Abstract
Aim:
The aim of the present study was to investigate the viability of lactic acid bacteria isolated from traditional cheeses and cocultured in Iranian white cheese during ripening.
Materials and Methods:
A total of 24 samples were isolated from 8 types of traditional cheeses in West Azerbaijan, Iran. Isolated species were cocultured with starter bacteria during the production of Iranian white cheese, and their viability was investigated up to 60 days of the refrigerated storage.
Results:
Of 118 isolates of Lactobacillus, 73 isolates (62%) were confirmed as facultative heterofermentative and 45 isolates (38%) as obligate homofermentative. Of the facultative heterofermentatives, 28 isolates (24%) were Lactobacillus plantarum, 24 isolates (20%) were Lactobacillus casei, and 21 isolates (18%) were Lactobacillus agilis. Obligate homofermentatives were Lactobacillus delbrueckii (21%), Lactobacillus helveticus (14%), and Lactobacillus salivarius (3%). L. plantarum, L. casei and L. helveticus were found in high enough levels(106 CFU/g).
Conclusion:
According to the obtained data, it is recommended that complex starters such as L. helveticus, L. plantarum, and L. casei can be used in industrial productions of cheese to obtain exclusive properties of traditional cheeses.
Keywords: heterofermentative, Lactobacillus, probiotic, starter, traditional cheeses
Introduction
Cheeses made from pasteurized milk have better hygienic quality and uniform texture than traditional ones; however, pasteurization has some devastating effects on the quality of the cheese as it can destroy the flavor-producing microorganisms. To overcome this problem, traditional starters are added after pasteurization [1]. Therefore, knowing the composition of lactic flora, naturally present in traditional cheeses, provides safe and standard starters while maintaining the essential characteristics [2].
Since West Azerbaijan Province (Iran) has environmental and ethnic diversity, there are varieties of traditional foods, particularly dairy products, in this region [3]. There are different kinds of cheese traditionally produced in this region. Urmia Koopeh Cheese is a fermented milk product and one of the most traditional and popular cheese in the West and North-West of Iran, Turkey, and Northern areas of Iraq. It is a semisoft sheep cheese and is produced without using any starter. Another well-known cheese in this area is Lighvan, which is a soft cheese (60% humidity) traditionally produced from raw sheep and goat milk without adding any starter culture [4], the fermentation also depends on the lactic flora of the raw milk. Lighvan cheese is one of the most common dairy products in Iran [5]. Shal cheese, which is also called fresh cheese, is produced traditionally in Urmia in West Azerbaijan province in large casts and has popularity in this region. Salty cheese is another milk product, traditionally made from boiled buttermilk along with salt. In this process, the clot-on-boiling is filtered, and consequently, the result is a salty cheese with particular aroma and flavor [5].
The traditional production of cheese and the replacement of traditional lactic acid bacteria with a variety of non-native microflora are a future possibility in dairy technology; therefore, it would be encouraging for researchers to collect different strains and species of lactic acid bacteria from different dairy products obtained from various regions.
The aim of the current study was to isolate Lactobacillus species from different traditional cheeses of West Azerbaijan province, Iran, to produce a white cheese with a uniform texture and a flavor similar to that of traditional cheeses as well as determining starter’s viability in dairy products.
Materials and Methods
Ethical approval
This study was approved by the Research Committee of Urmia University, Urmia, Iran.
Sampling
A total of 24 samples (250 g) were collected from 8 traditional cheeses of different geographical regions in West Azerbaijan (Urmia, Bukan, Mahabad, Sardasht, and Maku), Iran, as follow: Triplicate samples from five different types of Koopeh cheese (15 samples), Shal fresh cheese (3 samples), Lighvan cheese (3 samples), and Salty cheese (3 samples). Samples were kept at 4°C for further analysis.
