Abstract
Risk calculators may simplify the management of ocular hypertension and glaucoma patients and provide evidence-based treatment. Risk calculators are not new to medicine. Medical providers use risk calculators to guide decision-making for the risk of a chromosomal abnormality in neonates, osteoporosis in postmenopausal women, and cardiovascular disease in adults. This manuscript describes the current knowledge of risk calculators in ophthalmology. Clinicians should recognize that the current risk calculators do not include critical information guiding treatment such as life expectancy and patient’s willingness to commit to years of eye drops. Overall, eye care providers should consider the results of a risk calculator as supplemental information when treating an ocular hypertension or glaucoma patient.
Keywords: risk assessment, risk calculator, ocular hypertension treatment study, European Glaucoma Prevention study, Early Manifest Glaucoma trial, glaucoma, ocular hypertension
Introduction
Risk calculators may be an innovative approach to simplify the management of ocular hypertension and glaucoma patients and provide evidence-based treatment. This manuscript describes the what, where, how, and why of risk calculators in ophthalmology. It also identifies two websites to attain the latest risk calculators in several software versions.
Risk calculators in medicine
The Institute of Medicine’s book, Crossing the Quality Chasm, recommends safe, effective, efficient, and patient-centered health care in the 21st century1. Eye care providers need tools to practice this type of health care. A risk calculator may be such a tool.
Risk calculators are not new to medicine. Medical providers use demographic factors and prenatal blood tests to counsel pregnant women regarding the risk of a chromosomal abnormality when compared to the risk of amniocentesis. They use bone scans and demographic factors to help decide whether postmenopausal women require treatment to prevent osteoporosis. Another popular use occurs in internal medicine. The Framingham Heart Study developed a predictive equation to estimate the risk of cardiovascular disease with age, lipid levels, blood pressure, diabetes, and smoking history as explanatory variables.2 Medical providers use these results to decide whether to treat high blood pressure and cholesterol.
Using a risk calculator for ocular hypertension
Approximately 8% of adults over the age of 40 years in the United States have ocular hypertension3. While ocular hypertension is a common finding, eye care providers do not know which patients to treat or which patients to monitor without treatment. To address these issues, the Ocular Hypertension Treatment Study (OHTS) and European Glaucoma Prevention Study (EGPS) determined the baseline factors that predict the onset of primary open angle glaucoma in ocular hypertensive patients.4
Ocular hypertension for the combined OHTS and EGPS analysis4 included age between 30 to 80 years, IOP between 20 mm Hg and 32 mm Hg in both eyes, best-corrected visual acuity of 20/40 or better, normal automated visual fields, normal optic discs, and open angles on gonioscopy. Patients with secondary causes of high IOP (eg, pseudoexfoliation, pigment dispersion syndrome, corticosteroid use, trauma), other intraocular eye disease, history of refractive surgery, or other diseases possibly affecting the visual field (eg, central nervous syndrome tumors) were excluded. Patients with any evidence of diabetic retinopathy documented from a dilated ophthalmoscopic examination were also excluded from the study.
Trying to decide whether to treat an ocular hypertension patient is complex without a risk calculator. The newest OHTS multivariate regression contains five variables that are predictive of developing glaucoma from ocular hypertension: age, corneal thickness, IOP, PSD, and vertical C/D.4 Even if one simplifies the continuous variables of age, corneal thickness, IOP, and PSD into thirds and uses 9 different combinations for C/D (0.0–0.8), 729 (3×3×3×3×9) different results exist for ocular hypertension patients. This creates a large number of different combinations, which is difficult for clinicians to decipher when deciding whether to treat a particular ocular hypertension patient.
This difficulty was highlighted in a recent publication that estimates an ophthalmologist’s ability to predict the risk of glaucoma in ocular hypertensive patients.5 Ophthalmologists had the benefit of an oral review and written handouts summarizing the OHTS results. Ophthalmologists tended to underestimate the risk when compared to the actual risk found by a risk calculator. They also had a large range of predictions, sometimes differing from the actual risk by 40%. (Figure 1) In general, this study shows that eye care providers may frequently over- or undertreat their ocular hypertensive patients because of this difficulty in risk assessment.
Figure 1.
