Abstract
Introduction
Platin-induced peripheral neuropathy (PIPN) is a common cause of PN in cancer patients. The aim of this paper is to systematically review the current literature regarding PIPN, with a particular focus on epidemiological and clinical characteristics of painful PIPN, and to discuss relevant management strategies.
Methods
A systematic computer-based literature search was conducted on the PubMed database.
Results
This search strategy resulted in the identification of 353 articles. After the eligibility assessment, 282 articles were excluded. An additional 24 papers were identified by scanning the reference lists. In total, 95 papers met the inclusion criteria and were used for this review. The prevalence of neuropathic symptoms due to acute toxicity of oxaliplatin was estimated at 84.6%, whereas PN established after chemotherapy with platins was estimated at 74.9%. Specifically regarding pain, the reported prevalence of pain due to acute toxicity of oxaliplatin was estimated at 55.6%, whereas the reported prevalence of chronic peripheral neuropathic pain in PIPN was estimated at 49.2%.
Conclusion
Peripheral neuropathy is a common complication in patients receiving platins and can be particularly painful. There is significant heterogeneity among studies regarding the method for diagnosing peripheral neuropathy. Nerve conduction studies are the gold standard and should be performed in patients receiving platins and complaining of neuropathic symptoms post-treatment.
Keywords: Cancer, Chemotherapy, Pain, Platin, Polyneuropathy
Introduction
The term “peripheral neuropathy” (PN) refers to various disorders of the peripheral nervous system, including single and multiple (asymmetric) mononeuropathies, symmetrical involvement of many nerves (polyneuropathy), or the sole involvement of the dorsal root ganglia [1, 2].
PN is very prevalent in cancer patients [3] and can be a direct or an indirect complication of cancer or cancer-related treatment, a pre-existing comorbidity not related to cancer, or part of a paraneoplastic syndrome [4–7].
The vast majority of chemotherapy-induced PN (CIPN) is caused by neurotoxic chemotherapy schemes, with platins (cisplatin, oxaliplatin, carboplatin) constituting the leading source of treatment-induced PN in cancer. Contrary to the perception that painful neuropathies are largely caused by diabetes, other forms of PN can be particularly painful, leading to poor quality of life [2]. Therefore, platin-induced peripheral neuropathy (PIPN) should be considered a major cause of pain in cancer patients [8].
The aim of this paper is to systematically review the epidemiological and clinical characteristics of painful PIPN and provide an overview of relevant management strategies.
Methods
Literature Search Strategy
A systematic computer-based literature search was conducted August 15, 2017, on the PubMed database. For the search, we used three Medical Subject Headings (MeSH) terms in either the title or abstract, as follows: (1) “neuronopathy” or “ganglionopathy” or “neuropathy” or “polyneuropathy”; (2) “pain” or “painful”; (3) “chemotherapy” or “platin” or “platins” or “oxaliplatin” or “cisplatin”. Articles were limited to English language, species to human, and with full text available. We also perused the reference lists of the papers in order to identify papers not found through the search strategy.
Inclusion and Exclusion Criteria
Articles eligible for inclusion in the review were required to meet the following criteria:
Involved case series with platin-induced PN.
Studied human adult subjects.
The following were excluded:
Book chapters, reviews, letters to the editor, and editorials that did not provide new data.
Papers providing incomplete clinical or neurophysiological data about the single cases/case series.
Data Extraction
Data were extracted from each study in a structured coding scheme using Microsoft Excel, and included information on the article identification, year of publication, evaluation period, total number of subjects, gender, age, presence of pain in general, presence of pain secondary to the neuropathy, neurophysiological type of neuropathy, course of symptoms, type of cancer, and type of platin. We also collected information about the time point of diagnosis in each study, and whether they referred to acute toxicity or cumulative effect after completion of all cycles of chemotherapy. Papers referring to symptoms during chemotherapy without specifying the time point (such as which cycle) were not considered for analysis.
Statistical Analyses
A database was developed using IBM SPSS Statistics software (version 23.0 for Mac; IBM Corp., Armonk, NY, USA). Frequencies and descriptive statistics were examined for each variable. The primary outcome of interest was the proportion of patients who experienced pain because of PIPN.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Search Results
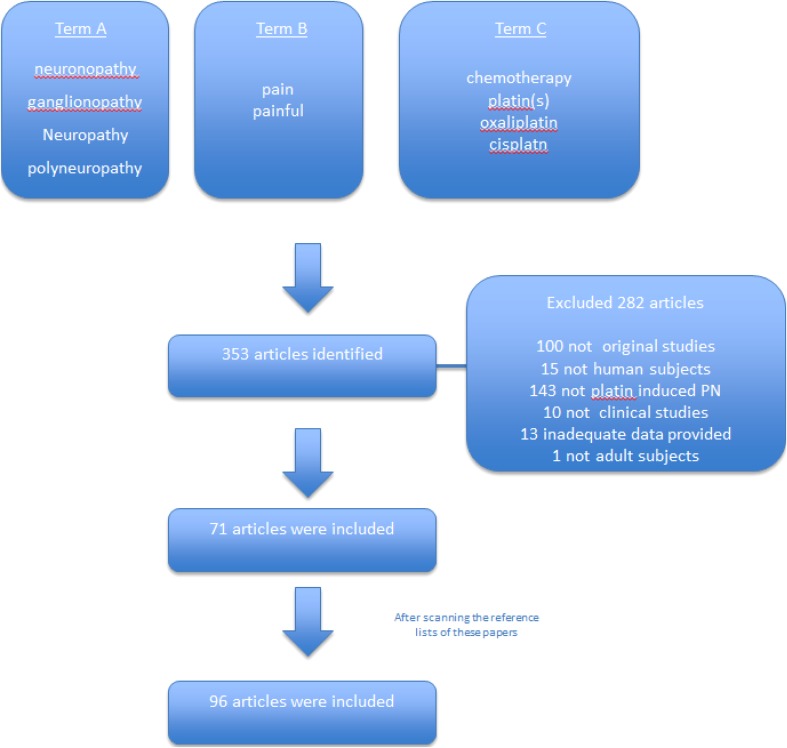
This search strategy resulted in the identification of 353 articles. A total of 282 articles were excluded during the eligibility assessment, and 25 additional papers were identified by scanning the reference lists. Therefore, 96 papers met the inclusion criteria and were used for this review [9–104]. These studies were published between 1980 and 2017. Figure 1 illustrates the study selection process.
Fig. 1.
PRISMA chart
Full clinical and neurophysiological data were further extracted from 54 papers [29–82] involving a total of 8159 patients (49.8% male) who received chemotherapy that included platins. The age of the patients ranged from 18 to 85 years (mean 65.9 years). The demographic and clinical characteristics of these patients are summarized in Table 1.
Table 1.
Description of studies included in the review
| Parameter | Value |
|---|---|
| Number of papers* | 54 |
| Total number of patients | 8159 |
| Number of patients per study, mean (SD) | 151.1 (419.6) |
| Male: Female | 1:1 |
| Mean age, in years | 65.9 |
| Diagnosis of PN | |
| Questionnaires only (%) | 12 (22.2) |
| Nerve conduction studies (%) | 3 (5.6) |
| Quantitative sensory testing (%) | 7 (13.0) |
| Clinical examination (%) | 8 (14.8) |
| Not reported (%) | 24 (44.4) |
| Platin-induced PN (%) | 73.3 |
| Painful PIPN (%) | 45.8 |
*Providing full clinical and neurophysiological data
SD standard deviation, PN peripheral neuropathy
Forty-six papers provided data for the prevalence of PN in patients who received platins in their chemotherapeutic scheme. Of these, 22 papers reported PN during chemotherapy, 14 reported PN after completion of chemotherapy, and three specifically reported the acute toxic effects of oxaliplatin. Seven papers did not clearly specify the time point where PN occurred. The prevalence of established PN after chemotherapy was estimated at 56.5%.
