subpopulation of CRF-IR cells and seem to be spatially segregated from the main ‘neuroendocrine’ group (Simmons & Swanson 2009). The ventral magnocellular division of the PVN contains fewer and less densely stained cells. The proportion of magnocellular neurons that co-express CRF is substantially higher in rats than in mice (Biag et al 2012). Occasional CRF-IR cells are reported in other nuclei (medial area (MPA), the supraoptic nucleus, the suprachiasmic nucleus, the arcuate nucleus, the mammillary nuclei, and the anterior, lateral, dorsomedial and ventromedial hypothalamic nuclei) (Wang et al 2011).
The boundaries of the hypothalamic nuclei are relatively less circumscribed in monkeys and humans (Saper 1990). Intensely stained CRF- immunoreactive (Koutcherov et al 2000, Mouri et al 1993, Paull et al 1984) and CRF-mRNA (Krolewski et al 2010) containing cells are found in the parvicellular subdivisions of the PVN, with scattered CRF-IR cells stretching laterally into magnocellular subdivision, as in rodent. The supraoptic nucleus has low levels of CRF mRNA (Krolewski et al 2010), not seen at the protein level (Mouri et al 1993).
4.6. Thalamus
The thalamus is a large complex structure, which is interconnected with vast areas of the cortex. In rats, many thalamic CRF-IR cells are densely distributed in the posterior midline thalamic nuclei (mTH) involved in limbic arousal of motor and visceral control or sensory integration (Merchenthaler et al 1984, Van der Werf et al 2002). Immuno-positive cells are mainly found the centromedian, the parafascicular, the rhomboid, and paracentral subnuclei. Some CRF-positive cells also populate the ventromedial, and ventroposterior subnuclei, which are basal ganglia relay nuclei. Further posteriorly, CRF-IR and CRF mRNA positive cells occupy the region near the peripeduncular nucleus (PPN), ‘suprageniculate’ thalamus, and medial geniculate nucleus (MGN), all of which are key nodes of the auditory relay system (Imaki et al 1991a). Interestingly, in mice, there are few CRF mRNA labeled cells in the thalamus outside of the MGN (Alon et al 2009, Kono et al 2017).
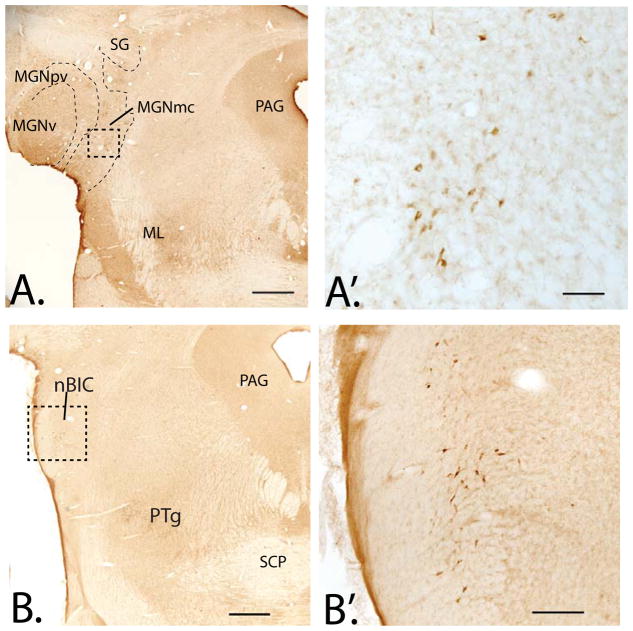
In monkey, as in the rat, CRF-IR cells are also located in the posterior mTH. However, there are also many CRF-IR cells in anterior (‘limbic’) mTH including the intralaminar nuclei, and reticular nucleus. Lighter distributions of CRF-IR neurons are seen in the anteromedial, anteroventral, and anterodorsal nucleus at posterior levels, as well as scattered CRF-IR cells in the mediodorsal nucleus and medial habenula (Foote & Cha 1988). As in the rat, CRF-IR cells are particularly abundant in the suprageniculate region where they form a column extending caudally into the nodes of the auditory relay system including the MGN (Fig. 3A, A′) and PPN in the midbrain.
Figure 3.
A. Low power photomicrograph of CRF-IR cells in monkey medial geniculate nucleus, magnocellular division (MGNmc), extending dorsally into the suprageniculate nucleus (SG). Subdivisions determined using adjacent sections immunostained for calbindin-D28k, not shown. A′. Higher power image of CRF-labeled cells in the MGNmc (boxed area of the in A). B. At slightly caudal levels of the pons, CRF-IR cells in the brachial nucleus of the inferior colliculus (nBIC), with higher magnification in B′. Abbreviations: ICnb; brachial nucleus of the inferior colliculus; ML, medial lemniscus; MGNpv, medial geniculate nucleus, posteroventral subdivision; MGNv, medial geniculate nucleus, ventral subdivision; PAG, periaqueductal gray; PTg, pedunculopontine tegmental nucleus; SCP, superior cerebellar peduncle. Scale bars: A and B= 1mm; A′= 100 μm, B′= 250 μm.
4.7. Periaqueductal gray (PAG) and deep mesencephalic nucleus (DMN)
The PAG is an important output for both active and passive defensive responding across species, and contains many CRF-positive cells in rats and mice, but somewhat surprisingly, not the primate (Bernard & Bandler 1998, Foote & Cha 1988, Kono et al 2017, Merchenthaler 1984, Olschowka et al 1982, Swanson et al 1983, Wang et al 2011)(Fudge et al., unpublished observations). The DMN, however, which is part of the reticular system and located just lateral to the PAG and dorsal to the midbrain DA neurons, contains many CRF-IR cells in all three species (Alon et al 2009, Foote & Cha 1988, Merchenthaler et al 1982, Sakanaka et al 1987a, Wang et al 2011).
