Abstract
Amyotrophic lateral sclerosis (ALS) and the behavioural variant of frontotemporal dementia (bvFTD) commonly share the presence of TDP-43 inclusions. Structural MRI studies demonstrated evidence for TDP-43 pathology spread, but while structural imaging usually reveals overt neuronal loss, perfusion imaging may detect more subtle neural activity alterations. We evaluated perfusion as an early marker for incipient pathology associated brain alterations in TDP-43 proteinopathies. Cortical thickness (CT) and perfusion measurements were obtained in ALS (N=18), pathologically and/or genetically confirmed bvFTD-TDP (N=12), and healthy controls (N=33).
BvFTD showed reduced frontotemporal CT, hypoperfusion encompassing orbitofrontal and temporal cortices, and hyperperfusion in motor and occipital regions. ALS did not show reduced CT, but exhibited hypoperfusion in motor and temporal regions, and hyperperfusion in frontal and occipital cortices. Frontotemporal hypoperfusion and reduced CT correlated with cognitive and behavioural impairment as investigated using Mini Mental State Examination (MMSE) and Philadelphia Brief Assessment of Cognition (PBAC) in bvFTD, and hypoperfusion in motor regions correlated with motor disability as measured by the ALS Functional Rating Scale-Revised (ALSFRS-R) in ALS.
Hypoperfusion marked early pathologically involved regions, while hyperperfusion characterized regions of late pathological involvement. Distinct perfusion patterns may provide early markers of pathology distribution in TDP-43 proteinopathies.
Keywords: Amyotrophic lateral sclerosis, Frontotemporal dementia, Magnetic Resonance Imaging, Perfusion, Pathology, TDP-43
1. Introduction
Approximately half of behavioural variant of frontotemporal dementia (bvFTD) and the vast majority of amyotrophic lateral sclerosis (ALS) patients share TAR DNA-binding protein 43 (TDP-43) as the main component in neuronal inclusions (Neumann et al., 2006) and this common source of pathology has supported the notion that these conditions are different clinical manifestations of the same proteinopathy. Neuropathological studies of TDP-43 pathology in bvFTD suggest that disease originates in the orbital frontal gyri, progresses toward middle frontal and temporal regions, and later encompasses motor areas followed by occipital cortex (Brettschneider et al., 2014). In ALS, cortical pathology appears to initiate in primary motor regions, spread to prefrontal cortex and parietal areas, and finally reach the anteromedial portions of the temporal lobe (Brettschneider et al., 2013). In the absence of quantifiable ways to measure TDP-43 in vivo, we focus our attention in this study on the use of structural and functional imaging modalities, to shed light on the anatomic patterns of TDP-43 related neurodegeneration.
Preliminary Magnetic Resonance Imaging (MRI) studies have demonstrated evidence for these neuropathological stages using diffusion tensor imaging (DTI) of white matter (WM) projections between hypothesized sites of TDP-43 progression (Kassubek et al., 2014). However, WM DTI only reflects severe degeneration (Caron et al., 2015) and similarly grey matter (GM) atrophy measured using T1-weighted structural MRI is generally thought to emerge only once there has been sufficient death in a neuronal population to be seen macroscopically (Popescu et al., 2015). Therefore, to date, structural MRI appears to detect only later-stage degeneration due to pathological accumulation.
Perfusion imaging provides a quantitative measure of cerebral blood flow (CBF), a physiological parameter reflecting tissue function. Reduced CBF may signal decreased neural activity before structural loss has become apparent, while increased CBF may reflect a compensatory activity increase in a neuronal population showing only mild pathology (Roquet et al., 2016). Thus perfusion may serve as an earlier marker of disease than structural MRI and we have indeed previously shown that perfusion alterations anticipate GM loss in a longitudinal study of language variants of frontotemporal lobar degeneration (FTLD) with likely TDP-43 pathology (Olm et al., 2016).
In this study, we evaluated perfusion imaging as an early physiological marker for brain alterations associated with increasing pathological accumulation across TDP-43 proteinopathies, including genetically and/or autopsy-confirmed TDP-43 bvFTD and ALS patients who are >95% likely to have TDP-43 pathology (Neumann et al., 2006; Ravits, 2014). In particular, we hypothesized that the spatial distribution of perfusion alterations would reflect the regional distribution of pathology reported in previous neuropathological studies of bvFTD (Brettschneider et al., 2014) and ALS (Brettschneider et al., 2013).
2. Material and methods
2.1 Subjects
We studied 30 patients with a high likelihood of TDP-43 proteinopathy including 18 ALS patients and 12 bvFTD patients, along with 33 demographically matched healthy controls. All participants had completed a written informed consent procedure under a protocol approved by the Institutional Review Board convened at the University of Pennsylvania.
All patients were clinically diagnosed by experienced neurologists (LM, LE, DJI, MG) in the Penn Comprehensive ALS Center and the Penn Frontotemporal Degeneration Center of the Department of Neurology at the University of Pennsylvania Perelman School of Medicine. Diagnosis was based on a consensus evaluation including: a) a semi-structured interview on neurologic history, b) a complete neurologic exam, c) an informant-based history, and d) a detailed neuropsychological assessment using the Philadelphia Brief Assessment of Cognition (PBAC) (Libon et al., 2011; see below) and/or clinical assessments of cognition including MMSE and verbal fluency. All ALS patients were defined according to El Escorial revised criteria (Brooks et al., 2000) (including possible, probable and definite). Three ALS cases were considered “suspected” according to original criteria (Brooks, 1994) at the time of MRI, but were retained in this study cohort since their clinical diagnosis later transitioned to either definite ALS (N=2) or possible ALS (N=1) over the course of their disease. BvFTD patients were evaluated using consensus criteria for the diagnosis of probable bvFTD (Rascovsky et al., 2011). For patients and healthy controls, exclusion criteria included vascular disease, medical diseases interfering with cognition, and primary psychiatric disorders. Motor disability in ALS patients was assessed using the ALS Functional Rating Scale-Revised (ALSFRS-R) (Cedarbaum et al., 1999). Global cognitive functioning was evaluated using the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) adjusted to account for motor impairment in ALS. Executive functions, memory, language and behavioural symptoms were evaluated using the Philadelphia Brief Assessment of Cognition (PBAC) (Libon et al., 2011). ALS patients were included only if there was no clinical evidence for cognitive impairment, determined for 13 out of 18 ALS patients using formal neuropsychological assessment as suggested by current revised clinical criteria (Strong et al., 2017), and based on cognitive assessment at clinical exam (including MMSE, verbal fluency, and negative informant report of behavioural and/or social deficits) for the remaining ALS patients (5 out of 18). Disease duration was calculated as the time in years from symptom onset to MRI scan.