In 16 studies, patients received only platins, not combined with another neurotoxic chemotherapeutic agent. Of these, four papers reported PN during chemotherapy, eight reported PN after completion of chemotherapy, and three specifically reported the acute toxic effects of oxaliplatin. One paper did not clearly specify the time point at which PN occurred. The prevalence of neuropathic symptoms due to acute toxicity of oxaliplatin was estimated at 84.6%, whereas established PN after chemotherapy with platins was estimated at 74.9%. Of note, in only two studies—which provided data about the prevalence of PIPN—was PN confirmed with a full neurophysiological assessment.
Specifically regarding pain, the reported prevalence of pain due to acute toxicity of oxaliplatin was estimated at 55.6%, whereas the reported prevalence of chronic peripheral neuropathic pain in PIPN was estimated at 49.2%.
Epidemiological Characteristics of PIPN
A meta-analysis of 31 studies providing data from 4179 patients established that the prevalence of CIPN was 68.1% (95% CI 57.7–78.4%) when measured in the first month after chemotherapy completion, 60.0% (95% CI 36.4–81.6%) at 3 months, and 30.0% (95% CI 6.4–53.5%) at 6 months or more [9]. However, these figures refer to a variety of chemotherapeutic agents. Moreover, the diagnosis of the neuropathy was not made via nerve conduction studies (NCS) in all cases; in some studies, neuropathy was based only on quantitative sensory testing (QST), neurological examination, and/or questionnaires [9]. Among only those studies in which NCS or QST was used for assessment of neuropathy, the prevalence of CIPN was higher: 73.3% (95% CI 58.6–87.3) within 1 month of chemotherapy cessation, 70.1% (95% CI 41.8–98.4) at 3 months, and 39.9% (95% CI 3.9–76.0) at 6 months or greater. A limitation of these figures, however, is that abnormal results obtained by QST without having performed NCS does not definitively establish the presence of PN, and thus the above-mentioned percentages reflect the presence of neuropathic symptoms rather than CIPN.
Specifically concerning platins, the incidence of neuropathic symptoms for cisplatin ranged from 49% to 100%, whereas carboplatin was reported to be less neurotoxic, with neuropathic symptoms observed in 13% to 42% of cases [10, 11]. The presence of acute oxaliplatin-induced neuropathic symptoms has been reported in 85–95% of patients, and these symptoms have been observed in a chronic persistent form in approximately 16–21% of patients [11, 12, 56].
Risk Factors for PIPN
Several risk factors have been associated with the development of painful PIPN. Wang et al. [32] reported that female sex, patient’s level of functioning (assessed by the Eastern Cooperative Oncology Group performance status scale), body mass index (BMI), and baseline opioid use were associated with increased severity of oxaliplatin-induced peripheral neuropathy [32]. Attal et al. [58] revealed a significant relationship between the severity of acute signs and symptoms of oxaliplatin neurotoxicity after three cycles of chemotherapy and the occurrence and severity of chronic residual neuropathic symptoms as assessed by QST and the Neuropathic Pain Symptom Inventory (NPSI) after 1 year.
Moreover, the duration of neuropathic symptoms was observed to increase as the cumulative dose of platin increased [69]. For example, Leonard et al. found that after the first cycle of chemotherapy, the median duration of dysesthesia was only 5 days, whereas it was 21 days in patients who received 12 cycles of chemotherapy. The median duration of paresthesia after cycle 1 was 7 days, but after cycle 12 was 21 days or longer [69]. Similarly, in many clinical trials sensory symptoms causing functional impairment have been found in only about 15% of patients after a cumulative dose of 780–850 mg/m2 but in 50% of patients at a cumulative dose of 1170 mg/m2 [13, 14].
Specifically concerning carboplatin, Takemoto et al. [88] observed a greater visual analogue scale (VAS) score when it was combined with paclitaxel than with docetaxel; additionally, as the number of chemotherapy cycles increased, the carboplatin–paclitaxel-induced neuropathic symptoms became more severe.
Concerning cisplatin, Bezjak et al. [95] demonstrated that in lung cancer survivors, sensory cisplatin-induced neuropathic symptoms were a late effect of cisplatin, persisting for at least 9 months after chemotherapy and affecting quality of life. Similar symptoms have been reported as a late effect of cisplatin in long-term survivors of testicular cancer, and the cumulative dose of cisplatin has been reported to be a major risk factor for the development of toxicity [28].
Not all studies reported screening for pre-existing neuropathy prior to chemotherapy with platins. Also, not all studies reported having excluded patients with other common risk factors for PN such as diabetes, excessive alcohol intake, gluten sensitivity [105, 106], or hereditary neuropathies. Therefore, such comorbidities may have contributed to the development of PN.
Predictors of PIPN
Cold allodynia and hyperalgesia of the hands after three cycles of oxaliplatin treatment was found to be predictive of severe chronic neuropathy [58]. Among a range of cold stimuli, pain induced by a 20 °C stimulus to the hand had the highest predictive value with regard to development of severe chronic neuropathy. The severity of chronic neuropathy was also found to correlate with the duration of cold-evoked symptoms, the intensity of acute neuropathic symptoms, and the intensity of cold-evoked pain [58].
Management
Management of platin induced peripheral neuropathic pain includes pharmacological and non-pharmacological approaches [8].
Antidepressants
Venlafaxine, a serotonin and norepinephrine reuptake inhibitor, was effective in the management of acute neuropathic symptoms in a small series of patients receiving oxaliplatin [100]. Venlafaxine also showed promising preliminary evidence of clinical effectiveness of this combination against chronic neuropathic symptoms in oxaliplatin-induced PN [97]. According to the EFFOX study, complete relief of neuropathic symptoms induced by oxaliplatin was achieved in 31.3% of patients, which is a significantly higher percentage than the 5.3% achieved with placebo [91].
Duloxetine, another serotonin and norepinephrine reuptake inhibitor, was also found to be effective in oxaliplatin-induced painful neuropathy [55]. In an open-label study, Yang et al. demonstrated that duloxetine could be used effectively in low doses (i.e. 60 mg/day) without impairment of renal or liver function—and importantly, without interfering with chemotherapy [55]. More recently, a large placebo-controlled randomized clinical trial showed that duloxetine was more effective than placebo in reducing the average PIPN pain score after a 5-week treatment period [101]. Overall, duloxetine has the largest volume of evidence supporting its use in the treatment of painful PIPN [107].
Nortriptyline, a tricyclic antidepressant, failed to demonstrate effectiveness for treating paresthesia or pain in cisplatin-induced neuropathic symptoms [98].
Anticonvulsants
Topiramate showed promising preliminary evidence of clinical effectiveness of this combination against chronic neuropathic symptoms in oxaliplatin-induced PN [97].
Carbamazepine, on the other hand, does not appear to be beneficial against acute oxaliplatin-induced painful neurotoxicity [99].
