Abstract
Chiral allylic amines are not only present in many bioactive compounds, but can also be readily transformed to other chiral amines. Therefore, the asymmetric synthesis of chiral allylic amines is highly desired. Herein, we report two types of Ni(II)-catalyzed asymmetric alkenylation of cyclic ketimines for the preparation of chiral allylic amines. When ketimines bear alkyl or alkoxycarbonyl groups, the alkenylation gives five- and six-membered cyclic α-tertiary allylic amine products with excellent yields and enantioselectivities under mild reaction conditions. A variety of ketimines can be used and the method tolerates some variation in alkenylboronic acid scope. Furthermore, with alkenyl five-membered ketimine substrates, an alkenylation/rearrangement reaction occurs, providing seven-membered chiral sulfamide products bearing a conjugated diene skeleton with excellent yields and enantioselectivities. Mechanistic studies reveal that the ring expansion step is a stereospecific site-selective process, which can be catalyzed by acid (Lewis acid or Brønsted acid).
Chiral allylic amines are often encountered in bioactive compounds and can be conveniently transformed into other chiral amines. Here, the authors report nickel-catalyzed enantioselective addition and addition/ring-expansion of alkenylboronic acids to ketimines providing a range of valuable chiral azaheterocycles.
Introduction
In recent years, the first-row late transition-metal-catalysts have attracted a lot of attention due to their low cost and abundance, meeting the requirements for sustainable chemical synthesis1–5. Addition of organometallic reagents to electron-deficient double bonds is one of the most powerful reactions for the construction of various molecular skeletons. To expand the viability of transition-metal catalysts for use in addition reactions of organoboron reagents and to lower the cost of transition-metal catalysts for use in industrial processes, the first-row late transition-metal-catalyzed addition of organoboron reagents to double bonds has become an attractive target6–24. Nickel, as a first-row transition-metal in the same group as that of palladium, has attracted much attention25, 26. However, nickel-catalyzed additions of organoboron reagents to electron-deficient double bonds have not been widely reported14–24.
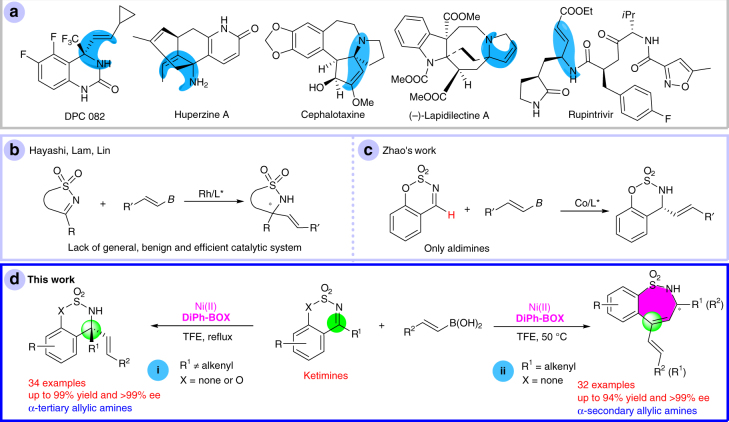
Chiral amines are important skeletons found in bioactive natural products and potent drugs, therefore their asymmetric synthesis has attracted much attention over the past few decades27–29. Chiral allylic amines are not only present in many bioactive compounds (Fig. 1a), but can also be readily transformed to other chiral amines via the functionalization of the additional olefin group. Therefore, the asymmetric synthesis of chiral allylic amines is highly desired. Herein, we report two types of Ni(II)-catalyzed asymmetric alkenylations of cyclic ketimines for the preparation of a series of chiral α-tertiary and α-secondary allylic amines under mild reaction conditions.
Fig. 1.
Addition of alkenylborons to imines for construction of chiral allylic amines. a Representative bioactive chiral allylic amines. b Rh-catalyzed addition of alkenylborons to ketimine. c Co-catalyzed addition of alkenylborons to aldimine. d This work: a general Ni(II)/BOX-catalyzed alkenylation and alkenylation/ring-expansion of cyclic ketimines. BOX = bisoxazoline
Results
Reaction discovery
Organoboron reagents, which can be conveniently prepared from industrial materials, have been widely used in transition-metal-catalyzed addition reactions30–36. Pioneered by the Hayashi group, Rh-catalyzed asymmetric additions of arylboron reagents to imines, including ketimines, have been well studied32–46. However, the addition of alkenylboron reagents to imines for the preparation of chiral allylic amines has not been widely reported, possibly due to their relatively low stability. Although Rh-catalyzed asymmetric additions of alkenylborons to aldimines have been studied by two groups47–49, a general, benign, and efficient catalytic system for similar reactions using ketimines is still highly desired (Fig. 1b)45–47. The Pd-catalyzed addition of alkenylboron reagents to double bonds, including imines, has not been described50–57. This may be due to potential side-reactions, such as homocouplings, Heck reactions, and aza-Wacker-type reactions. The Zhao group recently reported a Co(II)-catalyzed asymmetric addition of alkenylboronic acids to cyclic aldimines (Fig. 1c)58. However, the addition of alkenylboron reagents to ketimines, especially for the first-row late transition-metal-catalysis (including nonasymmetric process), remains highly desirable. We herein describe a general and highly efficient enantioselective addition of alkenylboronic acids to ketimines catalyzed by Ni(II) (Fig. 1d, reaction i). The preparation of seven-membered chiral sulfamides via the asymmetric addition of organoboron reagents to imines has not been previously reported, possibly due to the lack of a method for the synthesis of related substrates. Our methodology provides an alternative pathway to address this issue. We report a Ni(II)-catalyzed asymmetric cascade alkenylation/ring-expansion of unstrained five-membered cyclic ketimines via an α,α-dialkenyl substituted five-membered sulfamide intermediate (Fig. 1d, reaction ii), providing a series of seven-membered cyclic α-substituted chiral sulfamides59, 60. We found that in our reaction, the ring expansion step is a site-selective and stereospecific process.