Isolation of lactic acid bacteria
Amount of 25 g of each sample was aseptically weighted and homogenized with 225 ml of sodium citrate using a stomacher (Seward Stomacher 400 Circulator, UK). Samples were subsequently diluted (1:10) using sterile peptone water and 0.1 ml from each dilution was sub-cultured on Man Rogosa and Sharpe (MRS) agar (Merck, Germany) used for isolating lactic acid bacteria [6]. Plates were incubated at 30°C and 37°C for mesophilic lactic acid bacteria and at 42°C for thermophilic lactic acid bacteria for 48 h. MRS agar plates were incubated anaerobically using the gas pack systems (Anaerocult C, Merck, Germany). To differentiate homo- and heterofermentative strains, all the isolates were tested for gram reaction, catalase production, and carbohydrates fermentation (homofermentatives showed ribose +, sorbitol +, raffinose ±, lactose +, galactose +, maltose +, melibiose ±, rhamnose −, xylose ±, manose +, salicine +, sorbose +, glucose +, and sucrose + pattern, heterofermentatives showed ribose +, sorbitol ±, raffinose ±, lactose ±, galactose ±, maltose +, melibiose ±, rhamnose ±, xylose −, manose +, salicine ±, sorbose ±, glucose +, and sucrose±pattern). Cimon citrate test was also conducted as a differential test [7,8].
Iranian white cheese preparation
Starter (Chr. Hansen R 704: Lactococcus lactis Subsp., cremoris and L. lactis Subsp., diacetyl lactis) and isolated Lactobacillus species were added (0.5% w/v and 109 CFU/ml, respectively) to each vat of pasteurized cow milk (65°C for 30 min) separately. A solution of 20 g. 100/L CaCl2 was added to each batch, and the fermentation continued for 2-4 h until the pH reached to 6.1-6.2. Then, 0.001% w/v of rennet was added to each vat to coagulate the milk. To improve the efficiency of rennet, the milk temperature was maintained at about 35°C during the formation of cheese clots. After coagulation, the curds were divided into small cubes (1-2 cm3) and pressed. Cheese samples were allowed to rest in 20% w/v brine salt for 8 h and in 8% sterile brine-salt for 15 days at 12-14°C. Final ripening applied for 45 days at 4°C [9]. The manufacture of Iranian white cheese was performed in triplicate for each treatment. Lactobacillus count was performed 1 h after inoculation and on days 3, 7, 15, 30, 45, and 60.
Lactobacillus viable cell counts
For viability assessment of probiotic bacteria during 60 days of storage at refrigerator temperature, 10 g of cheeses were homogenized with 90 mL of sterile sodium citrate using a stomacher. Decimal dilutions in peptone water were prepared and plated on MRS agar (Merck, Germany). Plates were incubated at 37°C for 48 h [9]. Finally, colonies were Gram stained, and catalase production, carbohydrates fermentation, and Cimon citrate test were performed.
Results
A total of 118 Lactobacillus isolates were identified from 4 different kinds of cheese. Phenotypic characterization of isolates, such as gram reaction, catalase production, and carbohydrates fermentation (Table-1), resulted in the identification of 73 isolates (62%) of Lactobacillus as facultative heterofermentative and 45 isolates (38%) as obligate homofermentative.
Table-1.
Results of Gram staining and biochemical tests of Lactobacillus species.
| Tests | Bacterial strain | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Gram staining | + | + | + | + | + | + |
| Ribose | + | + | + | + | + | + |
| Sorbitol | + | + | + | − | + | + |
| Raffinose | + | − | + | − | − | + |
| Lactose | + | + | + | − | + | + |
| Galactose | + | + | + | + | − | + |
| Maltose | + | + | + | + | + | + |
| Melibiose | + | − | − | + | − | + |
| Rhamnose | − | − | − | + | − | − |
| Xylose | − | − | + | − | − | − |
| Manose | + | + | + | + | + | + |
| Salicine | + | + | + | − | + | + |
| Sorbose | + | + | + | + | − | + |
| Glucose | + | + | + | + | + | + |
| Sucrose | + | + | + | − | + | + |
| CO2 production from glucose | + | − | + | − | − | + |
| Growth at 30° | + | + | + | − | − | + |
| Growth at 37° | + | + | − | + | − | + |
| Growth at 42° | − | − | − | + | + | − |
| Catalase | − | − | − | − | − | − |
| Citrate | + | − | − | + | − | + |
1=Lactobacillus plantarum, 2=Lactobacillus casei, 3=Lactobacillus agilis, 4=Lactobacillus helveticus, 5=Lactobacillus delbrueckii, 6=Lactobacillus salivarius
Facultative heterofermentatives were composed of 28 isolates (24%) of Lactobacillus plantarum, 24 isolates (20%) of Lactobacillus Casei, and 21 isolates (18%) of Lactobacillus agilis. Obligate homofermentative Lactobacillus species were composed of 25 isolates (21%) of Lactobacillus delbrueckii, 16 isolates (14%) of Lactobacillus helveticus, and 4 isolates (3%) of Lactobacillus salivarius. 58 Lactobacillus isolates (49%) were identified at 30°C, 15 isolates (13%) at 37°C, and 45 isolates (38%) at 42°C. Distribution of Lactobacillus isolates in traditional cheeses is shown in Figure-1.