Research findings regarding the effectiveness of gabapentin for treating pain caused by PIPN remain controversial. In a phase III randomized, double-blind, placebo-controlled crossover trial, Rao et al. concluded that gabapentin failed to demonstrate any benefit [102], whereas an open-label study by Tsavaris et al. found that gabapentin monotherapy seemed to be well tolerated and useful for the management of chemotherapy-induced neuropathic pain [103]. However, both studies included patients with PN secondary to other chemotherapeutic agents in addition to platins. To date, no study has explored the efficacy of gabapentin alone in patients with PIPN.
Opioids
Liu et al. [89] reported that tramadol in combination with acetaminophen, administered in patients with colorectal or gastric adenocarcinoma, was effective in relieving oxaliplatin-induced peripheral neuropathic pain [89]. Interestingly, this study proposed that the A118G polymorphism of the mu-opioid receptor gene (OPRM1) was a possible mechanism for the reduced response to the combination of tramadol and acetaminophen [89], suggesting that management should be always be tailored to individual patient characteristics.
Topical Drugs
In an open-label study, Filipczak-Bryniarska et al. demonstrated that the high-dose capsaicin patch was effective in treating pain associated with oxaliplatin-induced neuropathy [104]. However, this finding should be interpreted with caution, given the limitations of the study design and small number of the participants.
Non-Pharmacological Approaches
There is some evidence that acupuncture may be beneficial for the treatment of PIPN. In a small case series, Donald et al. [87] reported that patients with oxaliplatin-induced painful neuropathy improved after acupuncture. Wong et al. [96] similarly described a small series of patients with symptoms of pain due to carboplatin-induced neuropathy who improved after acupuncture. A prospective pilot study by Hsieh et al. [30] showed that laser acupuncture relieved both cold and mechanical allodynia induced by oxaliplatin in gastrointestinal cancer survivors. To date, however, no large randomized controlled trial has been conducted to confirm the effectiveness of acupuncture in managing pain in PIPN. Therefore, the current evidence is weak.
Cunningham et al. [90] reported a case of almost complete resolution of the tingling, numbness, and pain of cisplatin-induced neuropathy with manual therapy (massage) in a patient with stage III esophageal adenocarcinoma. However, as this was based on a single case, this finding should be interpreted with extreme caution.
Henke et al. [84] reported that strength and endurance training in patients receiving platinum-based chemotherapy for lung cancer was effective in managing pain. The authors thus suggested that lung cancer patients should receive enhanced physical activity intervention during palliative chemotherapy.
Diagnosing and Monitoring PIPN
Large Fiber Neuropathy
The gold standard for diagnosing a large fiber neuropathy is NCS. Although centers have many different means of neurophysiologically determining the presence of PN, sensory conduction studies of sural and radial nerves are recommended for the diagnosis of mild, predominantly sensory axonal neuropathy [108]. This should be complemented with at least one motor study, commonly of the tibial nerve, to confirm motor involvement [109, 110]. In the case of PIPN, however, the neuropathy is sensory, affecting the dorsal root ganglia. In a sensory ganglionopathy, asymmetrical sensory nerve action potentials (SNAPs) or complete absence of SNAPs should be expected [111, 112].
Small Fiber Neuropathy
Patients often complain of disabling symptoms such as a burning sensation in the soles or the fingertips, which is a common manifestation of small fiber neuropathy (SFN). Nerve conduction studies assess only large fibers, and therefore, SFN cannot be excluded if NCS are normal.
The gold standard for a diagnosis of SFN is skin biopsy; however, this is an invasive technique and is thus usually avoided. QST is commonly used for assessment of SFN, but this is subjective. Alternatively, nerve morphology can be rapidly assessed using in vivo corneal confocal microscopy. This technique has been used for the detection of various types of neuropathy, including small fiber neuropathy [15]. Ferdousi et al. [35] showed that corneal confocal microscopy was effective in detecting small fiber neuropathy by a marked reduction in corneal nerve morphological parameters in patients with upper gastrointestinal cancer and oxaliplatin- or cisplatin-induced neuropathy.
Questionnaires
Several questionnaires have been used for the detection and assessment of PIPN. Particular interest has been focused on the development and validation of questionnaires regarding quality of life in patients with CIPN.
The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30), designed to assess quality of life for cancer patients, is widely used and consists of 30 items comprising five functional scales (physical, role, emotional, cognitive, and social), global health status, and nine symptom scales and single items (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties. It is also supplemented with specific modules for the different cancer types [16, 17, 83, 84, 95].
The EORTC QLQ-CIPN20 is a quality-of-life questionnaire developed to elicit patient experience of symptoms and functional limitations related to CIPN. It contains 20 items assessing sensory, motor, and autonomic symptoms [18, 86]. The CIPN20 can assess frequency and severity of painful CIPN in a wide range of oncology patient populations.
The L-BASIC [location-based assessment of sensory symptoms in cancer] instrument uses location-specific ratings of sensory symptoms in the cancer population [94]. It is structured such that patients provide a numeric score and an adjectival description for any sensory symptoms, including both pain and neuropathic sensations, present in each of 10 predefined body areas [94].
The Rasch-built Overall Disability Scale for CIPN (CIPN-R-ODS) is a Rasch-built disease-specific interval measure suitable for detecting disability and levels of activity and participation in patients with stable disease [85].
Τhe Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) questionnaire is the Functional Assessment of Cancer Therapy-General (FACT-G) instrument plus an 11-item subscale (Ntx subscale) [19]. It is a chemotherapy treatment effect-specific measurement tool used to evaluate the severity and impact of CIPN symptoms on functional status and health-related quality of life [19]. Questions on the Ntx subscale include the feeling of generalized weakness, numbness or tingling in the hands or feet, and difficulty with fine motor movements [19, 20].
The Total Neuropathy Score (TNS) was initially designed to evaluate diabetic neuropathy [21] and was later validated in patients with CIPN [22]. The TNS includes objective measures, such as pin prick, vibration threshold, and nerve conduction studies, combined with subjective report of sensory, motor, and autonomic items, and the instrument has been tested in a variety of tumor types [22–24, 93].
The chemotherapy-induced neuropathy-specific Neuropathic Pain Scale (NPS-CIN) is a six-item scale used to assess CIPN and related neuropathic pain severity [25, 92, 93].
The Patient Neurotoxicity Questionnaire, comprising two items, defines the incidence and severity of sensory and motor disturbances [26].
The Chemotherapy-Induced Peripheral Neuropathy Assessment Tool (CIPNAT) contains 36 items that evaluate the occurrence, severity, distress, and frequency of nine neuropathic symptoms, along with 14 items that evaluate neuropathic interference with activities [27].
Conclusion
This systematic review has identified the following key points:
PN is a common complication in patients receiving platins in their chemotherapeutic regime.
PIPN can be particularly painful. Acute toxicity occurs only with oxaliplatin. However, in a significant proportion of patients receiving platins, the pain persists and becomes chronic.
There is significant heterogeneity in the methods used to diagnose PN. In many studies, patients were diagnosed based only on questionnaires or clinical examination. Although the use of questionnaires may be adequate for characterizing and monitoring neuropathic symptoms, the gold standard for accurate diagnosis of an established peripheral neuropathy—from a neurological point of view—is NCS. Ideally, this should be performed as a baseline, before the chemotherapy, and should be repeated at the end or when symptoms occur. QST alone is not sufficient for establishing a diagnosis of PN, as it is not objective, and its role is more as an indicator of small fiber involvement; therefore, QST should be complemented with NCS.
