Abstract
There is a growing consensus that social cognition and behavior emerge from interactions across distributed regions of the “social brain”. Researchers have traditionally focused their attention on functional response properties of these gray matter networks and neglected the vital role of white matter connections in establishing such networks and their functions. In this article, we conduct a comprehensive review of prior research on structural connectivity in social neuroscience and highlight the importance of this literature in clarifying brain mechanisms of social cognition. We pay particular attention to three key social processes: face processing, embodied cognition, and theory of mind, and their respective underlying neural networks. To fully identify and characterize the anatomical architecture of these networks, we further implement probabilistic tractography on a large sample of diffusion-weighted imaging data. The combination of an in-depth literature review and the empirical investigation gives us an unprecedented, well-defined landscape of white matter pathways underlying major social brain networks. Finally, we discuss current problems in the field, outline suggestions for best practice in diffusion-imaging data collection and analysis, and offer new directions for future research.
Keywords: white matter, social cognition, face processing, mirroring, mentalizing, diffusion imaging, tractography
1. Introduction
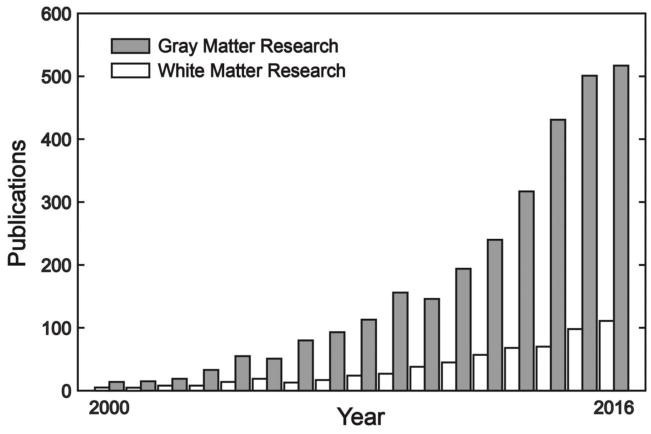
The history of social neuroscience shows an overwhelming emphasis on the functionality of gray matter, with a relative disregard of white matter (WM) (Fig. 1). However, few would deny the importance of WM for human cognition and behavior. It makes up half of the whole cerebral volume and plays a vital role in communications between cortical areas (Douglas Fields, 2008). Studies of human WM can provide insight into the organization of brain systems and the functions they perform (Wandell, 2016). Several WM structures have been well characterized for vision (e.g. optic tract, Rokem et al., 2017), sensorimotor (e.g. corticospinal tract, Ciccarelli et al., 2008), episodic memory (e.g. fornix, Thomas et al., 2011), and language (e.g. arcuate fasciculus, Dick et al., 2014; Friederici, 2015). However, current knowledge about the specific WM tracts underlying social cognition is limited.
Figure 1.
The proliferation of gray matter and white matter studies in social neuroscience. Both types of research have been rapidly increased over the past 15 year; however, the number of white matter studies per year is always less than 1/3 of the number of gray matter studies. The plotted data were extracted from https://www.ncbi.nlm.nih.gov/pubmed/ on 4/30/2017, using the search term “(social) AND (gray matter OR fMRI OR functional imaging)” for gray matter research (gray bars) and “(social) AND (white matter OR DTI OR diffusion imaging)” for white matter research (white bars).
The past few years have seen an increasing number of structural connectivity studies of social cognition, many of them propelled by the fast development of diffusion imaging techniques. Despite this, no dedicated review or meta-analysis exists in this field. Here we fill this void by providing a systematic review of existing studies (n=51) of WM related to three key social processes (face processing, embodied cognition, and theory of mind) and their respective underlying brain networks. In addition, to better understand the WM connectivity profile within each network, we carried out a matched empirical investigation on a large diffusion-weighted imaging dataset (n=103), using probabilistic tractography, to further define the tracts involved in social cognition. We then make conclusions based on the convergence of findings across the literature review and the empirical study. Finally, we outline current problems in the field, discuss emerging trends in methodology, and highlight new directions for future research. We begin by providing a brief overview of techniques used to measure WM in the human brain.
1.1 Techniques Used to Measure White Matter
There are three major tools currently used to measure WM connections in social neuroscience: diffusion-weighted MRI (dMRI), structural MRI (sMRI), and direct electrical stimulation (DES). In principle, dMRI is mainly used for characterizing macro- and microstructural properties of WM tracts, as well as for delineating long-range WM pathways between disparate brain regions. sMRI makes it possible to visualize and evaluate the macroscopic properties of local WM at high resolution, which is ideal for anatomical morphometry and detection of WM abnormalities and damage for clinical diagnosis. DES provides real-time causal investigations on the functional role of various WM tracts.
1.1.1 Diffusion-Weighted MRI (dMRI)
dMRI is the most popular and powerful technique for exploring WM anatomy and quantifying WM properties in the living human brain. The basic principle and concept behind this technique is that dMRI measures the random motion or diffusion of water molecules, which is restricted by tissue microstructure. When this microstructure is more organized, such as in WM, water diffusion is anisotropic, in that diffusion is less hindered parallel than perpendicular to WM fibers. Thus, by measuring the orientational dependence of water diffusion, dMRI infers the microstructure and properties of surrounding WM tissue (Jbabdi et al., 2015).
The simplest way to quantify the degree of anisotropic diffusion is the diffusion tensor model, which estimates the diffusion process by an ellipsoid, also known as tensor (hence the name origin of diffusion tensor imaging, DTI) (Soares et al., 2013). Several metrics can be derived from DTI in each voxel, including the mean diffusivity (MD), the degree of anisotropy (i.e. fractional anisotropy, FA) and two directional diffusivity measures (i.e. axial diffusivity, AD; radial diffusivity, RD). Variations in these metrics have been associated with alterations in the underlying WM microstructure. While FA is often used as a summary measure of local WM “integrity”, MD/AD/RD are useful indicators of WM maturation and dysfunction (Alexander et al., 2007). For example, MD is an inverse measure of the membrane density and sensitive to cellularity, edema and necrosis; AD has been reported to increase with brain maturation and decrease with axonal injury; RD is indicative of the degree of (de-)myelination and axonal diameters/density (Tromp, 2016). Four analysis strategies are typically applied to DTI metrics when researchers try to identify local WM differences across individuals or abnormalities in clinical populations: they can be compared locally in every voxel after registration to an anatomical atlas (voxel-based analysis), or averaged within a priori specific regions-of-interest (ROI-based analysis), or sampled along pathways after fiber tract reconstruction (tractography-based analysis), or analyzed based on the skeletonization of group registered FA maps (tract-based spatial statistics, TBSS) (Feldman et al., 2010; Soares et al., 2013; Travers et al., 2012). These strategies can also be applied to the investigation of anatomical correlates of numerous experimental and clinical conditions. An in-depth interpretation of DTI metrics (FA/MD/AD/RD) as well as the exploration of the relative strengths and weaknesses of each analysis approach is beyond the scope of this review but can be found in several review papers (Alexander et al., 2007; Feldman et al., 2010; Jones and Cercignani, 2010; Soares et al., 2013; Tromp, 2016).
Another advantage of dMRI techniques is the ability to visualize and characterize long-range WM pathways. To date, dMRI tractography is the only available tool to estimate the trajectories of WM fibers in vivo, by measuring the principal direction of water diffusivity on a voxel-by-voxel basis and piecing together information from contiguous voxels (Jbabdi et al., 2015). A long-range WM tract usually includes many fascicles and the computational estimate of a fascicle by tractography algorithms is called a streamline. There are two types of tractography algorithms: deterministic and probabilistic (Roberts et al., 2013; Rokem et al., 2017). The former is designed to trace a single path between two regions of interest, and thus is more suitable for identifying large WM fasciculi of the brain. Probabilistic tractography is more useful for quantitatively analyzing the connectivity between two regions based on the probability of a connection, taking into account that a single voxel might connect with more than one target voxel. Once dMRI tractography is completed for a particular WM pathway, one can inspect its macroscopic features (e.g. trajectory shape and volume), microstructural properties (e.g. FA/MD/AD/RD) and connectivity strength (e.g. probability or streamline count) (Soares et al., 2013). These approaches allow the researcher to compare equivalent WM pathways across individuals, even if the precise location of the tract varies (Feldman et al., 2010).
A fundamental limitation of dMRI is the indirect nature of its measurements. Since all estimates are based on water diffusivity, dMRI techniques provide only computational models of WM tissues with many theoretical assumptions about the underlying processes and structures. This makes dMRI error-prone and highly dependent on the data quality, the chosen diffusion model, and the analysis pipeline used (Jones et al., 2013). In addition, dMRI tractography does not provide information about the directionality (afferent or efferent) or functionality (inhibitory or excitatory) of a WM tract (Jones, 2010) and can be inaccurate when describing WM microstructure in regions with crossing/branching fibers or complex spatial arrangement (e.g. superficial WM fiber systems) (Feldman et al., 2010; Reveley et al., 2015).
1.1.2 Structural MRI (sMRI)
Conventional MRI techniques can also provide useful qualitative and quantitative measurements of WM structures in the brain. Rather than measuring water diffusion rate, sMRI collects MR signals (T1 or T2 relaxation) that vary across tissue types, since gray matter contains more cell bodies while WM is primarily composed of myelinated axons and glial cells. sMRI with morphometric analysis is used to measure the shape, size, myelination, and integrity of WM structures, which is very helpful for quantitative assessments of local WM changes in patient-control studies. One limitation of sMRI is that this technique only allows for voxel-level analysis, which restricts investigations to local WM characteristics. In addition, sMRI provides no information about microstructural properties of white matter (unless using very sophisticated modeling such as multi-compartment models) (Jbabdi et al., 2015). A recent trend is to use more quantitative MR sequence to directly measure WM tissue properties (e.g. magnetization transfer, T1/T2 relaxometry) (Alexander et al., 2011) and complement sMRI with dMRI to capture a comprehensive picture of WM maturation and integrity (Erus et al., 2015).
1.1.3 Direct Electrical Stimulation (DES)
dMRI and sMRI primarily use correlation analyses to reveal the relationship between WM tracts and behavior. Because correlation is not causation, structure-function relationships must be validated with techniques possessing stronger inferential power. DES is performed on patients during awake neurosurgery; it provides a rare and unique opportunity to gain insight into the function of various WM tracts (Duffau, 2015). In this technique, the neurosurgeon applies electrical stimulation to a well-defined WM tract, thus creating a “temporary lesion” by disrupting the function of that WM tract and consequently changes corresponding behavior. This technique provides real-time structure-function mapping with high spatial resolution, and has the strong advantages in scrutinizing the exact role (i.e. critical versus participatory) of a particular WM tract in specific mental process.
Like other techniques, DES has several inherent problems. First, because the technique is invasive (e.g. partial resection is required to access the WM beyond the cortex) and only restricted to special clinical groups (e.g. patients with gliomas), the sample sizes in DES studies are typically small. Second, some patients (e.g. with low-grade glioma) may have exhibited abnormal WM profiles for a prolonged period of time, thus confounding DES results with neuroplasticity and compensation effects. Third, the range of behavioral assessment is often limited in DES research, due to limited time available during surgery (Duffau, 2015).
1.2 Social Cognition and Brain Networks
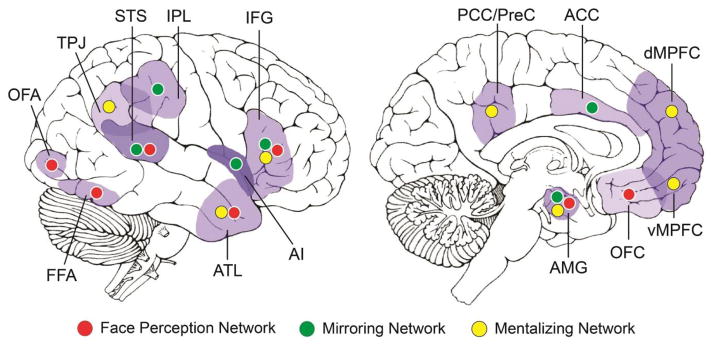
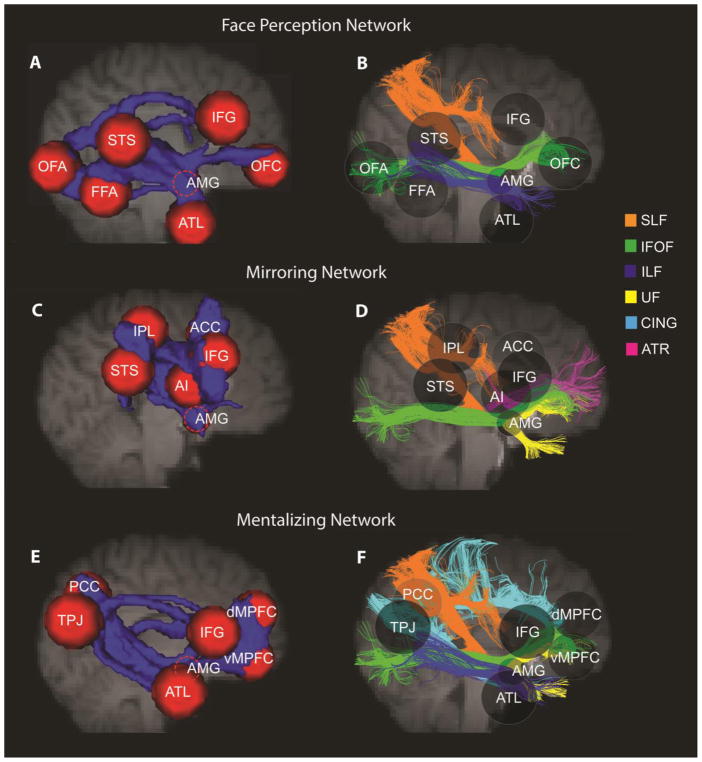
An extensive literature in social neuroscience suggests that there are at least three large-scale neural networks/circuits underlying social processes and interactions (Cross et al., 2016; Kennedy and Adolphs, 2012; Yang et al., 2015): the “face perception network” (Duchaine and Yovel, 2015; Gobbini and Haxby, 2007; Haxby et al., 2000), the “mirroring network” (Iacoboni, 2009a; Molenberghs et al., 2012; Rizzolatti and Craighero, 2004) and the “mentalizing network” (Mar, 2011; Schurz et al., 2014) (see Fig. 2). We briefly describe each network before turning to the WM review.
Figure 2.
Three major networks in the social brain. ACC, anterior cingulate cortex; AI, anterior insula; AMG, amygdala; ATL, anterior temporal lobe; dMPFC, dorsomedial prefrontal cortex; FFA, fusiform face area; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; OFC, orbitofrontal cortex; OFA, occipital face area; PCC/PreC, posterior cingulate cortex/precuneus; STS, superior temporal sulcus; TPJ, temporoparietal junction; vMPFC, ventromedial prefrontal cortex.
1.2.1 Face Processing and Face Perception Network
Social interactions often start with recognizing conspecific’s faces. This ability is arguably the most developed social skill in humans. Converging empirical evidence suggests that face perception is mediated by a widely distributed network of face-selective areas, each engaging in different aspects of face processing (Duchaine and Yovel, 2015; Gobbini and Haxby, 2007; Haxby et al., 2000). For example, posterior regions, such as the occipital face area (OFA), process low-level visual features and analyze facial parts (Pitcher et al., 2011); the fusiform face area (FFA) is involved in processing invariant facial features, such as identity and gender (Haxby et al., 2000), whereas the posterior superior temporal sulcus (STS) is more sensitive to changeable features, such as facial expression and lip movement (Gobbini and Haxby, 2007). Anterior regions, such as the amygdala (AMG), subserve emotional aspects of face representations (Mende-siedlecki et al., 2013); the anterior temporal lobe (ATL) stores conceptual knowledge related to faces, including names and biographical information (Collins & Olson, 2014; Wang et al., 2017); the inferior frontal gyrus (IFG) processes the semantic aspects of faces as well as gaze directions (Chan and Downing, 2011; Ishai, 2008), and the orbitofrontal cortex (OFC) evaluates rewarding aspects of faces, like facial attractiveness and trustworthiness (Mende-siedlecki et al., 2013; Troiani et al., 2016).
