Abstract
Introduction
Long-term survival is still rarely achieved with current systemic treatment in patients with breast cancer liver metastases (BCLM). Extended survival after hepatectomy was examined in a select group of BCLM patients.
Patients and methods
Hepatectomy for BCLM was performed in 139 consecutive patients between 1985 and 2012. Patients who survived < 5 years were compared to those who survived ≥ 5 years from first diagnosis of hepatic metastases. Predictive factors for survival were analyzed. Statistically cured, defined as those patients who their hazard rate returned to that of the general population, was analyzed.
Results
Of the 139, 43 patients survived ≥ 5 years. Significant differences between patient groups (< 5 vs. ≥ 5 years) were mean time interval between primary tumor and hepatic metastases diagnosis (50 vs. 43 months), mean number of resected tumors (3 vs. 2), positive estrogen receptors (54% vs. 79%), microscopic lymphatic invasion (65% vs. 34%), vascular invasion (63% vs. 37%), hormonal therapy after resection (34% vs. 74%), number of recurrence (40% vs. 65%) and repeat hepatectomy (1% vs. 42%), respectively. The probability of statistical cure was 14% (95% CI 1.4–26.7%) in these patients.
Conclusions
Hepatectomy combined with systemic treatment can provide a chance of long-term survival and even cure in selected patients with BCLM. Microscopic vascular/lymphatic invasion appears to be a novel predictor for long-term survival after hepatectomy for BCLM and should be part of the review when discussing multidisciplinary treatment strategies.
Electronic supplementary material
The online version of this article (10.1007/s10549-018-4714-1) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Liver metastases, Hepatectomy, Cure, And long-term survival
Introduction
Liver metastases in patients with breast cancer (BCLM) have historically been associated with the worst prognosis compared to other metastatic sites such as the lungs, bone, or brain, with 5-year survival rates of only 4–12% (median survival 4–21 months) [1–3]. Breast cancer in general is a major problem of public health for women worldwide with an estimated 1.7 million women diagnosed with breast cancer in 2012 [4]. A significant proportion of these patients (around 30%) will eventually develop metastatic disease (stage IV). Although systemic treatment for metastatic breast cancer has significantly improved in recent years, dissemination is still associated with poor survival.
Within current guidelines, patients with stage IV breast cancer are only eligible for palliative systemic treatment. The U.S. National Cancer Institute, among other influential organizations, does not mention liver resection as an option for metastatic breast cancer to the liver.
Considering the poor results achieved by current guidelines, the concept of oligimetastatic resection and the existence of unreachable tumor cells deep within tumors (by systemic agents) has become the driving force behind the advocates for resection of limited metastatic disease, especially if they are reactive to systemic treatment [5–7]. Patients with colorectal liver metastases, for example who undergo curative liver resection have seen remarkable results of 5-year survival rates between 30 and 40% and even 50% in selected cases with low surgical mortality or morbidity, something unthinkable for breast cancer liver metastases [8]. So far, only few small retrospective series have been reported regarding the resection of BCLM and there have been no randomized control trials [9–25].
At our institution, our highly selected patients with BCLM have routinely undergone surgical resection since 1985 with previously reported promising results [9]. In general, patients with limited disease experienced more favorable outcome after surgery compared to patients with more extensive tumor involvement. The real potential of prolonged survival or even “cure” in selected patients after hepatic resection is, however, still questioned and liver resection is still not offered as part of advanced breast cancer treatment strategy.
The purpose of this study was to analyze possible indications for and against liver resection in women with BCLM. Furthermore, to study the possibility of exceptional long-term survival, we focused on patients who underwent liver surgery combined with systemic treatment and survived less than 5 year and more than 5 years after liver metastases diagnosis (note: not time of liver resection) with survival beyond 5 years a rarity with current palliative guidelines. In addition, predictive factors and the possibility of “cure” were analyzed.
Patients and methods
Study population
All consecutive patients with BCLM, who underwent a partial hepatectomy at our center between January 1985 and December 2012, were selected. Patients were selected from our prospectively maintained institutional database, and each medical record was reviewed to update clinical and pathological data. Additional immunohistochemistry analysis was conducted in those patients with missing information and available tumor tissue. To compare with historical published studies, that reported on patients who were not operated, the starting point was defined as the moment of diagnosis of liver metastases. Patients who survived < 5 years were compared to those who survived > 5 years after first diagnosis of liver metastases.
Preoperative workup
To be considered for hepatic resection, all patients were required to have received stage-appropriate therapy for their primary tumor. Selection criteria for liver resection were previously presented [9]. In summary, liver resection was proposed to all patients with metastases confined to the liver (or associated to very limited and stable extrahepatic disease), provided that the tumor was controlled by systemic treatment and could be completely resected with a functional remnant liver of at least 30% of the total liver volume. Preoperatively, each patient underwent abdominal ultrasonography and abdominal and thoracic computed tomography (CT), as well as a bone radionuclide scan, to determine the extent of intra- and extrahepatic disease. In the more recent patients, MRI and FDG-PET were more routinely performed to better assess the extent of intra- and extrahepatic tumor spread.
Patients with single and easily resectable liver metastases underwent early surgery without chemotherapy in case of a prolonged disease-free interval (more than 6 months). Patients with large or multiple metastases and a short disease-free interval received preoperative chemotherapy for 2–3 months. Preoperative chemotherapy was furthermore routinely indicated for patients with concomitant extrahepatic disease. The aim of preoperative chemotherapy was to limit tumor spread, to reduce tumor volume, and to exclude patients with rapidly progressive metastatic disease in whom liver resection was unlikely to provide any survival benefit. Chemotherapy consisted of a combination of the classical cytostatic drugs (such as anthracyclines, pyrimidine analogs and taxanes), hormonal therapy (aromatase inhibitors and anti-estrogen agents) or targeted therapy (monoclonal antibodies) to achieve maximum response.
The decision for hepatectomy was taken in a multidisciplinary meeting including surgeons, medical oncologists and radiologists, when the overall surgical strategy could achieve complete tumor resection and the disease was controlled by chemotherapy.