4.8. Midbrain DA system and interpeduncular nucleus (IPN)
The question of CRF-positive cells in the midbrain DA system is controversial, with two studies reporting CRF-mRNA containing cells in mouse VTA (Grieder et al 2014, Korotkova et al 2006), but two other studies failing to find this (Alon et al 2009, Kono et al 2017). In rats, CRF-IR soma are reported just dorsal to the ventral tegmental area (VTA) and substantia nigra pars compacta (Merchenthaler 1984, Olschowka et al 1982), but this is not a consistent finding (Sakanaka et al 1987a, Swanson et al 1983). One possibility is that reported CRF-positive cells may actually be located in the midline tegmental region (i.e. reticular formation). No CRF-positive cells are seen in monkey VTA or substantia nigra (Foote & Cha 1988, unpublished data).
The IPN is largely GABAergic, and is a relay between excitatory inputs from the habenula and monoamine systems (Quina et al 2017, Herkenham & Nauta 1979, Nishikawa et al. 1986). It sits below the VTA between the cerebral peduncles (Fig. 5). CRF mRNA-positive cells are detected in this regulatory nucleus in mouse (Kono et al 2017), but not in rat (Merchenthaler 1984, Sakanaka et al 1987a, Swanson et al 1983) or monkey (Foote & Cha 1988).
Figure 5.
A–B. Dark-field photomicrographs showing CRF-IR fibers in the caudal midbrain. A. Labeled fibers are densest in the retrorubral field (RRF, or A8) with fewer labeled fibers in the ventral tegmental area (VTA, A10). B. Higher power image of RRF (boxed area in A). IPN, interpeduncular nucleus. Scale bar= 250 μm.
4.9. Pons and caudal brainstem
CRF-containing cells are found in many common brainstem nuclei across species including: the medial and lateral parabrachial nucleus (PB), the lateral dorsal tegmental nucleus (LDtg), locus coeruleus (LC), pedunculopontine tegmental nucleus (PTg), the inferior colliculus, the central linear nucleus, the median raphe nucleus (MR), the nucleus of the solitary tract (STn), and inferior olive. The main discrepancies across these regions in mouse, rat and monkey tend to be mainly related to relative densities of CRF-labeled cells (Alon et al 2009, Cha & Foote 1988, Cummings et al 1983, Foote & Cha 1988, Kono et al 2017, Merchenthaler et al, Olschowka et al 1982, Palkovits et al 1987, Powers et al 1987, Sakanaka et al 1987b, Swanson et al 1983), and may also arise because of different ages at assessment (e.g., Chang et al 1996). Rodents additionally have CRF-labeled cells in midline parts of the superior colliculus, the superior olive, and surrounding the cochlear nerve nuclei, which primates lack (Imaki et al 1991a, Sakanaka et al 1987b). Primates have a uniquely high number of CRF-IR cells in the brachium of the inferior colliculus, a tiny region that is in communication with the superior colliculus (Fig. 3B, B′) and is involved in sound-induced gaze control (Doubell et al 2000).
5. Beyond the VTA: a broader understanding of the ventral midbrain DA system
5.1 Traditional definitions of the DA subpopulations
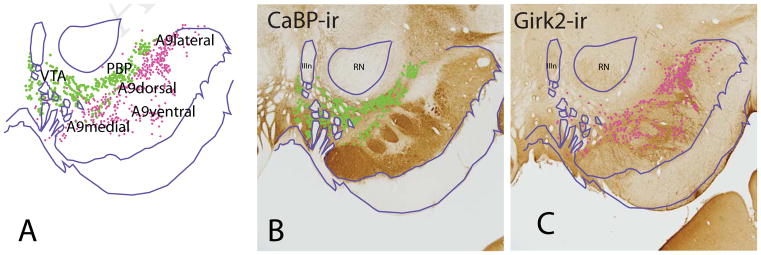
The DA neurons are traditionally divided into A10, A9, and A8 cell groups, defined morphologically and by anatomic location (Dahlstrom & Fuxe 1964, Olszewski & Baxter 2014). Across species, the A10 neurons are composed of the ventral tegmental area (VTA) and its various subnuclei, including the contiguous dorsal expanse of TH-positive neurons that stretch under the red nucleus known as the parabrachial pigmented nucleus (PBP). In monkey and human, the PBP was grouped previously in the A9 subgroup (or ‘A9 pars dorsalis’), based on its very large size and lateral position (Cho & Fudge 2010, Francois et al 1999, Olszewski & Baxter 2014, Williams & Goldman-Rakic 1998). However, accumulated evidence on cellular and histochemical features across species now indicate that the PBP, along with the midline VTA subnuclei, is more properly considered part of the A10 group (Halliday & Tork 1986, McRitchie et al 1996, Olszewski & Baxter 2014, Root et al 2016). All A10 DA cells, including the PBP, have high levels of the calcium binding protein, calbindin-D28k (CABP) (Haber et al 1995). In contrast, most A9 neurons of the substantia nigra, pars compacta (SNc), lack CaBP-IR, and instead contain relatively higher levels G-protein–regulated inward-rectifier potassium channel 2 (Girk2-IR) (Fig. 4). However, the distinction is not absolute, and some Girk2-IR cells occupy the overlying A10 group (Reyes et al 2012), and some CaBP-IR cells are found in the A9 (McRitchie & Halliday 1995). The A9 neurons are further divided into medial, dorsal, lateral and ventral groups (McRitchie et al 1996). In the caudal midbrain, A8 neurons (retrorubral field, RRF) emerge laterally, and like the A10 neurons, are CaBP-positive (not shown).
Figure 4.
A. Subdivisions of the primate ventral midbrain dopamine (DA) neurons depicted in adjacent sections. B. CalbindinD-28k (CaBP) positive cells populate the VTA and PBP (green). Note that the A9 cells are devoid of CaBP-IR. C. G protein-coupled inwardly-rectifying potassium channel 2 (Girk2) labeled cells (pink) are largely aggregated in the A9 neurons, which are further divided into medial, lateral, dorsal, and ventral subregions. Charts of CaBP-IR and Girk2-IR cells are overlaid in A, matching landmarks.
Abbreviations: IIIn, third nerve; RN, red nucleus.