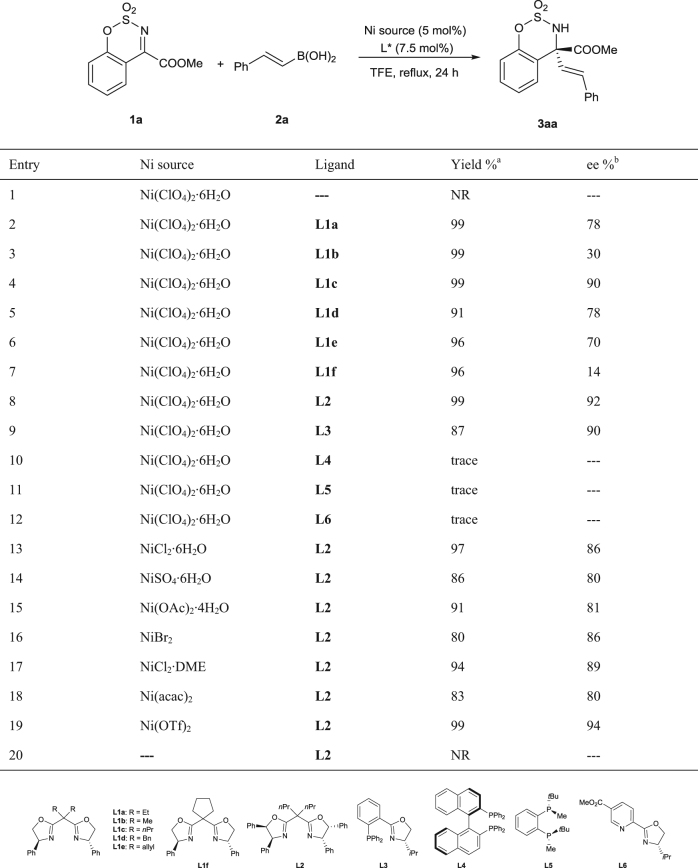
Investigation of reaction conditions
Our study began with the reaction of cyclic ketimine (1a) with phenyl alkenylboronic acid (2a) (Table 1). In the absence of ligand, no product was obtained when the reaction was heated at reflux in trifluoroethanol (TFE) for 24 h in air, and the starting materials were recovered intact. After screening various reaction conditions including solvent and reaction temperature, we found that the desired product could be obtained with excellent yield and moderate enantioselectivity by carrying out the reaction with 5.0 mol% of Ni(ClO4)2·6H2O, 7.5 mol% bisoxazoline ligand L1a in TFE at reflux for 24 h, under air (Table 1, entry 2. See Supplementary Table 1). The screening of substituents on the oxazoline rings of the ligand showed that the phenyl group gave the best results (see Supplementary Table 1). The effect of substituents on the bridge of the BOX ligand was also studied, and the best ee was obtained with ligand L1c bearing n-Pr groups (Table 1, entries 2–7). An additional phenyl group on both of the oxazoline rings (L2 = DiPh-BOX) led to an increase in ee from 90% to 92% (Table 1, entry 8 vs entry 4). Reactions with other types of ligands showed moderate or poor results (L3–L6, Table 1, entries 9–12). Finally, Ni(OTf)2 was found to be the best nickel source for the alkenylation (Table 1, entry 19). All other nickel salts resulted in a slight decrease in yield or ee (Table 1, entries 13–18). None of the desired product was obtained in the absence of Ni salt (Table 1, entry 20).
Table 1.
Reaction optimization
Reactions were carried out on a 0.20 mmol scale (1a) using trans-PhCH=CHB(OH)2 (2a) (0.30 mmol), 5 mol% nickel salt, 7.5 mol% ligand in unpurified TFE (2.0 mL) in a test tube for 24 h which was opened to air
aIsolated yields
bDetermined by HPLC using a chiral Enantiocol column. TFE = trifluoroethanol, NR = no reaction, ee = enantiomeric excess
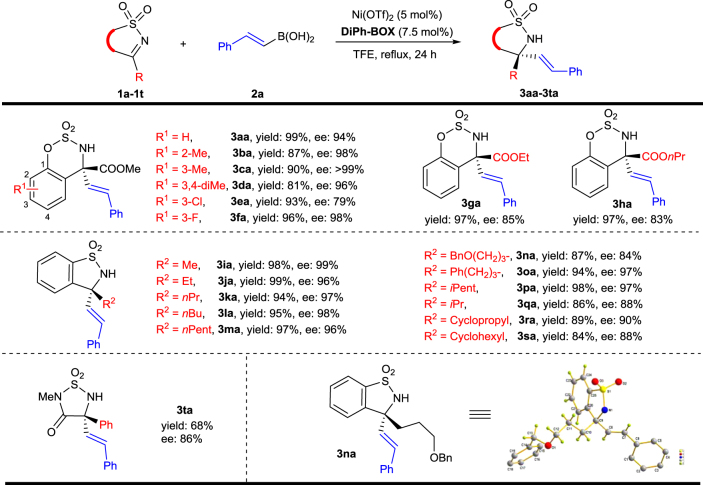
Scope of the asymmetric alkenylation of ketimines
With the optimized conditions in hand, the ketimine substrate scope was examined (Table 2). Substrates possessing methyl groups at different positions gave slightly higher ee’s (3ba–3da vs 3aa). Fluoride and chloride substituents have opposite effects on enantioselectivity (3ea and 3fa). Lower ee’s were obtained for substrates bearing bulkier ester groups (3ga and 3ha). To our delight, saccharin-derived ketimines are also compatible substrates for this reaction (3ia–3sa). Branched alkyl-substituted substrates provided the corresponding products with slightly lower ee’s and yields when compared to their linear alkyl-substituted counterparts (3ia–3sa). Our catalytic system could also be applied to substrate 1t46 giving a chiral α-tertiary amino acid derivative. The absolute configuration of the alkenylation product 3na was determined to be (R) by X-ray crystallographic analysis (Table 2).
Table 2.
Scope of ketimine substrates
Reactions were carried out on a 0.20 mmol scale using 5 mol% Ni(OTf)2, 7.5 mol% ligand and trans-PhCH = CHB(OH)2 (2a) (0.30 mmol) in TFE (2.0 mL) at reflux in a test tube for 24 h, which was opened to air. The yields are isolated yields and ee’s were determined by chiral HPLC
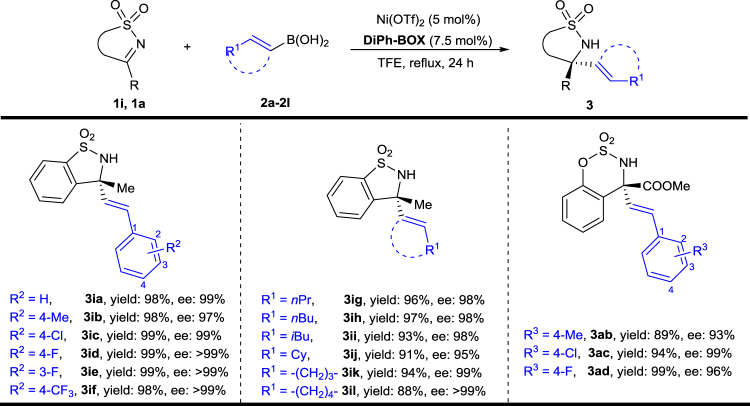
The alkenylboronic acid scope was also investigated, the results of which are summarized in Table 3. Firstly, substrate 1i was used as a ketimine for reaction with different alkenyl nucleophiles. The type of substituents on the benzene ring of the styrene-boronic acids have little influence on the results, and excellent yields and enantioselectivities could be obtained (3ia–3if). It is worth noting that the reaction proceeded smoothly when aliphatic group-substituted vinyl boronic acids were used as nucleophiles (3ig–3il). Disubstituted and trisubstituted vinyl boronic acids gave their corresponding products with good to excellent yields and enantioselectivities (3ig–3il). Substrate 1a was also tested as a substrate for the addition of several different styrene-derived boronic acids, and good results were obtained (3ab–3ad).
Table 3.