Figure-1.
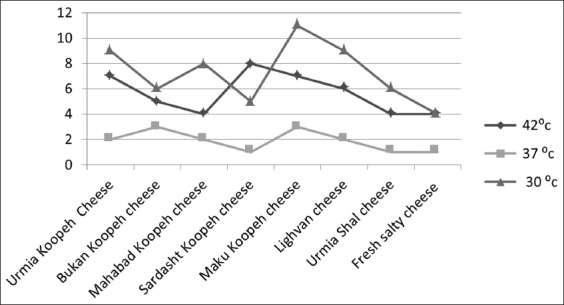
Lactobacillus isolates in various traditional cheeses at different temperatures.
Lactobacillus viability of probiotic white cheese samples during 60 days of storage is presented in Table-2. The viability of L. helveticus, L. plantarum, and L. casei was observed above 6 Log CFU/g, throughout 60 days of storage period. L. plantarum and L. casei were different from L. helveticus, and the differences were statistically significant (p<0.05). Other species declined below 6 log CFU/g after 30 days of storage (Table-2).
Table-2.
Lactobacillus viability of probiotic white cheese samples during storage time (mean values±SD).
| Lactobacillus species | Lactobacillus count (Log10 CFU/g) | ||||||
|---|---|---|---|---|---|---|---|
| Time (days) | |||||||
| 0 | 3 | 7 | 15 | 30 | 45 | 60 | |
| L. plantarum | 9.81±0.03Aa | 8.96±0.01Ab | 7.95±0.00Ac | 7.74±0.04Ad | 6.72±0.04Ae | 6.63±0.06Aef | 6.52±0.07Af |
| L. casei | 9.73±0.1Aa | 8.92±0.01ABb | 7.79±0.04Bc | 6.94±0.02Bd | 6.61±0.12Ae | 6.36±0.1Bf | 6.50±0.04Aef |
| L. agilis | 9.82±0.05Aa | 8.84±0.00BCb | 7.60±0.00Bc | 6.52±0.07Cd | 5.91±0.01Be | 5.72±0.04Cf | 5.52±0.07Bg |
| L. helveticus | 9.32±0.12Ba | 8.81±0.03Cb | 8.07±0.22Ac | 7.78±0.01Ac | 6.61±0.02Ade | 6.36±±0.10Be | 6.72±0.04Ad |
| L. delbrueckii | 9.44±0.12Ba | 8.52±0.07Db | 7.87±0.05ABc | 6.82±0.04Bd | 4.36±0.10Ce | 4.67±0.05Df | 4.56±0.07Ce |
| L. salivarius | 9.77±0.07Aa | 9.48±0.03Eb | 7.62±0.03Bc | 5.79±0.08Dd | 4.98±0.03De | 4.94±0.03Ee | 4.72±0.04Ce |
Same small letters within the same row indicate non-significant differences (p>0.05) based on Tukey’s Range Test. Same capital letters within the same column indicate non-significant differences (p>0.05) based on Tukey’s range test, SD=Standard deviation, L. plantarum=Lactobacillus plantarum, L. casei=Lactobacillus casei, L. agilis=Lactobacillus agilis, L. helveticus=Lactobacillus helveticus, L. delbrueckii=Lactobacillus delbrueckii, L. salivarius=Lactobacillus salivarius
Discussion
Knowing the microbial population of traditional dairy products results in better understanding of specific characteristics of such products [10]. The technology used in the different geographical area in producing traditional dairy products has a direct impact on the diversity of the microbial population [10,11]. Lactobacillus species are identified as dominant species in cheeses produced from raw milk because they can grow under severe selective conditions and cause desirable sensory properties due to their proteolytic activities [12]. In the current study, mesophilic strains of Lactobacillus were dominant lactic flora of traditional cheeses since 62% of Lactobacillus strains were isolated at 30°C and 37°C. In a study by Ahmadi et al. [13], on lactic acid bacteria of Lighvan cheese, mesophilic (85%, 46 isolates) and thermophilic (15%, 8 isolates) Lactobacillus were isolated, respectively. Previous studies indicated that facultative heterofermentative Lactobacillus, especially L. plantarum and L. casei, is the most typical isolated lactobacilli from cows and sheep milk [14], which can also produce specific flavor and aroma [15]. In a previous study on Fossa cheese, it was determined that L. plantarum, L. paracasei, and L. carvatus produce small peptides and volatile amino acids during activities of dipeptidase, aminopeptidase, endopeptidase, and proteinase enzymes, leading to the production of desirable flavor and aroma [16]. In other studies, these bacteria were identified as dominant species of Iranian Lighvan cheese, South African traditional fermented milk and Maasai traditional fermented milk (Kenya) which are all consistent with the results of this study [17-19]. These results confirm the important role of lactic acid bacteria isolated from traditional cheeses.