Prior autopsy studies suggest that TDP-43 is present in >95% of ALS patients (Neumann et al., 2006; Ravits, 2014), with the exception of familial SOD1 and FUS. Therefore we only included ALS cases in our cohort who were sporadic based on a three generation validated pedigree screening procedure (N=14) (Wood et al., 2013) or, if patients had a family history of any neurodegenerative disease (N=4), they were screened to confirm they were negative for a SOD1 or FUS mutation. This resulted in a final ALS sample of highly-likely TDP-43 pathology (N=18). Since TDP-43 is only present in about half of bvFTD (Rohrer, 2011), we restricted our inclusion of bvFTD cases to those individuals with either a neuropathological diagnosis of FTLD-TDP (MacKenzie et al., 2010) (N=3) and/or a known pathogenic genetic mutation associated with TDP-43 pathology including C9Orf72 repeat expansions (DeJesus-Hernandez et al., 2011; Renton et al., 2011) (N=9) or TARDBP mutations (Van Deerlin et al., 2008) (N=2). Also, since progranulin (GRN) mutations are exclusively associated with TDP-43 subtype A (Mackenzie et al., 2011; Irwin et al., 2015) that is not associated with the spectrum of motor neuron disease (MND) and FTD, we have also excluded bvFTD cases carrying a GRN mutation. Neuropathological and genetic procedures were performed as previously described (McMillan et al., 2014). In particular, we queried the Penn Brain Bank for autopsy confirmed bvFTD cases that had a primary neuropathological diagnosis of FTLD-TDP. Neuropathologic diagnoses were established according to consensus criteria (Mackenzie et al., 2010) by an expert neuropathologist using immunohistochemistry with established monoclonal antibodies specific for TDP-43 (mAbs p409/410 or 171) (Lippa et al., 2009). For genetic screening, DNA was extracted from peripheral blood or brain using manufacturer’s protocols (Flexigene, Qiagen; or QuickGene DNA, Autogen). Samples were genotyped for the C9orf72 hexanucleotide-repeat using a published modified repeat-primed polymerase-chain reaction and determined as an expansion if exceeding >30 repeats (Suh et al., 2015). Screening for additional mutations was performed by sequencing the coding regions of 45 genes previously associated with ALS and/or FTD (e.g., GRN, SOD1, FUS, TARDBP) along with other neurodegenerative diseases (e.g., PSEN1 for Alzheimer’s and LRRK2 for Parkinson’s Diseases). This was accomplished using a custom panel (MiND-Seq) that uses next-generation sequencing technology with capture using Haloplex enrichment custom kit (Agilent, Wilmington, DE, USA) according to the manufacturer’s protocol and sequencing on a Mi-Seq or Hi-Seq instrument (Illumina, San Diego, CA, USA). Alignment of sequence reads and variant calling were assessed by SureCall software (Agilent).
2.2 MRI acquisition and processing
Images were collected using a 3T Siemens Tim Trio scanner with an 8-channel head coil. Sessions started with a high-resolution T1-weighted MPRAGE structural scan, with TR= 1620 ms, TE= 3.09 ms, flip angle= 15, 192 x 256 matrix, and 1 x 1 x 1 mm voxels. We processed T1-weighted MRI images using Advanced Normalization Tools (ANTs) (Avants et al., 2011, 2008) including antsCorticalThickness (Tustison et al., 2014). Briefly, we deformed each dataset into a standard template space derived from scans of the Open Access Series of Imaging Studies (OASIS) dataset (Marcus et al., 2007). Afterwards, we segmented images into six tissue classes (cortex, white matter, CSF, subcortical gray structures, midbrain, and cerebellum) and generated probability maps of each tissue. Next, cortical thickness (CT) was calculated (Das et al., 2009). For analysis, CT images were then normalized to MNI152 space, smoothed using a 2 sigma full-width half-maximum Gaussian kernel, and downsampled to 2x2x2 mm3 resolution.
In the same scanning session as the T1-weighted acquisition, 80 volumes (40 label, 40 control) of a spin echo echoplanar pcASL sequence were collected with TR= 4300ms, TE= 20ms, labelling duration= 1500ms, post-label delay= 1500ms, matrix= 96 x 96, flip angle= 90, 2.5 x 2.5mm in-plane resolution, and 5 mm thick slices with a 1-mm slice gap, total: 20 slices. PcASL images were processed using the antsASLProcessing script in ANTsR. Using R (http://www.r-project.org/), a binary tag-control label, motion, and physiological confounds were regressed out of each volume (Avants et al., 2012). For further details of PcASL images processing please see our previous work (Olm et al., 2016). We then quantified CBF using the following formula:
where ΔM is the mean label-control difference, λ is the blood/tissue water partition coefficient (0.9 g/ml), α is the labeling efficiency for pcASL (85 %), M0 is the equilibrium magnetization estimated by averaging the control images, T1b is the T1 relaxation time of blood (1664 ms), w is the post-labeling delay, and τ is the labeling duration (1500 ms).
We used a partial volume correction (PVC) procedure to reduce potential confounds associated with large pcASL voxels likely containing multiple tissue classes. The CBF images were thus transformed to native-T1 space to take advantage of the higher spatial resolution when accounting for partial volume effects. As WM CBF is approximately 40 % of GM CBF and CSF should account for 0 % of CBF, we calculated partial volume-corrected CBF (CBFPVC) in every voxel with the previously reported formula: CBFPVC = CBF/ (GMP + 0.4 x WMP) (Johnson et al., 2005). Before dividing, we smoothed the (GMP + 0.4 x WMP) and CBF images. To account for individual global differences in perfusion, we also mean-centered the partial volume corrected CBF images, generating a “CBFcor” image. CBFcor images were normalized to MNI152 space and downsampled to 2mm3 resolution for analysis.
2.3 Statistical analysis
Demographic, clinical and neuropsychological variables were analysed using IBM SPSS version 23.0 to compute independent samples t-tests for continuous variables and Fisher’s exact test for categorical variables. Imaging comparisons were calculated using the randomise tool in FSL (http://fsl.fmrib.ox.ac.uk/fsl/randomise/). In particular, for both CT and cortical CBFcor images, we performed nonparametric, permutation tests (permutations = 10,000), which control for Type 1 errors (Winkler et al., 2014) for the following contrasts: bvFTD patients vs healthy controls, ALS patients vs healthy controls and bvFTD vs ALS patients, correcting for disease duration. In order to constrain our analysis to voxels that likely contain grey matter we considered only voxels with a mean CT > 0.4 mm3 for all comparisons. In order to exclude a potential significant impact of the mean-centering normalization procedure on hyperfusion estimation (Dukart et al., 2010), we additionally repeated all the analyses using the not mean-centered, partial volume corrected CBF images (CBFPVC). For the CT comparisons, we used threshold-free cluster enhancement and considered voxels significant if they surpassed a family-wise error corrected threshold of p < 0.05 with a volume greater than 650 mm3. For the CBFcor comparisons, clusters with an uncorrected threshold of p < 0.05 and a volume greater than 650 mm3 were considered significant. We chose to use different statistical thresholds for the T1- and pcASL-derived datasets due to differences in statistical properties of the two data types, including a reduced signal-to-noise ratio for pcASL relative to T1-weighted protocols. Finally, we performed univariate regressions to test the relationship between reduced CT, perfusion alterations and patients’ clinical impairment, correcting for disease duration. In particular, in bvFTD patients, we tested the correlation between CT and CBF values and: a) general cognitive impairment as investigated using the MMSE, and b) apathy and disinhibition as investigated using the PBAC apathy and disinhibition scales. In ALS patients we investigated the relationship between CT and CBF values and motor impairment as investigated using the ALSFRS-R (the largest cluster with an uncorrected threshold of p < 0.05 is reported for each comparison).