Scope of alkenylboronic acids
Ni(II)-catalyzed asymmetric alkenylation/ring-expansions
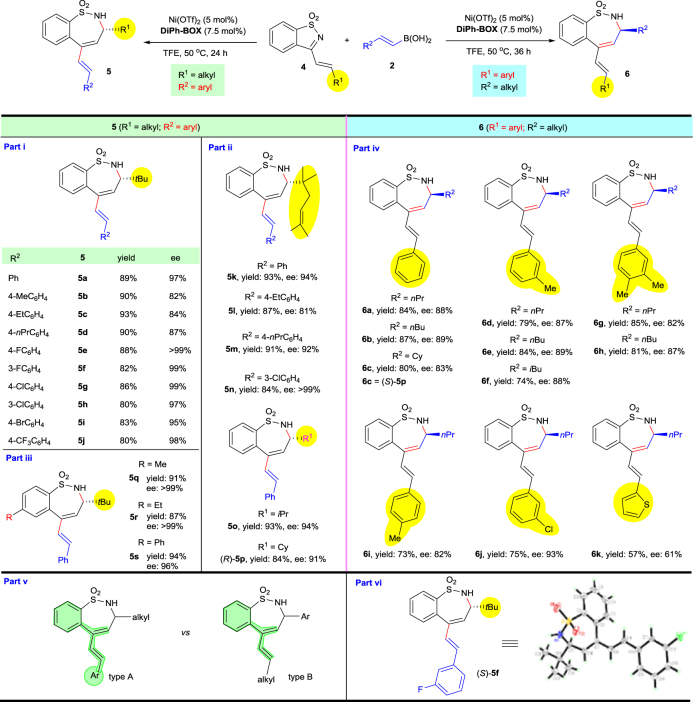
We were also interested in studying the regioselective and enantioselective issues of the addition of alkenylboronic acids to α,β-unsaturated cyclic ketimines. To our surprise, a seven-membered α-substituted chiral sulfamide (5a) bearing a conjugated diene system was obtained (Table 4). We next focused on developing this interesting asymmetric (n + 2)-ring expansion reaction.
Table 4.
Large π-conjugation-controlled site-selective asymmetric ketimine-alkenylation/ring-expansion
Reactions were carried out on a 0.20 mmol scale of 4 using 5 mol% Ni(OTf)2, 7.5 mol% ligand and 2 (0.30 mmol) in unpurified TFE (2.0 mL) at 50 oC in a test tube, which was open to air. Isolated yields and ee’s were determined by chiral HPLC
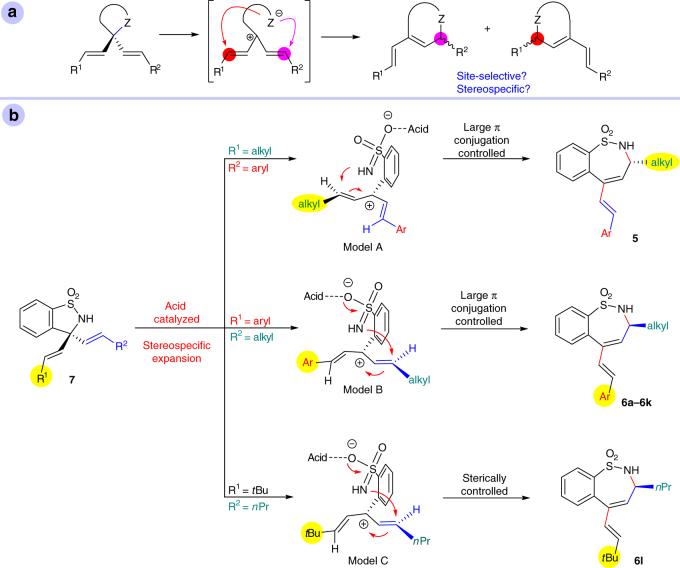
Three- and four-membered vinyl cyclic compounds have been well studied for the construction of five- and six-membered systems via (n + 2)-expansion due to their strong tendency to release their high ring strain61, 62. However, the (n + 2)-expansion of larger vinyl cyclic compounds remains challenging due to the fact that they possess less ring strain than their smaller ring counterparts, an essential driving force for ring expansion63. Furthermore, the asymmetric synthesis of chiral compounds via this ring (n + 2)-expansion has not been widely reported, possibly due to the lack of interactions between the alkenyl group and chiral catalyst that would allow for the remote control of the attacking enantioface at the β-carbon of the C=C bond64–66. Njardarson reported a chiral acid-catalyzed asymmetric ring (n + 2)-expansion of dialkenyl oxetane with only one substrate, requiring a low temperature and long reaction time to obtain the desired product with good ee and moderate yield67. Conversely, our catalytic system enables the ring-expansion of unstrained five-membered aza-rings (Table 4), providing a series of seven-membered α-substituted chiral sulfamides59, 60.
Scope of the asymmetric alkenylation/ring-expansions
After screening a series of parameters, the optimal reaction conditions were found to be when using Ni(OTf)2 as nickel source, DiPh-BOX (L2) as a chiral ligand, and performing the reaction in TFE solvent at 50 oC over 24 h (see Supplementary Table 2). It should be noted that other types of ligand were ineffective for this reaction. With the optimized conditions in hand, the alkenylboronic acid scope was examined (Table 4). Two series of catalytic reactions were carried out: the reaction of alkyl vinyl-substituted ketimines with styrenyl boronic acids (Table 4, Parts i–iii); and the reaction of styrenyl ketimines with alkyl-substituted vinyl boronic acids (Table 4, Part iv). Interestingly, the results showed that the site-selectivity of this cascade alkenylation/ring-expansion could be controlled by the driving force for the formation of a larger π-conjugated system, thus only isomer A was observed while none of the B type isomer was detected in the crude 1H NMR spectra (Table 4, Part v).
Firstly, different styrenyl boronic acids were tested with substrate 4a (Table 4, Part i). The results showed that styrenyl boronic acids bearing either electron-donating or electron-withdrawing substituents on the benzene ring gave the corresponding products with good to excellent yields and ee’s (5a–5j). The enantioselectivities for substrates bearing electron-donating substituents are comparatively lower than those for substrates bearing electron-withdrawing substituents, albeit good ee’s could still be obtained for all examples (5b–5d vs 5e–5j). The absolute configuration of 5f was confirmed to be (S) by X-ray crystallographic analysis (Table 4, Part vi).
Substrates with different alkyl vinyl substituents were also investigated and the results are summarized in Part ii of Table 4. When R1 is alkyl and R2 is aryl, the corresponding products 5k–5p were obtained with excellent yields and ee’s. Substituents on the benzene ring of the substrates have little influence on the reaction outcome (Part iii, 5q–5s).
The R1 group does not necessarily end up on the carbon atom α to the nitrogen of the product. When R1 was aryl and R2 was alkyl, the products 6, possessing the opposite configuration of 5 and with the R2 group at the α-position of the nitrogen, were obtained (Table 4, Part iv). Different substrates gave their corresponding products with good to excellent ee’s. The desired products were obtained with lower yields and required longer reaction times (6a–6j) compared to that of products 5, most likely due to the poor solubility of the substrates. Product 6k bearing a thienyl substituent was obtained in moderate yield and ee. The absolute configurations were confirmed to be opposite by comparison of the HPLC spectra and optical rotation data of 6c and (R)-5p.
