Abstract
Background:
Arsenic trioxide (As2O3) has shown effectiveness in the treatment of leukemia, but it is also associated with hepatotoxicity. Given antileukemic drug-induced oxidative stress and toxicity, this study focused on the mitigatory role of eugenol, a monoterpene compound from clove oil, in the hepatic tissue of Wistar rats.
Methods:
Twenty-four male Wistar rats (180-250 g) were randomly divided into 4 groups (6 rats per group): normal control rats, rats treated with As2O2 (4 mg/kg bwt), rats treated with eugenol (5 mg/kg bwt), and rats receiving co-treatment with As2O3 (4 mg/kg bwt) and eugenol (5 mg/kg bwt), all of which orally administered for a period of 30 days. The Tukey test (Origin version 7, Origin Lab Corporation, Northampton, USA) was applied to analyze the one-way analysis of variance (ANOVA) between the different groups. A P value less than 0.05 was considered significant.
Result:
Oral administration of As2O3 significantly induced hepatic damage, evident from increased levels of aspartate transaminase and alanine transaminase (P=0.01 and P<0.001, respectively). Moreover, a decrease in the activities of enzymatic and nonenzymatic antioxidants altered electrolyte concentration and increased the rate of lipid peroxidation (P=0.04) and the level of nitric oxide (P=0.01). Accumulation studies and histopathological analyses confirmed the biochemical variations. Co-treatment with eugenol (5 mg/kg bwt) exhibited hepatoprotective effects as manifested by the decreased rate of arsenic accumulation, lipid peroxidation, and nitric oxide level along with normalized levels of antioxidants and maintained histology of the liver.
Conclusion:
Eugenol may be used in combination with arsenic trioxide in chemotherapy to reduce oxidative damage to the hepatic system.
Keywords: Arsenic trioxide , Antioxidants , Eugenol , Hepatotoxicity , Oxidative stress
What’s Known
Arsenic trioxide is a promising drug used for the treatment of acute promyelocytic leukemia (APL).
Arsenic trioxide causes liver damage in experimental model rats.
What’s New
Eugenol can be used to prevent hepatocellular injury caused by the ALP drug arsenic trioxide.
Introduction
Drugs and some chemical agents are known to induce hepatotoxicity in humans, which is of great concern to clinicians. Arsenic has been considered a poison for a long time. In the 1970s, arsenic trioxide (As2O3) was introduced into the treatment of acute promyelocytic leukemia (APL). In APL there is a chromosomal translocation, t(15;17), resulting in the creation of a fusion between the retinoic acid receptor-alpha (RAR α) gene in chromosome 17 and the promyelocytic leukemia (PML) gene located in chromosome 15.1 The resulting PML-RAR α fusion protein blocks the expression of the genes required for normal myeloid differentiation.2 When arsenic trioxide is used in APL patients who have relapsed after trans retinoic acid treatment, it can induce differentiation.3 Oxidative damage has been deemed a key mechanism of the action of arsenic in the initial stage of apoptosis. Despite its broad therapeutic effectiveness, clinical trials of arsenic trioxide (Trisenox) are often limited because of its dose- dependent adverse effects on the heart, liver, and kidney. Mathews et al.4 reported arsenic trioxide-induced hepatotoxicity during APL therapy. Our previous study also reported that arsenic trioxide was able to cause serious biochemical and histopathological changes in the liver.5
Combination therapy is a frequent strategy to improve the clinical efficacy of arsenic trioxide. Accordingly, we chose a naturally occurring antioxidant compound, eugenol, for the present study. Eugenol (4 allyl 2 methoxyphenol) is a natural monoterpene component of clove oil. Eugenol possesses various biological properties, including antioxidant, anti-inflammatory, analgesic, antipyretic, antibacterial, antifungal, and antitumor activities.6 Eugenol is generally recognized as safe by the Food and Drug Administration.7 In traditional medicine, eugenol has been used against many gastrointestinal diseases. In addition, it has been widely used as a fragment and flavoring agent in food and cosmetic products.8.
The present work was undertaken with the main aim of evaluating the hepatoprotective property of eugenol over arsenic trioxide-induced toxicity.
