Abstract
N6-methyladenosine (m6A) is a chemical modification present in multiple RNA species, being most abundant in mRNAs. Studies on enzymes or factors that catalyze, recognize, and remove m6A have revealed its comprehensive roles in almost every aspect of mRNA metabolism, as well as in a variety of physiological processes. This review describes the current understanding of the m6A modification, particularly the functions of its writers, erasers, readers in RNA metabolism, with an emphasis on its role in regulating the isoform dosage of mRNAs.
Introduction
Over 150 RNA modifications have been identified as post-transcriptional regulatory marks in multiple RNA species, including messenger RNAs (mRNAs), transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), small non-coding RNAs, and long non-coding RNAs (lncRNAs).1–4 These modifications regulate several facets of RNA processing or metabolism, including alternative splicing,5–15 export,16–18 stability,19–25 and translation.26–32 N6-methyladenosine (m6A) is the most prevalent modification in the mRNA of many eukaryotic species, including yeast,33–35 plants,36–38 flies,39 and mammals.40–47 Although it was first discovered in the 1970s,44,48–50 detailed studies of its functions did not begin until around 2012, when transcriptome-wide profiling of m6A was made possible through antibody-based immunoprecipitation followed by high-throughput sequencing. More than 10,000 m6A peaks have been validated in over 25% of human transcripts. It was found to be on a consensus RNA motif of RRACH (R = A or G; H = A, U, or C), and enriched in long exons, near stop codons and 3’ untranslated regions (3’ UTRs).51,52
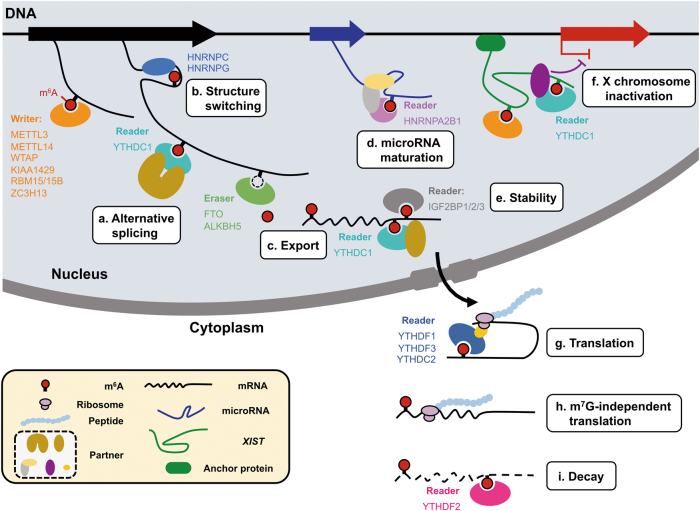
The abundance and effects of m6A on RNA are determined by the dynamic interplay between its methyltransferases (“writers”), binding proteins (“readers”), and demethylases (“erasers”) (Fig. 1). In this review, we provide a comprehensive summary about the biological functions of m6A writers, readers, and erasers, as well as the role of m6A in splicing regulation.
Fig. 1.
Diverse molecular functions of m6A. In eukaryotic cells, RNA m6A level is dynamically regulated by “writers” and “erasers”, and recognized by “readers” in direct or indirect ways. The diversity of cellular processes involving m6A is mainly contributed by various “readers”. The nuclear m6A modulates a mRNA alternative splicing,5,7–9,11–15,54,58–60 b secondary structure switching,5,11,105 c mRNA export,17,18 d pri-miRNA processing,104,135 e mRNA stability24 and f XIST-dependent X chromosome inactivation,57 while the cytoplasmic m6A (g and h) enhances mRNA translation efficiency26,27,29,32,106 and i accelerates mRNA decay.20,21,23,108
Methyltransferases/Writers
m6A is installed by a multicomponent methyltransferase complex consisting of Methyltransferase Like 3 (METTL3),53 METTL14,13,22,54,55 Wilms Tumor 1 Associated Protein (WTAP),56 KIAA1429,22 RNA Binding Motif Protein 15 (RBM15),57 and zinc finger CCCH domain-containing protein 13 (ZC3H13).58–60 METTL3 is the catalytic subunit, while METTL14 acts as the RNA-binding platform.61–63
METTL3
Purified METTL3 protein selectively methylates the GAC and AAC sequences in synthetic single-stranded RNA in vitro.15,64 METTL3 was also observed to localize onto nuclear speckles enriched with mRNA splicing factors as shown by in vivo immunofluorescence analysis, indicating a potential regulatory role of m6A in mRNA metabolism.13 Early studies used nuclear lysate to perform the methyltransferase assay, supporting the nuclear activity of METTL3. However, these studies also detected the activity of METTL3 in the cytoplasmic fraction, although at a lower level than that in the nuclear lysate.65 One recent study showed that cytoplasmic METTL3 acts to promote translation independent of its methyltransferase activity.66
As the core methyltransferase subunit, METTL3 has been demonstrated to modulate embryonic development,67,68 cell reprogramming47,68 and spermatogenesis,46 while its deletion in mice causes early embryonic lethality.69 METTL3 also regulates T cell homeostasis70 and endothelial-to-haematopoietic transition25,71 via methylation of specific target transcripts.
METTL3 is a highly conserved protein, with homologs in multiple species including Saccharomyces cerevisiae (IME4), Drosophila melanogaster (IME4) and Arabidopsis Thaliana (MTA). 33,36,39,67,72 In Arabidopsis thaliana, deficiency of the METTL3 homolog MTA affects development and growth,36,67 while in Saccharomyces cerevisiae, IME4 plays an essential regulatory role during meiosis and sporulation.33,35,73 In Drosophila, mutation of IME4 impairs neuronal functions and influences sex determination by modulating female-specific splicing of Sex-lethal (Sxl) gene.8,9
METTL14
METTL14 has been identified as another component of the m6A methyltransferase complex; both METTL3 and METTL14 are highly conserved in mammals, and form a stable heterodimer.13,54,55 Individually, METTL3 and METTL14 exhibit comparable weak methyltransferase activity in vitro. However, the METTL3-METTL14 complex has a much higher catalytic activity.54,55 A recent study on the crystal structure of the METTL3-METTL14 methyltransferase domain (MTD) complex illustrated that the primary function of METTL14 is not to catalyze methyl-group transfer but to offer an RNA-binding scaffold, allosterically activating and enhancing METTL3 the catalytic activity of METTL3.61–63 Two CCCH-type zinc finger domains (ZFDs) preceding the MTD in the N-terminus of METTL3 serve as the RNA target recognition domain.74
Similar to METTL3, METTL14 plays essential roles in diverse biological processes. Depletion of METTL14 causes a block in embryonic stem cell self-renewal and differentiation, embryonic developmental defects, and impaired gametogenesis in various organisms.55,69,75 METTL14 and METTL3 participate in neurogenesis by modulating cell-cycle progression of cortical neural progenitor cells in an m6A-dependent manner.40 METTL14 also plays a tumor-suppressor role in glioblastoma, and the depletion of METTL3 or METTL14 enhances growth and self-renewal of glioblastoma stem cells and promotes tumor progression.76 On the other hand, Weng et al. reported very recently that METTL14 is highly expressed in normal hematopoietic stem/progenitor cells and in various subtypes of acute myeloid leukemia (AML), and plays a critical oncogenic role in the development and maintenance of AML by blocking myeloid differentiation and promoting self-renewal of leukemia stem/initiating cells (LSCs/LICs).77 Thus, METTL14 might function in cancers in a tissue/cancer-type specific manner.
