Summary
Neural stem and precursor cell (NSPC) proliferation in the rodent adult hippocampus is essential to maintain stem cell populations and produce new neurons. Retinoic acid (RA) signaling is implicated in regulation of adult hippocampal neurogenesis, but its exact role in control of NSPC behavior has not been examined. We show RA signaling in all hippocampal NSPC subtypes and that inhibition of RA synthesis or signaling significantly decreases NSPC proliferation via abrogation of cell-cycle kinetics and cell-cycle regulators. RA signaling controls NSPC proliferation through hypoxia inducible factor-1α (HIF1α), where stabilization of HIF1α concurrent with disruption of RA signaling can prevent NSPC defects. These studies demonstrate a cell-autonomous role for RA signaling in hippocampal NSPCs that substantially broadens RA's function beyond its well-described role in neuronal differentiation.
Keywords: adult neurogenesis, retinoic acid, hippocampus, neural stem cells, HIF1α, VEGFA, cell cycle
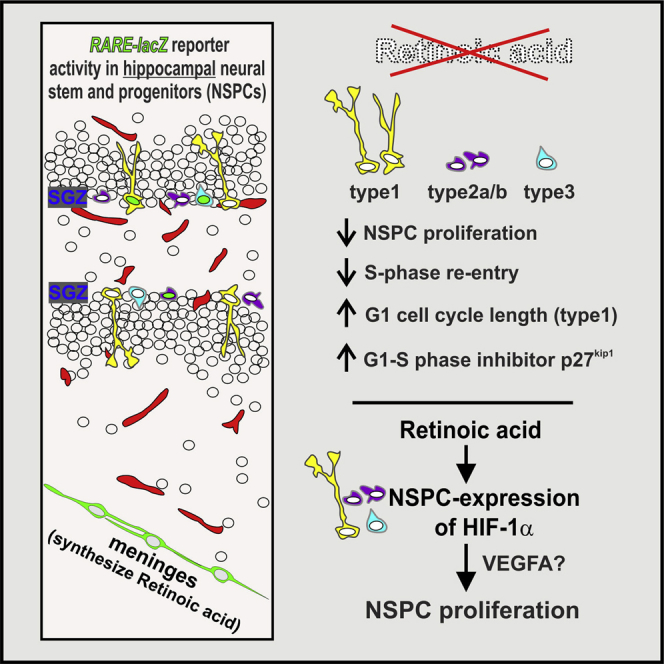
Graphical Abstract
Highlights
-
•
Retinoic acid (RA) signaling in mouse hippocampal neural stem and progenitors (NSPCs)
-
•
Systemic reduction in RA synthesis impairs NSPC proliferation and neurogenesis
-
•
HIF1α acts downstream of RA to regulate NSPC S-phase re-entry and cell-cycle genes
-
•
RA signaling is required cell autonomously in NSPCs for normal proliferation
In this article, Mishra and colleagues identify a critical role for retinoic acid (RA) signaling in adult hippocampal neural stem and progenitor cell (NSPC) proliferation via hypoxia inducible factor-1α. Using RA reporter mice and conditional disruption of RA signaling in adult NSPCs, the authors identify RA signaling in adult NSPCs and NSPC RA signaling is required for normal proliferative behavior.
Introduction
The generation of new neurons in the adult brain is required for certain aspects of learning, memory, and emotional processing (Deng et al., 2009, Revest et al., 2009). Adult neurogenesis is driven by neural stem and progenitor cells (NSPCs) located in the hippocampus, and impairments to these cells are associated with human neuropsychiatric and neurodegenerative disorders (Höglinger et al., 2004, Crews et al., 2010, Perry et al., 2012, Gomez-Nicola et al., 2014). NSPCs are located in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and generate neurons that reside in the granule cell layer and integrate into the hippocampal circuitry (Gage et al., 1998, van Praag et al., 2002). Cell extrinsic factors in the SGZ microenvironment critically regulate NSPC behavior and are produced both locally and from outside the niche. Cell extrinsic factors contributing to the NSPC microenvironment can be systemic factors delivered via blood vessels (Villeda et al., 2011, Villeda et al., 2014, Villeda and Wyss-Coray, 2013) or cerebrospinal fluid factors that cross into the subventricular zone (SVZ) niche at the ventricular surface (Silva-Vargas et al., 2016). Factors delivered at these niche interfaces influence neural stem cell (NSC) maintenance and neurogenesis. These discoveries broaden the repertoire of signals that could influence the NSC niche and highlight how far these signals could travel.
Retinoic acid (RA) is a bioactive metabolite of vitamin A that is present in the NSPC hippocampal microenvironment with a well-established role in developmental neurogenesis (Maden, 2007). While RA signaling is robust in the adult DG (Misner et al., 2001, Wagner et al., 2002, Goodman et al., 2012), RA is not synthesized by neural cells in the rodent hippocampus (Goodman et al., 2012). The meninges lining the ventral hippocampus express the retinol and retinal dehydrogenases required to produce RA and are the likely source of RA for the rodent hippocampus (Wagner et al., 2002, Goodman et al., 2012). Several studies suggest an important role for RA in adult hippocampal neurogenesis but show conflicting results. For example, rats on a chronic vitamin A deficient (VAD) diet, which prevents RA production systemically, showed decreased SGZ cell proliferation and diminished neurogenesis (Bonnet et al., 2008). Mice on a VAD diet also showed diminished neurogenesis (fewer proliferating neuroblasts, newborn granule cells, and neurons) but did not show reduced SGZ cell proliferation (Jacobs et al., 2006). A third study showed multi-week exposure to exogenous RA diminished cell proliferation in SGZ (Crandall et al., 2004). In addition to differing reports of RA's action on hippocampal NSPCs, no studies have looked at the cell-autonomous function of RA signaling in different NSPC subtypes and, as yet, there is no downstream mechanism for RA's action on NSPCs.
To examine the function of RA in adult neurogenesis, we disrupted RA synthesis systemically or RA signaling specifically in adult NSPCs. Our studies reveal an important role for RA in promoting NSPC proliferation through regulation of cell-cycle kinetics and cell-cycle proteins. We identified hypoxia inducible factor-1a (HIF1α) and its transcriptional target vascular endothelial growth factor-A (VEGFA) as key mediators of RA control of NSPC behavior. Our findings regarding RA are a significant departure from the dogma that RA acts mainly to promote neuronal differentiation and implicate RA as a hypoxia-independent regulator of HIF1α-VEGFA in the adult hippocampal niche.
Results
RA Signaling in Adult Hippocampal NSPCs
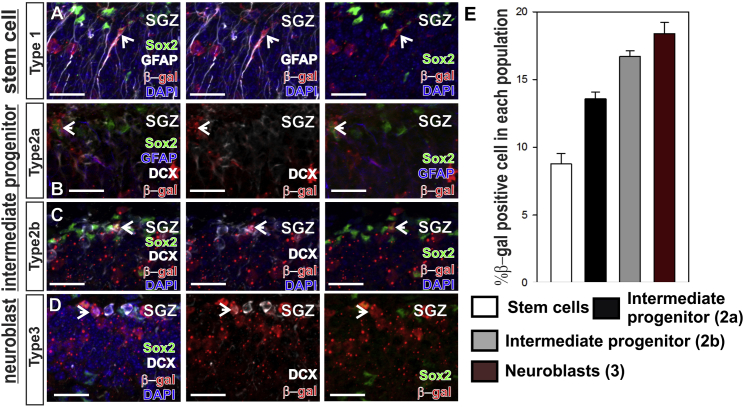
To examine RA signaling in NSPCs we used adult RARE-LacZ reporter mice where β-galactosidase protein (β-gal) expression is driven by multiple copies of an RA response element (RARE) (Rossant et al., 1991). β-gal+ cells indicate ongoing or recent RA signaling (β-gal protein is very stable, half-life of 24–48 hr; Gonda et al., 1989, McCutcheon et al., 2010). Co-labeling of β-gal with NSPC subtype specific markers was used to assess active RA signaling in each subtype. NSCs (type 1) were identified as SOX2+/GFAP+ (Figure 1A), type 2a progenitors were identified as SOX2+/GFAP−/DCX− (Figure 1B), type 2b progenitors were identified as SOX2+/DCX+ (Figure 1C), and type 3 neuroblasts were identified as SOX2−/DCX+ (Figure 1D) (Ferri et al., 2004, Kempermann et al., 2004, Komitova and Eriksson, 2004, Suh et al., 2007, Suh et al., 2009, Lugert et al., 2010, Bonaguidi et al., 2011, Ashton et al., 2012). We observed 8.8% of type 1 stem cells, 13.6% type 2a progenitors, 16.7% type 2b progenitors, and 18.4% type 3 progenitors were β-gal positive (Figure 1E, Table S2). Hence, at any given point, a portion of each NSPC subtype has RA signaling.
Figure 1.
Retinoic Acid Signaling in Adult Hippocampal NSPCs
(A–D) Arrows indicate β-gal (red) in subgranular zone (SGZ) of RARE-lacZ mice in (A) type 1 stem cells SOX2+ (green) and GFAP+ (white), (B) type 2a progenitors SOX2+GFAP−DCX− (GFAP, blue; DCX, white), (C) type 2b progenitors SOX2+DCX+, and (D) type 3 progenitors SOX2−DCX+. Scale bar, 20 μm.
(E) Quantification of %β-gal-positive NSPCs. Data represented as means ± SEM, n = 3.
