Abstract
Objective
To analyze, from the immunohistochemical perspective, the effects of hyaluronic acid of different molecular weights in an experimental model of osteoarthritis in rabbits.
Methods
Forty-four male California rabbits were randomly assigned to three different groups (PR, S, and P) and submitted to the resection of the anterior cruciate ligament of the right knee. Three weeks after the surgical procedure, three intra-articular weekly injections were carried out with low-molecular-weight native hyaluronic acid (Hyalgan®) to PR group, high molecular weight branched chain hyaluronic acid (Synvisc®) to group S, and saline solution 0.9% to group P. All animals were sacrificed 12 weeks after the surgical procedure, and the tibial plateaus of the infiltrated knees were then dissected. Histological sections of cartilage from the tibial plateau support areas were stained with immunohistochemical markers in order to investigate the amount of metalloproteases (MMPs 3 and 13) and their inhibitors (TIMPs 1 and 3). The staining intensity was quantified on a Zeiss Imager.Z2 Metasystems microscope and analyzed by Metafer4 Msearch software.
Results
The chondroprotective effect of the hyaluronic acids used in the study was demonstrated when compared to the control group. However, the comparison between them presented no significant statistical difference regarding chondroprotection.
Conclusion
The injection of saline solution demonstrated signs of OA development, while adding native hyaluronic acid of low molecular weight (Hyalgan®) and hyaluronic acid of high molecular weight (Synvisc®) protected the articular cartilage in this model of OA.
Keywords: Osteoarthritis, Hyaluronic acid, Experimental model of osteoarthritis, Immunohistochemistry
Resumo
Objetivo
Analisar do ponto de vista imuno-histoquímico os efeitos do ácido hialurônico de diferentes pesos moleculares em modelo experimental em coelhos.
Métodos
Foram alocados de modo aleatório 44 coelhos da raça California, machos, em três grupos (PR, S e P), e submetidos à ressecção do ligamento cruzado anterior do joelho direito. Decorridas três semanas do procedimento cirúrgico iniciaram-se as três injeções intra-articulares semanais de ácido hialurônico nativo de baixo peso molecular (Polireumin®) no grupo PR, ácido hialurônico de cadeia ramificada de alto peso molecular (Synvisc®) no grupo S e soro fisiológico 0,9% no grupo P. Todos os animais foram sacrificados após 12 semanas do ato cirúrgico e os platôs tibiais dos joelhos infiltrados foram dissecados. Cortes histológicos da cartilagem das áreas de apoio dos platôs tibiais foram corados com marcadores imuno-histoquímicos para pesquisa da quantidade de metaloproteases (MMPs-3,13) e seus inibidores (TIMPs-1,3). A intensidade de coloração foi quantificada em um aparelho de microscopia Zeiss Imager.Z2 Metasystems e analisada pelo software Metafer4 Msearch.
Resultado
O efeito condroprotetor dos ácidos hialurônicos usados no estudo foi demonstrado quando comparados com o grupo controle, porém feita a comparação entre si não houve diferença estatística significante quanto à condroproteção.
Conclusão
A injeção de solução salina demonstra sinais de desenvolvimento de OA enquanto que a adição de ácido hialurônico nativo de baixo peso molecular (Polireumin®) e ácido hialurônico de cadeia ramificada de alto peso molecular (Synvisc®) protegeram a cartilagem articular nesse modelo de OA.
Palavras-chave: Osteoartrite, Ácido hialurônico, Modelo experimental de osteoartrite, Imuno-histoquímica
Introduction
Osteoarthrosis is the most common joint affection in the knee; it is defined by specific structural alterations of the joint, including: focal degradation of the articular cartilage, inflammatory processes in the synovial tissue, biochemical changes in the synovial fluid and remodeling of the subchondral bone with osteophyte formation in the margins of the joint. It is currently defined as an inflammatory joint disease, and now it is termed osteoarthritis (OA), being no longer considered a simple joint degeneration.
