Abstract
Tapentadol is a novel pain reliever with apparently synergistic dual mechanisms of action, capable of addressing both nociceptive and neuropathic components of chronic pain. As an effective analgesic with good tolerability, tapentadol may be appropriate for patients suffering from severe chronic pain associated with low back pain (LBP) or osteoarthritis (OA). Efficacy studies of tapentadol in populations of patients with severe chronic LBP or OA pain suggest that tapentadol is non-inferior to oxycodone. Its tolerability, especially with respect to gastrointestinal (GI) side effects, may be better than that of other strong opioids in clinical trials and analyses of multiple trials. Patient satisfaction with tapentadol extended release for chronic noncancer pain syndromes is good. Although tapentadol has an opioid component with abuse liability, it appears to be a difficult opioid for tampering with less appeal to abusers than other opioids. For patients with severe LBP and OA pain, tapentadol appears to hold promise as a safe, effective therapeutic option.
Keywords: Chronic pain, Chronic pain control, Low back pain, Osteoarthritis pain, Severe chronic pain, Tapentadol
Introduction
More people in the US suffer from chronic pain than have diabetes, all cancers, and acquired immune deficiency syndrome (AIDS) combined. Altogether, 64.7 million Americans contend with those major diseases (diagnosed diabetics 29 million [1], all cancers 14.5 million [2], and HIV/AIDS 1.2 million [3])—far fewer than the US chronic pain population of 100 million [4]. In fact, the chronic pain population exceeds the population of 84 million Americans living with some form of cardiovascular disease (heart disease, stroke, sudden cardiac arrest, and so on) [5]. Despite its ubiquity, chronic pain has become the invisible epidemic, such that chronic pain patients often suffer in silence while clinicians wrestle with controversies surrounding the prolonged use of analgesics to treat chronic, noncancer pain. For those patients with severe chronic noncancer pain, options may seem limited. Indeed, clinicians who treat patients in severe pain may be reticent to prescribe strong pain relievers because of legal, regulatory, or societal concerns [6, 7]. The Centers for Disease Control and Prevention (CDC) recently issued guidelines regarding opioid prescribing that advocated conservative use of opioid analgesics for chronic noncancer pain; there is concern among some clinicians that these guidelines may limit noncancer pain patients’ access to opioid analgesia [8, 9]. As the role of opioid analgesics in the treatment of severe chronic noncancer pain remains controversial, chronic pain patients may become resigned to their suffering out of their inability to deal with an unhelpful healthcare system. For its part, the national media seems more interested in raising awareness about opioid addiction than the undertreatment of chronic pain in millions of Americans [10]. This creates a conundrum for the clinicians on the frontlines who treat patients with chronic, severe noncancer pain.
The purpose of our review is to consider tapentadol as an analgesic that can help to manage patients who present with severe chronic pain associated with either low back pain (LBP) or osteoarthritis (OA), the two most common chronic pain syndromes in the US [11]. According to the Centers for Disease Control and Prevention (CDC), about 25% of American adults have arthritis, and this number has increased about 20% since 2002 [12]. This article is timely, if not altogether overdue, because the population of patients with severe LBP or severe chronic pain from OA is large and likely to increase markedly in the coming years with the inversion of the age pyramid, more people surviving once deadly and debilitating diseases, and the growing prevalence of obesity. Obesity nearly doubles the rate of arthritis: 16.9% of normal-weight or underweight adults have arthritis compared with 29.6% of obese adults [13]. By the year 2040, the Centers for Disease Control and Prevention (CDC) predict that 26% of the adult population (> 18 years) will have doctor-diagnosed arthritis, of whom two-thirds will be female [14]. A subset of this rapidly growing chronic noncancer pain population will present with severe pain syndromes requiring strong analgesia and clinically appropriate and meaningful answers.
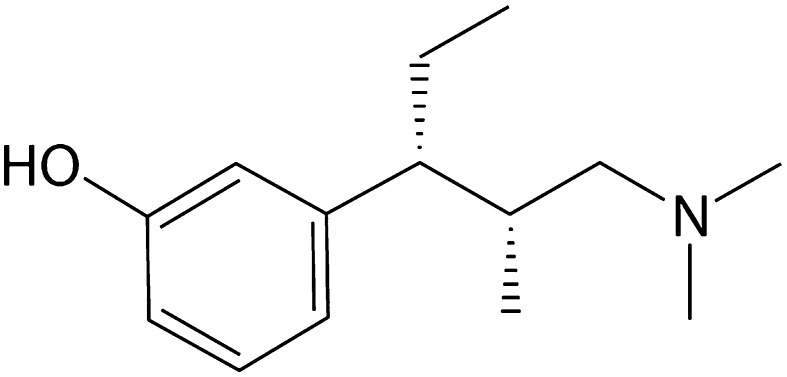
The introduction of tapentadol to the US market brought with it the recommendation that it be considered first in a new class of drugs tentatively described as MOR-NRI agents (µ-opioid receptor agonists and norepinephrine reuptake inhibitors) [15]. It has no official place on World Health Organization (WHO) pain ladder [16] as it is a newer product that was not on the market when WHO published its guidelines for cancer pain patients. Tapentadol is a unique product in that its dual analgesic mechanisms of action are combined in one molecule [17, 18]. In that way, it must be considered as an atypical opioid (Fig. 1). Tapentadol is effective in managing both nociceptive and neuropathic pain; it is approved for treating painful diabetic peripheral neuropathy and has been shown to be effective in treating other neuropathic pain [19] and phantom limb pain [20]. Tapentadol is available in immediate-release (IR) and prolonged-release (PR) formulations [21], making it an important new product to consider in the treatment of severe chronic pain associated with LBP or OA.
Fig. 1.
Molecular structure of tapentadol, described as an MOR-NRI agent (mu-opioid receptor agonist and norepinephrine reuptake inhibitor)
Review Methodology
The objective of our review was to review recent clinical trials, open-label studies, and meta-analyses of tapentadol with respect to its safety and efficacy, specifically in the populations of severe noncancer pain patients with cLBP or chronic OA pain. We searched the PubMed database for the broad keyword “tapentadol” for clinical trials and meta-analyses in the past 10 years and obtained 60 results. The authors eliminated articles dealing with animal studies, papers not available in English, and papers that studied populations other than patients with OA or cLBP (for example, acute pain or cancer pain). We also eliminated papers that were published prior to 2010 to focus on the latest research. This left 24 articles that were evaluated for this review. In addition, the references of these papers were also searched for other relevant support material that supplemented the article. Before reviewing the safety and efficacy of tapentadol for severe pain associated with cLBP and OA, a short review of the product, its mechanisms of action, and pain modulation are offered. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Tapentadol’s Dual Mechanisms of Action
Tapentadol’s two mechanisms of action both contribute to its analgesic effect. In a murine study, tapentadol showed agonist activity at the mu-opioid receptors, which was six times less potent than morphine in terms of G-protein-coupled inwardly rectifying K (GIRK) currents [22]. Tapentadol also inhibited the noradrenaline transporter with a similar potency as at the µ-opioid receptor. These two mechanisms had an additive effect in the individual locus coeruleus (LC) neurons of the rat [22]. Another study in rats found acute administration of tapentadol inhibited LC neurons in vivo, suggesting that both noradrenergic and opioid systems contribute to the inhibitory effect tapentadol exerts on the LC neurons [23].
It appeared that the antihyperalgesic activity of systemic tapentadol in mice occurred because of opioid spinal-suprapsinal synergy and intrinsic spinally mediated µ-opioid-receptor and norepinephrine reuptake inhibitor synergy [24]. In a rat model of neuropathic pain (spinal nerve ligation vs. sham surgery), tapentadol elevated spinal levels of norepinephrine [25]. Spinal noradrenaline likely plays a role in the descending pain inhibitory path and the modulation of nociceptive signals at the spinal level. In other preclinical studies, tapentadol increased noradrenaline levels but did not have the same effect on serotonin levels [26]. Morphine, on the other hand, slightly decreases the levels of both noradrenaline and serotonin [26]. This suggests that the noradrenergic component of tapentadol contributes to its analgesic mechanisms.
In a study to investigate how these two mechanisms of action interact, dose-response curves were generated in two pain models of rats (tail flick and spinal nerve ligation) for tapentadol with and without naloxone or yohimbe [an α(2)-adrenoceptor antagonist] [27]. The dose-effect relationship was compared according to the µ-opioid-receptor agonism, noradrenaline inhibition, and unblocked tapentadol. Tapentadol was shown to produce clear dose-dependent antinociception (analgesia) in both pain models in rats, and the dose-effect curves were shifted by both antagonists, which helped to differentiate the effect of µ-opioid-receptor agonism versus noradrenaline inhibition. Isobolographic analysis showed a marked synergistic interaction between these two mechanisms of action [27].