RA Stimulates NSPC Proliferation and HIF1α Mediates This Effect In Vitro
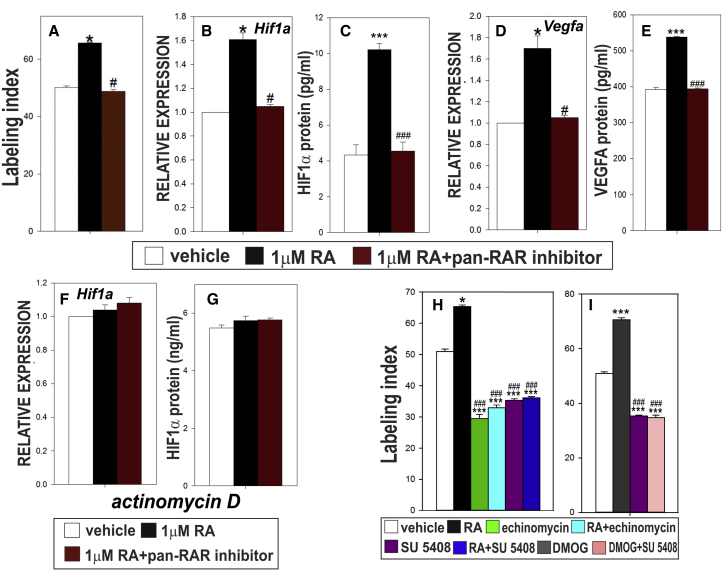
RA is well known to induce neuronal differentiation and, while recent studies implicate RA in regulating NSPC proliferation (Bonnet et al., 2008, Goodman et al., 2012, Takamura et al., 2017), the effect of RA on adult hippocampal NSPC proliferation remains largely unexplored. We treated rat hippocampal NSPC cultures with vehicle, RA, or RA + pan-RAR antagonist for 24 hr then pulsed with 5-ethynyl-2'-deoxyuridine (EdU) to calculate the labeling index (LI) as a readout of NSPC proliferation. RA treatment increased the LI and this effect was blocked by pan-RAR antagonist co-treatment (Figure 2A, Table S3). These data show RA is sufficient to stimulate hippocampal NSPC proliferation in vitro.
Figure 2.
RA Is Sufficient to Stimulate Adult Hippocampal NSPC Proliferation and HIF1α Mediates This Effect In Vitro
(A–G) Quantification (A) of labeling index (LI) in cultures of NSPCs. RT-PCR for (B) Hif1a and (D) Vegfa. ELISA quantification of (C) HIF1α and (E) VEGFA protein levels. Transcriptional inhibitor actinomycin D ablates RA-induced increase in (F) Hif1a gene expression and (G) HIF1α protein level.
(H) Quantification of LI for NSPC cultures treated with vehicle, RA, echinomycin, SU 5,408, RA + echinomycin or RA + SU 5,408.
(I) Quantification of LI for NSPC cultures treated with vehicle, DMOG, SU 5408, or DMOG + SU 5408.
Data represented as means ± SEM, ∗,#p ≤ 0.05, ∗∗∗,###p ≤ 0.001, n = 3.
Based on published reports and our previous work with RA in developmental neurogenesis, we suspected that RA may increase adult NSPC proliferation by stimulating HIF1α and its downstream target VEGFA (Jin et al., 2002, Segi-Nishida et al., 2008, Mazumdar et al., 2010, Amati et al., 2010, Antequera et al., 2012, Fournier et al., 2012, Kirby et al., 2015, Mishra et al., 2016). RA treatment of NSPC cultures increased HIF1α gene (Figure 2B; Table S3) and protein (Figure 2C; Table S3) expression, an effect blocked by pan-RAR antagonist treatment. Gene expression and protein levels of HIF1α target, VEGFA, showed a similar trend (Figures 2D and 2E; Table S3). Treatment with actinomycin D (a transcriptional inhibitor) resulted in no significant difference in HIF1α gene (Figure 2F; Table S3) or protein (Figure 2G; Table S3) expression between treatments. This suggests that RA likely increases HIF1α gene expression via transcription. We also tested if RA stimulation of HIF1α and VEGFA extended to astrocyte or neuronal cultures differentiated from NSPCs. RA did not alter HIF1α or VEGFA protein or gene expression in astrocytes, whereas neurons increased HIF1α and VEGFA in response to RA (Figure S1; Table S1). Altogether, our data identify RA as a regulator of HIF1α and VEGFA in adult hippocampal NSPCs and neurons.
To test if RA increases NSPC proliferation via HIF1α, cells were treated with vehicle, RA, echinomycin (inhibits HIF1α transcriptional activity; Kong et al., 2005), or RA + echinomycin. Echinomycin- and RA + echinomycin-treated cells showed significantly lower LI compared with vehicle (Figure 2H; Table S3). We next tested if RA increases NSPC proliferation via VEGFA. VEGFA stimulates NSPC proliferation via its kinase receptor VEGFR2 (Schänzer et al., 2004). We treated cells with vehicle, RA, VEGFR2 kinase inhibitor SU 5408, or RA + SU 5408. Cells treated with SU 5408 and RA + SU 5408 had a significantly lower LI compared with vehicle (Figure 2H; Table S3). HIF1α has many transcriptional targets therefore we tested if HIF1α increases NSPC proliferation via VEGFA. We treated cells with dimethyloxallyl glycine (DMOG) (to elevate HIF1α protein levels), SU 5408, or DMOG + SU 5408. DMOG stabilizes HIF1α protein by inhibiting prolyl hydrolase activity, an enzyme that promotes HIF1α degradation in normoxic conditions. DMOG increases HIF1α protein levels in hippocampal neurospheres (Chatzi et al., 2015). DMOG treatment increased LI compared with vehicle-treated cells and this effect was blocked by SU 5408 co-treatment (Figure 2I; Table S3). Altogether, these NSPC proliferation studies show (1) RA requires HIF1α and VEGFA signaling to increase NSPC proliferation in culture, and (2) VEGFA is likely the main target that mediates RA and HIF1α effects on cultured NSPCs in the treatment time periods (24 hr) used for these experiments.
RA Synthesis Is Required for Adult Hippocampal NSPC Proliferation via HIF1α
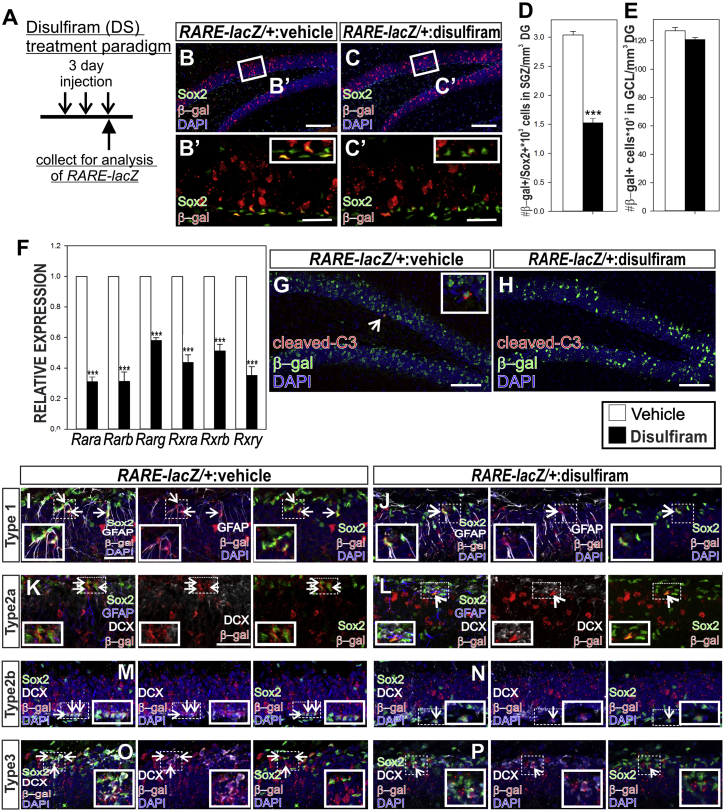
Our culture studies point to a key role for RA in stimulation of NSPC proliferation via HIF1α-VEGFA. Therefore, we tested the feasibility of using systemic manipulation of RA synthesis and HIF1α to test if RA is required for adult hippocampal NSPC proliferation in vivo. RARE-lacZ mice were treated with disulfiram (DS), a compound that blocks RA synthesizing enzymes and systemically decreases RA levels, including in the brain (Wagner et al., 2002, Wang et al., 2005, Xu et al., 2017). A 3-day treatment of DS decreased SGZ-located β-gal+/SOX2+ cell number (putative NSPCs) (Figure 3A) but did not alter the number of β-gal+ neurons in the GCL (Figures 3B–3E; Table S4). Possibly, the administration of DS for a short period of time affects NSPCs but not neurons or there is a difference in sensitivity between NSPCs and neurons to reduced RA levels. Along these lines, the dose of DS used is predicted to cause a moderate, ∼30%, decrease in RA levels in the brain (Xu et al., 2017). Transcript expression of RA receptors (RARs) and retinoid X receptors (RXRs) in whole hippocampus, of which Rarb is a direct transcriptional target of RA signaling (de Thé et al., 1990), were significantly decreased with DS (Figure 3F; Table S4). DS treatment did not alter the number of β-gal+ dying cells (identified by cleaved caspase3 labeling) in the SGZ (Figures 3G, and 3H). Using NSPC subtype specific markers, DS-exposed mice showed significantly reduced percentage of (1) β-gal+ stem cells (Figures 3I and 3J, Table S4), (2) β-gal+ type 2a cells (Figures 3K and 3L, Table S4), (3) β-gal+ type 2b cells (Figures 3M and 3N, Table S4), and (4) β-gal+ type 3 cells (Figures 3O and 3P, Table S2). Collectively, these data demonstrate DS treatment reduces NSPC RA signaling, thus validating it as a tool to test the role of RA in NSPC proliferation.