Hepatic resection
During surgery, abdominal exploration and liver ultrasonography were used to confirm tumor resectability and to evaluate the presence of extrahepatic disease. Parenchymal dissection was done using the ultrasonic dissector (Cavitron Ultrasonic Aspirator, Valleylab, Boulder, CO, USA) and a fenestrated bipolar forceps. The extent of hepatic resection was classified as minor (< 3 hepatic segments) or major (≥ 3 hepatic segments) according to Couinaud’s classification [26]. Clamping of the hepatic pedicle was used if needed to control intraoperative blood loss. Tumor-free resection margins were the objective in all cases and when needed radiofrequency ablation or cryoablation was performed in combination with liver resection to achieve potentially curative surgery. Suspicious lymph nodes on the hepatic pedicle (regional) were resected for pathological review when detected, as were lymph nodes of the celiac trunk or the superior mesenteric artery (distant). However, limited extrahepatic disease was not a contraindication for hepatic resection.
Postoperative outcome and follow-up
Postoperative mortality was defined as death within the first 60 days following surgery. Postoperative morbidity was defined as any postoperative adverse event, which occurred during the same period. Postoperative complications were divided into hepatic complications, which occurred within the field of liver resection (e.g., biliary fistula), and general complications, which occurred distant from the hepatic resection field (e.g., pneumonia).
All patients were regularly followed at our outpatient clinic, starting 1 month after surgery, then every 4 months for the first 2 years and every 6 months after 2 years. Follow-up consisted of a history, physical examination and radiological imaging. Abdominal ultrasound and abdominal and thoracic CT imaging were alternately performed.
Statistical analysis
Median follow-up time for the whole population was well beyond 5 years (108 months). As 5-year survival could be considered as a valuable turning point for the evaluation of outcome, the whole series was divided into two groups; patients who survived < 5 years versus patients who survived ≥ 5 years after first diagnosis of liver metastases. Categorical variables were compared between groups by the Chi square (χ2) test and continuous variables were compared using the independent-sample t test. Overall survival probabilities were estimated using the Kaplan–Meier method and were compared using the log-rank test. Univariate analysis was performed to determine factors related to a survival of patients who survived beyond 5 years by using the log-rank test. To identify independent predictors of long-term survival, all factors with an univariate significance of P < 0.10 were entered into a Cox proportional hazard model. To correct for missing values, multiple imputations were performed twenty times and pooled. Regressions are presented as original and imputed. All statistical analyses were performed with SPSS version 21.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was determined at P ≤ 0.05.
Cure model
When a patient’s observed hazard rate returns to that of the general population, that patient may be considered cured of the disease, because the risk of death is just as likely as for any member of the general population. [27] The estimation of expected survival and of expected hazard of the general population was derived from population-based survival tables obtained from the French National institute of Statistics and Economics, matched by age [28]. Survival time in our study group was defined as the period between hepatic resection (intervention to achieve cure) and date of death or last follow-up. The potential of cure was calculated using STATA software (StataCorp. 2011, College Station, TX: StataCorp LP) as described in several published works [27, 29–31].
Results
Study population
Between January 1985 and December 2012, 139 consecutive female patients underwent 162 hepatectomies for BCLM at our institution. Of these 139 patients, 120 (86%) underwent a single hepatectomy and 19 (14%) underwent a second hepatectomy. In 4 (3%) patients, a third hepatectomy was needed.
In total, 43 (31%) patients lived ≥ 5 years after first diagnosis of liver metastases. These 43 patients were compared to 96 (69%) patients who survived < 5 years after liver metastases diagnosis regardless of the number of hepatectomies.
Long-term overall and disease-free survival
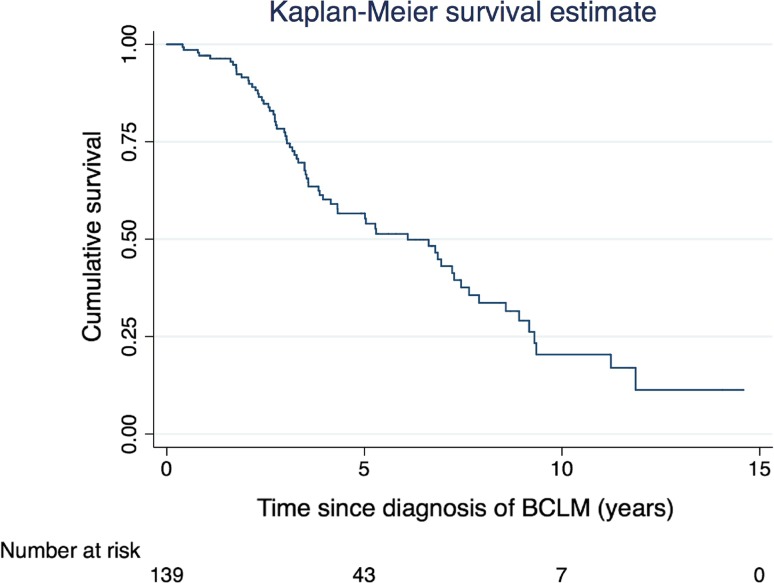
Median follow-up time was 108 months for the whole series. The 7- and 10-year overall survival in the ≥ 5 years group was 76% and 36%, respectively. Seven of the 43 patients (16%) are alive more than 10 years since liver metastases diagnosis (5% of the total population) with a longest survival of almost 15 years (175 months) (Figure 1) . Of the 43 patients who survived ≥ 5 years, 22 (51%) had no hepatic recurrence at last follow-up. Of the 96 patients that survived < 5 years, 58 patients (60%) had no hepatic recurrence at last follow-up.
Fig. 1.
Survival estimates
Median disease-free survival for the whole series was 33 months. Median disease-free survival was 25 and 43 months in the < 5 and ≥ 5 years groups, respectively (Supplemental Table 1).
Patient and tumor characteristics comparison
Patient and tumor characteristics of both groups are summarized in Table 1. Patients who survived ≥ 5 years had a shorter time interval between primary tumor and liver metastases diagnosis (43 ± 29 months vs. 50 ± 47 months, P = 0.026). The proportion of patients with concomitant extrahepatic disease at first hepatectomy was higher in patients who survived < 5 years (34% vs. 19%, P = 0.071).
Table 1.