WTAP
WTAP interacts with METTL3 and METTL14 to modulate the m6A levels of RNA transcripts.13,36,54,56 WTAP is a ubiquitously expressed nuclear protein that appears to play a role in both transcriptional and posttranscriptional regulation of certain cellular genes.78 As it lacks methyltransferase domains, WTAP shows no catalytic activity of m6A modification or effects on the activity of the METTL3-METTL14 complex in vitro, but it is required for their localization in nuclear speckles that are enriched with various precursor messenger RNA (pre-mRNA) processing factors.13,54
Depletion of WTAP induces tissue-dependent defects. Similar to zebrafish embryos depleted of METTL3, zebrafish embryos lacking WTAP undergo increased apoptosis.13 WTAP has been reported to be upregulated in > 30% AML cases and paly an oncogenic role in AML.41 Although it has not yet been investigated whether m6A is involved in the role of WTAP in AML, METTL14 expression was found to be increased in AML,77 implying that regulation of m6A levels by the m6A writer complex may be a key factor in AML oncogenesis.
KIAA1429
KIAA1429 was identified as another component of the m6A methyltransferase complex by both proteomic screening and cellular studies.22 Its Drosophila ortholog interacts with Drosophila WTAP and regulates alternative splicing of pre-mRNAs involved in sex determination.79,80 Depletion of KIAA1429 induced an about 4-fold decrease in m6A peak scores in human A549 cells,22 suggesting an important regulatory role of KIAA1429 in the methyltransferase complex. In support of this, Yue et al. recently reported that VIRMA/KIAA1429 recruits the catalytic core components (METTL3/METTL14/WTAP) to guide region-selective m6A methylation.81
RBM15/RBM15B
One recent study suggested that RBM15 and its paralogue RBM15B direct the methylation of adenosine residues in both mRNAs and the lncRNA target XIST. Immunoprecipitation analysis indicated that RBM15/RBM15B bind and recruit the WTAP-METTL3 complex to specific sites. Both RBM15 and RBM15B comprise three RNA recognition motif (RRM) domains, and combined analyses of iCLIP-seq data of RBM15/RBM15B and m6A-miCLIP-seq data revealed that RBM15/RBM15B binding sites are significantly enriched at the locations adjacent to m6A methylation sites.57 This study suggests a mechanism of selective activity of the methyltransferase towards XIST.
Consistently, the RBM15 homolog, Spenito (Nito), was shown to be a novel subunit of the methyltransferase complex required for m6A formation in mRNAs in Drosophila.8
ZC3H13
Most recently, there are three studies demonstrating that ZC3H13 is another component of m6A writer complex and regulates m6A methylation.58–60 Wen et al. revealed that mouse Zc3h13 is required for nuclear localization of the Zc3h13-WTAP-Virilizer-Hakai complex and regulates mouse embryonic stem cell (ESC) self-renewal through facilitating m6A methylation.58 Knuckles et al. showed that mouse Zc3h13 and its fly homolog, CG7358 (named as Flacc), serve as an adaptor between RBM15/Nito and WTAP/Fl(2)d, bridging RBM15/Nito to the m6A machinery to promote m6A deposition on mRNAs.59 They further found that Flacc regulates Drosophila sex determination and dosage compensation through modulating Sxl alternative splicing.59 Guo et al. also identified the CG7358 gene (named as Xio) as a component of the Drosophila sex determination pathway, and further demonstrated its role in controlling Sxl alternative splicing via m6A methylation.60
METTL16
The METTL3 homolog METTL16 (methyltransferase-like 16) controls cellular SAM level and installs m6A onto the U6 small nuclear RNA. METTL16 activity requires both the UACAGAGAA nonamer and a specific RNA structure.6
In addition to the above members, other subunits of the methyltransferase complex may exist to achieve precise post-transcriptional regulation through selectively recognizing candidate methylation sites.
Demethylases/Erasers
Although METTL3 was discovered as an m6A writer several decades ago, the identity of demethylases remained a mystery until 2011, when Jia et al. unveiled that fat mass and obesity-associated protein (FTO) exhibits efficient m6A demethylase activity.82 Another m6A demethylase, α-ketoglutarate-dependent dioxygenase alkB homolog 5 (ALKBH5), was soon discovered in 2013,17 and was found to be highly expressed in the testes. The demonstration of their demethylase activity provided the first evidence of reversible post-transcriptional modification in mRNAs.
FTO
FTO was originally reported as a demethylase for N3-methylthymidine in single-stranded DNA83 and for N3-methyluridine in single-stranded RNA84 in vitro. In 2011, Jia et al. found that FTO demethylates m6A in both DNA and RNA in vivo. Depletion of FTO induces significant increase in total m6A levels of polyadenylated RNA.82 As FTO oxidizes m6A to A, it generates N6-hydroxymethyladenosine (hm6A) as an intermediate product, and N6-formyladenosine (f6A) as a further oxidized product.85 The potential function of these oxidized labile intermediates needs further exploration. Intriguingly, a recent study demonstrated that FTO also displays demethylase activity towards m6Am that is exclusively located at the first encoded nucleotide after the 7-methylguanosine cap structure of a large number of mRNAs and reduces the stability of m6Am-containing mRNAs in vivo,86 suggesting that FTO could work on multiple substrates. Hence, further studies are needed to reveal functional relevance of different FTO substrates.