Figure 3.
Systemic Manipulation of RA Synthesis to Test Effects of RA on Adult Hippocampal NSPCs
(A) Schematic of experimental paradigm.
(B–C′). Confocal images of hippocampi immunolabeled with SOX2 (green), β-gal (red), and DAPI. Scale bars: (B and C) 100 μm, (B′ and C′) 50 μm.
(D and E) Quantification of β-gal+/SOX2+ cells in the SGZ representing NSPCs with RA signaling (D) and (E) β-gal+ cells in the GCL representing granule neurons.
(F–H) RT-PCR analysis (F) of Rar and Rxr genes in vehicle and DS-treated mice (G and H) Confocal images of immunolabeling for cleaved caspase-3 (C3; red) and β-gal (green) in RARE-lacZ SGZ from vehicle or DS-treated animals. Arrow in (G) indicates C3+/β-gal+ cells. Scale bar, 100 μm.
(I–P) Confocal images from vehicle and DS-treated RARE-lacZ mice depicting (I and J) β-gal+ type 1 stem cells (arrows), (K and L) β-gal+ type 2a intermediate progenitor (arrows), (M and N) β-gal+ type 2b intermediate progenitors (arrows), and (O and P) β-gal+ type 3 neuroblasts (arrows). Scale bar, 50 μm.
DS, disulfiram; SGZ, subgranular zone; GCL, granule cell layer. Data represented as mean ± SEM, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, n = 3.
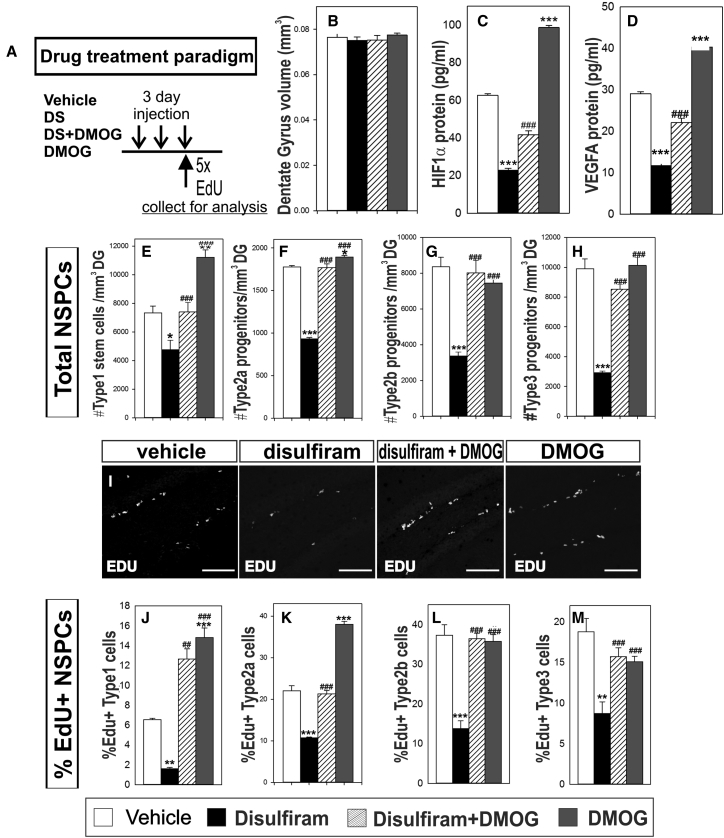
We next tested if systemic DMOG exposure could be used to elevate hippocampal HIF1α protein expression to test the function of HIF1α downstream of RA. Vehicle, DS, DS + DMOG, or DMOG injection regimens (Figure 4A) did not alter measured DG volume (Figure 4B; Table S5). DS treatment significantly decreased HIF1α and VEGFA protein levels (Figures 4C and 4D; Table S5), whereas co-treatment with DMOG significant increased HIF1α and VEGFA protein level compared with DS alone (Figures 4C and 4D; Table S5). DMOG increased HIF1α and VEGFA protein levels compared with vehicle (Figures 4C and 4D; Table S5). Taken together with DS and RARE-lacZ data, these data show (1) hippocampal HIF1α and VEGFA expression is dependent on RA synthesis and (2) systemic DS and DMOG exposure is effective to test RA and HIF1α pathways in adult hippocampal NSPC proliferation.
Figure 4.
RA-HIF1A Regulates Adult Hippocampal NSPC Proliferation In Vivo
(A) Schematic of experimental paradigm.
(B) Quantification of dentate gyrus (DG) volume in vehicle-, DS-, DS + DMOG-, or DMOG-treated mice.
(C and D) ELISA for HIF1α and VEGFA protein in hippocampus from vehicle-, DS-, DS + DMOG-, or DMOG-treated mice.
(E–H) Quantification of total number of NSPC subtypes type 1 (E), type 2a (F), type 2b (G) and type 3 (H) across all treatments.
(I) Confocal images showing EdU (white) labeling in the SGZ across all treatments.
(J–M) Quantification of percentage proliferating NSPC subtypes type 1 (J), type 2a (K), type 2b (L) and type 3 (M) in vehicle-, DS-, DS + DMOG-, or DMOG-treated mice.
Scale bar, 100 μm. Data represented as mean ± SEM, ∗p ≤ 0.05, ∗∗,##p ≤ 0.01, ∗∗∗,###p ≤ 0.001, n = 3.
For our studies of RA-HIF1α in hippocampal NSPCs proliferation, mice were injected with vehicle, DS, DS + DMOG, or DMOG and the total number of NSPCs was quantified using the same subtype markers used for our RARE-lacZ analysis. DS-treated mice showed significantly decreased total number of type 1 stem cells, type 2b, and type 3 progenitors. Compared with DS-treated mice, DS + DMOG-treated mice had significantly higher numbers of each NSPC subtype. Compared with vehicle treatment, DMOG alone significantly increased type 1 stem cells and type 2a cells but not type 2b or type 3 (Figures 4E–4H; Table S5).
Decreased NSPCs numbers could result from either decreased NSPC proliferation or increased cell death. Total number of cleaved caspase3 positive cells was not altered across treatments (Figures S2A–S2E; Table S1), indicating cell death is not a major factor. To quantify proliferation, mice were injected with EdU (Figure 4A) and EdU+ cells were observed in the SGZ in all treatments (Figure 4I). We quantified percentage EdU+ (%EdU+) NSPCs for each subtype (Figures 4J–4M; Table S5). DS-treated mice showed significantly reduced %EdU+ type 1 stem cells, %Edu+ type 2a, type 2b, and type 3 progenitors. Compared with DS treatment, DS + DMOG-treated mice had significantly higher number of %EdU+ stem cells, %EdU+ type 2a, type 2b, and type 3 progenitors. Compared with vehicle treatment, DMOG alone significantly increased %EdU+ stem cells, type 2a cells, but not type 2b or type 3 cells. Collectively, these data show RA synthesis is required for NSPC proliferation and suggests RA is a positive regulator of adult hippocampal NSPC proliferation via the HIF1α pathway.
We next tested if impaired NSPC proliferation altered neurogenesis. Using pulse-chase EdU assays (Figures S2F), we found DS decreased the number of newborn neuroblasts, immature neurons, and mature neurons, and restoring HIF1α protein level with DMOG improved neurogenesis to vehicle levels. DMOG treatment increased neurogenesis relative to vehicle (Figures S2G and S2H; Table S1). An important caveat is DS and DMOG have long half-lives, possibly remaining in the system for 5–7 days post-injection. Altered RA or HIF1α could affect other stages of adult hippocampal neurogenesis (survival or maturation). Thus the effects on neurogenesis may not be entirely due to altered proliferation but are likely a major contributing factor.