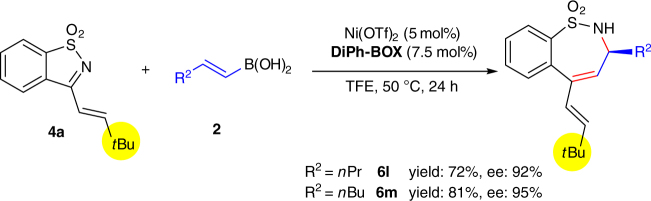
Surprisingly, when linear alkyl-substituted vinylboronic acids were used for reaction with 4a, the expansion of the ring occurred on the linear alkyl group side (Fig. 2), perhaps as a result of the steric hindrance of the bulky tBu group. Good yields and ee’s could also be obtained in both cases.
Fig. 2.
Sterically-controlled site-selective asymmetric alkenylation/ring-expansion of ketimine 4a. The results using linear alkyl-substituted vinylboronic acids as nucleophiles reaction with 4a indicate that the asymmetric alkenylation/ring-expansion can also be controlled by steric hindrance
Mechanistic considerations
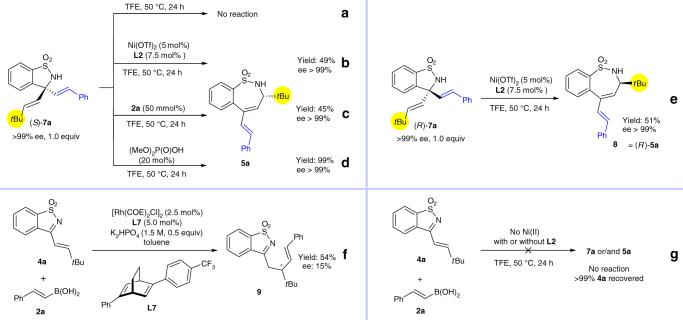
The results above indicate that nickel is capable of catalyzing the asymmetric alkenylation and alkenylation/ring-expansion of cyclic ketimines. The importance of this process prompted us to investigate the reaction mechanism. We propose that (S)-7a is the intermediate of the cascade reaction, which is the product resulting from alkenylation of the ketimine. Firstly, the stereochemistry and catalyst for the ring expansion step were studied using control experiments48, 49. Compound (S)-7a was stirred in TFE at 50 oC for 24 h. In the absence of catalyst and alkenylboronic acid, no ring expansion was observed (Fig. 3a). When the reaction was conducted with catalyst but no alkenylboronic acid, the product 5a was obtained with 49% yield and with no loss in ee (Fig. 3b). Interestingly, ring expansion product 5a could also be obtained in the presence of only the alkenylboronic acid or using (MeO)2P(O)OH as a catalyst, without any loss in ee (Fig. 3c, d). Furthermore, when ring expansion of (R)-7a was catalyzed by Ni catalyst, the enantiomeric product 8 was obtained (Fig. 3e). The above results indicate that the ring expansion step is an acid-promoted process, either by a Lewis acid or Brønsted acid59, 60, 64–66, and no matching/mismatching effect was observed between the catalyst and intermediate 7a. The ring expansion step for this reaction is a stereospecific process (see Supplementary Note 2 for preliminary DFT calculation results), suggesting that the chiral induction by the ligand occurs at the ketimine-alkenylation step, which is a regio-specific 1,2-addition process. It should be noted that Xu has found that the Rh-catalyzed asymmetric addition of arylboron to α,β-unsaturated cyclic ketimines gave regioselective 1,4-addition products68. The 1,4-addition product 9 was also obtained for the reaction of 4a and 2a, using rhodium as a catalyst (Fig. 3f). These reactions indicate the unique property of Ni-catalysts for addition reactions with alkenylborons compared to Rh-catalysts58, 69. Two control experiments were also conducted. Reactions of 4a with 2a in the absence of Ni(II) and with or without ligand L2, did not give rise to product 7a or 5a, with 4a being completely recovered (Fig. 3g). This also indicates that the alkenylation step requires the assistance of Ni(II) and the chiral ligand.
Fig. 3.
Control experiments. These experiments indicate that the ring expansion step is an acid-promoted stereospecific process
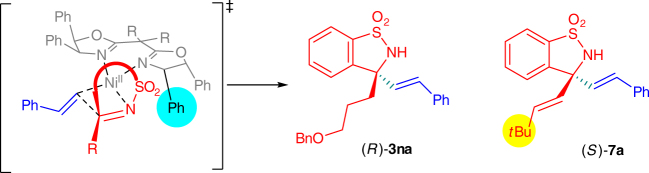
The stereochemical outcome can be explained using the model shown in Fig. 4, similar to that of Rh- and Pd-catalysis30–36, 50–57. The substrate coordinates with nickel and the sulfonyl group lies on the opposite side of diphenyl substituents on the oxazoline ring. This leads to (R)-3na as the major product and also leads to (S)-7a as the intermediate for producing 5a.
Fig. 4.
Model for explaining the origin of asymmetric induction. The alkenyl attacks the C=N bond from the Re-face to generate the products (R)-3na or (S)-7a
For α,α-dialkenyl heterocyclic compounds bearing two different alkenyl groups, control of the site-selectivity of the ring expansion and maintaining optical purity are challenging (Fig. 5a). Since 7 was confirmed to be the intermediate of the reaction for the preparation of seven-membered sulfamides from five-membered ketimines, our research shows that the aforementioned challenges can be overcome by choosing suitable substrates. A mechanistic explanation of this site-selective stereospecific process has been proposed (Fig. 5b). Ketimine substrate 4 undergoes Ni(II)/BOX-catalyzed addition of alkenylboronic acid to form dialkenyl sulfamide intermediate 7 following a mechanism similar to that shown in Fig. 4. Intermediate 7 is then activated immediately by the acid (Lewis acid or Brønsted acid) and the C–N bond is cleaved to generate a carbocation intermediate. When alkyl vinyl ketimine and styrenyl boronic acid are used (R1 = alkyl, R2 = aryl; Fig. 5b), carbocation model A is generated. When styrenyl ketimine and alkyl vinyl boronic acid are used (R1 = aryl, R2 = alkyl; Fig. 5b), carbocation model B is generated. In models A and B, the sulfamide anion attacks the alkyl-substituted carbon to yield the large π-conjugated ring system 5 or 6, respectively, with opposite configurations. When R1 is a tBu group and R2 is a nPr group, the sulfamide anion attacks the less sterically hindered carbon atom of model C, yielding 6l. The rotation of the single bond between the sulfonyl benzene and carbocation can lead to lower ee’s for the desired products (racemization process), however, it seems that this racemization process does not occur easily under the optimized reaction conditions due to that steric hindrance exists around the axis of this single bond (like that observed for axially-chiral biphenyl compounds) (see Supplementary Note 2 for preliminary DFT calculation results). In consideration of the experimental results shown in Table 4, it can be concluded that this reaction favors larger π-conjugation over lower steric hindrance.