FTO was first reported to be associated with increased body mass and obesity in human.87–90 It is widely expressed in all adult and fetal tissues and most highly expressed in the brain.83 FTO has been shown to function as an oncogene in both leukemia91,92 and glioblastoma.76 FTO expression is aberrantly upregulated by oncogenic proteins in certain subtypes of AML, where it promotes leukemogenesis and inhibits all-trans-retinoic acid (ATRA)-induced AML cell differentiation through reducing the m6A levels of a set of critical transcripts such as ASB2 and RARA.91 A recent extended study further revealed that FTO efficiently demethylates internal m6A, and the inhibition of FTO by R-2HG leads to increased m6A levels in mRNAs, but only small changes in cap m6Am.92 Notably, specific m6A sites in FTO target mRNA transcripts such as ASB2, RARA and MYC have been demonstrated with quantitative methods (i.e., luciferase reporter/mutagenesis assays and gene region-speific m6A qPCR) to be regulated by FTO.91,92 The possible mechanisms underlying the phenomenon that both FTO and METTL14 play oncogenic roles in AML have been discussed.77,93
FTO promotes cell growth and self-renewal of human glioblastoma stem cells, and is required for substantial tumor progression. Its deficiency prolongs the lifespan of glioblastoma stem cell-grafted mice through regulating the expression of critical genes.76 Nevertheless, another study testing a number of different AML cell lines failed to observe an obvious effect of FTO on AML cell viability.94
Additionally, FTO regulates mouse pre-adipocyte differentiation by regulating alternative splicing of pre-mRNAs of genes involved in adipogenesis.12 Finally, a recent study revealed that depletion of FTO results in upregulation of terminal mRNA exons,14 suggesting that FTO also regulates alternative polyA site usage and 3’UTR processing.
ALKBH5
As the second identified m6A demethylase, ALKBH5 displays m6A demethylation activity comparable to that of FTO. ALKBH5 may function in a sequence specific context, as it shows a preference for m6A within its consensus sequence rather than other methylated nucleotides in single-stranded RNA.17 ALKBH5 localizes to the nucleus and its depletion leads to global reduction of polyA RNAs in this cellular compartment,17 suggesting a role in the regulation of nuclear export of mRNA.
ALKBH5 is expressed in most tissues, being particularly abundant in the testes where it impacts mouse spermatogenesis and fertility.17 ALKBH5 regulates splicing and stability of mRNAs in the nuclei of spermatocytes and round spermatids by removing m6A from pre-mRNAs and allows the production of mRNAs containing longer 3’UTRs.95 Moreover, ALKBH5 plays an important role in the immune response to viral infections in macrophages. To inhibit interferon production, the nuclear protein DDX46 recruits ALKBH5 after viral infection to demethylate m6A-modified antiviral transcripts, thus sequestering them in the nucleus.96
ALKBH5 has also been shown to be important for cancer pathogenesis. A recent study revealed that overexpression of ALKBH5 is required for the proliferation and tumorigenesis of glioblastoma stem cells and predicts poor patient survival.97 Additionally, knockdown of ALKBH5 expression in MDA-MB-231 human breast cancer cells significantly reduced their capacity for tumor initiation attributing to the reduced of breast cancer stem cells.98
Given the varied distribution of the m6A demethylases across tissues and their essential roles in regulating m6A methylation, additional cell- or tissue-specific demethylases may exist to act on different RNA substrates.
m6A readers
Chemical modifications can directly affect properties of RNA transcripts, including charge, base-pairing, secondary structure, and protein-RNA interactions, which in turn shape gene expression by modulating RNA processing, localization, translation, and eventually, decay.1,3,4,99 Prominently, m6A also indirectly affects RNA processing by recruiting specific reader proteins. To date, several m6A reader proteins have been identified in mammalian cellular extracts using combined approaches of affinity chromatography and mass spectrometry.52 Investigations about these reader proteins have begun to elucidate the ultimate role of m6A in RNA processing.
Nuclear m6A readers
Several proteins selectively bind m6A-containing precursor RNAs in the nucleus. YTHDC1, the nuclear member of highly conserved YTH family proteins,100 locates in the nucleus and forms YT bodies at transcriptionally active sites adjacent to RNA processing speckles.101 Structural and binding studies revealed that YTHDC1 preferentially recognizes the GG(m6A)C sequences through its YTH domain.102
YTHDC1 promotes exon inclusion by selectively recruiting or inhibiting different splicing factors such as Serine-arginine repeat (SR) proteins.7 YTHDC1 also recognizes m6A on XIST and promotes XIST-mediated X chromosome silencing.57 In addition to its role in splicing regulation, very recent work in HeLa cells identified that YTHDC1 interacts with SRSF3 and nuclear RNA export factor 1 (NXF1) to facilitate the nuclear export of m6A-modified mRNAs,18 which expands the roles of YTHDC1-mediated m6A reading in mRNA metabolism regulation. In addition, YTHDC1 has been reported to be a potential tumor suppressor in endometrial cancer through its interaction with other splicing factors.101,103
Recent studies raised some controversies regarding whether the HNRNP family member HNRNPA2B1 is an m6A reader. Alarcon et al. reported that HNRNPA2B1 could directly bind m6A and regulate alternative splicing events and primary microRNA processing in concert with METTL3,104 whereas a structure-based study by Wu et al. revealed an “m6A switch” mechanism, instead of specific m6A binding, mediated by HNRNPA2B1.105 In addition, another two HNRNP proteins, HNRNPC11 and HNRNPG,5 function to regulate the processing of m6A-containing RNA transcripts. They do not bind to m6A directly, but instead, m6A serves as a structural switch to alter the RNA structure, which renders transcripts more accessible for binding by HNRNPC and HNRNPG.
Cytoplasmic m6A readers
After being processed from precursor transcripts, mature mRNAs containing m6A are further regulated in the cytoplasm by other members of the YTH family: YTHDF1, YTHDF2, YTHDF3, and YTHDC2.18,20,21,26,27,29,32,106,107
YTHDF1 was initially demonstrated to bind methylated mRNA transcripts at sites near the stop codon, and its overall distribution pattern is similar to that of m6A sites on mRNAs. Mechanistic study has demonstrated that YTHDF1 interacts with the translation initiation machinery and enhances the translation efficiency of its target RNAs.32
YTHDF2 co-localizes with both deadenylation and decapping enzyme complexes under normal conditions and directs its targets to processing bodies.21 By directly recruiting the CCR4-NOT deadenylase complex, YTHDF2 accelerates the degradation of m6A-modified transcripts.20 Data were presented to show that YTHDF1 and YTHDF3 promote deadenylation through the CCR4-NOT complex to a lesser extent than YTHDF2.20 In zebrafish, Ythdf2 deletion in embryos decelerates the decay of m6A-modified maternal mRNAs and impedes zygotic genome activation.23 Our recent study revealed that YTHDF2-mediated decay of transcripts of the arterial endothelial genes notch1a and rhoca contributes to the emergence of haematopoietic stem/progenitor cells (HSPCs) during endothelial-to-haematopoietic transition (EHT).25 In mice, YTHDF2 determines oocyte competence and early zygotic development by regulating maternal transcript dosage.108 In cells undergoing heat shock, YTHDF2 expression is increased, and its protein is translocated to the nucleus where YTHDF2 protects m6A residues in the 5’UTR of stress-induced transcripts from demethylation by FTO. Transcripts with enhanced 5’UTR methylation are selectively translated via a cap-independent mechanism.29
YTHDF3, together with YTHDF1, regulates mRNA translation by interacting with a common set of ribosomal proteins.26,27 In addition, YTHDF3 could also mediate mRNA decay by directly interacting with YTHDF2.26 Overall, YTHDF3 serves as a hub for fine-tuning the RNA accessibility of YTHDF1 and YTHDF2. YTHDF3 may also interact with other proteins to play additional cell-type specific roles on target transcripts.
