Abstract
Objectives
The principal objective of this study was to estimate the plasma levels of neutrophil gelatinase associated lipocalin (NGAL) in a cohort of patients with acute coronary syndromes (ACS) across their entire spectrum, and to correlate them with outcomes.
Methods
87 patients with acute coronary syndromes were included in the study. Apart from the routine work up and management, all patients underwent determination of plasma NGAL and serum high sensitivity C reactive protein (HSCRP) levels at admission. The patients were followed up through the hospital stay as well as for one month after discharge for clinical outcomes, and echocardiographic parameters of left ventricular function. Plasma NGAL was studied for its predictive power for various defined outcomes.
Results
Plasma NGAL levels were detectably elevated in 67% of patients with ACS without any significant proportion with renal dysfunction, sepsis or overt infection. Plasma NGAL was the strongest independent predictor of all cause hospital mortality in Cox regression multivariate analysis with an odds ratio of 8.353, p = 0.0237. Plasma NGAL did not correlate with HSCRP, or severity of coronary artery disease (CAD).
Conclusion
This is a small study that shows that plasma NGAL in patients admitted with ACS can predict hospital mortality and forms the basis for consideration of this molecule as a possible new risk marker in ACS meriting further and more extensive investigation.
Keywords: Neutrophil gelatinase associated lipocalin, Acute coronary syndrome, STEMI, NSTEMI, UA
1. Introduction
Neutrophil gelatinase associated lipocalin (NGAL), was first isolated by Kjeldsen et al. in the year 1993 from human neutrophils.1 Studies showed that the 135 kDa form of gelatinase was a complex of the 92 kDa gelatinase and a distinct 25 kDa protein. This 25 kDa protein was named NGAL which exists as a 25 kDa monomer – as well as a 45 kDa homodimer, and remains attached to gelatinase as a 135 kDa heterodimeric form.1 The monomeric form and sometimes the heterodimeric form are the predominant forms manufactured by the renal tubular epithelial cells – whereas the homodimeric form is usually specific to the neutrophils.2
NGAL levels maybe up-regulated in response to infections, inflammation, intoxication, ischemia, acute kidney injury (AKI) and even neoplastic transformation.3, 4, 5, 6, 7 It has been shown that urinary NGAL levels also depend on age, gender and hepatic function, and that they vary positively with inflammatory parameters.8
NGAL has also recently been associated, with heart failure and coronary artery disease (CAD), in a few studies.9, 10, 11, 12 The rise may be a manifestation of inflammation. Direct myocardial origin and seepage leading to elevation of NGAL levels, have also been proposed.13
Amongst patients with any form of acute coronary syndrome (ACS), we estimated the proportion with elevated NGAL levels, and studied whether elevated NGAL conferred any prognostic benefit in such patients.
2. Methods
2.1. Study design
This study was an observational cross-sectional study conducted in the Christian Medical College and Hospital, Vellore – a tertiary care hospital in south India with a dedicated twenty four hours emergency chest pain unit (CPU) and cardiac catheterization laboratory. Patients were recruited from the CPU at the time of admission, with informed consent. The diagnoses of the various forms of ACS were made according to standard guidelines.
Inclusion criteria:
-
1.
Patients with any form of ACS
-
2.
Age more than 18 years
-
3.
Valid consent
Exclusion criteria
-
1.
Any overt infection or sepsis
-
2.
Advanced hepatic failure
-
3.
Advanced renal failure (serum creatinine more than 2.65 mg/dL)
A total of 100 subjects were screened who met the criteria for recruitment. 13 subjects refused to give consent. Hence a total of 87 patients were included for final analysis.
2.2. Procedures
At admission, plasma NGAL and serum high sensitivity C reactive protein (HSCRP) were estimated. Hemodynamic, biochemical, renal, and clinical parameters were followed up. Hypertension was defined as the prior use of antihypertensives. Diabetes was defined as the use of hypoglycemic agents or an HBA1C more than 6.5%, or a fasting blood glucose ≥126 mg/dL or post prandial level ≥200 mg/dL. Estimated glomerular filtration rate (eGFR) was calculated by the Crockford Gault formula. Dyslipidemia was defined as prior treatment with cholesterol reducing medications or a total serum cholesterol ≥200 mg/dL or a serum LDL ≥70 mg/dL. Echocardiography was performed using a Vivid E9 (GE Vingmed Ultrasound, Horten, Norway) machine. The left ventricular ejection fraction (LVEF) was calculated by Simpson’s biplane method. Global longitudinal strain (GLS) was calculated for most patients. Angiographic details were noted for patients who underwent coronary angiography (CAG). SYNTAX and Gensini scores were calculated for all the performed angiograms.