1.2.2 Embodied Cognition and Mirroring Network
Social interactions also require individuals to rapidly and effortlessly grasp others’ intentions and emotions, and respond accordingly and appropriately. These social reciprocity skills are often linked to the so-called “mirroring network”, which mediates our capacity to share the meaning of actions and emotions through the embodied simulation mechanism (Gallese, 2007). By simulating observed action (or emotions) with one’s own motor (or affective) system, the mirroring mechanism provides the basis for action understanding (Rizzolatti et al., 2014; Rizzolatti and Craighero, 2004; Rizzolatti and Sinigaglia, 2010), imitation (Caspers et al., 2010; Iacoboni, 2009b), emotional recognition (Bastiaansen et al., 2009; Niedenthal et al., 2010; van der Gaag et al., 2007; Wood et al., 2016) and empathy (Bernhardt and Singer, 2012; Corradini and Antonietti, 2013; Gonzalez-Liencres et al., 2013; Iacoboni, 2009a; Shamay-Tsoory, 2011). In humans, the putative mirroring network is formed by a collection of areas (Bonini, 2017; Molenberghs et al., 2012; Mukamel et al., 2010), including the inferior frontal gyrus (IFG, which represents motor plans of actions; Rizzolatti et al., 2014), the inferior parietal lobule (IPL, which represents abstract action goal; Hamilton & Grafton, 2006), the posterior STS (which is theorized to serve as the sensory input of the network; Rizzolatti & Craighero, 2004), the anterior cingulate cortex (ACC, empathy for pain; Bernhardt & Singer, 2012), the anterior insula (AI, empathy for disgust; Bernhardt & Singer, 2012) and the amygdala (AMG, empathy for fear; Bastiaansen et al., 2009).
1.2.3 Theory of Mind and Mentalizing Network
Finally, the capacity to make accurate inferences about the mental states of other people (e.g. their thoughts, needs, desires, and beliefs) is important for predicting the behavior of others and for facilitating social interactions (Blakemore, 2008). This particular skill and its associated mental processes have often been referred to as “mentalizing” or “theory of mind” (ToM). A large number of neuroimaging and lesion studies have delineated an extensive brain network for mentalizing abilities (Fig. 2), mainly including the dorsal and ventral medial prefrontal cortex (dMPFC and vMPFC), the temporo-parietal junction (TPJ), the posterior cingulate cortex/precuneus (PCC/PreC), the ATL, the IFG and the AMG (Mar, 2011; Molenberghs et al., 2016; Schurz et al., 2014). The specific function of each region has not yet been clarified, but some (e.g. MPFC and TPJ) are consistently engaged irrespective of the mental state contents and the task modalities (Schurz et al., 2014), whereas the involvement of other regions seems to be more task-dependent (Carrington and Bailey, 2009; Molenberghs et al., 2016).
2. A Systematic Literature Review on White Matter in Social Neuroscience
2.1 Study Selection
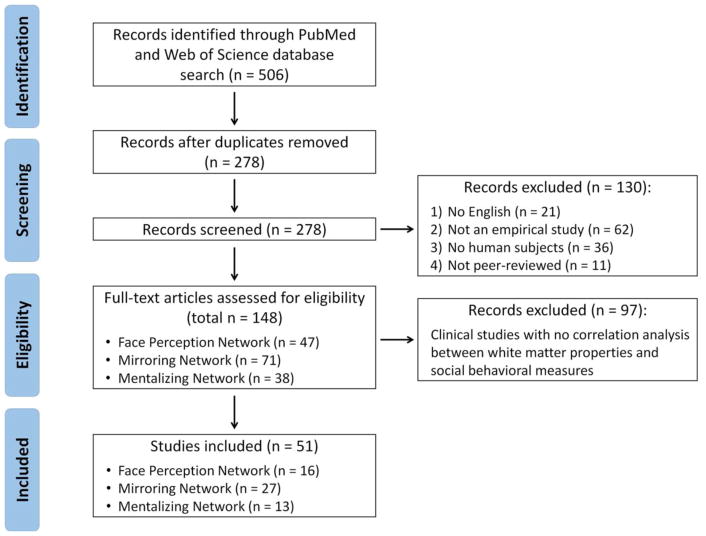
A step-wise procedure was used to identify white matter research of social cognition in the past decade. First, we used the following search terms on Pubmed and Web of Science on 4/30/2017: (“face” OR “embodied” OR “mirroring” OR “action perception” OR “action execution” OR “imitation” OR “empathy” OR “emotion recognition” OR “theory of mind” OR “mentalizing”) AND (“white matter” OR “tract” OR “pathway” OR “structural connectivity” OR “anatomical connectivity”) AND (“imaging” OR “MRI” OR “diffusion” OR “dMRI” OR “DTI” OR “tractography” OR “structural MRI” OR “morphometry” OR “direct electrical stimulation” OR “brain stimulation”). A total of 506 publications were identified (Fig. 3). After removing 228 duplicates between two databases, articles were assessed by reviewing their titles and abstracts for matching the following inclusion criteria: (1) written in the English language; (2) reported empirical results; (3) included human subjects; and (4) published in a peer-reviewed journal. It is important to note that this initial screen resulted in numerous clinical studies on social disorders that revealed abnormalities in WM structures in a patient groups compared to a healthy cohort. However, making a simple comparison between patients with a social disorder and a healthy group is not enough to establish specific associations between WM and social cognition, because the observed differences could be caused by patients’ non-social symptoms (e.g. repetitive movements in autism; Travers et al., 2012). Therefore, we excluded studies with simple patient-control comparisons and only included papers with correlation analyses between WM and social cognition measures. This yielded a final sample of 51 studies on 3745 subjects (see Fig. 3 and Tables 1–3 for details).
Figure 3.
PRISMA flow diagram of study selection procedure.
Table 1.
Summary of 16 studies linking white matter to the face perception network.
| Study | Modality | Gradient Directions/ B-value |
Analysis Methods |
Participants (N) |
Mean Age |
Social Cognition Measurements |
Associated Tracts |
Findings |
|---|---|---|---|---|---|---|---|---|
| Ethofer (2011) | DTI fMRI |
30/1000 | Probabilistic Tractography | healthy adults (N=22) | 26 | gaze perception task (fMRI) | right SLF | The posterior STS and AI are anatomically connected by the SLF and their functional connectivity is modulated by gaze shifts. |
| Ethofer (2013) | DTI fMRI |
30/1000 | Probabilistic Tractography | healthy adults (N=23) | 23 | face localizer (fMRI) | right SLF | Face-selective STS anatomically connects with anterior face areas (IFG or OFC) via the dorsal SLF. |
| Gschwind (2012) | DTI fMRI |
30/1000 | Probabilistic Tractography | healthy adults (N=22) | 28 | face localizer (fMRI) | right ILF, right IFOF, right SLF | The study reveals the WM connectivity between face-responsive regions (OFA, FFA, AMY and STS). Strong WM connectivity is found for EVC-OFA, OFA-FFA and EVC-AMY, all via ILF (with right hemisphere predominance). AMY has stronger connections with EVC than OFA/FFA. STS is not connected to FFA/OFA, but rather lined to more frontal regions via AF/SLF |
| Marstaller (2016) | DTI fMRI |
64/3000 | Probabilistic Tractography | healthy adults (N=28) | 26 | face expression matching task | Bilateral ILF | Facial expression recognition skills can be predicted by the structural connectivity between FFA and AMG. People who are faster at discriminating threat-related expressions showed higher FA in this pathway (via ILF) |
| Iidaka (2012) | DTI fMRI |
64/3000 | Probabilistic Tractography | healthy adults (N=30) | 22 | face localizer (fMRI); Autism quotient (AQ) | right ILF | The volume of STS-AMG connectivity is positively correlated with the AQ score in the healthy sample. |
| Pyles (2013) | dMRI-NT fMRI |
257/7000 | Deterministic Tractography | healthy adults (N=5) | 28 | face localizer (fMRI) | right ILF, right IFOF | The study reveals the WM connectivity between face-responsive regions (OFA, FFA, ATL and STS). Strong WM connectivity were found for OFA-FFA, FFA-ATL and OFA-ATL, all via ILF and IFOF (with right hemisphere predominance). No connection was found for STS-OFA, STS-FFA and STS-ATL |
| Saygin (2012) | DTI fMRI |
60/700 30/700 |
Probabilistic Tractography | healthy adults (N=23) | 28 | face localizer (fMRI) | N.A. | Anatomical connectivity patterns predict face selectivity in the FFA. |
| Scherf (2014) | DTI fMRI |
6/850 | Deterministic Tractography | healthy subjects aged 6–23 years (N=50) | 16 | face localizer (fMRI) | right ILF | The volume of FFA activation is positively correlated with the volume of right ILF, but negatively correlated with the MD in right ILF. |
| Tavor (2014) | DTI | 19/1000 | ROI-based, Deterministic Tractography | healthy adults (N=22) | 25 | face memory test | right ILF | Face memory accuracy is negatively correlated with FA (and positively correlated with RD) in the anterior portion of right ILF. |
| Unger (2016) | DTI | 64/1000 | Deterministic Tractography | healthy adults (N=28) | 22 | Cambridge task; Philadelphia face matching task; | right ILF, bilateral IFOF | Face memory accuracy is negative correlated with FA in right ILF and right IFOF, but positively correlated with FA in the left IFOF |
| Gomez (2015) | DTI fMRI |
30/900 96/2000 |
ROI-based, Probabilistic Tractography | developmental prosopagnosia patients (DP N=8; control N=18) | P=34 C=26 |
face localizer (fMRI); Benton test; Cambridge task; | local right FFA fibers (ILF is not involved) | Healthy controls show a positive correlation between FA in local WM of right FFA and face recognition and memory performance, whereas DP patients show a negative correlation. DP patients also exhibit lower MD values in local WM of FFA in both hemispheres. |
| Grossi (2014) | DTI | 32/1000 | Deterministic Tractography | progressive prosopagnosia patients (PP N=1; control N=7) | P=65 C=68 |
Benton test; Celebrity face recognition test; | right ILF | PP patients have a severe reduction of fibers (number of streamlines) in right ILF versus left ILF, while IFOFs are relatively symmetric. They also have higher MD in right ILF than controls. |
| Song (2015) | DTI fMRI |
61/1000 | Voxel-based, Deterministic Tractography, Probabilistic Tractography | developmental prosopagnosia patients (DP N=16; control N=16) | P=31 C=30 |
face localizer (fMRI); Cambridge task; celebrity face recognition test | local FFA fibers, bilaterally | DP patients show WM deficits only in local FFA (lower FA values), not in long-range tracts (IFOF and ILF). For both patients and controls, FA and MD in local FFA WM are correlated with face recognition performance. |
| Thomas (2008) | DTI | 6/850 | Deterministic Tractography | healthy adults across lifespan (N=28) | 42 | face matching task | right IFOF | A decrease in FA and volume in right IFOF by aging process is associated with a decrease in face discrimination accuracy |
| Thomas (2009) | DTI | 6/850 | Voxel-based, Deterministic Tractography | developmental prosopagnosia patients (DP N=6; control N=17) | P=58 C=56 |
celebrity face recognition test | right ILF, right IFOF | DP patients have lower FA in bilateral ILF and IFOF, compared to controls. For both groups, the FA and number of streamline in right ILF and IFOF are positively correlated with face recognition performance. |
| Valdés-Sosa (2011) | DTI sMRI fMRI |
12/1200 | Deterministic Tractography, Probabilistic Tractography | acquired prosopagnosia patients (AP N=1; control N=10) | P=73 C=70 |
neuropsychological tests on prosopagnosia | right ILF, right IFOF | This study reported an AP case who had disrupted FFA and ILF, but preserved anterior face-selective areas as well as covert face recognition skills. It suggests covert face skills might be subserved by the preserved IFOF connecting OFA to anterior face-selective areas. |
dMRI-NT: diffusion-weighted MRI with non-tensor modeling; C: controls; EVC: early visual cortices; P: patients; Other acronyms can be referred to from the abbreviations section in the main text.
Table 3.
Summary of 13 studies linking white matter to the mentalizing network.
| Study | Modality | Gradient Directions/ B-value |
Analysis Methods |
Participants (N) | Mean Age |
Social Cognition Measurements |
Associated Tracts |
Findings |
|---|---|---|---|---|---|---|---|---|
| Anderson (2015) | dMRI-NT | 30/1000 | Probabilistic Tractography | healthy young children (N=49) | N.A. | reading the mind in the eyes (RME) | left UF | FA in left UF is positively correlated with ToM task in 4 year olds, but not in 6 year olds. |
| Grosse Wiesmann (2017) | DTI | 60/1000 | Probabilistic Tractography TBSS | healthy young children (N=43) | N.A. | false belief task and implicit ToM task | bilateral SLF/AF and right IFOF | The emergence of explicit ToM ability between 3 and 4 years old is associated with FA increase in local WM of right TPJ, left MTG, right vMPFC and right PreC, as well as an increase in streamline density in bilateral SLF/AF and right IFOF. |
| Cabinio (2015) | DTI | 12/900 | TBSS | healthy adults (N=36) | 50 | reading the mind in the eyes (RME) | Bilateral UF, right SLF, IFOF, ILF, CC. | Age-related decline in ToM ability is associated with the decreased FA in bilateral UF, right SLF, IFOF, ILF, and genu of CC. |
| Charlton (2009) | DTI | 6/1000 | ROI-based | healthy adults (N=106) | 69 | strange stories test | N.A | For normal aging adults, ToM ability is positively correlated with the whole-brain mean FA and negatively correlated with the whole-brain mean MD. |
| Jalbrzikowski (2014) | DTI | 64/1000 | ROI-based, TBSS | patients with velocardiofacial syndrome (VCFS N=36; control N=29) | P=16 C=16 |
the awareness of social inference test | left IFOF and UF; Bilateral SLF and ILF | For both VCFS patients and controls, AD in the left IFOF and UF is positively correlated with ToM ability. For healthy controls only, AD in bilateral SLF and ILF is positively correlated with ToM ability. |
| Kana (2014) | DTI | 12/1000 | TBSS | patients with high-functioning autism (ASD N=15; control N=15) | P=21 C=22 |
comic strip vignettes | right SLF/AF and ILF | During the ToM task, ASD patients have lower TPJ activation and weaker functional connectivity between TPJ and premotor areas. DTI shows reduced FA in a site of right SLF/AF and ILF near TPJ in ASD patients. |
| Levin (2011) | DTI | 15/860 | Deterministic Tractography | children with traumatic brain injury (TBI N=49; control N=39) | P=13 C=12 |
social animations | local WM in mPFC and left IFG, left Cingulum | For both TBI patients and controls, ToM scores are positively correlated with FA in local WM of mPFC, left IFG, and left cingulum. |
| Scheibel (2011) | DTI fMRI |
32/1000 | ROI-based, Deterministic Tractography | adolescents with traumatic brain injury (TBI N=9; control N=9) patients with | P=16 C=17 |
social animations | bilateral UF and ILF; CC | For TBI adolescents, FA in the social brain WM (i.e. genu of CC, bilateral UF, bilateral ILF) is negatively correlated with ToM-related brain activations in mPFC and PCC. |
| Mike (2013) | sMRI | N.A. | Voxel-based | multiple sclerosis (MS N=49; control N=24) | P=40 C=37 |
reading the mind in the eyes (RME) | CC | For MS patients, ToM performance is negatively correlated with lesion volume in splenium of CC. |
| Herbet (2014) | sMRI | N.A. | Voxel-based | patients with surgical resection for diffuse low-grade glioma (DLGG N=93; control N=60) | 38 | reading the mind in the eyes (RME), comic strip vignettes | right SLF/AF and cingulum | For DLGG patients, damage to right SLF/AF is associated with impaired perceptual-based ToM performance (RME task) and damage to the right cingulum is associated with impaired inference-based mentalizing (comic strip vignettes) |
| Herbet (2015)_Brain Struct Funct | DES | N.A. | N.A. | patients with surgical resection for diffuse low-grade glioma (DLGG N=5) patients with | 38 | reading the mind in the eyes (RME) empathy | right SLF/AF | For DLGG patients, direct stimulation to WM underneath IFG (e.g. right SLF/AF) can disrupt perceptual-based ToM (RME task). |
| Herbet (2015)_Neuro psychologia | sMRI | N.A. | Voxel-based, ROI-based | surgical resection for diffuse low-grade glioma (DLGG N=107) | 41 | quotient (EQ)--cognitive empathy subscores | left Cingulum | For DLGG patients, disconnection of left cingulum is associated with low cognitive empathy. |
| Yordanova (2017) | DES | N.A. | N.A. | patients with surgical resection for diffuse low-grade glioma (DLGG N=27) | 39 | reading the mind in the eyes (RME) | right IFOF, SLF/AF, and cingulum | For DLGG patients, temporary disconnection of WM tracts, such as right IFOF, SLF/AF, and cingulum impairs perceptual-based ToM (RME task). |
C: controls; DLGG: diffuse low-grade glioma; dMRI-NT: diffusion-weighted MRI with non-tensor modeling; P: patients; RME: reading the mind in the eyes; TBI: traumatic brain injury; VCFS: velocardiofacial syndrome. Other acronyms can be referred to from the abbreviations section in the main text.
