Abstract
Hadron therapy (HT) with protons and carbon ions is an advanced radiotherapy technique. As the first report addressing this topic, the present study aimed to estimate the number of patients eligible for HT in Fars province and the whole of Iran. The data were collected through direct inspection of medical records of the patients treated at the Radiotherapy Department, Namazi Hospital, Shiraz, Iran in 2014. The patients who were treated with external-beam radiotherapy and declared to be a resident of Fars province were extracted from the medical records. After classification based on appropriate indications and factors, the number of eligible patients in Iran was calculated by scaling (Iran: Fars population). Of the 2,932 medical records, 1,943 patients were treated with external-beam radiotherapy, among which 1,536 were from Fars. The total number of patients eligible for HT in Fars was >351 cases/year (22.9% of the treated patients). The cancer site distribution of the eligible Fars residents was central nervous system primary tumors (n=31), brain metastases (n=64), eye (n=7), head and neck (n=28); thyroid (n=4), lung (n=17), breast (n=56), esophagus (n=5); pancreas (n=5), gastric (n=32), liver and bile duct (n=0), rectum and anus (n=26); prostate (n=27), bladder (n=8), cervix (n=6), soft tissue (n=17); kidney (n=1), Hodgkin lymphoma (n=9), non-Hodgkin lymphoma (n=4), and bone metastases (n=4). A total number of 5,756 cases/year was estimated for the entire country. Considering the experiences of other countries, these results suggest that establishing at least two HT centers (covering the northern and southern parts of Iran) is justified based on the total number of patients. Further accurate estimations and cost evaluations are recommended.
Keywords: Proton therapy , Carbon ion radiotherapy, Patient selection, Treatment costs, Hospitals
What’s Known
Hadron therapy is an advanced radiotherapy technique for inaccessible and resistant tumors as well as many pediatric indications.
There is currently no hadron therapy center in Iran.
What’s New
The total number of new patients eligible for hadron therapy in Fars and Iran is estimated to be at least 351 and 5,756 cases/year, respectively.
Based on the number of patients, establishing two hadron therapy centers in the northern and southern parts of Iran is justifiable.
Introduction
Hadron therapy (HT) is regarded as an advanced radiotherapy (RT) technique, where energetic light ions (heavy particles, in particular protons and carbon ions) are used to kill cancer cells. Due to the physical and radiobiological properties of these ions, some clinical advantages are observed in HT compared to the conventional X- or gamma-ray RT. Therefore, HT is usually indicated in the treatment of radioresistant neoplasms, tumors located near vital organs, pediatric patients, etc.1
The demand for hadron therapy is increasing sharply in recent years. Japan and China are the pioneers in this field. According to a recent report, other Asian countries such as India, Saudi Arabia, and Qatar intend to acquire this technology in the near future.2 Unfortunately, there are no HT centers in Iran despite a large number of cancer patients. Despite attempts in recent years to set up a reliable nationwide cancer registration system3 in Iran, there are still many limitations and bottlenecks in obtaining reliable and accurate data concerning the number of eligible patients for this treatment.4 This scenario instigated a study in Iran and Fars province (where the data were collected from) to indicate the extent of the need for HT. To the best of our knowledge, this is the first scientific report on the estimation of the number of eligible cases for HT in Iran.
Materials and Methods
In the present descriptive-analytical study, the data were collected from the medical records of the patients who referred to the Department of Radiotherapy and Oncology, Namazi Hospital, Shiraz, Iran in 2014. This department is the first and currently the sole RT center in Fars province and serves as the only RT and oncology referral center. It has the longest-established cancer registry office in the country. Fars province is in the southern part of Iran with Shiraz as its capital city. According to official reports by the Statistical Center of Iran, the population of Fars province in 2014 was 4,735,146, which accounts for 6.1% of the total population of the entire country.5
The number of patients was estimated in two stages, as described below.
i. Initially, the medical records of all patients who referred to this center were examined by the census method. Data, including patients’ national ID number, place of residence, diagnosis, and treatment, were collected and carefully recorded in a specially designed form by direct inspections of the records. The use of the national code prevented double entries in the collected data, and cases without pathological confirmation were excluded. Additionally, based on the declared place of residence, patients from regions other than Fars province were excluded.
ii. In the second stage, the refined data were categorized into different indications based on the anatomical location of the tumor. This classification method is according to a study in which the number of eligible patients for HT was estimated based on the data from five university hospitals in Germany, Italy, France, and Austria.6 The mean coefficients for each indication were extracted from the same study. Therefore, in the present study, previously-derived indications and mean coefficient values have been adapted and applied to our collected data. The estimated number of patients suitable for HT in six previous studies is summarized in table 1.