Small fiber neuropathy is gaining increasing attention in clinical practice; however, further studies are needed in patients receiving platins, as the neuropathic pain that patients experience during chemotherapy with platins is likely often secondary to small fiber involvement, with no involvement of the large fibers.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Vasiliki Brozou, Athina Vadalouca, and Panagiotis Zis have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/0AECF0606291D6F7.
References
- 1.Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62(4):310–318. doi: 10.1136/jnnp.62.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zis P, Sarrigiannis PG, Rao DG, Hewamadduma C, Hadjivassiliou M. Chronic idiopathic axonal polyneuropathy: a systematic review. J Neurol. 2016;263(10):1903–1910. doi: 10.1007/s00415-016-8082-7. [DOI] [PubMed] [Google Scholar]
- 3.Zis P, Varrassi G. Painful peripheral neuropathy and cancer. Pain Ther. 2017 doi: 10.1007/s40122-017-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchettini P, Formaglio F, Lacerenza M. Iatrogenic painful neuropathic complications of surgery in cancer. Acta Anaesthesiol Scand. 2001;45(9):1090–1094. doi: 10.1034/j.1399-6576.2001.450907.x. [DOI] [PubMed] [Google Scholar]
- 5.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL, American Society of Clinical Oncology Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 6.Zis P, Paladini A, Piroli A, McHugh PC, Varrassi G, Hadjivassiliou M. Pain as a first manifestation of paraneoplastic neuropathies: a systematic review and meta-analysis. Pain Ther. 2017 doi: 10.1007/s40122-017-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zis P, Rao DG, Wagner BE, Nicholson-Goult L, Hoggard N, Hadjivassiliou M. Cerebellar ataxia and sensory ganglionopathy associated with light-chain myeloma. Cerebellum Ataxias. 2017;5(4):1. doi: 10.1186/s40673-016-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vadalouca A, Raptis E, Moka E, Zis P, Sykioti P, Siafaka I. Pharmacological treatment of neuropathic cancer pain: a comprehensive review of the current literature. Pain Pract. 2012;12(3):219–511. doi: 10.1111/j.1533-2500.2011.00485.x. [DOI] [PubMed] [Google Scholar]
- 9.Seretny M, Curie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2467. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Mollman JE, Glover DJ, Hogan WM, Furman RE. Cisplatin neuropathy. Risk factors, prognosis, and protection by WR-2721. Cancer. 1988;61:2192–2195. doi: 10.1002/1097-0142(19880601)61:11<2192::aid-cncr2820611110>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Storey DJ, Sakala M, McLean CM, Phillips HA, Dawson LK, Wall LR, Fallon MT, Clive S. Capecitabine combined with oxaliplatin (CapOx) in clinical practice: How significant is peripheral neuropathy? Ann Oncol. 2010;21(8):1657–1661. doi: 10.1093/annonc/mdp594. [DOI] [PubMed] [Google Scholar]
- 13.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 14.Brienza S, Vignoud J, Itzakhi M, Krikorian A. 933 oxaliplatin (L-OHP®): global safety in 682 patients (PTS) Proc Am Soc Clin Oncol. 1995;14:209. [Google Scholar]
- 15.Tavakoli M, Marshall A, Pitceathly R, Gow D, Roberts ME, Malik RA. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010;223(1):245–250. doi: 10.1016/j.expneurol.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol. 2013;31(21):2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 17.Dearnaley DP, Fossa SD, Kaye SB, Cullen MH, Harland SJ, Sokal MP, Graham JD, Roberts JT, Mead GM, Williams MV, Cook PA, Stenning SP. Adjuvant bleomycin, vincristine and cisplatin (BOP) for high-risk stage I non-seminomatous germ cell tumours: a prospective trial (MRC TE17), MRC Testicular Tumour Working Party. Br J Cancer. 2005;92(12):2107–2113. doi: 10.1038/sj.bjc.6602624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lantéri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R. The development of an EORTC quality of life questionnaire to assess chemotherapyinduced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Calhoun EA, Welshman EE, Chang CH, Lurain JR, Fishman DA, Hunt TL, Cella D. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13(6):741–748. doi: 10.1111/j.1525-1438.2003.13603.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer. 2007;17:387–393. doi: 10.1111/j.1525-1438.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 21.Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, Joh T. Total neuropathy score: validation and reliability study. Neurology. 1999;10:1660–1664. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- 22.Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, Parma G, Lissoni A, Fei F, Cundari S, Zanna C. Grading of chemotherapy induced peripheral neurotoxicity using the Total Neuropathy scale. Neurology. 2003;61(9):1297–1300. doi: 10.1212/01.wnl.0000092015.03923.19. [DOI] [PubMed] [Google Scholar]
- 23.Cavaletti G, Frigeni B, Lanzani F, Piatti M, Rota S, Briani C, Zara G, Plasmati R, Pastorelli F, Caraceni A, Pace A, Manicone M, Lissoni A, Colombo N, Bianchi G, Zanna C. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: Comparison with the National Cancer Institute Common Toxicity Scale. J Peripher Nerv Syst. 2007;12(3):210–215. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 24.Cavaletti G, Jann S, Pace A, Plasmati R, Siciliano G, Briani C, Cocito D, Padua L, Ghiglione E, Manicone M, Giussani G. Multi-center assessment of the Total Neuropathy Score for chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2006;11(2):135–141. doi: 10.1111/j.1085-9489.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 25.Wampler MA, Miaskowski C, Hamel K, Byl N, Rugo H, Topp LS. The modified total neuropathy score: a clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. Support Oncol. 2006;4(8):W9–W16. [Google Scholar]
- 26.Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, Ohsumi S, Makino H, Mukai H, Katsumata N, Sunada Y, Watanabe T, Hausheer FH. Feasibility and validity of the patient neurotoxicity questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer. 2009;17:1483–1491. doi: 10.1007/s00520-009-0613-7. [DOI] [PubMed] [Google Scholar]
- 27.Tofthagen CS, McMillan SC, Kip KE. Development and psychometric evaluation of the chemotherapy-induced peripheral neuropathy assessment tool. Cancer Nurs. 2011;34:E10–E20. doi: 10.1097/NCC.0b013e31820251de. [DOI] [PubMed] [Google Scholar]
- 28.Bokemeyer C, Berger C, Kuczyk M, Schmoll HJ. Evaluation of long-term toxicity after chemotherapy for testicular cancer. J Clin Oncol. 1996;14(11):2923–2932. doi: 10.1200/JCO.1996.14.11.2923. [DOI] [PubMed] [Google Scholar]
