Much has been written about the escalating crisis of opioid-overdose deaths in the United States and its mounting social and economic costs. Although political and public health leaders have begun to confront this urgent problem, hidden beneath it lies another danger: the increasing spread of hepatitis C virus (HCV) associated with injection-opioid use.
The discovery and understanding of HCV and its complications and the recent development of highly effective treatments with cure rates of greater than 90% are triumphs of modern medicine. But this success has fostered a false sense of security: a “curable” disease is deemed a “conquered” disease that no longer warrants high-priority investment. Opioid-related HCV infection and its sequelae, however, affect growing numbers of people.
Of the estimated 3.5 million Americans with chronic hepatitis C, most are baby boomers born between 1945 and 1965, the vast majority of whom acquired HCV decades ago from blood transfusions, contaminated medical equipment, or parenteral drug use. Most people with HCV don’t know they have it, since HCV-related liver disease often causes few clinical signs or symptoms until its late stages. HCV-related mortality has been increasing for decades — a trend that is especially pronounced for HCV-associated liver cancer. From 2013 on, the number of HCV-related deaths in the United States has exceeded the number of deaths associated with HIV and 59 other infectious diseases combined. Public health actions to prevent HCV-related disease and death are focused on testing baby boomers and other people at risk for HCV and connecting infected people to proper medical care and curative treatment.
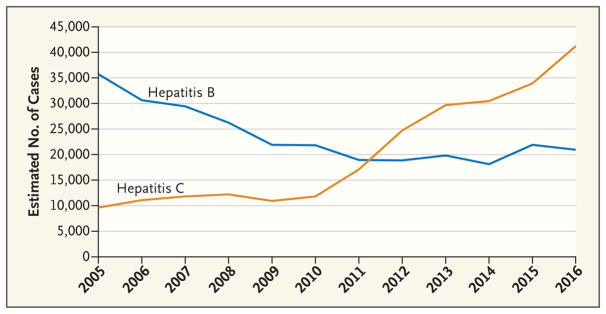
After the discovery of HCV in 1989 and the implementation of HCV screening for all blood products, the estimated number of new cases fell by more than 90% in the 1990s, then remained stable for years. Since 2009, however, the number of new HCV cases has risen dramatically (see graph). This increase has been driven largely by transmission among white adults in their 20s and 30s, particularly those living in nonurban areas.1 Many of these people initially became addicted to prescribed oral opioids and later switched to intravenous opioid use, which carries a high risk of HCV infection. The growing number of women of childbearing age with HCV has contributed to an increased number of babies born with the virus.2
Transmission of other blood-borne infections, particularly HIV and hepatitis B virus (HBV), is also increasing among injection-drug users, albeit at a slower rate. The opioid epidemic has also been linked to increasing rates of syphilis and other sexually transmitted infections, microbial endocarditis, and other infections associated with unsafe drug injection.3
The social and economic costs of the HCV epidemic could be staggering. Most injection-drug users who become infected with HCV do so as young adults. Such people are at risk for chronic hepatitis C and could face years of hefty health care expenses; left untreated, they may transmit HCV to others. The cost of caring for people with HCV places further strain on an already fragile health care system. Furthermore, because young adults are entering their most productive years, HCV will affect the economic productivity of the country for years to come.
The cost of treating HCV has fallen dramatically since the licensure of direct-acting antivirals (DAAs) beginning in 2014. Medication costs continue to be an issue, however, and restrictions imposed by public and private payers keep these drugs out of reach for many people. To address rising rates of HCV-related disease, barriers to treatment could be minimized by reducing drug costs, reforming restrictive health insurance policies, and combating the stigma that may influence policymakers and clinicians.
The opioid crisis and related HCV epidemic underscore the importance of continued investment in HCV research. More accessible HCV testing could prevent transmission and HCV-related disease by facilitating early identification of infection and linkage to care. Studies are needed to assess implementation of routine HCV testing in facilities that provide risk-reduction services such as medication-assisted treatment and syringe programs for injection-drug users, in clinical settings where HCV prevalence is often high (e.g., emergency departments, urgent care clinics, and prison clinics), and in community health centers that provide preventive services to young adults, including pregnant women. Population-based screening of young adults may be a reasonable strategy for identifying infected people and connecting them to treatment, as has been done among baby boomers. Development of new virologic-detection assays, available as point-of-care tests, will improve detection of new infections while simplifying clinical monitoring.