Materials and Methods
Animals and Ethics
Male Wistar rats were purchased from the Pharmacology Unit, Nagarjuna Herbal Concentrates Ltd., Thodupuzha, Idukki, Kerala, India, and were acclimatized for 6 days. The experiments were conducted as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The study protocol was approved by the Institutional Animal Ethics committee (IAEC), School of Biosciences, Mahatma Gandhi University, Kottayam, Kerala, India (Approval #B29122014/3).
Experimental Design
Twenty-four adult male rats were randomly selected for the study. Their body weight ranged between 180 and 250 g. Rats from the same species (Wistar) were selected and only healthy rats were subjected to treatment. They were maintained in the same atmospheric and nutritional conditions throughout the study. The rats were divided into 4 groups, each containing 6 rats, in order to obtain statistically significant data. The study groups were classified as follows: Group I was treated as the normal control and was orally given autoclaved water and normal diet only, Group II received daily oral administration of a single dose of arsenic trioxide (4 mg/kg bwt), Group III received oral administration of eugenol (5 mg/kg bwt), and Group IV was taken as a combined group and given a single dose of arsenic trioxide (4 mg/kg bwt) and eugenol (5 mg/kg bwt) every day for a period of 30 days.
Biochemical Analysis
After 30 days of experimental period, all the animals were euthanized and decapitated and their blood was collected and centrifuged at 3000 rpm for 20 minutes. Thereafter, the clear serum obtained was used for the determination of serum enzymes. The liver was removed immediately, washed in ice-cooled 0.15 M of NaCl, and homogenized by using Tris HCl buffer (0.2 M, pH 7.4). The homogenate was used for further biochemical analyses. Total arsenic deposition in the liver tissue and tissue electrolytes (calcium, sodium, and potassium) were analyzed by standard inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Optima 2000 DV ICP-OES, Perkin Elmer, Inc., Waltham, Massachusetts, USA). The activity of serum aspartate transaminase (AST) and alanine transaminase (ALT) was assayed spectrophotometrically according to the standard procedures using commercially available diagnostic kits (Agappe Diagnostic Ltd., Ernakulam, Kerala, India).
Glutathione (GSH), a nonenzymatic antioxidant, was measured in the tissue homogenate according to the Ellman method.9 Glutathione S-transferase (GST) was assayed by the method of Habig et al.10 and changes in optical activity were read at 340 nm for 5 minutes. The activity of glutathione peroxidase (GPx) was determined by the method of Rotruck et al.11 The color that developed was read at 412 nm against a reagent blank, and the results were expressed as µg of GSH consumed/mg protein. Catalase activity in the sample was measured according to the method of Aebi12 by measuring the decrease in the absorbance of H2O2 at 240 nm. The activity of thiobarbituric acid reactive substances (TBARS) was estimated by the method of Beuge and Aust.13 The concentration of nitric oxide in the form of nitrate was determined using the Griess reagent, and the amount of nitrate present in various samples was measured at 540 nm.14 Hematoxylin and eosin was employed to examine histopathological changes under the microscope (Phase contrast microscope [Inea, Olympus]). The microphotographs were taken using a Moticam-1000 camera at an original magnification of 100X.
Statistical Analysis
The data were analyzed using the statistical package program Origin, version 7 (Origin Lab Corporation, Northampton, USA). The statistical analyses between the different groups were performed with one-way ANOVA by Tukey test analysis. All the data were expressed as mean±standard deviation (SD). The results were considered significant at a P value less than 0.05.