Patient and tumor characteristics comparison n = 139
| < 5-years group | % | ≥ 5-years group | % | p | |
|---|---|---|---|---|---|
| Primary breast tumor§ | |||||
| Adenocarcinoma | |||||
| Ductal | 51 | 86 | 26 | 93 | 0.490 |
| Lobular | 8 | 14 | 2 | 7 | |
| Differentiation | |||||
| Well | 3 | 5 | 1 | 3 | 0.503 |
| Moderate | 34 | 61 | 22 | 73 | |
| Poor | 19 | 34 | 7 | 23 | |
| Surgical removal | |||||
| Breast conserving | 42 | 45 | 25 | 60 | 0.140 |
| Mastectomy | 51 | 55 | 17 | 40 | |
| Receptor status | |||||
| ER positive | |||||
| No | 9 | 20 | 3 | 14 | 0.737 |
| Yes | 36 | 80 | 19 | 86 | |
| PR positive | |||||
| No | 15 | 35 | 7 | 35 | 1.000 |
| Yes | 28 | 65 | 13 | 65 | |
| Her2/Neu positive | |||||
| No | 24 | 69 | 9 | 60 | 0.746 |
| Yes | 11 | 31 | 6 | 40 | |
| ER/PR negative | |||||
| No | 36 | 84 | 18 | 90 | 0.706 |
| Yes | 7 | 16 | 2 | 10 | |
| Systemic treatment | |||||
| Neoadjuvant chemo | |||||
| No | 93 | 97 | 41 | 95 | 0.645 |
| Yes | 3 | 3 | 2 | 5 | |
| Adjuvant chemo | |||||
| No | 39 | 41 | 19 | 44 | 0.713 |
| Yes | 57 | 59 | 24 | 56 | |
| Post op hormonal therapy | |||||
| No | 66 | 69 | 26 | 61 | 0.341 |
| Yes | 30 | 31 | 17 | 39 | |
| Post op radiotherapy | |||||
| No | 32 | 33 | 13 | 30 | 0.845 |
| Yes | 64 | 67 | 30 | 70 | |
| Breast cancer liver metastases | |||||
| Sync | |||||
| Synchronous | 7 | 7 | 4 | 10 | 0.738 |
| Metachronous | 87 | 93 | 38 | 90 | |
| Mean interval months between primary and BCLM ± SD | 50 ± 47 months | 43 ± 29 months | 0.026* | ||
| Mean number of BCLM ± SD | 2 ± 2 | 2 ± 2 | 0.803 | ||
| Mean maximum tumor size ± SD, mm | 33 ± 17 | 37 ± 20 | 0.312 | ||
| Distribution | |||||
| Bilateral | 29 | 32 | 17 | 44 | 0.232 |
| Unilateral | 62 | 68 | 22 | 56 | |
| Concomitant extra-hepatic disease | |||||
| No | 63 | 66 | 35 | 81 | 0.071 |
| Yes | 33 | 34 | 8 | 19 | |
| Preoperative chemotherapy | |||||
| No | 30 | 31 | 11 | 26 | 0.551 |
| Yes | 66 | 69 | 32 | 74 | |
| Hepatectomy | |||||
| Mean age at hepatectomy ± SD | 53 ± 11 years | 48 ± 10 years | 0.208 | ||
| Timing of hepatectomy | |||||
| Year < 2000 | 36 | 38 | 17 | 40 | 0.852 |
| Year > 2000 | 60 | 62 | 26 | 60 | |
| Extent of resection | |||||
| Limited resection (< 3 segments) | 38 | 40 | 19 | 44 | 0.710 |
| Major resection (≥ 3 segments) | 58 | 60 | 24 | 56 | |
| Type of resection | |||||
| Anatomical | 32 | 33 | 17 | 40 | 0.657 |
| Wedge | 35 | 37 | 13 | 30 | |
| Anatomical+wedge | 29 | 30 | 13 | 30 | |
| Histopathology | |||||
| Mean number of resected metastases ± SD | 3 ± 2 | 2 ± 2 | 0.023* | ||
| Solitary tumor | |||||
| No | 57 | 63 | 19 | 46 | 0.090 |
| Yes | 34 | 37 | 22 | 54 | |
| Mean maximum size ± SD, mm (M) | 30 ± 26 | 26 ± 16 | 0.374 | ||
| Resection margin | |||||
| R0 | 52 | 58 | 25 | 66 | 0.435 |
| R+ | 38 | 42 | 13 | 34 | |
| Hormonal receptor status | |||||
| ER− | 40 | 46 | 7 | 21 | 0.021 |
| ER+ | 48 | 54 | 26 | 79 | |
| PR− | 57 | 65 | 24 | 73 | 0.516 |
| PR+ | 31 | 35 | 9 | 27 | |
| Her2/neu− | 60 | 70 | 23 | 72 | 1.000 |
| Her2/neu+ | 26 | 30 | 9 | 28 | |
| Triple negative (ER, PR, HER2/NEU) | |||||
| No | 66 | 75 | 28 | 85 | 0.329 |
| Yes | 22 | 25 | 5 | 15 | |
| Lymphatic embolus | |||||
| No | 28 | 35 | 21 | 66 | 0.003* |
| Yes | 53 | 65 | 11 | 34 | |
| Vascular embolus | |||||
| No | 33 | 37 | 24 | 63 | 0.011* |
| Yes | 56 | 63 | 14 | 37 | |
| Regional lymph node invasion | |||||
| Negative for tumor cells | 9 | 41 | 4 | 57 | 0.667 |
| Positive for tumor cells | 13 | 59 | 3 | 43 | |
| Distant lymph node invasion | |||||
| Negative for tumor cells | 12 | 75 | 1 | 100 | 1.000 |
| Positive for tumor cells | 4 | 25 | 0 | 0 | |
| Post hepatectomy | |||||
| Postoperative chemotherapy | |||||
| No | 35 | 37 | 9 | 21 | 0.078 |
| Yes | 61 | 64 | 34 | 79 | |
| Hormonal therapy after resection | |||||
| No | 63 | 66 | 11 | 26 | 0.000* |
| Yes | 33 | 34 | 32 | 74 | |
| Monoclonal therapy after resection | |||||
| No | 72 | 75 | 25 | 58 | 0.071 |
| Yes | 24 | 25 | 18 | 42 | |
| Recurrence | |||||
| No | 58 | 60 | 15 | 35 | 0.006* |
| Yes | 38 | 40 | 28 | 65 | |
| Repeat hepatectomy | |||||
| No | 95 | 99 | 25 | 58 | 0.000* |
| Yes | 1 | 1 | 18 | 42 | |
§Referring center for hepatectomy, primary usually is treated in a different hospital, *p-value < 0.05
The mean number of tumors resected in the ≥ 5 years group was 2 compared to 3 in < 5 years group (P = 0.023). More patients in the ≥ 5 years group had a solitary liver metastasis compared to the < 5 years group (54% vs. 37%, P = 0.090). Estrogen receptor positive tumors were more prevalent in the ≥ 5 years group (79% vs. 54%, P = 0.021). The proportion of patients with microscopic lymphatic and vascular invasion was higher in patients who survived < 5 years (lymphatic; 65% vs. 34%, P = 0.003, vascular; 63% vs. 37%, P = 0.011). Patients who lived ≥ 5 years more frequently received hormonal therapy after hepatic resection (74% vs. 34%, P < 0.000).