Pharmacokinetics of Tapentadol
Tapentadol is rapidly absorbed with absolute oral bioavailability of approximately 32% after a single dose for tapentadol IR 86 mg (mg) [95% confidence interval (CI) 29.4–34.8%, n = 24] and 32% (95% CI 28.0–35.9%, n = 18) for tapentadol PR 86 mg [28]. When tapentadol PR was compared with tapentadol IR (both doses 86 mg), tapentadol PR exhibited a lower Cmax value (22.6 ng/ml vs. 64.2 ng/ml), longer time to Cmax (5.0 vs. 1.5 h), higher half-value duration (12.5 vs. 3.6 h), and longer mean residence time (10.6 vs. 6.0 h) [28].
The major metabolic pathway for tapentadol is conjugation with glucoronic acid to produce glucuronides; the major metabolite is tapentadol-O-glucuronide [28, 29]. None of the metabolites appear to contribute to the analgesic action. In a pharmacokinetic study of tapentadol PR (polyethylene-oxide-based tablets) of doses ranging from 50 to 250 mg in healthy subjects, maximum concentrations (Cmax) were typically observed 5 h after dosing with terminal half-life values ranging from 4.4 to 5.9 h [30]. Trough concentrations increased during repeat dosing and achieved a steady state after the third dose. When tapentadol PR 250 mg was consumed with a high-fat meal, Cmax and the area under the concentration-time curve (AUC) increased by an average of less than 17%. Thus, tapentadol PR can be considered to have consistent pharmacokinetic parameter values after single and repeated dosing and may be administered with or without food [30].
Descending Pain Control
Pain perception is governed by multiple modulatory systems that are able to control how a noxious stimulus is “translated” into a painful sensation. Pain transmission is conducted along afferent “ascending” pathways and modulated by efferent “descending” pathways, which may be inhibitory or facilitating. An important surrogate for endogenous pain control is conditioned pain modulation (CPM), which essentially defines the body’s own ability to inhibit a pain signal along descending pathways. CPM can be used as a surrogate biomarker in studies of healthy adults by applying a nociceptive stimulus on one part of the body and, at the same time, applying a secondary, conditioning, tonic nociceptive stimulus remote from that primary site. In healthy individuals, the conditioning stimulus inhibits the sensation of pain from the primary site [31, 32]. In a study of healthy volunteers, subjects were administered tapentadol IR formulation 100 mg, morphine IR 40 mg, or placebo, and the results were obtained at 60–90 and 120–150 min after ingestion. CPM was detectable in patients treated with tapentadol or placebo, with mean treatment differences of tapentadol compared with morphine of 18.2% (95% confidence interval 3.4–32.9%) and 19.5% (95% confidence interval 5.7–33.2%) at 60–90 and 120–150 min, respectively (p = 0.001). Morphine in this study appeared to affect CPM, while neither tapentadol nor CPM affected this endogenous system [33].
Tapentadol’s Efficacy in Treating Severe Pain Associated with cLBP or OA
The long-term use of opioids for managing severe pain from cLBP or chronic OA are not thoroughly studied, and the scope and logistic involved in conducting such broad long-term studies make them unlikely. However, the short-term use of opioids in the treatment of chronic pain has been studied in trials of sound design and provides good evidence of analgesic efficacy. In an update of a Cochrane database review first published in 2007 [34], Chaparro and colleagues compared 15 trials of opioids versus placebo for cLBP (15 trials, n = 5540). In six trials, tapentadol among other “strong opioids” (oxycodone, oxymorphone, morphine, hydromorphone) was better than placebo for controlling pain and improving function [35]. Below are summaries of individual clinical trials and pooled analyses or meta-analyses that examined the use of tapentadol for severe pain associated with cLBP or chronic OA.
Clinical Trials
Head-To-Head Clinical Trials
The following trials evaluated tapentadol and oxycodone or an oxycodone product (oxycodone/naltrexone fixed-dose combination product) for noninferiority. The comparisons were not sufficiently powered to make direct comparisons for judging superiority.
Patients with severe chronic low back pain (cLBP) with a neuropathic component (n = 258 in the safety set, 256 in the full analysis set) were randomized into a tapentadol group (50 mg of tapentadol PR twice a day) or oxycodone/naloxone PR group (10/5 mg twice a day) [36]. Patients were titrated over a 21-day period (maximum doses achieved were 250 mg of tapentadol PR and 40/20 mg oxycodone/naloxone), and then that dose was maintained over 9 weeks. The primary endpoint was the effectiveness of the drug in reducing pain over baseline (as measured on an 11-point scale). Tapentadol PR was found to be noninferior to oxycodone/naloxone [an upper limit < 1.3 was needed to establish noninferiority, a 97.5% repeated confidence interval (− 1.820 to − 1.084, p < 0.001), and pain scores using painDETECT, and the Neuropathic Pain Symptom Inventory showed significantly greater reduction with tapentadol PR versus oxycodone/nalxoone PR (p ≤ 0.005 for all)]. Adverse events from studies described here appear in Table 1. Tapentadol PR was associated with a 40% lower rate of constipation than oxycodone/naloxone [37].
Table 1.
Adverse events from key clinical trials, open-label studies, pooled analyses, or meta-analyses of clinical trials in which various adverse events were reported for tapentadol taken from studies dating from 2010 to present
| Adverse event | Study | n | Rate of adverse events | ||
|---|---|---|---|---|---|
| Tapentadol | Active comparator | Placebo | |||
| Gastrointestinal disorders | Baron 2016 | 258 | 44.6% | 51.6% O/N | NA |
| Steigerwald 2012 (knee OA) | 200 | 38.5% | NA | NA | |
| Nausea | Schwittay 2013 | 3222 | 2.5% | NA | NA |
| Steigerwald 2013 | 54 | 24.1% | W1: 46.0% | NA | |
| Galves 2013 | 136 | 15.2% | NA | NA | |
| Schwartz 2011 | 588 dpn | 13.8% | NA | 6.2% | |
| Wild 2010 | 1117 | 18.1% | 33.2% O | NA | |
| Lange 2010 | 2968 | 20.7% | 36.2% O | 7.4% | |
| Buynak 2010 | 981 | 20.1% | 34.5% O | 9.1% | |
| Baron 2016 | 258 | 22.3% | 18.0% O/N | NA | |
| Steigerwald 2012 (knee OA) | 200 | 13.0% | NA | NA | |
| Vertigo/dizziness | Schwittay 2013 | 3222 | 1.6% | NA | NA |
| Steigerwald 2013 | 54 | 9.3% for dizziness 5.6% for vertigo |
W1: 12.7% dizziness W1: 4.8% for vertigo |
NA | |
| Galves 2013 | 136 | 12.8% | NA | NA | |
| Schwartz 2011 | 588 dpn | 7.7% | NA | 1.6% | |
| Wild 2010 | 1117 | 14.8% | 19.3% | NA | |
| Lange 2010 | 2968 | 17.2% | 21.0% O | 6.3% | |
| Buynak 2010 | 981 | 11.9% | 17.1% | 5.6% | |
| Baron 2016 | 258 | 18.5% | 17.2% O/N | ||
| Steigerwald 2012 (knee OA) | 200 | 12.0% | NA | NA | |
| Vomiting | Schwittay 2013 | 3222 | 0.7% | NA | NA |
| Steigerwald 2013 | 54 | 0 | W1:3.2% | NA | |
| Schwartz 2011 | 588 dpn | 6.6% | NA | 1.0% | |
| Wild 2010 | 1117 | 7.0% | 13.5% | NA | |
| Lange 2010 | 2968 | 8.2% | 21.0% O | 2.9% | |
| Buynak 2010 | 981 | 9.1% | 19.2% O | 1.6% | |
| Baron 2016 | 258 | 7.7% | 16.4% O/N | NA | |
| Steigerwald 2012 (knee OA) | 200 | 5.0% | NA | NA | |
| Diarrhea | Schwittay 2013 | 3222 | 0.5% | NA | NA |
| Schwartz 2011 | 588 dpn | 8.2% | NA | 4.1% | |
| Wild 2010 | 1117 | 7.9% | 5.4% O | NA | |
| Lange 2010 | 2968 | 5.2% | 5.1% | 5.8% | |
| Buynak 2010 | 981 | 6.0% | 2.4% | 7.2% | |
| Steigerwald 2012 (knee OA) | 200 | 5.5% | NA | NA | |
| Constipation | Schwittay 2013 | 3222 | 0.4% | NA | NA |
| Steigerwald 2013 | 54 | 7.4% | W1: 31.7% | NA | |
| Galves 2013 | 136 | 12.0% | NA | NA | |
| Schwartz 2011 | 588 dpn | 6.1% | NA | 1.0% | |
| Wild 2010 | 1117 | 22.6% | 38.6% O | NA | |
| Lange 2010 | 2968 | 16.9% | 33.0% O | 6.9% | |
| Buynak 2010 | 981 | 13.8% | 26.8% O | 5.0% | |
| Baron 2016 | 258 | 15.4% | 25.8% O/N | NA | |
| Steigerwald 2012 (knee OA) | 200 | 10.5% | NA | NA | |
| Insomnia | Schwittay 2013 (sleeping disorders in general) | 3222 | 0.4% | NA | NA |