The pathology is a major cause of activity limitation, physical restraint, overuse of healthcare services, and reduced quality of life, especially in people over 45 years.1 Recent researches indicate an increase in the prevalence of this condition, as 27 millions of adults in the United States over 25 years old show clinical signs of OA in the hand, knee, or hip, an increase from the 21 million in 1995. In the population above 45 years, 19–28% present radiologically-confirmed OA; it is considered the most common chronic arthritis in the world.2
In Brazil, disease costs are harder to estimate due to lack of official statistics, but given that the incidence of OA is directly proportional to population aging, national costs also tend to increase. As in 2016 the Brazil had 207 million inhabitants, 22.69% of whom in the range of young individuals and 8.17% in that of elderly individuals. According to projections of the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística [IBGE]), by 2030 the country will have 223 million inhabitants, 17.59% in the youth age group and 13.44% in the elderly group, that is, a relative increase of 64% in the elderly population in the country.3
OA is the result of several factors in joint dysfunction; its functional failure is characterized by cartilage degeneration, joint inflammation, and simultaneous proliferation of bone, cartilage, and connective tissue.4, 5, 6 Among the various treatment modalities currently available, intra-articular injections of hyaluronic acid (HA) have demonstrated beneficial effects in the control of knee OA symptoms (gonarthrosis).7
HA, administered as intra-articular injections, may potentiate the regenerative effects of endogenous HA on articular cartilage, restore the viscoelasticity of synovial fluid, contribute to the synthesis of endogenous HA and other components of the extracellular matrix by synoviocytes, and prevent the degradation of proteoglycans and extracellular matrix collagen fibers. HA also stimulates chondrocyte metabolism and prevents its apoptosis; it also inhibits chondral degradation and inflammatory joint responses.8 These effects are attributed not only to HA's ability to reduce OA-related symptoms, but also to its interference in the progression of inflammatory processes and joint degeneration.9, 10
In order to evaluate the effects of these substances on OA, the authors proposed the use of an already-established experimental model of OA that resembles that observed in the human species, the section of the anterior cruciate ligament (ACL) of the knee of rabbits (stifle) mimics the morphological and biochemical changes observed in human OA, which allows an accurate reproduction of the results obtained.11, 12
Material and methods
The Research Ethics Committee of the Health Sciences Division of this University evaluated and approved the research protocol used in this experiment (CEP/SD Registry: 001.004 SI 06-06).
A total of 44 California male rabbits were housed prior to and during procedures in the vivarium, in 40 × 30 cm rectangular cages with two animals each with standard feed and water ad libitum. They were kept in light control (12-h light–dark cycle), with controlled temperature (25 ± 1 °C), humidity, and noise level; the mean weight of each animal was 3.5 kg. All animals were initially submitted to ACL section on two days of work by three researchers. For experiment standardization, the procedure was performed on the right knee. The surgical procedure consisted of preoperative anesthesia with 10 mg/kg of ketamine hydrochloride (Dopalen®) and 50 mg/kg of xylazine hydrochloride (Anasedan®) in a single syringe, administered intramuscularly (IM) in the midsection of the semimembranous and semitendinous muscles of the posterior right limb. At the same time, 14,400 UI of penicillin and 6 mg of streptomycin (Pentabiótico Veterinário Reforçado® – Eurofarma) were injected as antibiotic prophylaxis, and Flunamine® (Bayer) at a dosage of 2.2 mg/kg IM, for post-operative analgesia.
The rabbits remained housed in their respective cages after the operation, without load restriction of the operated limbs. Two animals presented surgical site infection and were excluded from the study.
Three weeks after surgical procedure, the intra-articular injections were started. The volume of HA was 0.3 mL, similar to that used in small human joints.13 In the present study, the high molecular weight (6 × 106 Da) branched-chain HA of avian origin (Synvisc®) and the low molecular weight HA (5–7 × 105 Da) of avian origin (gallinaceous crest; Polireumin® – international name brand: Hyalgan®) were used. Both HAs are routinely used in intra-articular injections in humans.14 The groups were divided as follows:
Group P: control, three weekly injections of isotonic saline solution (0.9% saline).
Group PR: three weekly injections of native HA (Polireumin®).
Group S: three weekly injections of branched HA (Synvisc®).
All animals were euthanized 12 weeks after surgery. The medial tibial plateaus were aseptically resected and immersed in 10% formalin vials. The vials were labeled for identification of the groups and sent to the Department of Pathology Anatomy of the University.
Ten histological slides of each group were made and automatically digitized on a Carl Zeiss Imager.Z2 Metasystems microscope, with Metafer MSearch4 software, with post-assembly with VSlide. Subsequently, the cartilage regions were selected with the MetaViewer software's snapshot tool. The photos were analyzed in the ImageJ software, using the RGB Stack tool.
In the quantification of DAB marking, the ImageJ software (NIH) was used, with labeled or immunolabelled and DAB-stained blades. In the software, the cartilage was sectioned from the remainder of the sample; then color deconvolution was used to separate the brown color from the stain. Subsequently, the marked area of the cartilage was quantified in percentages.