After hepatic resection, the proportion of hepatic recurrences was higher in the ≥ 5 years group (65% vs. 40% (P = 0.006). Repeat hepatectomy was performed in 1 of the patients who survived < 5 years and in 18 patients who survived ≥ 5 years (1% vs. 42%, P < 0.000).
Short-term outcome
There were more general complications in the < 5 years group than in the ≥ 5 years group (25% vs. 10%, P = 0.061). The mean hospital stay was similar in both groups; 11 vs. 10 days (P = 0.420). The 60-day mortality was 2% after hepatic resection for the whole series (Table 2).
Table 2.
Comorbidity
| < 5-year group | % | ≥ 5-year group | % | p | |
|---|---|---|---|---|---|
| Morbiditya | |||||
| No | 63 | 66 | 28 | 72 | 0.684 |
| Yes | 32 | 34 | 11 | 28 | |
| General complications | |||||
| No | 71 | 75 | 35 | 90 | 0.062 |
| Yes | 24 | 25 | 4 | 10 | |
| Hepatic complications | |||||
| No | 83 | 87 | 32 | 82 | 0.425 |
| Yes | 12 | 13 | 7 | 18 | |
| Biliary leakage | 2 | 17 | 4 | 57 | |
| Biliary leakage+infected collection | 1 | 8 | 1 | 14 | |
| Biliary leakage+noninfected collection | 1 | 8 | 0 | 0 | |
| Hemorrhage | 0 | 0 | 1 | 14 | |
| Infected collection | 4 | 33 | 0 | 0 | |
| Noninfected collection | 3 | 25 | 1 | 14 | |
| Mean hospital stay, days ± SD (M) | 11 + 7 | 10 + 4 | 0.420 | ||
n = 139
aGeneral and/or hepatic complication
Predictive factors of long-term survival
At univariate analysis, factors significantly related to better survival in those patient who lived longer than 5 years were: time interval between primary tumor and hepatic metastases equal or more than 18 months, resected metastasis size smaller than 35 mm, absence of microscopic vascular invasion or combination of vascular and lymphatic invasion, absence of chemotherapy after hepatic resection (Table 3).
Table 3.
Univariate and multivariate analysis of overall survival in patients that survived 5 years or longer after hepatectomy since date of diagnosis
| n | % | 7 years (%) | 10 years (%) | Median (Mo) | Log rank | P d | P e | Hazard ratio | (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver metastases | |||||||||||
| Time of appearance | |||||||||||
| Synchronous | 4 | 9 | 0 | 0 | – | 0.462 | |||||
| Metachronous | 38 | 88 | 74 | 37 | – | ||||||
| Interval primary tumor and metastasis | |||||||||||
| < 18 months | 6 | 14 | 0 | 0 | 82 | 0.01 | 0.035 | NS | |||
| ≥ 18 months | 35 | 81 | 81 | 40 | 111 | ||||||
| Tumor number | |||||||||||
| Solitaire | 20 | 47 | 81 | 52 | 142 | 0.157 | |||||
| > 1 | 23 | 53 | 72 | 21 | 110 | ||||||
| Maximal tumor size | |||||||||||
| < 30 mm | 14 | 33 | 93 | 0 | 94 | 0.680 | |||||
| ≥ 30 mm | 23 | 53 | 76 | 50 | 111 | ||||||
| Distribution | |||||||||||
| Unilateral | 22 | 51 | 84 | 52 | 134 | 0.187 | |||||
| Bilateral | 17 | 40 | 61 | 12 | 107 | ||||||
| Segments involved | |||||||||||
| 1 | 18 | 42 | 79 | 47 | 102 | 0.999 | |||||
| > 1 | 20 | 47 | 79 | 36 | 110 | ||||||
| Chemo tx pre hepatectomya | |||||||||||
| No | 11 | 26 | 80 | 48 | 94 | 0.430 | |||||
| Yes | 32 | 74 | 75 | 30 | 110 | ||||||
| Hormone tx pre hepatectomyb | |||||||||||
| No | 36 | 84 | 71 | 32 | 102 | 0.108 | |||||
| Yes | 7 | 16 | 100 | 50 | 107 | ||||||
| Targeted tx pre hepatectomyc | |||||||||||
| No | 36 | 84 | 76 | 36 | 110 | 0.985 | |||||
| Yes | 7 | 16 | 75 | 0 | 86 | ||||||
| Extra hepatic metastases | |||||||||||
| No | 35 | 81 | 77 | 37 | 110 | 0.644 | |||||
| Yes | 8 | 19 | 73 | 29 | 91 | ||||||
| Sites of Extrahepatic disease | |||||||||||
| Brain | 1 | 2 | |||||||||
| Bone | 2 | 5 | |||||||||
| Lung | 4 | 9 | |||||||||
| Lymph Node | 1 | 2 | |||||||||
| First hepatectomy | |||||||||||
| Age (E3) | |||||||||||
| < 50 years. | 26 | 60 | 77 | 29 | 102 | 0.613 | |||||
| ≥ 50 years | 17 | 40 | 75 | 44 | 111 | ||||||
| Hepatic resection (E3) | |||||||||||
| Minor (< 3) | 17 | 40 | 81 | 37 | 111 | 0.978 | |||||
| Major (≥ 3) | 26 | 60 | 72 | 33 | 120 | ||||||
| Type of resection (E3) | |||||||||||
| Anatomical | 19 | 44 | 88 | 62 | 134 | 0.287 | |||||
| Both | 24 | 56 | 66 | 23 | 107 | ||||||
| Not Anatomical | |||||||||||
| Tumor number (E3) | 17 | 40 | 74 | 41 | 110 | 0.900 | |||||
| Solitaire | 13 | 30 | 69 | 46 | 91 | ||||||
| > 1 | 13 | 30 | 100 | 21 | 111 | ||||||
| Maximal tumor size (E3) | |||||||||||
| < 35 mm | 26 | 60 | 74 | 19 | 102 | 0.033 | NS | NS | |||