5.2. DA subpopulation diversity: DA to non-DA cell ratios
DA subpopulation diversity begins with the fact that each DA cell group is embedded among GABAergic neurons, which function as interneurons (Johnson & North 1992, Omelchenko & Sesack 2009), and also projection neurons (Carr & Sesack 2000, Van Bockstaele & Pickel 1995). Each subpopulation has differing ratios of DA-ergic to non-DA-ergic neurons, and these ratios differ between rodents and primates (e.g., Francois et al 1999, German & Manaye 1993, Hirsch et al 1992, Nair-Roberts et al 2008, Poirier et al 1983, Swanson 1982). The ratio of DA-ergic to GABAergic neurons in each region has important implications for how a given afferent projection can influence each cell group. For example, extended amygdala afferent projections likely contact different ratios of GABA: DAergic neurons depending on the subpopulation targeted (e.g. PBP versus A8 neuronal group). Additionally, non-dopaminergic neurons may be purely GABAergic, or be a mix with glutamatergic neurons, also depending on the subregion (Yamaguchi et al 2007, Yamaguchi et al 2013).
5.3. DA subpopulation diversity: ‘multiplexed’ and non- ‘multiplexed’ cells
Recent anatomic data show that the basic division of ‘DA’, ‘GABA’, and ‘glutamatergic’ cells is yet more complex: some cells are ‘multiplexed’. That is, some TH-positive neurons co-contain either GABA or glutamate (Anderegg et al 2015, Hnasko et al 2012, Mingote et al 2015, Root et al 2016, Sulzer et al 1998, Yamaguchi et al 2007, Yamaguchi et al 2013, Zhang et al 2015), and some GABAergic neurons may co-express glutamate (Barker et al 2016, Root et al 2015, Yoo et al 2016). In rat VTA, ‘multiplexed’ DA neurons (i.e. those containing both TH and glutamatergic markers) project to major forebrain targets, including ventral striatum, lateral habenula, basal forebrain and amygdala (Kawano et al 2006, Taylor et al 2014, Yamaguchi et al 2011, Zhang et al 2015). In mice, the neuronal phenotype (transmitter type) associated with specific projections appears to play a role in distinct behaviors (e.g. Adamantidis et al 2011, Lammel et al 2012, Lerner et al 2015). It is important to note that there are, however, some conflicting findings across studies, e.g. with respect to the proportions of specific cell types occupying specific regions, and whether GABA/glutamate neurons are detected (Root et al 2015, Taylor et al 2014, Yoo et al 2016). Additionally, there appear to be some species differences in the concentrations of different cell types between mice and rats (Barker et al 2016).
Important for translation to human disease, cellular characteristics of the ventral midbrain were also recently examined in marmoset and human midbrain. The proportion of ‘pure’ DA neurons throughout the midbrain, including PBP, is higher overall in marmoset and human, compared to both rodent species. As in mice and rats, some DA cells co-contain glutamate and there is a small subpopulation of glutamate-only cells (Root et al 2016). However, in contrast to rodents, the PBP (rather than medial VTA) contains highest proportion of DA/glutamate cells (18% of all TH+ cells). Yet, with a relative overall increase in TH-only cells, TH-glutamatergic cells may form a relatively smaller overall contribution in monkey than in rodent. Although newer molecular techniques are not yet available in monkeys, it is important to begin to investigate cellular features in the DA subregion, and their associated paths using available methods, in order to bridge rodent mechanistic studies and human neuroimaging results.
6. CRF afferents to diverse DA subpopulations: what are the potential sources?
6.1. Using anatomic work to inform functional studies
Most pharmacology and behavioral work on CRF-DA interactions have focused on CRF influences on the VTA, as the seat of the classic ‘mesolimbic’ path. Yet the anatomy shows that CRF fibers stretch mediolaterally across the entire dorsal expanse of ventral midbrain DA cells, with particularly high concentrations encroaching on dorsal DA neuronal clusters in the nonhuman primate (Foote & Cha 1988), and on the retrorubral field (A8 neurons) in both rodents and primates (Fig. 5). Given newer understanding of the complexities of DA subpopulations, a broader perspective on sources of CRF innervation is in order.
6.2. Afferent inputs to the DA system: potential sources of CRF
The ventral midbrain region encompassing the DA neurons receives multiple inputs from a broad distribution of brain regions (Geisler et al 2007, Geisler & Zahm 2005, Lerner et al 2015, Phillipson 1979, Watabe-Uchida et al 2012, Zahm et al 2011)(Fig. 6). The topography of these inputs is important since anatomic location correlates with specific input and output paths, which in turn predict the specific physiologic properties of DA cells, including responses to salient stimuli (Beier et al 2015, Lammel et al 2012, Lerner et al 2015, Zhang et al 2015). In monkeys, differential DA responses to aversive, appetitive, and novel stimuli follow a mediolateral gradient across the ventral midbrain (Matsumoto & Hikosaka 2009, Mirenowicz & Schultz 1996, Ungless et al 2004), although work mapping these physiologic properties onto specific circuits is still lacking.
Figure 6.