3. Results
Main demographic, clinical and neuropsychological features are summarized in Table 1. No statistically significant differences were observed between patient groups and healthy controls for age, sex and education (Table 1). ALS patients did not differ from healthy controls for global cognition as evaluated using the MMSE, while bvFTD showed an expected significantly reduced MMSE relative to both healthy controls and ALS cases (Table 1). BvFTD patients also showed significantly more severe behavioural symptoms and greater language impairment relative to ALS cases. Disease duration was not significantly different between the two patient groups, even if ALS cases exhibited, as expected, a relatively shorter disease duration.
Table 1.
Demographic, clinical and neuropsychological features of healthy controls, ALS and bvFTD patient groups.
| Healthy controls | ALS | bvFTD | |
|---|---|---|---|
| N | 33 | 18 | 12 |
| Age | 59.69 (9.74) | 62.01 (8.39) | 62.62 (6.18) |
| Sex [F/M] | 16/17 | 9/9 | 4/8 |
| Education | 15.36 (2.45) | 14.77 (2.69) | 17.25 (2.55) |
| MMSE | 29.56 (0.55) | 28.18 (1.87) | 22.40 (7.13) * # |
| ALSFRS-r | - | 36.70 (5.62) | - |
| Disease duration [Y] | - | 3.66 (2.68) | 5.33 (2.01) |
| PBAC- Letter “F” fluency | - | 14.84 (3.95) | 11.50 (4.66) |
| PBAC- Digit span backward [0–7] | - | 4.48 (0.97) | 3.87 (0.78) |
| PBAC- Naming [0–6] | - | 6.00 (0.00) | 5.50 (0.50) # |
| PBAC- Digit span forward [0–8] | - | 6.08 (1.38) | 5.12 (0.78) |
| PBAC- Apathy [3-0]* | - | 3.00 (0.00) | 2.00 (0.71)# |
| PBAC- Disinhibition [3-0]* | - | 2.92 (0.27) | 1.50 (0.71)# |
| PBAC- Agitation [3-0]* | - | 2.92 (0.27) | 2.13 (1.05) |
| PBAC- Empathy [3-0]* | - | 3.00 (0.00) | 1.38 (0.86)# |
| PBAC- Ritualistic behavior [3-0]* | - | 3.00 (0.00) | 1.38 (0.86)# |
| PBAC- Self Insight [3-0]* | - | 2.85 (0.53) | 1.13 (1.17)# |
Behaviors are scored using the following scale: 3=not observed, 2=mild, 1=moderate, 0=severe.
Values are means and standard deviations. Significant differences between healthy controls and patient groups are indicated by *, significant differences between bvFTD and ALS patients are indicated by # (p < 0.05 using independent samples t test for continuous variables and Fisher’s exact test for categorical variables). Abbreviations: ALS= Amyotrophic lateral sclerosis; ALSFRS-r= Amyotrophic lateral sclerosis functional rating scale revised; bvFTD= behavioral variant of frontotemporal dementia; F=female; M=male; MMSE= Mini Mental State Examination; N= number; PBAC= Philadelphia Brief Assessment of Cognition; Y= years.
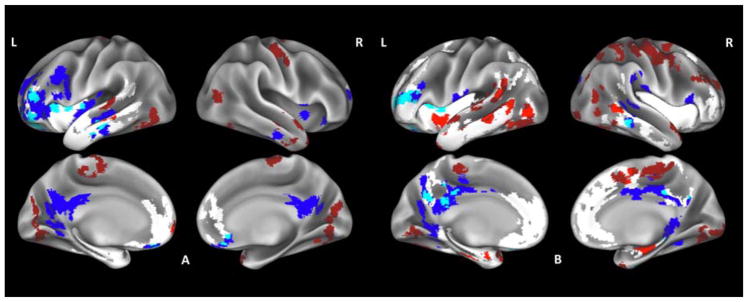
Relative to controls, bvFTD patients showed reduced CT encompassing prefrontal cortex bilaterally, left superior, middle and inferior temporal gyri and insula (Fig. 1.A., Supplementary table 1). Areas of hypoperfusion partially overlapping with reduced CT included the orbitofrontal cortex bilaterally, left dorsolateral prefrontal cortex, left middle temporal gyrus as well as left superior frontal cortex and insula. Hypoperfusion not overlapping with reduced CT was detected in posterior cingulate cortex bilaterally, right insula and right middle temporal gyrus. Clusters of hyperperfusion partially overlapping with reduced CT were observed in the left superior frontal and middle temporal gyri, while hyperperfusion not overlapping with reduced CT encompassed primary motor and occipital cortex bilaterally as well as right temporal pole.
Fig. 1.
Whole-brain perfusion and cortical thickness (CT) comparisons of bvFTD patients relative to healthy controls (A) and relative to ALS patients (B). Regions where patients display significant reduced CT are shown in white, hypoperfusion is shown in blue, hyperperfusion is shown in red, overlapping reduced CT and hypoperfusion is shown in light blue and overlapping reduced CT and hyperperfusion is shown in light red.
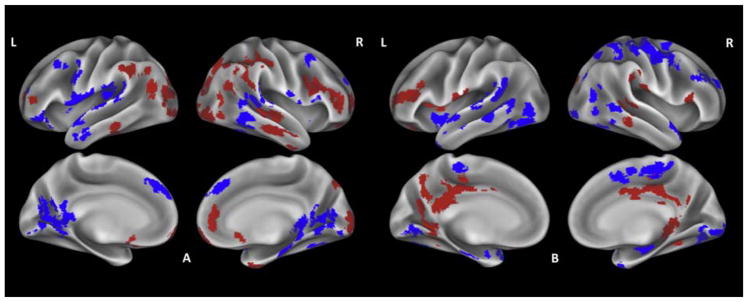
ALS patients did not show significant reduced CT relative to controls (Fig. 2.A., Supplementary table 2). Hypoperfusion was observed in the premotor cortex, superior frontal gyrus, superior and middle temporal gyrus, and lingual gyrus bilaterally as well as in the left primary motor cortex and inferior frontal gyrus and right fusiform gyrus. Hyperperfusion encompassed inferior frontal, inferior temporal and angular gyrus and occipital pole bilaterally as well as right superior frontal gyrus and temporal pole.