2.3. Follow up
The patients were followed up at one month from discharge on an out-patient basis or in the CPU, if they underwent readmission, or by telephone on failure to follow up. A repeat echocardiography was performed and patients were interviewed for clinical events.
2.4. Outcomes
-
1.
Proportion of patients with acute coronary syndromes with elevated plasma NGAL levels
-
2.Endpoints in hospital:
-
a.Cardiogenic shock
-
b.Mechanical and electrical complications
-
c.In-hospital all-cause mortality
-
d.Left ventricular function
-
e.TIMI risk percentage
-
a.
-
3.
Correlation of NGAL with angiographic severity of CAD
-
4.
Correlation of plasma NGAL with biomarkers − troponins, CKMB, HSCRP
-
5.Endpoints at one month follow up:
-
a.Total all-cause mortality up till one month
-
b.Major adverse cardiac and cerebrovascular events (MACCE) up till one month
-
c.Left ventricular function
-
a.
2.5. Laboratory considerations
The quantitative assay that was used for NGAL was the NGAL test (Bio Porto Diagnostics, Denmark), which is a particle enhanced turbidimetric immunoassay. The minimum level of detection is 10 ng/mL. In accordance with established literature, values of NGAL above the 75th centile in our population distribution were considered high (>207 ng/mL).12 HSCRP was measured by means of particle enhanced immunonephelometry.
2.6. Statistical analysis
Eighty seven patients were included. Continuous variables were analyzed by the student T test in normally distributed data and the Mann Whitney test in cases of skewed data. Categorical variables were analysed with Chi square tests and odds ratios (OR). Kaplan Meir survival analysis was done. The Cox regression analysis model was used for multivariate analysis. For statistical analysis, p < 0.05 was considered to be of statistical significance. SPSS for Windows version 17.0 (SSPS Inc., Chicago, Illinois) was used for analysis.
3. Results
3.1. Distribution of NGAL
The mean value of NGAL in patients with STEMI was 159.88 ng/mL whereas the mean value amongst patients with UA/NSTEMI was 150.74 ng/mL (p = 0.893). Patients who had mild renal failure at admission (serum creatinine less than 2.65 mg/dL), had a mean NGAL value of 277.8 ng/mL, and patients who did not, had a mean NGAL value of 122.09 ng/mL (p = 0.018) (Table 1, Table 2).
Table 1.
Baseline characteristics.
| Parameters | Low NGAL ≤207 μg/L | High NGAL >207 μg/L | p-value |
|---|---|---|---|
| N = 65 | N = 22 | ||
| Age (years) | 55.97 | 63.82 | 0.008* |
| Male (%) | 72.30 | 77.27 | 0.648 |
| Hypertension (%) | 47.69 | 63.63 | 0.196 |
| Diabetes mellitus (%) | 53.84 | 45.45 | 0.496 |
| Current smoker (%) | 56.92 | 77.27 | 0.179 |
| Dyslipidemia (%) | 95.38 | 72.72 | 0.003* |
| LDL (mg/dL) | 109.48 | 100.36 | 0.278 |
| Duration of symptoms (h) | 12.32 | 13.07 | 0.860 |
| Troponin T at admission (μg/L) | 739.48 | 1461.32 | 0.228 |
| CKMB mass at admission (ng/mL) | 51.33 | 58.03 | 0.805 |
| HSCRP at admission (mg/L) | 22.15 | 19.68 | 0.810 |
| Serum creatinine at admission (mg/dL) | 1.17 | 1.43 | 0.027* |
| Estimated glomerular filtration rate (ml/min) | 65.72 | 49.95 | 0.002* |
| Left ventricular ejection fraction (mean%) | 43.98 | 42.68 | 0.580 |
| Global longitudinal strain | −11.47 | −10.89 | 0.527 |
| Percutaneous coronary intervention (%) | 58.40 | 54.50 | 0.806 |
Significant p-values.
Table 2.
Mean NGAL values in major population groups.
| Mean NGAL (ng/mL) | p-value | |
|---|---|---|
| STEMI | 159.88 | 0.893 |
| UA/NSTEMI | 150.74 | |
| Mild renal failure at admission | 277.8 | 0.018* |
| No renal failure at admission | 122.09 |
Significant p-values.
The levels of NGAL are normally undetectable. Here, however, detectable levels of NGAL were found in 67% of the population. This by itself can be considered to be a significant finding. Amongst patients with UA/NSTEMI, NGAL was detectable in 57.8% of the patients – the proportion was non-significantly higher at 69.1% in STEMI patients.