- 29.Andriamamonjy M, Delmotte JB, Savinelli F, Beaussier H, Coudoré F. Quantification of chronic oxaliplatin-induced hypesthesia in two areas of the hand. J Clin Neurophysiol. 2017;34(2):126–131. doi: 10.1097/WNP.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh YL, Chou LW, Hong SF, Chang FC, Tseng SW, Huang CC, Yang CH, Yang CC, Chiu WF. Laser acupuncture attenuates oxaliplatin-induced peripheral neuropathy in patients with gastrointestinal cancer: a pilot prospective cohort study. Acupunct Med. 2016;34(5):398–400. doi: 10.1136/acupmed-2016-011112. [DOI] [PubMed] [Google Scholar]
- 31.Zhu T, Liu CL, Zhang YF, Liu YH, Xu FP, Zu J, Zhang GC, Li XR, Liao N. Wang K.A phase II trial of dose-dense (biweekly) paclitaxel plus carboplatin as neoadjuvant chemotherapy for operable breast cancer. Breast Cancer Res Treat. 2016;156(1):117–124. doi: 10.1007/s10549-016-3735-x. [DOI] [PubMed] [Google Scholar]
- 32.Wang XS, Shi Q, Dougherty PM, Eng C, Mendoza TR, Williams LA, Fogelman DR, Cleeland CS. Prechemotherapy touch sensation deficits predict oxaliplatin-induced neuropathy in patients with colorectal cancer. Oncology. 2016;90(3):127–135. doi: 10.1159/000443377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagao S, Iwasa N, Kurosaki A, Nishikawa T, Hanaoka T, Hasegawa K, Fujiwara K. The efficacy of low-dose paclitaxel added to combination chemotherapy of carboplatin and gemcitabine or pegylated liposomal doxorubicin. Int J Gynecol Cancer. 2016;26(3):443–448. doi: 10.1097/IGC.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 34.Ventzel L, Jensen AB, Jensen AR, Jensen TS, Finnerup NB. Chemotherapy-induced pain and neuropathy: a prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain. 2016;157(3):560–568. doi: 10.1097/j.pain.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 35.Ferdousi M, Azmi S, Petropoulos IN, Fadavi H, Ponirakis G, Marshall A, Tavakoli M, Malik I, Mansoor W, Malik RA. Corneal confocal microscopy detects small fibre neuropathy in patients with upper gastrointestinal cancer and nerve regeneration in chemotherapy induced peripheral neuropathy. PLoS One. 2015;10(10):e0139394. doi: 10.1371/journal.pone.0139394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochenduszko S, Puskulluoglu M, Konopka K, Fijorek K, Urbanczyk K, Budzynski A, Matlok M, Lazar A, Sinczak-Kuta A, Pedziwiatr M, Krzemieniecki K. Comparison of efficacy and safety of first-line palliative chemotherapy with EOX and mDCF regimens in patients with locally advanced inoperable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma: a randomized phase 3 trial. Med Oncol. 2015;32(10):242. doi: 10.1007/s12032-015-0687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pachman DR, Qin R, Seisler DK, Smith EM, Beutler AS, Ta LE, Lafky JM, Wagner-Johnston ND, Ruddy KJ, Dakhil S, Staff NP, Grothey A, Loprinzi CL. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (alliance) J Clin Oncol. 2015;33(30):3416–3422. doi: 10.1200/JCO.2014.58.8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makielski RJ, Lubner SJ, Mulkerin DL, Traynor AM, Groteluschen D, Eickhoff J, LoConte NK. A phase II study of sorafenib, oxaliplatin, and 2 days of high-dose capecitabine in advanced pancreas cancer. Cancer Chemother Pharmacol. 2015;76(2):317–323. doi: 10.1007/s00280-015-2783-y. [DOI] [PubMed] [Google Scholar]
- 39.Hu XC, Zhang J, Xu BH, Cai L, Ragaz J, Wang ZH, Wang BY, Teng YE, Tong ZS, Pan YY, Yin YM, Wu CP, Jiang ZF, Wang XJ, Lou GY, Liu DG, Feng JF, Luo JF, Sun K, Gu YJ, Wu J, Shao ZM. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16(4):436–446. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 40.Ezendam NP, Pijlman B, Bhugwandass C, Pruijt JF, Mols F, Vos MC, Pijnenborg JM, van de Poll-Franse LV. Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: results from the population-based PROFILES registry. Gynecol Oncol. 2014;135(3):510–517. doi: 10.1016/j.ygyno.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Padman S, Lee J, Kumar R, Slee M, Hakendorf P, Richards A, Koczwara B, Kichenadasse G, Sukumaran S, Roy A, Vatandoust S, Karapetis CS. Late effects of oxaliplatin-induced peripheral neuropathy (LEON)–cross-sectional cohort study of patients with colorectal cancer surviving at least 2 years. Support Care Cancer. 2015;23(3):861–869. doi: 10.1007/s00520-014-2423-9. [DOI] [PubMed] [Google Scholar]
- 42.Du J, Hu C, Zhang Y, Hu B, Wang F, Zhang Y. A retrospective study of paclitaxel combining nedaplatin chemotherapy for esophageal cancer. Anticancer Drugs. 2015;26(1):101–105. doi: 10.1097/CAD.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 43.de Carvalho Barbosa M, Kosturakis AK, Eng C, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Wang XS, Cleeland CS, Dougherty PM. A quantitative sensory analysis of peripheral neuropathy in colorectal cancer and its exacerbation by oxaliplatin chemotherapy. Cancer Res. 2014;74(21):5955–5962. doi: 10.1158/0008-5472.CAN-14-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor SE, Beck TL, Krivak TC, Zorn KK, Kelley JL, Edwards RP. Oxaliplatin salvage for recurrent ovarian cancer: a single institution’s experience in patient populations with platinum resistant disease or a history of platinum hypersensitivity. Gynecol Oncol. 2014;134(1):68–72. doi: 10.1016/j.ygyno.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 45.Leal AD, Qin R, Atherton PJ, Haluska P, Behrens RJ, Tiber CH, Watanaboonyakhet P, Weiss M, Adams PT, Dockter TJ, Loprinzi CL. Alliance for Clinical Trials in Oncology North Central Cancer Treatment Group/Alliance trial N08CA-the use of glutathione for prevention of paclitaxel/carboplatin-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled study. Cancer. 2014;120(12):1890–1897. doi: 10.1002/cncr.28654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krøigård T, Schrøder HD, Qvortrup C, Eckhoff L, Pfeiffer P, Gaist D, Sindrup SH. Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies. Eur J Neurol. 2014;21(4):623–629. doi: 10.1111/ene.12353. [DOI] [PubMed] [Google Scholar]