YTHDC2, the largest member of the YTH family, also preferentially binds m6A within the consensus motif and can enhance the translation efficiency while decreasing the bundance of its target mRNAs.106,107,109,110 YTHDC2 has also been reported to play a role in spermatogenesis, in which it can interact with the meiosis-specific protein MEIOC to affect the stability of target transcripts during meiosis prophase I.111,112 Both male and female Ythdc2 knockout mice display infertility, demonstrating defects in meiotic prophase I, suggesting its essential roles in spermatogenesis and oogenesis.106 Importantly, YTHDC2 is much larger than other YTH proteins (~160 kDa vs ~60 kDa), and contains multiple helicase domains and two Ankyrin repeats. These unique features may endow YTHDC2 with a variety of functions, including regulatory effects on RNA binding and RNA structure, and recruitment of or binding with other protein complex members.106,113 Intriguingly, a lack of m6A binding activity was reported by a CLIP study of YTHDC2 in HEK cells.57,114 The controversial findings that YTHDC2 acts as a reader of m6A in mouse testes but lacks m6A binding activity in HEK cells suggest a cell- or tissue-specific role of YTHDC2; it is also possible that YTHDC2 may indirectly regulate m6A-containing transcripts through interaction with other factors.
Methylated RNA-binding 1 (Mrb1), another YTH domain containing protein, has been reported to bind m6A in yeast.31 Additionally, several studies based on RNA pull-down approaches detected other m6A interactors, including ELAV like RNA binding protein 1 (ELAVL1, also known as HuR),55,97,115 FMR1,116,117 LRPPRC,117 and also IGF2BP family proteins.24 However, in most cases, whether these proteins directly bind to m6A or whether they are part of an m6A binding ribonucleoprotein complex needs further clarification.
Functions in RNA metabolism
Recent accumulative studies revealed that m6A methylation regulates almost every aspect of mRNA metabolism, from expression and pre-mRNA processing in the nucleus to translation and mRNA decay in the cytoplasm.1,3,4,10,118 m6A has been reported to be associated with alternative polyadenylation (APA),119,120 which is coupled to the splicing of the last intron. m6A-involved regulation mediated by METTL3,121 ALKBH517 and YTHDC118 has been shown to modulate mRNA export from the nucleus to the cytoplasm. Several distinct mechanisms by which m6A promotes mRNA translation have been demonstrated including the YTHDF1-eIF3 pathway,32 the cap-independent translation29,122 and IGF2BPs-mediated translation.24 In the aspect of mRNA stability control, earlier studies generally considered m6A as a destabilizer facilitating mRNA degradation mainly through YTHDF2,20,21,52,55 while a recent study revealed a distinct function of m6A mediated by IGF2BP proteins in promoting the stability and storage of the mRNA targets.24 In addition, m6A could also alter RNA folding and structure,5,11,28,73,123–127 and sort transcripts into a fast track for mRNA metabolism.7,18,21,26,27,32 In the rest of this review, we focus on our current understanding of the roles of m6A in RNA splicing regulation and discuss the discrepancy and challenges in the field.
m6A modulates RNA splicing
Processing of pre-mRNAs to mature mRNAs consists of three main steps: 5’capping, 3’polyadenylation and splicing. Splicing of the pre-mRNA, involving precise excision of introns and joining of exons in the nucleus,128 is an important process in gene expression and can increase the gene product diversity. Cis-regulatory RNA elements and trans-regulatory splicing factors participate in the process of alternative splicing regulation via specific interactions between trans-factors and cis-element sequences, which makes splicing regulation a dynamic and complicated process.129 Cis-regulatory RNA elements can be either intronic or exonic sequences and can promote (splicing enhancers) or inhibit (splicing silencers) splicing activity.130 Trans-regulatory splicing factors regulate splicing by forming spliceosomes with different categories of U snRNAs on pre-mRNAs and orchestrating with cis-regulatory elements.131 To date, exonic and intronic splicing enhancers (abbreviated as ESE and ISE, respectively) bound by the SR proteins of splicing activators as well as exonic and intronic splicing silencers (ESS and ISS, respectively) bound by the splicing repressor hnRNP proteins have been characterized and documented.130 In addition to RNA-protein interactions, RNA-RNA base pairing, chromatin modifications, small RNAs, and RNA polymerase II complex have also been reported to regulate alternative splicing patterns.128,130 Recently, several lines of evidence indicated that m6A also serves as an important pre-mRNA splicing regulator.
The predicted role of m6A as a splicing regulator is initially based on the early observations that m6A sites are concentrated in the introns of pre-mRNAs and were more abundant in pre-mRNAs than in mature mRNA.132–134 One previous study indicated that pre‑mRNAs are methylated at a level of ~4 m6A residues per mRNA, whereas for mature mRNAs, the number is ~2 m6A residues per mRNA,132 suggesting that methylation occurs in the nucleus and the removal of introns results in the loss of total m6A content per mRNA. Additionally, m6A is more likely to be found in introns and exons that undergo alternative splicing.52 Accumulating evidence from specific individual mRNAs supports the idea that m6A truly regulates splicing events, and its presence in either intronic regions or exonic regions plays important roles in alternative splicing.6–13,52,118,124,135
The localization of writers, readers and erasers of m6A to nuclear speckles, the locations for mRNA splicing and storage of splicing factors,7,12,13,17,82 also supports the link of m6A with splicing. Firstly, PAR-CLIP analysis showed that mRNAs undergoing alternative splicing have more METTL3 binding sites and N6-adenosine methylation sites.13,52 CLIP-seq of METTL3-METTL14 by another group revealed that 29-34% of their binding sites are in intronic regions.54 Mettl3 depletion in mouse embryonic stem cells generally favors exon skipping and intron retention.69 A recent study also demonstrated that METTL3 functions in spermatogenesis by regulating the alternative splicing of spermatogonial differentiation and meiosis initiation- related mRNAs. Mettl3 deletion led to aberrant splicing of important spermatogenesis-regulating genes, such as Sohlh1 and Dazl, by altering the m6A modification of their transcripts.46 mRNAs that exhibit multiple isoforms due to alternative splicing are significantly more likely to contain m6A and be bound by METTL3 than mRNAs with only one spliced isoform.13,52 The m6A signaling-deficient Ime4/Yt521-B null mutant flies exhibit aberrant alternative splicing of Sxl.8,9,15 These results indicate that the recruitment of METTL3 to pre-mRNA is a co-transcriptional event with potential effects on either promoting or influencing splicing. WTAP is also considered as a splicing factor that binds to WT1 in Drosophilia,136 and its targets are mainly enriched in alternatively spliced exons rather than constitutively spliced exons.13 The finding that deficiency of WTAP results in altered mRNA isoforms also supports the idea that WTAP and m6A methylation affects mRNA splicing.13 Moreover, a recent study reported an interesting phenomenon that the m6A methyltransferase METTL16 regulates the expression of the SAM synthetase MAT2A by the enhanced splicing of a retained intron in the presence of its methylation substrate, a vertebrate conserved hairpin (hp1) in the MAT2A 3’UTR.6