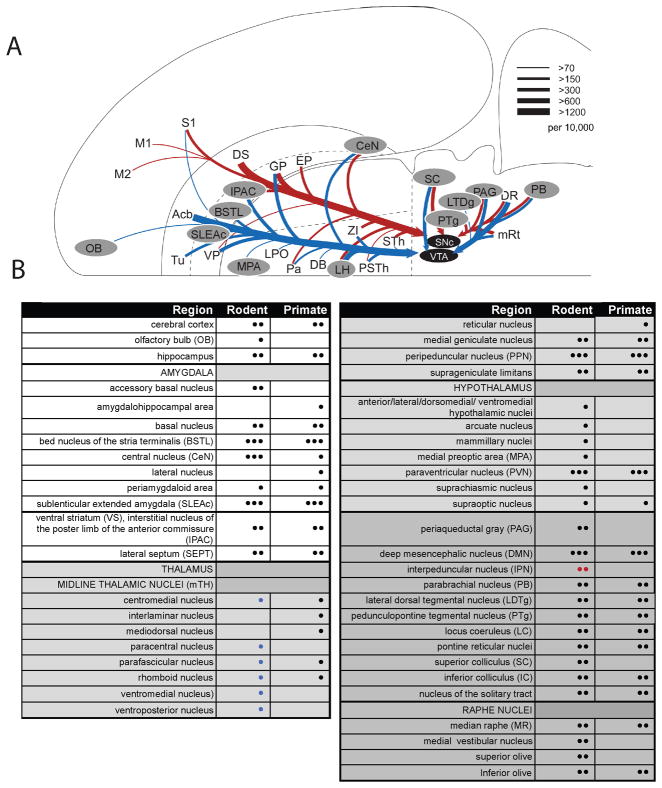
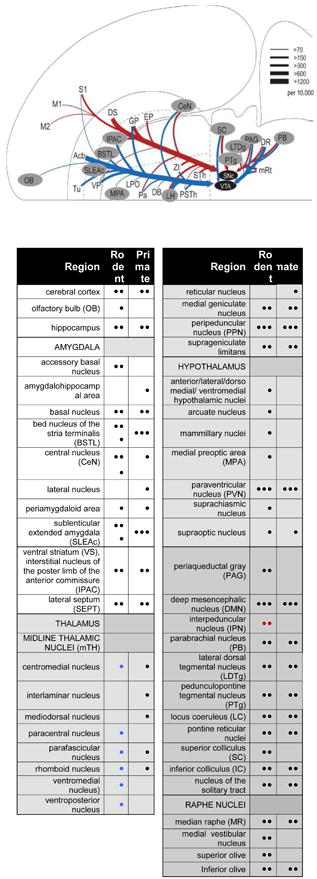
A. Many of the known afferent sources to the midbrain dopamine neurons, adapted from (Watabe-Uchida et al 2012) with permission. Blue indicates predominant inputs to VTA while red indicates predominant inputs to A9 dopamine neurons, in mouse. gray-highlighted regions depict brain regions that also contain CRF-IR cells. Dopamine afferent sources from DS to VTA and Acb to SNc were omitted for clarity. Line thickness corresponds to the number of input neurons in each area (inputs per 10,000 total inputs; per Watabe-Uchida et al 2012). B. Relative distribution of CRF cells in rodent and primate. • = few, •• = moderate, ••• = robust as translated from the literature. For rodent, blue symbols correspond to “rat only”, red symbols correspond to “mouse only”.
Abbreviations not shown in the Table: DB, diagonal band of Broca; DS, dorsal striatum; EP, endopeduncular nucleus; GP, globus pallidus; LPO, lateral preoptic area; M1, M2, primary and secondary motor cortex; PSTh, parasubthalamic nuclei; S1, primary sensory cortex; VP, ventral pallidum; ZI, zona incerta; sTH, subthalamic nuclei.
DA responses are further modulated if CRF can directly excite them via CRFR1/PLC Phospholipase-C)-PKC (Protein Kinase C) signaling (Paul 2017, Wanat et al 2008) resulting in increases in neuronal excitability (Riegel & Williams 2008). Although CRFR1 mRNA positive DA cells appear relatively more concentrated in the lateral VTA (PBP) and dorsal SNc (mouse) (Refojo et al 2011), a subpopulation or circuit-based analysis has not been done (Oliva & Wanat 2016). CRF-labeled fibers overlap the dorsal DA cells from the VTA to the lateral SNc and A8 subregion in rats and monkeys (Dabrowska et al 2016, Foote & Cha 1988, Fudge et al 2017). At the ultrastructural level, CRF-positive contacts in the VTA are mainly asymmetric (excitatory) on presumptive DAergic dendrites. Both excitatory and inhibitory-type CRF synapses are found on TH-negative neurons. Since excitatory afferents to the VTA are hypothesized to underlie stress-mediated DA release in the prefrontal cortex, CRF-co-transmission is proposed to possibly enhance this response (Enrico et al 1998, Kalivas et al 1987). The sources of this excitatory drive are unclear. Most excitatory input in the VTA appears to be subcortical, based on relatively high numbers of vesicular glutamate transporter 2 (subcortical) labeled terminals compared to those containing vesicular glutamate transporter 1(cortical) (Omelchenko & Sesack 2007).
Comparing the multiple afferent sources to the DA system with known nodes of the CRF system, as reviewed above, reveals possible origins of CRF innervation beyond the well-known BSTL, CeN, and PVN. Among established DA inputs, the ones that may include CRF as a co-transmitter are: the ventral striatum, midline thalamic nuclei (mTh), periaqueductal gray (PAG)(in rodents), peripeduncular nucleus (PPN), pedunculopontine tegmental nucleus (PTg), parabrachial nucleus (PB), locus coeruleus (LC; i.e. its CRF+, non-noradrenergic component), lateral dorsal tegmental nucleus (LDTg), median raphe, and the reticular fields in the pons (pontine reticular nuclei) and mesencephalon (DMN) (Fig. 6A, B). (Although it is theoretically possible that CRF-containing cells in the cortex project to the midbrain DA neurons, these CRF-positive interneurons more likely play a role in intrinsic cortical activity).
Conversely, within the midbrain, the extended amygdala (BSTL, SLEA, and CeN) and PVN may not be the most robust input to all DA subregions. Although CRF-positive fibers are densely distributed throughout the dorsal ventral midbrain, most data indicate that the BSTL and CeN project lateral to the medial VTA subnuclei, indicating that these CRF sources act on circuits outside the classic ‘mesolimbic’ paths (Fudge et al 2017, Gonzales & Chesselet 1990, Zahm et al 2011). Thus, the high concentrations of CRF-positive fibers in the classic VTA may derive from additional sources, including those reviewed above.