Fig. 2.
Whole-brain perfusion comparisons of ALS patients relative to healthy controls (A), and relative to bvFTD patients (B). Hypoperfusion is shown in blue, hyperperfusion is shown in red.
When we directly compared the two patient groups, bvFTD cases showed widespread reduced CT relative to ALS mainly involving frontal and temporal cortices as well as insula bilaterally (Fig. 1.B, Supplementary table 3). Areas of hypoperfusion in bvFTD partially overlapping with reduced CT included posterior cingulate cortex bilaterally as well as left prefrontal and orbitofrontal cortex, and right inferior temporal gyrus. Areas of hypoperfusion not overlapping with reduced CT included the fusiform gyrus bilaterally, right inferior frontal and supramarginal gyrus. Clusters of overlapping hyperperfusion and reduced CT were located in the right prefrontal cortex, left middle temporal gyrus, temporal pole and insula. Widespread hyperperfusion not overlapping with reduced CT was found in right motor cortices, right occipital pole and left superior and middle temporal gyri.
Relative to bvFTD patients, ALS cases showed no significant reduced CT (Fig. 2.B, Supplementary table 3). Hypoperfusion mainly encompassed motor and temporal cortex bilaterally, while hyperperfusion was observed in posterior cingulate cortex and inferior frontal gyrus bilaterally, left dorsolateral and orbitofrontal cortex and right inferior temporal gyrus.
When we repeated all the analyses using the not mean-centered CBFPVC images the majority of clusters of hyperperfusion remained significant in all comparisons (Supplementary tables 1–3).
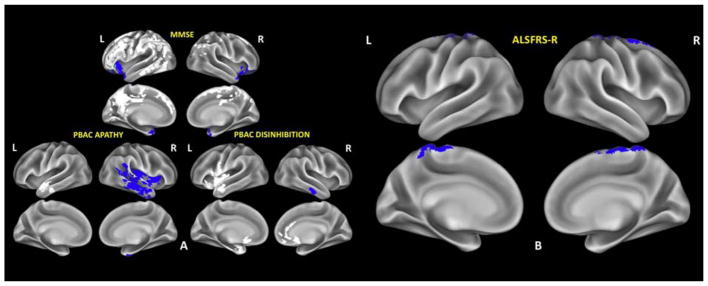
In bvFTD, reduced MMSE scores correlated with hypoperfusion in the inferior frontal cortex and temporal pole bilaterally and widespread frontotemporoparietal CT reductions. Reduced PBAC apathy scores were associated with right temporal hypoperfusion and left temporal CT reductions, while reduced PBAC disinhibition scores were mainly associated with right temporal hypoperfusion and widespread bilateral CT reductions (Fig. 3.A, Supplementary table 1). In ALS, reduced ALSFRS-R correlated with hypoperfusion in primary motor cortex and postcentral gyrus bilaterally and in right supplementary motor cortex (Fig. 3.B, Supplementary table 2).
Fig. 3.
Whole-brain perfusion and cortical thickness (CT) regression analyses in bvFTD patients (A) and ALS patients (B). Regions where hypoperfusion is significantly correlated with reduced MMSE, reduced PBAC apathy and reduced PBAC disinhibition scales scores (in bvFTD) and reduced ALSFRS-R (in ALS) are shown in blue, regions where reduced CT is significantly correlated with reduced MMSE, reduced PBAC apathy and reduced PBAC disinhibition scales scores (in bvFTD) are shown in white.
4. Discussion
The neuroanatomic distribution of perfusion alterations in our sample of TDP-43 proteinopathies converges with the regional distribution of pathology proposed in autopsy studies, with hypoperfusion in anatomic regions thought to have high pathological burden and hyperperfusion characterizing anatomic areas of likely more modest pathological accumulation. We discuss in detail below the interaction between perfusion alterations and CT reductions in both bvFTD and ALS and how these neuroimaging modalities can provide in vivo markers of anatomic disease consistent with TDP-43 pathological progression.
4.1 Perfusion changes in areas of Reduced Cortical Thickness in bvFTD
In bvFTD patients, widespread CT reductions were detected in frontotemporal cortices and insula, in agreement with their clinical phenotype (Du et al., 2007; McMillan et al., 2012). Notably, we observed more widespread CT reductions in the left hemisphere, a finding that might be related to the selective inclusion of TDP-43 confirmed bvFTD. While imaging studies of bvFTD typically include right hemisphere disease, prior studies focused on confirmed TDP-43 samples have emphasized greater left sided atrophy relative to other pathological subtypes (Rohrer et al., 2011). Many areas of reduced CT also had evidence for hypoperfusion. Interestingly, some regions of CT reductions did not show altered perfusion, and this is in accordance with a previous perfusion study we performed in language variants of FTLD (Olm et al., 2016). Since partial volume correction normalizes CBF for the amount of GM density, it is possible that the observed areas of reduced CT without altered perfusion are actually reflecting a proportional decline in perfusion and thickness, but longitudinal studies are warranted to confirm this hypothesis.
Hypoperfusion overlapping with reduced CT was observed in regions known to show severe damage related to early pathological accumulation in bvFTD, including the orbitofrontal cortex bilaterally, left frontotemporal regions and insula. It has been suggested recently that concurrent hypoperfusion and reduced CT may indicate that a specific neuronal population has excessively reduced activity relative to the amount of thinning (Olm et al., 2016). One possible explanation is that there may be a floor effect to the amount of possible cortical thinning since an area of neuronal depletion will still have some thickness associated with gliosis and related cellular structural elements, even though hypoperfusion may continue to decline due to the loss of neuronal activity. Accordingly, in the bvFTD cohort, this pattern was mainly observed in areas of significant neuronal depletion known from autopsy studies to be areas of early pathological accumulation in this phenotype, including anterior prefrontal cortex and left temporal regions (Brettschneider et al., 2014). Clusters of hyperperfusion overlapping with reduced CT were also detected. While we propose that hyperperfusion in absence of CT reductions might signal the activity enhancement of a mild diseased neural population, the overlap of hyperperfusion and CT reductions in other regions might reflect a different underlying mechanism, such as neuroinflammation. Neuroinflammation has indeed been previously associated with both hypermetabolism (partially explained by the high metabolic demand of microglia) and CT reductions related to increasing pathology (Brendel et al., 2017; Fleischman et al., 2010). However, the biological mechanisms sustaining hyperperfusion are still not fully understood and future studies are warranted to better investigate this aspect.