Secondly, m6A erasers also affect splicing. Our previous report demonstrated that enhanced levels of m6A in response to FTO depletion in mouse pre-adipocytes promote the RNA binding ability of serine- and arginine-rich splicing factor 2 (SRSF2), leading to increased inclusion of target exons.12 A recent study in the human 293T cell line revealed that FTO binds pre-mRNAs in the nucleus and triggers inclusion of alternatively spliced exons. Upon FTO depletion, m6A was strongly enriched at intronic as well as exonic regions surrounding skipped exons, leading to exon skipping events.14 ALKBH5 deficiency also significantly influenced the nuclear speckle localization of several splicing factors and altered more than 3000 mRNA isoforms, suggesting its effects on splicing.17 A recent study further showed that ALKBH5-mediated m6A erasure in the nuclei of spermatocytes and round spermatids regulates splicing and stability of long 3’UTR mRNAs.95
Thirdly, m6A readers could also regulate splicing. Direct binding of m6A-decorated alternatively spliced exons by the m6A reader protein YTHDC1 facilitates their inclusion into mRNA. YTHDC1 promotes SRSF3 (driving exon inclusion) but antagonizes SRSF10 (driving exon exclusion) binding to mRNAs and affects mRNA splicing, leading to exon inclusion events.7 Moreover, YTHDC1-mediated m6A signaling plays a role in regulating the splicing of replication transcription activator (RTA) pre-mRNA encoded by the Kaposi’s sarcoma-associated herpesvirus (KSHV).137 HNRNP family proteins could affect splicing through forming ribonucleoprotein granules. Recent studies demonstrated that HNRNPA2B1 and HNRNPC are active splicing regulators correlated with m6A modification.11,104 Specially, HNRNPA2B1 has been reported to directly bind to m6A, leading to the regulation of alternative splicing events similar to those regulated by METTL3,104 as well as facilitating the processing of primary microRNAs (pri-miRNAs) to mature miRNAs.104,135 Additionally, m6A also regulates pre-mRNA processing by altering the local RNA structure to facilitate the binding of some HNRNP proteins, such as HNRNPC and HNRNPG.5,11
Thus, m6A modification appears to change the mRNA isoform diversity by regulating alternative splicing. Based on the accumulating evidence that both exonic and intronic m6A sites regulate alternative splicing, we propose a model depicting that m6A modification serves as an elastic system in splicing regulation (Fig. 2), in which the rapid and dynamic changes in both levels and modification sites of m6A can elaborately regulate mRNA isoform dosage through the combined actions of m6A writers, readers and erasers: A, m6A sites located in alternatively spliced (AS) exons mainly lead to exon inclusion through m6A-dependent molecular mechanisms regulating alternative splicing. B, m6A sites buried into intronic regions could promote either exon inclusion or skipping.
Fig. 2.
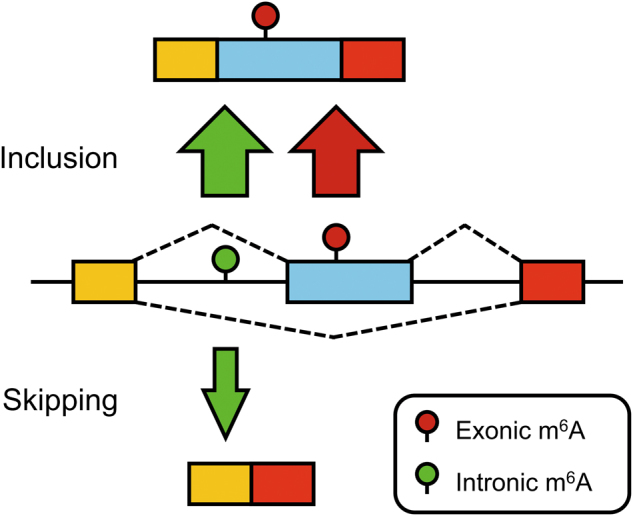
Schematic summary of the roles of m6A in regulating mRNA splicing. Exonic and most intronic m6A sites promotes exon inclusion while a small proportion of intronic m6A sites can also lead to exon skipping through a refined buffering system composed of its writers, readers, erasers as well as other splicing-related factors
Though many current reports support the association of m6A with RNA splicing regulation, one recent study from Ke et al.138 reported that the vast majority of exons harboring m6A in wild-type mouse stem cells are correctly spliced in cells lacking METTL3, suggesting that m6A is not obligatory for most of the splicing events. In their study, 99 exons from 2000 alternatively spliced cassette exons containing m6A were observed to have significantly different inclusion levels upon Mettl3 depletion using Quantas software with FDR < 5% and ΔPSI ≥ 0.1. Ke et al. also examined the prevalence and distribution of m6A within intronic sequences by carrying out m6A-CLIP on pre-mRNAs and found that adenosine residues in introns have a smaller chance of being methylated compared with adenosine residues in exons.138 However, Liu et al. recently identified 39,060 m6A-switches among HNRNPC-binding sites and the majority (87%) of m6A-switches occur within introns.11 Pendleton et al. also found that most of the METTL16-dependent m6A peaks were in introns or spanned intron-exon boundaries.6
Several possibilities may explain different conclusions. The first possibility is that the analyses are done in different scales (at a global scale or with individual mRNAs). For the MBNL protein,139 a well-established splicing factor, only 6% of exons with MBNL binding clusters showed significant changes in splicing upon MBNL deficiency. This low proportion is attributed to the high-affinity binding of many RNA-binding proteins (RBPs) to thousands of different positions in the transcriptome, whereas probably only a subset of the interactions are truly associated with their specific functions.140 Considering that more and more m6A readers are being validated, m6A in different regions along mRNAs may play diverse regulatory roles at the post-transcriptional level, which can also explain why the splicing effect of m6A can be found on some specifically modified mRNAs but not all modified exons. Furthermore, not only exonic m6A on cassete exons but also m6A on flanking exons or introns may regulate alternative splicing, which is supported by the recent findings that many of the METTL3 binding sites are located in introns,13,54 illustrating that intronic sequences may be a major target of nuclear methylation. m6A may be introduced into introns at a relatively high level, which is then followed by rapid intron excision and degradation.