Table 1.
Estimated values of hadron therapy indications reported in previous studies
| Publication | Year | Center | Reported number |
|---|---|---|---|
| Mayer et al.6 | 2004 | MedAustron, Austria | 2,044 cases/year |
| Orecchia et al.7 | 1997 | CNAO, Italy | 10,825 cases/year |
| Engels et al.8 | 1999 | MedAustron, Austria | 13,145 cases/year |
| Baron et al.9 | 2004 | ETOILE, France | 5,320 indications/year for carbon ions |
| Krengli et al.10 | 2004 | CNAO, Italy | 3,694 cases/year for protons 1,885 cases/year for carbon ions |
| Patin et al.11 | 2013 | Rhône-Alpes, France | 8.5 (incidence/100,000 inhabitants/year) |
Results
Of the total of 2,932 cases in the archives of 2014, medical records of 1,943 patients who underwent conventional RT were used for data analysis. Among them, 1,536 records (male: 628 (41%) and female: 908 (59%)) of those residing in Fars province were selected. The age distribution of the patients is shown in figure 1. Then, 1,536 indications suitable for HT were classified, as shown in table 2. In this table, the category of indications is shown in the first column, and their corresponding distribution in Fars province and the coefficients used in the previous study6 are shown in the adjacent columns. Multiplying each coefficient by the corresponding number in the previous column determines the absolute number of indications (last column) suitable for HT. Hence, the total number of patients who reside in Fars and are eligible for HT is 351 cases/year. Multiplying this value by the proportion of the Fars population with respect to the entire country (1/16.4), the estimated eligible patients in the whole country (i.e., 16.4×351) is 5,756 cases per year.
Figure1.
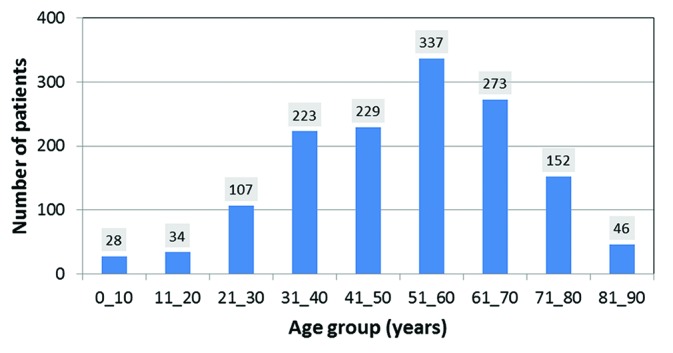
Age distribution of patients treated at the Radiotherapy Department of Namazi Hospital and residing in Fars province in 2014.
Table 2.
Distribution of patients in terms of indications appropriate for hadron therapy in Iran and fars province
| Category | Number of residents in fars treated with radiotherapy (% of all radiotherapy patients) | Suitable for hadrons based on ref.6 (%) | Number of patients eligible for hadron therapy living in fars |
|---|---|---|---|
| Primary central nervous system tumors | 156 (74) | 20 | 31 |
| Brain metastases | 64 (85) | 100 | 64 |
| Eye tumors | 10 (83) | 67 | 7 |
| Head and neck tumors | 112 (79) | 25 | 28 |
| Thyroid cancer | 8 (72) | 45 | 4 |
| Lung metastases | 17 (89) | 100 | 17 |
| Breast cancer | 563 (79) | 10 | 56 |
| Esophagus cancer | 18 (62) | 28 | 5 |
| Pancreas cancer | 24 (82) | 20 | 5 |
| Gastric cancer | 74 (77) | 43 | 32 |
| Liver and bile duct cancer | 0 (0) | 46 | 0 |
| Rectum and anus cancer | 126 (84) | 21 | 26 |
| Prostate cancer | 98 (88) | 28 | 27 |
| Bladder cancer | 36 (88) | 22 | 8 |
| Cervix cancer | 26 (76) | 23 | 6 |
| Soft tissue tumors | 46 (75) | 38 | 17 |
| Kidney cancer | 4 (37) | 14 | 1 |
| Hodgkin lymphoma | 48 (69) | 19 | 9 |
| Non-Hodgkin lymphoma | 20 (68) | 19 | 4 |
| Bone metastases | 82 (83) | 5 | 4 |
| Total | 1,536 (79) | 693 | 351 |
Discussion
Despite the frequent and relatively reliable data available on the construction of HT centers and patients who have been treated, limited access to the methods for estimating the number of patients eligible for HT still poses a challenge. Most of these reports have been published between 1998 and 2002.6-10 A literature review has been reported in a recent study published in 2013.11 Given the significance of finding a useful method for estimating the number of patients, who are eligible for HT, some of these studies are discussed in more detail.