In this study, L. plantarum was the dominant Lactobacilli of traditional cheeses, especially Urmia Koopeh cheese. Of 118 isolated Lactobacillus strains, 28 isolates (24%) were L. plantarum. These mesophilic bacteria are able to fermentate citrate and proteolysis during cheese curing [20]. Results of similar studies have shown such differences in the microbial composition of fermented foods, using culture-dependent and independent methods [21].
Lactic acid bacteria are responsible for potential usefulness of fermented milk products [22]. Many researchers have studied the antibacterial activity of lactic acid bacteria and their ability to produce antimicrobial substances and destroying pathogens [23,24].
In recent years, various preservatives including natural additives have been introduced to overcome pathogenic microorganisms [25,26]. The effect of lactic acid bacteria in preventing the growth of pathogens plays an important role in reducing the use of chemical additives and preservatives. It also improves consumer’s satisfaction by obtaining healthier and ready-to-use foods [27]. There have been several studies on the enrichment of fermented milk products with probiotics, such as enrichment of Cheddar [28], Manchego [29], Cottage [30], Turkish Beyaz [31], Argentinean [32], and white cheese [33].
Among probiotic bacteria, L. plantarum, L. casei, and L. helveticus were present in high enough levels (106 CFU/g threshold) throughout the storage, which is required for probiotic activity and having satisfactory viability (count decreases during 60 days <3 log order) in cheese. In the current study, L. agilis, L. delbrueckii, and L. salivarius reduced at least 4 logs during 60 days of the storage and reached to the final count of <5.52±0.07 log CFU/g. The adverse conditions such as low pH, lack of carbohydrates, and probably unfavorable ambient temperature during 60 days of the storage might be responsible for this reduction [34]. This reduction in Lactobacillus population, constructed various strategies such as microencapsulation with different materials to protect microorganisms for the gradual release of microorganism in cheese [35].
Conclusion
Results of this study indicated that bacterial isolates obtained from traditional cheeses could be used in industrial cheese production to obtain exclusive properties of traditional cheeses. The outcome of the study also showed that L. plantarum, L. casei, and L. helveticus are good combinations of starter cultures with acceptable viability.
Authors’ Contributions
AE supervised the project. MH carried out the experiment. AA wrote the manuscript with support from Dr. Ehsani and Hashemi. MA contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by the vice chancellor of research, Urmia University, Urmia, Iran under Grant No. 1587.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Montel M.C, Buchin S, Mallet A, Delbes-Paus C, Vuitton D.A, Desmasures N, Berthier F. Traditional cheeses:Rich and diverse microbiota with associated benefits. Int. J. Food. Microbiol. 2014;177:136–154. doi: 10.1016/j.ijfoodmicro.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Durlu-Ozkaya F, Xanthopoulos V, Tunail N, Litopoulou-Tzanetaki E. Technologically important properties of lactic acid bacteria isolates from Beyaz cheese made from raw ewes'milk. J. Appl. Microbiol. 2001;91:861–870. doi: 10.1046/j.1365-2672.2001.01448.x. [DOI] [PubMed] [Google Scholar]
- 3.Incheh K.H.G, Hassanzadazar H, Forouzan S.H, Banafshehchin E.L, Mozafarian E.L, Aminzare M, Hashemi M. A survey on the quality of traditional butters produced in West Azerbaijan province, Iran. Int. Food Res. J. 2017;24:327–332. [Google Scholar]