The Mann–Whitney's U test was used to assess the normality of the sample and to test the heterogeneity of two ordinal samples; Student's t-test was used to assess statistical significance between the groups.
Results
The decalcified and immunohistochemistry-stained slices showed the presence of metalloproteases (MMP) 3 and 13 in a lower amount in the PR and S groups (Fig. 1) when compared with the P group (control; Fig. 2). In relation to the tissue inhibitors of metalloproteases (TIMP) 1 and 3, an increase in the PR and S groups was observed in relation to the P group; all these evidences were statistically significant.
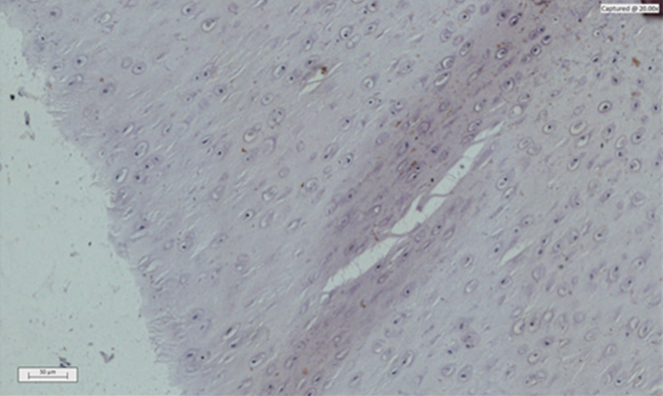
Fig. 1.
Photomicrograph of a histological section of immunomodulated articular cartilage of MMP 3 – Synvisc®.
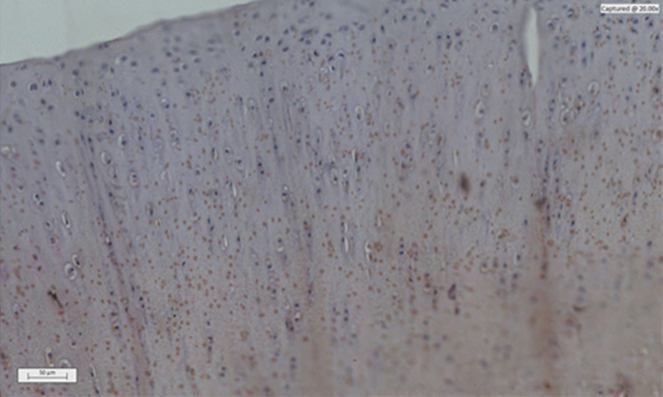
Fig. 2.
Photomicrograph of a histological section of immunomodulated articular cartilage of MMP 3 – Placebo.
In the comparison between the PR and S groups in the measurement of the number of MMPs and TIMPs, an increase in TIMPs and a decrease in MMPs was observed in the PR group; a slight tendency of greater chondroprotection was found in the PR group, but without a statistically significant difference between the two groups.
The evaluation of the staining intensity of the stained and immunolabelled histological sections using Zeiss Imager Z2 Metasystems microscopy device generated results that were arranged in tables and graphs. Table 1, Table 2 compare the P group with the S and PR groups regarding the MMPs (3 and 13), while Table 3, Table 4, Table 5, Table 6 compare them regarding the TIMPs (1 and 3); Fig. 3, Fig. 4, Fig. 5, Fig. 6 represent the actual intensity of the staining measured in each group.
Table 1.
Comparison of the P group vs. the S group for MMP13.
| S MMP13 | P MMP13 | |
|---|---|---|
| Mean | 1.491 | 2.931 |
| Variance | 0.124832222 | 0.35641 |
| Observations | 10 | 10 |
| Mean difference hypothesis | 0 | |
| gL | 15 | |
| T-test | −6.564182248 | |
| p(T ≤ t) one-tailed | 4.48725E−06 | |
| Critical one-tailed t | 1.753050356 | |
| p(T ≤ t) two-tailed | 8.9745E−06 | |
| Critical two-tailed t | 2.131449546 |
T test, two samples with unequal variances.
Table 2.
Comparison of the P group vs. the PR group for MMP13.
| P MMP13 | PR MMP13 | |
|---|---|---|
| Mean | 2.931 | 1.414 |
| Variance | 0.35641 | 0.187671111 |
| Observations | 10 | 10 |
| Mean difference hypothesis | 0 | |
| gL | 16 | |
| T-test | 6.503599012 | |
| p(T ≤ t) one-tailed | 3.6375E−06 | |
| Critical one-tailed t | 1.745883676 | |
| p(T ≤ t) two-tailed | 7.275E−06 | |
| Critical two-tailed t | 2.119905299 |
T test, two samples with unequal variances.