Fig. 5.
Challenges for ring-expansion and proposed mechanism. a Challenges for ring-expansion of α,α-dialkenyl heterocyclic compounds bearing two different alkenyl groups. b Proposed mechanism for ring-expansion step
Synthetic utility of alkenylation products
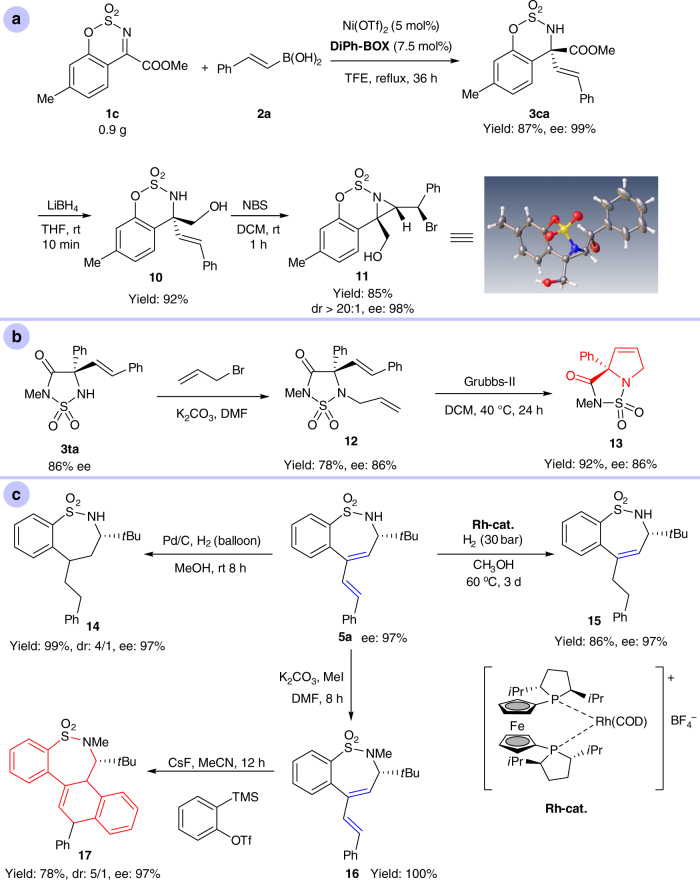
A gram-scale reaction was conducted and the product 3ca was obtained in good yield with excellent enantioselectivity (Fig. 6a). The ester group of 3ca was reduced with LiBH4 conveniently to 10. A bromoamination of the olefin group of 10 then gave cyclization product 11, a chiral aziridine bearing three carbon stereocenters, including a tetrasubstituted carbon atom. Product 3aa was reduced to a tetrasubstituted amino alcohol by LiAlH4 with excellent yield and enantioselectivity (see Supplementary Note 3). The alkenylated amino acid product 3ta was also converted to interesting skeletons (Fig. 6b). After allylation of the nitrogen of the sulfamide, a metathesis reaction catalyzed by Grubbs-II catalyst was carried out, which furnished a chiral 2,2-disubstituted pyrrolidine 13 without any loss in ee. This skeleton, which contains at least one aryl substituent, represents an important class of pharmaceutically relevant compounds70.
Fig. 6.
Synthetic utility of alkenylation products. a Gram-scale reaction and transformations of 3ca. b Transformations of 3ta. c Further transformations of 5a
Several transformations of seven-membered sulfamide product 5a were also performed (Fig. 6c). Both C=C bonds of 5a could be hydrogenated using Pd/C as a catalyst. Product 14 was obtained with no loss in ee but with moderate dr (diastereoisomer ratio). Interestingly, when [Rh(iPr-Ferphos)(cod)]BF4 was used as a catalyst, the disubstituted C=C bond could be hydrogenated selectively to generate 15 with no reduction in ee. A Diels–Alder reaction was also conducted. Firstly, 5a was quantitatively methylated to 16 with MeI. Subsequently, the corresponding benzyne was generated in situ from 2-(trimethylsilyl)phenyl trifluoro-methanesulfonate, which was reacted with 16 to give the tetracyclic compound 17 with 5:1 dr and 97% ee.
Discussion
In summary, we have developed a general enantioselective addition of alkenylboronic acids to ketimines enabled by a Ni(II)/BOX-catalytic system. A relatively wide substrate scope of cyclic ketimines and alkenylboronic acids are compatible with our reaction conditions. The desired chiral α-tertiary allylic amines can be obtained in good yields (up to 99%) and enantioselectivities (up to >99% ee). A Ni(II)-catalyzed asymmetric cascade alkenylation/ring-expansion reaction of alkenyl cyclic ketimines was also developed. A series of seven-membered chiral sulfamides were synthesized with good yields (up to 94%) and enantioselectivities (up to >99% ee) under mild reaction conditions. This reaction is also an efficient method for the preparation of trisubstituted conjugated dienes. Mechanistic studies showed that the alkenylation is the enantioselectivity-determining step, while the ring expansion step is a stereospecific process. The site-selectivity of the ring rearrangement expansion can be controlled by the formation of a large π-conjugated system or by steric interactions. Transformations of products for both types of reactions were conducted to show the potential applications of our methodologies.
Methods
Procedure for asymmetric alkenylations of ketimines
A test tube (100 mL) was charged with Ni(OTf)2 (3.6 mg, 0.010 mmol, 0.050 equiv), L2 (8.1 mg, 0.015 mmol, 0.075 equiv), and unpurified TFE (1.0 mL). The solution was stirred at reflux for 5 min, then substrate (0.20 mmol, 1.0 equiv) and alkenylboronic acid (0.30 mmol, 1.5 equiv) were added into the tube. The wall of the tube was rinsed with an additional portion of TFE (1.0 mL). After stirring at reflux for 24 h in air, the reaction mixture was cooled to room temperature and the solvent was removed by rotary evaporation. The residue was purified by preparative TLC on silica gel (petroleum ether/EtOAc = 5/1) to give the product.
Procedure for asymmetric alkenylation/ring-expansions
A test tube (100 mL) was charged with Ni(OTf)2 (3.6 mg, 0.010 mmol, 0.050 equiv), L2 (8.1 mg, 0.015 mmol, 0.075 equiv), and unpurified TFE (1.0 mL). The solution was stirred at 50 oC for 20 min, then substrate (0.20 mmol, 1.0 equiv) and alkenylboronic acid (0.30 mmol, 1.5 equiv) were added into the tube. The wall of the tube was rinsed with an additional portion of TFE (1.0 mL). After stirring at 50 oC for 24 h or 36 h in air, the reaction mixture was cooled to room temperature and the solvent was removed by rotary evaporation. The residue was purified by preparative TLC on silica gel (petroleum ether/EtOAc = 5/1) to give the product.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information file. For the experimental procedures, and NMR and HPLC analysis of the compounds in this article, see Supplementary Methods and charts in the Supplementary Information file. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (3na: CCDC 1486078, 5f: CCDC 1556426, 11: CCDC 1816920). These data could be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Electronic supplementary material
Acknowledgments
We thank the National Natural Science Foundation of China (Nos. 21302124, 21620102003, and 21772119), Science and Technology Commission of Shanghai Municipality (No. 15JC1402200), and Shanghai Municipal Education Commission (No. 201701070002E00030) for the financial support.