4.2 Perfusion changes in the Absence of Reduced Cortical Thickness in bvFTD
We also observed perfusion changes in absence of CT reduction. These may be associated with altered neuronal activity in areas lacking sufficient pathology associated degeneration to be already manifested as structural damage (Rajagopalan and Pioro, 2014). In bvFTD patients, we observed hypoperfusion without reduced CT in posterior cingulate cortex bilaterally, right insula and right inferior temporal gyrus, all regions known from previous MRI studies to show progressive atrophy during the course of the disease (Du et al., 2007; Meyer et al., 2017; Tan et al., 2013). These findings are also in accordance with previous SPECT studies reporting hypoperfusion of the cingulate cortex and temporal regions in FTD (Guedj et al., 2007; McMurtray et al., 2006). Notably, we found that not only reduced CT, but also hypoperfusion in frontal and temporal regions without overlapping atrophy significantly correlated with greater cognitive impairment and behavioural symptoms in bvFTD, strengthening the hypothesis that hypoperfusion may be associated with clinically relevant regions of neurodegeneration.
We also observed hyperperfusion in absence of reduced CT. This phenomenon has been previously associated with early compensatory mechanisms for some initial decline in a neuronal population at risk for disease (Hu et al., 2010) and here we further propose that it might indicate activity enhancement in response to mild incipient pathology. In bvFTD, hyperperfusion was indeed mainly observed in primary motor cortex and cuneus bilaterally. These regions are known from autopsy studies to show only late pathological involvement (Brettschneider et al., 2014) and may be upregulated to help maintain functioning until advanced disease stages. Notably, while some studies have suggested increased activity to occur in contralateral homologues of diseased regions to provide compensatory support (Andoh and Martinot, 2008), our findings of bilateral occipital and motor hyperperfusion in bvFTD seem to favour the hypothesis of a regional activity increase due to mild pathological burden that remains to be manifested as reduced CT.
4.3 Perfusion changes in the Absence of Reduced Cortical Thickness in ALS
No significant CT reductions were observed in ALS patients. This is not an uncommon finding. Some previous MRI studies have indeed reported reduced CT in motor and extramotor brain regions in ALS (Agosta et al., 2016; Walhout et al., 2014), while others have not detected GM loss (Abrahams et al., 2005), presumably partially reflecting the relative proportions of upper motor neuron and lower motor neuron dysfunction in patients included in these studies. While many patients with predominant lower motor neuron disease may lack significant cortical thinning (Spinelli et al., 2016; Walhout et al., 2014), longitudinal imaging studies suggest that some of these patients may exhibit progressive cortical atrophy during the course of the disease (Schuster et al., 2014). Notably however, we detected both hyper- and hypoperfusion, suggesting that perfusion might be a sensitive marker for altered brain activity in absence of structural loss as detectable using CT analysis, even if future longitudinal studies are needed to fully address this hypothesis.
Hypoperfusion was observed in motor and premotor regions which have been proposed as the epicenters of pathology spread in ALS (Brettschneider et al., 2013) and show progressive structural decline during the course of the disease (Agosta et al., 2009; Kwan et al., 2012), but also in superior frontal and supramarginal gyrus, in accordance with the sequential propagation of pathology (Brettschneider et al., 2013). Overall, hypoperfusion was observed in several extramotor brain regions including the temporal, fusiform and lingual gyrus, pericalcarine cortex and precuneus bilaterally. Reduced perfusion in temporal and fusiform gyri is in agreement with the progressive involvement of extramotor brain regions reported in previous structural longitudinal MRI studies in ALS (Verstraete et al., 2014). Our results also converge with findings from recent functional imaging studies looking at measures of brain networks organization in ALS. In particular, decreased network degree centrality in lingual, calcarine and fusiform gyrus (Zhou et al., 2016) has been recently reported in ALS and related to altered visual processing (Lulé et al., 2010), strengthening the notion that ALS is a multisystem disorder extending beyond motor cortices. An alternative explanation for hypoperfusion might be diaschisis, where altered WM projections from a structurally and/or functionally impaired region may result in reduced functioning of an otherwise intact projection area (Baron et al., 1981). However, in this work we have not directly investigated WM alterations and future studies using WM analyses are warranted to test this hypothesis. It is noteworthy, however, that we specifically identified hypoperfusion in motor regions to be associated with motor impairment in our ALS cohort, reinforcing the hypothesis that reduced perfusion mainly manifests in clinically relevant regions.
Hyperperfusion was observed in inferior frontal and orbitofrontal regions, right temporal gyri and occipital cortex bilaterally. Anterior frontal and right temporal regions are highly involved in executive and memory functions, and the enhanced activity of these regions is in line with the absence of frontotemporal cognitive dysfunction in our ALS sample. These results are also in agreement with a recent PET study reporting increased right temporal metabolism in sporadic ALS patients with no cognitive impairment relative to healthy controls (Cistaro et al., 2014). Interestingly, we also observed bilateral occipital hyperperfusion in our ALS cohort. Pathological studies report occipital cortex to be relatively spared by TDP-43 pathology in ALS (Brettschneider et al., 2013) and this would strengthen the hypothesis, as in bvFTD cases, of elevated perfusion helping to maintain functioning until advanced disease stages. In line with this possible explanation, the occipital activity enhancement we observed would also fit well with the increased occipital functional connectivity reported in recent MRI studies in ALS (Geevasinga et al., 2017), even if the compensatory nature of these processes is still under debate.
4.4 Conclusions
We demonstrated that perfusion alterations extend beyond neuroanatomic loci of reduced CT in bvFTD and are evident in ALS even in the absence of reduced CT. This suggests that perfusion imaging, relative to structural imaging, may serve as an early physiological marker for altered neural activity related to the distribution of underlying TDP-43 pathology.
While our results are largely convergent with pathological staging systems, some divergent findings may be attributed to methodological differences between neuropathological and neuroimaging studies (e.g., microsampling of unilateral 70 μm sections representative of large anatomic regions vs whole-brain imaging, different sample sizes and distinct disease stages at the time of examination). In this context, more post-mortem and clinico-pathological correlation studies are necessary to establish the convergence between in vivo neuroimaging and ex vivo neuropathological observations (Chen et al., 2018).
The present work has some limitations. Firstly, this is a cross-sectional study and longitudinal analyses are needed to explore disease progression in areas showing different perfusion patterns. The second limitation deals with the relatively small sample size, which is due to the selective inclusion of bvFTD patients with either a neuropathological diagnosis and/or a known genetic mutation associated with TDP-43. Additionally, while the majority of our ALS cases had detailed neuropsychological assessments, a limitation of this retrospective study is the use of PBAC and/or clinical evaluations to assess cognitive impairment. Future studies using more sensitive tools that also better control for potential motor confounds, such as the now widely-used Edinburgh Cognitive Assessment Scale (ECAS) (Abrahams et al., 2014), are necessary to more carefully evaluate cognition in ALS. As regards our MRI approach it is noteworthy that while structural damage was not evident in our CT analysis of ALS, we can’t completely rule out the possibility that different grey matter analyses, such as voxel-based morphometry or surface-based CT measurements using FreeSurfer (Fischl and Dale, 2000), would be more sensitive in detecting neurodegeneration. Partial volume effects might also be important confounds in perfusion MRI analyses and improved methods of correction are still needed. Moreover, even if the relatively low signal-to-noise ratio of perfusion data has constrained the analyses to a less strict statistical threshold, our MRI results are notably in line with pathological findings in both bvFTD and ALS.