Table 3.
Comparison of the P group vs. the S group for TIMP 1.
| S TIMP1 | P TIMP1 | |
|---|---|---|
| Mean | 3.227 | 1.017 |
| Variance | 0.376467778 | 0.091734444 |
| Observations | 10 | 10 |
| Mean difference hypothesis | 0 | |
| gL | 13 | |
| T-test | 10.21352223 | |
| p(T ≤ t) one-tailed | 7.03657E−08 | |
| Critical one-tailed t | 1.770933396 | |
| p(T ≤ t) two-tailed | 1.40731E−07 | |
| Critical two-tailed t | 2.160368656 |
T test, two samples with unequal variances.
Table 4.
Comparison of the P group vs. the PR group for TIMP 1.
| P TIMP1 | PR TIMP1 | |
|---|---|---|
| Mean | 1.017 | 3.032 |
| Variance | 0.091734444 | 0.189884444 |
| Observations | 10 | 10 |
| Mean difference hypothesis | 0 | |
| gL | 16 | |
| T-test | −12.00726678 | |
| p(T ≤ t) one-tailed | 1.01975E−09 | |
| Critical one-tailed t | 1.745883676 | |
| p(T ≤ t) two-tailed | 2.0395E−09 | |
| Critical two-tailed t | 2.119905299 |
T test, two samples with unequal variances.
Table 5.
Comparison of the P group vs. the S group for TIMP 3.
| S TIMP3 | P TIMP3 | |
|---|---|---|
| 4.82 | 2.15 | |
| Mean | 3.798888889 | 2.234444444 |
| Variance | 0.851136111 | 0.499002778 |
| Observations | 9 | 9 |
| Mean difference hypothesis | 0 | |
| gL | 15 | |
| T-test | 4.039170415 | |
| p(T ≤ t) one-tailed | 0.000535294 | |
| Critical one-tailed t | 1.753050356 | |
| p(T ≤ t) two-tailed | 0.001070589 | |
| Critical two-tailed t | 2.131449546 |
T test, two samples with unequal variances.
Table 6.
Comparison of the P group vs. the PR group for TIMP 3.
| PR TIMP3 | P TIMP3 | |
|---|---|---|
| Mean | 3.901 | 2.14 |
| Variance | 0.860832222 | 0.631577778 |
| Observations | 10 | 10 |
| Mean difference hypothesis | 0 | |
| gL | 18 | |
| T-test | 4.558429902 | |
| p(T ≤ t) one-tailed | 0.000121767 | |
| Critical one-tailed t | 1.734063607 | |
| p(T ≤ t) two-tailed | 0.000243534 | |
| Critical two-tailed t | 2.10092204 |
T test, two samples with unequal variances.
Fig. 3.
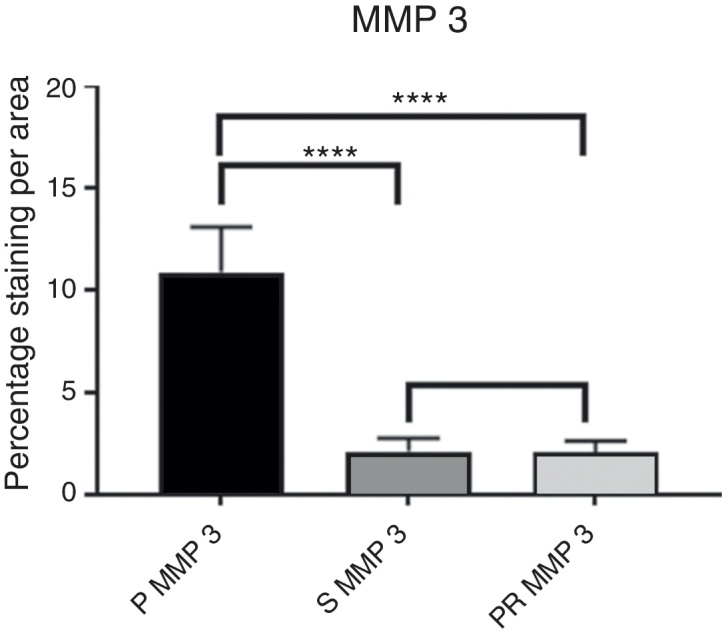
Intensity of MMP3 immunostaining.
Fig. 4.