| Steigerwald 2013 | 54 | 3.7% | W1: 1.6% | NA | |
| Galves 2013 | 136 | 12.8% | NA | NA | |
| Schwartz 2011 | 588 dpn | 5.1% | NA | 3.6% | |
| Wild 2010 | 1117 | 6.7% | 4.0% O | NA | |
| Buynak 2010 | 981 | 4.1% | 7.6% | 2.8% | |
| Restlessness | Schwittay 2013 | 3222 | 0.4% | NA | NA |
| Schwartz 2011 | 588 dpn | 5.6% | NA | 4.1% | |
| Somnolence | Schwittay 2013 | 3222 | 0.4% | NA | NA |
| Wild 2010 | 1117 | 14.9% | 11.2% O | NA | |
| Lange 2010 | 2968 | 11.6% | 16.8% | 3.5% | |
| Buynak 2010 | 981 | 13.2% | 16.2% | 2.5% | |
| Steigerwald 2012 (knee OA) | 200 | 7.0% | NA | NA | |
| Headache | Schwittay 2013 | 3222 | 0.3% | NA | NA |
| Galves 2013 | 136 | 14.4% | NA | NA | |
| Schwartz 2011 | 588 dpn | 5.1% | NA | 5.2% | |
| Wild 2010 | 1117 | 13.3% | 7.6% O | NA | |
| Lange 2010 | 2968 | 14.9% | 13.2% O | 13.2% | |
| Buynak 2010 | 981 | 11.9% | 17.1% O | 5.6% | |
| Baron 2016 | 258 | 7.7% | 3.9% O/N | NA | |
| Steigerwald 2012 (knee OA) | 200 | 6.5% | NA | NA | |
| Fatigue | Schwittay 2013 | 3222 | 0.3% | NA | NA |
| Steigerwald 2013 | 54 | 7.4% | W1: 17.5% | NA | |
| Galves 2013 | 136 | 10.4% | NA | NA | |
| Wild 2010 | 1117 | 9.7% | NA | NA | |
| Lange 2010 | 2968 | 11.6% | 16.8% O | 3.5% | |
| Buyank 2010 | 981 | 6.6% | 7.3% | 4.1% | |
| Baron 2016 | 258 | 30.0% | 24.2% O/N | NA | |
| Steigerwald 2012 (knee OA) | 200 | 10.5% | NA | NA | |
| Vision disturbances | Schwittay 2013 | 3222 | 0.3% | NA | NA |
| Dry mouth | Steigerwald 2013 | 54 | 5.6% | W1: 17.5% | NA |
| Galves 2013 | 136 | 6.4% | NA | NA | |
| Wild 2010 | 1117 | 9.1% | 4.5% O | NA | |
| Lange 2010 | 2968 | 6.8% | 4.0% O | 2.2% | |
| Buynak 2010 | 981 | 8.2% | 3.7% O | 2.2% | |
| Baron 2016 | 258 | 6.9% | 5.5% O/N | NA | |
| Steigerwald 2012 (knee OA) | 200 | 10.0% | NA | NA | |
| Pruritus | Steigerwald 2013 | 54 | 0 | NA | NA |
| Wild 2010 | 1117 | 5.4% | NA | NA | |
| Lange 2010 | 2968 | 5.2% | 13.4% O | Not reported | |
| Buynak 2010 | 981 | 7.2% | 16.8% O | 1.9% | |
| Baron 2016 | 258 | 6.2% | 8.6% O/N | NA | |
| Gastritis | Steigerwald 2013 | 54 | 3.7% | NA | NA |
| Upper abdominal pain | Galves 2013 | 136 | 5.6% | NA | NA |
| Hyperhidrosis | Galves 2013 | 136 | 8.0% | NA | NA |
| Lange 2010 | 2968 | 5.3% | 5.3% | 0.9% | |
| Buynak 2010 | 981 | 3.8% | 5.2% O | 0 | |
| Baron 2016 | 258 | 6.2% | 10.2% O/N | NA | |
| Anxiety | Schwartz 2011 | 588 dpn | 9.2% | NA | 4.1% |
| Bone pain | Schwartz 2011 | 588 dpn | 4.1% | NA | 5.2% |
| Myalgia | Schwartz 2011 | 588 dpn | 6.6% | NA | 7.3% |
| Dyspepsia | Buynak 2010 | 981 | 5.0% | 1.8% | 2.5% |
| Nervous system disorders | Baron 2016 | 258 | 29.2% | 27.3% O/N | NA |
| Skin and subcutaneous tissue disorders | Baron 2016 | 258 | 12.3% | 18.8% O/N | NA |
| Infections and infestations | Baron 2016 | 258 | 14.6% | 8.6% O/N | NA |
| Nasopharyngitis | Baron 2016 | 258 | 6.2% | 3.9% O/N | NA |
| Steigerwald 2012 (knee OA) | 200 | 8.0% | NA | NA | |
The first number refers to results at week 1 treatment with tapentadol extended release; the second number refers to results at 12 weeks
dpn Diabetic peripheral neuropathy patients only, NA not applicable to this study/paper, O oxycodone controlled release, OE opioid-experienced patient population, Wk weeks, W1 week 1 when, in this study, patients were taking another WHO step III strong opioid
Tapentadol PR significantly reduced average pain intensity from baseline to week 12 versus placebo in a study of patients with moderate-to-severe chronic pain associated with knee OA (n = 1023) [38]. Oxycodone CR likewise reduced pain significantly versus baseline throughout the maintenance period but not at week 12. Significantly more tapentadol than placebo patients achieved a 50% or greater improvement in pain intensity (32.0% vs. 24.3%, p = 0.027); the converse was true for oxycodone, where significantly fewer oxycodone patients achieved ≥ 50% pain relief than placebo at 12 weeks (17.3% vs. 24.3%, p = 0.023) [38].
In an active- and placebo-controlled, double-blind study of tapentadol PR versus oxycodone to treat chronic LBP (including but not limited to severe pain), tapentadol significantly reduced the average pain intensity compared with placebo at week 12 (p < 0.001) and throughout the 12-week maintenance period (p < 0.001), as did oxycodone CR (p < 0.001 for both); there was no significant difference in effectiveness between agents [39].
A post hoc analysis was conducted of two multicenter, randomized, double-blind trials of tapentadol IR to oxycodone IR in patients with moderate-to-severe pain from OA [40]. One study was conducted over 10 days and found tapentadol patients had greater pain relief and tolerability (PRT) than oxycodone patients (the difference was only significant for the 50-mg formulation). In a 90-day trial, tapentadol IR had a significantly higher proportion of days meeting PRT criteria than oxycodone IR.
Open-Label Studies
In a study of patients with severe chronic low back pain (cLBP) with a neuropathic component treated with tapentadol PR 300 mg/day showed significant improvements in pain control, better relief of neuropathic symptoms, and improved quality of life over baseline. Treatment-emergent adverse effects in this study were ≤ 5.1% [41].
In an open-label study of patients with severe chronic knee pain due to OA (n = 82), tapentadol PR (50–250 mg twice a day) was more effective than other WHO step III “strong opioids” in patients who had previously responded to the latter; the responder rate to tapentadol PR was 94.3% at 6 weeks with a mean total daily dose of 232.7 ± 145.37 mg [42].
In an open-label phase IIIb clinical trial of 136 patients with severe cLBP, tapentadol PR 50–250 mg provided at least comparable pain relief to other strong opioids, and patients taking other opioids could be successfully converted to tapentadol [43]. In that study, patients taking tapentadol PR achieved significant improvements in pain relief over baseline in both pain intensity and neuropathic symptoms at weeks 6 and 12 (p < 0.05).
A phase IIIb clinical trial evaluated patients with severe chronic knee OA that could not be adequately managed with WHO step I or step II analgesics [44]. In this open-label trial, patients were titrated over 5 weeks on tapentadol PR (50–250 mg twice daily) and then maintained for 7 weeks with tapentadol IR 50 mg permitted throughout the study (no more than twice daily and at least 4 h apart). Pain intensity was measured at baseline and then at the end of the study based on an 11-point rating scale. The mean change from baseline to week 6 in pain intensity was − 3.4 (2.10, p < 0.0001) for the 195 patients evaluated. Further, significant decreases in pain intensity occurred at weeks 6, 8, and 12 (p < 0.0001, for all).
Pooled Data and Meta-Analyses
Four randomized clinical trials (n = 4094 patients total) of patients with OA or back pain treated with tapentadol were analyzed. When compared with oxycodone, tapentadol reduced pain by an additional 0.24 points on a 0–10 pain scale compared with oxycodone (95% confidence interval 0.43–0.05) and was associated with a 50% risk reduction of discontinuation of treatment because of adverse events (95% confidence interval 42–60%). Thus, overall, tapentadol ER could be associated with greater reduction in pain intensity compared with oxycodone or placebo and had a better safety profile and greater tolerability than oxycodone [45].
Data were pooled from four 15-week phase III studies comparing tapentadol PR with an active comparator in patients with either moderate-to-severe chronic OA of the knee, LBP, or painful diabetic peripheral neuropathy (DPN). The combined results found that tapentadol PR (100–250 mg twice daily) was effective in controlling moderate-to-severe knee OA pain, LBP, and diabetic peripheral neuropathy, with relief comparable to that provided by oxycodone CR (20–50 mg twice daily) for chronic knee OA and LBP. Results were durable over 1 year, and tapentadol PR was associated with greater tolerability, particularly for gastroinestinal adverse effects [46].