| ≥ 35 mm | 12 | 28 | 92 | 69 | 142 | ||||||
| Resection margin (E3) | |||||||||||
| R0 | 25 | 58 | 82 | 41 | 111 | 0.209 | |||||
| R1 | 13 | 30 | 69 | 19 | 91 | ||||||
| R2 | 0 | 0 | – | – | – | ||||||
| Hormone receptor status (E3) | |||||||||||
| ER− | 7 | 16 | 86 | 86 | (124) | 0.713 | |||||
| ER+ | 26 | 60 | 83 | 30 | 110 | ||||||
| No tumor cells | 4 | 9 | 67 | 0 | (98) | ||||||
| PR− | 24 | 56 | 76 | 33 | 107 | 0.543 | |||||
| PR+ | 9 | 21 | 100 | 37 | 112 | ||||||
| No tumor cells | 4 | 9 | 67 | 0 | (98) | ||||||
| HER2− | 23 | 53 | 76 | 37 | 102 | 0.465 | |||||
| HER2+ | 9 | 21 | 100 | 42 | 111 | ||||||
| No tumor cells | 4 | 9 | 67 | 0 | (98) | ||||||
| Double neg (ER−, PR−) (E3) | |||||||||||
| No | 8 | 19 | 100 | 23 | 110 | 0.877 | |||||
| Yes | 25 | 58 | 77 | 39 | 107 | ||||||
| No tumor cells | 4 | 9 | 67 | 0 | (98) | ||||||
| Triple neg (ER−, PR−, HER2−) (E3) | |||||||||||
| No | 28 | 65 | 84 | 34 | 110 | 0.874 | |||||
| Yes | 5 | 12 | 80 | 0 | (81) | ||||||
| No tumor cells | 4 | 9 | 67 | 0 | (98) | ||||||
| Vascular invasion (E3) | |||||||||||
| No | 24 | 56 | 92 | 47 | 111 | 0.06 | NS | NS | |||
| Yes | 14 | 33 | 64 | 13 | 91 | ||||||
| Lymphatic Invasion (E3) | |||||||||||
| No | 21 | 49 | 95 | 50 | 111 | 0.149 | |||||
| Yes | 11 | 26 | 80 | 15 | 102 | ||||||
| Vascular and/or lymphatic Invasion (E3) | |||||||||||
| No | 19 | 44 | 95 | 54 | 134 | 0.078 | NS | 0.023 | 3.485 | 1.184 | 10.250 |
| Yes | 19 | 44 | 70 | 20 | 91 | ||||||
| Lymph Node Invasion (E3) | |||||||||||
| No | 4 | 9 | 75 | 50 | 87 | 0.222 | |||||
| Yes | 3 | 7 | 0 | 0 | 79 | ||||||
| Chemo tx post hepatectomya (E3−>) | |||||||||||
| No | 9 | 21 | 88 | 73 | 142 | 0.077 | NS | NS | |||
| Yes | 34 | 79 | 73 | 22 | 107 | ||||||
| Hormone tx post hepatectomyb (E3−>) | |||||||||||
| No | 11 | 26 | 89 | 64 | 142 | 0.088 | 0.007 | 0.023 | 4.197 | 1.217 | 14.471 |
| Yes | 32 | 74 | 72 | 24 | 107 | ||||||
| Targeted tx post hepatectomyc (E3−>) | |||||||||||
| No | 25 | 58 | 83 | 46 | 111 | 0.209 | |||||
| Yes | 18 | 42 | 64 | 0 | 110 | ||||||
| Radiofrequency ablation, cryo ablation or arterial embolization (E3) | |||||||||||
| No | 38 | 88 | 79 | 41 | 110 | 0.159 | |||||
| Yes | 5 | 12 | 40 | 0 | 82 | ||||||
| Post hepatectomy course | |||||||||||
| Chemo tx peri hepatectomya (E2− > E3−>) | |||||||||||
| No | 0 | 0 | 100 | 50 | 94 | 0.802 | |||||
| Yes | 40 | 93 | 74 | 34 | 110 | ||||||
| Hormone tx peri hepatectomyb (E2− > E3− >) | |||||||||||
| No | 9 | 21 | 88 | 63 | 142 | 0.137 | |||||
| Yes | 34 | 79 | 73 | 25 | 107 | ||||||
| Targeted tx peri hepatectomyc (E2− > E3−>) | |||||||||||
| No | 23 | 53 | 87 | 48 | 111 | 0.110 | |||||
| Yes | 20 | 47 | 59 | 0 | 110 | ||||||
| Hepatic Recurrence (E2.2) | |||||||||||
| No | 15 | 35 | 86 | 53 | (116) | 0.216 | |||||
| Yes | 28 | 65 | 71 | 29 | 102 | ||||||
| Interval first hepatectomy to recurence (E3− > E2.2) | |||||||||||
| < 12 months | 6 | 14 | 60 | 40 | 86 | 0.670 | |||||
| ≥ 12 months | 12 | 28 | 73 | 26 | 110 | ||||||
| Tumor number | |||||||||||
| Solitair | 10 | 23 | 77 | 43 | 111 | 0.481 | |||||
| > 1 | 17 | 40 | 71 | 21 | 102 | ||||||
| Repeat hepatectomy | |||||||||||
| No | 10 | 23 | 64 | 0 | 87 | 0.650 | |||||
| Yes | 18 | 42 | 74 | 47 | 112 | ||||||
n = 43
E1 primary tumor, E2 diagnosis hepatic metastases, E3 hepatectomy, E4 extra hepatic metastases, E2.2 hepatic recurrence
aAntracyclines, pyrimidine, taxanes, platinum, vinca; single or in combinations
bAromatase inhibitor and anti-estrogen
cMonoclonal antibodies, () = estimated mean+ = in ER+ or PR+ patients
dMultivariate
emultivariate with 20 × imputation of missing values, tx therapy, NS not significant p > 0.05
Multivariate analysis identified two factors associated with long-term survival in patients who lived ≥ 5 years. Patients with microscopic vascular and/or lymphatic invasion had a three and half fold chances of dying compared to patients with no vascular or lymphatic invasion (7-year survival 70% vs. 95%, respectively, HR 3.485, range 1.184-10.250, P = 0.023). Patients who received hormonal therapy after hepatic resection had a four fold chance of dying compared to those that did not receive hormonal therapy (7-year survival 72% vs. 89%, respectively, HR 4.197, range 1.217–14.471, P = 0.023).