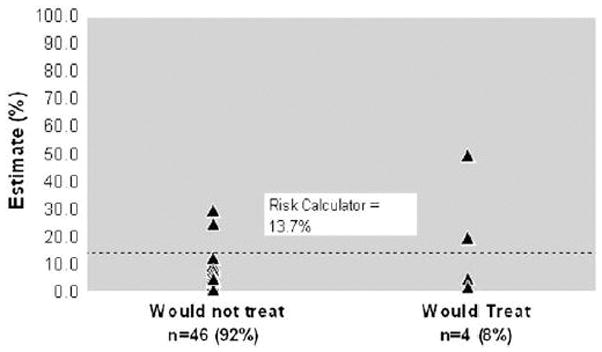
Scatterplot of ophthalmologists’ estimate of risk (%) and whether or not they would treat. The dashed line within the figure represents the risk calculator estimate. Reproduced from Mansberger, et al. J Glaucoma. 2006 Oct;15(5):426–31.
Benefits to Patients
A risk calculator may provide benefits to patients regarding compliance. The OHTS risk calculator provides clinicians an individualized estimate of susceptibility to developing glaucoma in five years for a patient; thus risk calculators encourage treatment to be patient-centered rather than population-based. In other words, patients are more likely to adhere to therapy if they have a more definitive expectation of risk, rather than something vague, such as “higher or lower” risk. Thus, risk calculation may strengthen the physician-patient relationship and enhance compliance.
Clearly, providers should avoid treating every ocular hypertension patient. A risk calculator can help select those patients who will most benefit from treatment because of their high risk of developing glaucoma. It can also determine which patients have a low risk of developing glaucoma and should not be treated. Finally, when a calculator determines a borderline risk, the provider’s experience and the patient’s input can provide guidance regarding whether to treat.
Benefits to society of a Risk Calculator
Risk calculators may simultaneously save money and decrease blindness. For example, some providers treat all ocular hypertensive patients based on the OHTS study results. This produces excess costs to society. The five-year cumulative probability of developing glaucoma was 9.5% in the untreated group and 4.4% in the treated group. This results in a relative risk reduction of 54%((9.5–4.4)÷9.5) and an absolute risk reduction of 5.1% (9.5–4.4). In this example, the number needed to treat (NNT) is 20 (1/0.051) persons to prevent 1 person from developing glaucoma. If one uses visual field loss (a outcome more likely to be associated with decreased quality of life) as the criteria for glaucoma, the NNT increases to 42 persons! The main reason for these high NNT values is low risk of developing glaucoma for the majority of ocular hypertension patients.
The excess 5-year cost to prevent one ocular hypertensive patient from developing glaucoma is $54,000 with an NNT of 20 and $113,000 with an NNT of 42 (assuming effective monotherapy with a $20 beta-adrenergic antagonist and two extra office visits per year). Substituting an $80 prostaglandin analogue for treatment increases the cost to $126,000 with an NNT of 20 and $264,600 with an NNT of 42. Clinicians need to consider these costs as well as side effects, efficacy, and convenience when choosing an ocular hypotensive medication for treatment.
An economic analyses6 has shown that not all patients require treatment of ocular hypertension, just those with higher risk. This manuscript showed a probability of developing glaucoma of greater than 2% per year (or 10% over 5 years) as the threshold for cost-effective treatment of ocular hypertension patients.
Validation of Risk Calculators
The development of risk calculators involves use of statistical methods to develop models for prediction of outcome using one or more explanatory variables. However, a model that is derived from a particular dataset is not guaranteed to work on a different group of patients. In fact, the performance of regression models (or risk calculators) used as diagnostic or prediction tools is generally better on the dataset on which the model has been constructed (derivation set) compared to the performance of the same model on new data. Therefore, before risk calculators can be successfully incorporated into clinical practice they need to be validated on different populations. By validation we mean establishing that the risk calculator works satisfactorily for patients other than those from whose data the model was derived.
In 2005, we published the results on the development and validation of a risk calculator to assess the risk of an ocular hypertensive patient to develop glaucoma.7 The risk calculator was derived based on the results published by the OHTS4, 8 and incorporated the variables that were described by that study as being significantly associated with the risk of developing glaucoma over time, that is, age, IOP, CCT, PSD, vertical cup/disc ratio and diabetes mellitus. The risk calculator was designed to estimate the chance of an ocular hypertensive patient to develop glaucoma if left untreated for 5 years. It was subsequently validated on an independent population of 126 patients with ocular hypertension who were followed as part of a prospective longitudinal study conducted at the University of California San Diego (DIGS – Diagnostic Innovations in Glaucoma Study).