Results
Figure 1 shows that treatment with As2O3 caused a significant increase in the concentration of arsenic in the hepatic tissue (P=0.01). Co-administration with eugenol significantly decreased the accumulation of arsenic (P=0.02). Figure 2 indicates that the level of the tissue electrolyte calcium was increased in the arsenic-treated rats (P<0.001). Eugenol administration helped to maintain the normal range of calcium (P=0.01), whereas the concentrations of potassium (P<0.001) and sodium (P=0.01) were reduced in the arsenic-treated ones (figure 3). Co-treatment with eugenol significantly maintained the level of potassium (P=0.03) and sodium (P<0.001). Figure 4 shows that the oral administration of arsenic trioxide caused an abnormal liver function in the treated rats. The levels of liver marker enzymes such as AST and ALT were significantly increased during arsenic trioxide treatment, when compared with the control rats (P=0.01 and P<0.001 for AST and ALT, respectively). Administration of eugenol (5 mg/kg) with arsenic significantly normalized the level of these enzymes (P=0.03 for AST and P<0.001 for ALT). Here we compare the normal control group and the arsenic trioxide group represented as ‘a’ (P value indicated as “P1”) and the arsenic trioxide group and the arsenic trioxide+eugenol group represented as ‘b’ (P value indicated as “P2”). A significant decline in the level of GSH was noticed in the liver tissue of the arsenic-treated rats as compared to the controls (P1<0.001). A significantly decreased (P<0.05) activity of nonenzymatic antioxidants like GST (P1=0.04), GPx (P1<0.001), and catalase (P1<0.001) was also observed in the arsenic-induced group. Co-treatment with eugenol and arsenic significantly restored the level of the nonenzymatic antioxidant GSH (P<0.001) and the enzymatic antioxidants GST (P2=0.01), GPx (P2<0.001), and CAT (P2<0.001) when compared with the group treated with arsenic alone (table 1). The rats treated with arsenic trioxide treatment showed a significant (P=0.04) rise in the hepatic lipid peroxidation marker, TBARS, as compared to the control group (figure 5). Treatment with eugenol caused a significant reduction in the TBARS level (P=0.03). Figure 6 shows that arsenic administration resulted in a drastic elevation in the hepatic nitric oxide level as compared to the corresponding controls (P=0.01). Eugenol therapy resulted in a significant decrease in the nitric oxide level in the combined group compared with the arsenic-treated one (P<0.001). The histopathological studies also supported the biochemical analysis. The liver of the control (figure 7A) and eugenol-administered (figure 7C) rats showed normal hepatic structures, characterized by polygonal-shaped hepatocytes with well-defined boundaries and slight staining acidophilic cytoplasms with large and centrally located nuclei. The hepatic tissue of the arsenic-induced group (figure 7B) revealed that the normal architecture of the liver was completely lost in the rats treated with arsenic trioxide, with the appearance of hemorrhage, cholangio fibrosis, and focal necrosis. The liver of the eugenol- and arsenic-administered rats (figure 7D) exhibited restored normal hepatic architectures.
Figure1.
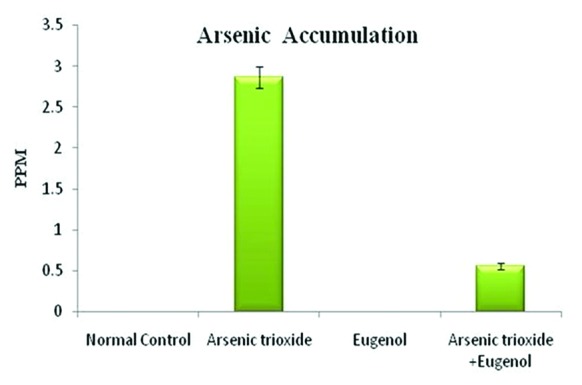
Effects of eugenol on arsenic accumulation in the liver. Here the experimental group comprises normal control, arsenic trioxide (4 mg/kg bwt), eugenol (5 mg/kg bwt), and arsenic trioxide (4 mg/kg bwt)+eugenol (5 mg/kg bwt). Data are presented as mean±SD and n=6. ‘a’ indicates a significant difference between the normal control group and the arsenic trioxide group (P=0.01). ‘b’ indicates a significant difference between the arsenic trioxide group and the arsenic trioxide+eugenol group (P=0.02).
Figure2.
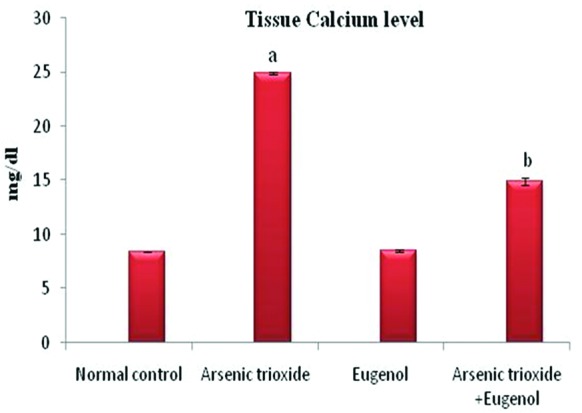
Effects of eugenol on the tissue calcium in the liver. Here the experimental group comprises normal control, arsenic trioxide (4 mg/kg bwt), eugenol (5 mg/kg bwt), and arsenic trioxide (4 mg/kg bwt)+eugenol (5 mg/kg bwt). Data are presented as mean±SD and n=6. ‘a’ represents a significant difference between the normal control group and the arsenic trioxide group. ‘b’ represents a significant difference between the arsenic trioxide and the arsenic trioxide+eugenol group. ‘a’ indicates P<0.001 and ‘b’ indicates P=0.01.