Research is now needed to guide development of care models that simplify HCV testing and treatment by enabling primary care clinicians, including midlevel practitioners, to routinely provide these services. Studies suggest that at-risk groups can have high rates of treatment completion and virologic cure.4 Although reinfection is a major concern when treating people with a history of injection-drug use, implementing concurrent risk-reduction measures, behavioral counseling, and other social-support services can dramatically reduce reinfection rates. Greater implementation of these measures could prevent not only new cases of hepatitis C, but also HIV infection and other sequelae.
Theoretical models suggest that combining HCV treatment with risk-reduction measures is the most effective way to prevent transmission among injection-drug users. The efficacy of treatment as a means of prevention has already been documented for HIV.5 Field trials could generate the evidence needed to develop effective HCV-prevention programs, particularly in communities with new substance-use and HCV epidemics. Studies are also needed to examine how HCV therapies can interrupt other modes of transmission, including mother-to-child transmission and sexual transmission, particularly among HIV-infected men who have sex with men.
In addition, we believe the development of an effective HCV vaccine should be a high priority. Increases in HCV infection could have been mitigated if an effective vaccine were available, just as universal childhood HBV vaccination and vaccination of at-risk adults reduced the spread of HBV infection among injection-drug users. Young injection-drug users have the highest incidence of HCV infection during their initial years of drug use. An effective vaccine could reduce the risk of HCV transmission if it were routinely provided to people before the onset of high-risk behaviors. Research and investment in HCV vaccine development have been inadequate, however, and such a project faces many challenges and scientific hurdles. A concerted and coordinated research effort by the public and private sectors could help achieve this goal.
Major strides have been made in understanding HCV infection and associated diseases. Yet many unmet needs and knowledge gaps remain. For instance, we still don’t know enough about how HCV establishes productive infection, how it persists in an otherwise healthy person, what the mechanisms involved in an effective immune response are, which molecular and genetic changes in people with chronic HCV infection are associated with liver cancer, and how infection alters host metabolism and organ-system functions to elicit pathologic responses and disease processes. Although excellent research tools exist to study HCV infection, a convenient and relevant animal model is still needed.
Current knowledge of the genetic and other determinants of HCV-related disease progression and cancer development is still rudimentary. Without a solid understanding of these processes and validated biomarkers, the goal of tailoring treatment to individual patients’ needs will remain elusive. Despite the development of highly effective medications, many treatment challenges persist. People infected with HCV genotype 3 and people with liver cirrhosis are least likely to have a response to treatment. Drug resistance can emerge in patients with multiple treatment failures, and there is theoretical concern regarding the spread of multi-drug-resistant HCV in such patients, especially among injection-drug users. Thus, many important questions pertaining to hepatitis C remain, and more research investments are needed to find the answers.
The opioid epidemic is a stark reminder of the consequences of a societal problem that remained hidden for years, in part because of the stigma associated with drug use and the reluctance to confront it as a public health problem. The concurrent spread of HCV, if not controlled, will similarly have public health and financial repercussions for decades to come.
Figure 1. Estimated Number of New Hepatitis B and Hepatitis C Infections in the United States, by Year.
Data are from the Centers for Disease Control and Prevention and are adjusted for the expected number of people with acute hepatitis B virus and HCV infections who become symptomatic, seek medical care, and are reported to state or local public health surveillance.
Footnotes
Disclosure forms provided by the authors are available at NEJM.org.
References
- 1.Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108:175–81. doi: 10.2105/AJPH.2017.304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick SW, Bauer AM, Warren MD, Jones TF, Wester C. Hepatitis C virus infection among women giving birth — Tennessee and United States, 2009–2014. MMWR Morb Mortal Wkly Rep. 2017;66:470–3. doi: 10.15585/mmwr.mm6618a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larney S, Peacock A, Mathers BM, Hickman M, Degenhardt L. A systematic review of injecting-related injury and disease among people who inject drugs. Drug Alcohol Depend. 2017;171:39–49. doi: 10.1016/j.drugalcdep.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018 Jan 5; doi: 10.1016/S2468-1253(17)30404-1. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 5.Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]