A pooled analysis from three randomized, double-blind phase II studies comparing tapentadol PR with an active comparator for moderate-to-severe chronic knee OA or LBP (n = 2968 and 2974 patients for efficacy and tolerability, respectively) found that 100–250 mg twice daily of tapentadol ER was well tolerated and provided similar pain relief as that of oxycodone CR but with better gastrointestinal (GI) tolerability [47].
In a systematic review of trials comparing tapentadol to other agents for treating severe chronic pain, tapentadol was significantly better in relieving pain intensity, achieving levels of 30% and 50% pain relief, improving patient global impression of change, and enhancing quality of life when compared directly with oxycodone [48]. A pooled analysis of four 15-week phase 3 studies of tapentadol PR in the treatment of moderate-to-severe chronic pain associated with a variety of conditions (OA, LBP, and diabetic peripheral neuropathy) found tapentadol PR at doses of 100–250 mg was associated with effective analgesia and good tolerability with lower rates of discontinuations and adverse events than oxycodone CR 50–250 mg [46].
In a pooled analysis (three randomized double-blind trials, n = 2968 for efficacy, n = 2974 for safety) of patients with moderate-to-severe chronic pain associated with LBP or OA, tapentadol ER (100–250 mg twice daily) provided similar pain relief and tolerability as oxycodone CR (20–50 mg) over 3 weeks (titration) followed by 12 weeks (maintenance). Tapentadol and oxycodone effectiveness was similar regardless of baseline pain intensity levels, prior opioid experience, gender, or body mass index (BMI) [47].
Effectiveness in Treating Neuropathic Pain
Neuropathic pain occurs in the setting of cLBP at a rate of 65–77% [49], but not all clinical trials of tapentadol for cLBP evaluated its specific effectiveness in treating the neuropathic component of cLBP. Indeed, the neuropathic pain contribution to the more severe forms of cLBP may be underdiagnosed and thus go untreated [50]. Since neuropathic pain involves both the ascending and descending (noradrenergic) pain pathways, opioids may be ineffective in that they modulate pain solely via the ascending pain pathways [51–53]. Thus, the noradrenergic activity of tapentadol could be thought to contribute to neuropathic pain control. In a head-to-head comparison study of tapentadol PR compared with oxycodone/naloxone PR, the painDETECT score of pain control at 9 weeks versus baseline showed that tapentadol patients achieved significantly greater neuropathic pain relief than the oxycodone/naloxone patients (p = 0.002). Both groups achieved significant neuropathic pain relief over baseline, but the neuropathic pain reduction was significantly greater in the tapentadol group [37].
Although not a study of LBP or OA patients, a randomized-withdrawal, placebo-controlled trial of 588 patients with painful diabetic neuropathy exhibits the potential effectiveness of tapentadol in controlling neuropathic pain. Patients enrolled in this study were dissatisfied with their current pain treatment (opioid and nonopioid), had an average pain intensity score of ≥ 5 on an 11-point numerical rating scale, and ≥ 3-month use of an analgesic to manage their neuropathy [54]. Upon entering the study, patients were titrated to an optimal dose of tapentadol PR (100–250 mg) in an open-label phase of 3 weeks. Patients who achieved at least a one-point reduction on the pain scale (n = 395) advanced to the randomization phase where they were assigned either placebo or an optimal fixed dose of tapentadol extended release for 12 weeks. The tapentadol ER group showed significantly improved pain scores over baseline compared with placebo (p < 0.001), and 60.5% of patients had ≥ 30% improvement in pain intensity over the open-label phase.
Quality of Life
Quality of life (QoL) is an important metric for any long-term therapy and is particularly relevant to patients, although QoL data are not always reported for chronic pain treatments. In a study of severe cLBP patients randomized to compare tapentadol PR versus oxycodone/naloxone PR, QoL scores were analyzed using the SF-12 summary and domain scores. In both groups, there were significant improvements in QoL at 9 weeks compared with baseline, but the tapentadol PR group had significantly greater QoL improvements than the oxycodone/naloxone group at 9 weeks for the mean physical component score and six domain scores [36].
A noninterventional prospective study (n = 3134) of patients with various types of severe chronic pain (82% had low back pain) found tapentadol PR was effective in various pain syndromes for patients who had previously been administered strong opioids [55]. All of the patients included in this study opted to change from another opioid agent to tapentadol. The most common reasons for wanting to switch from another opioid to tapentadol was found to be inadequate analgesia (91%) and reduced QoL (70%) [55].
Productivity and Function
Using clinical trial data for pain outcomes in a study of tapentadol PR versus oxycodone controlled release (CR) versus placebo, a validated methodology was employed to assess differences in at-work productivity among the groups. Based on an assumed annual salary of $100,000 per participant, it could be imputed that patients taking tapentadol ER or oxycodone CR had improved productivity compared with placebo patients (1.95% for tapentadol ER vs. 1.51% placebo, p = 0.001 or 1.96% for oxycodone CR vs. 1.40% for placebo, p < 0.001) [56]. The mean net savings per subject were calculated to be $450 (p < 0.01) for tapentadol ER compared with placebo or $560 (p = 0.001) for tapentadol ER compared with oxycodone CR.
Long-term tapentadol therapy
The role of long-term opioid therapy for cLBP remains controversial. Moderate-to-severe chronic noncancer pain may be treated effectively by opioids, but high-quality evidence is limited for the long-term efficacy and tolerability of opioids in these populations [57, 58]. Tapentadol PR has been reported in the literature to be safe and effective in chronic noncancer pain patients treated up to 3 or 4 months [38, 39, 44, 59, 60], and it was reported to be well tolerated and effective in patients with chronic OA or cLBP for up to 2 years [61]. In the initial 1-year phase of this study, 22.1% of tapentadol PR and 36.8% of oxycodone CR patients experienced treatment-emergent adverse events (TEAEs) that led to discontinuation of the study drug. Indeed, the overall incidence of several TEAEs favored tapentadol PR over oxycodone CR: overall GI effects (8.6% vs. 21.5% for tapentadol PR versus oxycodone CR, respectively), nausea (3.4% vs. 12.1%), constipation (1.6% vs. 7.2%), and vomiting (2.6% vs. 6.7%) [61]. In another long-term tolerability study of tapentadol PR, pain relief occurred within about 4 weeks of onset of treatment and remained durable for 2 years [62], suggesting that patients did not acquire tolerance to tapentadol PR [63].
A panel of pain experts convened in Europe to discuss tapentadol PR after it had been on the market for 5 years and been used to treat over 4 million patients globally [64]. Long-term safety and tolerability of tapentadol therapy for patients with moderate-to-severe hip or knee OA or cLBP could be confirmed for up to 1 year. Thus, pain control with tapentadol offered durable relief with no evidence of acquired tolerability over time [64].
Safety
Opioid-associated side effects can limit treatment, cause morbidity, and distress patients. Because opioid-associated side effects are dose related, tolerant patients who need high doses of opioid analgesics are at elevated risk for such adverse events. Tapentadol’s tolerability holds promise for lowering side effect rates in the long-term setting of chronic pain [17, 65]. Tapentadol may provide a favorable efficacy-to-side-effect ratio that may benefit those being treated for severe chronic pain [66]. See Table 1 for an overview of tapentadol-associated adverse events.
In a quantitative systematic review of nine clinical trials (n = 7948) of tapentadol or oxycodone, the risk of several typical opioid-associated adverse events was lower with tapentadol than oxycodone (risk ratios for tapentadol were 0.61 for nausea, 0.50 for vomiting, 0.47 for constipation, 0.86 for dizziness, 0.76 for somnolence, and 0.46 for pruritus) [67]. However, tapentadol conferred a higher risk of dry mouth and dyspepsia compared with oxycodone. In the safety portion of a noninterventional study of severe chronic pain patients (n = 3222) switched from a strong opioid to extended-release tapentadol, adverse events were reported in 6.7% of patients (446 total adverse drug reactions) [55]. The majority of these adverse events (91%) were considered not serious, and no life-threatening AEs occurred.
In the safety analysis of a study of severe chronic pain patients with knee OA (n = 54), patients at week 1 were taking a “strong opioid” (not tapentadol) and by week 12 had completed a course of tapentadol PR [42]. Many adverse events decreased during the tapentadol phase (nausea from 46.0% to 24.1%, constipation from 31.7% to 7.4%, dry mouth from 17.5% to 5.6%, fatigue from 17.5% to 7.4%, dizziness from 12.7% to 9.3%, pruritus from 4.8% to 0, and vomiting from 3.2% to 0). A few of the adverse events in this study increased: vertigo from 4.8% to 5.6%, gastritis from 3.2% to 3.7%, and insomnia from 1.6% to 3.7%. In this study, 55.6% of patients took concomitant medications to treat adverse events for either tapentadol or their previous opioid agent [42].