Figure3.
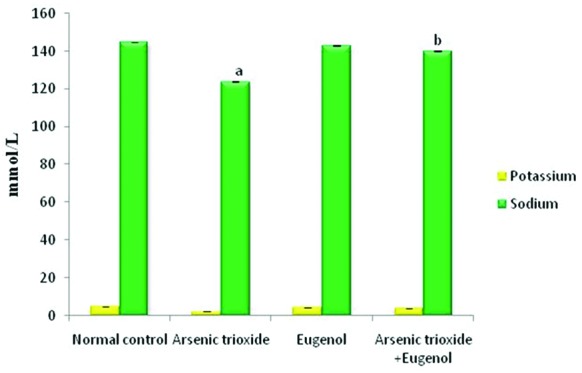
Effects of eugenol on tissue potassium and sodium levels in the liver. Here the experimental group comprises normal control, arsenic trioxide (4 mg/kg bwt), eugenol (5 mg/kg bwt), and arsenic trioxide (4 mg/kg bwt)+eugenol (5 mg/kg bwt). Data are presented as mean±SD and n=6. ‘a’ represents a significant difference between the normal control group and the arsenic trioxide group (P<0.001 for potassium and P=0.01 for sodium). ‘b’ indicates a significant difference between the arsenic trioxide and the arsenic trioxide+eugenol (P=0.03 for potassium and P<0.001 for sodium).
Figure4.
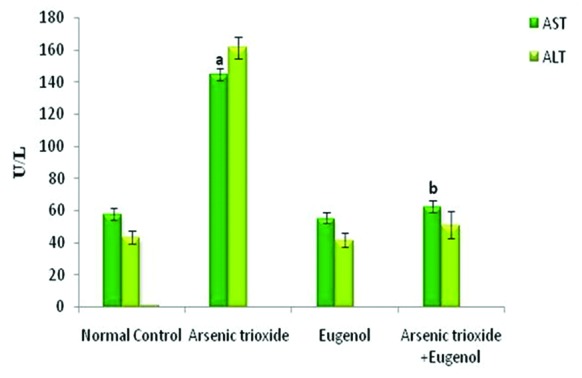
Changes in the liver markers AST and ALT on arsenic-induced toxicity. Here the experimental group comprises normal control, arsenic trioxide (4 mg/kg bwt), eugenol (5 mg/kg bwt), and arsenic trioxide (4 mg/kg bwt)+eugenol (5 mg/kg bwt). Data are presented as mean±SD and n=6. ‘a’ represents a significant difference between the normal control and the arsenic trioxide (P=0.01 for AST and P<0.001 for ALT). ‘b’ indicates a significant difference between the arsenic trioxide group and the arsenic trioxide+eugenol group (P=0.03 for AST and P<0.001 for ALT). AST: Aspartate aminotransferase; ALT: Alanine aminotransferase
Table 1.
Effects of eugenol on the hepatic antioxidant system
| Parameters | Normal Control | Arsenic Trioxide | Eugenol | Arsenic Trioxide+Eugenol |
|---|---|---|---|---|
| GSH (µM/g tissue) | 52.04±0.72 | 23.72±1.24a | 40.92±0.81 | 42.38±0.18b |
| GST(µM of CDNB- GSH conjugate formed/min/mg protein) | 2.54±0.56 | 1.49±0.99a | 2.18±0.06 | 2.72±0.03b |
| GPx (µg of GSH consumed/min/mg protein) | 12.16±3.22 | 7.38±0.87a | 11.08±0.16 | 8.92±0.01b |
| CAT (µmoles of H2O2 consumed/min/mg protein) | 39.66±0.14 | 16.07±0.51a | 38.00±0.18 | 33.40±0.11b |
GSH: Glutathione; GST: Glutathione S transferase; GPx: Glutathione peroxidase; CAT: Catalase; Table 1 depicts the effects of eugenol on the hepatic antioxidant system. Here the experimental group comprises normal control, arsenic trioxide (4 mg/ kg bwt), eugenol (5 mg/kg bwt), and arsenic trioxide (4 mg/ kg bwt)+eugenol (5 mg/ kg bwt). Data are presented as mean±SD and n=6. ‘a’ indicates a significant difference between the normal control group and the arsenic trioxide group. ‘b’ indicates a significant difference between the arsenic trioxide group and the arsenic trioxide+eugenol group.