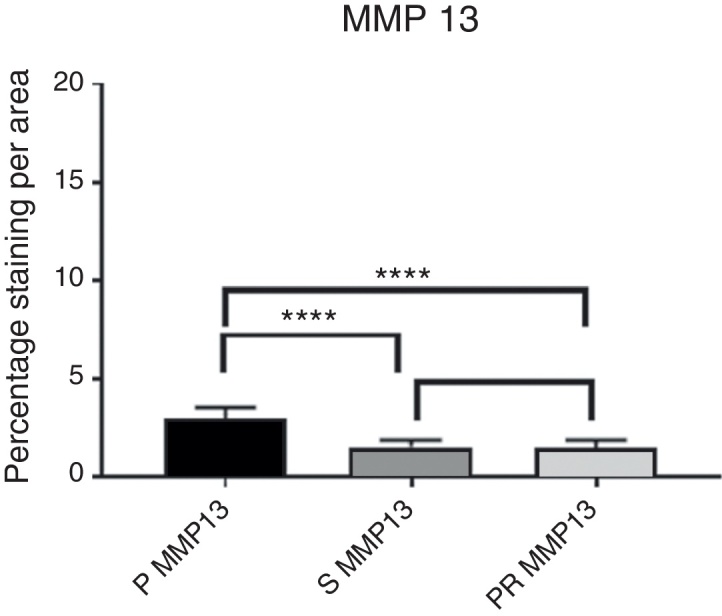
Intensity of MMP13 immunostaining.
Fig. 5.
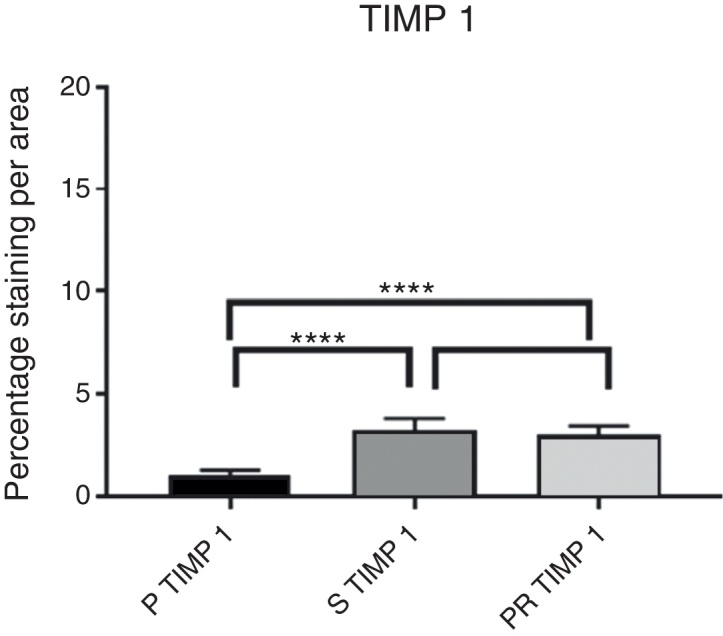
Intensity of TIMP1 immunostaining.
Fig. 6.
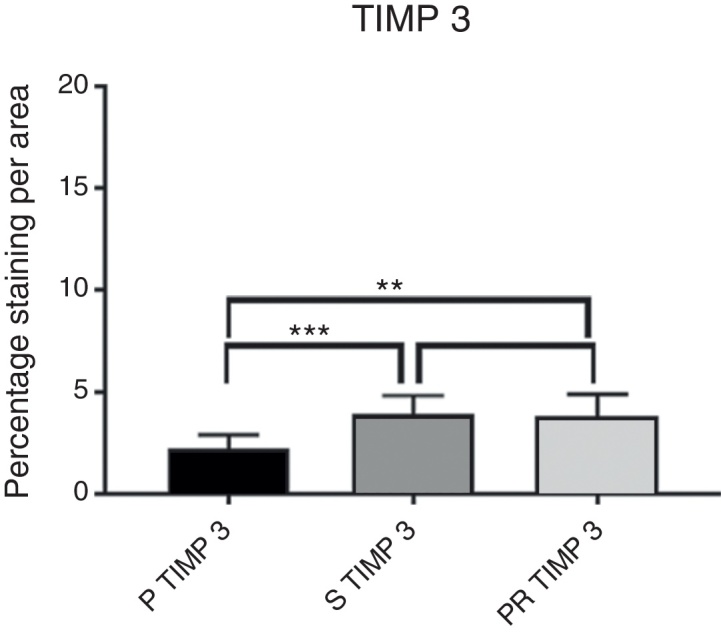
Intensity of TIMP3 immunostaining.