Several steps were taken to validate the OHTS-derived model. In the first step, the importance of the prognostic variables that had been previously identified by the OHTS study was evaluated on the new data set (DIGS data set). All the variables had similar performance, except for diabetes mellitus, which was not significantly associated with the risk of developing glaucoma in the DIGS data. Subsequently, the predictive performance of the model was investigated on the new data set. The ability of the OHTS-derived risk calculator to discriminate DIGS subjects who developed glaucoma from those who did not was reasonably good with a c-index of approximately 0.7. The c-index is a measure of the discriminating ability of a model (similar to the area under the Receiver Operating Characteristic [ROC] curve) and a c-index of 0.7 indicates that, in approximately 70% of the cases, the model allocated a higher predicted probability for a subject who actually developed glaucoma than for a subject who did not. The closer the c-index gets to 1, the better the discriminating ability of the model. The values of c-index found for the OHTS-derived risk calculator when applied to DIGS subjects were similar to those found when risk models such as the Framingham coronary prediction scores are used to predict coronary heart disease events.4, 9 D’Agostino et al. reported c-indexes ranging from 0.63 to 0.83 when the Framingham functions were applied to 6 different cohorts of patients.4
The OHTS-derived risk calculator also had a good calibration when applied to the DIGS data set (Figure 2). Checking calibration is another important step in validating a predictive model. A reliable or well-calibrated model will give predicted probabilities that agree numerically with the actual outcomes. For example, suppose a group of 100 ocular hypertensive patients. If the model assigns an average probability of 12% for conversion to glaucoma for this group of subjects, it is expected that approximately 12 subjects will convert to glaucoma over time. That is, for a well-calibrated model, the predicted probabilities of conversion to glaucoma will agree closely with the observed probabilities of conversion. Figure 2 shows that the OHTS-derived risk calculator performed well on the DIGS data set. For patients in whom the model predicted a high chance of converting to glaucoma, there was a high observed conversion rate; whereas for patients in whom the model predicted a low conversion rate, there was a low observed conversion rate.
FIGURE 2.
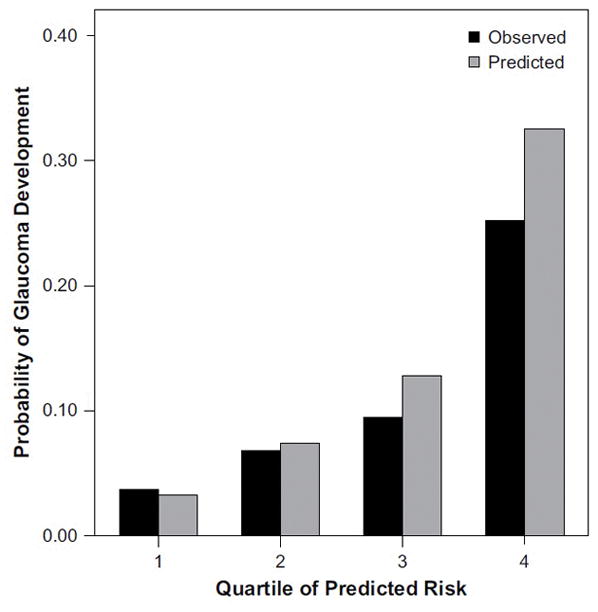
Comparison of observed and predicted 5-year incidence of glaucoma when the OHTS-derived risk calculator was applied to the DIGS (Diagnostic Innovations in Glaucoma Study) data set. Predictied probabilities agreed closed with observed outcomes. Reproduced from Medeiros et al. Arch Ophthalmol 2005; 123:1351–60.