2.2 Methodological Summary
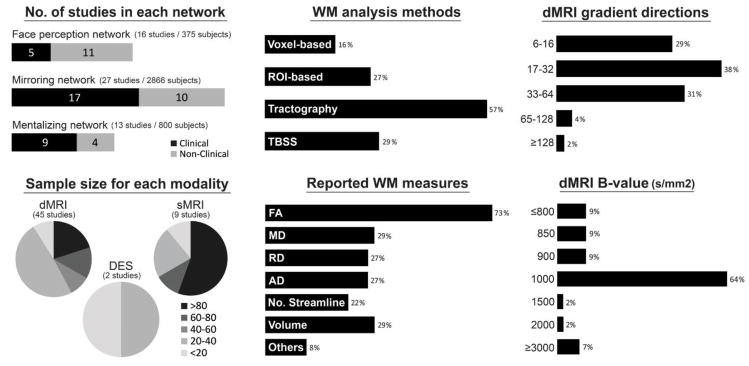
Fig. 4 summarizes a couple of key features in the literature. As can be seen, the sample size varies across studies (ranging from 5 to 766) and depends on technique modality. In addition, it is clear that the most common data analysis method was tractography-based and the most common dMRI measure was FA, although many studies used overlapping measures and methods. Fig. 4 also illustrates the frequency of two critical data acquisition parameters used in dMRI research: the “gradient directions” and the “b-value”. The number of diffusion-encoding gradient directions defines the number of orientations at which diffusion signals are sampled. As the number increases, more diffusion-weighted images are used for the calculation of the diffusion tensor model, resulting in more accurate estimation of microstructural indices related to the tensor. Although more directions is better, this comes at a cost as a large number of gradient directions elongates the scan time. The b-value represents the degree of diffusion weighting and determines the strength and duration of the diffusion gradients. The ability to delineate WM fasciculi oriented in different directions improves as the b-value increases, but higher b-values (e.g. >3000) come at a cost of lower signal-to-noise ratio (Jones et al., 2013). As we can see from Fig. 4, most dMRI studies in the literature used 17–32 gradient directions with a b-value of 1000 s/mm2.
Figure 4.
Key features of the 51 empirical studies surveyed in the present paper, including number of clinical/non-clinical studies for each social brain network, the sample size for each technique modality, how WM measures were analyzed and reported in the studies, and the diffusion data acquisition parameters (the gradient directions and b-values). Note: Percentage might add up to more than 100% because of studies often using more than one type of analysis method, measure, or acquisition protocol. AD, axial diffusivity; DES, direct electrical stimulation; dMRI, diffusion magnetic resonance imaging; FA, fractional anisotropy; MD, mean diffusivity; No. Streamline, number of streamline; RD, radial diffusivity; sMRI, structural magnetic resonance; TBSS, tract-based spatial statistics; WM, white matter.
We found that more than half of the studies, particularly of the mirroring and mentalizing network, are based on clinical populations. They included major psychiatric and neurological disorders characterized by prominent social impairments, such as autism spectrum disorder, behavioral-variant frontotemporal dementia, and prosopagnosia, as well as those with secondary impairments in social cognition, such as schizophrenia, amyotrophic lateral sclerosis, mild cognitive impairment, traumatic brain injury, Parkinson’s disease, velocardiofacial syndrome, and multiple sclerosis. In terms of social cognitive measurements, the whole literature has employed several behavioral paradigms to probe each social function (see Table 1–3). For example, face processing skills were measured by celebrity face recognition tasks, face matching tasks (e.g. Benton tests, Philadelphia battery), and face memory tasks (e.g. Cambridge tests); empathy was assessed by the “empathy quotient” and “interpersonal reactivity index”; mentalizing abilities were evaluated by “false belief” stories, cartoon animations, comic strip vignettes, and the “reading the mind in the eyes” task. Such a wide variety of seemingly disparate disorders as well as diverse behavioral paradigms provides an excellent opportunity for exploring the relationship between WM tracts and social functions.
2.3 Major Findings
2.3.1 Face Perception Network
Two WM tracts are repeatedly reported in the face perception literature: the inferior longitudinal fasciculus (ILF) and the inferior fronto-occipital fasciculus (IFOF) (see Table 1). They are the main associative bundles that project through occipito-temporal cortex, connecting the occipital lobe to the temporal, and frontal lobes, respectively (Rokem et al., 2017). The ILF is a monosynaptic pathway connecting ventral extrastriate regions, and in some cases portions of the inferior parietal lobe, to the anterior temporal lobe, the hippocampus, and the amygdala (Catani et al., 2003). The IFOF begins in the ventral occipital cortex, continues medially through the temporal cortex dorsal to the uncinate fasciculus, and terminates in the inferior frontal, medial prefrontal, and orbitofrontal cortex (Catani and Thiebaut de Schotten, 2008). dMRI tractography combined with functional face localizer confirmed that the ILF connects multiple pairs of face perception network nodes including the OFA-FFA, OFA-ATL, FFA-ATL, FFA-AMG and STS-AMG (Gschwind et al., 2012; Iidaka et al., 2012; Pyles et al., 2013), while the IFOF connects the OFA-IFG (Valdés-Sosa et al., 2011). Converging evidence, described below, indicates that these two tracts are critically important for face processing.
First, the early development of the right ILF is associated with the emergent functional properties of the face perception network. Using both DTI and fMRI, Scherf et al., (2014) investigated whether developmental differences in the structural properties of bilateral ILF were related to developmental differences in the functional characteristics of the face-processing regions connected by ILF (e.g. OFA, FFA). Across children, adolescents, and adults (ages 6–23 years), they found bilateral ILF exhibited an age-related increase in volume, and those individuals with larger right ILF volumes also exhibited larger right FFA volumes. This suggests a tight relationship between the structural refinements of right ILF and functional selectivity in the developing face perception network.
Similarly, age-related declines in face perception skills have been linked to degeneration of the right IFOF. Thomas et al., (2008) used DTI to scan subjects across a wide age range (18–86 years) and also measured individual performance on face perception tasks. They observed that the right IFOF was the only tract that decreased in volume as a function of age, and subjects with smaller volumes and lower FA values in the right IFOF exhibited worse performance on the face matching task. This evidence indicates that the right IFOF is vulnerable to the aging process, and age-related decreases in the structural properties of this tract might be responsible for decrements in face processing abilities in aging adults.
Moreover, disruptions in ILF and IFOF are associated with face blindness in prosopagnosia. Developmental prosopagnosia (DP) is a social disorder characterized by a lifelong impairment in face recognition despite normal sensory vision and intelligence. Interestingly, DP patients exhibit normal patterns of fMRI activation in response to faces in posterior parts of the face perception network (e.g. OFA, FFA; Behrmann et al., 2005), but reduced activation in anterior nodes (e.g. ATL; Avidan et al., 2014). Based on this observation, it had been suggested that the impairments in DP might arise, not from a dysfunction of cortical parts of the face perception network, but from a failure to propagate signals from the intact posterior components to the compromised anterior components of the network. As the two major tracts that project through the posterior to anterior regions of the face network, the ILF and IFOF are top candidates for testing. As such, Thomas et al., (2009) scanned a group of DP patients and measured the severity of their face recognition deficits. Relative to the control group, the integrity in the right ILF and IFOF in DP patients was remarkably compromised (i.e. lower FA and volume) and the extent of this compromise was correlated with individual face perception deficits. This finding was interpreted as evidence for DP as a “disconnection syndrome”, i.e. face blindness occurs because intact posterior face processing regions are unable to communicate via the ILF and IFOF with more anterior regions. Similar findings were also observed in other types of prosopagnosia where patients exhibited severe fiber reductions in the right ILF (Grossi et al., 2014; Valdés-Sosa et al., 2011).
Other face processing abilities are also related to the ILF and IFOF, although the underlying mechanisms are unclear. Unger et al., (2016) showed that face memory accuracy was negatively correlated with FA in the right ILF and IFOF, but positively correlated with FA in the left IFOF. Tavor et al., (2014) reported similar findings and further indicated that the anterior part of right ILF explained the most inter-individual variation of face memory performance. Moreover, individual differences in processing facial communicative signals can be predicted by the structural connectivity between face-selective areas (e.g. FFA or STS) and amygdala (AMG) via the ILF. People who are better at discerning threat-related facial expressions showed higher FA in the FFA-AMG connectivity (Marstaller et al., 2016), and people who have better social communication skills had larger volumes of the STS-AMG pathway (Iidaka et al., 2012).
Aside from the ILF and IFOF, the superior longitudinal fasciculus (SLF) seems to be a third WM tract that subserves face processing. Anatomically, the SLF connects superior-posterior face-selective regions, such as the STS, with anterior-inferior face-selective regions (IFG and OFC) (Ethofer et al., 2013; Gschwind et al., 2012). Functionally, the SLF has been associated with gaze processing (Ethofer et al., 2011) and face-voice integration (Ethofer et al., 2013).
It is important to note that almost every reported WM correlate of face processing skills is in the right hemisphere. This lateralization of WM function is consistent with the significant right-hemisphere predominance in the face perception literature: fMRI studies typically show larger face activations in the right, relative to the left hemisphere, and behavioral studies show better performance for faces presented in the left than the right visual fields (Tavor et al., 2014). Some studies speculate that the left ILF is more specialized for face tasks requiring access to language, such as face naming, while the right ILF may have functions more aligned with strictly visuospatial functions, such as face discrimination (Unger et al., 2016).
2.3.2 Mirroring Network
Converging evidence suggests that the superior longitudinal fasciculus (SLF) is the most important WM tract for embodied social cognition (see Table 2). The SLF is a large association bundle composed of medial and lateral fibers connecting the frontal, parietal, and temporal lobes (Kamali et al., 2014). This WM tract has a known role in language and spatial attention (Merchant, 2011) and has recently been identified to be the main fiber pathway for the fronto-parietal mirroring network (Hamzei et al., 2016; Hecht et al., 2013; Iacoboni and Dapretto, 2006; Parlatini et al., 2017). Several studies indicate that the SLF is functionally associated with imitation, empathy, and emotion recognition abilities. For example, Hecht et al., (2013) found that the evolved imitation skills across species (macaques, chimpanzees, and humans) can be explained by increased SLF connections supporting the fronto-parietal mirroring network. The empathy quotient is positively correlated with FA values in the SLF bilaterally, most extensively in the right SLF (Chou et al., 2011; Parkinson and Wheatley, 2014; Takeuchi et al., 2013). The SLF is also associated with individual’s emotion recognition ability, regardless of whether the task is face-based (Crespi et al., 2014; Philippi et al., 2009; Radoeva et al., 2012), story-based (Crespi et al., 2016), or voice-based (Ethofer et al., 2013, 2012). When the right SLF is disrupted by brain lesion (Philippi et al., 2009) or psychiatric disorders (Crespi et al., 2016, 2014; Radoeva et al., 2012; Saito et al., 2017), the integrity of SLF is also positively correlated with patients’ emotion recognition skills.
Table 2.
Summary of 27 studies linking white matter to the mirroring network.
| Study | Modality | Gradient Directions/ B-value |
Analysis Methods |
Participants (N) | Mean Age |
Social Cognition Measurements |
Associated Tracts |
Findings |
|---|---|---|---|---|---|---|---|---|
| Imitation | ||||||||
|
| ||||||||
| Hamzei (2016) | DTI fMRI |
61/1000 | Probabilistic Tractography | healthy adults (N=116) | 26 | imitation tasks (fMRI) | left SLF/AF, left extreme capsule | There are two anatomical pathways between frontal and parietal mirroring areas: the SLF III and AF (the dorsal stream) and the extreme capsule (the ventral stream) |
| Hecht (2013) | DTI | 60/1000 | Probabilistic Tractography | healthy adults (N=30) | 20 | N.A. | SLF, extreme capsule, ILF, all bilateral | In humans, SLF III connects IFG and IPL; SLF III, AF and extreme capsule connect IFG and STS; ILF connects IPL and STS. |
| Fishman (2015) | DTI fMRI |
61/1000 | Probabilistic Tractography | Children with autism (ASD N=35; control N=35) | P=14 C=13 |
N.A. | left SLF | For ASD children, reduced FA and increased MD are found in WM tracts within the mirroring network (i.e. SLF) and this reduced WM integrity is correlated with individual ASD symptom severity. |
|
| ||||||||
| Emotion Recognition | ||||||||
|
| ||||||||
| Ethofer (2012) | DTI fMRI |
30/1000 | Probabilistic Tractography | healthy adults (N=22) | 26 | passively listen to emotional voices (fMRI) | bilateral SLF and extreme capsule | Vocal emotion recognition is subserved by bilateral SLF and extreme capsule connecting IPL and IFG. |
| Ethofer (2013) | DTI fMRI |
30/1000 | Probabilistic Tractography | healthy adults (N=23) | 23 | emotional recognition task (fMRI) | right SLF | Face/voice emotion recognition engages posterior STS and IFG, and these two regions are connected by dorsal SLF. |
| Taddei (2012) | DTI EEG |
35/1000 | ROI-based, Deterministic Tractography, TBSS | healthy children (N=20) | 15 | passive facial emotion observation (EEG) | left UF, bilateral ILF and IFOF | N400 amplitudes in response to angry faces are negatively correlated with FA in left UF and bilateral ILF and IFOF. |
| Unger (2016) | DTI | 64/1000 | Deterministic Tractography | healthy adults (N=28) | 22 | facial emotion recognition task | right ILF, bilateral IFOF | The AD in right ILF and the FA in bilateral IFOF are negatively correlated with face emotion recognition performance. |
| Baggio (2012) | DTI | 30/1000 | ROI-based, TBSS | patients with Parkinson’s disease (PD N=39; control N=23) | P=63 C=61 |
Ekman facial emotion recognition | right IFOF, left IFOF/ILF, CC | For PD patients, FA in three WM tracts are positively correlated with the sadness identification performance: frontal portion of right IFOF (near IFG), left ILF/IFOF (near STS) and CC (right forceps minor). |
| Crespi (2014) | DTI | 32/1000 | ROI-based, TBSS | patients with amyotrophic lateral sclerosis (ALS N=22; control N=55) | P=60 C=62 |
Ekman facial emotion recognition | right IFOF, right ILF | For ALS patients, the accuracy of facial emotion recognition (especially negative emotions) shows a positive correlation with FA values of both the right ILF and IFOF. |
| Crespi (2016) | DTI | 32/1000 | TBSS | patients with amyotrophic lateral sclerosis (ALS N=13; control N=14) | P=59 C=56 |
story-based emotion attribution task | right IFOF and UF, left SLF and CC | For ALS patients, emotion attribution scores are positively correlated with FA in right IFOF and UF, left SLF, CC (genu and forceps minor). |
| Downey (2015) | DTI | 64/1000 | TBSS | patients with behavioral variant frontotemporal dementia (bvFTD N=44; control N=37) | P=64 C=63 |
the awareness of social inference test | right ATR and UF, fornix | For bvFTD patients, emotion identification score is negatively correlated with AD, RD, and MD in fornix and right ATR; sarcasm identification score is negatively correlated with AD, RD, and MD in right UF. |
| Fujie (2008) | DTI | 12/700 | Deterministic Tractography | patients with mild cognitive impairment (MCI N=16; control N=16) | P=71 C=71 |
facial emotion recognition task | left UF | For MCI patients, FA values of the left UF are positively correlated with the performance of face emotional recognition for sadness, fear, and surprise. |
| Genova (2015) | DTI | 12/1000 | TBSS | patients with traumatic brain injury (TBI N=42; control N=23) | P=35 C=39 |
facial emotion identification test | right ILF and IFOF | For TBI patients, the face emotion discrimination score is positively correlated with FA in right IFOF, but negatively correlated with AD, MD, and RD in right ILF. |
| Philippi (2009) | sMRI | N.A. | Voxel-based, ROI-based | patients with brain-damage (N=103; control N=18) | P=52 C=56 |
facial emotion recognition task | right ILF, IFOF and SLF | Damage to the right IFOF, ILF and SLF predicts facial emotion recognition impairment. Damage to right IFOF specifically impairs fear recognition |
| Jalbrzikowski (2014) | DTI | 64/1000 | ROI-based, TBSS | patients with velocardiofacial syndrome (VCFS N=36; control N=29) | P=16 C=16 |
Penn emotion recognition test | left IFOF and UF. | For VCFS patients, AD in the left IFOF and UF is positively correlated with emotional recognition performance, especially fear recognition. |
| Radoeva (2012) | DTI | 15/800 | ROI-based | patients with velocardiofacial syndrome (VCFS N=33; control N=16) | P=18 C=18 |
facial emotion recognition task | right SLF and IFOF | For VCFS patients, emotion recognition score is positively correlated with AD in right SLF and right IFOF. |