Multiple studies have reported that tapentadol PR has lower rates of nausea, vomiting, dizziness, and constipation compared with oxycodone [38, 39, 59, 61] and morphine [68]. For example, the odds of a patients with moderate-to-severe cLBP experiencing constipation or the composite endpoint of nausea and vomiting was significantly lower for tapentadol ER than oxycodone CR (p < 0.001) [39].
Gastrointestinal (GI) Side Effects
With respect to other strong opioids, tapentadol may have superior GI tolerability [48, 69–71]. A randomized, parallel-group, double-blind, placebo-controlled study of the acute effects of oral tapentadol versus oral oxycodone on gastric, small bowel, and colonic transit was conducted in 38 healthy volunteers. This study found oxycodone and tapentadol had significantly delayed gastric and small bowel transit time compared with placebo but not for colonic transit [72]. Tapentadol significantly delayed the gastric emptying half-life and small bowel transit but in a manner similar to that of oxycodone. Compared with oxycodone/naloxone PR, tapentadol was noninferior with respect to its impact on bowel function and had significantly lower rates of constipation and vomiting [37].
Side effects such as nausea and vomiting are more prevalent in opioid-naïve than opioid-experienced patients, but these effects may subside over the course of a few days or weeks [73]. Nausea and vomiting are among the most common side effects of opioid therapy and are greatly disliked by patients [74]. The risk of nausea and vomiting varies among opioid analgesic agents; the elevated risk per drug exposure for adverse events was three to four times lower for tapentadol IR compared with oxycodone IR, and the risk per drug exposure of adverse events with oxycodone was, in turn, about 60 times lower than for oxymorphone [75]. In a double-blind study of end-stage joint disease patients, tapentadol IR was compared with oxycodone IR over a 14-day treatment period, showing better GI tolerability for tapentadol compared with oxycodone for nausea, vomiting, and constipation at comparable doses (tapentadol IR 50 or 75 mg versus oxycodone IR 10 mg) [76].
In a long-term clinical trial of patients with chronic LBP or OA pain, patients received at least one dose of the study drug (tapentadol PR 100–250 mg) or oxycodone CR (20–50 mg) for up to 1 year. A total of 1117 patients received at least one dose of a study drug [61]. The overall rate of TEAEs was 85.7% vs. 90.6% in the tapentadol versus oxycodone groups, respectively. Discontinuation because of adverse events occurred in 22.1% vs. 36.8% of tapentadol versus oxycodone patients. Specifically, GI adverse events that led to drug discontinuation occurred in 8.6% (tapentadol) vs. 21.5% (oxycodone) of patients [61].
In a randomized, double-blind, active-controlled, placebo-controlled, parallel-arm phase III study, TEAEs occurred in 75.9% of tapentadol PR patients compared with 87.4% of oxycodone CR patients, with a 61.1% rate for placebo patients [38]. Specifically GI-related TEAEs occurred in 43.0% of tapentadol, 67.3% of oxycodone, and 26.1% of placebo patients [38].
In cLBP patients, tapentadol PR patients experienced fewer TEAEs than oxycodone CR patients (n = 981); GI adverse events (constipation, nausea, vomiting) occurred in 43.7% of tapentadol, 61.9% of oxycodone, and 26.3% of placebo patients [39]. In this study, tapentadol patients were significantly less likely to experience constipation or the composite endpoint of nausea and/or vomiting than oxycodone patients (p < 0.001 both).
In a secondary post hoc analysis based on a questionnaire of patient-reported bowel function, patients treated for 10 (n = 518) or 90 days (n = 457) with tapentadol IR had less impairment in bowel function than those treated with oxycodone IR, including having a lower proportion of days without a bowel movement (p < 0.05), less risk of hard stools (p < 0.001), and less moderate-to-severe straining during a bowel movement (p < 0.001) [77]. Moreover, tapentadol IR patients consumed less laxative during the study compared with patients taking oxycodone IR (p < 0.001).
Tapentadol IR (50 and 75 mg) was superior in GI tolerability to oxycodone IR (10 mg) for commonly reported adverse events such as nausea, vomiting, and constipation. Patients in the tapentadol group had significantly more spontaneous bowel movements over a 14-day period (9.0 vs. 6.7, respectively) with significantly lower rates of nausea and vomiting [76]. In this study, results were similar with tapentadol IR and oxycodone IR as well as tapentadol PR and oxycodone CR.
In a post hoc analysis of a phase III 90-day double-blind, flexible-dose study comparing tapentadol IR 50 or 100 mg to oxycodone IR 10 or 15 mg (every 4-6 h, as needed), significantly fewer patients in the tapentadol group discontinued treatment because of constipation compared with oxycodone IR (1.5% vs. 5.9%, p = 0.0023). Likewise, significantly fewer tapentadol patients discontinued treatment owing to nausea and/or vomiting than those taking oxycodone (5.9% vs. 14.7%, p = 0.0003) [78]. Both treatment groups achieved similar levels of effective pain control.
Androgen Deficiency
Opioid-induced androgen deficiency (OPIAD) has been reported with long-term opioid exposure. From a total of three clinical studies with healthy subjects, tapentadol has less effect on sex hormone concentrations compared with pure opioid analgesics, morphine, and oxycodone. In a single-dose comparison study of tapentadol versus morphine, mean total testosterone concentrations at 6 h after dosing were comparable between placebo (8.6 nmol/l) and two doses of tapentadol IR, 43 mg (8.8 nmol/l) and 86 mg (9.3 nmol/l), but were reduced after 30 mg morphine IR (5.4 nmol/l) [79]. The dual mechanisms of action of tapentadol (µ-opioid receptor and norepinephrine reuptake inhibitor) appear to contribute to this reduced impact on serum androgen concentrations compared with those agents, such as morphine, that act solely on the µ-opioid receptor.
Sleep Disorders
Sleep and pain have an apparent but not yet fully elucidated relationship. In a noninterventional study of 3134 patients with severe chronic pain (majority LBP), tapentadol PR was reported to be associated with improved sleep quality. At the outset of this study, only 4.9% of patients reported they slept through the night, but at the end of the 3-month observation period, 23.5% slept all night without waking [55]. In a study of patients with severe chronic pain from knee OA (n = 63), patients taking tapentadol PR significantly increased the mean number of hours slept per night over baseline (6.6 h), resulting in 7.0 h of sleep per night at week 6 (p < 0.05) [42].
An interesting and clinically relevant “pain relief/tolerability” composite endpoint from a study by Merchant and colleagues evaluated outcomes as ≥ 30% pain relief with no nausea, vomiting, or constipation and without discontinuations [80]. In a post hoc meta-analysis of three randomized, double-blinded clinical trials (n = 1977), at 12 weeks tapentadol PR patients were more likely to have achieved this composite endpoint than oxycodone CR patients (odds ratio 3.15, 95% confidence interval 2.47–4.00, p < 0.001) [80].
Tapentadol and Patient Satisfaction
Patient satisfaction is an increasingly important metric in modern patient-centric healthcare paradigms. In an open-label study by Steigerwald and colleagues, “excellent” patient satisfaction scores occurred in none of 62 patients at baseline (all taking a strong opioid other than tapentadol), but rose to 5.5% at 6 and 27.8% at 12 weeks. “Excellent” or “very good” patient satisfaction occurred in no patients at baseline, 54.6% at week 6, and 61.1% at week 12 [42]. The metric of “overall improvement” may be related to patient satisfaction. In a study of 378 acute pain patients, a greater overall improvement was reported with tapentadol IR compared with oxycodone IR, although pain intensity scores, patient satisfaction, and pain relief were statistically similar between groups [81].
Abuse Liability of Tapentadol
The nonmedical use of prescription analgesics is a problematic, widespread, and unintended consequence of their availability. Tapentadol is an “atypical” opioid, and, as such, appears to have an atypical abuse profile. To be sure, tapentadol is associated with physical and psychologic dependency in long-term use [82]. In a survey from the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System College Survey Program (n = 13,514), nonmedical use of tapentadol IR occurred in 0.7% of patients; nonmedical use peaked in the fourth quarter of 2009 and declined over the next 2 years to 0.4% [83]. A database study found the risk of abuse was greater for oxycodone than tapentadol in patients who received a prescription opioid and were followed for 1 year [84].
Tamper-resistant and abuse-deterrent formulations (ADFs) of opioid analgesics are growing in importance [85–87]. In a study of 25 experienced, healthy subjects who abused oxycodone CR formulation, subjects were asked to defeat tamper-resistant tapentadol PR and oxycodone CR products. Investigators evaluated the tampering behavior, drug desirability, and yield of active drug in solution [88]. Subjects found tampered oxycodone more desirable than tampered tapentadol and were able to extract significantly more active drug from the oxycodone than tapentadol products (37.02% vs. 3.52%, respectively, p = 0.008). Moreover, tampering with tapentadol took more time than manipulating oxycodone [88].
Tapentadol Use in Special Populations
Geriatric Patients
In general, geriatric patients may be administered tapentadol PR with no dosage adjustments [89]. The mean AUC (mean exposure) to tapentadol was shown in a study to be similar for geriatric patients (65–78 years of age) compared with younger patients (19–43 years), with maximum concentrations 16% lower in elderly versus younger patients [90].