In summary, our study indicates that perfusion measurement can mark common and distinct targets of neurodegeneration across the spectrum of TDP-43 proteinopathies. We observed similar frontotemporal and posterior cingulate perfusion reductions as well as posterior cortical hyperperfusion in both bvFTD and ALS. This suggests partially overlapping brain alterations to occur in TDP-43 proteinopathies and contributes to explain the clinical continuum between these conditions, even if future studies with ALS-FTD patients are necessary to fully address this hypothesis. Notably, in line with their different clinical manifestations, we also detected divergent physiological alterations in the two phenotypes, with bvFTD patients manifesting reduced perfusion in regions supporting cognition and elevated perfusion in motor regions, and ALS patients showing the opposite pattern. We conclude that perfusion measurement may provide a sensitive in vivo marker for characterizing important early mechanisms involved in disease pathogenesis and progression.
Supplementary Material
ALS and bvFTD are commonly linked by TDP-43 molecular pathology.
Perfusion imaging detects early alterations even in the absence of cortical atrophy.
Hypoperfusion can be observed in regions associated with early TDP-43 pathology.
Hypoperfusion in core areas is associated with clinical impairment.
Hyperperfusion characterizes regions associated with late TDP-43 pathology.
Acknowledgments
This work was supported in part by the National Institutes of Health (AG043503, AG017586, NS088341, P41 RR002305), the ALS Association and the Dana Foundation.
Footnotes
Declarations of interest:
P.M.F., C.J., C.A.O. K.P., D.J.I., J.A.D., M.G. and C.T.M. report no conflicts of interest.
F. A. is Section Editor of NeuroImage: Clinical; has received speaker honoraria from Biogen Idec, ExceMED – Excellence in Medical Education, and ACCMED (Accademia Nazionale di Medicina); and receives or has received research supports from the Italian Ministry of Health, AriSLA (Fondazione Italiana di Ricerca per la SLA), and the European Research Council. M. F. is Editor-in-Chief of the Journal of Neurology; serves on a scientific advisory board for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Biogen Idec, ExceMED, Novartis, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Teva Pharmaceutical Industries, Novartis, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, Alzheimer’s Drug Discovery Foundation (ADDF), the Jacques and Gloria Gossweiler Foundation (Switzerland), and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams S, Goldstein LH, Suckling J, Ng V, Simmons A, Chitnis X, Atkins L, Williams SCR, Leigh PN. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol. 2005;252:321–331. doi: 10.1007/s00415-005-0646-x. https://doi.org/10.1007/s00415-005-0646-x. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Front Degener. 2014;15:9–14. doi: 10.3109/21678421.2013.805784. https://doi.org/10.3109/21678421.2013.805784. [DOI] [PubMed] [Google Scholar]
- Agosta F, Ferraro PM, Riva N, Spinelli EG, Chiò A, Canu E, Valsasina P, Lunetta C, Iannaccone S, Copetti M, Prudente E, Comi G, Falini A, Filippi M. Structural brain correlates of cognitive and behavioral impairment in MND. Hum Brain Mapp. 2016;37:1614–1626. doi: 10.1002/hbm.23124. https://doi.org/10.1002/hbm.23124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Gorno-Tempini ML, Pagani E, Sala S, Caputo D, Perini M, Bartolomei I, Fruguglietti ME, Filippi M. Longitudinal assessment of grey matter contraction in amyotrophic lateral sclerosis: a tensor based morphometry study. Amyotroph Lateral Scler. 2009;10:168–74. doi: 10.1080/17482960802603841. https://doi.org/10.1080/17482960802603841. [DOI] [PubMed] [Google Scholar]
- Andoh J, Martinot JL. Interhemispheric compensation: A hypothesis of TMS-induced effects on language-related areas. Eur Psychiatry. 2008;23:281–288. doi: 10.1016/j.eurpsy.2007.10.012. https://doi.org/10.1016/j.eurpsy.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric Diffeomorphic Image Registration with Cross- Correlation: Evaluating Automated Labeling of Elderly and Neurodegenerative Brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. https://doi.org/10.1007/s11103-011-9767-z.Plastid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. https://doi.org/10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Lakshmikanth SKDJ, et al. Robust cerebral blood flow reconstruction from perfusion imaging with an open-source, multi-platform toolkit 2012 [Google Scholar]
- Baron JC, Bousser MG, Comar D, Castaigne P. “Crossed cerebellar diaschisis” in human supratentorial brain infarction. Trans Am Neurol Assoc. 1981;105:459–61. https://doi.org/10.2174/1385272820666160905104. [PubMed] [Google Scholar]
- Brendel M, Focke C, Blume T, Peters F, Deussing M, Probst F, Jaworska A, Overhoff F, Albert N, Lindner S, von Ungern-Sternberg B, Bartenstein P, Haass C, Kleinberger G, Herms J, Rominger A. Time Courses of Cortical Glucose Metabolism and Microglial Activity Across the Life-Span of Wild-Type Mice: A PET Study. J Nucl Med. 2017 doi: 10.2967/jnumed.117.195107. jnumed.117.195107. https://doi.org/10.2967/jnumed.117.195107. [DOI] [PubMed]
- Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, Fang L, Van Deerlin VM, Ludolph AC, Lee VMY, Braak H, Trojanowski JQ. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) Acta Neuropathol. 2014;127:423–439. doi: 10.1007/s00401-013-1238-y. https://doi.org/10.1007/s00401-013-1238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VMY, Braak H, Trojanowski JQ. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. https://doi.org/10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR. El escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci. 1994;124:96–107. doi: 10.1016/0022-510x(94)90191-0. https://doi.org/10.1016/0022-510X(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2000;1:293–299. doi: 10.1080/146608200300079536. https://doi.org/10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Caron I, Micotti E, Paladini A, Merlino G, Plebani L, Forloni G, Modo M, Bendotti C. Comparative magnetic resonance imaging and histopathological correlates in two SOD1 transgenic mouse models of amyotrophic lateral sclerosis. PLoS One. 2015:10. doi: 10.1371/journal.pone.0132159. https://doi.org/10.1371/journal.pone.0132159. [DOI] [PMC free article] [PubMed]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. https://doi.org/10.1016/S0022-510X(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Chen J, Kostenko V, Pioro EPTB. MR Imaging-based Estimation of Upper Motor Neuron Density in Patients with Amyotrophic Lateral Sclerosis: A Feasibility Study. Radiology. 2018 doi: 10.1148/radiol.2018162967. https://doi.org/10.1148/radiol.2018162967. [DOI] [PMC free article] [PubMed]