In 2007, we validated the OHTS derived risk calculator in a large independent European sample of patients with ocular hypertension4. These analyses were based on nearly 5 years of follow-up data from 717 OHTS patients who did not receive hypotensive treatment and 406 patients in placebo group of the European Glaucoma Prevention Study (EGPS). The European sample provided the strongest evidence to date of the generalizeability of the OHTS derived risk calculator to other patient populations with ocular hypertension. Not only did independent analyses of the EGPS data identify the same predictors that increased the risk of POAG (older age, higher IOP, thiner CCT, larger cup-to-disc ratio, and higher PSD), but the risk coefficients of each of these predictors did not differ from each other (p values of 0.53, 0.49, 0.89, 0.96 and 0.55 respectively). In these pooled analyses, diabetes was not found to be a risk factor for the development of POAG and was dropped from the risk model. The risk model from the pooled OHTS/EGPS sample of over 1,100 ocular hypertensive patients demonstrated excellent fit with the observed data with a c-statistic of 0.74 and the calibration statistic was 7.05.4 The large sample substantially increases the statistical precision of coefficient estimates and thereby reduces the errors of estimating risk.
To simplify application of the risk model to an individual patient with ocular hypertension, one can obtain the risk calculator free of charge at http://ohts.wustl.edu/risk (web-based and Adobe Acrobat®) and www.deverseye.org/grc_web/grc.cfm (web-based, Palm®, C++, Pocket PC ®) and MAC® versions). As new factors for the development of glaucoma are identified, the risk calculator will need to be modified.
Risk Assessment for Progressive Glaucoma
The Early Manifest Glaucoma Trial (EMGT) randomized patients with early glaucoma either to argon laser trabeculoplasty plus betaxolol (n = 129) or to monitoring without immediate treatment (n = 126)10. These were newly diagnosed glaucoma patients, found during a community glaucoma screening. The rate of progression was 45% in the treated group versus 62% in the untreated group. The treatment reduced intraocular pressure approximately 20% and decreased the risk of worsening glaucoma by 50%.11 The study identified elevated IOP, exfoliation, bilateral disease, worse mean deviation with perimetry and older age as baseline risk factors for glaucoma progression, and central corneal thickness (CCT) was not found to be a risk factor. During follow-up, disc hemorrhages increased the risk of progression.
The EMGT recently published the predictors of long-term progression (mean 8 years) in patients with early glaucoma.12 Their results are similar to those reported above, but now showed an increased risk of glaucomatous progression with thin CCT and decreased perfusion pressure. The results were interesting in that statistical interaction between CCT and IOP occurred- with CCT only a risk factor in those with an IOP >21, not those with IOP <21. Overall, interpretation of these results is more difficult than the OHTS study and the study has a smaller sample size. Therefore, a risk assessment equation may be less precise.
An EMGT risk calculator could identify the patients at highest risk for glaucomatous progression. These patients could be monitored closely and treated more aggressively when compared to a patient who is unlikely to progress. Finally, risk calculators could identify those who would benefit from a specific type of treatment, such as ocular hypotensive medications, argon laser trabeculoplasty, and surgery. Overall, this may decrease the risk of blindness in glaucoma patients. The authors of the EMGT have not yet released a risk assessment equation.
Caveats of risk calculators
A recent study suggested that in a critical care setting, clinicians may not change their treatment based on a risk calculator.13 This randomized, clinical trial showed that even when a risk model predicted an ICU patient would die within a week, doctors rarely used this information to obtain an end-of-life recommendation from the family13. The authors suggested that the doctors were unwilling to apply results from the calculator due to “inertia” and “lack of incentives” with the current situation. We believe that implementation of risk assessment would differ between a critical care setting and an ophthalmologist’s office, but providing a risk assessment tool does not guarantee adoption by clinicians.
Risk assessment and calculation has several other limitations. Risk calculators are based on the best available information, thus their use should be restricted to patients that are similar to the inclusion criteria of the study. For example, with regards to a risk calculator based on the OHTS, ophthalmologists should not assume that eyes with secondary causes of ocular hypertension, such as pseudoexfoliation, juvenile open angle glaucoma, chronic angle closure glaucoma, pigmentary dispersion syndrome, etc, will have similar risk factors to the OHTS study population. A risk calculator can be imprecise if the representative patient has a rare combination of characteristics such as diabetes, a smaller C/D, a thicker cornea, and older age. This rare combination creates a lack of similar patients, inadequate sample size for comparison, and a large confidence interval for the log odds.