The current technical limitation in the quantifications of m6A modification might also result in different conclusions on whether m6A affects alternative splicing. Unlike m5C that can be directly quantified by C-to-T transition after bisufite treatment, m6A modification can only be quantified so far as the enrichment score by IP/input51,52 or detected as mutation or truncation products following a UV cross-linking procedure.119,141,142 The real methylation level cannot be estimated at the transcriptome level, thus it is hard to evaluate whether the effect on splicing is caused by relative m6A levels on specific sites. Besides, details of the methodology may also impact the sensitivity and efficiency of detection, such as sequencing depth, analysis pipeline and parameters used. Short reads and low converage result in less read-covered junctions.143 The boundaries of cutoff on inclusion change and FDR139,144–146 are quite different among labs and there is no gold standard for answering how many alternative splicing events can be considered as direct effects. All of these factors may have contributed to the controversy about the effect of m6A on splicing at the transcriptome level. Moreover, given the highly dynamic regulation of m6A by its methyltransferase and demethylases, potential removal of m6A in spliced exons cannot be ruled out. Thus, future systematic quantitative analysis of m6A in pre-mRNAs at single site resolution will be promising to provide a clearer view of the mechanism underlying m6A-mediated regulation of pre-mRNA splicing.
Concluding remarks
Dynamic transcriptomic m6A modification is orchestrated by its writers and erasers, while functions of m6A in RNA metabolisms are carried out by its readers. Although some inconsistency in the current literature needs further detailed investigation, multiple lines of evidence support its significance in regulating both RNA metabolism and distinct biological processes. Recently, efforts have been made to directly detect m6A sites in RNA by engineering DNA polymerase mutant capable of increasing misincorporation opposite m6A147 or by using the nanopore technology for direct detection of RNA modifications.148 Developing the future direct sequencing technology for RNA m6A will be a key step to understand its dynamics and functions in vivo.
Acknowledgements
The authors apologize to colleagues whose work was not cited owing to space limitation. This work was supported by the National Key R&D Program of China (2016YFC0900300), the Ministry of Science and Technology of the People’s Republic of China (MOST2014CB964902), the National Natural Science Foundation of China (NSFC; 31625016 and 31500659), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB14030300 and QYZDY-SSW-SMC027), and Shanghai Municipal Science and Technology Major Project (2017SHZDZX01). Y.Y. is supported by the Youth Innovation Promotion Association (CAS 2018133). P.J.H. is supported by the NIH Medical Scientist National Research Service Award (T32 GM007281).
Competing interests
The authors declare no competing interests.
References
- 1.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu N, Pan T. N6-methyladenosine-encoded epitranscriptomics. Nat. Struct. Mol. Biol. 2016;23:98–102. doi: 10.1038/nsmb.3162. [DOI] [PubMed] [Google Scholar]
- 3.Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell. Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachtergaele S, He C. The emerging biology of RNA post-transcriptional modifications. RNA Biol. 2017;14:156–163. doi: 10.1080/15476286.2016.1267096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu N, et al. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–6063. doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pendleton KE, et al. The U6 snRNA m6A Methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169:824–835. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao W, et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol. Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Lence T, et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–247. doi: 10.1038/nature20568. [DOI] [PubMed] [Google Scholar]
- 9.Haussmann IU, et al. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, et al. Dynamic m6A modification and its emerging regulatory role in mRNA splicing. Sci. Bull. 2015;60:21–32. [Google Scholar]
- 11.Liu N, et al. N6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ping XL, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartosovic M, et al. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3’-end processing. Nucleic Acids Res. 2017;45:11356–11370. doi: 10.1093/nar/gkx778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kan L, et al. The m6A pathway facilitates sex determination in Drosophila. Nat. Commun. 2017;8:15737. doi: 10.1038/ncomms15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m5C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roundtree IA, et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. eLife. 2017;6:e31311. doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishima Y, Tomari Y. Codon usage and 3’ UTR length determine maternal mRNA stability in zebrafish. Mol. Cell. 2016;61:874–885. doi: 10.1016/j.molcel.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Du H, et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz S, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao BS, et al. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature. 2017;542:475–478. doi: 10.1038/nature21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell. Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, et al. m6A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- 26.Shi H, et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li A, et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi J, et al. N6-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat. Struct. Mol. Biol. 2016;23:110–115. doi: 10.1038/nsmb.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, et al. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominissini D, et al. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodi Z, Bottley A, Archer N, May ST, Fray RG. Yeast m6A methylated mRNAs are enriched on translating ribosomes during meiosis, and under rapamycin treatment. PLoS ONE. 2015;10:e0132090. doi: 10.1371/journal.pone.0132090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clancy MJ, Shambaugh ME, Timpte CS, Bokar JA. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 2002;30:4509–45018. doi: 10.1093/nar/gkf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah JC, Clancy MJ. IME4, a gene that mediates MAT and nutritional control of meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992;12:1078–1086. doi: 10.1128/mcb.12.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bodi Z, Button JD, Grierson D, Fray RG. Yeast targets for mRNA methylation. Nucleic Acids Res. 2010;38:5327–5335. doi: 10.1093/nar/gkq266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong SL, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haugland RA, Cline MG. Post-transcriptional modifications of oat coleoptile ribonucleic acids. 5’-terminal capping and methylation of internal nucleosides in poly(A)-rich RNA. Eur. J. Biochem. 1980;104:271–277. doi: 10.1111/j.1432-1033.1980.tb04425.x. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy TD, Lane BG. Wheat embryo ribonucleates. XIII. Methyl-substituted nucleoside constituents and 5’-terminal dinucleotide sequences in bulk poly(AR)-rich RNA from imbibing wheat embryos. Can. J. Biochem. 1979;57:927–931. doi: 10.1139/o79-112. [DOI] [PubMed] [Google Scholar]
- 39.Hongay CF, Orr-Weaver TL. Drosophila Inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc. Natl Acad. Sci. USA. 2011;108:14855–14860. doi: 10.1073/pnas.1111577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon KJ, et al. Temporal control of mammalian cortical neurogenesis by m6A methylation. Cell. 2017;171:877–889. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bansal H, et al. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia. 2014;28:1171–1174. doi: 10.1038/leu.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schibler U, Kelley DE, Perry RP. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J. Mol. Biol. 1977;115:695–714. doi: 10.1016/0022-2836(77)90110-3. [DOI] [PubMed] [Google Scholar]