- Cistaro A, Pagani M, Montuschi A, Calvo A, Moglia C, Canosa A, Restagno G, Brunetti M, Traynor BJ, Nobili F, Carrara G, Fania P, Lopiano L, Valentini MC, Chiò A. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur J Nucl Med Mol Imaging. 2014;41:844–852. doi: 10.1007/s00259-013-2667-5. https://doi.org/10.1007/s00259-013-2667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Avants BB, Grossman M, Gee JC. Registration based cortical thickness measurement. Neuroimage. 2009;45:867–879. doi: 10.1016/j.neuroimage.2008.12.016. https://doi.org/10.1016/j.neuroimage.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NCA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GYR, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. https://doi.org/10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K, Miller BL, Weiner MW. Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain. 2007;130:1159–66. doi: 10.1093/brain/awm016. https://doi.org/10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J, Mueller K, Horstmann A, Vogt B, Frisch S, Barthel H, Becker G, Möller HE, Villringer A, Sabri O, Schroeter ML. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. Neuroimage. 2010;49:1490–1495. doi: 10.1016/j.neuroimage.2009.09.017. https://doi.org/10.1016/j.neuroimage.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. https://doi.org/10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA, Arfanakis K, Kelly JF, Rajendran N, Buchman AS, Morris MC, Barnes LL, Bennett DA. Regional brain cortical thinning and systemic inflammation in older persons without dementia. J Am Geriatr Soc. 2010 doi: 10.1111/j.1532-5415.2010.03049.x. https://doi.org/10.1111/j.1532-5415.2010.03049.x. [DOI] [PMC free article] [PubMed]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. https://doi.org/10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geevasinga N, Korgaonkar MS, Menon P, Van den Bos M, Gomes L, Foster S, Kiernan MC, Vucic S. Brain functional connectome abnormalities in amyotrophic lateral sclerosis are associated with disability and cortical hyperexcitability. Eur J Neurol. 2017;24:1507–1517. doi: 10.1111/ene.13461. https://doi.org/10.1111/ene.13461. [DOI] [PubMed] [Google Scholar]
- Guedj E, Le Ber I, Lacomblez L, Dubois B, Verpillat P, Didic M, Salachas F, Vera P, Hannequin D, Lotterie JA, Puel M, Decousus M, Thomas-Antérion C, Magne C, Vercelletto M, Bernard AM, Golfier V, Pasquier J, Michel BF, Namer I, Sellal F, Bochet J, Volteau M, Brice A, Meininger V, Habert MO FTD/FTD-MND*, F.R.N. Brain spect perfusion of frontotemporal dementia associated with motor neuron disease. Neurology. 2007;69:488–90. doi: 10.1212/01.wnl.0000266638.53185.e7. https://doi.org/10.1212/WNL.0b013e318281cbfe. [DOI] [PubMed] [Google Scholar]
- Hu WT, Wang Z, Lee VMY, Trojanowski JQ, Detre JA, Grossman M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology. 2010;75:881–888. doi: 10.1212/WNL.0b013e3181f11e35. https://doi.org/10.1212/WNL.0b013e3181f11e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, Lee VMY, Trojanowski JQ. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2015 doi: 10.1007/s00401-014-1380-1. https://doi.org/10.1007/s00401-014-1380-1. [DOI] [PMC free article] [PubMed]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of Cerebral Hypoperfusion in Alzheimer Disease and Mild Cognitive Impairment Measured with Arterial Spin-labeling MR Imaging: Initial Experience1. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. https://doi.org/10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J, Müller HP, Del Tredici K, Brettschneider J, Pinkhardt EH, Lulé D, Böhm S, Braak H, Ludolph AC. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain. 2014;137:1733–1740. doi: 10.1093/brain/awu090. https://doi.org/10.1093/brain/awu090. [DOI] [PubMed] [Google Scholar]
- Kwan JY, Meoded A, Danielian LE, Wu T, Floeter MK. Structural imaging differences and longitudinal changes in primary lateral sclerosis and amyotrophic lateral sclerosis. NeuroImage Clin. 2012;2:151–160. doi: 10.1016/j.nicl.2012.12.003. https://doi.org/http://dx.doi.org/10.1016/j.nicl.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Rascovsky K, Gross RG, White MT, Xie SX, Dreyfuss M, Boller A, Massimo L, Moore P, Kitain J, Coslett HB, Chatterjee A, Grossman M. The Philadelphia Brief Assessment of Cognition (PBAC): A validated screening measure for dementia. Clin Neuropsychol. 2011;25:1314–1330. doi: 10.1080/13854046.2011.631585. https://doi.org/10.1080/13854046.2011.631585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa CF, Rosso AL, Stutzbach LD, Neumann M, Lee VMY, Trojanowski JQ. Transactive response DNA-binding protein 43 burden in familial Alzheimer disease and Down syndrome. Arch Neurol. 2009;66:1483–1488. doi: 10.1001/archneurol.2009.277. https://doi.org/10.1001/archneurol.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lulé D, Diekmann V, Müller HP, Kassubek J, Ludolph AC, Birbaumer N. Neuroimaging of multimodal sensory stimulation in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2010;81:899–906. doi: 10.1136/jnnp.2009.192260. https://doi.org/10.1136/jnnp.2009.192260. [DOI] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DMA, Lee VMY. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011 doi: 10.1007/s00401-011-0845-8. https://doi.org/10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed]
- MacKenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJM, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DMA. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathologica. 2010:1–4. doi: 10.1007/s00401-009-0612-2. https://doi.org/10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed]
- Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19:1498–507. doi: 10.1162/jocn.2007.19.9.1498. https://doi.org/10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- McMillan CT, Brun C, Siddiqui S, Churgin M, Libon D, Yushkevich P, Zhang H, Boller A, Gee J, Grossman M. White matter imaging contributes to the multimodal diagnosis of frontotemporal lobar degeneration. Neurology. 2012;78:1761–1768. doi: 10.1212/WNL.0b013e31825830bd. https://doi.org/10.1212/WNL.0b013e31825830bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan CT, Toledo JB, Avants BB, Cook PA, Wood EM, Suh E, Irwin DJ, Powers J, Olm C, Elman L, McCluskey L, Schellenberg GD, Lee VMY, Trojanowski JQ, Van Deerlin VM, Grossman M. Genetic and neuroanatomic associations in sporadic frontotemporal lobar degeneration. Neurobiol Aging. 2014;35:1473–1482. doi: 10.1016/j.neurobiolaging.2013.11.029. https://doi.org/10.1016/j.neurobiolaging.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtray AM, Chen AK, Shapira JS, Chow TW, Mishkin F, Miller BL, Mendez MF. Variations in regional SPECT hypoperfusion and clinical features in frontotemporal dementia. Neurology. 2006;66:517–522. doi: 10.1212/01.wnl.0000197983.39436.e7. https://doi.org/10.1212/01.wnl.0000197983.39436.e7. [DOI] [PubMed] [Google Scholar]