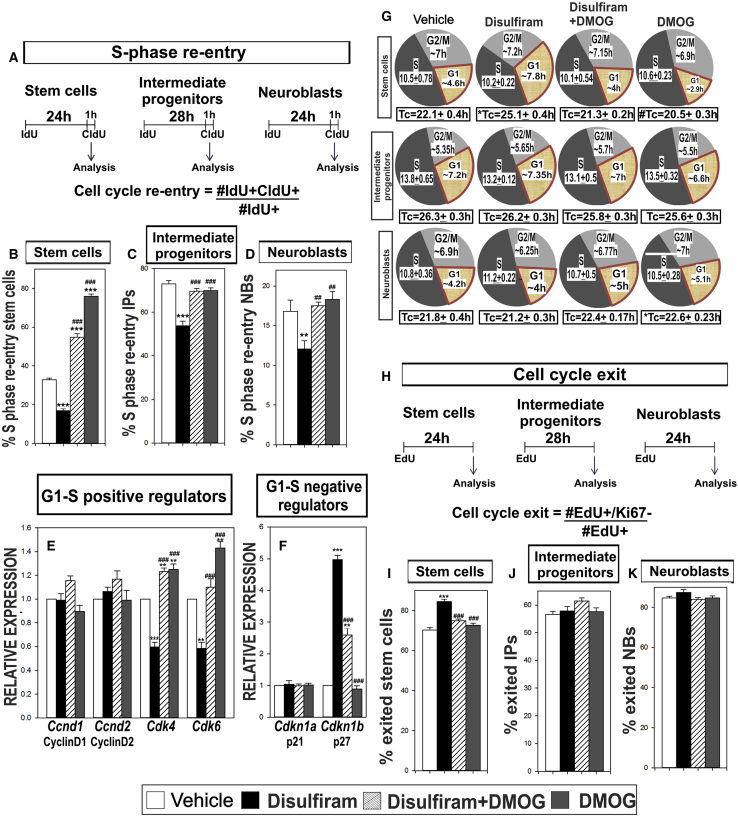
RA-HIF1α Signaling Regulates NSPC Proliferation via Regulating S-Phase Re-entry of NSPCs
Diminished NSPC proliferation could result from impaired cell-cycle kinetics, including S-phase re-entry, cell-cycle length, or cell-cycle length exit. To quantify the rate of S-phase re-entry after completing a cell cycle, we utilized a published double thymidine analog approach (Brandt et al., 2012, Farioli-Vecchioli et al., 2014) (Figure 5A). Tbr2 was used to identify intermediate progenitor (IP) cells (type 2a, type 2b) (Hodge et al., 2008, Roybon et al., 2009), and DCX was used to label neuroblasts (NBs; type 3) (Brown et al., 2003). Some Tbr2 positive cells are also DCX positive; however, the percentage is low (Hevner et al., 2006, von Bohlen und Halbach, 2011, Hodge et al., 2012). Different NSPC markers were used to accommodate labeling of two thymidine analogs. We measured percentage S-phase (%S-phase) re-entry for each NSPC subtype (Figure 5A). DS significantly decreased %S-phase re-entry of stem cells. Treatment with DS + DMOG or DMOG significantly increased %S-phase re-entry compared with vehicle and DS-treated mice (Figure 5B; Table S6). Similar analysis with IPs showed a significant decrease in %S-phase re-entry with DS treatment. Though %S-phase re-entry of IPs was significantly increased in DS + DMOG-treated compared with DS-treated mice, DMOG alone did not significantly alter %S-phase re-entry (Figure 5C; Table S6). We observed a similar trend in DCX+ NBs (Figure 5D; Table S6). Taken together, our data indicate RA-HIF1α signaling is required for S-phase re-entry of NSPCs in the adult hippocampus and points to impaired S-phase re-entry as an underlying cause of decreased NSPC proliferation seen in DS-treated mice.
Figure 5.
RA-HIF1A Regulates Adult Hippocampal NSPC Cell-Cycle Kinetics
(A) Experimental paradigm for calculating %S-phase re-entry.
(B–D) Quantification for %S-phase re-entry in (B) stem cells, (C) Tbr2+ intermediate progenitors, and (D) DCX+ neuroblasts.
(E and F) RT-PCR gene expression analysis (E) of G1-S positive regulators and (F) G1-S negative regulators.
(G) Cell-cycle length analysis using double thymidine analog method in NSPC subtypes.
(H) Schema of % cell-cycle exit.
(I–K) Quantification of cell-cycle exit in (I) stem cells, (J) intermediate progenitors, and (K) neuroblasts.
Data represented as mean ± SEM, ∗p ≤ 0.05, ∗∗,##p ≤ 0.01, ∗∗∗,###p ≤ 0.001, n = 3.
RA-HIF1α Signaling Regulates G1-S Transition of NSPCs
We next tested if decreased S-phase re-entry is due to impaired G1-S transition during cell-cycle progression. We quantified gene expression of G1-S positive regulators Cyclin D1 (Lange et al., 2009, Artegiani et al., 2011), Cyclin D2 (Kowalczyk et al., 2004, Garthe et al., 2014), CDK4 (Lange et al., 2009, Artegiani et al., 2011) and CDK6 (Beukelaers et al., 2011), and G1-S negative regulators p21Cip1 (Pechnick et al., 2008) and p27Kip1 (Doetsch et al., 2002, Qiu et al., 2009, Andreu et al., 2015) in whole hippocampus of vehicle-, DS-, DS + DMOG-, and DMOG-treated mice. Ccnd1 and Ccnd2 gene expression did not show any difference across treatments. Expression of Cdk4 and Cdk6 was significantly decreased in DS-treated mice. DS + DMOG treatment increased Cdk4 and Cdk6 gene expression compared with DS-treated mice. DMOG-treated mice showed significantly higher Cdk4 and Cdk6 gene expression compared with all treatments (Figure 5E; Table S6). Gene expression of p21Cip1 was unaltered across treatments (Figure 5F; Table S6). DS treatment significantly increased in p27Kip1 gene expression. DS + DMOG significantly reduced p27Kip1 compared with DS-treated mice. DMOG alone did not alter p27Kip1 relative to vehicle (Figure 5F; Table S6). These data demonstrate impaired RA-HIF1α signaling alters gene expression of positive and negative regulator of G1-S transition.
We reasoned if there was decreased G1-S transition as suggested by our data thus far, we might observe prolonged G1 length in NSPC subtypes. To test this, we used an established double labeling protocol (Brandt et al., 2012) to measure S-phase length (TS) and total cell-cycle length (TC) of all NSPC types. Using this analysis, we obtained estimates of G1 and G2/M lengths using previously described calculations (Brandt et al., 2012, Fischer et al., 2014). Our analysis identified elongated G1 phase and cell-cycle length of stem cells but not IPs or NBs in DS-treated mice. G1 and cell-cycle length of stem cells significantly decreased in DS + DMOG-treated compared with DS-treated mice. DMOG treatment significantly decreased G1 and cell-cycle length in stem cells compared with vehicle. IPs had no significant difference in G1 or cell-cycle length. However, G1 length and cell-cycle length of NBs was significantly longer in DS + DMOG- and DMOG-treated mice compared with vehicle-treated mice (Figure 5G, Table S6). Collectively, our data point to RA-HIF1α regulating G1-S transition of NSPCs via control of G1-S cell-cycle regulators. Furthermore, our data indicate stem cells are potentially more responsive to this pathway compared with other NSPCs subtypes.
Impaired RA-HIF1α Increases Cell-Cycle Exit of Stem Cells
Decreased S-phase re-entry could result from NSPCs increasing cell-cycle exit. To test this, we used an EdU pulse-chase assay then labeled sections with EdU and Ki-67, which identifies all proliferating SGZ cells (Figure 5H). DS treatment significantly increased cell-cycle exit in stem cells. Cell-cycle exit of stem cells in DS + DMOG- and DMOG-treated mice were comparable with vehicle but significantly lower than in DS-treated mice (Figure 5I; Table S6). Cell-cycle exit of IPs (Figure 5J; Table S6) and NBs (Figure 5K; Table S6) was not significantly altered in any treatments. Taken together, our data point to RA-HIF1α as required for cell-cycle re-entry of stem cells that are already in the cell cycle. Conceivably, impaired G1-S-phase transition increases cell-cycle exit and, presumably, a premature return to quiescence in this population.