An analysis of pooled data from three similarly designed double-blind placebo- and active-controlled trials of tapentadol PR versus oxycodone CR in 210 elderly patients (≥ 75 years) with severe cLBP or severe chronic knee OA found that both tapentadol ER and oxycodone CR resulted in significant pain reductions at 15 weeks compared with baseline (p = 0.0075), but there was no significant difference between tapentadol and oxycodone [91]. The rate of GI TEAEs was significantly lower in the tapentadol group than the oxycodone group (p ≤ 0.0206); in this study, more oxycodone than tapentadol patients discontinued treatment.
A post hoc data analysis of 90-day randomized phase III, double-blind clinical trial data of 849 patients with moderate-to-severe LBP or OA pain administered either 50 or 100 mg of tapentadol IR or 10 to 15 mg of oxycodone IR every 4–6 h found that rates of constipation (19.0% vs. 35.6%) and nausea or vomiting (30.4% vs. 51.1%) were significantly lower with tapentadol IR than oxycodone IR in patients over age 65 years (p < 0.05) [92]. Among the patients over age 65, fewer tapentadol IR patients discontinued therapy because of GI adverse events compared with oxycodone IR patients (15.8% vs. 24.4%, p = 0.190). Both analgesics provided similar effective pain relief, and no age-related differences in analgesic benefit were observed.
Treatment of all patients, but particularly geriatric patients, must take into account the potential risks imposed by polypharmacy. The reliable pharmacokinetic profile of tapentadol may offer particular advantages in this setting. Metabolized mainly by phase 2 glucuronidation, tapentadol has a low potential for interactions related to phase 2 metabolism. Tapentadol neither inhibits nor induces cytochrome P450 (CYP450) enzymes and has low plasma-protein binding (~ 20%) and no metabolites [90]. Many analgesics, such as acetaminophen, acetylsalicylic acid, and naproxen, as well as other drugs such as omeprazole, may be administered concomitantly with tapentadol with no drug-drug interactions likely. However, pharmacodynamic drug-drug interactions may occur with the concomitant use of other central nervous system (CNS) depressants, including benzodiazepines, antipsychotics, opioids, alcohols, and antihistamines [89].
Since geriatric patients suffer from higher rates of constipation than younger patients, generally due in part to age-related changes in anorectal physiology, opioid-induced constipation can emerge as a treatment-limiting side effect in this population [92]. For this reason, the lower rates of constipation observed with tapentadol, in general and in the elderly populations, is an important factor in analgesic selection.
Patients with Renal or Hepatic Dysfunction
Tapentadol has not yet been evaluated for use in patients with severely compromised renal or hepatic function [18] and thus should not be used in this population. Patients with mild hepatic dysfunction may be administered tapentadol PR with no dosage adjustments, but those with moderate hepatic impairment should be started at the lowest possible dose strength and should not be administered tapentadol more than once over a 24-h period [90].
Hypertensives
In a study of 1464 chronic pain patients with hypertension who received either tapentadol PR, oxycodone CR, or placebo to manage their chronic knee OA or cLBP, tapentadol PR (100–250 mg twice daily) was not associated with any clinically meaningful changes in blood pressure or heart rate [93].
Conclusion
Tapentadol is a novel agent about which much can still be learned. It appears to offer similar effectiveness in the treatment of severe chronic non-cancer pain syndromes associated with LBP and OA as oxycodone, but with better tolerability. Indeed, its lower incidence of side effects makes tapentadol a particularly promising drug in that long-term analgesic therapy can be compromised by adverse events. The abuse liability of tapentadol does not appear to be greater (and might be less) than that of other strong opioids. Tapentadol extended release is a promising new analgesic agent, in particular for treating chronic severe pain associated with LBP and OA.
Acknowledgements
Funding
No funding or sponsorship was received for this study or the article processing charges.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Joseph V. Pergolizzi, Robert Taylor, Jr, Jo Ann LeQuang, Robert B. Raffa, and John Bisney have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to 10.6084/m9.figshare.6021903.
References
- 1.Centers for Disease Control and Prevention. Diabetes: working to reverse the US epidemic, at a glance 2016. Diabetes 2016; https://www.cdc.gov/chronicdisease/resources/publications/aag/diabetes.htm. Accessed Feb 24, 2017.
- 2.American Cancer Society. Cancer prevalence: how many people have cancer. Cancer Basics. 2016; https://www.cancer.org/cancer/cancer-basics/cancer-prevalence.html. Accessed Feb 24, 2017.
- 3.Centers for Disease Control and Prevention. HIV in the United States: at a glance. HIV/AIDs. 2016; https://www.cdc.gov/hiv/statistics/overview/ataglance.html. Accessed Feb 24, 2017.
- 4.Relieving pain in America: a blueprint for transforming prevention, care, education, and research. 2011. http://www.iom.edu/~/media/Files/Report%20Files/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research/Pain%20Research%202011%20Report%20Brief.pdf. Accessed July 17, 2013. [PubMed]
- 5.Johns Hopkins Medicine. Cardiovascular disease statistics. Health Library. 2017; http://www.hopkinsmedicine.org/healthlibrary/conditions/cardiovascular_diseases/cardiovascular_disease_statistics_85,P00243/. Accessed Feb 24, 2017.
- 6.Auret K, Schug SA. Underutilisation of opioids in elderly patients with chronic pain: approaches to correcting the problem. Drugs Aging. 2005;22(8):641–654. doi: 10.2165/00002512-200522080-00002. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease C, Prevention CDC grand rounds: prescription drug overdoses—a US epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10–13. [PubMed] [Google Scholar]
- 8.Dowell D, Haegerich T. R C. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 9.Pergolizzi JV, Jr, Raffa RB, LeQuang JA. The Centers for Disease Control and Prevention opioid guidelines: potential for unintended consequences and will they be abused? J Clin Pharm Ther. 2016;41(6):592–593. doi: 10.1111/jcpt.12444. [DOI] [PubMed] [Google Scholar]
- 10.Cassels A. The opioid crisis: facts that news coverage is missing. Health News Review. 2016; http://www.healthnewsreview.org/2016/01/the-opioid-crisis-facts-that-news-coverage-is-missing/. Accessed Feb 24, 2017.
- 11.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Arthritis in America. Vital Signs. 2017; https://www.cdc.gov/vitalsigns/arthritis/index.html. Accessed Mar 21, 2017.
- 13.Centers for Disease Control and Prevention. NIHS arthritis surveillance: arthritis prevalence in women and men. 2010. http://www.cdc.gov/arthritis/data_statistics/national_nhis.htm#excess. Accessed Jan 8, 2014.
- 14.Centers for Disease Control and Prevention. Arthritis: National Statistics. Arthritis. 2016. https://www.cdc.gov/arthritis/data_statistics/national-statistics.html. Accessed Feb 24, 2017.
- 15.Kress HG. Tapentadol and its two mechanisms of action: is there a new pharmacological class of centrally-acting analgesics on the horizon? Eur J Pain (London, England). 2010;14(8):781–83. [DOI] [PubMed]
- 16.World Health Organization. WHO’s pain ladder for adults. 1988. http://www.who.int/cancer/palliative/painladder/en/. Accessed May 7, 2013.