Discussion
HA, a polysaccharide of the high-viscosity glycosaminoglycan group, has been used in medical practice for over 50 years. This substance contributes to joint homeostasis and has a molecular weight of approximately 0.5–3 × 109 Da in the normal joint; it is found in lower concentration and decreased molecular weight in the synovial fluid of joints with OA.15
In the 1960s, Balazs pioneered the concept of viscosupplementation. He believed that the ideal viscosupplementation would have specific criteria: the permeability of the substance, not being immunogenic, having molecular weight similar to that of synovial fluid, and having a long half-life.16 Intra-articular injections of different HAs are used as chondroprotectors in the treatment of OA8, 9, 17; in 1997, the Food and Drug Administration (FDA) approved the use of intra-articular HA knee injections in the United States.
HA's mechanism of action has been the subject of numerous studies; it has mechanical effects on the best distribution of forces, decreases pressure by axial weight, and improves the rheological functions of synovial fluid.18
Studies comparing the efficiency of HAs of different molecular weights have been published in recent decades. The data obtained are discrepant due to their results and models of evaluation, with a slight predominance of positive results when HAs with higher molecular weight are used.19
In clinical practice, high (Synvisc®) and low molecular weight HA (Polireumin®) presented a lower progression of joint space narrowing in patients with initial OA development.20, 21 There is a preference for HA of high molecular weight for the treatment of OA based on studies such as those made by Atamaz et al.22 and Wobig et al.,23 who studied patients with OA and compared HAs of different molecular weights with a saline placebo, intra-articularly injected. In the present study, better results were obtained with the use of HAs of higher molecular weight, in both clinical and non-histological criteria. A meta-analysis conducted by Altman et al.24 also confirmed these data.
However, according to Karlsson et al.,25 who studied HAs of different molecular weights in intra-articular injections in humans with OA, no significant differences were observed between HAs of different molecular weights in clinical and non-histological criteria.
These controversies led to this study, in which the possible chondroprotective effect of a high molecular weight hyaluronate was compared with that of a low weight hyaluronate. For this, an experimental model of OA was used.
In the present study, the management with the animals and the surgical dissection of the rabbits’ knees were easy. The skin incision, the medial capsulotomy with visualization of the ACL and its transection were quick and convenient procedures. The plateaus of the knees that were submitted to this procedure presented signs of macroscopic lesion, especially those of the P group (placebo – 0.9% saline).26 Subsequently, a histological analysis was performed with specific stains for proteoglycans (toluidine blue and alcian blue), which demonstrated the chondroprotective character of HA in relation to the placebo, but without significant statistical alteration between the different molecular weights.27 The justification for the continuation of the immunohistochemical study was to increase the metabolic knowledge on OA, to discover its relations with the main MMPs and TIMPs, and to assess whether HA molecular weight impacts the chondroprotection factor.
Proteoglycans are extracellular proteins linked to glycosaminoglycans, whose function is to provide rigidity to the cell matrix, resist compression and fill spaces; they are the main components of cartilage, attracting water molecules to the tissue. MMPs are enzymes that destroy proteoglycans and, consequently, the cartilage structure. In turn, TIMPs inhibit metalloproteases and play an important role in chondroprotection. Due to the importance of MMPs and TIMPs in cartilage physiology, and because they are an evaluation factor in OA, the dosage of MMP-3, MMP-13, TIMP-1, and TIMP-3, which are the main ones involved in OA, was measured.28, 29
The data found were consistent with the literature on the subject18, 29: MMP-3 and MMP-13 presented increased staining values in the P group, while TIMP-1 and TIMP-3 values decreased in relation to the other groups, demonstrating an increased catabolic activity, i.e., an ongoing cartilaginous destruction. In the S (Synvisc®) and PR (Polireumin®) groups, a decrease in the specific staining of MMP-3 and MMP-13 and an increase in TIMP-1 and TIMP-3 was observed, suggesting a lower cartilaginous catabolic action. The data observed in the present study confirms the hypothesis that HAs have a chondroprotective action.
The numerical values of the staining intensity of the MMP-3 and MMP-13 of the PR group were higher than those of the S group, while the values of TIMP-1 and TIMP-3 are lower, demonstrating a tendency of superior chondroprotection in the PR group. However, when comparing the staining values found in the S and PR groups, no significant statistical differences were observed in the staining intensity of the MMPs and TIMPs of the knees injected with high molecular weight HA (Synvisc®) and those injected with low molecular weight HA (Polireumin®); therefore, no superior chondroprotective effect can be assigned to low or high molecular weight HA.