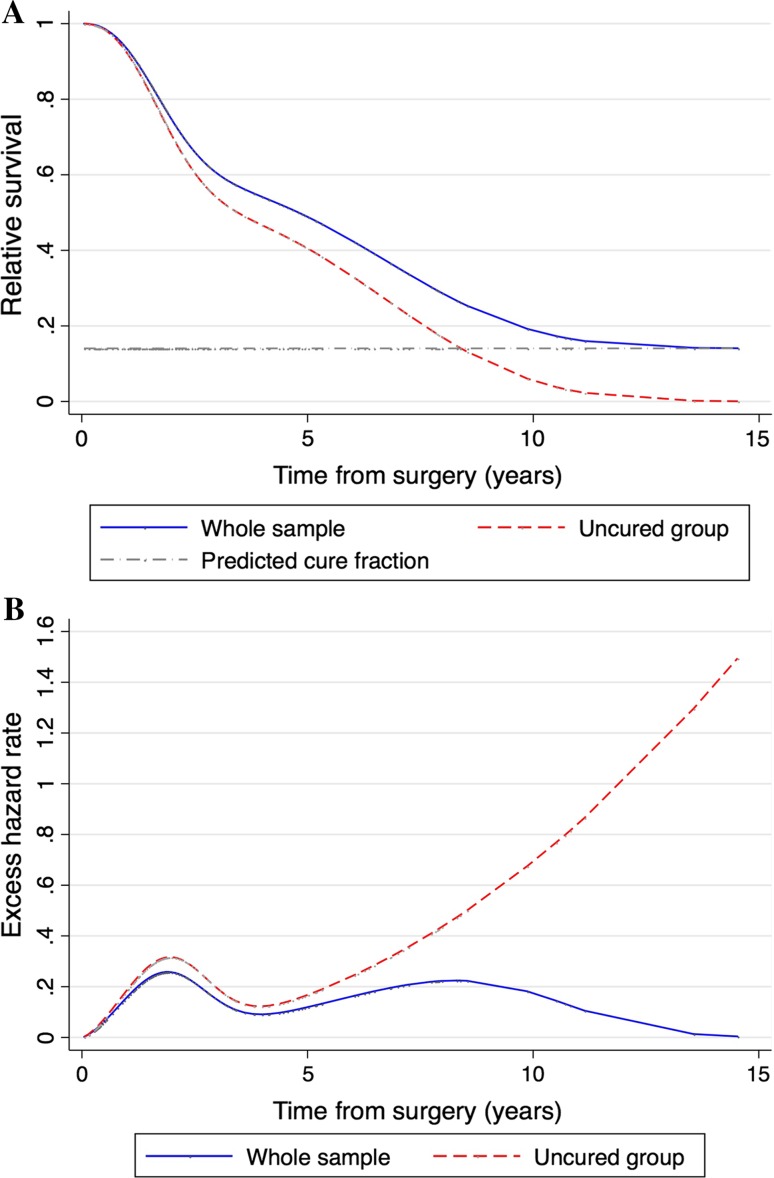
Probability of cure
In the entire study population, the probability of being cured of BCLM by hepatic resection combined with systemic treatment was 14% (95% CI 1.4–26.7%) (Fig. 2a). The excess of hazard after surgery started from a 2% increased risk of death early after surgical resection with respect to the general population (Fig. 2b). In the first two postoperative years, the excess hazard increased to approximately 25.8% in the entire group and was up to 32.9% in non-cured patients. After a parallel trajectory of risk of death up to 8.3 years, the entire group demonstrated a progressive reduction in the hazard while the hazard for non-cured patients progressively increased after the 4th year after surgery. The excess of hazard in the entire group decreased towards the general population hazard at 13.6 years after hepatic resection, indicating that after this time point, a patient still alive could be considered cured with 99% certainty.
Fig. 2.
Cure model results. a Relative survival of the entire group of patients and the uncured patients. b Excess hazard rate of the entire study group and the uncured patients
Discussion
Long-term survival of BCLM is almost never achieved when liver metastases remain unresected [32–37]. Following hepatectomy, the 3- and 5-year survival rates were 58 and 47%, respectively, for the whole series. Of the 43 patients who survived beyond 5 years, 76% survived 7 years and even 35% survived 10 years after the time of first diagnosis. These results support the indication of hepatic resection for BCLM in selected patients and suggest that some of these patients can even be cured. This strategy also relates with current developments in local treatment of oligometastasis in other cancers such as colorectal carcinomas as part of a more individual tailored approach. The value of liver resection in these patients is also reflected in the fact that even though a portion of patients developed hepatic recurrences, long-term survival can still be achieved when repeat hepatectomy is performed.
Although systemic treatment of breast cancer patients has developed in the past decades, survival of patients with BCLM is still poor with 5-year survival rates of only 4–12% when applying current guidelines. Resection of BCLM remains controversial and is not generally accepted. Few articles have been published describing the possible benefit of surgery in mostly small study populations varying from 2 to 115 patients with a 5-year survival rate ranging from 27 to 50%.
Our series, representing a small proportion of the total number of patients with stage IV breast cancer, is without a doubt a selected group of patients. However, these results have never been expected before, based on the historical reported survival of breast cancer patients with hepatic metastases. This series is the only series to date focusing on the possibility of real long-term survival of BCLM after hepatic resection in an experienced hepatobiliary center. Two different approaches were implemented to highlight key factor that might help selecting patients with better chances of survival and a statistical cure model was constructed that compares the hazard rate of the general population to that of this series over time.
Predictive factors of ≥ 5 year survival were: interval more than 18 months between primary breast tumor and diagnosis of hepatic metastases, size of resected metastases < 35 mm, absence of microscopic vascular and lymphatic invasion, absence of hormonal therapy after resection and repeat hepatectomy in case of tumor recurrence.
Time interval and tumor size are well-documented predictors for overall survival and are related to tumor biology. In several publications, a time interval of 1–2 years after removal of the primary tumor was reported as a significant factor of survival [38].
Sadot et al. compared 69 operated patients to 98 systemically treated in a single center looking at historically selected patients for treatment groups [39]. Even though they concluded that hepatic resection was not associated with survival advantages (median OS: 50 vs 45 months; 5-year OS: 38% vs 39%), a significant recurrence-free interval was seen.