| Mike (2013) | sMRI | N.A. | Voxel-based | patients with multiple sclerosis (MS N=49; control N=24) | P=40 C=37 |
facial emotion recognition test | left fornix; right ILF and IFOF, bilateral UF; CC | For MS patients, facial emotion recognition performance is negatively correlated with lesion volume in genu and splenium of CC, right ILF and IFOF, bilateral UF, and left fornix. |
| Saito (2017) | DTI | 51/900 | Deterministic Tractography | patients with schizophrenia (SZ N=16; control N=16) | P=20 C=22 |
perception of emotional intimacy | left SLF | For SZ patients, MD in IPL-IFG connection (via SLF) is positively correlated with the disrupted perception of emotional intimacy (e.g. inability to feel intimacy). |
|
| ||||||||
| Empathy | ||||||||
|
| ||||||||
| Chou (2011) | DTI | 13/900 | TBSS | healthy adults (N=80) | 26 | empathy quotient (EQ) | local WM in left IPL and STS; right ATR and SLF; left ILF | WM underlying empathy is sex-dependent. EQ score is positively correlated with FA in multiple regions (left IPL and STS) and tracts (right ATR and SLF, left ILF) in females, but negatively correlated with FA in these regions/tracts in males. |
| Mueller 2013) | DTI sMRI fMRI |
20/1000 | TBSS | patients with high-functioning autism (ASD N=12; control N=12) | P=36 C=33 |
questionnaires of empathy and emotionality | N.A. | WM in local TPJ is correlated with emotionality. But no measures correlate with empathy scores |
| Nakagawa (2015) | DTI sMRI |
32/1000 | Voxel-based ROI-based | healthy adults (N=776) | 20 | empathy quotient (EQ) | local WM in bilateral IPL, right AI/IFG, and left TPJ/STS | For lonely individuals, the EQ score is positively correlated with local WM density in bilateral IPL, right AI/IFG, and left TPJ/STS |
| Parkinson (2014) | DTI | 32/1000 | TBSS | healthy adults (N=64) | 19 | interpersonal reactivity index | IFOF, SLF, UF, ATR, CC, CST, all bilaterally; right ILF | The ‘empathic concern’ subscales of emotional empathy is positively correlated with FA in right ILF, bilateral SLF, IFOF, UF, ATR, CST, and CC (forceps minor) |
| Takeuchi (2013) | DTI sMRI |
32/1000 | Voxel-based | healthy adults (N=567) | 21 | empathy quotient (EQ) | local WM in social regions, left SLF, ILF and IFOF, fornix, CC | EQ score is positively correlated with local WM volume in multiple regions (right IFG, IPL, TPJ, PCC, and MPFC) and tracts (left ILF, left IFOF, fornix, genu of CC). The EQ score is also positively correlated with FA in left SLF. |
| Fujino (2014) | DTI | 81/1500 | TBSS | patients with schizophrenia (SZ N=69; control N=69) | P=37 C=34 |
interpersonal reactivity index (IRI) | CC, left IFOF, left ATR | For SZ patients, the ‘personal distress’ subscales of empathy are negatively correlated with FA in the splenium of the CC, and the ‘fantasy’ subscales are positively correlated with FA in the left IFOF and ATR. |
| Herbet (2015)_Neuro psychologia | sMRI | N.A. | Voxel-based, ROI-based | patients with surgical resection for diffuse low-grade glioma (DLGG N=107) | 41 | empathy quotient (EQ) | right UF and IFOF | For DLGG patients, disconnection of right UF predicts a low subjective empathy and disconnection of right IFOF predicts a high subjective empathy. |
| Oishi (2015) | DTI sMRI |
6/1000 | ROI-based | patients with acute ischemic stroke (N=30) | N.A. | emotional empathy task | right UF | Damage to the right UF is negatively correlated with emotional empathy performance. |
| Olszewski (2017) | dMRI-NT | 64/700 | Deterministic Tractography | patients with velocardiofacial syndrome (VCFS N=57; control N=30) | P=21 C=21 |
trait emotional intelligence questionnaire (empathy subsets) | right ATR and UF | For VCFS patients, empathy scores is positively correlated with RD in right ATR and negatively correlated with the number of streamlines in right UF. |
bvFTD: behavioral-variant frontotemporal dementia; C: controls; DLGG: diffuse low-grade glioma; dMRI-NT: diffusion-weighted MRI with non-tensor modeling; P: patients; TBI: traumatic brain injury; SZ: schizophrenia; VCFS: velocardiofacial syndrome. Other acronyms can be referred to from the abbreviations section in the main text.
Other robust associations between WM and embodied cognition have been identified in three limbic tracts: the uncinate fasciculus (UF), the anterior thalamic radiation (ATR), and the fornix. The UF is a hook-shaped ventral associative bundle that links medial temporal areas (e.g. ATL, AMG) to portions of frontal cortices (both medial and lateral OFC) (Catani and Thiebaut de Schotten, 2008). It has been linked to episodic memory, semantic memory, and social-emotional processing (Von Der Heide et al., 2013). The ATR is a major projection from the thalamus, which carries reciprocal connections from the hypothalamus and limbic structures (e.g. AMG, hippocampus) to the prefrontal cortex and anterior cingulate cortex (Catani et al., 2013). It has been primarily implicated in affective processing and emotion regulation (Downey et al., 2015). The fornix is a core limbic tract directly connecting the hippocampus to the mammillary bodies and hypothalamus. It is mainly involved in episodic memory and evaluative processing (Catani and Thiebaut de Schotten, 2008). Disruption of these limbic tracts has been commonly observed in clinical disorders, such as the behavioral variant frontotemporal dementia, mild cognitive impairment, and velocardiofacial syndrome (Daianu et al., 2016; Liu et al., 2017; Perlstein et al., 2014), and these patients typically exhibit severe impairments in empathy and emotion recognition abilities (Jalbrzikowski et al., 2012; Kessels et al., 2007; Lough et al., 2006; Spoletini et al., 2008). Abnormal diffusivity (AD, RD, and MD) has been reported in the right ATR, UF, and fornix in frontotemporal dementia patients and correlates with disrupted understanding of emotion and sarcasm (Downey et al., 2015). Reduced FA in the left UF in patients with mild cognitive impairment correlates with impaired emotion recognition and expression (Fujie et al., 2008). For patients with velocardiofacial syndrome, one study reported that empathy scores correlated with radial diffusivity in the right ATR and negatively correlated with the number of streamlines in right UF (Olszewski et al., 2017); the patients’ emotional recognition performance for fear expression was also positively correlated with axial diffusivity in the left UF (Jalbrzikowski et al., 2014). In addition, for patients with multiple sclerosis, facial emotion recognition performance is negatively correlated with lesion volume in bilateral UF, as well as the left fornix (Mike et al., 2013). For patients with acute ischemic stroke or surgical resection for a diffuse low-grade glioma, disconnection of the right UF predicted low empathy ability (Herbet et al., 2015b; Oishi et al., 2015). For schizophrenia patients, subscales of empathy positively correlated with FA in the left ATR (Fujino et al., 2014). Finally, the integrity of these three limbic tracts not only predicts socio-emotional functioning in pathological circumstances, but also in normal individuals. For instance, higher FA in the UF or ATR has been associated with higher levels of empathy among healthy individuals (Parkinson and Wheatley, 2014), and larger WM volume in the fornix is associated with higher empathy quotient scores (Takeuchi et al., 2013).
The ILF and IFOF also appear to be important for emotional recognition and empathy. Two prior studies with large samples of patients with focal brain lesions reported that damage to the right ILF or IFOF correlates with impairments in facial emotion recognition, more specifically in the recognition of fear, anger, and sadness (Genova et al., 2015; Philippi et al., 2009). For patients with Parkinson’s disease, decreased FA in bilateral IFOF and the left ILF was associated with impaired sadness identification performance (Baggio et al., 2012). Additionally, the inter-individual variation of emotion recognition and empathy abilities among healthy adults can be predicted by the microstructure of the right ILF and bilateral IFOF (Parkinson and Wheatley, 2014; Unger et al., 2016). Little is known, however, about how the IFOF and ILF are implicated in embodied cognition, because the core mirroring network (STS, IPL, and IFG) is usually thought to be part of the dorsal stream (Hamzei et al., 2016). Since the two tracts directly project from early visual cortices to affective mirroring areas (i.e. AMG, AI, and ACC) (Gschwind et al., 2012; Sarubbo et al., 2013), it is possible that they engage in rapid evaluative processing paralleled with the basic embodied simulation process to facilitate accurate recognition of emotions. Another possibility stems from the nature of the biased behavioral paradigm used in the literature: almost all emotion recognition tasks are face-based, such as the Ekman Face Test, and it is already established that the IFOF and ILF are essential for face processing (Rokem et al., 2017).
Interestingly, sex differences in empathy may be reflected in sex differences in WM microstructure of the aforementioned tracts. Research on the “empathizing-systemizing” theory (Baron-Cohen, 2009) suggests that females generally perform better on emotion recognition and empathy tasks, whereas males excel in mental rotation, spatial navigation, and mathematics (i.e. systemizing). Two studies have shown that empathizing skill is positively correlated with microstructure in bilateral SLF, right ATR, right fornix, left ILF, and left IFOF in females, but negatively correlated with microstructure in these tracts in males (Chou et al., 2011; Takeuchi et al., 2013).
2.3.3 Mentalizing Network
The literature suggests that the cingulum and a portion of the SLF, the arcuate fasciculus, are two pivotal WM tracts for mentalizing abilities (see Table 3). The cingulum is a large association fiber pathway that encircles the corpus callosum, going from the medial prefrontal cortex/anterior cingulate cortex through the posterior cingulate cortex/precuneus, and from there to the medial temporal structures proximal to the hippocampus. It is part of the limbic system and is broadly involved in attention, memory, and emotional processing (Catani and Thiebaut de Schotten, 2008). Given that the cingulum provides strong structural connections between the MPFC and PCC, it has been argued as the main structural skeleton of the default mode network (van den Heuvel et al., 2008) and the mentalizing network (Yordanova et al., 2017). The arcuate fasciculus (AF) has long been implicated in language processing, as it connects Wernicke’s area to Broca’s area in the left hemisphere; however, the function of the right AF remains unclear. It has recently been proposed that the right AF might subserve mentalizing (Herbet et al., 2014), since the tract connects frontal cortices with the right TPJ, a region responsible for thinking about others’ thoughts and intentions (Saxe and Wexler, 2005).
Several clinical studies have reported that mentalizing abilities are compromised when the cingulum or right AF is disrupted. For children with traumatic brain injury, the severity of the ToM impairment is positively correlated with the degree of axonal injury in the left cingulum (Levin et al., 2011). Individuals with high-functioning autism had lower right TPJ activation, weaker functional connectivity between the TPJ and frontal areas during the ToM task, and most critically, reduced WM integrity in the right AF near the TPJ (Kana et al., 2014). Perhaps the most compelling evidence comes from two studies using direct electrical stimulation of WM tracts during neurosurgery—the only technique that allows for direct information on the functional role of WM tracts in cognition. Both studies found that virtual disconnection of the right AF or cingulum severely impairs the accuracy of mental state attribution (Herbet et al., 2015a; Yordanova et al., 2017). This suggests that proper functioning of these tracts is essential for normal mentalizing abilities.
There is some evidence that these two tracts might be specialized for different mentalizing processes. Studies on patients with gliomas revealed that damage to the right cingulum is associated with impaired performance on inference-based tasks (e.g. comic strip vignettes), whereas damage to the right AF is associated with impaired performance on perceptual-based ToM tasks (e.g. “reading the mind in the eyes”) (Herbet et al., 2014). Considering that the cingulum and AF connect different nodes of the mentalizing network (the cingulum mainly projects to medial nodes, such as MPFC and PCC, while the AF projects to lateral nodes, such as the TPJ and IFG), this double dissociation in terms of WM function resonates with previous fMRI studies showing that the MPFC engages most in inference-based ToM tasks, whereas the IFG only activates during perceptual-based ToM tasks (Schurz et al., 2014).
Substantial evidence in fMRI research suggests a critical role of the amygdala in ToM, especially for face-based mental state inferences (Mar, 2011). This may be due to the amygdala’s role in guiding attention to the eye region of the face, which may be an important first step in the process of interpreting the mental states of others (Adolphs and Spezio, 2006). However, the amygdala does not operate in isolation: WM tracts connecting the amygdala to other mentalizing areas may also contribute to ToM processes. Several studies have shown that amygdala-related WM tracts (i.e. UF, IFOF, and ILF) are important for accurate mentalizing. For example, impaired ToM skills in patients with velocardiofacial syndrome are associated with WM microstructural alterations in the left IFOF, left UF, and bilateral ILF (Jalbrzikowski et al., 2014). Transient disconnection of the right IFOF by direct electrical stimulation impairs performance on the “reading the mind in the eyes” task (Yordanova et al., 2017). Cross-sectional research also supports the crucial role of these amygdala-related WM tracts in lifespan changes in ToM abilities. Using TBSS, Grosse Wiesmann et al., (2017) found that the emergence of explicit ToM abilities between 3 and 4 years of age is associated with an increase in streamline density in the right IFOF and bilateral SLF/AF. Another study revealed that variation in the microstructure of the left UF positively correlates with inter-individual variance of “reading the mind in the eyes” task performance in 4-year-olds, but not in 6-year-olds, suggesting that the UF might be more important for the emergence, but not maintenance, of ToM function (Anderson et al., 2015). In addition, age-related declines in ToM abilities throughout the lifespan have been associated with decreased FA in bilateral UF, right IFOF, and right SLF (Cabinio et al., 2015).
2.4 Summary
To summarize, three major tracts in the right hemisphere have been implicated in face processing: the ILF, the IFOF, and the SLF. Studies in young children and older adults, as well as patients with prosopagnosia, all attest to the crucial role these tracts play in skilled face perception. The literature on imitation, empathy, and emotional recognition identifies the SLF as the most critical tract for embodied cognition, and it has also been identified as the primary fiber pathway for the mirroring network. The ILF/IFOF and three limbic tracts (UF, ATR, and fornix) have also shown robust associations with embodied social processes. Disruption of these tracts causes severe impairments in empathy and emotion recognition abilities across a variety of clinical disorders. Finally, WM research on ToM suggests that the cingulum and the AF are essential for mentalizing abilities. This claim is bolstered by strong evidence from direct electrical stimulation studies. Additionally, changes in ToM abilities across the lifespan are associated with amygdala-related WM tracts (i.e. UF, IFOF, and ILF). Bear in mind that our literature review tries to draw conclusions more generally from the entire body of WM studies, rather than from any single finding.
We believe our review is just the beginning to unveil the functionality of these major associative WM tracts in social processing. We still have very limited knowledge about their domain specificity and generality. For example, our review implicates the ILF in face processing, empathy, emotion recognition, and mentalizing abilities, and the past literature also suggests its critical roles in object recognition, reading and language processing (Ashtari, 2012; Catani and Thiebaut de Schotten, 2008). This seemingly nonspecific role of the ILF in a variety of social and non-social processes may not be surprising, considering that the ILF is a large fasciculus reaching up to 12cm in length and that different fiber bundles enter and exit the fasciculus at various positions. As such, the properties of WM tissue vary systematically along the trajectory of the ILF, potentially yielding distinct functional subcomponents that support discrete cognitive functions. For example, Tavor et al., (2014) reported the anterior portion of the ILF is associated with face memory abilities, whereas the middle and posterior portions are associated with scene memory abilities (also see Gomez et al., 2015 and Song et al., 2015). These findings suggest the existence of segregated segments or pathways within the ILF, each specialized for distinct functions (e.g. face vs. scene processing). This logic also applies to other tracts that have been associated with multiple social and non-social functions (e.g. UF, SLF, and IFOF) (Hecht et al., 2015; Olson et al., 2015; Von Der Heide et al., 2013).