- 17.Pergolizzi J, Alon E, Baron R, et al. Tapentadol in the management of chronic low back pain: a novel approach to a complex condition? J Pain Res. 2011;4:203–210. doi: 10.2147/JPR.S19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasero C. Tapentadol for multimodal pain management. J Perianesth Nurs. 2011;26(5):343–346. doi: 10.1016/j.jopan.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Games G, Hutchison A. Tapentadol-ER for the treatment of diabetic peripheral neuropathy. Consult Pharm. 2013;28(10):672–675. doi: 10.4140/TCP.n.2013.672. [DOI] [PubMed] [Google Scholar]
- 20.Kern KU, Bialas P, Fangmann D. Prolonged-release tapentadol for phantom pain. A case series. Schmerz (Berlin, Germany). 2013;27(2):174–81. [DOI] [PubMed]
- 21.Taylor R, Pergolizzi JV, Raffa RB. Tapentadol extended release for chronic pain patients. Adv Ther. 2013;30(1):14–27. doi: 10.1007/s12325-013-0002-y. [DOI] [PubMed] [Google Scholar]
- 22.Sadeghi M, Tzschentke TM, Christie MJ. μ-Opioid receptor activation and noradrenaline transport inhibition by tapentadol in rat single locus coeruleus neurons. Br J Pharmacol. 2015;172(2):460–468. doi: 10.1111/bph.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres-Sanchez S, Alba-Delgado C, Llorca-Torralba M, Mico JA, Berrocoso E. Effect of tapentadol on neurons in the locus coeruleus. Neuropharmacology. 2013;72:250–258. doi: 10.1016/j.neuropharm.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 24.Christoph T, Schroder W, Tallarida RJ, De Vry J, Tzschentke TM. Spinal-supraspinal and intrinsic mu-opioid receptor agonist-norepinephrine reuptake inhibitor (MOR-NRI) synergy of tapentadol in diabetic heat hyperalgesia in mice. J Pharmacol Exp Ther. 2013;347(3):794–801. doi: 10.1124/jpet.113.207704. [DOI] [PubMed] [Google Scholar]
- 25.Meske DS, Xie JY, Oyarzo J, Badghisi H, Ossipov MH, Porreca F. Opioid and noradrenergic contributions of tapentadol in experimental neuropathic pain. Neurosci Lett. 2014;562:91–96. doi: 10.1016/j.neulet.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzschentke TM, Folgering JH, Flik G, De Vry J. Tapentadol increases levels of noradrenaline in the rat spinal cord as measured by in vivo microdialysis. Neurosci Lett. 2012;507(2):151–155. doi: 10.1016/j.neulet.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Schroder W, Tzschentke TM, Terlinden R, et al. Synergistic interaction between the two mechanisms of action of tapentadol in analgesia. J Pharmacol Exp Ther. 2011;337(1):312–320. doi: 10.1124/jpet.110.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gohler K, Brett M, Smit JW, Rengelshausen J, Terlinden R. Comparative pharmacokinetics and bioavailability of tapentadol following oral administration of immediate- and prolonged-release formulations. Int J Clin Pharmacol Ther. 2013;51(4):338–348. doi: 10.5414/CP201722. [DOI] [PubMed] [Google Scholar]
- 29.Singh DR, Nag K, Shetti AN, Krishnaveni N. Tapentadol hydrochloride: a novel analgesic. Saudi J Anaesth. 2013;7(3):322–326. doi: 10.4103/1658-354X.115319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zannikos PN, Smit JW, Stahlberg HJ, Wenge B, Hillewaert VM, Etropolski MS. Pharmacokinetic evaluation of tapentadol extended-release tablets in healthy subjects. J Opioid Manag. 2013;9(4):291–300. doi: 10.5055/jom.2013.0171. [DOI] [PubMed] [Google Scholar]
- 31.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain (London, England). 2010;14(4):339. [DOI] [PubMed]
- 32.Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain. 2014;155(4):663–665. doi: 10.1016/j.pain.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Martini C, van Velzen M, Drewes A, Aarts L, Dahan A, Niesters M. A randomized controlled trial on the effect of tapentadol and morphine on conditioned pain modulation in healthy volunteers. PLoS One. 2015;10(6):e0128997. doi: 10.1371/journal.pone.0128997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshpande A, Furlan A, Mailis-Gagnon A, Atlas S, Turk D. Opioids for chronic low-back pain. Cochrane Database Syst Rev (Online). 2007(3):CD004959. [DOI] [PubMed]
- 35.Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev (Online). 2013;8:CD004959. [DOI] [PMC free article] [PubMed]
- 36.Baron R, Likar R, Martin-Mola E, et al. Effectiveness of tapentadol prolonged release (PR) compared with oxycodone/naloxone PR for the management of severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, phase 3b/4 study. Pain Pract. 2016;16(5):580–599. doi: 10.1111/papr.12308. [DOI] [PubMed] [Google Scholar]
- 37.Baron R, Jansen JP, Binder A, et al. Tolerability, safety, and quality of life with tapentadol prolonged release (PR) compared with oxycodone/naloxone PR in patients with severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, phase 3b/4 trial. Pain Pract. 2016;16(5):600–619. doi: 10.1111/papr.12361. [DOI] [PubMed] [Google Scholar]
- 38.Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of Tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010;30(8):489–505. doi: 10.2165/11533440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Buynak R, Shapiro DY, Okamoto A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled Phase III study. Expert Opin Pharmacother. 2010;11(11):1787–1804. doi: 10.1517/14656566.2010.497720. [DOI] [PubMed] [Google Scholar]
- 40.Kavanagh S, Kwong WJ, Hammond GC, Nelson W, Upmalis D, Yang M. Pain relief and tolerability balance of immediate release tapentadol or oxycodone treatment for patients with moderate to severe osteoarthritis or low back pain. Pain Med (Malden, Mass). 2012;13(9):1110–20. [DOI] [PubMed]
- 41.Baron R, Kern U, Muller M, Dubois C, Falke D, Steigerwald I. Effectiveness and tolerability of a moderate dose of tapentadol prolonged release for managing severe, chronic low back pain with a neuropathic component: an open-label continuation arm of a randomized phase 3b study. Pain Pract. 2015;15(5):471–486. doi: 10.1111/papr.12199. [DOI] [PubMed] [Google Scholar]
- 42.Steigerwald I, Schenk M, Lahne U, Gebuhr P, Falke D, Hoggart B. Effectiveness and tolerability of tapentadol prolonged release compared with prior opioid therapy for the management of severe, chronic osteoarthritis pain. Clin Drug Investig. 2013;33(9):607–619. doi: 10.1007/s40261-013-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galvez R, Schafer M, Hans G, Falke D, Steigerwald I. Tapentadol prolonged release versus strong opioids for severe, chronic low back pain: results of an open-label, phase 3b study. Adv Ther. 2013;30(3):229–259. doi: 10.1007/s12325-013-0015-6. [DOI] [PubMed] [Google Scholar]
- 44.Steigerwald I, Muller M, Kujawa J, Balblanc JC, Calvo-Alen J. Effectiveness and safety of tapentadol prolonged release with tapentadol immediate release on-demand for the management of severe, chronic osteoarthritis-related knee pain: results of an open-label, phase 3b study. J Pain Res. 2012;5:121–138. doi: 10.2147/JPR.S30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos J, Alarcao J, Fareleira F, Vaz-Carneiro A, Costa J. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev (Online). 2015;5:CD009923. [DOI] [PMC free article] [PubMed]
- 46.Afilalo M, Morlion B. Efficacy of tapentadol ER for managing moderate to severe chronic pain. Pain Physician. 2013;16(1):27–40. [PubMed] [Google Scholar]
- 47.Etropolski M, Lange B, Goldberg J, Steup A, Rauschkolb C. A pooled analysis of patient-specific factors and efficacy and tolerability of tapentadol extended release treatment for moderate to severe chronic pain. J Opioid Manag. 2013;9(5):343–356. doi: 10.5055/jom.2013.0177. [DOI] [PubMed] [Google Scholar]
- 48.Riemsma R, Forbes C, Harker J, et al. Systematic review of tapentadol in chronic severe pain. Curr Med Res Opin. 2011;27(10):1907–1930. doi: 10.1185/03007995.2011.611494. [DOI] [PubMed] [Google Scholar]
- 49.Freynhagen R, Baron R, Gockel U, Tolle T. painDETECT: a new screening quesitonnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 50.Baron R, Binder A, Attal N, Casale R, Dickenson AH, Treede RD. Neuropathic low back pain in clinical practice. Eur J Pain (London, England). 2016;20(6):861–73. [DOI] [PMC free article] [PubMed]
- 51.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartleson JD. Evidence for and against the use of opioid analgesics for chronic nonmalignant low back pain: a review. Pain Med (Malden, Mass). 2002;3(3):260–71. [DOI] [PubMed]
- 53.Hanna M, O’Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur J Pain (London, England). 2008;12(6):804–13. [DOI] [PubMed]
- 54.Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin. 2011;27(1):151–162. doi: 10.1185/03007995.2010.537589. [DOI] [PubMed] [Google Scholar]
- 55.Schwittay A, Schumann C, Litzenburger BC, Schwenke K. Tapentadol prolonged release for severe chronic pain: results of a noninterventional study involving general practitioners and internists. J Pain Palliat Care Pharmacother. 2013;27(3):225–234. doi: 10.3109/15360288.2013.816406. [DOI] [PubMed] [Google Scholar]
- 56.Lerner D, Chang H, Rogers WH, et al. Imputing at-work productivity loss using results of a randomized controlled trial comparing tapentadol extended release and oxycodone controlled release for osteoarthritis pain. J Occup Environ Med. 2012;54(8):933–938. doi: 10.1097/JOM.0b013e31825f31a1. [DOI] [PubMed] [Google Scholar]
- 57.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2–guidance. Pain Physician. 2012;15(3 Suppl):S67–S116. [PubMed] [Google Scholar]
- 58.Boudreau D, Von Korff M, Rutter C, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1165–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lange B, Kuperwasser B, Okamoto A, et al. Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain. Adv Ther. 2010;27(6):381–399. doi: 10.1007/s12325-010-0036-3. [DOI] [PubMed] [Google Scholar]
- 60.Steigerwald I, Muller M, Davies A, et al. Effectiveness and safety of tapentadol prolonged release for severe, chronic low back pain with or without a neuropathic pain component: results of an open-label, phase 3b study. Curr Med Res Opin. 2012;28(6):911–936. doi: 10.1185/03007995.2012.679254. [DOI] [PubMed] [Google Scholar]
- 61.Wild JE, Grond S, Kuperwasser B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010;10(5):416–427. doi: 10.1111/j.1533-2500.2010.00397.x. [DOI] [PubMed] [Google Scholar]
- 62.Etropolski M, Van Hove I, Ashworth J, Haufel T. Effiacy and tolerability of tapentadol extended release (ER) in patients with moderate to severe osteoarthritis or low back pain over 2 years of treatments. In: 64th Annual Postgraduate Assembly in Anesthesiology (PGA); December 10–14, 2010; New York, NY.