- 43.Wei CM, Moss B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry. 1977;16:1672–1676. doi: 10.1021/bi00627a023. [DOI] [PubMed] [Google Scholar]
- 44.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horowitz S, Horowitz A, Nilsen TW, Munns TW, Rottman FM. Mapping of N6-methyladenosine residues in bovine prolactin mRNA. Proc. Natl Acad. Sci. USA. 1984;81:5667–5671. doi: 10.1073/pnas.81.18.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu K, et al. Mettl3-mediated m6A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27:1100–1114. doi: 10.1038/cr.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen T, et al. m6A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell. 2015;16:289–301. doi: 10.1016/j.stem.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Lavi S, Shatkin AJ. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc. Natl Acad. Sci. USA. 1975;72:2012–2016. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furuichi Y, et al. Methylated, blocked 5 termini in HeLa cell mRNA. Proc. Natl Acad. Sci. USA. 1975;72:1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams JM, Cory S. Modified nucleosides and bizarre 5’-termini in mouse myeloma mRNA. Nature. 1975;255:28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- 51.Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 53.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell. Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 2012;8:e1002732. doi: 10.1371/journal.pgen.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patil DP, et al. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen J, et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol. Cell. 2018;69:1028–1038. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knuckles P, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo, J., Tang, H. W., Li, J., Perrimon, N. & Yan D. Xio is a component of the Drosophila sex determination pathway and RNA N6-methyladenosine methyltransferase complex. Proc. Natl Acad. Sci. USA115, 3674–3679 (2018). [DOI] [PMC free article] [PubMed]
- 61.Śledź P, Jinek M. Structural insights into the molecular mechanism of the m6A writer complex. eLife. 2016;5:e18434. doi: 10.7554/eLife.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol. Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, et al. Structural basis of N6-adenosine methylation by the METTL3–METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 64.Rottman FM, Bokar JA, Narayan P, Shambaugh ME, Ludwiczak R. N6-adenosine methylation in mRNA: substrate specificity and enzyme complexity. Biochimie. 1994;76:1109–1114. doi: 10.1016/0300-9084(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 65.Harper JE, Miceli SM, Roberts RJ, Manley JL. Sequence specificity of the human mRNA N6-adenosine methylase in vitro. Nucleic Acids Res. 1990;18:5735–5741. doi: 10.1093/nar/18.19.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m6A methyltransferase METTL3 promotes translation in human cancer cells. Mol. Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bodi Z, et al. Adenosine methylation in Arabidopsis mRNA is associated with the 3’ end and reduced levels cause developmental defects. Front. Plant Sci. 2012;3:48. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aguilo F, et al. Coordination of m6A mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell. 2015;17:689–704. doi: 10.1016/j.stem.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geula S, et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 70.Li HB, et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548:338–342. doi: 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lv J, et al. Endothelial-specific m6A modulates mouse hematopoietic stem and progenitor cell development via Notch signaling. Cell Res. 2018;28:249–252. doi: 10.1038/cr.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bujnicki JM, Feder M, Radlinska M, Blumenthal RM. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA: m6A methyltransferase. J. Mol. Evol. 2002;55:431–444. doi: 10.1007/s00239-002-2339-8. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz S, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421. doi: 10.1016/j.cell.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang, J. et al. Solution structure of the RNA recognition domain of METTL3-METTL14 N6-methyladenosine methyltransferase. Protein Cell10.1007/s13238-018-0518-7 (2018). [DOI] [PMC free article] [PubMed]
- 75.Lin Z, et al. Mettl3-/Mettl14-mediated mRNA N6-methyladenosine modulates murine spermatogenesis. Cell Res. 2017;27:1216–1230. doi: 10.1038/cr.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui Q, et al. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weng H, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification. Cell Stem Cell. 2018;22:191–205.e9. doi: 10.1016/j.stem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horiuchi K, et al. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J. Biol. Chem. 2013;288:33292–33302. doi: 10.1074/jbc.M113.500397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Granadino B, Campuzano S, Sanchez L. The Drosophila melanogaster fl(2)d gene is needed for the female-specific splicing of Sex-lethal RNA. EMBO J. 1990;9:2597–2602. doi: 10.1002/j.1460-2075.1990.tb07441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ortega A, et al. Biochemical function of female-lethal (2)D/Wilms’ tumor suppressor-1-associated proteins in alternative pre-mRNA splicing. J. Biol. Chem. 2003;278:3040–3047. doi: 10.1074/jbc.M210737200. [DOI] [PubMed] [Google Scholar]
- 81.Yue Y, et al. VIRMA mediates preferential m6A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerken T, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jia G, et al. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 2008;582:3313–3319. doi: 10.1016/j.febslet.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fu Y, et al. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat. Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mauer J, et al. Reversible methylation of m6Am in the 5’ cap controls mRNA stability. Nature. 2017;541:371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dina C, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 88.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scuteri A, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao X, Yang Y, Sun BF, Zhao YL, Yang YG. FTO and obesity: mechanisms of association. Curr. Diab. Rep. 2014;14:486. doi: 10.1007/s11892-014-0486-0. [DOI] [PubMed] [Google Scholar]
- 91.Li Z, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su R, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m6A/MYC/CEBPA Signaling. Cell. 2018;172:90–105. doi: 10.1016/j.cell.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deng X, et al. Role of N6-methyladenosine modification in cancer. Curr. Opin. Genet. Dev. 2017;48:1–7. doi: 10.1016/j.gde.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vu LP, et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang C, et al. ALKBH5-dependent m6A demethylation controls splicing and stability of long 3’-UTR mRNAs in male germ cells. Proc. Natl Acad. Sci. USA. 2018;115:E325–E333. doi: 10.1073/pnas.1717794115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng Q, Hou J, Zhou Y, Li Z, Cao X. The RNA helicase DDX46 inhibits innate immunity by entrapping m6A-demethylated antiviral transcripts in the nucleus. Nat. Immunol. 2017;18:1094–1103. doi: 10.1038/ni.3830. [DOI] [PubMed] [Google Scholar]
- 97.Zhang S, et al. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang C, et al. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7:64527–64542. doi: 10.18632/oncotarget.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu N, Pan T. N6-methyladenosine-encoded epitranscriptomics. Nat. Struct. Mol. Biol. 2016;23:98–102. doi: 10.1038/nsmb.3162. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Z, et al. The YTH domain is a novel RNA binding domain. J. Biol. Chem. 2010;285:14701–14710. doi: 10.1074/jbc.M110.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang B, et al. Alternative splicing-related factor YT521: an independent prognostic factor in endometrial cancer. Int. J. Gynecol. Cancer. 2010;20:492–499. doi: 10.1111/IGC.0b013e3181d66ffe. [DOI] [PubMed] [Google Scholar]
- 102.Xu C, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat. Chem. Biol. 2014;10:927–929. doi: 10.1038/nchembio.1654. [DOI] [PubMed] [Google Scholar]