RA Signaling Functions Cell Autonomously in NSPCs to Control Proliferation
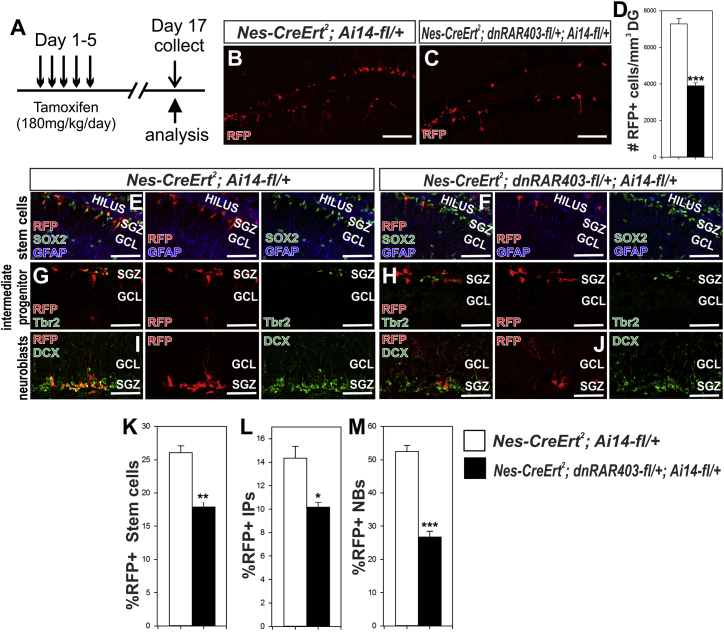
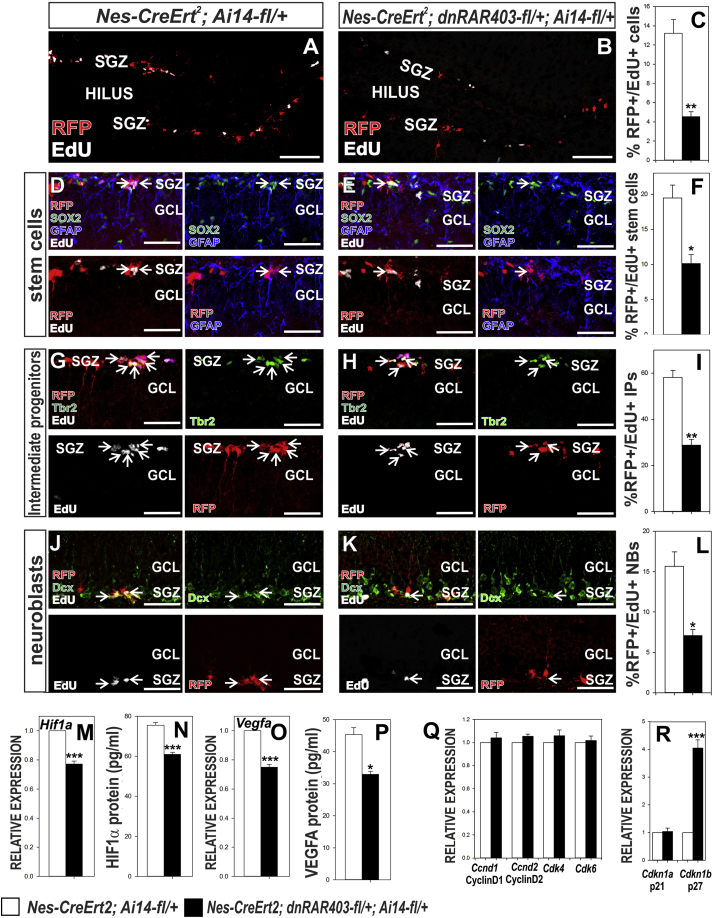
DS effects are systemic; therefore, we cannot make firm conclusions about the cell-autonomous function of RA signaling in NSPCs as DS may alter non-NSPC hippocampal cell types. To examine a cell-autonomous role of RA signaling in NSPCs, we utilized tamoxifen-inducible Nestin-CreERT2 (Nes-CreERT2; Lagace et al., 2007) with a conditional, dominant-negative RARα allele (dnRAR403-flox; Rosselot et al., 2010) to disrupt NSPC RA signaling in the adult NSPCs (Figure 6A). Nestin-CreERT2+/−;Ai14-fl/+ mice were designated as control animals and Nestin-CreERT2;Ai14-fl/+;dnRAR403fl/+ were designated as mutant animals. Recombined NSPCs were identified by tdTomato expression, a red fluorescent protein (RFP) expressed in Cre recombined cells. Disrupted RA signaling in recombined NSPCs reduced the total number of RFP+ cells in the SGZ (Figures 6B–6D; Table S7). This reduction was not due to increased cell death, as shown by no change in RFP+/cleaved caspase-3+ cells (Figures S3A and S3B; Table S1). Additionally, there were no macroscopic changes in hippocampal morphology, as shown by no significant difference in the measured DG volume (Figures S3C). We quantified the percentage of RFP+ cells that co-labeled with the same stem cell, IP, or NB markers used for NSPC cell-cycle analysis. Our data showed a significant decrease in percentage of RFP+ (%RFP+) stem cells (Figures 6E, 6F, and 6K; Table S7), IPs (Figures 6G, 6H, and 6L; Table S7), and NBs (Figures 6I, 6J, and 6M; Table S7). This analysis shows that NSPC RA signaling is required for maintaining proper NSPC number. We quantified the percentage of RFP+/EdU+ cells in the SGZ of control and mutant mice. Mutants had significantly lower %RFP+/EdU+ cells in the SGZ compared with controls (Figures 7A–7C; Table S8). We also observed mutants had significantly lower %RFP+/EdU+ stem cells (Figures 7D–7F; Table S8), IPs (Figures 7G–7I; Table S8), and NBs (Figures 7J–7L; Table S8). These data show that disrupting NSPC RA signaling diminishes cell proliferation of all hippocampal NSPCs subtypes.
Figure 6.
Disruption of RA Signaling in Adult NSPCs Decreases Their Population
(A) Paradigm of tamoxifen injection and collection of experimental animals.
(B–D) Representative images depicting RFP+ cells in the (B) control and (C) mutant SGZ. Quantification of RFP+ cells in the DG of each genotype is in (D). Scale bar, 100 μm.
(E–M) Fewer RFP+ (E and F) stem cells, intermediate progenitors (G and H ), and (I and J) neuroblasts are obsevred in the mutant SGZ as in compared to control animals. Quantification of RFP+ cells (K) stem cells, (L) intermediate progenitors (IP) and (M) neuroblasts (NB) in the two genotypes. Scale bar, 50 μm. Data represented as mean ± SEM, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, n = 3.
Figure 7.
Cell-Autonomous Disruption of NSPC RA Signaling Affects Proliferation and HIF1α, VEGFA, and p27 Expression
(A–C) Representaitive images of RFP+ (red)/EdU+ (white) cells in SGZ of (A) control and (B) mutant hippocampus. Quantification of %RFP+/EdU+ in each genotype is depicted in (C) Scale bar, 100 μm.
(D–L) Representaitive images of RFP+/EdU+ (D and E) stem cells, (G and H) intermediate progenitors and (J and K) neuroblasts in control and mutant animals. Quatification of %RFP+/EdU+ (F) stem cells, (I) intermediate progenitors (IP), and (L) neuoblasts (NB) in the DG of control and mutant animals. Arrows in (D), (E), (G), (H), (J), and (K) indicate EdU+ cells expression subtype markers. Scale bar, 50 μm.
(M–R) Graphs depict quantification of HIF1α (M) gene (RT-PCR) and (N) protein (ELISA) expression in whole hippocampus of indicated genotypes. VEGFA (O) gene and (P) protein expression in whole hippocampus. Gene expression of (Q) G1-S positive and (R) negative regulators.
Data represented as mean ± SEM, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, n = 3.
We next tested if HIF1α and VEGFA gene expression and protein levels are altered in the hippocampus of mutant animals. We observed a significant reduction in HIF1α and VEGFA gene (Figures 7M and 7O; Table S8) and protein (Figures 7N and 7P; Table S8) expression in mutants compared with controls. However, we used whole hippocampus; therefore, further studies are required to show that disruption of NSPC RA signaling alters NSPC HIF1α and VEGFA. We next looked at gene expression of G1-S transition positive and negative regulators in whole hippocampus from control and mutant mice. We did not observe any significant difference in the gene expression of G1-S transition positive regulators (Figure 7Q; Table S8) or G1-S negative regulator p21cip1 (Figure 7R; Table S8). We observed a significant increase in expression of a negative regulator of G1-S transition, p27kip1 (Figure 7R; Table S8). Taken together, our data suggest that NSPC RA signaling regulates HIF1α-VEGFA and, possibly, affects NSPCs proliferation by inhibiting p27 expression. Of note, we used RNA isolated from whole hippocampus for gene expression analysis. Cell-cycle regulator gene expression would only be predicted to be affected in a small percentage of cells in the sample. Therefore, detection of gene changes just in recombined NSPCs could be difficult, leaving open the possibility that Cdk4/6 are affected in mice with conditional disruption of NSPC RA signaling.
Discussion
RA has a vital role in adult hippocampal function and here we describe a previously unknown, cell-autonomous function for RA signaling in adult NSPCs. We find systemic disruption in RA synthesis causes a decrease in NSPC numbers and proliferation. We show impaired NSPC proliferation is due to elongated cell-cycle length, decreased S-phase entry, and increased cell-cycle exit; this was coincident with changes in cell-cycle regulators that would prevent G1-S-phase transition and push cells out of the cell cycle. We identify HIF1α as a target of RA signaling in adult hippocampal NSPCs and a major mediator of RA's effects on NSPC proliferation. Finally, using conditional disruption of RA signaling in adult NSPCs, we show that RA signaling functions cell autonomously to regulate NSPC proliferation and, potentially, HIF1α and VEGFA expression. These discoveries are a significant shift from the traditional view that RA primarily promotes neuronal differentiation.
Many studies have looked at RA signaling activity in the hippocampus and its functions in hippocampal neurogenesis and circuitry (reviewed in McCaffery et al., 2006, Shearer et al., 2012). Despite this, no studies have specifically looked for RA signaling in NSPCs, and studies of RA's effects on NSPCs are conflicting. Some studies conclude that RA promotes hippocampal neurogenesis by increasing neuronal differentiation of NSPCs and improving survival of newly born neurons (Crandall et al., 2004, Jacobs et al., 2006, Goodman et al., 2012). If this were its only function, the expectation might be that RA signaling would be limited to type 3 neuroblasts that are the cusp of neuronal differentiation and newborn neurons. However, using NSPC specific markers, we show active RA signaling in type 1 stem cells, type 2a and type 2b IPs, and type 3 neuroblasts. Our studies do not rule out functions for RA in neuronal differentiation and neuronal survival. Instead, they show that RA's effects are much broader than previously appreciated and include roles in promoting NSPC proliferation. Studies using VAD to systemically reduce RA levels have suggested an important role for RA in promoting NSPC proliferation, reporting decreased EdU+ cell number in the hippocampal SGZ in VAD rats (Bonnet et al., 2008). Our work provides a more complete and nuanced picture of RA's effects on NSPC subtype proliferation. For example, though all NSPC subtypes have decreased numbers and EdU incorporation when RA synthesis is blocked, only type 1 stem cells have an elongated G1 phase. Further, our S-phase re-entry analysis shows that proliferating NSPCs are much less likely to re-enter the cell cycle when RA levels are reduced. This observation, along with our data showing decreased expression of G1-to-S-phase regulators and increased expression of cell-cycle inhibitor p27, strongly point to RA promoting proliferation of NSPCs through modulating expression of cell-cycle regulators.