There is no publication that reported on the survival impact of microscopy invasion into vascular or lymphatic structures. Besides the widely accepted residual classification (R0, R1 and R2) for microscopic invasion into the surgical field, we believe that this characteristic should be part of a standard histological review in order to further explore its utility as possible selection criteria for further intervention.
The negative long-term survival effect of postoperative hormonal treatment (idiopathic menopausal state) has never been presented or investigated given the historically documented marginal survival rate of these patients with conventional systemic treatment only. It is known that (early) menopausal state is related to an increased risk of a variety of diseases including cardiovascular disease and this might explain this finding [40]. Given the rise in acceptance of hepatic resection for breast cancer liver metastases, more investigation in a larger cohort will shed more light into whether the benefit of hormonal therapy outweighs the risks in this subset of patients.
Among the patients that survived beyond 5 years we found a higher rate of recurrences but also a higher rate of repeat hepatectomy. This result confirms the importance of an aggressive approach through multiple hepatectomies when surgically possible. The potential benefit of repeat hepatectomy has been presented in a previous published work [41].
It remains controversial to explore the idea of cure given the complexity of the disease and the notion that total eradication of disseminated cell is very unlikely. A cure model was used to compare the hazard rate of our highly selected group of patients to that of the general population. We found that 19 patients (14%) of the whole series had their chance of death reduced to that of the general population. This outcome has never been reported for breast cancer liver metastases patients treated by systemic treatment alone. This result further strengthens the indication for hepatic resection in patients with BCLM.
Our results might be based on our highly selected population but should not be dismissed as selection bias since these results are rarely achieved by conventional palliative therapy. Our study should serve as a guide to select women who might and might not benefit from an aggressive approach and stimulate future studies.
In conclusion, we believe that hepatectomy for BCLM should be considered in all patients when technically feasible and responding to systemic treatment. The current study shows that hepatectomy provides a chance of extreme long-term survival or even statistical cure in selected patients without increased morbidity. Something that was unthinkable within the current palliative approach to BCLM patients. Accurate selection of patients for hepatectomy remains crucial.
Available data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to this manuscript. APR served as the lead author, conducting data analysis and leading manuscript preparation and writing. MS was pathologist involved in tissues review. RvH, DW CCB. provided feedback, and edits during the process. BP, DC, DC, FFM provided surgical and clinical guidance, feedback, and edits during the process. RA was the PI for the project, and provided substantive guidance through the development, analysis, and writing of the manuscript. All authors have approved this work.
Funding
The Netherlands Organization for Scientific Research (NWO) provided funding for a research fellow (Grant number: 017.009.053).
Compliance with ethical standards
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
Institutional review board approval was waived in accordance with French law for retrospective studies.
Informed consent
All patient at our institution are systemically ask to consent the use of anonymous data for analysis and publication. No identifiable data was collected.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10549-018-4714-1) contains supplementary material, which is available to authorized users.
References
- 1.Ge Q-D, Lv N, Kong Y-N, Xie X-H, He N, Xie X-M, et al. Clinical characteristics and survival analysis of breast cancer molecular subtypes with hepatic metastases. Asian Pac J Cancer Prev. 2012;13(10):5081–5086. doi: 10.7314/APJCP.2012.13.10.5081. [DOI] [PubMed] [Google Scholar]
- 2.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507–514. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly SM, Richards MA, Rubens RD. Liver metastases from breast cancer: The relationship between clinical, biochemical and pathological features and survival. Eur J Cancer Clin Oncol. 1990;26(5):574–577. doi: 10.1016/0277-5379(90)90080-D. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S, Martling A, Lindholm J, Holm T, Palmer G. Remaining cancer cells within the fibrosis after neo-adjuvant treatment for locally advanced rectal cancer. Eur J Surg Oncol. 2015;41(9):1204–1209. doi: 10.1016/j.ejso.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Sebagh M, Allard MA, Cunha AS, Ruiz A, Araujo R, Lemoine A, et al. A proposed new method for assessing the pathological response to chemotherapy in resected colorectal liver metastases. Br J Cancer. 2014;111(3):470–476. doi: 10.1038/bjc.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang F, Wu G, Yang K. Oligometastasis and oligo-recurrence: more than a mirage. Radiat Oncol (London, England) 2014;9:230. doi: 10.1186/s13014-014-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2001;235(6):759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam R, Aloia T, Krissat J, Bralet MP, Paule B, Giacchetti S, et al (2006) Is liver resection justified for patients with hepatic metastases from breast cancer?. Ann Surg 244(6):897–907; discussion -8 [DOI] [PMC free article] [PubMed]
- 10.Caralt M, Bilbao I, Cortes J, Escartin A, Lazaro JL, Dopazo C, et al. Hepatic resection for liver metastases as part of the “oncosurgical” treatment of metastatic breast cancer. Ann Surg Oncol. 2008;15(10):2804–2810. doi: 10.1245/s10434-008-0072-2. [DOI] [PubMed] [Google Scholar]
- 11.Kollmar O, Moussavian MR, Richter S, Bolli M, Schilling MK. Surgery of liver metastasis in gynecological cancer—indication and results. Onkologie. 2008;31(7):375–379. doi: 10.1159/000135516. [DOI] [PubMed] [Google Scholar]
- 12.Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, Scotte M. Liver resection for breast cancer metastasis: does it improve survival? Surg Today. 2008;38(4):293–299. doi: 10.1007/s00595-007-3617-2. [DOI] [PubMed] [Google Scholar]