- 4.Aminifar M, Emam-Djomeh Z. Changes of texture, microstructure and free fatty acid contents of lighvan cheese during accelerated ripening with lipase. J. Agric. Sci. Technol. 2014;16:113–123. [Google Scholar]
- 5.Ehsani A, Mahmoudi R, Hashemi M, Raeisi M. Identification ofLactobacillusspecies isolated from traditional cheeses of west azerbaijan. Iran. J. Med. Microbiol. 2014;8:38–43. [Google Scholar]
- 6.Milani E, Shahidi F, Mortazavi S.A, Saeedi M. Isolation and identification of lactic acid bacteria in kurdish cheese during ripening using16S rRNAgene sequence analysis. J. Food Process Preserv. 2017;41:e13009. [Google Scholar]
- 7.Tajik H, Aminzare M, Raad T.M, Hashemi M, Azar H.H, Raeisi M, Naghili H. Effect ofZataria multifloraBoiss essential oil and grape seed extract on the shelf life of raw buffalo patty and fate of inoculatedListeria monocytogenes. J. Food Process Preserv. 2015;39:3005–3013. [Google Scholar]
- 8.Mannan S.J, Rezwan R, Rahman M.S, Begum K. Isolation and biochemical characterization ofLactobacillusspecies from yogurt and cheese samples in dhaka metropolitan area. Bangladesh Pharm. J. 2017;20:27–33. [Google Scholar]
- 9.Krasaekoopt W, Bhandari B, Deeth H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy. 2004;14:737–743. [Google Scholar]
- 10.Colombo E, Franzetti L, Frusca M, Scarpellini M. Phenotypic and genotypic characterization of lactic acid bacteria isolated from artisanal italian goat cheese. J. Food Protect. 2010;73:657–662. doi: 10.4315/0362-028x-73.4.657. [DOI] [PubMed] [Google Scholar]
- 11.Hatamikia M, Bahmani M, Azar H.H, Sepahvand R, Parsaei P, Aminzare M. Microbial contamination of commercial and traditional doogh dairy products in lorestan province of Iran. J. Food Qual. Hazards Control. 2016;3:114–116. [Google Scholar]
- 12.Diezhandino I, Fernández D, González L, McSweeney P.L.H, Fresno J.M. Microbiological, physico-chemical and proteolytic changes in a Spanish blue cheese during ripening (Valdeón cheese) Food Chem. 2015;168:134–141. doi: 10.1016/j.foodchem.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi S.M, Khamiri M, Khosroshahi A, Nezhad M.K. Isolation and identification of Lacic acid bacteria from Lighvan cheeses. J. Agric. Sci. Nat. Res. 2009;3:80–91. [Google Scholar]
- 14.Centeno J, Cepeda A, Rodriguez-Otero J. Lactic acid bacteria isolated from Arzu´a cows'milk cheese. Int. Dairy J. 1996;6:65–78. [Google Scholar]
- 15.Mannu L, Comunian R, Scintu M.F. MesophilicLactobacilliin Fiore Sardo cheese:PCR-identification and evolution during cheese ripening. Int. Dairy J. 2000;10:383–389. [Google Scholar]
- 16.De Angelis M, Corsetti A, Tosti N, Rossi J, Corbo M, Gobbetti M. Characterization of non-starter lactic acid bacteria from Italian ewe cheeses based on phenotypic, genotypic, and cell wall protein analyses. Appl. Environ. Microbiol. 2001;67:2011–2020. doi: 10.1128/AEM.67.5.2011-2020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beukes E.M, Bester B.H, Mostert J.F. The microbiology of South African traditional fermented milks. Int. J. Food Microbiol. 2001;63:189–197. doi: 10.1016/s0168-1605(00)00417-7. [DOI] [PubMed] [Google Scholar]
- 18.Mathara J.M, Schillinger U, Kutima P.M, Mbugua S.K, Holzapfel W.H. Isolation, identification and characterisation of the dominant microorganisms of kule naoto:The Maasai traditional fermented milk in Kenya. Int. J. Food Microbiol. 2004;94:269–278. doi: 10.1016/j.ijfoodmicro.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Abdi R, Sheikh-Zeinoddin M, Soleimanian-Zad S. Identification of lactic acid bacteria isolated from traditional Iranian Lighvan cheese. Pak. J. Biol. Sci. 2006;9:99–103. [Google Scholar]