- 47.Guo Y, Jones D, Palmer JL, Forman A, Dakhil SR, Velasco MR, Weiss M, Gilman P, Mills GM, Noga SJ, Eng C, Overman MJ, Fisch MJ. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: a randomized, double-blind, placebo-controlled trial. Support Care Cancer. 2014;22(5):1223–1231. doi: 10.1007/s00520-013-2075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffith KA, Couture DJ, Zhu S, Pandya N, Johantgen ME, Cavaletti G, Davenport JM, Tanguay LJ, Choflet A, Milliron T, Glass E, Gambill N, Renn CL, Dorsey SG. Evaluation of chemotherapy-induced peripheral neuropathy using current perception threshold and clinical evaluations. Support Care Cancer. 2014;22(5):1161–1169. doi: 10.1007/s00520-013-2068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol. 2013;31(21):2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 50.Sohal DP, Metz JM, Sun W, Giantonio BJ, Plastaras JP, Ginsberg G, Kochman ML, Teitelbaum UR, Harlacker K, Heitjan DF, Feldman MD, Drebin JA, O’Dwyer PJ. Toxicity study of gemcitabine, oxaliplatin, and bevacizumab, followed by 5-fluorouracil, oxaliplatin, bevacizumab, and radiotherapy, in patients with locally advanced pancreatic cancer. Cancer Chemother Pharmacol. 2013;71(6):1485–1491. doi: 10.1007/s00280-013-2147-4. [DOI] [PubMed] [Google Scholar]
- 51.Sun V, Otis-Green S, Morgan R, Wakabayashi M, Hakim A, Callado ME, Yang E, Ferrell B, Grant M. Toxicities, complications, and clinical encounters during intraperitoneal chemotherapy in 17 women with ovarian cancer. Eur J Oncol Nurs. 2013;17(3):375–380. doi: 10.1016/j.ejon.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen DL, Nørgaard H, Vestermark LW, Pfeiffer P, Jensen BK, Nelausen KM, Bergenfeldt M, Hermann KL, Jensen BV. Intrahepatic and systemic therapy with oxaliplatin combined with capecitabine in patients with hepatic metastases from breast cancer. Breast. 2012;21(4):556–561. doi: 10.1016/j.breast.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, Le-Lindqwister NA, Soori GS, Jaslowski AJ, Kelaghan J, Novotny PJ, Lachance DH, Loprinzi CL. Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: north Central Cancer Treatment Group trial N08C1. Cancer. 2012;118(20):5171–5178. doi: 10.1002/cncr.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stavraka C, Ford A, Ghaem-Maghami S, Crook T, Agarwal R, Gabra H, Blagden S. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol. 2012;125(1):59–64. doi: 10.1016/j.ygyno.2011.12.421. [DOI] [PubMed] [Google Scholar]
- 55.Yang YH, Lin JK, Chen WS, Lin TC, Yang SH, Jiang JK, Chang SC, Lan YT, Lin CC, Yen CC, Tzeng CH, Wang WS, Chiang HL, Teng CJ, Teng HW. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: an open-label pilot study. Support Care Cancer. 2012;20(7):1491–1497. doi: 10.1007/s00520-011-1237-2. [DOI] [PubMed] [Google Scholar]
- 56.Tofthagen C, McAllister RD, McMillan SC. Peripheral neuropathy in patients with colorectal cancer receiving oxaliplatin. Clin J Oncol Nurs. 2011;15(2):182–188. doi: 10.1188/11.CJON.182-188. [DOI] [PubMed] [Google Scholar]
- 57.Zweifel M, Jayson GC, Reed NS, Osborne R, Hassan B, Ledermann J, Shreeves G, Poupard L, Lu SP, Balkissoon J, Chaplin DJ, Rustin GJ. Phase II trial of combretastatin A4 phosphate, carboplatin, and paclitaxel in patients with platinum-resistant ovarian cancer. Ann Oncol. 2011;22(9):2036–2041. doi: 10.1093/annonc/mdq708. [DOI] [PubMed] [Google Scholar]
- 58.Attal N, Bouhassira D, Gautron M, Vaillant JN, Mitry E, Lepère C, Rougier P, Guirimand F. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain. 2009;144(3):245–252. doi: 10.1016/j.pain.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 59.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins JN, Seay TE, Fehrenbacher L, O’Reilly S, Chu L, Azar CA, Wolmark N. Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27(20):3385–3390. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carlomagno C, Farella A, Bucci L, D’Armiento FP, Pesce G, Pepe S, Cannella L, Pacelli R, De Stefano A, Solla R, D’Armiento MR, De Placido S. Neo-adjuvant treatment of rectal cancer with capecitabine and oxaliplatin in combination with radiotherapy: a phase II study. Ann Oncol. 2009;20(5):906–912. doi: 10.1093/annonc/mdn719. [DOI] [PubMed] [Google Scholar]
- 61.Chin SN, Pinto V, Rosen B, Oza A, Dodge J, Murphy J, Mackay H. Evaluation of an intraperitoneal chemotherapy program implemented at the Princess Margaret Hospital for patients with epithelial ovarian carcinoma. Gynecol Oncol. 2009;112(3):450–454. doi: 10.1016/j.ygyno.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki R, Yamamoto M, Saka H, Taniguchi H, Shindoh J, Tanikawa Y, Nomura F, Gonda H, Imaizumi K, Hasegawa Y, Shimokata K. A phase II study of carboplatin and paclitacel with meloxicam. Lung Cancer. 2009;63(1):72–76. doi: 10.1016/j.lungcan.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Zhu X, Leaw J, Gu W, Qian Y, Du H, Wang B, Hong X, Yin J. Phase II clinical trial of advanced and metastatic gastric cancer based on continuous infusion of 5-fluorouracil combined with epirubicin and oxaliplatin. J Cancer Res Clin Oncol. 2008;134(9):929–936. doi: 10.1007/s00432-008-0376-4. [DOI] [PubMed] [Google Scholar]
- 64.Boige V, Malka D, Elias D, Castaing M, De Baere T, Goere D, Dromain C, Pocard M, Ducreux M. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008;15(1):219–226. doi: 10.1245/s10434-007-9581-7. [DOI] [PubMed] [Google Scholar]
- 65.Binder A, Stengel M, Maag R, Wasner G, Schoch R, Moosig F, Schommer B, Baron R. Pain in oxaliplatin-induced neuropathy–sensitisation in the peripheral and central nociceptive system. Eur J Cancer. 2007;43(18):2658–2663. doi: 10.1016/j.ejca.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 66.Land SR, Kopec JA, Cecchini RS, Ganz PA, Wieand HS, Colangelo LH, Murphy K, Kuebler JP, Seay TE, Needles BM, Bearden JD, 3rd, Colman LK, Lanier KS, Pajon ER, Jr, Cella D, Smith RE, O’Connell MJ, Costantino JP, Wolmark N. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25(16):2205–2211. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 67.Dragovich T, Mendelson D, Kurtin S, Richardson K, Von Hoff D, Hoos A. A Phase 2 trial of the liposomal DACH platinum L-NDDP in patients with therapy-refractory advanced colorectal cancer. Cancer Chemother Pharmacol. 2006;58(6):759–764. doi: 10.1007/s00280-006-0235-4. [DOI] [PubMed] [Google Scholar]