It is also worth noting that healthy WM in the corpus callosum (CC) appears to be important for social cognition, as our literature review shows apparent involvement of the CC in both embodied cognition (Baggio et al., 2012; Crespi et al., 2016; Fujino et al., 2014; Mike et al., 2013; Parkinson and Wheatley, 2014; Takeuchi et al., 2013) and ToM (Cabinio et al., 2015; Mike et al., 2013; Scheibel et al., 2011). This is consistent with research on autism and agenesis of the corpus callosum, which both reveal that corpus callosum abnormalities can cause severe impairments in social functioning in the real world (Paul et al., 2007; Travers et al., 2012). One appealing hypothesis (Kennedy and Adolphs, 2012) is that social cognition is contingent upon rapid and reliable communication between social brain areas that are spatially separate, such as language-related areas in the left hemisphere and face processing areas in the right hemisphere. Given the highly interactive, real-time nature of social behavior, there is substantial pressure to integrate contralateral processing as efficiently as possible; therefore, social cognition requires considerable amounts of myelinated corpus callosum connections across hemispheres.
3. Elucidating Anatomical Architecture of Social Brain Networks
The above literature review has informed us of several important WM tracts for social cognition. However, the exact architecture of interconnections between social brain regions still remains unknown. Unraveling this connectivity profile is extremely useful when we interpret results, because once we find a correlation between a WM tract and a social behavior/disorder, we would like to infer what underlying neural communications (e.g. AMG-MPFC interaction) are potentially involved or disrupted. A second motivation is to bridge the conceptual gap between two major analysis methods used in the DTI literature. The TBSS method tends to report findings based on the tract name listed in a standard brain atlas (e.g. SLF, ATR), whereas the tractography-based studies frequently report results in terms of pathways and seed ROIs (e.g. STS-IFG pathway). It is difficult to compare findings from these two methods without knowing the tract composition of each pathway. Last, sample sizes are often small in this literature and many findings have not been replicated. For these reasons, we conducted an empirical analysis on an existing dataset, described below.
We performed probabilistic tractography on a large in-house DTI dataset (103 healthy young adult subjects) accumulated from previous studies (Alm et al., 2016, 2015; Hampton et al., 2016; Metoki et al., 2017; Unger et al., 2016). All studies used the same MR procedures and parameters. We choose probabilistic tractography because it enables us to estimate the likelihood/probability of every voxel involved in the trajectory of a defined WM pathway (Behrens et al., 2007). By overlaying this probabilistic map on a standard WM atlas (i.e. ICBM-DTI-81 atlas, Mori et al., 2008), we were able to extract the contribution of each known WM tract to each social brain pathway (see detailed methods description in Supplementary Materials and Methods). In short, our goal was to build the connectivity matrix between putative regions in each social brain network and elucidate the fiber tract composition for each pathway (see Fig. 5 and Table 4–6).
Figure 5.
Social brain white matter tracts. Using probabilistic tractography, we reconstructed the WM skeleton, across 103 subjects, between putative regions in each social brain network (A)(C)(E), and we summarize the major white matter tracts for each network based on the literature review and the present tractography (B)(D)(F). In the left column, each red sphere represents a gray matter region of interest (ROI) and the blue represents the tractography-reconstructed WM pathways between ROIs. In the right column, transparent spheres are retained to use as landmarks. Different white matter tracts are represented by different colored streamlines.
Table 4.
The connectivity matrix for the face perception network. All numbers are tract composition percentage (%). Only tracts with percentages larger than 5% are shown. Tract percentages greater than 50% are presented in bold font. The labels across the top and left side denote gray matter regions. White matter tracts are listed inside the connectivity matrix.
| OFC | AMG | ATL | FFA | IFG | OFA | STS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OFC | |||||||||||||
|
|
|||||||||||||
| AMG | CC | 38.32 | |||||||||||
| UF | 28.75 | ||||||||||||
| IFOF | 18.49 | ||||||||||||
| ATR | 13.96 | ||||||||||||
|
|
|||||||||||||
| ATL | UF | 49.56 | ILF | 36.73 | |||||||||
| IFOF | 29.84 | UF | 30.80 | ||||||||||
| CC | 10.02 | CING | 15.75 | ||||||||||
| ATR | 6.66 | IFOF | 15.75 | ||||||||||
|
|
|||||||||||||
| FFA | IFOF | 59.65 | ILF | 45.00 | |||||||||
| UF | 29.81 | IFOF | 41.67 | ILF | 69.26 | ||||||||
| ATR | 7.84 | CST | 13.33 | IFOF | 27.98 | ||||||||
|
|
|||||||||||||
| IFG | CC | 37.50 | ATR | 94.92 | ATR | 61.90 | SLF | 98.18 | |||||
| IFOF | 23.14 | UF | 15.84 | ||||||||||
| UF | 20.82 | ILF | 11.72 | ||||||||||
| ATR | 18.55 | IFOF | 10.53 | ||||||||||
|
|
|||||||||||||
| OFA | IFOF | 71.71 | IFOF | 61.46 | ILF IFOF |
49.86 46.40 |
CC IFOF |
65.41 33.72 |
IFOF | 90.64 | |||
| UF | 13.90 | CC | 19.58 | ||||||||||
| ILF | 10.09 | ILF | 16.19 | ||||||||||
|
|
|||||||||||||
| STS |
IFOF ILF |
55.53 16.18 |
IFOF | 55.06 | ILF | 43.90 | SLF | 36.58 | SLF | 96.73 |
IFOF ILF |
52.60 32.32 |
|
| ILF | 23.93 | IFOF | 30.69 | ILF | 34.67 | ||||||||
| SLF | 12.99 | SLF | 19.16 | IFOF | 25.84 | ||||||||
Gray matter regions: AMG=amygdala; ATL=anterior temporal lobe; FFA=fusiform face area; IFG=inferior frontal gyrus; OFA=occipital face area; OFC=orbitofrontal cortex face patch; STS=superior temporal sulcus. White matter tracts: ATR=anterior thalamic radiations; CC=corpus callosum; CING=cingulum bundle; IFOF=inferior frontal occipital fasciculus; ILF=inferior longitudinal fasciculus; SLF=superior longitudinal fasciculus; UF=uncinate fasciculus.
Table 6.
The connectivity matrix for the mentalizing network. All numbers are tract composition percentage (%). Only tracts with percentages larger than 5% are shown. Tract percentages greater than 50% are presented in bold font. The labels across the top and left side denote gray matter regions. White matter tracts are listed inside the connectivity matrix.
| dMPFC | PCC | AMG | ATL | IFG | TPJ | vMPFC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dMPFC | |||||||||||||
|
|
|||||||||||||
| PCC | CING | 62.59 | |||||||||||
| CC | 37.41 | ||||||||||||
|
|
|||||||||||||
| AMG | IFOF | 56.12 | |||||||||||
| ATR | 56.34 | CING | 13.56 | ||||||||||
| CC | 35.93 | ILF | 10.87 | ||||||||||
| UF | 7.60 | ||||||||||||
|
|
|||||||||||||
| ATL | CC | 42.29 | |||||||||||
| ILF | 18.42 | IFOF | 49.01 | IFOF | 41.86 | ||||||||
| IFOF | 16.84 | ILF | 34.35 | ILF | 40.48 | ||||||||
| UF | 12.14 | UF | 8.18 | UF | 16.76 | ||||||||
| ATR | 8.48 | ||||||||||||
|
|
|||||||||||||
| IFG | CC | 50.72 |
IFOF CING |
78.41 7.58 |
ATR IFOF |
75.58 14.49 |
IFOF | 71.84 | |||||
| ATR | 27.38 | UF | 13.57 | ||||||||||
| IFOF | 17.43 | ILF | 11.73 | ||||||||||
|
|
|||||||||||||
| TPJ | SLF IFOF CC |
44.90 33.08 18.46 |
SLF | 40.61 | IFOF | 41.24 |
ILF SLF IFOF |
51.18 29.48 16.30 |
SLF | 99.81 | |||
| CING | 26.14 | UF | 29.93 | ||||||||||
| IFOF | 17.91 | ILF | 15.59 | ||||||||||
| ATR | 10.31 | SLF | 11.63 | ||||||||||
|
|
|||||||||||||
| vMPFC | CC | 90.87 |
CING CC |
92.01 7.99 |
CC UF IFOF ATR |
36.32 30.00 20.49 11.90 |
IFOF | 36.85 |
CC ATR IFOF UF |
53.25 18.12 15.56 13.06 |
IFOF UF ATR CC |
42.99 30.70 16.84 9.47 |
|
| UF | 31.65 | ||||||||||||
| ILF | 13.54 | ||||||||||||
| CC | 11.04 | ||||||||||||
| ATR | 6.72 | ||||||||||||
Gray matter regions: AMG=amygdala; ATL=anterior temporal lobe; dMPFC = dorsomedial prefrontal cortex; IFG=inferior frontal gyrus; PCC=posterior cingulate cortex; TPJ=temporo-parietal junction; vMPFC=ventromedial prefrontal cortex. White matter tracts: ATR=anterior thalamic radiations; CC=corpus callosum; CING=cingulum bundle; IFOF=inferior frontal occipital fasciculus; SLF=superior longitudinal fasciculus; UF=uncinate fasciculus.
3.1 Results
For the face perception network, probabilistic tractography classified 30.43% of WM voxels into the tracts listed in the ICBM-DTI-81 atlas. Among all classified tracts, the SLF occupied the most WM volume in the face network (32.35%), followed by the IFOF (27.32%), ILF (23.81%), CC (6.17%), ATR (5.56%), and UF (5.35%). When we more closely examined which specific pathways these tracts mainly subserved (see Table 4), we found that the SLF constituted a large proportion of two dorsal pathways projecting to the IFG (FFA-IFG: 98.18%; STS-IFG: 96.73%). This means that 98.18% of the voxels in the FFA-IFG pathway were classified as SLF, so were 96.73% of the voxels in the STS-IFG pathways. The IFOF was observed to mediate communications between posterior core face areas (OFA, FFA, STS) and anterior amygdala-frontal face areas (OFA-IFG: 90.64%; OFA-OFC: 71.71%; OFA-AMG: 61.46%; FFA-OFC: 59.65%; STS-OFC: 55.53%; STS-AMG: 55.06%), and the ILF was found to support both short and long pathways along the ventral stream (FFA-ATL: 69.26%; OFA-FFA: 65.41%; OFA-ATL: 49.86%). In addition, the CC appeared to take part in two pathways with the OFC (AMG-OFC: 38.32%; IFG-OFC: 37.50%), and the ATR subserved connections between medial temporal cortex and IFG (AMG-IFG: 94.92%; ATL-IFG: 61.90%). Finally, the UF was found to be involved in connections between ATL, amygdala and OFC (ATL-OFC: 49.56%; ATL-AMG: 30.80%; AMG-OFC: 28.75%).
For the mirroring network, only 16.46% of the WM voxels could be classified by the tracts listed in the ICBM-DTI-81 atlas. Among them, the SLF was the most dominant tract, occupying 83.86% of WM voxels in the mirroring network, with the rest labelled as the IFOF (6.72%), corticospinal tract (CST, 5.17%), ATR (1.96%), and UF (1.47%). For the tract composition of each pathway in the mirroring network, Table 5 shows that the SLF mediated all pathways between perisylvian regions (STS-IFG: 99.68%; IPL-IFG: 99.54%; STS-IPL: 96.35%) and played an important role in most ACC connections (IPL-ACC: 99.57%; STS-ACC: 99.00%; IFG-ACC: 96.10%). Albeit in smaller proportions, the IFOF was found to be part of insula-related pathways (AMG-AI: 28.42%; STS-AI: 11.63%; AI-ACC: 11.06%), and the CST was part of amygdala-related pathways (IPL-AMG: 22.21%; AMG-AI: 14.63%). The ATR and UF were mainly involved in AMG-ACC (81.25%) and AMG-AI pathway (10.85%), respectively.
Table 5.
The connectivity matrix for the mirroring network. All numbers are tract composition percentage (%). Only tracts with percentages larger than 5% are shown. Tract percentages greater than 50% are presented in bold font. The labels across the top and left side denote gray matter regions. White matter tracts are listed inside the connectivity matrix.
| ACC | AI | AMG | IFG | IPL | STS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC | |||||||||||
|
|
|||||||||||
| AI | SLF | 86.20 | |||||||||
| IFOF | 11.06 | ||||||||||
|
|
|||||||||||
| AMG | SLF | 44.19 | |||||||||
| ATR | 81.25 | IFOF | 28.42 | ||||||||
| ILF | 7.55 | CST | 14.63 | ||||||||
| UF | 10.85 | ||||||||||
|
|
|||||||||||
| IFG | SLF | 96.10 | SLF | 94.75 | SLF | 85.06 | |||||
| ATR | 8.79 | ||||||||||
|
|
|||||||||||
| SLF | 99.57 | SLF | 84.87 | SLF | 75.54 | SLF | 99.54 | ||||
| IPL | CST | 8.04 | CST | 22.21 | |||||||
|
|
|||||||||||
| STS | SLF | 99.00 |
SLF IFOF |
83.91 11.63 |
SLF IFOF CST |
81.45 7.00 6.73 |
SLF | 99.68 | SLF | 96.35 | |
Gray matter regions: ACC=anterior cingulate cortex; AI=anterior insula; AMG=amygdala; IFG=inferior frontal gyrus; IPL=inferior parietal lobe; STS=superior temporal sulcus. White matter tracts: ATR=anterior thalamic radiations; CST=cortico-spinal tract; IFOF=inferior frontal occipital fasciculus; SLF=superior longitudinal fasciculus; UF=uncinate fasciculus.
Last, for the mentalizing network, probabilistic tractography revealed that 32.04% of WM voxels were atlas-listed tracts. The CC had the largest proportion of volume (32.58%), followed by the IFOF (18.80%), SLF (16.39%), ATR (14.93%), ILF (7.14%), and UF (5.79%). As shown in Table 6, large percentages of voxels in major frontal pathways were labeled as the CC (vMPFC-dMPFC: 90.87%, vMPFC-IFG: 53.25%; IFG-dMPFC: 50.72%). The IFOF mediated several projections to the PCC (PCC-IFG: 78.41%; PCC-AMG: 56.12%; PCC-ATL: 49.01%), as well as to the ATL (ATL-IFG: 71.84%; ATL-AMG: 41.86%). The ILF was also part of ATL-related pathways (TPJ-ATL: 51.18%; ATL-AMG: 40.48%; ATL-PCC: 34.35%). The SLF exclusively subserved the connection between the TPJ and IFG (99.81%), and the ATR was the main tract for amygdala-frontal connections (AMG-IFG: 75.58%; AMG-dMPFC: 56.34%). In addition, the UF was engaged in vMPFC connections with the ATL (31.65%) and AMG (30.00%). Finally, although the cingulum (CING) occupied a small percentage of WM in the network (4.33%), it is the most dominant tract connecting all distant medial mentalizing areas (PCC-vMPFC: 92.01%; PCC-dMPFC: 62.59%).
These results, which are summarized in Fig. 5, are in line with previous studies using similar tractography methods. For the face perception network, there is a dorsal and a ventral pathway. The dorsal pathway runs from face-selective STS to the IFG via portions of the SLF (Ethofer et al., 2013; Gschwind et al., 2012). The ventral pathway runs from OFA and FFA to the ATL and AMG via the ILF and IFOF, and extends even more anteriorly into face-selective frontal regions via the IFOF (Gschwind et al., 2012; Pyles et al., 2013). For the mirroring network, Hecht et al., (2013) and Hamzei et al., (2016) revealed that the SLF mediates two main pathways linking core mirroring areas (e.g. IPL-IFG, STS-IFG), and the ILF is involved in the STS-IPL connection. No DTI study thus far has directly elucidated the WM architecture of the mentalizing network; however, since the mentalizing network shares much overlap with the default mode network (DMN) both anatomically and functionally (Buckner et al., 2008; Li et al., 2014; Mars et al., 2012), we can glean insights from that line of research. Most DMN studies show that the dorsal cingulum mediates the MPFC-PCC pathway, while the ventral cingulum supports communications between the PCC and ATL/AMG (Greicius et al., 2009; Sethi et al., 2015; van den Heuvel et al., 2009, 2008). The TPJ connects with the ATL/AMG via the ILF (Horn, Ostwald, Reisert, & Blankenburg, 2014), and with the MPFC ventrally via the IFOF and dorsally via the SLF (Grosse Wiesmann et al., 2017; van den Heuvel et al., 2009). In the present study, we observed these reported structural connections and beyond that, we identified the whole connectivity profile of each social brain network, as well as the tract composition of each social pathway. In addition, our tractography results converge well with our literature review. The ILF, IFOF, and SLF are the most reported tracts for face processing in the literature, and in our tractography analyses they are the top 3 tracts occupying the majority of WM voxels in the face network. Past studies suggest that the SLF is the most important tract for embodied cognition, and our tractography results also support its dominant role in the mirroring network (i.e. 83.86% of WM voxels belong to the SLF). Finally, for the mentalizing network, the same tracts (i.e. cingulum, SLF, UF, IFOF, and ILF) were found in both the ToM literature and the current analyses.