To begin to identify a mechanism of action for RA signaling on NSPC proliferation, we worked from our studies of RA in brain development, where we showed that RA increased VEGFA in neocortical progenitors (Mishra et al., 2016) and follow-up studies identified a parallel increase in protein and transcript of VEGFA regulator, HIF1α. HIF1α is a potent transcriptional regulator of VEGFA (Pagès and Pouysségur, 2005) and RA has been shown to increase HIF1α (Fernández-Martínez et al., 2011, Fernández-Martínez et al., 2012, Liang et al., 2014). Importantly, HIF1α is implicated in regulating hippocampal NSPC behavior (Mazumdar et al., 2010). Studies of HIF1α regulation and activity in adult NSC niches point to niche hypoxia as the main regulator of HIF1α expression. NSC niches are hypoxic and have increased HIF1α stabilization (Mazumdar et al., 2010, Roitbak et al., 2011), and hypoxia is sufficient to induce cell proliferation in HIF1α-dependent cultured SVZ NSPCs (Qi et al., 2017). Our studies identify RA as a hypoxia-independent regulator of HIF1α in adult hippocampal NSPCs, likely working in parallel with hypoxia in the NSC niche. Most importantly, our rescue experiments using DMOG to restore HIF1α demonstrate that HIF1α is likely the primary mediator of RA's effects on NSPC proliferation. Our animal and culture data point to HIF1α target VEGFA as a key mediator downstream of RA. Hippocampal NSPCs express and release VEGFA, which acts as an NSPC mitogen (Kirby et al., 2015). Conditional deletion of Vegfa in adult hippocampal NSPCs results in decreased NSC numbers and proliferation, pointing to a key role of niche produced VEGFA in NSC maintenance (Kirby et al., 2015). WNT-β-catenin signaling is implicated downstream of HIF1α in regulation of stem cell proliferation (Mazumdar et al., 2010). However, studies using embryonic NSPCs point to hypoxia inducing WNT-β-catenin activity independent of HIF1α (Braunschweig et al., 2015), thus further studies are needed to probe this connection.
Systemic manipulation of RA synthesis or HIF1α stabilization has the potential to affect all cells in the hippocampus. We show RA has a stimulatory effect on HIF1α and VEGFA expression in hippocampal neurons differentiated from NSPCs but not astrocytes. However, it is unclear if hippocampal neurons are a significant source of VEGFA for the niche, regulated by RA or other signals, since VEGFA-GFP reporter studies did not detect reporter activity in hippocampal granule cells (Kirby et al., 2015). The dose of DS used appears to only partially reduce RA synthesis and, thus, RA levels in the hippocampus such that only the NSPCs showed a decrease in RA signaling, whereas the number of granule cells with RA signaling was unchanged. However, RARE-lacZ activity is only one measure of RA signaling and neuronal RA signaling could still be impaired in hippocampal granule cells by DS. This is important as RA has important functions in granule cell synapse plasticity and hippocampal circuitry (Aoto et al., 2008, Maghsoodi et al., 2008, Jiang et al., 2012), and neuronal activity is a major stimulant of NSPC proliferation (reviewed in Pineda and Encinas, 2016). Also important to consider is effects on the vascular niche. HIF1α and VEGFA, impaired by DS treatment, regulate blood vessels in NSC niches (Roitbak et al., 2008, Li et al., 2014, Licht et al., 2016). The strongest support we have for RA acting directly on NSPCs is our data (1) showing RA signaling in NSPC subtypes and (2) showing conditional disruption of RA signaling in adult NSPCs decreases proliferation and HIF1α and VEGFA expression. Demonstrating a cell-autonomous effect of RA in hippocampal NSPCs is important for future studies understanding the mechanism of RA's effect on proliferation. However, it is equally important to understand the impact of systemic decreases in RA or RA signaling on the whole hippocampus and how this could affect neurogenesis. This is especially valuable information for evaluating RA therapeutics used to treat cancer (Chakrabarti et al., 2016) or in clinical trials for neurological disorders like Alzheimer disease (Shudo et al., 2009, Fukasawa et al., 2012, Chakrabarti et al., 2016) and to better elucidate the impact of chronic deficiency of vitamin A on the brains of millions of people worldwide (Shearer et al., 2012, Stoney and McCaffery, 2016, Wirth et al., 2017).
Experimental Procedures
Animals
Mice were housed in specific-pathogen-free facilities approved by Assessment and Accreditation of Laboratory Animal Care and were handled in accordance with protocols approved by the Institutional Animal Care and Use Committee. Mice were housed under a 12 hr light/dark cycle and were given food and water ad libitum. All mouse strains used in this study and chemicals administered via intraperitoneal injections are listed in Table S1.
Tissue Collection and Processing
Mice were anesthetized with sodium pentobarbital (40 mg/kg) and perfused with 0.1 M PBS followed by 4% paraformaldehyde (PFA). Brains were harvested and postfixed overnight in 4% PFA at 4°C and then cryoprotected with 20% sucrose in PBS at 4°C. Brains were frozen in optimal cutting temperature compound and 40 μm cryosections were obtained for all experiments.
Immunohistochemistry
Brain sections were subjected to heat mediated antigen retrieval using citric acid (pH 6) for 15 min and blocked with 10% lamb serum for 1 hr at room temperature. Sections were incubated overnight at 4°C with primary antibodies listed in Table S1. Sections were washed in PBS and incubated with species-appropriate fluorophore conjugated secondary antibodies (AlexaFluor, Invitrogen 1:500) for 2 hr at room temperature before mounting. For some experiments, sections were counterstained with DAPI (1 μg/mL).
In Vivo Cell Proliferation Analysis
For cell proliferation analysis, mice were injected with EdU (150 mg/kg) five times every 2 hr over 10 hr on the day of collection as previously described (Morshead et al., 1998). Brain sections were immunostained with a combination of markers to identify NSCs and progenitors cells. Click-iT 647 Imaging Kit (#C10340, Thermo Fisher Scientific) was used to detect EdU. Percentage of proliferating cells was determined as total EdU+ stem or progenitor cells divided by the total stem or progenitor cell population. Description of cell-cycle analysis is provided in Supplemental Information.
Imaging and Stereological Analysis
Brain sections were imaged using confocal microscopy using a Zeiss 780 laser scanning microscope. Single-plane confocal images and Z stacks were obtained to image cells immunostained with combination of markers. Cells labeled with marker(s) to identify NSPCs and various thymidine analogs were counted throughout the rostro-caudal extent of DG using one in every six series of 40 μm coronal sections as previously described (Lagace et al., 2007, Lagace et al., 2010). The total number of cells in all experimental groups was normalized to volume of DG analyzed. Volume of DG was calculated as previously described (Lagace et al., 2007, Lagace et al., 2010). Briefly, area was calculated by tracing the outline of each blade of DG in each section. Subsequently, areas of all the sections were added and multiplied with section thickness (40 μm) and inverse of sampling fraction (six) to obtain DG volume analyzed. We analyzed three animals (n = 3) per treatment condition or genotype for all imaging based analysis.
Hippocampal NSC Cultures and Cell Proliferation Assay
Adult rat hippocampal NSCs (#SCR022, Millipore) were seeded onto poly-L-ornithine and laminin coated 24-well plates as a monolayer culture. Cells were cultured in NSC expansion medium (#SCM009, Millipore) containing DMEM/F12; B27 supplement; 2 mM L-glutamine; and 1× penicillin, streptomycin, and fungizone. Culture medium was supplemented with basic fibroblast growth factor (20 ng/mL) to maintain cells in the undifferentiated state. All chemicals and their concentrations used in cell culture experiments are listed in Table S1. Following 24 hr treatment, NSCs were incubated with 10 μM EdU for 3 hr. Cells were fixed with 4% PFA, washed with 1× PBS, and permeabilized with 0.5% Triton X-100. EdU incorporation was detected according to manufacturer's instructions (Click-iT EdU Alexa Fluor 647 kit). Post-EdU detection, cells were incubated overnight with anti-SOX2 and nuclei were labeled with DAPI. LI was calculated as EdU+/SOX2+/total SOX2+ cells. All measurements were performed using ImageJ software on a minimum of ten 20×fields per coverslip. Three independent experiments (n = 3) were analyzed for all treatments.
Statistical Analysis
To detect statistically significant differences in mean values between two groups, Student’s t test was used. For analysis that compared more than two groups, a one-way ANOVA with Tukey's post hoc analysis was used to detect statistically significant differences between treatment conditions using pairwise analysis. The SEM is reported on all graphs. Results from all experiments are provided in Tables S1, S2, S3, S4, S5, S6, S7, and S8.
Author Contributions
S.M. and J.A.S. conceived the study. S.M., K.K.K., and N.L.M. performed experiments. S.M. and J.A.S. prepared the figures and wrote the manuscript.
Acknowledgments
This work was supported by the Children's Hospital of Colorado Research Foundation (start-up funds to J.A.S.) and the NIH/National Institute of Neurological Disorders and Stroke (R01 NS098273 to J.A.S.). The senior author would like to acknowledge the contributions of Marie Siegenthaler and dedicate this work to her memory.
Published: May 24, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and eight tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.04.024.
Supporting Citations
The following reference appears in the Supplemental Information: Brandt et al., 2014.