Author contributions
M.Q. conducted most of the synthetic experiments. X.W. and L.W. conducted part of the synthetic experiments. I.D.G. conducted the DFT computational study. W.Z., G.Y., and M.Q. wrote the manuscript. G.Y. and W.Z. directed the project. All authors contributed to discussions.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-04645-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guoqiang Yang, Email: gqyang@sjtu.edu.cn.
Wanbin Zhang, Email: wanbin@sjtu.edu.cn.
References
- 1.Bullock, R. M. (Ed) Catalysis Without Precious Metals (Wiley-VCH, Weinheim, 2010).
- 2.Gosmini C, Bégouin J, Moncomble A. Cobalt-catalyzed cross-coupling reactions. Chem. Commun. 2008;44:3221–3233. doi: 10.1039/b805142a. [DOI] [PubMed] [Google Scholar]
- 3.Pellissier H, Clavier H. Enantioselective cobalt-catalyzed transformations. Chem. Rev. 2014;114:2775–2823. doi: 10.1021/cr4004055. [DOI] [PubMed] [Google Scholar]
- 4.Gandeepan P, Cheng CH. Cobalt catalysis involving π components in organic synthesis. Acc. Chem. Res. 2015;48:1194–1206. doi: 10.1021/ar500463r. [DOI] [PubMed] [Google Scholar]
- 5.Zhu SF, Zhou QL. Iron-catalyzed transformations of diazo compounds. Natl. Sci. Rev. 2014;1:580–603. doi: 10.1093/nsr/nwu019. [DOI] [Google Scholar]
- 6.Wada R, Oisaki K, Kanai M, Shibasaki M. Catalytic enantioselective allylboration of ketones. J. Am. Chem. Soc. 2004;126:8910–8911. doi: 10.1021/ja047200l. [DOI] [PubMed] [Google Scholar]
- 7.Shi SL, Xu LW, Oisaki K, Kanai M, Shibasaki M. Identification of modular chiral bisphosphines effective for Cu(I)-catalyzed asymmetric allylation and propargylation of ketones. J. Am. Chem. Soc. 2010;132:6638–6639. doi: 10.1021/ja101948s. [DOI] [PubMed] [Google Scholar]
- 8.Takatsu K, Shintani R, Hayashi T. Copper-catalyzed 1,4-addition of organoboronates to alkylidene cyanoacetates: mechanistic insight and application to asymmetric catalysis. Angew. Chem. Int. Ed. 2011;50:5548–5552. doi: 10.1002/anie.201008196. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida M, Ohmiya H, Sawamura M. Enantioselective conjugate addition of alkylboranes catalyzed by a copper–N-heterocyclic carbene complex. J. Am. Chem. Soc. 2012;134:11896–11899. doi: 10.1021/ja304481a. [DOI] [PubMed] [Google Scholar]
- 10.Wu C, et al. Asymmetric conjugate addition of organoboron reagents to common enones using copper catalysts. J. Am. Chem. Soc. 2016;138:742–745. doi: 10.1021/jacs.5b11441. [DOI] [PubMed] [Google Scholar]
- 11.Karthikeyan J, Jeganmohan M, Cheng CH. Cobalt-catalyzed addition reaction of organoboronic acids with aldehydes: highly enantioselective synthesis of diarylmethanols. Chem. – Eur. J. 2010;16:8989–8992. doi: 10.1002/chem.201001160. [DOI] [PubMed] [Google Scholar]
- 12.Chen MH, Mannathan S, Lin PS, Cheng CH. Cobalt(II)-catalyzed 1,4-addition of organoboronic acids to activated alkenes: an application to highly cis-stereoselective synthesis of aminoindane carboxylic acid derivatives. Chem. – Eur. J. 2012;18:14918–14922. doi: 10.1002/chem.201202575. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Ma C, Lee YX, Huang RZ, Zhao Y. Cobalt-catalyzed allylation of heterobicyclic alkenes: ligand-induced divergent reactivities. Angew. Chem. Int. Ed. 2015;54:13696–13700. doi: 10.1002/anie.201506003. [DOI] [PubMed] [Google Scholar]
- 14.Lin PS, Jeganmohan M, Cheng CH. Nickel-catalyzed Mizoroki–Heck- versus Michael-type addition of organoboronic acids to α,β-unsaturated alkenes through fine-tuning of ligands. Chem. Asian J. 2007;2:1409–1416. doi: 10.1002/asia.200700128. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Du X, He R, Ci Z, Bao M. Nickel salt-catalyzed addition reaction of arylboronic acids to aromatic aldehydes. Tetrahedron Lett. 2009;50:406–408. doi: 10.1016/j.tetlet.2008.11.018. [DOI] [Google Scholar]
- 16.Chen W, et al. Ligand-free nickel-catalysed 1,4-addition of arylboronic acids to α,β-unsaturated carbonyl compounds. Adv. Synth. Catal. 2015;357:1474–1482. doi: 10.1002/adsc.201400761. [DOI] [Google Scholar]
- 17.Clarke C, Incerti-Pradillos CA, Lam HW. Enantioselective nickel-catalyzed anti-carbometallative cyclizations of alkynyl electrophiles enabled by reversible alkenylnickel E/Z isomerization. J. Am. Chem. Soc. 2016;138:8068–8071. doi: 10.1021/jacs.6b04206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirakawa E, Yasuhara Y, Hayashi T. Nickel-catalyzed conjugate addition of arylboron reagents to α,β-unsaturated carbonyl compounds with the aid of a catalytic amount of an alkyne. Chem. Lett. 2006;35:768–769. doi: 10.1246/cl.2006.768. [DOI] [Google Scholar]
- 19.Hirano K, Yorimitsu H, Oshima K. Nickel-catalyzed 1,4-addition of trialkylboranes to α,β-unsaturated esters: dramatic enhancement by addition of methanol. Org. Lett. 2007;9:1541–1544. doi: 10.1021/ol070288y. [DOI] [PubMed] [Google Scholar]
- 20.Hirano K, Yorimitsu H, Oshima K. Nickel-catalysed reactions with trialkylboranes and silacyclobutanes. Chem. Commun. 2008;44:3234–3241. doi: 10.1039/b803172j. [DOI] [PubMed] [Google Scholar]
- 21.Sieber JD, Morken JP. Asymmetric Ni-catalyzed conjugate allylation of activated enones. J. Am. Chem. Soc. 2008;130:4978–4983. doi: 10.1021/ja710922h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maekawa T, Sekizawa H, Itami K. Controlled alcohol–carbonyl interconversion by nickel catalysis. Angew. Chem. Int. Ed. 2011;50:7022–7026. doi: 10.1002/anie.201102092. [DOI] [PubMed] [Google Scholar]
- 23.Chau ST, Lutz JP, Wu K, Doyle AG. Nickel-catalyzed enantioselective arylation of pyridinium ions: harnessing an iminium ion activation mode. Angew. Chem. Int. Ed. 2013;52:9153–9156. doi: 10.1002/anie.201303994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong YC, Gandeepan P, Mannathan S, Lee WT, Cheng CH. Alkene-assisted nickel-catalyzed regioselective 1,4-addition of organoboronic acid to dienones: a direct route to all-carbon quaternary centers. Org. Lett. 2014;16:2806–2809. doi: 10.1021/ol500838h. [DOI] [PubMed] [Google Scholar]
- 25.Fu GC. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 2017;3:692–700. doi: 10.1021/acscentsci.7b00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamaru, Y. (Ed) Modern Organonickel Chemistry (Wiley-VCH, Weinheim, 2005).