We also encountered some unexpected findings, most of which related to the corpus callosum (CC). For example, the connectivity matrix of the face perception network (Table 4) revealed that the AMG-OFC pathway was mainly subserved by the CC (38.32%), followed by the UF (28.75%). Since all ROIs defined in our tractography were in the right hemisphere, this finding conflicts with our knowledge of brain anatomy, since the right AMG and OFC should be primarily connected by the right UF, rather than the CC (Catani and Thiebaut de Schotten, 2008). Similar erroneous findings can also be manifested in MPFC-related mentalizing pathways (Table 6), such as spuriously high probability of CC involvement in the dMPFC-vMPFC connection (90.87%). In addition, we did not expect the CST implicated in the mirroring network (5.17%) because this tract is primarily involved in motor functions and thus has often been used as a non-social control tract in the literature (Anderson et al., 2015).
These problems might arise from our atlas-based approach, which computes the tract composition of each pathway by overlaying the probabilistic map onto a standard WM atlas. This method typically works well for WM connections with simple fiber configurations but is prone to produce artefactual results when the pathway travels through structures with high uncertainty of fiber orientations (i.e. crossing fiber sites), as is seen in the CC and CST. Moreover, it is important to bear in mind that the tract percentage numbers in the connectivity matrices only reflects the relative contribution of each atlas-listed tract for the pathway; they could completely change from one atlas to another. The ICBM-DTI-81 atlas we used for the current analysis includes 48 tracts (Mori et al., 2008), which means we can only elucidate the tract composition based on these tracts. This is why the connectivity matrix of the mirroring network in Table 5 shows the sole engagement of the SLF for the STS-IFG and STS-IPL pathways but in fact the literature suggests considerable involvement of other unlisted tracts for these two pathways such as the extreme/external capsule and the middle longitudinal fasciculus (Hecht et al., 2013). Since the ICBM-DTI-81 is currently the best atlas in use, future research should focus on developing more fine-grained WM atlases that include more segmented tracts and labels. This will also profoundly increase the value and accuracy of other atlas-based methods (e.g. TBSS).
It may seem odd that our probabilistic tractography results indicated that 70–85% of WM voxels in social brain networks were unclassified WM tissue, or in other words, do not belong to any atlas-listed long-range tracts. This is likely due to the fact that long-range WM tracts only comprise 4–10% of the whole human WM connectome and the majority of WM consists of short association fibers in superficial WM (e.g. U-shaped fibers) that lie immediately beneath the gray matter and connect adjacent gyri (Jbabdi et al., 2015; Schuz and Braitenberg, 2002; Sotiropoulos and Zalesky, 2017; Wandell, 2016). However it is technically difficult to study local regional fibers. The spatial arrangements around sulci or gyri are complicated and most dMRI tractography algorithms are unsuitable for reconstructing them (Feldman et al., 2010; Reveley et al., 2015). Researchers usually have to adopt voxel-based or ROI-based approaches to estimate local WM properties associated with social cognition, or use novel fiber clustering algorithms (Zhang et al., 2014) or sophisticated ensemble tractography approach (Takemura et al., 2016) to accurately identify and characterize U-shaped fiber system. In the future, we anticipate more investigations of local WM function, especially those near social brain regions, as the present literature clearly indicates such local WM can be critical to social processing in both healthy and clinical population (Chou et al., 2011; Gomez et al., 2015; Nakagawa et al., 2015; Song et al., 2015; Takeuchi et al., 2013).
4. Limitations of the Current Review
We would be remiss if we didn’t point out some limitations of our review. First, we defined three social brain networks for paper classification. Although these networks are conceptually specialized for distinct social processes, they are not mutually exclusive. They overlap in regards to anatomy (e.g. AMG, IFG, STS/TPJ, see Fig. 2) and interact with each other during many social tasks (Barrett and Satpute, 2013; Greven and Ramsey, 2017; Sperduti et al., 2014; Spunt et al., 2011; Zaki et al., 2010).
Second, the way we classified each social task or process into three brain networks might be debatable. The challenge is that social processes are interdependent and multifaceted, and we are far from having an agreed-upon taxonomy or factor structure (Happé et al., 2017). For instance, some studies believe the “reading the mind in the eyes” task is measuring the mirroring network (Herbet et al., 2015a), while others argue the task is probing the mentalizing network (Mike et al., 2013). Some researchers might categorize studies of “cognitive empathy”, a subtype of empathy, into the mirroring network group, while others would sort them into the mentalizing network because the term is conceptually interchangeable with perspective-taking and ToM (Shamay-Tsoory, 2011). Even when empathy as a whole belongs to embodied cognition, not all of its sub-components (e.g. “empathic concern”, “fantasy”, see “interpersonal reactivity index”, Davis, 1983) are subserved by the same neural network (Kanske et al., 2015; Lamm and Majdandžić, 2015) or tracts (Fujino et al., 2014; Parkinson and Wheatley, 2014).
Third, our summary of WM tracts for each social brain network could be biased by the fact that the tracts that were investigated in some studies (particularly those with ROI-based or tractography analysis) were pre-selected by the authors, and we don’t necessarily know what other tracts might have emerged if they had exhaustively examined the whole brain when correlating with social behavioral measures.
Fourth, we defined ROIs for our probabilistic tractography using mean MNI coordinates from prior meta-analysis studies. This analytic choice was imposed on us by using an existing dataset that lacked certain features. Ideally, functionally-defined gray matter regions should supplement dMRI, allowing for the creation of functional seeds for precise fiber tracking (Sotiropoulos and Zalesky, 2017).
Finally, several social processes were not covered by the current review due to the small number of studies in these domains examining WM indexes. Individual studies exist examining self-processing (Chavez and Heatherton, 2017), personality (Cohen et al., 2008), social reward (Bjornebekk et al., 2012), peer influence (Kwon et al., 2014), in-group bias (Baumgartner et al., 2015), social communication skills (Lo et al., 2017), social decision-making (Barbey et al., 2014), and social network size (Hampton et al., 2016). Future reviews should include and discuss these in order to provide a larger overview of the WM basis for social cognition.
5. Problems and Recommendations for Best Practices
Our review of the literature indicates that there are a large number of studies on WM in the realm of social cognition. However, at times, the reviewed findings were inconclusive and even contradictory to other findings. For example, Thomas et al., (2009) used DTI to evaluate the structural integrity of long-range visual tracts (i.e. ILF and IFOF) in individuals with developmental prosopagnosia (DP). They found that these patients showed reductions in the integrity of the right ILF and these reductions were positively correlated with individual face perception deficits. In contrast, Gomez et al., (2015) did not detect any WM integrity reductions in the right ILF in DP subjects; instead, they found that DP arises from the local WM difference in the right FFA, rather than any long-range WM tracts.
A few explanations can account for these discrepancies. One likely factor is the low statistical power in both studies (n=6 in Thomas et al., 2009; n=8 in Gomez et al., 2015). Another possibility comes from differences in data quality and analysis methods. Thomas et al., (2009) collected diffusion data with only 6 gradient directions and analyzed the data with simplistic deterministic tractography, while Gomez et al., (2015) employed 30 gradient directions and probabilistic tractography. These interpretations are further supported by a recent study (Song et al., 2015) using a larger sample size (n=16), optimized scanning parameters (61 directions), and multiple analysis methods (deterministic tractography, probabilistic tractograpphy, and voxel-wise comparison). Consistent with Gomez et al., (2015), they found no differences on any of the WM measures in the right ILF between the DP and control group, but local WM differences in the right FFA accounted for the face perception deficit in DP. Thus, it seems that small sample size, poor data acquisition, and the relatively simple tractography method prevented the findings in Thomas et al., (2009) from being replicated.
In summary, as the sample size, data quality, and analysis methods are significantly improving, we expect replicability in this field to similarly improve (De Santis et al., 2014; Jones et al., 2013; Poldrack et al., 2017). In Table 7, we describe common problems in the field, and offer some practical solutions to these challenges.
Table 7.
Nine recommendations for future dMRI studies of social cognition
| Recommendations | Rationales and comments |
|---|---|
| 1. Test for conceptual generality of structure-function relationship | Different social processes are inherently intertwined, thus most behavioral measures and paradigms might tap multiple constructs, making interpretation difficult (Happé et al., 2017). Moreover, our review of the literature shows that disparate tasks are used to test identical constructs, making it difficult to generalize across studies. Leverage can be gained by using a multi-measurement approach, that hones in on the construct of interest, for each study. |
| 2. Test for specificity of structure-function relationship | To do this, control tasks (e.g. non-social tasks with equivalent cognitive demands) and control tracts (non-social tracts such as CST) should also be included in a study’s design and analysis. |
| 3. Acquire robust data with sufficient measurement and statistical power | Poor data quality in dMRI (e.g. noise, artifacts, and data under-sampling) often leads to errors in tensor estimation and, consequently, in diffusion maps that give rise to fiber reconstructions with erroneous orientations or lengths. Some general guidelines for best practices in data acquisition include: minimally sampling along 30 unique gradient directions (60+ directions is better) and using a b-value of at least 1000 s/mm2 (even better, multiple b-values with some up to 2000–3000 s/mm 2) (Jones et al., 2013). In addition, white matter research requires a sufficiently large sample size (n>30) to robustly reveal the relationship between fiber tracts and inter-subject variability of behavior (De Santis et al., 2014). In our literature review, more than one third of the studies used protocols that did not meet these standards (Fig. 4), which significantly undermines their reliability. The findings from these studies should not be weighted very heavily when determining a consensus. |
| 4. Use advanced analytic methods to analyze diffusion data | Human white matter is extremely difficult to model, due to its high density (e.g. more than 10,000 pathways with distinct origins and terminations, Jbabdi et al., 2015), complex trajectory patterns (e.g. 90% of white matter voxels contains crossing fibers, Jeurissen et al., 2013), and intricate organizations in its cortical origins/terminations (e.g. superficial white matter bundles running parallel to the cortex, impeding the detection of fibers entering the cortex, Reveley et al., 2015). Despite these complexities, 50% of tractography studies in our literature review used a single tensor model with a deterministic tracing algorithm, which only calculated a single principle diffusion direction in each voxel. This method is too simplistic for characterizing white matter microstructure in crossing fiber voxels and can lead to erroneous fiber trajectory reconstructions. Optimal approaches include high-angular-resolution diffusion imaging with multi-shell acquisition (i.e. multiple b-values) and more complex biophysical models such as multi-tensor models and ball-and-stick models, or utilizing advanced “model-free” techniques such as Q-Ball Imaging and diffusion spectral imaging (Wandell, 2016). These sophisticated tools are much easier to implement with recent advances in scanner technology and are becoming popular in other fields of neuroscience such as vision research (e.g. Rokem et al., 2017). Thus far, there are only three social neuroscience studies employing these optimal tools (Anderson et al., 2015; Olszewski et al., 2017; Pyles et al., 2013). Using these methods will significantly improve reliability and reproducibility compared to the tensor model. |
| 5. Control for confounding variables by matching or regressing | White matter measurements can be measurably affected by participants’ age (Cabinio et al., 2015; Charlton et al., 2009), gender (Chou et al., 2011), handedness (McKay et al., 2017), socioeconomic status (Ursache and Noble, 2016), intelligence (Penke et al., 2012) and head motion (Yendiki et al., 2014). These factors should be matched between groups or regressed out. |
| 6. Avoid simplistic interpretations of FA | Although FA may reflect fiber integrity, it can also be confounded by factors that do not necessarily reflect white matter integrity, such as partial volume effects (i.e., signal mixing of white matter, gray matter, and cerebrospinal fluid), or heterogeneity in the orientation of axons (Jbabdi et al., 2015). Therefore, any notion of being able to relate FA to behavioral performance in a linear fashion is flawed, because higher FA is not always associated with superior social performance (Imfeld et al., 2009; Unger et al., 2016). When exploring the white matter correlates of individual differences, researchers should not misinterpret that those participants with higher FA have “better” structure connectivity. Even in a situation in which higher FA indeed reflects superior white matter connectivity, it is impossible to discern whether a pathway is inhibitory or excitatory. Therefore, lower or higher FA values must be interpreted in the context of the known functions of a pathway and its connecting regions (Roberts et al., 2013). |
| 7. Don’t just focus on FA; provide information about other white matter indices | Most studies in the literature only report results based on FA (Figure 4), which may be insufficient to fully characterize the underlying changes in white matter microstructure. Additional dMRI measures, including MD, AD, RD, and volume, may be more informative regarding the specific nature of white matter changes and dysfunction. It has been argued that all DTI measures (e.g. MD, AD, RD, etc.) should be routinely analyzed and reported even if some are not statistically significant (Alexander et al., 2007). We strongly support this practice and believe reliable interpretation of dMRI research requires a complete and comprehensive report of white matter measures. |
| 8. Provide detailed information about white matter anatomy | Large tracts like the SLF are composed of distinct subsections (e.g. SLF I—SLF III and AF, Kamali et al., 2014) and for each subsection, many fasciculi enter and exit at different points such that not all WM bundles traverse the full length of the tract. Since different subsections of a tract might be responsible for different cognitive functions (Metoki et al., 2017; Tavor et al., 2014), it is important for researchers to report sufficient information about the precise location of their results (anterior or posterior portion of the ILF; genu or splenium of the CC; parahippocampal portion of the cingulum). |
| 9. Consider using functionally defined seed regions for tractography | This will help to identify subtracts of large fasciculi that are specific to particular social processes (e.g. the pathway between face-selective STS and IFG for social gaze perception). |
6. Future Directions
Many social functions have only received a cursory examination in regards to their white matter. A deeper understanding of the structural networks underlying social bonding, for instance, would be useful for understanding several social disorders. More generally, we feel that research on WM can open new avenues for testing social neuroscience theories. Theories that are most amenable to this endeavor are ones that propose some sort of ordered processing. For instance, the Haxby model is considered the most dominant neural theory of face processing (Gobbini and Haxby, 2007; Haxby et al., 2000). According to this model, the OFA, FFA, and STS constitute the core system that subserves the visual analysis of faces, and more anterior brain regions (ATL, AMG, and OFC) comprise the extended system that gleans other information from faces, such as their emotional and personal significance. This model postulates a hierarchical structure such that the OFA projects to both the FFA and STS, and each plays a different role in face processing (Bernstein and Yovel, 2015). After being processed by the core system, information is then sent to the extended system to extract biographical knowledge, analyze emotional information, or to evaluate facial attractiveness.
However, recent WM research challenges this framework. Gschwind et al., (2012) revealed several direct WM pathways between early visual cortices and face-selective regions without any mediation by the OFA, and some of these pathways were even stronger than connections with the OFA. This signifies that face processing might not proceed in sequence, but rather in a parallel and interactive fashion. The observation of multiple pathways to each face-selective region also helps explain cases of neuropsychological patients who have lost bilateral OFA but FFA and STS remain sensitive to faces (Duchaine and Yovel, 2015). Moreover, several studies demonstrated that direct structural connections only exist between the OFA and FFA, but not between the OFA and STS or between the FFA and STS (Gschwind et al., 2012; Pyles et al., 2013). This suggests that the OFA and FFA are tightly connected, but the STS seems to be more isolated within the core system. Taken together, these findings warrant a revised framework to the Haxby model (Bernstein and Yovel, 2015; Duchaine and Yovel, 2015).
This example illustrates how investigations of structural connectivity can provide unique insights into the underlying organization of various social networks. This approach has only been applied to a small number of theories, such as the dual-stream model of empathy (Herbet et al., 2015b; Parkinson and Wheatley, 2014) and mentalizing (Herbet et al., 2014). Future research should continue to test theoretical models in social neuroscience from the perspective of structural connectivity.