Figure5.
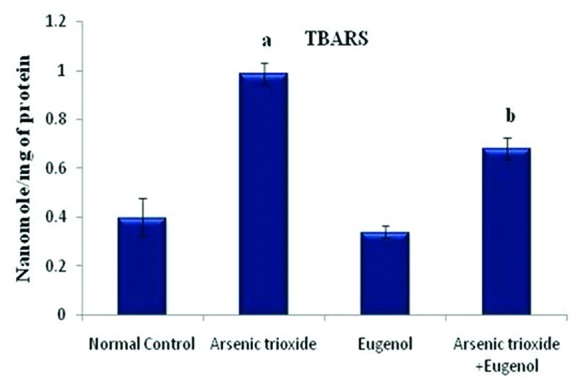
Effects of eugenol on the lipid peroxidation rate in the hepatic tissue. Here the experimental group comprises normal control, arsenic trioxide (4 mg/kg bwt), eugenol (5 mg/kg bwt), and arsenic trioxide (4 mg/kg bwt)+eugenol (5 mg/kg bwt). Data are presented as mean±SD and n=6. ‘a’ represents a significant difference between the normal control and the arsenic trioxide (P=0.04). ‘b’ represents a significant difference between the arsenic trioxide group and the arsenic trioxide+eugenol group (P=0.03).
Figure6.
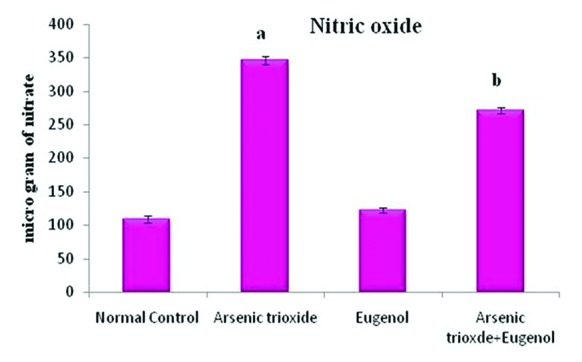
Effects of eugenol on the arsenic trioxide-induced nitric oxide level in the liver tissue. Here the experimental group comprises normal control, arsenic trioxide (4 mg/kg bwt), eugenol (5 mg/kg bwt), and arsenic trioxide (4 mg/kg bwt)+eugenol (5 mg/kg bwt). Data are presented as mean±SD and n=6. ‘a’ indicates a significant difference between the normal control group and the arsenic trioxide group (P=0.01). ‘b’ represents a significant difference between the arsenic trioxide group and the arsenic trioxide+eugenol group (P<0.001).
Figure7.
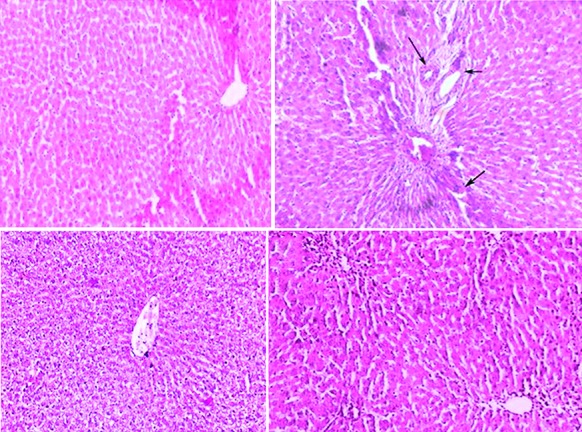
Effects of eugenol on arsenic trioxide-induced oxidative stress in the histology of the liver. (A) Hematoxylin and eosin (100×)-stained microscopic section of the liver of the normal control rats shows a normal hepatic morphology. (B) Hematoxylin and eosin (100X)-stained microscopic section of the hepatic tissue of the arsenic (4 mg/kg)-treated rats shows the appearance of hemorrhage (↙), cholangio fibrosis (←), and focal necrosis (↘). (C) Eugenol (5 mg/kg)-administered rats show a normal hepatic structure and morphology. (D) Hematoxylin and eosin (100×)-stained microscopic section of the liver of the rats treated with a combination of eugenol (5 mg/kg) and arsenic trioxide (4 mg/kg) shows a normal structure without any hepatic parenchymal degeneration, hyperplasia, and vascular lesions.