7. The search for specificity in CRF-DA circuits
While interactions between CRF and DA have long been known to alter DA cellular responses and behavior, it is now recognized that the ventral midbrain DA cells participate in highly specific input/output circuits. The source and role of CRF in these circuits may be quite specific, depending on the DA subpopulation affected. In primates, CRF-containing afferents from the extended amygdala project mainly to the PBP, the lateral-most wing of the A10, and the A8 neuronal group of the retrorubral field. The PBP and A8 DA neurons match the location of the ‘salience-detecting’ neurons in the primate (Matsumoto & Hikosaka 2009, Matsumoto & Takada 2013). By placing bidirectional tracers into these DA subpopulations, (or, presumptive ‘salience’ neurons), we found that retrogradely labeled cells in the extended amygdala predicted anterogradely labeled fibers in specific striatal zones, namely in the rostral central striatum and lateral shell. These regions are functionally defined by converging inputs from the orbitofrontal cortex, dorsal anterior cingulate, and dorsolateral prefrontal circuits (for review (Haber 2014)). We thus refer to this striatal region as ‘limbic-associative striatum’ due to highly interleaved projections from cortical regions that play roles in flexibly altering cognitive strategies when options or potential opportunities change (O’Doherty 2004, Rushworth et al 2011). In contrast, the ventromedial striatum and medial shell of the nucleus accumbens (which receives relatively more inputs from the ventromedial cingulate (e.g. area 25) (Haber et al 2006)) and the dorsolateral striatum (which receives input from premotor and motor cortices (Calzavara et al 2007)), are relatively excluded from the extended amygdala-DA-striatal circuit. Together these projection patterns suggest that extended amygdala CRF-DA interactions in the region of putative ‘salience’-encoding neurons can influence striatal coding of cognitive ‘switches’ related to ongoing monitoring of salient features in the external milieu. An important next step is to determine the primary transmitter expressed in the CRF-enriched extended amygdala-DA path, and the precise cellular targets of these projections in the PBP and A8 neuronal subpopulations.
It is equally important to discover additional sources of CRF that target specific DA subpopulations. As we have tried to show, there is theoretically a wide number of subcortical and brainstem regions that are potentially important sources of CRF to sustain or amplify neural responses in the ventral midbrain. Uncovering newer and more precise anatomic models of CRF influences on a heterogeneous DA system should help elucidate the effects of stress and CRF on diverse behaviors regulated by DA such as approach, attention, impulsivity, anhedonia, and repetitive behaviors.
Highlights.
Corticotropin-releasing factor (CRF) is a neuropeptide shown to play a role in acute and prolonged stress responses that promote adaptation in mammals.
CRF influences on dopamine (DA) physiologic responses, thought to originate in the extended amygdala to ventral tegmental area (VTA) path, have been used to model stress-induced behaviors associated with drug addiction, mood disorders, and psychosis
First described over forty years ago, the issue of CRF localization in the brain is revisited, with comparison of available data among the species
We discuss an array of CRF cell populations that are positioned to modulate DA subpopulations including, and beyond, the VTA
Acknowledgments
We appreciate assistance with histological work from Nanette Alcock. We also are grateful for comments of Talie Baram, MD, PhD on a draft of manuscript. The work was supported by the National Institute of Mental Health, ROIMH-63291-16 (JLF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–35. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon T, Zhou L, Perez CA, Garfield AS, Friedman JM, Heisler LK. Transgenic mice expressing green fluorescent protein under the control of the corticotropin-releasing hormone promoter. Endocrinology. 2009;150:5626–32. doi: 10.1210/en.2009-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg A, Poulin JF, Awatramani R. Molecular heterogeneity of midbrain dopaminergic neurons--Moving toward single cell resolution. FEBS Lett. 2015;589:3714–26. doi: 10.1016/j.febslet.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni FA, Palkovits M, Makara GB, Linton EA, Lowry PJ, Kiss JZ. Immunoreactive corticotropin-releasing hormone in the hypothalamoinfundibular tract. Neuroendocrinology. 1983;36:415–23. doi: 10.1159/000123492. [DOI] [PubMed] [Google Scholar]
- Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–67. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–23. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behavioural Brain Research. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Zhang S, Morales M. Multiplexed neurochemical signaling by neurons of the ventral tegmental area. J Chem Neuroanat. 2016;73:33–42. doi: 10.1016/j.jchemneu.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JL, Foote SL. Distribution of corticotropin-releasing factor-like immunoreactivity in squirrel monkey (Saimiri sciureus) amygdala. Journal of Comparative Neurology. 1992;323:91–102. doi: 10.1002/cne.903230108. [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, et al. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell. 2015;162:622–34. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Bandler R. Parallel circuits for emotional coping behaviour: new pieces in the puzzle. J Comp Neurol. 1998;401:429–36. [PubMed] [Google Scholar]
- Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom FE, Battenberg EL, Rivier J, Vale W. Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regulatory Peptides. 1982;4:43–8. doi: 10.1016/0167-0115(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. Journal of Neuroscience. 1999;19:3723–30. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–9. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield SR. The stress response: a new perspective. Psychosom Med. 1979;41:661–72. doi: 10.1097/00006842-197912000-00008. [DOI] [PubMed] [Google Scholar]
- Burlet A, Tonon MC, Tankosic P, Coy D, Vaudry H. Comparative immunocytochemical localization of corticotropin releasing factor (CRF-41) and neurohypophysial peptides in the brain of Brattleboro and Long-Evans rats. Neuroendocrinology. 1983;37:64–72. doi: 10.1159/000123517. [DOI] [PubMed] [Google Scholar]
- Calzavara R, Mailly P, Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. Eur J Neurosci. 2007;26:2005–24. doi: 10.1111/j.1460-9568.2007.05825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–80. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–23. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. Journal of Comparative Neurology. 1989;281:320–33. doi: 10.1002/cne.902810212. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. Journal of Comparative Neurology. 1986;246:478–99. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Cortes R, Hokfelt T. Effect of reserpine and colchicine on neuropeptide mRNA levels in the rat hypothalamic paraventricular nucleus. Brain Research. Molecular Brain Research. 1991;9:57–69. doi: 10.1016/0169-328x(91)90130-p. [DOI] [PubMed] [Google Scholar]