7. Concluding Remarks
Research on structural connectivity in social neuroscience is a promising field for insights into the anatomical basis of social cognition, social behavior, and social disorders. In the present article, we comprehensively reviewed past literature to summarize the reported WM structures associated with social cognition and also empirically employed probabilistic tractography to elucidate major WM tracts scaffolding social brain networks. These two approaches demonstrated a converging group of tracts critical for face processing (the ILF, IFOF and SLF), embodied cognition (the SLF, UF, ATR and IFOF), and ToM (the cingulum, SLF/AF, UF, IFOF and ILF) (Fig. 5). In addition, our review introduces multiple facets of research on structural connectivity in social neuroscience, covering a wide array of approaches and applications. However, the main bottleneck of this exciting field is still the limited sample size, poor data quality, and simplistic analysis methods, which could be potentially addressed by utilizing large open datasets, such as the Human Connectome Project (Van Essen et al., 2013) and UK biobank (Miller et al., 2016). Nevertheless, we are optimistic that the current paradigm shift towards connectivity will bring with it higher-quality data and a larger corpus of findings relevant to white matter and social neuroscience.
Supplementary Material
Highlights.
First paper to highlight the importance of white matter on social cognition
Systematic review of existing white matter research in social neuroscience
The connectivity profiles of face, mirroring, and mentalizing networks are elucidated
The field is bottlenecked by limited sample size, poor data quality, and simplistic methods
Acknowledgments
This work was supported by a National Institute of Health grant to I. Olson [RO1 MH091113]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Spezio M. Chapter 20 Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Tromp DPM, Zakszewski E, Field AS. Characterization of Cerebral White Matter Properties Using Quantitative Magnetic Resonance Imaging Stains. Brain Connect. 2011;1:423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion Tensor Imaging of the Brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm KH, Rolheiser T, Mohamed FB, Olson IR. Fronto-temporal white matter connectivity predicts reversal learning errors. Front Hum Neurosci. 2015:9. doi: 10.3389/fnhum.2015.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm KH, Rolheiser T, Olson IR. Inter-individual variation in fronto-temporal connectivity predicts the ability to learn different types of associations. Neuroimage. 2016;132:213–224. doi: 10.1016/j.neuroimage.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LC, Rice K, Chrabaszcz J, Redcay E. Tracking the Neurodevelopmental Correlates of Mental State Inference in Early Childhood. Dev Neuropsychol. 2015;40:379–394. doi: 10.1080/87565641.2015.1119836. [DOI] [PubMed] [Google Scholar]
- Ashtari M. Anatomy and functional role of the inferior longitudinal fasciculus: A search that has just begun. Dev Med Child Neurol. 2012;54:6–7. doi: 10.1111/j.1469-8749.2011.04122.x. [DOI] [PubMed] [Google Scholar]
- Avidan G, Tanzer M, Hadj-Bouziane F, Liu N, Ungerleider LG, Behrmann M. Selective dissociation between core and extended regions of the face processing network in congenital prosopagnosia. Cereb Cortex. 2014;24:1565–1578. doi: 10.1093/cercor/bht007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio HC, Segura B, Ibarretxe-Bilbao N, Valldeoriola F, Marti MJ, Compta Y, Tolosa E, Junqué C. Structural correlates of facial emotion recognition deficits in Parkinson’s disease patients. Neuropsychologia. 2012;50:2121–2128. doi: 10.1016/j.neuropsychologia.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Paul EJ, Chau A, Solomon J, Grafman JH. Lesion mapping of social problem solving. Brain. 2014;137:2823–2833. doi: 10.1093/brain/awu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. Autism: The empathizing-systemizing (E-S) theory. Ann N Y Acad Sci. 2009;1156:68–80. doi: 10.1111/j.1749-6632.2009.04467.x. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. Large-scale brain networks in affective and social neuroscience: Towards an integrative functional architecture of the brain. Curr Opin Neurobiol. 2013;23:361–372. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen JACJ, Thioux M, Keysers C. Evidence for mirror systems in emotions. Philos Trans R Soc B Biol Sci. 2009;364:2391–2404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Nash K, Hill C, Knoch D. Neuroanatomy of intergroup bias: A white matter microstructure study of individual differences. Neuroimage. 2015;122:345–354. doi: 10.1016/j.neuroimage.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Marotta JJ, Kimchi R. Detailed Exploration of Face-related Processing in Congenital Prosopagnosia: 1. Behavioral Findings. J Cogn Neurosci. 2005;17:1130–1149. doi: 10.1162/0898929054475154. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Singer T. The Neural Basis of Empathy. Annu Rev Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Bernstein M, Yovel G. Two neural pathways of face processing: A critical evaluation of current models. Neurosci Biobehav Rev. 2015;55:536–546. doi: 10.1016/j.neubiorev.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Westlye LT, Fjell AM, Grydeland H, Walhovd KB. Social reward dependence and brain white matter microstructure. Cereb Cortex. 2012;22:2672–2679. doi: 10.1093/cercor/bhr345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bonini L. The Extended Mirror Neuron Network. Neurosci. 2017;23:56–67. doi: 10.1177/1073858415626400. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabinio M, Rossetto F, Blasi V, Savazzi F, Castelli I, Massaro D, Valle A, Nemni R, Clerici M, Marchetti A, Baglio F. Mind-reading ability and structural connectivity changes in aging. Front Psychol. 2015;6:1–10. doi: 10.3389/fpsyg.2015.01808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp. 2009;30:2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37:1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Chan AWY, Downing PE. Faces and Eyes in Human Lateral Prefrontal Cortex. Front Hum Neurosci. 2011;5:1–10. doi: 10.3389/fnhum.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez RS, Heatherton TF. Structural integrity of frontostriatal connections predicts longitudinal changes in self-esteem. Soc Neurosci. 2017;12:280–286. doi: 10.1080/17470919.2016.1164753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KH, Cheng Y, Chen IY, Lin CP, Chu WC. Sex-linked white matter microstructure of the social and analytic brain. Neuroimage. 2011;54:725–733. doi: 10.1016/j.neuroimage.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Jan-Christoph Schoene-Bake, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2008;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- Collins JA, Olson IR. Beyond the FFA: The role of the ventral anterior temporal lobes in face processing. Neuropsychologia. 2014;61:65–79. doi: 10.1016/j.neuropsychologia.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini A, Antonietti A. Mirror neurons and their function in cognitively understood empathy. Conscious Cogn. 2013;22:1152–1161. doi: 10.1016/j.concog.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Crespi C, Cerami C, Dodich A, Canessa N, Arpone M, Iannaccone S, Corbo M, Lunetta C, Scola E, Falini A, Cappa SF. Microstructural white matter correlates of emotion recognition impairment in Amyotrophic Lateral Sclerosis. Cortex. 2014;53:1–8. doi: 10.1016/j.cortex.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Crespi C, Cerami C, Dodich A, Canessa N, Iannaccone S, Corbo M, Lunetta C, Falini A, Cappa SF. Microstructural correlates of emotional attribution impairment in non-demented patients with amyotrophic lateral sclerosis. PLoS One. 2016;11:1–14. doi: 10.1371/journal.pone.0161034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ES, Ramsey R, Liepelt R, Prinz W, de Hamilton AFC. The shaping of social perception by stimulus and knowledge cues to human animacy. Philos Trans R Soc B Biol Sci. 2016;371:20150075. doi: 10.1098/rstb.2015.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daianu M, Mendez MF, Baboyan VG, Jin Y, Melrose RJ, Jimenez EE, Thompson PM. An advanced white matter tract analysis in frontotemporal dementia and early-onset Alzheimer???s disease. Brain Imaging Behav. 2016;10:1038–1053. doi: 10.1007/s11682-015-9458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–126. doi: 10.1037/0022-3514.44.1.113. [DOI] [Google Scholar]
- De Santis S, Drakesmith M, Bells S, Assaf Y, Jones DK. Why diffusion tensor MRI does well only some of the time: Variance and covariance of white matter tissue microstructure attributes in the living human brain. Neuroimage. 2014;89:35–44. doi: 10.1016/j.neuroimage.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Bernal B, Tremblay P. The language connectome: New pathways, new concepts. Neuroscientist. 2014;20:453–467. doi: 10.1177/1073858413513502. [DOI] [PubMed] [Google Scholar]
- Douglas Fields R. White Matter Matters. Sci Am. 2008;298:54–61. doi: 10.1038/scientificamerican0308-54. [DOI] [PubMed] [Google Scholar]
- Downey LE, Mahoney CJ, Buckley AH, Golden HL, Henley SM, Schmitz N, Schott JM, Simpson IJ, Ourselin S, Fox NC, Crutch SJ, Warren JD. White matter tract signatures of impaired social cognition in frontotemporal lobar degeneration. NeuroImage Clin. 2015;8:640–651. doi: 10.1016/j.nicl.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine B, Yovel G. A Revised Neural Framework for Face Processing. Annu Rev Vis Sci. 2015;1:393–416. doi: 10.1146/annurev-vision-082114-035518. [DOI] [PubMed] [Google Scholar]
- Duffau H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol. 2015;11:255–265. doi: 10.1038/nrneurol.2015.51. [DOI] [PubMed] [Google Scholar]
- Erus G, Battapady H, Satterthwaite TD, Hakonarson H, Gur RE, Davatzikos C, Gur RC. Imaging patterns of brain development and their relationship to cognition. Cereb Cortex. 2015;25:1676–1684. doi: 10.1093/cercor/bht425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Gschwind M, Kreifelts B, Wildgruber D, Vuilleumier P. Emotional voice areas: Anatomic location, functional properties, and structural connections revealed by combined fMRI/DTI. Cereb Cortex. 2012;22:191–200. doi: 10.1093/cercor/bhr113. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Wiethoff S, Bisch J, Schlipf S, Wildgruber D, Kreifelts B. Functional responses and structural connections of cortical areas for processing faces and voices in the superior temporal sulcus. Neuroimage. 2013;76:45–56. doi: 10.1016/j.neuroimage.2013.02.064. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Gschwind M, Vuilleumier P. Processing social aspects of human gaze: A combined fMRI-DTI study. Neuroimage. 2011;55:411–419. doi: 10.1016/j.neuroimage.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Feldman HM, Yeatman JD, Lee ES, Barde LHF, Gaman-Bean S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. J Dev Behav Pediatr. 2010;31:346–356. doi: 10.1097/DBP.0b013e3181dcaa8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Chapter 10 – White-matter pathways for speech and language processing. Handbook of Clinical Neurology. 2015:177–186. doi: 10.1016/B978-0-444-62630-1.00010-X. [DOI] [PubMed] [Google Scholar]
- Fujie S, Namiki C, Nishi H, Yamada M, Miyata J, Sakata D, Sawamoto N, Fukuyama H, Hayashi T, Murai T. The role of the uncinate fasciculus in memory and emotional recognition in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;26:432–439. doi: 10.1159/000165381. [DOI] [PubMed] [Google Scholar]
- Fujino J, Takahashi H, Miyata J, Sugihara G, Kubota M, Sasamoto A, Fujiwara H, Aso T, Fukuyama H, Murai T. Impaired empathic abilities and reduced white matter integrity in schizophrenia. Prog Neuro-Psychopharmacology Biol Psychiatry. 2014;48:117–123. doi: 10.1016/j.pnpbp.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Gallese V. Before and below “theory of mind”: embodied simulation and the neural correlates of social cognition. Philos Trans R Soc B Biol Sci. 2007;362:659–669. doi: 10.1098/rstb.2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova HM, Rajagopalan V, Chiaravalloti N, Binder A, Deluca J, Lengenfelder J. Facial affect recognition linked to damage in specific white matter tracts in traumatic brain injury. Soc Neurosci. 2015;10:27–34. doi: 10.1080/17470919.2014.959618. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Gomez J, Pestilli F, Witthoft N, Golarai G, Liberman A, Poltoratski S, Yoon J, Grill-Spector K. Functionally Defined White Matter Reveals Segregated Pathways in Human Ventral Temporal Cortex Associated with Category-Specific Processing. Neuron. 2015;85:216–228. doi: 10.1016/j.neuron.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Liencres C, Shamay-Tsoory SG, Brune M. Towards a neuroscience of empathy: Ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neurosci Biobehav Rev. 2013;37:1537–1548. doi: 10.1016/j.neubiorev.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven IM, Ramsey R. Person perception involves functional integration between the extrastriate body area and temporal pole. Neuropsychologia. 2017;96:52–60. doi: 10.1016/j.neuropsychologia.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Grosse Wiesmann C, Schreiber J, Singer T, Steinbeis N, Friederici AD. White matter maturation is associated with the emergence of Theory of Mind in early childhood. Nat Commun. 2017;8:14692. doi: 10.1038/ncomms14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi D, Soricelli A, Ponari M, Salvatore E, Quarantelli M, Prinster A, Trojano L. Structural connectivity in a single case of progressive prosopagnosia: The role of the right inferior longitudinal fasciculus. Cortex. 2014;56:111–120. doi: 10.1016/j.cortex.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Gschwind M, Pourtois G, Schwartz S, Van De Ville D, Vuilleumier P. White-matter connectivity between face-responsive regions in the human brain. Cereb Cortex. 2012;22:1564–1576. doi: 10.1093/cercor/bhr226. [DOI] [PubMed] [Google Scholar]
- Hamilton AFDC, Grafton ST. Goal Representation in Human Anterior Intraparietal Sulcus. J Neurosci. 2006;26:1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton WH, Unger A, Von Der Heide RJ, Olson IR. Neural connections foster social connections: a diffusion-weighted imaging study of social networks. Soc Cogn Affect Neurosci. 2016;11:721–727. doi: 10.1093/scan/nsv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F, Vry MS, Saur D, Glauche V, Hoeren M, Mader I, Weiller C, Rijntjes M. The Dual-Loop Model and the Human Mirror Neuron System: An Exploratory Combined fMRI and DTI Study of the Inferior Frontal Gyrus. Cereb Cortex. 2016;26:2215–2224. doi: 10.1093/cercor/bhv066. [DOI] [PubMed] [Google Scholar]
- Happé F, Cook JL, Bird G. The Structure of Social Cognition: In(ter)dependence of Sociocognitive Processes. Annu Rev Psychol. 2017;68:243–267. doi: 10.1146/annurev-psych-010416-044046. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/S1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hecht EE, Gutman DA, Bradley BA, Preuss TM, Stout D. Virtual dissection and comparative connectivity of the superior longitudinal fasciculus in chimpanzees and humans. Neuroimage. 2015;108:124–137. doi: 10.1016/j.neuroimage.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht EE, Gutman DA, Preuss TM, Sanchez MM, Parr LA, Rilling JK. Process versus product in social learning: Comparative diffusion tensor imaging of neural systems for action execution-observation matching in macaques, chimpanzees, and humans. Cereb Cortex. 2013;23:1014–1024. doi: 10.1093/cercor/bhs097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Bonnetblanc F, Moritz-Gasser S, Menjot De Champfleur N, Duffau H. Inferring a dual-stream model of mentalizing from associative white matter fibres disconnection. Brain. 2014;137:944–959. doi: 10.1093/brain/awt370. [DOI] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Moritz-Gasser S, Bonnetblanc F, Duffau H. Interfering with the neural activity of mirror-related frontal areas impairs mentalistic inferences. Brain Struct Funct. 2015a;220:2159–2169. doi: 10.1007/s00429-014-0777-x. [DOI] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Moritz-Gasser S, Menjot de Champfleur N, Costi E, Bonnetblanc F, Duffau H. A disconnection account of subjective empathy impairments in diffuse low-grade glioma patients. Neuropsychologia. 2015b;70:165–176. doi: 10.1016/j.neuropsychologia.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Horn A, Ostwald D, Reisert M, Blankenburg F. The structural-functional connectome and the default mode network of the human brain. Neuroimage. 2014;102:142–151. doi: 10.1016/j.neuroimage.2013.09.069. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Imitation, Empathy, and Mirror Neurons. Annu Rev Psychol. 2009a;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neurobiology of imitation. Curr Opin Neurobiol. 2009b;19:661–5. doi: 10.1016/j.conb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Miyakoshi M, Harada T, Nakai T. White matter connectivity between superior temporal sulcus and amygdala is associated with autistic trait in healthy humans. Neurosci Lett. 2012;510:154–158. doi: 10.1016/j.neulet.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Ishai A. Let’s face it: It’s a cortical network. Neuroimage. 2008;40:415–419. doi: 10.1016/j.neuroimage.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M, Carter C, Senturk D, Chow C, Hopkins JM, Green MF, Galván A, Cannon TD, Bearden CE. Social cognition in 22q11.2 microdeletion syndrome: Relevance to psychosis? Schizophr Res. 2012;142:99–107. doi: 10.1016/j.schres.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Villalon-Reina JE, Karlsgodt KH, Senturk D, Chow C, Thompson PM, Bearden CE. Altered white matter microstructure is associated with social cognition and psychotic symptoms in 22q11.2 microdeletion syndrome. Front Behav Neurosci. 2014;8:1–18. doi: 10.3389/fnbeh.2014.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S, Sotiropoulos SN, Haber SN, Van Essen DC, Behrens TE. Measuring macroscopic brain connections in vivo. Nat Neurosci. 2015;18:1546–1555. doi: 10.1038/nn.4134. [DOI] [PubMed] [Google Scholar]