The literature comparing the different molecular weights of hyaluronates in this experimental model is scarce. Shimizu et al.,30 in a study in rabbits, concluded that low molecular weight HA were superior to those of higher molecular weight. The injected dose used that study was not presented, which would allow a better comparison with the present study.
Ghosh and Guidolin,31 who used an experimental model of ACL transection in dogs, obtained better results with HAs of lower molecular weight. In an in vitro study, the same authors observed better results with the use of HAs of higher molecular weight, contrary to their animal studies, since those HA would be better stimulate the production of cellular matrix components, which could be partially explained by the fact that lower molecular weight HA penetrates the extracellular matrix more easily, maximizing its concentration and promoting its interaction with the target cells of the synovium. Moreover, there is evidence that the binding of HA molecules to cell receptors is molecular weight-dependent.
In summary, the data observed in the present study confirm the findings of Karlsson et al.,25 who assessed the effects of hyaluronates of different molecular weights on intra-articular injections in humans with OA, indicating that chondroprotection was not affected by the different molecular weight of HAs.
The present study has limitations, such as the fact that the animals were not stimulated to gait and were not free, with restricted ambulation; another limitation is the lower thickness of the rabbit cartilage relative to that of humans. In the study by Guidolin, in which the animals analyzed were ambulant, gait with unstable knee led to a higher rate of OA progression.31
In clinical practice, the study demonstrated the safe and effective use of HA, regardless of its molecular weight, regarding adverse effects and its chondroprotective nature in mild and moderate OA.
Conclusion
Animals injected with saline solution presented signs of OA, while the injection of low molecular weight HA (Polireumin®) or high molecular weight branched chain HA (Synvisc®) protected the joint cartilage in this OA model.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Study conducted at the Universidade Federal do Paraná (UFPR), Departamento de Ortopedia e Traumatologia, Curitiba, PR, Brazil.
References
- 1.Hochberg M.C. Osteoarthritis year 2012 in review: clinical. Osteoarthr Cartil. 2012;20(12):1465–1469. doi: 10.1016/j.joca.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Neogi T., Zhang Y. Osteoarthritis prevention. Curr Opin Rheumatol. 2011;23(2):185–191. doi: 10.1097/BOR.0b013e32834307eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasil. Instituto Brasileiro de Geografia e Estatística. Projeção da população do Brasil e das Unidades da Federação. Available from: www.ibge.gov.br/apps/populacao/projecao/ [accessed 20.01.17].
- 4.Lotz M. Osteoarthritis year 2011 in review: biology. Osteoarthr Cartil. 2012;20(3):192–196. doi: 10.1016/j.joca.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezende M.U., Campos C.G. Viscossuplementação. Rev Bras Ortop. 2012;47(2):160–164. [Google Scholar]
- 6.Zhang W., Nuki G., Moskowitz R.W., Abramson S., Altman R.D., Arden N.K. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr Cartil. 2010;18(4):476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 7.ltman R.D., Moskowitz R., Hyalgan Study Group Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. J Rheumatol. 1998;25(11):2203–2212. [PubMed] [Google Scholar]
- 8.Schiavinato A., Finesso M., Cortivo R., Abatangelo G. Comparison of the effects of intra-articular injections of Hyaluronan and its chemically cross-linked derivative (Hylan G-F20) in normal rabbit knee joints. Clin Exp Rheumatol. 2002;20(4):445–454. [PubMed] [Google Scholar]
- 9.Yoshimi T., Kikuchi T., Obara T., Yamaguchi T., Sakakibara Y., Itoh H. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop Relat Res. 1994;298:296–304. [PubMed] [Google Scholar]
- 10.Hulmes D.J., Marsden M.E., Strachan R.K., Harvey R.E., McInnes N., Gardner D.L. Intra-articular hyaluronate in experimental rabbit osteoarthritis can prevent changes in cartilage proteoglycan content. Osteoarthr Cartil. 2004;12(3):232–238. doi: 10.1016/j.joca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz R.W. Experimental models of osteoarthritis. In: Altman R.D., Buckwalter J.A., Goldberg V.M., Hochberg M.C., editors. Osteoarthritis: diagnosis and medical/surgical management. 2nd ed. Saunders; Philadelphia: 1992. pp. 213–252. [Google Scholar]