- Cha CI, Foote SL. Corticotropin-releasing factor in olivocerebellar climbing-fiber system of monkey (Saimiri sciureus and Macaca fascicularis): parasagittal and regional organization visualized by immunohistochemistry. J Neurosci. 1988;8:4121–37. doi: 10.1523/JNEUROSCI.08-11-04121.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Yi SJ, Baram TZ. Developmental profile of corticotropin releasing hormone messenger RNA in the rat inferior olive. Int J Dev Neurosci. 1996;14:69–76. doi: 10.1016/0736-5748(95)00072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001;21:7171–81. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Molet J, Gunn BG, Ressler K, Baram TZ. Diversity of Reporter Expression Patterns in Transgenic Mouse Lines Targeting Corticotropin-Releasing Hormone-Expressing Neurons. Endocrinology. 2015;156:4769–80. doi: 10.1210/en.2015-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. Journal of Neuroscience. 1983;3:1607–19. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Fudge JL. Heterogeneous dopamine populations project to specific subregions of the primate amygdala. Neuroscience. 2010;165:1501–18. doi: 10.1016/j.neuroscience.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–3. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–68. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Martinon D, Moaddab M, Rainnie DG. Targeting corticotropin-releasing factor (CRF) projections from the oval nucleus of the BNST using cell-type specific neuronal tracing studies in mouse and rat brain. J Neuroendocrinol. 2016 doi: 10.1111/jne.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62(supl. 232):1–55. [PubMed] [Google Scholar]
- De Olmos J. The Amygdala. In: Paxinos G, Mai JK, editors. The Human Nervous System. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- Deutch AY, Bean AJ, Bissette G, Nemeroff CB, Robbins RJ, Roth RH. Stress-induced alterations in neurotensin, somatostatin and corticotropin-releasing factor in mesotelencephalic dopamine system regions. Brain Res. 1987;417:350–4. doi: 10.1016/0006-8993(87)90462-8. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. discussion 02–3. [DOI] [PubMed] [Google Scholar]
- Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, et al. Cloning and characterization of human urocortin. Endocrinology. 1996;137:3896. doi: 10.1210/endo.137.9.8756563. [DOI] [PubMed] [Google Scholar]
- Doubell TP, Baron J, Skaliora I, King AJ. Topographical projection from the superior colliculus to the nucleus of the brachium of the inferior colliculus in the ferret: convergence of visual and auditory information. Eur J Neurosci. 2000;12:4290–308. [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol. 2008;583:186–93. doi: 10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrico P, Bouma M, de Vries JB, Westerink BH. The role of afferents to the ventral tegmental area in the handling stress-induced increase in the release of dopamine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Brain Res. 1998;779:205–13. doi: 10.1016/s0006-8993(97)01132-3. [DOI] [PubMed] [Google Scholar]
- Fellmann D, Bugnon C, Bresson JL, Gouget A, Cardot J, et al. The CRF neuron: immunocytochemical study. Peptides. 1984;5(Suppl 1):19–33. doi: 10.1016/0196-9781(84)90261-4. [DOI] [PubMed] [Google Scholar]
- Foote SL, Cha CI. Distribution of corticotropin-releasing-factor-like immunoreactivity in brainstem of two monkey species (Saimiri sciureus and Macaca fascicularis): an immunohistochemical study. J Comp Neurol. 1988;276:239–64. doi: 10.1002/cne.902760208. [DOI] [PubMed] [Google Scholar]
- Francois C, Yelnik J, Tande D, Agid Y, Hirsch EC. Dopaminergic cell group A8 in the monkey: anatomical organization and projections to the striatum. Journal of Comparative Neurology. 1999;414:334–47. [PubMed] [Google Scholar]
- Fudge JL, Kelly EA, Pal R, Bedont JL, Park L, Ho B. Beyond the Classic VTA: Extended Amygdala Projections to DA-Striatal Paths in the Primate. Neuropsychopharmacology. 2017;42:1563–76. doi: 10.1038/npp.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–25. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–43. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. Journal of Comparative Neurology. 2005;490:270–94. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J Comp Neurol. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- Gonzales C, Chesselet M-F. Amygdalonigral pathway: An anterograde study in the rat with phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1990;297:182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- Grieder TE, Herman MA, Contet C, Tan LA, Vargas-Perez H, et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci. 2014;17:1751–8. doi: 10.1038/nn.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Baram TZ. Stress and Seizures: Space, Time and Hippocampal Circuits. Trends Neurosci. 2017;40:667–79. doi: 10.1016/j.tins.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Cox CD, Chen Y, Frotscher M, Gall CM, et al. The Endogenous Stress Hormone CRH Modulates Excitatory Transmission and Network Physiology in Hippocampus. Cereb Cortex. 2017;27:4182–98. doi: 10.1093/cercor/bhx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282C:248–57. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland N. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. Journal of Neuroscience. 2000;20(6):2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–76. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Ryoo H, Cox C, Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: Comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol. 1995;362:400–10. doi: 10.1002/cne.903620308. [DOI] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009;29:6535–44. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Tork I. Comparative anatomy of the ventromedial mesencephalic tegmentum in the rat, cat, monkey and human. J Comp Neurol. 1986;252:423–45. doi: 10.1002/cne.902520402. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJH. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–48. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Mouatt A, Faucheux B, Bonnet A-M, Javoy-Agid F, et al. Dopamine, tremor, and parkinson’s disease. Lancet. 1992;340:126–20. doi: 10.1016/0140-6736(92)90457-e. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–85. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Miczek KA. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology (Berl) 2016;233:163–86. doi: 10.1007/s00213-015-4151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–6. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Imaki J, Imaki T, Vale W, Sawchenko PE. Distribution of corticotropin-releasing factor mRNA and immunoreactivity in the central auditory system of the rat. Brain Res. 1991a;547:28–36. doi: 10.1016/0006-8993(91)90571-c. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991b;11:585–99. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Nahon JL, Sawchenko PE, Vale W. Widespread expression of corticotropin-releasing factor messenger RNA and immunoreactivity in the rat olfactory bulb. Brain Res. 1989;496:35–44. doi: 10.1016/0006-8993(89)91050-0. [DOI] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–37. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. Journal of Physiology. 1992;450:455–68. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SA, Knigge KM. Corticotropin Releasing-Factor - Immunocytochemical Localization in Rat-Brain. Neuroscience Letters. 1983;35:135–41. doi: 10.1016/0304-3940(83)90540-2. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. Journal of Comparative Neurology. 1989;280:603–21. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Interactions between neuropeptides and dopamine neurons in the ventromedial mesencephalon. Neurosci Biobehav Rev. 1985;9:573–87. doi: 10.1016/0149-7634(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer LG. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther. 1987;242:757–63. [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, et al. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–92. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Kiss JZ. Dynamism of chemoarchitecture in the hypothalamic paraventricular nucleus. Brain Research Bulletin. 1988;20:699–708. doi: 10.1016/0361-9230(88)90080-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. Journal of Neuroscience. 2008;28:7837–46. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono J, Konno K, Talukder AH, Fuse T, Abe M, et al. Distribution of corticotropin-releasing factor neurons in the mouse brain: a study using corticotropin-releasing factor-modified yellow fluorescent protein knock-in mouse. Brain Struct Funct. 2017;222:1705–32. doi: 10.1007/s00429-016-1303-0. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–80. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–85. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kostich WA, Grzanna R, Lu NZ, Largent BL. Immunohistochemical visualization of corticotropin-releasing factor type 1 (CRF1) receptors in monkey brain. J Comp Neurol. 2004;478:111–25. doi: 10.1002/cne.20271. [DOI] [PubMed] [Google Scholar]