- 13.Thelen A, Benckert C, Jonas S, Lopez-Hanninen E, Sehouli J, Neumann U, et al. Liver resection for metastases from breast cancer. J Surg Oncol. 2008;97(1):25–29. doi: 10.1002/jso.20911. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann K, Franz C, Hinz U, Schirmacher P, Herfarth C, Eichbaum M, et al. Liver resection for multimodal treatment of breast cancer metastases: identification of prognostic factors. Ann Surg Oncol. 2010;17(6):1546–1554. doi: 10.1245/s10434-010-0931-5. [DOI] [PubMed] [Google Scholar]
- 15.Rubino A, Doci R, Foteuh JC, Morenghi E, Fissi S, Giorgetta C, et al. Hepatic metastases from breast cancer. Updat surg. 2010;62(3–4):143–148. doi: 10.1007/s13304-010-0026-7. [DOI] [PubMed] [Google Scholar]
- 16.Cassera MA, Hammill CW, Ujiki MB, Wolf RF, Swanstrom LL, Hansen PD. Surgical management of breast cancer liver metastases. HPB. 2011;13(4):272–278. doi: 10.1111/j.1477-2574.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott DE, Brouquet A, Mittendorf EA, Andreou A, Meric-Bernstam F, Valero V, et al. Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery. 2012;151(5):710–716. doi: 10.1016/j.surg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan XF, Dong NN, Zhang T, Li Q. Comparison of surgical outcomes in patients with colorectal liver metastases versus non-colorectal liver metastases: a Chinese experience. Hepatol Res. 2012;42(3):296–303. doi: 10.1111/j.1872-034X.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- 19.Groeschl RT, Nachmany I, Steel JL, Reddy SK, Glazer ES, de Jong MC, et al. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg. 2012;214(5):769–777. doi: 10.1016/j.jamcollsurg.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 20.van Walsum GA, de Ridder JA, Verhoef C, Bosscha K, van Gulik TM, Hesselink EJ, et al. Resection of liver metastases in patients with breast cancer: survival and prognostic factors. Eur J Surg Oncol. 2012;38(10):910–917. doi: 10.1016/j.ejso.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Dittmar Y, Altendorf-Hofmann A, Schule S, Ardelt M, Dirsch O, Runnebaum IB, et al. Liver resection in selected patients with metastatic breast cancer: a single-centre analysis and review of literature. J Cancer Res Clin Oncol. 2013;139(8):1317–1325. doi: 10.1007/s00432-013-1440-2. [DOI] [PubMed] [Google Scholar]
- 22.Kostov DV, Kobakov GL, Yankov DV. Prognostic factors related to surgical outcome of liver metastases of breast cancer. J breast cancer. 2013;16(2):184–192. doi: 10.4048/jbc.2013.16.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polistina F, Costantin G, Febbraro A, Robusto E, Ambrosino G. Aggressive treatment for hepatic metastases from breast cancer: results from a single center. World J Surg. 2013;37(6):1322–1332. doi: 10.1007/s00268-013-1986-9. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Park JS, Lee SA, Kim JK, Jeong J, Yoon DS, et al. Does liver resection provide long-term survival benefits for breast cancer patients with liver metastasis? A single hospital experience. Yonsei Med J. 2014;55(3):558–562. doi: 10.3349/ymj.2014.55.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinrich M, Weiss C, Schuld J, Rau BM. Liver resections of isolated liver metastasis in breast cancer: results and possible prognostic factors. HPB surg. 2014;2014:893829. doi: 10.1155/2014/893829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couinaud C. Variations of the right bile ducts. The futility of complete anatomical classifications. Chirurgie; memoires de l’Academie de chirurgie. 1993;1993; 119(6-7):354-6 [PubMed]
- 27.Lambert PC, Thompson JR, Weston CL, Dickman PW. Estimating and modeling the cure fraction in population-based cancer survival analysis. Biostatistics. 2007;8(3):576–594. doi: 10.1093/biostatistics/kxl030. [DOI] [PubMed] [Google Scholar]
- 28.French population life tables. http://www.insee.fr/ Accessed 21 Sept 2015
- 29.Cucchetti A, Ferrero A, Cescon M, Donadon M, Russolillo N, Ercolani G, et al. Cure model survival analysis after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2015;22(6):1908–1914. doi: 10.1245/s10434-014-4234-0. [DOI] [PubMed] [Google Scholar]
- 30.Spolverato G, Vitale A, Cucchetti A, Popescu I, Marques HP, Aldrighetti L, et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121:3998–4006. doi: 10.1002/cncr.29619. [DOI] [PubMed] [Google Scholar]
- 31.Lambert PC. Modeling of the cure fraction in survival studies. Stata J. 2007;7(3):351–375. [Google Scholar]
- 32.Abuzallouf S, Motawy M, Thotathil Z. Baseline staging of newly diagnosed breast cancer–Kuwait cancer control center experience. Med Princ Pract. 2007;16(1):22–24. doi: 10.1159/000096135. [DOI] [PubMed] [Google Scholar]
- 33.Schuetz F, Diel IJ, Pueschel M, von Holst T, Solomayer EF, Lange S, et al. Reduced incidence of distant metastases and lower mortality in 1072 patients with breast cancer with a history of hormone replacement therapy. Am J Obstet Gynecol. 2007;196(4):342. doi: 10.1016/j.ajog.2006.10.901. [DOI] [PubMed] [Google Scholar]
- 34.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 35.Pogoda K, Niwinska A, Murawska M, Pienkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol (Northwood, London, England) 2013;30(1):388. doi: 10.1007/s12032-012-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berman AT, Thukral AD, Hwang WT, Solin LJ, Vapiwala N. Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin Breast Cancer. 2013;13(2):88–94. doi: 10.1016/j.clbc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Follana P, Barriere J, Chamorey E, Largillier R, Dadone B, Mari V, et al. Prognostic factors in 401 elderly women with metastatic breast cancer. Oncology. 2014;86(3):143–151. doi: 10.1159/000357781. [DOI] [PubMed] [Google Scholar]
- 38.Duan XF, Dong NN, Zhang T, Li Q. The prognostic analysis of clinical breast cancer subtypes among patients with liver metastases from breast cancer. Int J Clin Oncol. 2013;18(1):26–32. doi: 10.1007/s10147-011-0336-x. [DOI] [PubMed] [Google Scholar]
- 39.Sadot E, Lee SY, Sofocleous CT, Solomon SB, Gonen M, Peter Kingham T, et al. Hepatic resection or ablation for isolated breast cancer liver metastasis: a case-control study with comparison to medically treated patients. Ann Surg. 2016;264(1):147–154. doi: 10.1097/SLA.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis S. Do endocrine treatments for breast cancer have a negative impact on lipid profiles and cardiovascular risk in postmenopausal women? Am Heart J. 2007;153(2):182–188. doi: 10.1016/j.ahj.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz A, Castro-Benitez C, Sebagh M, Giacchetti S, Castro-Santa E, Wicherts DA, et al. Repeat hepatectomy for breast cancer liver metastases. Ann Surg Oncol. 2015;22(3):1057–1066. doi: 10.1245/s10434-015-4785-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.