- 12.Sah R.L., Yang A.S., Chen A.C., Hant J.J., Halili R.B., Yoshioka M. Physical properties of rabbit articular cartilage after transaction of the anterior cruciate ligament. J Orthop Res. 1997;15(2):197–203. doi: 10.1002/jor.1100150207. [DOI] [PubMed] [Google Scholar]
- 13.Artmed; Porto Alegre: 2011. Infiltrações no aparelho locomotor: técnicas para realização com e sem o auxílio de imagem. [Google Scholar]
- 14.Adams M.E., Atkinson M.H., Lussier A.J., Schulz J.I., Siminovitch K.A., Wade J.P. The role of viscosupplementation with hylan G-F 20 (Synvisc) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthr Cartil. 1995;3(4):213–225. doi: 10.1016/s1063-4584(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 15.Dahl L.B., Dahl I.M., Engström-Laurent A., Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44(12):817–822. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balazs E.A., Denlinger J.L. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9. [PubMed] [Google Scholar]
- 17.Wang C.T., Lin Y.T., Chiang B.L., Lin Y.H., Hou S.M. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthr Cartil. 2006;14(12):1237–1247. doi: 10.1016/j.joca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki A., Sasaki K., Konttinen Y.T., Santavirta S., Takahara M., Takei H. Hyaluronate inhibits the interleukin-1beta-induced expression of matrix metalloproteinase (MMP)-1 and MMP-3 in human synovial cells. Tohoku J Exp Med. 2004;204(2):99–107. doi: 10.1620/tjem.204.99. [DOI] [PubMed] [Google Scholar]
- 19.Lo G.H., LaValley M., McAlindon T., Felson D.T. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290(23):3115–3121. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 20.Jubb R.W., Piva S., Beinat L., Dacre J., Gishen P. A one-year, randomised, placebo (saline) controlled clinical trial of 500-730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract. 2003;57(6):467–474. [PubMed] [Google Scholar]
- 21.Wang Y., Hall S., Hanna F., Wluka A.E., Grant G., Marks P. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011;12:195. doi: 10.1186/1471-2474-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atamaz F., Kirazli Y., Akkoc Y. A comparison of two different intra-articular hyaluronan drugs and physical therapy in the management of knee osteoarthritis. Rheumatol Int. 2006;26(10):873–878. doi: 10.1007/s00296-005-0096-x. [DOI] [PubMed] [Google Scholar]
- 23.Wobig M., Bach G., Beks P., Dickhut A., Runzheimer J., Schwieger G. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: a comparison of hylan G-F 20 and a lower-molecular-weight hyaluronan. Clin Ther. 1999;21(9):1549–1562. doi: 10.1016/s0149-2918(00)80010-7. [DOI] [PubMed] [Google Scholar]
- 24.Altman R.D., Bedi A., Karlsson J., Sancheti P., Schemitsch E. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2016;44(8):2158–2165. doi: 10.1177/0363546515609599. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson J., Sjögren L.S., Lohmander L.S. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology (Oxford) 2002;41(11):1240–1248. doi: 10.1093/rheumatology/41.11.1240. [DOI] [PubMed] [Google Scholar]
- 26.Albano M.B., Vidigal L., de Oliveira M.Z., Namba M.M., da Silva J.L., Pereira Filho F.A. Macroscopic analyses of the effects of hyaluronates and corticosteroids on induced osteoarthritis in rabbits’ knees. Rev Bras Ortop. 2015;45(3):273–278. doi: 10.1016/S2255-4971(15)30368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira M.Z., Albano M.B., Namba M.M., Cunha L.A.M., Gonçalves R.R.L., Trindade E.S. Efeito de ácidos hialurônicos como condroprotetores em modelo experimental de osteoartrose. Rev Bras Ortop. 2014;49(1):62–68. doi: 10.1016/j.rboe.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mero A., Campisi M., Favero M., Barbera C., Secchieri C., Dayer J.M. A hyaluronic acid-salmon calcitonin conjugate for the local treatment of osteoarthritis: chondro-protective effect in a rabbit model of early OA. J Control Release. 2014;187:30–38. doi: 10.1016/j.jconrel.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K., Goomer R.S., Harwood F., Kubo T., Hirasawa Y., Amiel D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta(IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthr Cartil. 1999;7(2):182–190. doi: 10.1053/joca.1998.0207. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu C., Kubo T., Hirasawa Y., Coutts R.D., Amiel D. Histomorphometric and biochemical effect of various hyaluronans on early osteoarthritis. J Rheumatol. 1998;25(9):1813–1819. [PubMed] [Google Scholar]
- 31.Ghosh P., Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32(1):10–37. doi: 10.1053/sarh.2002.33720. [DOI] [PubMed] [Google Scholar]