- 27.Eckelbarger JD, Wilmot JT, Gin DY. Strain-release rearrangement of N-vinyl-2-arylaziridines. Total synthesis of the anti-leukemia alkaloid (−)-deoxyharringtonine. J. Am. Chem. Soc. 2006;128:10370–10371. doi: 10.1021/ja063304f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cozzi PG, Hilgraf R, Zimmermann N. Enantioselective catalytic formation of quaternary stereogenic centers. Eur. J. Org. Chem. 2007;2007:5969–5994. doi: 10.1002/ejoc.200700318. [DOI] [Google Scholar]
- 29.Shibasaki M, Kanai M. Asymmetric synthesis of tertiary alcohols and α-tertiary amines via Cu-catalyzed C–C bond formation to ketones and ketimines. Chem. Rev. 2008;108:2853–2873. doi: 10.1021/cr078340r. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi T, Yamasaki K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 2003;103:2829–2844. doi: 10.1021/cr020022z. [DOI] [PubMed] [Google Scholar]
- 31.Fagnou K, Lautens M. Rhodium-catalyzed carbon–carbon bond forming reactions of organometallic compounds. Chem. Rev. 2003;103:169–196. doi: 10.1021/cr020007u. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida, K. & Hayashi, T. in Modern Rhodium-Catalyzed Organic Reactions (ed Evans, P. A.) Ch. 3 (Wiley-VCH, Weinheim, 2005).
- 33.Miyaura N. Metal-catalyzed reactions of organoboronic acids and esters. Bull. Chem. Soc. Jpn. 2008;81:1535–1553. doi: 10.1246/bcsj.81.1535. [DOI] [Google Scholar]
- 34.Edwards HJ, Hargrave JD, Penrose SD, Frost CG. Synthetic applications of rhodium catalysed conjugate addition. Chem. Soc. Rev. 2010;39:2093–2105. doi: 10.1039/b919762c. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Xu MH. Simple sulfur–olefins as new promising chiral ligands for asymmetric catalysis. Chem. Commun. 2014;50:3771–3782. doi: 10.1039/C3CC49431D. [DOI] [PubMed] [Google Scholar]
- 36.Dong HQ, Xu MH, Feng CG, Sun XW, Lin GQ. Recent applications of chiral N-tert-butanesulfinyl imines, chiral diene ligands and chiral sulfur–olefin ligands in asymmetric synthesis. Org. Chem. Front. 2015;2:73–89. doi: 10.1039/C4QO00245H. [DOI] [Google Scholar]
- 37.Shintani R, Takeda M, Tsuji T, Hayashi T. Rhodium-catalyzed asymmetric arylation of N-tosyl ketimines. J. Am. Chem. Soc. 2010;132:13168–13169. doi: 10.1021/ja106114q. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura T, Noishiki A, Tsui GC, Hayashi T. Asymmetric synthesis of (triaryl)methylamines by rhodium-catalyzed addition of arylboroxines to cyclic N-sulfonyl ketimines. J. Am. Chem. Soc. 2012;134:5056–5059. doi: 10.1021/ja300697c. [DOI] [PubMed] [Google Scholar]
- 39.Jung HH, Buesking AW, Ellman JA. Highly functional group compatible Rh-catalyzed addition of arylboroxines to activated N-tert-butanesulfinyl ketimines. Org. Lett. 2011;13:3912–3915. doi: 10.1021/ol201438k. [DOI] [PubMed] [Google Scholar]
- 40.Luo Y, Hepburn HB, Chotsaeng N, Lam HW. Enantioselective rhodium-catalyzed nucleophilic allylation of cyclic imines with allylboron reagents. Angew. Chem. Int. Ed. 2012;51:8309–8313. doi: 10.1002/anie.201204004. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Jiang T, Xu MH. Simple branched sulfur–olefins as chiral ligands for Rh-catalyzed asymmetric arylation of cyclic ketimines: highly enantioselective construction of tetrasubstituted carbon stereocenters. J. Am. Chem. Soc. 2013;135:971–974. doi: 10.1021/ja3110818. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Li Y, Xu MH. Rhodium-catalyzed highly enantioselective arylation of cyclic diketimines: efficient synthesis of chiral tetrasubstituted 1,2,5-thiadiazoline 1,1-dioxides. Org. Lett. 2014;16:3962–3965. doi: 10.1021/ol501770q. [DOI] [PubMed] [Google Scholar]
- 43.Chen YJ, Chen YH, Feng CG, Lin GQ. Enantioselective rhodium-catalyzed arylation of cyclic N-sulfamidate alkylketimines: a new access to chiral β-alkyl-β-aryl amino alcohols. Org. Lett. 2014;16:3400–3403. doi: 10.1021/ol501464e. [DOI] [PubMed] [Google Scholar]
- 44.Takechi R, Nishimura T. Rhodium-catalyzed asymmetric addition of arylboronic acids to cyclic N-sulfonyl ketimines towards the synthesis of α,α-diaryl-α-amino acid derivatives. Org. Biomol. Chem. 2015;13:4918–4924. doi: 10.1039/C5OB00431D. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, et al. Enantioselective rhodium-catalyzed dearomative arylation or alkenylation of quinolinium salts. Angew. Chem. Int. Ed. 2016;55:3776–3780. doi: 10.1002/anie.201511663. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Yu YN, Xu MH. Simple open-chain phosphite-olefin as ligand for Rh-catalyzed asymmetric arylation of cyclic ketimines: enantioselective access to gem-diaryl α-amino acid derivatives. ACS Catal. 2016;6:661–665. doi: 10.1021/acscatal.5b02403. [DOI] [Google Scholar]
- 47.Luo Y, Carnell AJ, Lam HW. Enantioselective rhodium-catalyzed addition of potassium alkenyltrifluoroborates to cyclic imines. Angew. Chem. Int. Ed. 2012;51:6762–6766. doi: 10.1002/anie.201202136. [DOI] [PubMed] [Google Scholar]
- 48.Brak K, Ellman JA. Asymmetric synthesis of α-branched allylic amines by the Rh(I)-catalyzed addition of alkenyltrifluoroborates to N-tert-butanesulfinyl aldimines. J. Am. Chem. Soc. 2009;131:3850–3851. doi: 10.1021/ja9002603. [DOI] [PubMed] [Google Scholar]
- 49.Brak K, Ellman JA. Asymmetric Rh(I)-catalyzed addition of MIDA boronates to N-tert-butanesulfinyl aldimines: development and comparison to trifluoroborates. J. Org. Chem. 2010;75:3147–3150. doi: 10.1021/jo100318s. [DOI] [PubMed] [Google Scholar]