- 20.Terzic-Vidojevic A, Vukasinovic M, Veljovic K, Ostojic M, Topisirovic L. Characterization of microflora in homemade semi-hard white Zlatar cheese. Int. J. Food Microbiol. 2007;114:36–42. doi: 10.1016/j.ijfoodmicro.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Randazzo C.L, Vaughan E.E, Caggia C. Artisanal and experimental Pecorino Siciliano cheese:Microbial dynamics during manufacture assessed by culturing and PCR-DGGE analyses. Int. J. Food. Microbiol. 2006;109:1–8. doi: 10.1016/j.ijfoodmicro.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Parvez S, Malik K, Ah Kang S, Kim H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006;100:1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- 23.Singh P, Prakash A. Screening of lactic acid bacteria for antimicrobial properties againstListeria monocytogenesisolated from milk products at Agra region. Int. J. Food Saf. 2009;11:81–87. [Google Scholar]
- 24.Naghili H, Tajik H, Raiesi M, Ghasemmahdi H, Moradi M, Zare M.A, Mehdizade T, Hasanazar H, Hariri F. Inhibitory potential of metabolites fromLactobacillus caseiagainst phenotypic characteristic ofSalmonella typhimuriumLT2. Med. Lab. J. 2016;10:7–13. [Google Scholar]
- 25.Aminzare M, Abbasi Z, Amiri E, Hashemi M, Raeisi M, Mousavi N, Hassanzadazar H. Colibacillosis phytotherapy:An overview on the most important world medicinal plants effective onEscherichia coli. J. Pharm. Sci. Res. 2017;9:629–636. [Google Scholar]
- 26.Hashemi M, Ehsani A, Hassani A, Afshari A, Aminzare M, Sahranavard T, Azimzadeh Z. Phytochemical, antibacterial, antifungal and antioxidant properties ofAgastache foeniculumessential oil. J. Chem. Health Risks. 2017;7:95–104. [Google Scholar]
- 27.Naghili H, Tajik H, Aliakbarlu J, Zare P, Ghasemmahdi H, Raiesi M, Amin Zare M. Application ofLactobacillus caseiand Temperature to control ofSalmonella typhimoriumunderin vitroconditions. Urmia Med. J. 2014;24:1005–1015. [Google Scholar]
- 28.Ryan P.M, Burdikova Z, Beresford T, Auty M.A.E, Fitzgerald G.F, Ross R.P, Sheehan J.J, Stanton C. Reduced-fat cheddar and swiss-type cheeses harboring exopolysaccharide-producing probioticLactobacillus mucosaeDPC 6426. J. Dairy Sci. 2015;98:8531–8544. doi: 10.3168/jds.2015-9996. [DOI] [PubMed] [Google Scholar]
- 29.Salazar-Montoya J.A, González-Cuello R, Flores-Girón E, Ramos-Ramírez E.G. Effect of free and microencapsulatedLactococcus lactison composition and rheological properties of Manchego-type cheeses during ripening. Food Res. Int. 2018;105:59–64. doi: 10.1016/j.foodres.2017.10.067. [DOI] [PubMed] [Google Scholar]
- 30.Jeon E.B, Son S.H, Jeewanthi R.K.C, Lee N.K, Paik H.D. Characterization ofLactobacillus plantarumLb41, an isolate from kimchi and its application as a probiotic in cottage cheese. Food Sci. Biotechnol. 2016;25:1129–1133. doi: 10.1007/s10068-016-0181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasımoğlu A, Göncüoğlu M, Akgün S. Probiotic white cheese withLactobacillusacidophilus. Int. Dairy J. 2004;14:1067–1073. [Google Scholar]
- 32.Bergamini C, Hynes E, Quiberoni A, Suarez V, Zalazar C. Probiotic bacteria as adjunct starters:Influence of the addition methodology on their survival in a semi-hard Argentinean cheese. Food Res. Int. 2005;38:597–604. [Google Scholar]
- 33.Yerlikaya O, Ozer E. Production of probiotic fresh white cheese using co-culture withStreptococcus thermophilus. Food Sci. Technol. 2014;34:471–477. [Google Scholar]
- 34.Ong L, Shah N.P. Probiotic cheddar cheese:Influence of ripening temperaturves on survival of probiotic microorganisms, cheese composition and organic acid profiles. LWT Food Sci. Technol. 2009;42:1260–1268. [Google Scholar]
- 35.Kailasapathy K, Masondole L. Survival of free and microencapsulatedLactobacillus acidophilusandBifidobacterium lactisand their effect on texture of feta cheese. Aust.J. Dairy Technol. 2005;60:252. [Google Scholar]