- 63.Sanchez Del Aguila MJ, Schenk M, Kern KU, Drost T, Steigerwald I. Practical considerations for the use of tapentadol prolonged release for the management of severe chronic pain. Clin Ther. 2015;37(1):94–113. doi: 10.1016/j.clinthera.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Baron R, Eberhart L, Kern K-U, et al. Tapentadol prolonged release for chronic pain: a review of clinical trials and five years of routine clinical practice data. Pain Pract. 2017;17(5):678–700. doi: 10.1111/papr.12515. [DOI] [PubMed] [Google Scholar]
- 65.Pergolizzi J, Alegre C, Blake D, et al. Current considerations for the treatment of severe chronic pain: the potential for tapentadol. Pain Pract. 2012;12(4):290–306. doi: 10.1111/j.1533-2500.2011.00487.x. [DOI] [PubMed] [Google Scholar]
- 66.Varrassi G, Marinangeli F, Piroli A, Coaccioli S, Paladini A. Strong analgesics: working towards an optimal balance between efficacy and side effects. Eur J Pain (London, England). 2010;14(4):340–42. [DOI] [PubMed]
- 67.Merker M, Dinges G, Koch T, Kranke P, Morin AM. Undesired side effects of tapentadol in comparison to oxycodone. A meta-analysis of randomized controlled comparative studies. Schmerz (Berlin, Germany). 2012;26(1):16–26. [DOI] [PubMed]
- 68.Kress HG, Koch ED, Kosturski H, et al. Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain. Pain Physician. 2014;17(4):329–343. [PubMed] [Google Scholar]
- 69.Raffa RB, Buschmann H, Christoph T, et al. Mechanistic and functional differentiation of tapentadol and tramadol. Expert Opin Pharmacother. 2012;13(10):1437–1449. doi: 10.1517/14656566.2012.696097. [DOI] [PubMed] [Google Scholar]
- 70.Vadivelu N, Timchenko A, Huang Y, Sinatra R. Tapentadol extended-release for treatment of chronic pain: a review. J Pain Res. 2011;4:211–218. doi: 10.2147/JPR.S14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartrick CT, Rozek RJ. Tapentadol in pain management: a mu-opioid receptor agonist and noradrenaline reuptake inhibitor. CNS Drugs. 2011;25(5):359–370. doi: 10.2165/11589080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 72.Jeong ID, Camilleri M, Shin A, et al. A randomised, placebo-controlled trial comparing the effects of tapentadol and oxycodone on gastrointestinal and colonic transit in healthy humans. Aliment Pharmacol Ther. 2012;35(9):1088–1096. doi: 10.1111/j.1365-2036.2012.05040.x. [DOI] [PubMed] [Google Scholar]
- 73.Coluzzi F, Pappagallo M. Opioid therapy for chronic noncancer pain: practice guidelines for initiation and maintenance of therapy. Minerva Anesthesiol. 2005;71:425–33. [PubMed]
- 74.Smith HS, Laufer A. Opioid induced nausea and vomiting. Eur J Pharmacol. 2014;722:67–78. doi: 10.1016/j.ejphar.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 75.Xu XS, Etropolski M, Upmalis D, Okamoto A, Lin R, Nandy P. Pharmacokinetic and pharmacodynamic modeling of opioid-induced gastrointestinal side effects in patients receiving tapentadol IR and oxycodone IR. Pharm Res. 2012;29(9):2555–2564. doi: 10.1007/s11095-012-0786-5. [DOI] [PubMed] [Google Scholar]
- 76.Etropolski M, Kelly K, Okamoto A, Rauschkolb C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Adv Ther. 2011;28(5):401–417. doi: 10.1007/s12325-011-0018-0. [DOI] [PubMed] [Google Scholar]
- 77.Kwong WJ, Hammond G, Upmalis D, Okamoto A, Yang M, Kavanagh S. Bowel function after tapentadol and oxycodone immediate release (IR) treatment in patients with low back or osteoarthritis pain. Clin J Pain. 2013;29(8):664–672. doi: 10.1097/AJP.0b013e318274b695. [DOI] [PubMed] [Google Scholar]
- 78.Vorsanger G, Xiang J, Okamoto A, Upmalis D, Moskovitz B. Evaluation of study discontinuations with tapentadol inmmediate release and oxycodone immediate release in patients with low back or osteoarthritis pain. J Opioid Manag. 2010;6(3):169–179. doi: 10.5055/jom.2010.0015. [DOI] [PubMed] [Google Scholar]
- 79.Eichenbaum G, Gohler K, Etropolski M, et al. Does tapentadol affect sex hormone concentrations differently from morphine and oxycodone? An initial assessment and possible implications for opioid-induced androgen deficiency. J Opioid Manag. 2015;11(3):211–227. doi: 10.5055/jom.2015.0270. [DOI] [PubMed] [Google Scholar]
- 80.Merchant S, Provenzano D, Mody S, Ho KF, Etropolski M. Composite measure to assess efficacy/gastrointestinal tolerability of tapentadol ER versus oxycodone CR for chronic pain: pooled analysis of randomized studies. J Opioid Manag. 2013;9(1):51–61. doi: 10.5055/jom.2013.0147. [DOI] [PubMed] [Google Scholar]
- 81.Vorsanger GJ, Klopfer AM, Xiang J, Benson CJ, Moskovitz BL, Rosenthal NR. Immediate-release tapentadol or oxycodone for treatment of acute postoperative pain after elective arthroscopic shoulder surgery: a randomized, phase IIIb study. J Opioid Manag. 2013;9(4):281–290. doi: 10.5055/jom.2013.0170. [DOI] [PubMed] [Google Scholar]
- 82.Guay DR. Is tapentadol an advance on tramadol? Consult Pharm. 2009;24(11):833–840. doi: 10.4140/TCP.n.2009.833. [DOI] [PubMed] [Google Scholar]
- 83.Dart RC, Bartelson BB, Adams EH. Non-medical use of tapentadol immediate release by college students. Clin J Pain. 2014;30(8):685–692. doi: 10.1097/AJP.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 84.Cepeda M, Fife D, Vo L, Mastrogiovanni G, Yuan Y. Comparison of opioid doctor shopping for tapentadol and oxycodone: a cohort study. J Pain Palliat Care Pharmacother. 2013;14:158–164. doi: 10.1016/j.jpain.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 85.Bannwarth B. Will abuse-deterrent formulations of opioid analgesics be successful in achieving their purpose? Drugs. 2012;72(13):1713–1723. doi: 10.2165/11635860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 86.Romach MK, Schoedel KA, Sellers EM. Update on tamper-resistant drug formulations. Drug Alcohol Depend. 2013;130(1–3):13–23. doi: 10.1016/j.drugalcdep.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 87.Pergolizzi JV, Jr, LeQuang JA. Abuse-deterrent formulations of opioid analgesics. Pain Pract. 2014;4(3):204–206. doi: 10.1111/papr.12093. [DOI] [PubMed] [Google Scholar]
- 88.Vosburg SK, Jones JD, Manubay JM, Ashworth JB, Shapiro DY, Comer SD. A comparison among tapentadol tamper-resistant formulations (TRF) and OxyContin(R) (non-TRF) in prescription opioid abusers. Addiction (Abingdon, England). 2013;108(6):1095–1106. [DOI] [PMC free article] [PubMed]
- 89.Sanchez Del Aguila MJ, Schenk M, Kern KU, Drost T, Steigerwald I. Practical considerations for the use of tapentadol prolonged release for the management of severe chronic pain. Clin Ther. 2014;37(1):94–113. doi: 10.1016/j.clinthera.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 90.Janssen Ortho L. Nucynta 50 mg, tapentadol tablet. Gurabo, PR, USA; 2010.
- 91.Biondi DM, Xiang J, Etropolski M, Moskovitz B. Tolerability and efficacy of tapentadol extended release in elderly patients >/= 75 years of age with chronic osteoarthritis knee or low back pain. J Opioid Manag. 2015;11(5):393–403. doi: 10.5055/jom.2015.0289. [DOI] [PubMed] [Google Scholar]
- 92.Vorsanger G, Xiang J, Biondi D, et al. Post hoc analyses of data from a 90-day clinical trial evaluating the tolerability and efficacy of tapentadol immediate release and oxycodone immediate release for the relief of moderate to severe pain in elderly and nonelderly patients. Pain Res Manag journal de la societe canadienne pour le traitement de la douleur. 2011;16(4):245–51. [DOI] [PMC free article] [PubMed]
- 93.Biondi DM, Xiang J, Etropolski M, Moskovitz B. Evaluation of blood pressure and heart rate in patients with hypertension who received tapentadol extended release for chronic pain: a post hoc, pooled data analysis. Clin Drug Investig. 2014;34(8):565–576. doi: 10.1007/s40261-014-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.