Supplemental Information
References
- Amati F., Diano L., Campagnolo L., Vecchione L., Cipollone D., Bueno S., Prosperini G., Desideri A., Siracusa G., Chillemi G. Hif1α down-regulation is associated with transposition of great arteries in mice treated with a retinoic acid antagonist. BMC Genomics. 2010;11:497. doi: 10.1186/1471-2164-11-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu Z., Khan M.A., González-Gómez P., Negueruela S., Hortigüela R., San Emeterio J., Ferrón S.R., Martínez G., Vidal A., Fariñas I. The cyclin-dependent kinase inhibitor p27kip1 regulates radial stem cell quiescence and neurogenesis in the adult hippocampus. Stem Cells. 2015;33:219–229. doi: 10.1002/stem.1832. [DOI] [PubMed] [Google Scholar]
- Antequera D., Portero A., Bolos M., Orive G., Hernández R.M., Pedraz J.L., Carro E. Encapsulated VEGF-secreting cells enhance proliferation of neuronal progenitors in the hippocampus of AβPP/Ps1 mice. J. Alzheimers Dis. 2012;29:187–200. doi: 10.3233/JAD-2011-111646. [DOI] [PubMed] [Google Scholar]
- Aoto J., Nam C.I., Poon M.M., Ting P., Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani B., Lindemann D., Calegari F. Overexpression of Cdk4 and CyclinD1 triggers greater expansion of neural stem cells in the adult mouse brain. J. Exp. Med. 2011;208:937–948. doi: 10.1084/jem.20102167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton R.S., Conway A., Pangarkar C., Bergen J., Lim K.-I., Shah P., Bissell M., Schaffer D.V. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci. 2012;15:1399–1406. doi: 10.1038/nn.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukelaers P., Vandenbosch R., Caron N., Nguyen L., Belachew S., Moonen G., Kiyokawa H., Barbacid M., Santamaria D., Malgrange B. Cdk6-dependent regulation of G(1) length controls adult neurogenesis. Stem Cells. 2011;29:713–724. doi: 10.1002/stem.616. [DOI] [PubMed] [Google Scholar]
- Bonaguidi M.A., Wheeler M.A., Shapiro J.S., Stadel R.P., Sun G.J., Ming G.L. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet E., Touyarot K., Alfos S., Pallet V., Higueret P., Abrous D.N. Retinoic acid restores adult hippocampal neurogenesis and reverses spatial memory deficit in vitamin A deprived rats. PLoS One. 2008;3:e3487. doi: 10.1371/journal.pone.0003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M.D., Hübner M., Storch A. Brief report: adult hippocampal precursor cells shorten S-phase and total cell cycle length during neuronal differentiation. Stem Cells. 2012;30:2843–2847. doi: 10.1002/stem.1244. [DOI] [PubMed] [Google Scholar]
- Brandt M.D., Ellwardt E., Storch A. Short- and long-term treatment with modafinil differentially affects adult hippocampal neurogenesis. Neuroscience. 2014;278:267–275. doi: 10.1016/j.neuroscience.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Braunschweig L., Meyer A.K., Wagenführ L., Storch A. Oxygen regulates proliferation of neural stem cells through Wnt/β-catenin signalling. Mol. Cell. Neurosci. 2015;67:84–92. doi: 10.1016/j.mcn.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Brown J.P., Couillard-Despres S., Cooper-Kuhn C.M., Winkler J., Aigner L., Kuhn H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Chakrabarti M., McDonald A.J., Will Reed J., Moss M.A., Das B.C., Ray S.K. Molecular signaling mechanisms of natural and synthetic retinoids for inhibition of pathogenesis in Alzheimer's disease. J. Alzheimers Dis. 2016;50:335–352. doi: 10.3233/JAD-150450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi C., Schnell E., Westbrook G.L. Localized hypoxia within the subgranular zone determines the early survival of newborn hippocampal granule cells. eLife. 2015;4:e08722. doi: 10.7554/eLife.08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall J., Sakai Y., Zhang J., Koul O., Mineur Y., Crusio W.E., McCaffery P. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc. Natl. Acad. Sci. USA. 2004;101:5111–5116. doi: 10.1073/pnas.0306336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L., Adame A., Patrick C., Delaney A., Pham E., Rockenstein E., Hansen L., Masliah E. Increased BMP6 levels in the brains of Alzheimer's disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J. Neurosci. 2010;30:12252–12262. doi: 10.1523/JNEUROSCI.1305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M.M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- Deng W., Saxe M.D., Gallina I.S., Gage F.H. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Verdugo J.M.-G., Caille I., Alvarez-Buylla A., Chao M.V., Casaccia-Bonnefil P. Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis. J. Neurosci. 2002;22:2255–2264. doi: 10.1523/JNEUROSCI.22-06-02255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farioli-Vecchioli S., Mattera A., Micheli L., Ceccarelli M., Leonardi L., Saraulli D., Costanzi M., Cestari V., Rouault J.-P., Tirone F. Running rescues defective adult neurogenesis by shortening the length of the cell cycle of neural stem and progenitor cells. Stem Cells. 2014;32:1968–1982. doi: 10.1002/stem.1679. [DOI] [PubMed] [Google Scholar]
- Fernández-Martínez A.B., Jiménez M.I.A., Cazaña F.J.L. Retinoic acid increases hypoxia-inducible factor-1α through intracrine prostaglandin E 2 signaling in human renal proximal tubular cells HK-2. Biochim. Biophys. Acta. 2012;1821:672–683. doi: 10.1016/j.bbalip.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Fernández-Martínez A.B., Jiménez M.I.A., Hernández I.S., García-Bermejo M.L., Manzano V.M., Fraile E.A., de Lucio-Cazaña F.J. Mutual regulation of hypoxic and retinoic acid related signaling in tubular proximal cells. Int. J. Biochem. Cell Biol. 2011;43:1198–1207. doi: 10.1016/j.biocel.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Ferri A.L., Cavallaro M., Braida D., Di Cristofano A., Canta A., Vezzani A., Ottolenghi S., Pandolfi P.P., Sala M., DeBiasi S., Nicolis S.K. SOX2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Fischer T.J., Walker T.L., Overall R.W., Brandt M.D., Kempermann G. Acute effects of wheel running on adult hippocampal precursor cells in mice are not caused by changes in cell cycle length or S phase length. Front. Neurosci. 2014;8:314. doi: 10.3389/fnins.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier N.M., Lee B., Banasr M., Elsayed M., Duman R.S. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology. 2012;63:642–652. doi: 10.1016/j.neuropharm.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa H., Nakagomi M., Yamagata N., Katsuki H., Kawahara K., Kitaoka K., Miki T., Shudo K. Tamibarotene: a candidate retinoid drug for Alzheimer's disease. Biol. Pharm. Bull. 2012;35:1206–1212. doi: 10.1248/bpb.b12-00314. [DOI] [PubMed] [Google Scholar]
- Gage F., Kempermann G., Palmer T., Peterson D., Ray J. Multipotent progenitor cells in the adult dentate gyrus. J. Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Garthe A., Huang Z., Kaczmarek L., Filipkowski R., Kempermann G. Not all water mazes are created equal: cyclin D2 knockout mice with constitutively suppressed adult hippocampal neurogenesis do show specific spatial learning deficits. Genes Brain Behav. 2014;13:357–364. doi: 10.1111/gbb.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nicola D., Suzzi S., Vargas-Caballero M., Fransen N.L., Al-Malki H., Cebrian-Silla A., Garcia-Verdugo J.M., Riecken K., Fehse B., Perry V.H. Temporal dynamics of hippocampal neurogenesis in chronic neurodegeneration. Brain. 2014;137:2312–2328. doi: 10.1093/brain/awu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda D.K., Bachmair A., Wünning I., Tobias J.W., Lane W.S., Varshavsky A. Universality and structure of the N-end rule. J. Biol. Chem. 1989;264:16700–16712. [PubMed] [Google Scholar]
- Goodman T., Crandall J.E., Nanescu S.E., Quadro L., Shearer K., Ross A., McCaffery P. Patterning of retinoic acid signaling and cell proliferation in the hippocampus. Hippocampus. 2012;22:2171–2183. doi: 10.1002/hipo.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner R.F., Hodge R.D., Daza R.A.M., Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci. Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hodge R.D., Nelson B.R., Kahoud R.J., Yang R., Mussar K.E., Reiner S.L., Hevner R.F. Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J. Neurosci. 2012;32:6275–6287. doi: 10.1523/JNEUROSCI.0532-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge R.D., Kowalczyk T.D., Wolf S.A., Encinas J.M., Rippey C., Enikolopov G., Kempermann G., Hevner R.F. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J. Neurosci. 2008;28:3707–3717. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger G.U., Rizk P., Muriel M.P., Duyckaerts C., Oertel W.H., Caille I., Hirsch E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Lie D.C., DeCicco K.L., Shi Y., DeLuca L.M., Gage F.H., Evans R.M. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Yu Q., Gong M., Chen L., Wen E.Y., Bi Y., Zhang Y., Shi Y., Qu P., Liu Y.X. Vitamin A deficiency impairs postnatal cognitive function via inhibition of neuronal calcium excitability in hippocampus. J. Neurochem. 2012;121:932–943. doi: 10.1111/j.1471-4159.2012.07697.x. [DOI] [PubMed] [Google Scholar]