Discussion
The aim of the present investigation was to evaluate and explore the hepatoprotective role of eugenol, a monoterpene compound, against arsenic trioxide. The toxicity of a wide range of chemicals and drugs is associated with oxidative stress mechanism.15 Our previous studies revealed that eugenol had an excellent antioxidant activity.16
Our ICP-OES analysis revealed that arsenic trioxide treatment caused accumulation of arsenic in the hepatic tissue. Quatrehomme et al.17 reported that arsenic was rapidly distributed to the organs or tissues that contained proteins with sulfhydryl groups and accumulated in the liver, kidneys, spleen, and adrenal glands. Methylation converts arsenic into less toxic elements and transforms it into the form of monomethyl arsonic acid and dimethyl arsonic acid.2 Nonetheless, co-treatment with the phenolic compound eugenol reduces the accumulation rate of arsenic in the hepatic tissue. The present study demonstrated that the level of the tissue electrolyte calcium was increased, while the potassium and sodium levels were decreased in the arsenic-treated group compared with the normal rats. Williams et al.18 reported that in the liver tissue, there was an alteration of electrolyte concentration during oxidative stress. Combination therapy with the natural phenolic compound eugenol showed a distinguished normalization of these electrolytes.
AST and ALT are the premier parameters that help to detect hepatic damage.19 During arsenic trioxide treatment, both AST and ALT levels rise in serum. In liver injury, the transport function of hepatocytes is disturbed, resulting in the leakage of the plasma membrane and elevation in the serum levels of AST and ALT.20 In human APL trials, toxicity has been mainly allied to serum hepatic enzyme changes.21 Normalization of the above-mentioned enzyme level in the rats treated with eugenol clearly established its hepatoprotective effects.
Hepatic GSH plays a crucial role in both scavenging reactive oxygen species and detoxification of drugs.22 Because the liver is rich in GSH, it is a major site of arsenic detoxification.23 In the hepatic tissue, GSH stimulates arsenic detoxification processes by modulating arsenic methylation24 and increasing the activity of GPx.25 Hepatobiliary excretion of arsenic is also processed through GSH.26 Yokota et al.27 reported that eugenol had the capacity to induce the antioxidant enzyme system for xenobiotic metabolism in the liver. GSH depletion may facilitate the promotion of oxidative stress and the initiation of the apoptosis signaling pathway.28 Arsenic trioxide was found to inhibit GST activity significantly in the liver and kidney.25 Uthus et al.29 observed that arsenic deprivation decreased hepatic GST activity. GPx plays a major role in the reduction of H2O2 and hydroperoxide to nontoxic products.30 The decrease in the activity of GPx during the period of arsenic treatment in this experiment was an indication of the overproduction of peroxides. Catalase is a heme protein that catalyzes the reduction of H2O2, thereby protecting the cell from oxidative damage by H2O2 and OH.31 The activity of catalase was decreased in the arsenic-treated group compared with the normal control rats. Combination therapy with eugenol maintained the level of catalase compared with the arsenic group. Our previous study report on eugenol established its antioxidant property.16
Lipid peroxidation plays an important role in oxidative stress injury to the liver, with the role being determined indirectly by evaluating the enhanced levels of malondialdehyde and reduced levels of GSH.22 In the current study, we observed a higher level of malondialdehyde and a lower level of GSH in the hepatic tissue of the arsenic-administered rats. Horton et al.31 explained arsenic-induced lipid peroxidation by the depletion of GSH in the liver. The antioxidant properties of eugenol may be the core reason behind the lower hepatic lipid peroxidation rate and the increased amount of GSH in the liver. Our results revealed that the levels of nitric oxide in the rats’ hepatic tissue were significantly enhanced by arsenic trioxide. A previous report by Choi et al.32 showed that eugenol was able to suppress inducible nitric oxide synthase protein expression. Eugenol has nitric oxide scavenging properties in vitro.33 GSH also has the capacity to scavenge reactive nitrogen species.28 Administration of eugenol effectively overcomes these problems during combination therapy.