3.2. Cardiac biomarkers
There was a statistically significant correlation between admission troponin T and plasma NGAL values with a Pearson correlation coefficient of 0.227 (p = 0.034). NGAL values did not correlate significantly with CK-MB mass.
3.3. Echocardiographic parameters
There was no statistically significant correlation between the LVEF and NGAL. 81 of the 87 recruited patients (93.1%) had their GLS measured. The GLS in the patients with STEMI and UA/NSTEMI were no different. GLS correlated well with Simpson’s ejection fraction with a correlation coefficient of −0.777 (p < 0.001) – it did not correlate with NGAL.
3.4. Coronary anatomy and interventions
There were no significant differences in the mean NGAL, amongst patients with different infarct related arteries. Correlations between SYNTAX and plasma NGAL as well as between Gensini scores and plasma NGAL were not found to be significant. However, SYNTAX and Gensini scores correlated significantly – correlation coefficient being 0.744 (p < 0.001). There was no significant difference in the proportion or outcome of patients undergoing PCI in the high and low NGAL groups.
3.5. Clinical outcomes
3.5.1. All-cause mortality
High NGAL was able to significantly predict all-cause in-hospital mortality with a significant OR of 6.078 (95% confidence intervals 1.318–28.033), p = 0.023. High NGAL again, also significantly predicted all cause total mortality at the end of one month [OR 5.8 (95% confidence intervals 1.449–23.209); p = 0.014].
3.5.2. Cardiogenic shock
High NGAL significantly predicted cardiogenic shock at admission [OR 5.719 (95% confidence interval 1.439–22.725), p = 0.015]. There were a total of 21 (24.1%) patients who suffered cardiogenic shock at some point in time during their admission. High NGAL was able to significantly predict this outcome as well [OR 4.091 (95% confidence interval 1.417–11.814), p = 0.010]. Electrical, mechanical complications and hospitalization duration were not significantly predicted by NGAL.
3.5.3. Major adverse cardiac and cerebrovascular events (MACCE)
MACCE included any of the following: non-fatal ACS, angina, heart failure, cerebrovascular accidents and death from any cause. There were a total of 15 MACCE events. Heart failure was the commonest. NGAL did not predict MACCE at one month (Table 3).
Table 3.
Statistically significant odds ratios of high NGAL in predicting outcomes.
| Parameter | OR | 95% confidence interval | p-value |
|---|---|---|---|
| All cause hospital mortality | 6.078 | 1.318–28.033 | 0.023 |
| Total all-cause mortality at 30 days | 5.8 | 1.449–23.209 | 0.014 |
| Cardiogenic shock at presentation | 5.719 | 1.439–22.725 | 0.015 |
| Cardiogenic shock any time in hospital | 4.091 | 1.417–11.814 | 0.010 |
3.6. Thrombolysis in myocardial infarction (TIMI) risk stratification system
TIMI risk percentage and NGAL-correlated with statistical significance with a correlation coefficient of 0.333 (p = 0.002). NGAL was superior to HSCRP and admission troponin T in predicting TIMI risk which determines adverse cardiac events and mortality in the period around an ACS. Hence a solitary plasma NGAL value may be enough for risk stratification, obviating the need to calculate the cumbersome TIMI risk score.
3.7. HSCRP
HSCRP >3 mg/L confers high risk in cardiovascular disease. NGAL failed to correlate significantly with HSCRP. This may indicate that variations in the values of NGAL in these patients may arise from a mechanism unrelated to the inflammatory cascade. Hence plasma NGAL could be a potential risk predictor in ACS, independent of the inflammatory cascade.
3.8. Survival analysis
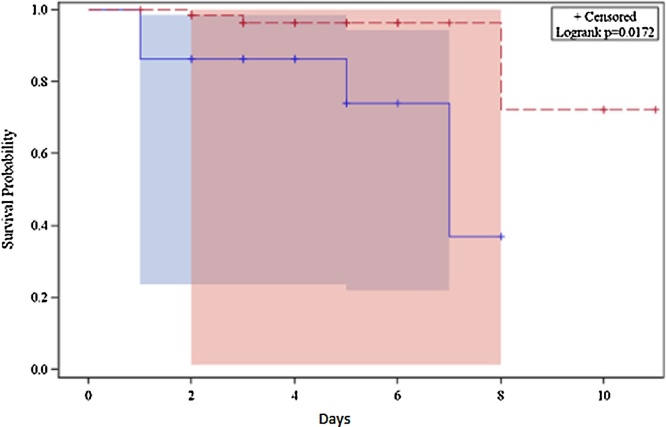
A Kaplan Meir survival analysis was drawn for the duration of hospital stay for all the patients and stratified according to low and high NGAL values. The survival curves revealed a definite and statistically significant divergence with a significant log-rank p value of 0.0172 (Fig. 1).