- 50.Dai H, Yang M, Lu X. Palladium(II)-catalyzed one-pot enantioselective synthesis of arylglycine derivatives from ethyl glyoxylate, p-toluenesulfonyl isocyanate and arylboronic acids. Adv. Synth. Catal. 2008;350:249–253. doi: 10.1002/adsc.200700362. [DOI] [Google Scholar]
- 51.Zhang Q, et al. Palladium-catalyzed addition of arylboronic acids to N-tosylarylimines. Synlett. 2008;2008:935–939. doi: 10.1055/s-2008-1042932. [DOI] [Google Scholar]
- 52.Ma GN, Zhang T, Shi M. Catalytic enantioselective arylation of N-tosylarylimines with arylboronic acids using C2-symmetric cationic N-heterocyclic carbene Pd2+ diaquo complexes. Org. Lett. 2009;11:875–878. doi: 10.1021/ol802861s. [DOI] [PubMed] [Google Scholar]
- 53.Marques CS, Burke AJ. Catalytic enantioselective addition of phenylboronic acid and phenylboroxine to N-tosylimines: PdII and RhI catalysis. Eur. J. Org. Chem. 2010;2010:1639–1643. doi: 10.1002/ejoc.200901139. [DOI] [Google Scholar]
- 54.Chen J, et al. Palladium(II)-catalyzed enantioselective arylation of α-imino esters. J. Org. Chem. 2012;77:8541–8548. doi: 10.1021/jo301423e. [DOI] [PubMed] [Google Scholar]
- 55.Yang G, Zhang W. A palladium-catalyzed enantioselective addition of arylboronic acids to cyclic ketimines. Angew. Chem. Int. Ed. 2013;52:7540–7544. doi: 10.1002/anie.201302861. [DOI] [PubMed] [Google Scholar]
- 56.Jiang C, Lu Y, Hayashi T. High performance of a palladium phosphinooxazoline catalyst in the asymmetric arylation of cyclic N-sulfonyl ketimines. Angew. Chem. Int. Ed. 2014;53:9936–9939. doi: 10.1002/anie.201406147. [DOI] [PubMed] [Google Scholar]
- 57.Schrapel C, Peters R. Exogenous-base-free palladacycle-catalyzed highly enantioselective arylation of imines with arylboroxines. Angew. Chem. Int. Ed. 2015;54:10289–10293. doi: 10.1002/anie.201501846. [DOI] [PubMed] [Google Scholar]
- 58.Huang Y, Huang RZ, Zhao Y. Cobalt-catalyzed enantioselective vinylation of activated ketones and imines. J. Am. Chem. Soc. 2016;138:6571–6576. doi: 10.1021/jacs.6b02372. [DOI] [PubMed] [Google Scholar]
- 59.Tollefson MB, et al. A novel class of apical sodium co-dependent bile acid transporter inhibitors: the 1,2-benzothiazepines. Bioorg. Med. Chem. Lett. 2003;13:3727–3730. doi: 10.1016/j.bmcl.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Kiefer L, et al. Design and synthesis of cyclic sulfonamides and sulfamates as new calcium sensing receptor agonists. Bioorg. Med. Chem. Lett. 2010;20:7483–7487. doi: 10.1016/j.bmcl.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Ilardi EA, Njardarson JT. Ring expansions of vinyloxiranes, -thiiranes, and -aziridines: synthetic approaches, challenges, and catalytic success stories. J. Org. Chem. 2013;78:9533–9540. doi: 10.1021/jo401776s. [DOI] [PubMed] [Google Scholar]
- 62.Njardarson JT. Catalytic ring expansion adventures. Synlett. 2013;24:787–803. doi: 10.1055/s-0032-1318326. [DOI] [Google Scholar]
- 63.Bersch HW, Hoff D, Schon D. Syntheses and rearrangements of tertiary α-arylallylamines. Arch. Pharm. 1978;311:1029–1042. doi: 10.1002/ardp.19783111210. [DOI] [Google Scholar]
- 64.Ruider SA, Müller S, Carreira EM. Ring expansion of 3-oxetanone-derived spirocycles: facile synthesis of saturated nitrogen heterocycles. Angew. Chem. Int. Ed. 2013;52:11908–11911. doi: 10.1002/anie.201306563. [DOI] [PubMed] [Google Scholar]
- 65.Yang BM, et al. Organocatalytic asymmetric tandem Nazarov cyclization/semipinacol rearrangement: rapid construction of chiral spiro[4.4]nonane-1,6-diones. J. Am. Chem. Soc. 2015;137:8344–8347. doi: 10.1021/jacs.5b04049. [DOI] [PubMed] [Google Scholar]
- 66.Lukamto DH, Gaunt MJ. Enantioselective copper-catalyzed arylation-driven semipinacol rearrangement of tertiary allylic alcohols with diaryliodonium salts. J. Am. Chem. Soc. 2017;139:9160–9163. doi: 10.1021/jacs.7b05340. [DOI] [PubMed] [Google Scholar]
- 67.Guo B, Schwarzwalder G, Njardarson JT. Catalytic ring expansion of vinyl oxetanes: asymmetric synthesis of dihydropyrans using chiral counterion catalysis. Angew. Chem. Int. Ed. 2012;51:5675–5678. doi: 10.1002/anie.201201367. [DOI] [PubMed] [Google Scholar]
- 68.Zhang YF, Chen D, Chen WW, Xu MH. Construction of cyclic sulfamidates bearing two gem-diaryl stereocenters through a rhodium-catalyzed stepwise asymmetric arylation protocol. Org. Lett. 2016;18:2726–2729. doi: 10.1021/acs.orglett.6b01183. [DOI] [PubMed] [Google Scholar]
- 69.Yap C, et al. Enantioselective nickel-catalyzed intramolecular allylic alkenylations enabled by reversible alkenylnickel E/Z isomerization. Angew. Chem. Int. Ed. 2017;56:8216–8220. doi: 10.1002/anie.201703380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calaza MI, Cativiela C. Stereoselective synthesis of quaternary proline analogues. Eur. J. Org. Chem. 2008;2008:3427–3448. doi: 10.1002/ejoc.200800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information file. For the experimental procedures, and NMR and HPLC analysis of the compounds in this article, see Supplementary Methods and charts in the Supplementary Information file. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (3na: CCDC 1486078, 5f: CCDC 1556426, 11: CCDC 1816920). These data could be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.