Histological studies have also supported the curative effects of the monoterpenoid compound eugenol on arsenic-induced hepatotoxicity. This might be due to increased cellular activity and nuclear interruption in the mechanism of arsenic-based xenobiotic metabolism. Hepatocyte necrosis due to arsenic exposure might indicate oxidative stress on the liver. Our previous study revealed that arsenic trioxide (4 mg/kg bwt) caused alterations in the hepatic system.5 Histopathological findings of the rats’ livers co-treated with eugenol (5 mg/kg) showed a well-preserved normal morphology of the hepatic system. Thus, eugenol protected the liver against damage caused by arsenic, inhibited lipid peroxidation, and conferred more potential to the antioxidant system. Indeed, our results illustrated a near-normal restoration of the architecture of the hepatic system. Our study had several limitations because the exact mechanism behind the hepatoprotective effects of eugenol has yet to be identified. Given the dearth of data on molecular markers in the hepatic system, we suggest that further research be conducted to determine the exact pathway and the involvement of molecular targets.
Conclusion
Administration of eugenol improved antioxidant status and reduced the lipid peroxidation rate and arsenic deposition in the hepatic tissue of the rats in the present study. This monoterpenoid compound helped to maintain tissue electrolytes, liver marker enzyme levels, and normal architecture of the hepatic tissue. Accordingly, our study reveals that eugenol treatment mitigates arsenic intoxication-related oxidative damage, which could be due its antioxidant nature that combines free radical scavenging and metal-chelating properties.
Acknowledgement
This work was supported by the Department of Biotechnology (DBT), New Delhi through DBT-MSUB/IPLSARE (#BT/PR4800/INF/22/152/2012). We thank Dr. Prakash Kumar B, Project Coordinator, DBT-MSUB/IPLSARE, for providing the necessary facilities for our work.
Conflict of Interest:None declared.
References
- 1.de The H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347:558–61. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 2.Miller WH, Jr , Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–903. [PubMed] [Google Scholar]
- 3.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–8. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 4.Mathews V, Desire S, George B, Lakshmi KM, Rao JG, Viswabandya A, et al. Hepatotoxicity profile of single agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia, its impact on clinical outcome and the effect of genetic polymorphisms on the incidence of hepatotoxicity. Leukemia. 2006;20:881–3. doi: 10.1038/sj.leu.2404165. [DOI] [PubMed] [Google Scholar]
- 5.Mathews V, Binu P, Paul MS, Abhilash M, Manju A, Nair RH. Hepatoprotective efficacy of curcumin against arsenic trioxide toxicity. Asian Pacific Journal of Tropical Biomedicine. 2012;2:S706–S11. [Google Scholar]
- 6.Fouad AA, Yacoubi MT. Mechanisms underlying the protective effect of eugenol in rats with acute doxorubicin cardiotoxicity. Arch Pharm Res. 2011;34:821–8. doi: 10.1007/s12272-011-0516-2. [DOI] [PubMed] [Google Scholar]
- 7.Dutta B, Borborah K, Borthakur S. Aromatic plants containing essential oil component-linalool, eugenol and methyl chavicol reported from North-East India. J Nat Prod Plant Resour. 2007;5:6–10. [Google Scholar]
- 8.Fujisawa S, Atsumi T, Kadoma Y, Sakagami H. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177:39–54. doi: 10.1016/s0300-483x(02)00194-4. [DOI] [PubMed] [Google Scholar]
- 9.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 10.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–9. [PubMed] [Google Scholar]
- 11.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 12.Aebi Hi, Bergmeyer H. Methods of enzymatic analysis. Vol 2. New York, NY: Academic Press; 1974. pp. 673–8. [Google Scholar]