- Koutcherov Y, Mai JK, Ashwell KW, Paxinos G. Organization of the human paraventricular hypothalamic nucleus. Journal of Comparative Neurology. 2000;423:299–318. [PubMed] [Google Scholar]
- Krolewski DM, Medina A, Kerman IA, Bernard R, Burke S, et al. Expression patterns of corticotropin-releasing factor, arginine vasopressin, histidine decarboxylase, melanin-concentrating hormone, and orexin genes in the human hypothalamus. J Comp Neurol. 2010;518:4591–611. doi: 10.1002/cne.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, et al. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011;21:1803–17. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–62. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(Pt B):351–9. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–7. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. Noradrenaline in basic models of depression. European Neuropsychopharmacology. 1997;7(Suppl 1):S11–6. doi: 10.1016/s0924-977x(97)00415-x. discussion S71–3. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, et al. Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell. 2015;162:635–47. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Foote SL, Cha CI. Corticotropin-releasing factor immunoreactivity in monkey neocortex: an immunohistochemical analysis. J Comp Neurol. 1989;290:599–613. doi: 10.1002/cne.902900412. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–40. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2016;41:335–56. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. Journal of Neuroscience. 2008;28:8908–13. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Jasnow AM, Dabrowska J, Hazra R, et al. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol Psychiatry. 2010;67:1212–6. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Powers RE, Dellovade TL, Price DL. The bed nucleus-amygdala continuum in human and monkey. J Comp Neurol. 1991;309:445–85. doi: 10.1002/cne.903090404. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–41. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Takada M. Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron. 2013;79:1011–24. doi: 10.1016/j.neuron.2013.07.002. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Is there an amygdala and how far does it extend? An anatomical perspective. Ann N Y Acad Sci. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- McRitchie DA, Halliday GM. Calbindin D28K-containing neurons are restricted to the medial substantia nigra in humans. Neuroscience. 1995;65:87–91. doi: 10.1016/0306-4522(94)00483-l. [DOI] [PubMed] [Google Scholar]
- McRitchie DA, Hardman CD, Halliday GM. Cytoarchitectural distribution of calcium binding proteins in midbrain dopaminergic regions of rats and humans. Journal of Comparative Neurology. 1996;364:121–50. doi: 10.1002/(SICI)1096-9861(19960101)364:1<121::AID-CNE11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–26. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–63. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5(Suppl 1):53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Vigh S, Petrusz P, Schally AV. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. American Journal of Anatomy. 1982;165:385–96. doi: 10.1002/aja.1001650404. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Vigh S, Schally AV, Stumpf WE, Arimura A. Immunocytochemical Localization of Corticotropin Releasing-Factor (Crf)-Like Immunoreactivity in the Thalamus of the Rat. Brain Research. 1984;323:119–22. doi: 10.1016/0006-8993(84)90272-5. [DOI] [PubMed] [Google Scholar]
- Mingote S, Chuhma N, Kusnoor SV, Field B, Deutch AY, Rayport S. Functional Connectome Analysis of Dopamine Neuron Glutamatergic Connections in Forebrain Regions. J Neurosci. 2015;35:16259–71. doi: 10.1523/JNEUROSCI.1674-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–51. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Mouri T, Itoi K, Takahashi K, Suda T, Murakami O, et al. Colocalization of corticotropin-releasing factor and vasopressin in the paraventricular nucleus of the human hypothalamus. Neuroendocrinology. 1993;57:34–9. doi: 10.1159/000126339. [DOI] [PubMed] [Google Scholar]
- Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125:2056–65. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–31. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–36. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Oliva I, Wanat MJ. Ventral Tegmental Area Afferents and Drug-Dependent Behaviors. Front Psychiatry. 2016;7:30. doi: 10.3389/fpsyt.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschowka JA, O’Donohue TL, Mueller GP, Jacobowitz DM. The distribution of corticotropin releasing factor-like immunoreactive neurons in rat brain. Peptides. 1982;3:995–1015. doi: 10.1016/0196-9781(82)90071-7. [DOI] [PubMed] [Google Scholar]
- Olszewski J, Baxter D. Cytoarchitecture of the Human Brainstem. Basel: Karger; 2014. [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–74. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. Regulation of synaptic transmission by CRF receptors. Rev Neurosci. 2006;17:279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Leranth C, Gorcs T, Young WS., 3rd Corticotropin-releasing factor in the olivocerebellar tract of rats: demonstration by light- and electron-microscopic immunohistochemistry and in situ hybridization histochemistry. Proc Natl Acad Sci U S A. 1987;84:3911–5. doi: 10.1073/pnas.84.11.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul EDCA. Neural Circuitry of Stress, Fear, and Anxiety: Focus on Extended Amygdala Corticotropin-Releasing Factor Systems. In: Fink G, editor. Stress: Neuroendocrinology and Neurobiology. Univeristy of Melbourne; Australia: Academic Press; 2017. pp. 83–96. [Google Scholar]
- Paull WK, Phelix CF, Copeland M, Palmiter P, Gibbs FP, Middleton C. Immunohistochemical localization of corticotropin releasing factor (CRF) in the hypothalamus of the squirrel monkey, Saimiri sciureus. Peptides. 1984;5(Suppl 1):45–51. doi: 10.1016/0196-9781(84)90264-x. [DOI] [PubMed] [Google Scholar]
- Peng J, Long B, Yuan J, Peng X, Hong N, et al. A quantittative analysis of the distribution of CRH neurons in whole mouse brain. Frontiers in Neuroanatomy. 2017;11:1–12. doi: 10.3389/fnana.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: A horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–44. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Piekut DT, Joseph SA. Co-existence of CRF and vasopressin immunoreactivity in parvocellular paraventricular neurons of rat hypothalamus. Peptides. 1986;7:891–8. doi: 10.1016/0196-9781(86)90111-7. [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Bonci A. Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron. 2015;86:1145–57. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Poirier LJ, Giguere M, Marchand R. Comparative morphology of the substantia nigra and ventral tegmental area in the monkey, cat and rat. Brain Res Bull. 1983;11:371–97. doi: 10.1016/0361-9230(83)90173-9. [DOI] [PubMed] [Google Scholar]
- Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, et al. A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Front Neurosci. 2015;9:487. doi: 10.3389/fnins.2015.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RE, Desouza EB, Walker LC, Price DL, Vale WW, Young WS. Corticotropin-Releasing Factor as a Transmitter in the Human Olivocerebellar Pathway. Brain Research. 1987;415:347–52. doi: 10.1016/0006-8993(87)90218-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–59. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Hokfelt BT, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Amsterdam: Elsevier; 1987. pp. 279–381. [Google Scholar]
- Quina LA, Harris J, Zeng H, Turner EE. Specific connections of the interpeduncular subnuclei reveal distinct components of the habenulopeduncular pathway. J Comp Neurol. 2017;525:2632–56. doi: 10.1002/cne.24221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–25. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–7. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- Reyes S, Fu Y, Double K, Thompson L, Kirik D, et al. GIRK2 expression in dopamine neurons of the substantia nigra and ventral tegmental area. J Comp Neurol. 2012;520:2591–607. doi: 10.1002/cne.23051. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–70. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier CL, Plotsky PM. Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Annu Rev Physiol. 1986;48:475–94. doi: 10.1146/annurev.ph.48.030186.002355. [DOI] [PubMed] [Google Scholar]
- Rivier J, Spiess J, Vale W. Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci U S A. 1983;80:4851–5. doi: 10.1073/pnas.80.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Ronan PJ, Summers CH. Molecular Signaling and Translational Significance of the Corticotropin Releasing Factor System. Brain as a Drug Target. 2011;98:235–92. doi: 10.1016/B978-0-12-385506-0.00006-5. [DOI] [PubMed] [Google Scholar]
- Root DH, Hoffman AF, Good CH, Zhang S, Gigante E, et al. Norepinephrine activates dopamine D4 receptors in the rat lateral habenula. J Neurosci. 2015;35:3460–9. doi: 10.1523/JNEUROSCI.4525-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Wang HL, Liu B, Barker DJ, Mod L, et al. Glutamate neurons are intermixed with midbrain dopamine neurons in nonhuman primates and humans. Scientific Reports. 2016:6. doi: 10.1038/srep30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–69. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–38. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987a;260:256–98. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin-releasing factor-containing afferents to the inferior colliculus of the rat brain. Brain Res. 1987b;414:68–76. doi: 10.1016/0006-8993(87)91326-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Hypothalamus. In: Paxinos G, editor. The Human Nervous System. San Diego, CA: Academic Press, Inc; 1990. pp. 389–413. [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. discussion 21–9. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. Journal of Comparative Neurology. 1983;218:121–44. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci U S A. 1984;81:1883–7. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. Journal of Neuroscience. 1993;13:900–13. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S, Morimoto Y, Furutani Y, Notake M, Takahashi H, et al. Isolation and sequence analysis of the human corticotropin-releasing factor precursor gene. EMBO J. 1983;2:775–9. doi: 10.1002/j.1460-2075.1983.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda S, Nakai Y, Kitazawa S, Sunayama H. Immunocytochemical observations of corticotropin-releasing-factor-containing neurons in the rat hypothalamus with special reference to neuronal communication. Acta Anat (Basel) 1985;124:58–64. doi: 10.1159/000146097. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J Comp Neurol. 2009;516:423–41. doi: 10.1002/cne.22126. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends in Neuroscience. 1999;22:521–27. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, et al. Dopamine neurons make Glutamatergic synapses in vitro. J Neurosci. 1998;18(12):4588–602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Biochemical switching in hypothalamic circuits mediating responses to stress. In: Holstege G, editor. Progress in Brain Research. Elsevier Science; 1991. pp. 181–200. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506:616–26. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol. 2014;522:3308–34. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilders FJ, Berkenbosch F. CRF and catecholamines; their place in the central and peripheral regulation of the stress response. Acta Endocrinol Suppl (Copenh) 1986;276:63–75. doi: 10.1530/acta.0.111s0063. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–7. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–21. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–40. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Van Praag HM. New evidence of serotonin-deficient depressions. Neuropsychobiology. 1977;3:56–63. doi: 10.1159/000117590. [DOI] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, et al. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33:17569–76. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Research Bulletin. 1992;28:447–54. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–70. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–94. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J Comp Neurol. 2008;509:302–18. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Goebel-Stengel M, Stengel A, Wu SV, Ohning G, Tache Y. Comparison of CRF-immunoreactive neurons distribution in mouse and rat brains and selective induction of Fos in rat hypothalamic CRF neurons by abdominal surgery. Brain Res. 2011;1415:34–46. doi: 10.1016/j.brainres.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–73. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol Psychiatry. 2017;81:31–42. doi: 10.1016/j.biopsych.2016.03.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex. 1998;8:321–45. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Milner TA. Delta opioid receptors colocalize with corticotropin releasing factor in hippocampal interneurons. Neuroscience. 2011;179:9–22. doi: 10.1016/j.neuroscience.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Research. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–86. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–18. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–90. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Morales M. Glutamate neurons in the substantia nigra compacta and retrorubral field. Eur J Neurosci. 2013;38:3602–10. doi: 10.1111/ejn.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–43. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, et al. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun. 2016;7:13697. doi: 10.1038/ncomms13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Cheng AY, Lee TJ, Ghobadi CW, Schwartz ZM, et al. Inputs to the midbrain dopaminergic complex in the rat, with emphasis on extended amygdala-recipient sectors. J Comp Neurol. 2011;519:3159–88. doi: 10.1002/cne.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, et al. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci. 2015;18:386–92. doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]