- Meyer S, Mueller K, Stuke K, Bisenius S, Diehl-Schmid J, Jessen F, Kassubek J, Kornhuber J, Ludolph AC, Prudlo J, Schneider A, Schuemberg K, Yakushev I, Otto M, Schroeter ML. Predicting behavioral variant frontotemporal dementia with pattern classification in multi-center structural MRI data. NeuroImage Clin. 2017;14:656–662. doi: 10.1016/j.nicl.2017.02.001. https://doi.org/10.1016/j.nicl.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar Ha, Trojanowski JQ, Lee VMY. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. https://doi.org/10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Olm CA, Kandel BM, Avants BB, Detre JA, Gee JC, Grossman M, McMillan CT. Arterial spin labeling perfusion predicts longitudinal decline in semantic variant primary progressive aphasia. J Neurol. 2016;263:1927–1938. doi: 10.1007/s00415-016-8221-1. https://doi.org/10.1007/s00415-016-8221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu V, Klaver R, Voorn P, Galis-de Graaf Y, Knol D, Twisk J, Versteeg A, Schenk G, Van der Valk P, Barkhof F, De Vries H, Vrenken H, Geurts J. What drives MRI-measured cortical atrophy in multiple sclerosis? Mult Scler. 2015:1–11. doi: 10.1177/1352458514562440. https://doi.org/10.1177/1352458514562440. [DOI] [PubMed]
- Rajagopalan V, Pioro EP. Comparing brain structural MRI and metabolic FDG-PET changes in patients with ALS-FTD: “the chicken or the egg?” question. J Neurol Neurosurg Psychiatry. 2014:1–7. doi: 10.1136/jnnp-2014-308239. https://doi.org/10.1136/jnnp-2014-308239. [DOI] [PubMed]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. https://doi.org/10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravits J. Focality, stochasticity and neuroanatomic propagation in ALS pathogenesis. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.07.021. https://doi.org/10.1016/j.expneurol.2014.07.021. [DOI] [PubMed]
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. https://doi.org/10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD. Behavioural variant frontotemporal dementia-defining genetic and pathological subtypes. Journal of Molecular Neuroscience. 2011:583–588. doi: 10.1007/s12031-011-9542-2. https://doi.org/10.1007/s12031-011-9542-2. [DOI] [PubMed]
- Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, Beck J, Hardy J, De Silva R, Warrington E, Troakes C, Al-Sarraj S, King A, Borroni B, Clarkson MJ, Ourselin S, Holton JL, Fox NC, Revesz T, Rossor MN, Warren JD. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain. 2011;134:2565–2581. doi: 10.1093/brain/awr198. https://doi.org/10.1093/brain/awr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquet D, Sourty M, Botzung A, Armspach JP, Blanc F. Brain perfusion in dementia with Lewy bodies and Alzheimer’s disease: an arterial spin labeling MRI study on prodromal and mild dementia stages. Alzheimers Res Ther. 2016;8:29. doi: 10.1186/s13195-016-0196-8. https://doi.org/10.1186/s13195-016-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C, Kasper E, Machts J, Bittner D, Kaufmann J, Benecke R, Teipel S, Vielhaber S, Prudlo J. Longitudinal course of cortical thickness decline in amyotrophic lateral sclerosis. J Neurol. 2014;261:1871–1880. doi: 10.1007/s00415-014-7426-4. https://doi.org/10.1007/s00415-014-7426-4. [DOI] [PubMed] [Google Scholar]
- Spinelli EG, Agosta F, Ferraro PM, Riva N, Lunetta C, Falzone YM, Comi G, Falini A, Filippi M. Brain MR Imaging in Patients with Lower Motor Neuron–Predominant Disease. Radiology. 2016;280:545–556. doi: 10.1148/radiol.2016151846. https://doi.org/10.1148/radiol.2016151846. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, Mioshi E, Roberts-South A, Benatar M, HortobáGyi T, Rosenfeld J, Silani V, Ince PG, Turner MR. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Front Degener. 2017;18:153–174. doi: 10.1080/21678421.2016.1267768. https://doi.org/10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh ER, Lee EB, Neal D, Wood EM, Toledo JB, Rennert L, Irwin DJ, McMillan CT, Krock B, Elman LB, McCluskey LF, Grossman M, Xie SX, Trojanowski JQ, Van Deerlin VM. Semi-automated quantification of C9orf72 expansion size reveals inverse correlation between hexanucleotide repeat number and disease duration in frontotemporal degeneration. Acta Neuropathol. 2015;130:363–372. doi: 10.1007/s00401-015-1445-9. https://doi.org/10.1007/s00401-015-1445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RH, Pok K, Wong S, Brooks D, Halliday GM, Kril JJ. The pathogenesis of cingulate atrophy in behavioral variant frontotemporal dementia and Alzheimer’s disease. Acta Neuropathol Commun. 2013;1:30. doi: 10.1186/2051-5960-1-30. https://doi.org/10.1186/2051-5960-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, Kandel BM, van Strien N, Stone JR, Gee JC, Avants BB. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166–179. doi: 10.1016/j.neuroimage.2014.05.044. https://doi.org/10.1016/j.neuroimage.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, Steinbart E, McCluskey L, Grossman M, Neumann M, Wu IL, Yang WS, Kalb R, Galasko DR, Montine TJ, Trojanowski JQ, Lee VMY, Schellenberg GD, Yu CE. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. https://doi.org/10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete E, Veldink JH, van den Berg LH, Van den Heuvel MP. Structural brain network imaging shows expanding disconnection of the motor system in amyotrophic lateral sclerosis. Hum Brain Mapp. 2014;35:1351–1361. doi: 10.1002/hbm.22258. https://doi.org/10.1002/hbm.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhout R, Westeneng HJ, Verstraete E, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH. Cortical thickness in ALS: towards a marker for upper motor neuron involvement. J Neurol Neurosurg Psychiatry. 2014;86:1–7. doi: 10.1136/jnnp-2013-306839. https://doi.org/10.1136/jnnp-2013-306839. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. https://doi.org/10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood EM, Falcone D, Suh E, Irwin DJ, Chen-Plotkin AS, Lee EB, Xie SX, Van Deerlin VM, Grossman M. Development and validation of pedigree classification criteria for frontotemporal lobar degeneration. JAMA Neurol. 2013;70:1411–1417. doi: 10.1001/jamaneurol.2013.3956. https://doi.org/10.1001/jamaneurol.2013.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Hu X, Hu J, Liang M, Yin X, Chen L, Zhang J, Wang J. Altered brain network in amyotrophic lateral sclerosis: A resting graph theory-based network study at voxel-wise level. Front Neurosci. 2016:10. doi: 10.3389/fnins.2016.00204. https://doi.org/10.3389/fnins.2016.00204. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.