- 13.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 14.Lepoivre M, Chenais B, Yapo A, Lemaire G, Thelander L, Tenu JP. Alterations of ribonucleotide reductase activity following induction of the nitrite-generating pathway in adenocarcinoma cells. J Biol Chem. 1990;265:14143–9. [PubMed] [Google Scholar]
- 15.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 16.Binu P, Harikumaran Nair R. Screening of Eugenol - A Monoterpene, as an antioxidant. Int J Adv Res. 2015;3:1527–33. [Google Scholar]
- 17.Quatrehomme G, Ricq O, Lapalus P, Jacomet Y, Ollier A. Acute arsenic intoxication: forensic and toxicologic aspects (an observation) J Forensic Sci. 1992;37:1163–71. [PubMed] [Google Scholar]
- 18.Williams JA, Withrow CD, Woodbury DM. Effects of ouabain and diphenylhydantoin on transmembrane potentials, intracellular electrolytes, and cell pH of rat muscle and liver in vivo. J Physiol. 1971;212:101–15. doi: 10.1113/jphysiol.1971.sp009312. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson EM, Okpako DT, Evans FJ. Pharmacological Methods in Phytotherapy Research, Vol. 1: Selection, Preparation, and Pharmaceutical Evaluation of Plant Materaisls Selection, preparation and pharmacological evaluation of plant material. Chichester: John Wiley & Sons; 1998. pp. 15–23. [Google Scholar]
- 20.Mohammadyari A, Razavipour ST, Mohammadbeigi M, Negahdary M, Ajdary M. Exploring vivo toxicity assessment of copper oxide nanoparticle in Wistar rats. Journal of Biology and Today’s World. 2014;3:124–8. [Google Scholar]
- 21.Singh S, Rana SV. Amelioration of arsenic toxicity by L-Ascorbic acid in laboratory rat. J Environ Biol. 2007;28:377–84. [PubMed] [Google Scholar]
- 22.DeLeve LD, Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther. 1991;52:287–305. doi: 10.1016/0163-7258(91)90029-l. [DOI] [PubMed] [Google Scholar]
- 23.Vahter M, Marafante E, Dencker Tissue distribution and retention of 74As-dimethylarsinic acid in mice and rats. Arch Environ Contam Toxicol. 1984;13:259–64. doi: 10.1007/BF01055275. [DOI] [PubMed] [Google Scholar]
- 24.Lee TC, Wei ML, Chang WJ, Ho IC, Lo JF, Jan KY, et al. Elevation of glutathione levels and glutathione S-transferase activity in arsenic-resistant Chinese hamster ovary cells. In Vitro Cell Dev Biol. 1989;25:442–8. doi: 10.1007/BF02624629. [DOI] [PubMed] [Google Scholar]
- 25.Gyurasics A, Varga F, Gregus Z. vGlutathione-dependent biliary excretion of arsenic. Biochem Pharmacol. 1991;42:465–8. doi: 10.1016/0006-2952(91)90306-p. [DOI] [PubMed] [Google Scholar]
- 26.Yokota A, Shigeoka S, Onishi T, Kitaoka S. Selenium as Inducer of Glutathione Peroxidase in low-CO(2)-Grown Chlamydomonas reinhardtii. Plant Physiol. 1988;86:649–51. doi: 10.1104/pp.86.3.649. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med. 2009;30:29–41. doi: 10.1016/j.mam.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Uthus EO. Diethyl maleate, an in vivo chemical depletor of glutathione, affects the response of male and female rats to arsenic deprivation. Biol Trace Elem Res. 1994;46:247–59. doi: 10.1007/BF02789300. [DOI] [PubMed] [Google Scholar]
- 29.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 30.Chance B, Oshino N. Analysis of the catalase--hydrogen peroxide intermediate in coupled oxidations. Biochem J. 1973;131:564–7. doi: 10.1042/bj1310564. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horton AA, Fairhurst S. Lipid peroxidation and mechanisms of toxicity. Crit Rev Toxicol. 1987;18:27–79. doi: 10.3109/10408448709089856. [DOI] [PubMed] [Google Scholar]
- 32.Choi MS, Lee SH, Cho HS, Kim Y, Yun YP, Jung HY, et al. Inhibitory effect of obovatol on nitric oxide production and activation of NF-kappaB/MAP kinases in lipopolysaccharide-treated RAW 264. 7cells. Eur J Pharmacol 2007;556:181–9. doi: 10.1016/j.ejphar.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 33.Kar Mahapatra S, Chakraborty SP, Majumdar S, Bag BG, Roy S. Eugenol protects nicotine-induced superoxide mediated oxidative damage in murine peritoneal macrophages in vitro. Eur J Pharmacol. 2009;623:132–40. doi: 10.1016/j.ejphar.2009.09.019. [DOI] [PubMed] [Google Scholar]