- 103.Hirschfeld M, et al. Hypoxia-dependent mRNA expression pattern of splicing factor YT521 and its impact on oncological important target gene expression. Mol. Carcinog. 2014;53:883–892. doi: 10.1002/mc.22045. [DOI] [PubMed] [Google Scholar]
- 104.Alarcón CR, et al. HNRNPA2B1 is a mediator of m6A -dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu B, et al. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat. Commun. 2018;9:420. doi: 10.1038/s41467-017-02770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hsu PJ, et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–1127. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bailey AS, et al. The conserved RNA helicase YTHDC2 regulates the transition from proliferation to differentiation in the germline. eLife. 2017;6:e26116. doi: 10.7554/eLife.26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ivanova I, et al. The RNA m6A reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Mol. Cell. 2017;67:1059–1067. doi: 10.1016/j.molcel.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wojtas MN, et al. Regulation of m6A transcripts by the 3’ → 5’ RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol. Cell. 2017;68:374–387. doi: 10.1016/j.molcel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 110.Jain D, et al. ketu mutant mice uncover an essential meiotic function for the ancient RNA helicase YTHDC2. eLife. 2018;7:e30919. doi: 10.7554/eLife.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abby E, et al. Implementation of meiosis prophase I programme requires a conserved retinoid-independent stabilizer of meiotic transcripts. Nat. Commun. 2016;7:10324. doi: 10.1038/ncomms10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soh YQS, et al. Meioc maintains an extended meiotic prophase I in mice. PLoS Genet. 2017;13:e1006704. doi: 10.1371/journal.pgen.1006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tanabe A, et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett. 2016;376:34–42. doi: 10.1016/j.canlet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 114.Patil DP, Pickering BF, Jaffrey SR. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell. Biol. 2018;28:113–127. doi: 10.1016/j.tcb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hosono Y, et al. Oncogenic role of THOR, a conserved cancer/testis long non-coding RNA. Cell. 2017;171:1559–1572. doi: 10.1016/j.cell.2017.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Edupuganti RR, et al. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat. Struct. Mol. Biol. 2017;24:870–878. doi: 10.1038/nsmb.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arguello AE, DeLiberto AN, Kleiner RE. RNA chemical proteomics reveals the N6-methyladenosine (m6A)-regulated protein-RNA interactome. J. Am. Chem. Soc. 2017;139:17249–17252. doi: 10.1021/jacs.7b09213. [DOI] [PubMed] [Google Scholar]
- 118.Adhikari S, Xiao W, Zhao YL, Yang YG. m6A: Signaling for mRNA splicing. RNA Biol. 2016;13:756–759. doi: 10.1080/15476286.2016.1201628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ke S, et al. A majority of m6A residues are in the last exons, allowing the potential for 3’ UTR regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Molinie B, et al. m6A-LAIC-seq reveals the census and complexity of the m6A epitranscriptome. Nat. Methods. 2016;13:692–698. doi: 10.1038/nmeth.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fustin JM, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 122.Meyer KD, et al. 5’UTR m6A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Batista PJ, et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roost C, et al. Structure and thermodynamics of N6-methyladenosine in RNA: a spring-loaded base modification. J. Am. Chem. Soc. 2015;137:2107–2115. doi: 10.1021/ja513080v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spitale RC, et al. Structural imprints in vivo decode RNA regulatory mechanisms. Nature. 2015;519:486–490. doi: 10.1038/nature14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wan Y, et al. Landscape and variation of RNA secondary structure across the human transcriptome. Nature. 2014;505:706–709. doi: 10.1038/nature12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou KI, et al. N6-methyladenosine modification in a long noncoding RNA hairpin predisposes its conformation to protein binding. J. Mol. Biol. 2016;428:822–833. doi: 10.1016/j.jmb.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat. Rev. Mol. Cell. Biol. 2017;18:437–451. doi: 10.1038/nrm.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Braunschweig U, Gueroussov S, Plocik AM, Graveley BR, Blencowe BJ. Dynamic integration of splicing within gene regulatory pathways. Cell. 2013;152:1252–1269. doi: 10.1016/j.cell.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee Y, Rio DC. Mechanisms and regulation of alternative pre-mRNA splicing. Annu. Rev. Biochem. 2015;84:291–323. doi: 10.1146/annurev-biochem-060614-034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 132.Salditt-Georgieff M, et al. Methyl labeling of HeLa cell hnRNA: a comparison with mRNA. Cell. 1976;7:227–237. doi: 10.1016/0092-8674(76)90022-2. [DOI] [PubMed] [Google Scholar]
- 133.Carroll SM, Narayan P, Rottman FM. N6-methyladenosine residues in an intron-specific region of prolactin pre-mRNA. Mol. Cell. Biol. 1990;10:4456–4465. doi: 10.1128/mcb.10.9.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stoltzfus CM, Dane RW. Accumulation of spliced avian retrovirus messenger-rna is inhibited in S-adenosylmethionine-depleted chicken-embryo fibroblasts. J. Virol. 1982;42:918–931. doi: 10.1128/jvi.42.3.918-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Penn JK, et al. Functioning of the Drosophila Wilms’-tumor-1-associated protein homolog, Fl(2)d, in sex-lethal-dependent alternative splicing. Genetics. 2008;178:737–748. doi: 10.1534/genetics.107.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ye F, Chen ER, Nilsen TW. Kaposi’s sarcoma-associated herpesvirus utilizes and manipulates RNA N6-Adenosine methylation to promote lytic replication. J. Virol. 2017;91:e00466–17. doi: 10.1128/JVI.00466-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ke S, et al. m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017;31:990–1006. doi: 10.1101/gad.301036.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang ET, et al. Transcriptome-wide regulation of pre-mRNA splicing and mRNA localization by muscleblind proteins. Cell. 2012;150:710–724. doi: 10.1016/j.cell.2012.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.König J, et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat. Struct. Mol. Biol. 2010;17:909–915. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen K, et al. High-resolution N6-methyladenosine m6A map using photo-crosslinking-assisted m6A sequencing. Angew. Chem. Int. Ed. Engl. 2015;54:1587–1590. doi: 10.1002/anie.201410647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Linder B, et al. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Norton S, Vaquero-Garcia J, Lahens NF, Grant GR, Barash FC. Outlier detection for improved differential splicing quantification from RNA-Seq experiments with replicates. Bioinformatics. 2018;34:1488–1497. doi: 10.1093/bioinformatics/btx790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goodwin M, et al. MBNL sequestration by toxic RNAs and RNA misprocessing in the myotonic dystrophy brain. Cell Rep. 2015;12:1159–1168. doi: 10.1016/j.celrep.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Charizanis K, et al. Muscleblind-like 2-mediated alternative splicing in the developing brain and dysregulation in myotonic dystrophy. Neuron. 2012;75:437–450. doi: 10.1016/j.neuron.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Taliaferro JM, et al. Distal alternative last exons localize mRNAs to neural projections. Mol. Cell. 2016;61:821–833. doi: 10.1016/j.molcel.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aschenbrenner J, et al. Engineering of a DNA polymerase for direct m6A sequencing. Angew. Chem. Int. Ed. Engl. 2017;57:417–421. doi: 10.1002/anie.201710209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Garalde DR, et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods. 2018;15:201–206. doi: 10.1038/nmeth.4577. [DOI] [PubMed] [Google Scholar]