- Jones DK. Challenges and limitations of quantifying brain connectivity in vivo with diffusion MRI. Imaging Med. 2010;2:341–355. doi: 10.2217/iim.10.21. [DOI] [Google Scholar]
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23:803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219:269–281. doi: 10.1007/s00429-012-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Hu CP, Deshpande HD, Colburn JS. Functional brain networks and white matter underlying theory-of-mind in autism. Soc Cogn Affect Neurosci. 2014;9:98–105. doi: 10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Bockler A, Trautwein FM, Singer T. Dissecting the social brain: Introducing the EmpaToM to reveal distinct neural networks and brain-behavior relations for empathy and Theory of Mind. Neuroimage. 2015;122:6–19. doi: 10.1016/j.neuroimage.2015.07.082. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels RPC, Gerritsen L, Montagne B, Ackl N, Diehl J, Danek A. Recognition of facial expressions of different emotional intensities in patients with frontotemporal lobar degeneration. Behav Neurol. 2007;18:31–6. doi: 10.1155/2007/868431. doi: http://dx.doi.org/10.1155/2007/868431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MS, Vorobyev V, Moe D, Parkkola R, Hamalainen H. Brain structural correlates of risk-taking behavior and effects of peer influence in adolescents. PLoS One. 2014;9:20–24. doi: 10.1371/journal.pone.0112780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Majdandžić J. The role of shared neural activations, mirror neurons, and morality in empathy - A critical comment. Neurosci Res. 2015;90:15–24. doi: 10.1016/j.neures.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Hanten G, Li X, Chu ZD, Vásquez AC, Cook L, Yallampalli R, Hunter JV. Mental State Attributions and Diffusion Tensor Imaging After Traumatic Brain Injury in Children. Dev Neuropsychol. 2011;36:273–287. doi: 10.1080/87565641.2010.549885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mai X, Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front Hum Neurosci. 2014;8:1–15. doi: 10.3389/fnhum.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang P, Yin L, Shu N, Zhao T, Xing Y, Li F, Zhao Z, Li K, Han Y. White matter abnormalities in two different subtypes of amnestic mild cognitive impairment. PLoS One. 2017;12:1–12. doi: 10.1371/journal.pone.0170185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YC, Chen YJ, Hsu YC, Tseng WYI, Gau SSF. Reduced tract integrity of the model for social communication is a neural substrate of social communication deficits in autism spectrum disorder. J Child Psychol Psychiatry Allied Discip. 2017;58:576–585. doi: 10.1111/jcpp.12641. [DOI] [PubMed] [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44:950–958. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mar RA. The Neural Bases of Social Cognition and Story Comprehension. Annu Rev Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MFS. On the relationship between the “default mode network” and the “social brain. Front Hum Neurosci. 2012;6:1–9. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marstaller L, Burianova H, Reutens DC. Individual differences in structural and functional connectivity predict speed of emotion discrimination. Cortex. 2016;85:65–74. doi: 10.1016/j.cortex.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Mende-siedlecki P, Said CP, Todorov A. The social evaluation of faces: A meta-analysis of functional neuroimaging studies. Soc Cogn Affect Neurosci. 2013;8:285–299. doi: 10.1093/scan/nsr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant RE. Encyclopedia of Clinical Neuropsychology. Springer New York; New York, NY: 2011. Superior Longitudinal Fasciculus; pp. 2435–2436. [DOI] [Google Scholar]
- Metoki A, Alm KH, Wang Y, Ngo CT, Olson IR. Never forget a name : white matter connectivity predicts person memory. Brain Struct Funct. 2017 doi: 10.1007/s00429-017-1458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mike A, Strammer E, Aradi M, Orsi G, Perlaki G, Hajnal A, Sandor J, Banati M, Illes E, Zaitsev A, Herold R, Guttmann CRG, Illes Z. Disconnection mechanism and regional cortical atrophy contribute to impaired processing of facial expressions and theory of mind in multiple sclerosis: A structural MRI study. PLoS One. 2013:8. doi: 10.1371/journal.pone.0082422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, Bartsch AJ, Jbabdi S, Sotiropoulos SN, Andersson JLR, Griffanti L, Douaud G, Okell TW, Weale P, Dragonu I, Garratt S, Hudson S, Collins R, Jenkinson M, Matthews PM, Smith SM. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19:1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Johnson H, Henry JD, Mattingley JB. Understanding the minds of others: A neuroimaging meta-analysis. Neurosci Biobehav Rev. 2016;65:276–291. doi: 10.1016/j.neubiorev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr Biol. 2010;20:750–6. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Takeuchi H, Taki Y, Nouchi R, Sekiguchi A, Kotozaki Y, Miyauchi CM, Iizuka K, Yokoyama R, Shinada T, Yamamoto Y, Hanawa S, Araki T, Hashizume H, Kunitoki K, Sassa Y, Kawashima R. White matter structures associated with loneliness in young adults. Sci Rep. 2015;5:17001. doi: 10.1038/srep17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal PM, Mermillod M, Maringer M, Hess U. The Simulation of Smiles (SIMS) model: Embodied simulation and the meaning of facial expression. Behav Brain Sci. 2010;33:417–433. doi: 10.1017/S0140525X10000865. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria AV, Hsu J, Tippett D, Mori S, Hillis AE. Critical role of the right uncinate fasciculus in emotional empathy. Ann Neurol. 2015;77:68–74. doi: 10.1002/ana.24300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Von Der Heide RJ, Alm KH, Vyas G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski AK, Kikinis Z, Gonzalez CS, Coman IL, Makris N, Gong X, Rathi Y, Zhu A, Antshel KM, Fremont W, Kubicki MR, Bouix S, Shenton ME, Kates WR. The social brain network in 22q11.2 deletion syndrome: a diffusion tensor imaging study. Behav Brain Funct. 2017;13:4. doi: 10.1186/s12993-017-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson C, Wheatley T. Relating anatomical and social connectivity: White matter microstructure predicts emotional empathy. Cereb Cortex. 2014;24:614–625. doi: 10.1093/cercor/bhs347. [DOI] [PubMed] [Google Scholar]
- Parlatini V, Radua J, Dell’Acqua F, Leslie A, Simmons A, Murphy DG, Catani M, Thiebaut de Schotten M. Functional segregation and integration within fronto-parietal networks. Neuroimage. 2017;146:367–375. doi: 10.1016/j.neuroimage.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Perlstein MD, Chohan MR, Coman IL, Antshel KM, Fremont WP, Gnirke MH, Kikinis Z, Middleton FA, Radoeva PD, Shenton ME, Kates WR. White matter abnormalities in 22q11.2 deletion syndrome: Preliminary associations with the Nogo-66 receptor gene and symptoms of psychosis. Schizophr Res. 2014;152:117–123. doi: 10.1016/j.schres.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to Association Fiber Tracts Impairs Recognition of the Facial Expression of Emotion. J Neurosci. 2009;29:15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Exp Brain Res. 2011;209:481–493. doi: 10.1007/s00221-011-2579-1. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline JB, Vul E, Yarkoni T. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18:115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyles JA, Verstynen TD, Schneider W, Tarr MJ. Explicating the Face Perception Network with White Matter Connectivity. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0061611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoeva PD, Coman IL, Antshel KM, Fremont W, McCarthy CS, Kotkar A, Wang D, Shprintzen RJ, Kates WR. Atlas-based white matter analysis in individuals with velo-cardio-facial syndrome (22q11.2 deletion syndrome) and unaffected siblings. Behav Brain Funct. 2012;8:38. doi: 10.1186/1744-9081-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, Leopold DA, Ye FQ. Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc Natl Acad Sci. 2015;112:E2820–E2828. doi: 10.1073/pnas.1418198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Cattaneo L, Fabbri-Destro M, Rozzi S. Cortical Mechanisms Underlying the Organization of Goal-Directed Actions and Mirror Neuron-Based Action Understanding. Physiol Rev. 2014;94:655–706. doi: 10.1152/physrev.00009.2013. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 2010;11:264–74. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Anderson EJ, Husain M. White Matter Microstructure and Cognitive Function. Neurosci. 2013;19:8–15. doi: 10.1177/1073858411421218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokem A, Bock AS, Scherf KS, Wandell BA, Bridge H. The visual white matter : The application of diffusion MRI and fiber tractography to vision science. J Vis. 2017;17:1–30. doi: 10.1167/17.2.4.doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Kubicki M, Koerte I, Otsuka T, Rathi Y, Pasternak O, Bouix S, Eckbo R, Kikinis Z, von Hohenberg CC, Roppongi T, Del Re E, Asami T, Lee SH, Karmacharya S, Mesholam-Gately RI, Seidman LJ, Levitt J, McCarley RW, Shenton ME, Niznikiewicz MA. Impaired white matter connectivity between regions containing mirror neurons, and relationship to negative symptoms and social cognition, in patients with first-episode schizophrenia. Brain Imaging Behav. 2017:1–9. doi: 10.1007/s11682-017-9685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: Anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 2013;218:21–37. doi: 10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: The role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Wilde EA, McClelland MM, Hanten G, Krawczyk DC, Cook LG, Chu ZD, Vásquez AC, Yallampalli R, Lin X, Hunter JV, Levin HS. Brain activation during a social attribution task in adolescents with moderate to severe traumatic brain injury. Soc Neurosci. 2011;6:582–598. doi: 10.1080/17470919.2011.588844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Thomas C, Doyle J, Behrmann M. Emerging structure-function relations in the developing face processing system. Cereb Cortex. 2014;24:2964–2980. doi: 10.1093/cercor/bht152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Schuz A, Braitenberg V. The human cortical white matter: quantitative aspects of cortico-cortical long-range connectivity. In: Shuez A, Miller R, editors. Cortical Areas: Unity and Diversity. Taylor & Francis; London: 2002. pp. 377–384. [DOI] [Google Scholar]
- Sethi A, Gregory S, Dell’Acqua F, Periche Thomas E, Simmons A, Murphy DGM, Hodgins S, Blackwood NJ, Craig MC. Emotional detachment in psychopathy: Involvement of dorsal default-mode connections. Cortex. 2015;62:11–19. doi: 10.1016/j.cortex.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The Neural Bases for Empathy. Neurosci. 2011;17:18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. 2013;7:1–14. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Garrido L, Nagy Z, Mohammadi S, Steel A, Driver J, Dolan RJ, Duchaine B, Furl N. Local but not long-range microstructural differences of the ventral temporal cortex in developmental prosopagnosia. Neuropsychologia. 2015;78:195–206. doi: 10.1016/j.neuropsychologia.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos SN, Zalesky A. Building connectomes using diffusion MRI: Why, how and but. NMR Biomed. 2017:1–23. doi: 10.1002/nbm.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperduti M, Guionnet S, Fossati P, Nadel J. Mirror Neuron System and Mentalizing System connect during online social interaction. Cogn Process. 2014;15:307–316. doi: 10.1007/s10339-014-0600-x. [DOI] [PubMed] [Google Scholar]
- Spoletini I, Marra C, Di Iulio F, Gianni W, Sancesario G, Giubilei F, Trequattrini A, Bria P, Caltagirone C, Spalletta G. Facial Emotion Recognition Deficit in Amnestic Mild Cognitive Impairment and Alzheimer Disease. Am J Geriatr Psychiatry. 2008;16:389–398. doi: 10.1097/JGP.0b013e318165dbce. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Satpute AB, Lieberman MD. Identifying the What, Why, and How of an Observed Action: An fMRI Study of Mentalizing and Mechanizing during Action Observation. J Cogn Neurosci. 2011;23:63–74. doi: 10.1162/jocn.2010.21446. [DOI] [PubMed] [Google Scholar]
- Takemura H, Caiafa CF, Wandell BA, Pestilli F. Ensemble Tractography. PLoS Comput Biol. 2016;12:1–22. doi: 10.1371/journal.pcbi.1004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Thyreau B, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R. White matter structures associated with empathizing and systemizing in young adults. Neuroimage. 2013;77:222–236. doi: 10.1016/j.neuroimage.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Tavor I, Yablonski M, Mezer A, Rom S, Assaf Y, Yovel G. Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. Neuroimage. 2014;86:123–130. doi: 10.1016/j.neuroimage.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Thomas AG, Koumellis P, Dineen RA. The Fornix in Health and Disease: An Imaging Review. RadioGraphics. 2011;31:1107–1121. doi: 10.1148/rg.314105729. [DOI] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung K, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009;12:29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung KJ, Peterson MA, Behrmann M. Reduction in White Matter Connectivity, Revealed by Diffusion Tensor Imaging, May Account for Age-related Changes in Face Perception. J Cogn Neurosci. 2008;20:268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp DPM, Destiche D, Doran S, Bigler ED, Lange N, Lainhart JE, Alexander AL. Diffusion Tensor Imaging in Autism Spectrum Disorder: A Review. Autism Res. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Dougherty CC, Michael AM, Olson IR. Characterization of Face-Selective Patches in Orbitofrontal Cortex. Front Hum Neurosci. 2016;10:279. doi: 10.3389/fnhum.2016.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp D. DTI Scalars (FA, MD, AD, RD) - How do they relate to brain structure? The Winnower. 2016:1–8. doi: 10.15200/WINN.146119.94778. [DOI] [Google Scholar]
- Unger A, Alm KH, Collins JA, O’Leary JM, Olson IR. Variation in White Matter Connectivity Predicts the Ability to Remember Faces and Discriminate Their Emotions. J Int Neuropsychol Soc. 2016;22:180–190. doi: 10.1017/S1355617715001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-Sosa M, Bobes MA, Quiñones I, Garcia L, Valdes-Hernandez PA, Iturria Y, Melie-Garcia L, Lopera F, Asencio J. Covert face recognition without the fusiform-temporal pathways. Neuroimage. 2011;57:1162–1176. doi: 10.1016/j.neuroimage.2011.04.057. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H. Microstructural Organization of the Cingulum Tract and the Level of Default Mode Functional Connectivity. J Neurosci. 2008;28:10844–10851. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl RCW, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. Facial expressions: What the mirror neuron system can and cannot tell us. Soc Neurosci. 2007;2:179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K. The WU-Minn Human Connectome Project: An overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA. Clarifying Human White Matter. Annu Rev Neurosci. 2016;39:103–128. doi: 10.1146/annurev-neuro-070815-013815. [DOI] [PubMed] [Google Scholar]
- Wang Y, Collins JA, Koski J, Nugiel T, Metoki A, Olson IR. A Dynamic Neural Architecture for Social Knowledge Retrieval. Proc Natl Acad Sci. 2017;114:1–46. doi: 10.1073/pnas.1621234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A, Rychlowska M, Korb S, Niedenthal P. Fashioning the Face: Sensorimotor Simulation Contributes to Facial Expression Recognition. Trends Cogn Sci. 2016;20:227–240. doi: 10.1016/j.tics.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Yang DYJ, Rosenblau G, Keifer C, Pelphrey KA. An integrative neural model of social perception, action observation, and theory of mind. Neurosci Biobehav Rev. 2015;51:263–275. doi: 10.1016/j.neubiorev.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova YN, Duffau H, Herbet G. Neural pathways subserving face-based mentalizing. Brain Struct Funct. 2017;0:1–19. doi: 10.1007/s00429-017-1388-0. [DOI] [PubMed] [Google Scholar]
- Zaki J, Hennigan K, Weber J, Ochsner KN. Social Cognitive Conflict Resolution: Contributions of Domain-General and Domain-Specific Neural Systems. J Neurosci. 2010;30:8481–8488. doi: 10.1523/JNEUROSCI.0382-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Chen H, Guo L, Li K, Li L, Zhang S, Shen D, Hu X, Liu T. Characterization of U-shape streamline fibers: Methods and applications. Med Image Anal. 2014;18:795–807. doi: 10.1016/j.media.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.