Fig. 1.
Kaplan Meir survival analysis in the high and low NGAL groups (blue line indicates high NGAL and red line indicates low NGAL).
3.9. ROC curve
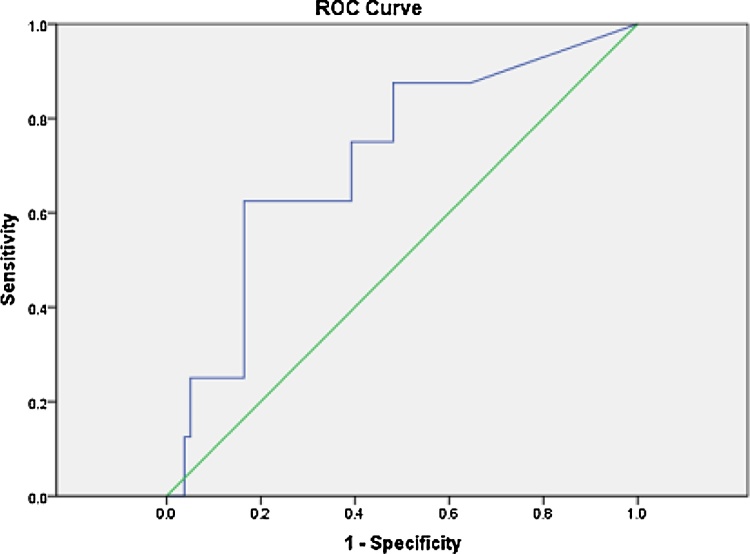
A receiver operating characteristic (ROC) curve was drawn for hospital mortality and NGAL values. The area under the curve (AUC) was found to be 71.5% with a significant p value of 0.046 indicating that NGAL was a fair predictor of hospital mortality. From this ROC curve, the cut off chosen for determining high NGAL had a sensitivity of 62.5% and a specificity of 78.5% for the prediction of in hospital all-cause mortality. It was determined from the same curve, that if the NGAL cut off was taken at 215 ng/mL the sensitivity for predicting hospital mortality would have been 62.5% and the specificity would have been 83.5% – this would have been the best NGAL cut off for predicting in-hospital mortality (Fig. 2).
Fig. 2.
ROC curve for NGAL to predict hospital mortality.
In order to attempt an improvement in the predictive power of NGAL for in hospital all-cause mortality, NGAL was combined with various echocardiographic and angiographic variables and ROC curves were redrawn; however, these ROC curves could not better the predictive power of NGAL used independently. This was probably due to the fact that the study was not powered towards these analyses; however, they could be subjects of further investigation.
3.10. Multivariate analysis
In the Cox regression analysis model constructed for predicting all-cause hospital mortality, the covariates that were included were HSCRP, NGAL, troponin T and mild renal failure at admission. NGAL emerged as the single strongest predictor of all cause in-hospital mortality, with an adjusted odds ratio of 8.353 (95% confidence interval 1.328–52.522, p = 0.0237).
4. Discussion
The NGAL molecule that is usually undetectable in blood was found to be elevated in 67% of our cohort. Only a small minority had mild renal failure and patients with sepsis and acute infections were excluded – thus, this high proportion of patients with detectable plasma NGAL becomes a significant finding. Its elevation was seen across the spectrum of ACS. Recently there have been few reports looking at using NGAL as an early marker of renal dysfunction, especially in patients with contrast induced nephropathy (CIN).14 There appears to be significant elevation of NGAL in an ACS per se, which appears to be independent of renal dysfunction. Also in the multivariate model we see that NGAL is an independent predictor of death even after adjusting for renal failure – this points to the fact that NGAL is probably associated with the pathophysiology of coronary ischemia, independent of renal mechanisms. Hence, in the context of an ACS at least, NGAL may not be a good marker of CIN owing to the fact that the levels may be elevated due to myocardial ischemia per se and not necessarily indicate renal dysfunction.
In view of the lack of correlation of plasma NGAL with serum HSCRP in our study it may be assumed that the mechanism of NGAL elevation could be independent of the level of inflammation within the body. This is significant as this makes NGAL a suitable non inflammatory risk discriminator in ACS. This may make it a tool suitable in situations such as sepsis where the current cardiac biomarkers are less accurate in cardiovascular risk stratification.