- Jin K., Zhu Y., Sun Y., Mao X.O., Xie L., Greenberg D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Jessberger S., Steiner B., Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kirby E.D., Kuwahara A.A., Messer R.L., Wyss-Coray T. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc. Natl. Acad. Sci. USA. 2015;112:4128–4133. doi: 10.1073/pnas.1422448112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M., Eriksson P.S. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci. Lett. 2004;369:24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Kong D., Park E.J., Stephen A.G., Calvani M., Cardellina J.H., Monks A., Fisher R.J., Shoemaker R.H., Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- Kowalczyk A., Filipkowski R.K., Rylski M., Wilczynski G.M., Konopacki F.A., Jaworski J., Ciemerych M.A., Sicinski P., Kaczmarek L. The critical role of cyclin D2 in adult neurogenesis. J. Cell Biol. 2004;167:209–213. doi: 10.1083/jcb.200404181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace D.C., Donovan M.H., DeCarolis N.A., Farnbauch L.A., Malhotra S., Berton O., Nestler E.J., Krishnan V., Eisch A.J. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl. Acad. Sci. USA. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace D.C., Whitman M.C., Noonan M.A., Ables J.L., DeCarolis N.A., Arguello A.A., Donovan M.H., Fischer S.J., Farnbauch L.A., Beech R.D. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C., Huttner W.B., Calegari F. Cdk4/CyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5:320–331. doi: 10.1016/j.stem.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Li L., Candelario K.M., Thomas K., Wang R., Wright K., Messier A., Cunningham L.A. Hypoxia inducible factor-1α (HIF-1α) is required for neural stem cell maintenance and vascular stability in the adult mouse SVZ. J. Neurosci. 2014;34:16713–16719. doi: 10.1523/JNEUROSCI.4590-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Guo S., Yang L. Effects of all-trans retinoic acid on VEGF and HIF-1α expression in glioma cells under normoxia and hypoxia and its anti-angiogenic effect in an intracerebral glioma model. Mol. Med. Rep. 2014;10:2713–2719. doi: 10.3892/mmr.2014.2543. [DOI] [PubMed] [Google Scholar]
- Licht T., Rothe G., Kreisel T., Wolf B., Benny O., Rooney A.G., Ffrench-Constant C., Enikolopov G., Keshet E. VEGF preconditioning leads to stem cell remodeling and attenuates age-related decay of adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA. 2016;113:E7828–E7836. doi: 10.1073/pnas.1609592113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert S., Basak O., Knuckles P., Haussler U., Fabel K., Götz M., Haas C.A., Kempermann G., Taylor V., Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Maghsoodi B., Poon M.M., Nam C.I., Aoto J., Ting P., Chen L. Retinoic acid regulates RARalpha- mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proc. Natl. Acad. Sci. USA. 2008;105:16015–16020. doi: 10.1073/pnas.0804801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J., O'Brien W.T., Johnson R.S., LaManna J.C., Chavez J.C., Klein P.S., Simon M.C. O2 regulates stem cells through Wnt/β-catenin signalling. Nat. Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery P., Zhang J., Crandall J.E. Retinoic acid signaling and function in the adult hippocampus. J. Neurobiol. 2006;66:780–791. doi: 10.1002/neu.20237. [DOI] [PubMed] [Google Scholar]
- McCutcheon S.C., Jones K., Cumming S.A., Kemp R., Ireland-Zecchini H., Saunders J.C., Houghton C.A., Howard L.A., Winton D.J. Characterization of a heat resistant ß-glucosidase as a new reporter in cells and mice. BMC Biol. 2010;8:89. doi: 10.1186/1741-7007-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S., Choe Y., Pleasure S.J., Siegenthaler J.A. Cerebrovascular defects in Foxc1 mutants correlate with aberrant WNT and VEGF-A pathways downstream of retinoic acid from the meninges. Dev. Biol. 2016;420:148–165. doi: 10.1016/j.ydbio.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misner D.L., Jacobs S., Shimizu Y., de Urquiza A.M., Solomin L., Perlmann T., De Luca L.M., Stevens C.F., Evans R.M. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc. Natl. Acad. Sci. USA. 2001;98:11714–11719. doi: 10.1073/pnas.191369798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead C.M., Craig C.G., van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- Pagès G., Pouysségur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene–a concert of activating factors. Cardiovasc. Res. 2005;65:564–573. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Pechnick R.N., Zonis S., Wawrowsky K., Pourmorady J., Chesnokova V. p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc. Natl. Acad. Sci. USA. 2008;105:1358–1363. doi: 10.1073/pnas.0711030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E.K., Johnson M., Ekonomou A., Perry R.H., Ballard C., Attems J. Neurogenic abnormalities in Alzheimer's disease differ between stages of neurogenesis and are partly related to cholinergic pathology. Neurobiol. Dis. 2012;47:155–162. doi: 10.1016/j.nbd.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J.R., Encinas J.M. The contradictory effects of neuronal hyperexcitation on adult hippocampal neurogenesis. Front. Neurosci. 2016;10:74. doi: 10.3389/fnins.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C., Zhang J., Chen X., Wan J., Wang J., Zhang P., Liu Y. Hypoxia stimulates neural stem cell proliferation by increasing HIF-1α expression and activating Wnt/β-catenin signaling. Cell. Mol. Biol. (Noisy-le-grand) 2017;63:12–19. doi: 10.14715/cmb/2017.63.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Takagi Y., Harada J., Topalkara K., Wang Y., Sims J.R., Zheng G., Huang P., Ling Y., Scadden D.T. p27Kip1 constrains proliferation of neural progenitor cells in adult brain under homeostatic and ischemic conditions. Stem Cells. 2009;27:920–927. doi: 10.1002/stem.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest J.M., Dupret D., Koehl M., Funk-Reiter C., Grosjean N., Piazza P.V., Abrous D.N. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Roitbak T., Li L., Cunningham L.A. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J. Cereb. Blood Flow Metab. 2008;28:1530–1542. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitbak T., Surviladze Z., Cunningham L.A. Continuous expression of HIF-1α in neural stem/progenitor cells. Cell. Mol. Neurobiol. 2011;31:119–133. doi: 10.1007/s10571-010-9561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J., Zirngibl R., Cado D., Shago M., Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Rosselot C., Spraggon L., Chia I., Batourina E., Riccio P., Lu B., Niederreither K., Dolle P., Duester G., Chambon P. Non-cell-autonomous retinoid signaling is crucial for renal development. Development. 2010;137:283–292. doi: 10.1242/dev.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybon L., Hjalt T., Stott S., Guillemot F., Li J.-Y., Brundin P. Neurogenin2 directs granule neuroblast production and amplification while neuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS One. 2009;4:e4779. doi: 10.1371/journal.pone.0004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schänzer A., Wachs F.-P., Wilhelm D., Acker T., Cooper-Kuhn C., Beck H., Winkler J., Aigner L., Plate K.H., Kuhn H.G. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segi-Nishida E., Warner-Schmidt J.L., Duman R.S. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc. Natl. Acad. Sci. USA. 2008;105:11352–11357. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer K.D., Stoney P.N., Morgan P.J., McCaffery P.J. A vitamin for the brain. Trends Neurosci. 2012;35:733–741. doi: 10.1016/j.tins.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Shudo K., Fukasawa H., Nakagomi M., Yamagata N. Towards retinoid therapy for Alzheimer's disease. Curr. Alzheimer Res. 2009;6:302–311. doi: 10.2174/156720509788486581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V., Maldonado-Soto A.R., Mizrak D., Codega P., Doetsch F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell. 2016;19:643–652. doi: 10.1016/j.stem.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Stoney P.N., McCaffery P. A vitamin on the mind: new discoveries on control of the brain by vitamin A. World Rev. Nutr. Diet. 2016;115:98–108. doi: 10.1159/000442076. [DOI] [PubMed] [Google Scholar]
- Suh H., Deng W., Gage F.H. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Suh H., Consiglio A., Ray J., Sawai T., D'Amour K.A., Gage F.H. In vivo fate analysis reveals the multipotent and self-renewal capacities of SOX2(+) neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura R., Watamura N., Nikkuni M., Ohshima T. All-trans retinoic acid improved impaired proliferation of neural stem cells and suppressed microglial activation in the hippocampus in an Alzheimer's mouse model. J. Neurosci. Res. 2017;95:897–906. doi: 10.1002/jnr.23843. [DOI] [PubMed] [Google Scholar]
- van Praag H., Schinder A.F., Christie B.R., Toni N., Palmer T.D., Gage F.H. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S.A., Wyss-Coray T. The circulatory systemic environment as a modulator of neurogenesis and brain aging. Autoimmun. Rev. 2013;12:674–677. doi: 10.1016/j.autrev.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Villeda S.A., Plambeck K.E., Middeldorp J., Castellano J.M., Mosher K.I., Luo J., Smith L.K., Bieri G., Lin K., Berdnik D. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G., Stan T.M., Fainberg N., Ding Z., Eggel A. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011;345:1–19. doi: 10.1007/s00441-011-1196-4. [DOI] [PubMed] [Google Scholar]
- Wagner E., Luo T., Dräger U.C. Retinoic acid synthesis in the postnatal mouse brain marks distinct developmental stages and functional systems. Cereb. Cortex. 2002;12:1244–1253. doi: 10.1093/cercor/12.12.1244. [DOI] [PubMed] [Google Scholar]
- Wang T.W., Zhang H., Parent J.M. Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development. 2005;132:2721–2732. doi: 10.1242/dev.01867. [DOI] [PubMed] [Google Scholar]
- Wirth J.P., Petry N., Tanumihardjo S.A., Rogers L.M., McLean E., Greig A., Garrett G.S., Klemm R.D., Rohner F. Vitamin A supplementation programs and country-level evidence of vitamin A deficiency. Nutrients. 2017;9 doi: 10.3390/nu9030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Li C., Gao X., Xia K., Guo H., Li Y., Hao Z., Zhang L., Gao D., Xu C. Excessive UBE3A dosage impairs retinoic acid signaling and synaptic plasticity in autism spectrum disorders. Cell Res. 2017;28:48–68. doi: 10.1038/cr.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.