Clinicians need to understand that a risk calculator provides a mean risk based on a group of patients with similar characteristics. Rare combinations in OHTS, such as a cup-to-disc ratio less than 0.2, an intraocular pressure above 29 mm Hg, or an age of above 70 years, will result in larger confidence intervals around individual estimates and therefore less precise estimates.14
The OHTS patients may not accurately represent a typical ocular hypertension patient in an eye care provider office and they may have a lower or higher probability of developing glaucoma. These differences may occur because the OHTS patients received free medications and clinical visits, were reliable visual field takers, and were apparently compliant with their medications and follow-up. These characteristics are typical of research study patients, but uncommon in the clinical realm. Thus, validation in additional patient populations and everyday clinical settings would enhance the ability to generalize the risk calculators.
Finally, clinicians should use more than the results of a risk calculator to decide whether or not to treat a patient. The current risk calculators do not include important information guiding treatment such as medical health and life expectancy, patient’s willingness to commit to years of eye drops, cost, and the effect of quality of life with treatment. Overall, eye care providers should consider the results of a risk calculator as supplemental information when treating ovular hypertension or glaucoma patient.
Conclusion
Predicting the development of glaucoma from ocular hypertension is a cornerstone to deciding on whether or not to treat. Risk calculators may be an innovative approach to simplify the management of ocular hypertension and glaucoma patients and provide evidence-based treatment. They provide benefits to patients, clinicians, and society as a whole. However, eye care providers should recognize that risk assessment is still evolving and requires refinement. Therefore, eye care providers should consider the result of a risk calculator as supplemental information when deciding whether to treat.
Methods of Literature Search
The authors performed a Pubmed search using the terms ocular hypertension, glaucoma, risk calculator, risk assessment, prediction model, from the period 1970 until 2007. They included pertinent articles from their personal collection if not contained within the above search. Their search did not include any articles written in a language other than English.
Acknowledgments
Support: NEI 3 K23 EY0155501-01 (SLM), NEI EY09341, EY09307 (MG), Merck Research Laboratories (MG), Research to Prevent Blindness (MG)
Footnotes
Financial Interests: Steven L. Mansberger, MD, MPH (none), Felipe Medeiros, MD, PhD (none), Mae Gordon, PhD (none)
References
- 1.Institute of Medicine (U.S.) Crossing the quality chasm : a new health system for the 21st century. Washington, D.C: National Academy Press; 2001. Committee on Quality of Health Care in America; p. xx.p. 337. [PubMed] [Google Scholar]
- 2.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 3.Tielsch JM, Katz J, Singh K, et al. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol. 1991;134:1102–10. doi: 10.1093/oxfordjournals.aje.a116013. [DOI] [PubMed] [Google Scholar]
- 4.Gordon MO, Torri V, Miglior S, et al. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–9. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansberger SL, Cioffi GA. The probability of glaucoma from ocular hypertension determined by ophthalmologists in comparison to a risk calculator. J Glaucoma. 2006;15:426–31. doi: 10.1097/01.ijg.0000212258.02702.0c. [DOI] [PubMed] [Google Scholar]
- 6.Kymes SM, Kass MA, Anderson DR, Miller JP, Gordon MO. Management of ocular hypertension: a cost-effectiveness approach from the Ocular Hypertension Treatment Study. Am J Ophthalmol. 2006;141:997–1008. doi: 10.1016/j.ajo.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–60. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 8.Coleman AL, Gordon MO, Beiser JA, Kass MA. Baseline risk factors for the development of primary open-angle glaucoma in the Ocular Hypertension Treatment Study. Am J Ophthalmol. 2004;138:684–5. doi: 10.1016/j.ajo.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Hong Y, D’Agostino RB, Sr, et al. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. Jama. 2004;291:2591–9. doi: 10.1001/jama.291.21.2591. [DOI] [PubMed] [Google Scholar]
- 10.Leske MC, Heijl A, Hyman L, Bengtsson B. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology. 1999;106:2144–53. doi: 10.1016/s0161-6420(99)90497-9. [DOI] [PubMed] [Google Scholar]
- 11.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 12.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 13.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT) The SUPPORT Principal Investigators. Jama. 1995;274:1591–8. [PubMed] [Google Scholar]
- 14.Hosmer DW, Lemeshow S. Applied survival analysis : regression modeling of time to event data. New York: Wiley; 1999. p. xiii.p. 386. [Google Scholar]