In univariate analyses, plasma NGAL was noted to be significantly associated with all-cause mortality (in hospital and at one month follow up) and cardiogenic shock with significant odds ratios. Interestingly, plasma NGAL was also found to correlate significantly with TIMI risk percentages. In the multivariate analysis, NGAL appeared to be solely and significantly predicting all cause hospital mortality with an odds ratio of 2.89 (p = 0.0237). Survival analysis by Kaplan Meir curves showed that the graph for the patients with high NGAL diverged significantly from the graph for the patients with low NGAL with a p value of 0.0172 from a very early stage of the acute coronary event. The ROC curve drawn for NGAL correlating with all cause in-hospital mortality showed a fair predictive power of the test. These results were independent of factors like renal failure and invasive revascularizations done in the patient groups. The implications of all the above results may be that the plasma NGAL molecule could potentially behave as a robust, independent and significant predictor of clinical outcomes in hospital and one month follow up, amongst patients with ACS and hence worth studying further in the future. The plasma levels of NGAL at admission were not found to significantly differ within the different spectra of ACS. The levels also did not predict the severity of CAD both in terms of the angiographic numbers of vessels involved or angiographic risk scores like SYNTAX and Gensini.
4.1. Study limitations
Though this study unexpectedly yielded encouraging results, it was an observational study. The sample size was not large. The biological plausibility needs to be studied further. Mild renal dysfunction as a cause of NGAL elevation may still be possible. The MACCE rate and the duration of follow up were probably inadequate to do statistically sound analyses for outcomes at follow up. Also instead of a single sample, serial samples of the NGAL molecule would have better elucidated the time related kinetics of the molecule.
4.2. Conclusions
The following conclusions can be drawn from the study:
-
1.
A large proportion of patients with ACS have detectable NGAL values in their plasma which appear unrelated to sepsis, infections or renal failure.
-
2.
NGAL does not correlate with the inflammatory marker HSCRP making the inflammatory state of the body an unlikely cause for the elevation of NGAL.
-
3.
NGAL significantly predicts the risk of clinical outcomes in hospital and one month follow up, and correlates significantly with TIMI risk percentages.
-
4.
NGAL does not discriminate between the spectra of acute coronary syndromes.
-
5.
NGAL does not predict the severity of CAD.
What is already known?
NGAL is up-regulated in various inflammatory states of the body as well as in renal failure.
What this study adds?
NGAL is elevated in ACS as well and this rise appears to be by a non-inflammatory mechanism. NGAL also provides prognostic benefit in ACS.
Conflict of interest
None of the authors have any conflicts of interest to declare.
Contributor Information
Anandaroop Lahiri, Email: anandaroop_lahiri@yahoo.com.
Anoop George Alex, Email: alexanoop@gmail.com.
Paul V. George, Email: paulgeorgev@hotmail.com.
References
- 1.Kjeldsen L., Johnsen A.H., Sengeløv H., Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 2.Cai L., Rubin J., Han W., Venge P., Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol CJASN. 2010;5:2229–2235. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz D.H., Willie S.T., Armen R.S., Bratt T., Borregaard N., Strong R.K. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry (Mosc) 2000;39:1935–1941. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- 4.Kjeldsen L., Cowland J.B., Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000;1482:272–283. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- 5.Cowland J.B., Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 6.Mishra J., Ma Q., Prada A. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol JASN. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 7.Mishra J., Dent C., Tarabishi R. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 8.Tomonaga Y., Szucs T., Ambühl P., Nock S., Risch M., Risch L. Insights on urinary NGAL obtained in a primary care setting. Clin Chim Acta Int J Clin Chem. 2012;413:733–739. doi: 10.1016/j.cca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Yndestad A., Landrø L., Ueland T. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229–1236. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 10.Kafkas N., Demponeras C., Zoubouloglou F., Spanou L., Babalis D., Makris K. Serum levels of gelatinase associated lipocalin as indicator of the inflammatory status in coronary artery disease. Int J Inflamm. 2012;2012:189797. doi: 10.1155/2012/189797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickstein K., Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet. 2002;360:752–760. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg S., Pedersen S.H., Mogelvang R. Prognostic utility of neutrophil gelatinase-associated lipocalin in predicting mortality and cardiovascular events in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2012;60:339–345. doi: 10.1016/j.jacc.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Hemdahl A.-L., Gabrielsen A., Zhu C. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26:136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 14.McCullough P.A., Williams F.J., Stivers D.N. Neutrophil gelatinase-associated lipocalin: a novel marker of contrast nephropathy risk. Am J Nephrol. 2012;35(6):509–514. doi: 10.1159/000339163. [DOI] [PubMed] [Google Scholar]