- 68.Hu JB, Sun XN, Yang QC, Xu J, Wang Q, He C. Three-dimensional conformal radiotherapy combined with FOLFOX4 chemotherapy for unresectable recurrent rectal cancer. World J Gastroenterol. 2006;12(16):2610–2614. doi: 10.3748/wjg.v12.i16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leonard GD, Wright MA, Quinn MG, Fioravanti S, Harold N, Schuler B, Thomas RR, Grem JL. Survey of oxaliplatin-associated neurotoxicity using an interview-based questionnaire in patients with metastatic colorectal cancer. BMC Cancer. 2005;16(5):116. doi: 10.1186/1471-2407-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dearnaley DP, Fossa SD, Kaye SB, Cullen MH, Harland SJ, Sokal MP, Graham JD, Roberts JT, Mead GM, Williams MV, Cook PA, Stenning SP, MRC Testicular Tumour Working Party Adjuvant bleomycin, vincristine and cisplatin (BOP) for high-risk stage I non-seminomatous germ cell tumours: a prospective trial (MRC TE17) Br J Cancer. 2005;92(12):2107–2113. doi: 10.1038/sj.bjc.6602624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fujiwara K, Suzuki S, Ishikawa H, Oda T, Aotani E, Kohno I. Preliminary toxicity analysis of intraperitoneal carboplatin in combination with intravenous paclitaxel chemotherapy for patients with carcinoma of the ovary, peritoneum, or fallopian tube. Int J Gynecol Cancer. 2005;15(3):426–431. doi: 10.1111/j.1525-1438.2005.15304.x. [DOI] [PubMed] [Google Scholar]
- 72.Marsland TA, Garfield DH, Khan MM, Look RM, Boehm KA, Asmar L. Sequential versus concurrent paclitaxel and carboplatin for the treatment of advanced non-small cell lung cancer in elderly patients and patients with poor performance status: results of two Phase II, multicenter trials. Lung Cancer. 2005;47(1):111–120. doi: 10.1016/j.lungcan.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Sabbatini P, Aghajanian C, Leitao M, Venkatraman E, Anderson S, Dupont J, Dizon D, O’Flaherty C, Bloss J, Chi D, Spriggs D. Intraperitoneal cisplatin with intraperitoneal gemcitabine in patients with epithelial ovarian cancer: results of a phase I/II Trial. Clin Cancer Res. 2004;10(9):2962–2967. doi: 10.1158/1078-0432.ccr-03-0486. [DOI] [PubMed] [Google Scholar]
- 74.Yasufuku T, Shigemura K, Matsumoto O, Arakawa S, Fujisawa M. Combination chemotherapy with weekly paclitaxel or docetaxel, carboplatin, and estramustine for hormone-refractory prostate cancer. J Infect Chemother. 2010;16(3):200–205. doi: 10.1007/s10156-010-0047-7. [DOI] [PubMed] [Google Scholar]
- 75.Martinez-Monge R, Jurado M, Aristu JJ, Moreno M, Cambeiro M, Perez-Ochoa A, Lopez-Garcia G, Alcazar JL. Intraoperative electron beam radiotherapy during radical surgery for locally advanced and recurrent cervical cancer. Gynecol Oncol. 2001;82(3):538–543. doi: 10.1006/gyno.2001.6329. [DOI] [PubMed] [Google Scholar]
- 76.Kern W, Beckert B, Lang N, Stemmler J, Beykirch M, Stein J, Goecke E, Waggershauser T, Braess J, Schalhorn A, Hiddemann W. Phase I and pharmacokinetic study of hepatic arterial infusion with oxaliplatin in combination with folinic acid and 5-fluorouracil in patients with hepatic metastases from colorectal cancer. Ann Oncol. 2001;12(5):599–603. doi: 10.1023/a:1011186708754. [DOI] [PubMed] [Google Scholar]
- 77.Greenberg HS, Chamberlain MC, Glantz MJ, Wang S. Adult medulloblastoma: multiagent chemotherapy. Neuro Oncol. 2001;3(1):29–34. doi: 10.1215/15228517-3-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mulatero C, McClaren BR, Mason M, Oliver RT, Gallagher CJ. Evidence for a schedule-dependent deleterious interaction between paclitaxel, vinblastine and cisplatin (PVC) in the treatment of advanced transitional cell carcinoma. Br J Cancer. 2000;83(12):1612–1616. doi: 10.1054/bjoc.2000.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Makhija S, Sabbatini P, Aghajanian C, Venkatraman E, Spriggs DR, Barakat R. Intraperitoneal cisplatin and intravenous paclitaxel in the treatment of epithelial ovarian cancer patients with a positive second look. Gynecol Oncol. 2000;79(1):28–32. doi: 10.1006/gyno.2000.5890. [DOI] [PubMed] [Google Scholar]
- 80.Plaxe SC, Braly PS, Freddo JL, McClay E, Christen RD, Kirmani S, Kim S, Heath D, Howell SB. Phase I and pharmacokinetic study of intraperitoneal ormaplatin. Gynecol Oncol. 1993;51(1):72–77. doi: 10.1006/gyno.1993.1249. [DOI] [PubMed] [Google Scholar]
- 81.Greenspan A, Treat J. Peripheral neuropathy and low dose cisplatin. Am J Clin Oncol. 1988;11(6):660–662. doi: 10.1097/00000421-198812000-00014. [DOI] [PubMed] [Google Scholar]
- 82.Sugiyama T, Okamoto A, Enomoto T, Hamano T, Aotani E, Terao Y, Suzuki N, Mikami M, Yaegashi N, Kato K, Yoshikawa H, Yokoyama Y, Tanabe H, Nishino K, Nomura H, Kim J, Kim BG, Pignata S, Alexandre J, Green J, Isonishi S, Terauchi F, Fujiwara K, Aoki D. Randomized phase III trial of irinotecan plus cisplatin compared with paclitaxel plus carboplatin as first-line chemotherapy for ovarian clear cell carcinoma: JGOG3017/GCIG Trial. J Clin Oncol. 2016;34(24):2881–2887. doi: 10.1200/JCO.2016.66.9010. [DOI] [PubMed] [Google Scholar]
- 83.Brotto L, Brundage M, Hoskins P, Vergote I, Cervantes A, Casado HA, Poveda A, Eisenhauer E, Dongsheng Tu. Randomized study of sequential cisplatin-topotecan/carboplatin-paclitaxel versus carboplatin-paclitaxel: effects on quality of life. Support Care Cancer. 2014;22:95–101. doi: 10.1007/s00520-015-2873-8. [DOI] [PubMed] [Google Scholar]
- 84.Henke CC, Cabri J, Fricke L, Pankow W, Kandilakis G, Feyer PC, de Wit M. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer. 2014;22:95–101. doi: 10.1007/s00520-013-1925-1. [DOI] [PubMed] [Google Scholar]
- 85.Binda D, Vanhoutte EK, Cavaletti G, Cornblath DR, Postma TJ, Frigeni B, Alberti P, Bruna J, Velasco R, Argyriou AA, Kalofonos HP, Psimaras D, Ricard D, Pace A, Galiè E, Briani C, Dalla Torre C, Lalisang RI, Boogerd W, Brandsma D, Koeppen S, Hense J, Storey D, Kerrigan S, Schenone A, Fabbri S, Rossi E, Valsecchi MG, Faber CG, Merkies IS, CI-PeriNomS study group. Galimberti S, Lanzani F, Mattavelli L, Piatti ML, Bidoli P, Cazzaniga M, Cortinovis D, Lucchetta M, Campagnolo M, Bakkers M, Brouwer B, Boogerd W, Grant R, Reni L, Piras B, Pessino A, Padua L, Granata G, Leandri M, Ghignotti I, Plasmati R, Pastorelli F, Heimans JJ, Eurelings M, Meijer RJ, Grisold W, Lindeck Pozza E, Mazzeo A, Toscano A, Russo M, Tomasello C, Altavilla G, Penas Prado M, Dominguez Gonzalez C, Dorsey SG. Rasch-built Overall Disability Scale for patients with chemotherapy-induced peripheral neuropathy (CIPN-R-ODS) Eur J Cancer. 2013;49(13):2910–2918. doi: 10.1016/j.ejca.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Lavoie Smith EM, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQCIPN20 Questionnaire. Qual Life Res. 2013;22:2787–2799. doi: 10.1007/s11136-013-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donald GK, Tobin I, Stringer J. Evaluation of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Acupunct Med. 2011;29(3):230–233. doi: 10.1136/acupmed.2011.010025. [DOI] [PubMed] [Google Scholar]
- 88.Takemoto S, Ushijima K, Honda K, Wada H, Terada A, Imaishi H, Kamura T. Precise evaluation of chemotherapy-induced peripheral neuropathy using the visual analogue scale: a quantitative and comparative analysis of neuropathy occurring with paclitaxel-carboplatin and docetaxel-carboplatin therapy. Int J Clin Oncol. 2012;17(4):367–372. doi: 10.1007/s10147-011-0303-6. [DOI] [PubMed] [Google Scholar]
- 89.Liu YC, Wang WS. Human mu-opioid receptor gene A118G polymorphism predicts the efficacy of tramadol/acetaminophen combination tablets (ultracet) in oxaliplatin-induced painful neuropathy. Cancer. 2012;118(6):1718–1725. doi: 10.1002/cncr.26430. [DOI] [PubMed] [Google Scholar]
- 90.Cunningham JE, Kelechi T, Sterba K, Barthelemy N, Falkowski P, Chin SH. Case report of a patient with chemotherapy-induced peripheral neuropathy treated with manual therapy (massage) Support Care Cancer. 2011;19(9):1473–1476. doi: 10.1007/s00520-011-1231-8. [DOI] [PubMed] [Google Scholar]
- 91.Durand JP, Deplanque G, Montheil V, Gornet JM, Scotte F, Mir O, Cessot A, Coriat R, Raymond E, Mitry E, Herait P, Yataghene Y, Goldwasser F. Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: results of EFFOX, a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2012;23:200–205. doi: 10.1093/annonc/mdr045. [DOI] [PubMed] [Google Scholar]
- 92.Lavoie Smith EM, Cohen JA, Pett MA, Beck SL. The validity of neuropathy and neuropathic pain measures in patients with cancer receiving taxanes and platinums. Oncol Nurs Forum. 2011;38(2):133–142. doi: 10.1188/11.ONF.133-142. [DOI] [PubMed] [Google Scholar]
- 93.Smith EM, Cohen JA, Pett MA, Beck SL. The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nurs. 2010;33(3):173–183. doi: 10.1097/NCC.0b013e3181c989a3. [DOI] [PubMed] [Google Scholar]
- 94.Burkey AR, Kanetsky PA. Development of a novel location-based assessment of sensory symptoms in cancer patients: preliminary reliability and validity assessment. J Pain Symptom Manag. 2009;37(5):848–862. doi: 10.1016/j.jpainsymman.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bezjak A, Lee CW, Ding K, Brundage M, Winton T, Graham B, Whitehead M, Johnson DH, Livingston RB, Seymour L, Shepherd FA. Quality-of-life outcomes for adjuvant chemotherapy in early-stage non-small-cell lung cancer: results from a randomized trial, JBR.10. J Clin Oncol. 2008;26(31):5052–5059. doi: 10.1200/JCO.2007.12.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong R, Sagar S. Acupuncture treatment for chemotherapy-induced peripheral neuropathy—a case series. Acupunct Med. 2006;24(2):87–91. doi: 10.1136/aim.24.2.87. [DOI] [PubMed] [Google Scholar]
- 97.Durand JP, Alexandre J, Guillevin L, Goldwasser F. Clinical activity of venlafaxine and topiramate against oxaliplatin-induced disabling permanent neuropathy. Anticancer Drugs. 2005;16(5):587–591. doi: 10.1097/00001813-200506000-00015. [DOI] [PubMed] [Google Scholar]
- 98.Hammack JE, Michalak JC, Loprinzi CL, Sloan JA, Novotny PJ, Soori GS, Tirona MT, Rowland KM, Jr, Stella PJ, Johnson JA. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98(1–2):195–203. doi: 10.1016/s0304-3959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 99.Wilson RH, Lehky T, Thomas RR, Quinn MG, Floeter MK, Grem JL. Acute oxaliplatin-induced peripheral nerve hyperexcitability. J Clin Oncol. 2002;20(7):1767–1774. doi: 10.1200/JCO.2002.07.056. [DOI] [PubMed] [Google Scholar]
- 100.Durand JP, Brezault C, Goldwasser F. Protection against oxaliplatin acute neurosensory toxicity by venlafaxine. Anticancer Drugs. 2003;14(6):423–425. doi: 10.1097/00001813-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 101.Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL, Alliance for Clinical Trials in Oncology Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110(9):2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 103.Tsavaris N, Kopterides P, Kosmas C, Efthymiou A, Skopelitis H, Dimitrakopoulos A, Pagouni E, Pikazis D, Zis PV, Koufos C. Gabapentin monotherapy for the treatment of chemotherapy-induced neuropathic pain: a pilot study. Pain Med. 2008;9(8):1209–1216. doi: 10.1111/j.1526-4637.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 104.Filipczak-Bryniarska I, Krzyzewski RM, Kucharz J, Michalowska-Kaczmarczyk A, Kleja J, Woron J, Strzepek K, Kazior L, Wordliczek J, Grodzicki T, Krzemieniecki K. High-dose 8% capsaicin patch in treatment of chemotherapy-induced peripheral neuropathy: single-center experience. Med Oncol. 2017;34(9):162. doi: 10.1007/s12032-017-1015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zis P, Rao DG, Sarrigiannis PG, Aeschlimann P, Aeschlimann DP, Sanders D, Grünewald RA, Hadjivassiliou M. Transglutaminase 6 antibodies in gluten neuropathy. Dig Liver Dis. 2017;49(11):1196–1200. doi: 10.1016/j.dld.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 106.Thawani SP, Brannagan TH, 3rd, Lebwohl B, Green PH, Ludvigsson JF. Risk of neuropathy among 28,232 patients with biopsy-verified celiac disease. JAMA Neurol. 2015;72(7):806–811. doi: 10.1001/jamaneurol.2015.0475. [DOI] [PubMed] [Google Scholar]
- 107.Pachman DR, Watson JC, Loprinzi CL. Therapeutic strategies for cancer treatment related peripheral neuropathies. Curr Treat Options Oncol. 2014;15(4):567–580. doi: 10.1007/s11864-014-0303-7. [DOI] [PubMed] [Google Scholar]
- 108.Rutkove SB, Kothari MJ, Raynor EM, Levy ML, Fadic R, Nardin RA. Sural/radial amplitude ratio in the diagnosis of mild axonal polyneuropathy. Muscle Nerve. 1997;20(10):1236–1241. doi: 10.1002/(sici)1097-4598(199710)20:10<1236::aid-mus5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 109.Buschbacher RM. Tibial nerve motor conduction to the abductor hallucis. Am J Phys Med Rehabil. 1999;78(6 Suppl):S15–S20. doi: 10.1097/00002060-199911001-00004. [DOI] [PubMed] [Google Scholar]
- 110.Zis P, Grünewald RA, Chaudhuri RK, Hadjivassiliou M. Peripheral neuropathy in idiopathic Parkinson’s disease: a systematic review. J Neurol Sci. 2017;15(378):204–209. doi: 10.1016/j.jns.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 111.Camdessanché JP, Jousserand G, Ferraud K, Vial C, Petiot P, Honnorat J, Antoine JC. The pattern and diagnostic criteria of sensory neuronopathy: a case-control study. Brain. 2009;132(Pt 7):1723–1733. doi: 10.1093/brain/awp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zis P, Hadjivassiliou M, Sarrigiannis PG, Barker ASTJE, Rao DG. Rapid neurophysiological screening for sensory ganglionopathy: a novel approach. Brain Behav. 2017. 10.1002/brb3.880 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.