In a study by Baron et al., the statistics for one-day referral of eligible patients for HT in five RT centers in the east of France were reported. Assuming the treatment period for such patients lasts 5-7 weeks and applying a factor of 10, the total number of patients admitted per year was estimated at 5,320. This estimate showed a fairly good agreement with the actual number of patients (6,300). Finally, the authors concluded 770 appropriate indications for HT.9 Krengli et al. studied the predicted equipment and facility requirements for the CNAO installation in Italy by considering the capacity to treat 900 patients per year. The study showed that, on a yearly basis, 25,000 new cases are added to the number of cancer patients in Italy. Given that 10-15% of such patients would probably need to undergo HT, the number of candidates for HT was estimated at 3,000-4,000 patients.10 Mayer et al. conducted a feasibility study of patients as candidates for the Austrian National Center for HT (MedAustron). All patients who were candidates for RT (a total of 3,783 patients in three months) were studied. The total number of such patients was multiplied by 4 to derive 15,132 patients eligible for HT during a year.6 They estimated the mean values from five European university hospitals and multiplied the calculated values by indications collected during a whole year and found that 2,044 patients were eligible for HT. Accordingly, they underlined the urgent need for establishing a national HT in Austria.6 Note that, the mean coefficients were calculated in the same manner (third column in table 2) as in the present study. The need for an HT center in Rhône-Alpes (France) was evaluated in 2010. The patients’ data were collected from 34 centers and the estimated value of 8.5 (incidence/100,000 inhabitants/year) was calculated. Without commenting on the need for establishing an HT center in the region, the authors recommended further research to be carried out in the region under analysis.11 In another study published in 2013, the feasibility study of an HT center in Belgium was performed in collaboration with several universities, hospitals, and leading experts.12
The present study estimated appropriate indications for HT in Fars province and the whole of Iran by inspecting the medical records of patients treated at Namazi Hospital in 2014. Based on the presented data, the total number of eligible patients for HT in Fars was 351 cases per year, accounting for 29.4% of the treated patients in 2014. A total number of 5,756 cases per year was estimated at the nationwide level. Indications eligible for HT are also discussed in table 2.
Although the exact figures are not accurately known, it is well established that the utilization rate of RT in Iran is below the worldwide average. This means that, at present, a smaller fraction of Iranian cancer patients undergo RT compared to developed countries. Therefore, the total number of patients used in the current analysis is an underestimate of the real number requiring RT. Consequently, the number requiring hadron therapy is also an underestimate.
In addition to the evaluation and approximation of the number of patients (as carried out in the present study), other factors should also be taken into consideration for the establishment and development of HT centers. Although the design and operational stages of these centers are almost the same in all countries, other factors can play important roles in the success of HT projects, including the existence of strong infrastructures that can greatly increase the chance of success.13 As developing countries are moving towards implementing HT,2 some important recommendations need to be considered by such countries, including the establishment of a strong RT infrastructure, annual patient throughput, accurate patient estimates that allow a project to be financially sustainable, and even the assessment of the number of treatment sessions that can be done per day. To maximize health benefits, it is recommended to implement work plans and feasibility studies. This issue is particularly important in the sense that in most reports the final cost is estimated up to $100M and the maintenance costs of these centers should also be added.13,14 However, it should be emphasized that new technologies such as laser accelerators1,15 and the use of linear accelerators16 have reduced the costs. Also, the introduction of single-room centers1,17 can be very useful for research and development purposes too. Therefore, substantial cost reduction seems feasible. Moreover, providing training programs for radiation oncologists, medical physicists, and radiotherapy technologists alongside a skilled management team is highly recommended.13
To sum up, in the present study, an evaluation of the number of patients eligible for HT was conducted for the first time in Iran; supported by data from previous studies in other countries. Based on the number of patients and benefiting from the experiences of HT centers in other countries, the need for at least two HT centers was indicated. Geographically spreading the two centers is sensible for better patient access. However, given an increase in the incidence rate of cancer in Iran, further research that includes health economic evaluation is required in this field.
Conclusion
Given the estimated number of total indications for HT in Fars province and Iran, establishing at least two HT centers (covering the northern and southern parts of Iran) is justified in terms of patient numbers. However, there is a need for further detailed studies to provide more accurate data for the entire county by considering cost-effectiveness and other economic and cultural factors.
Acknowledgement
The study was conducted through joint collaboration between the Radiation Oncology Department of Namazi Hospital and the Research Center for Protection against Ionizing and Non-Ionizing Radiations. The project was supported by the Research Deputy of Shiraz University of Medical Sciences (number: 93-01-75-8402).
Conflict of Interest:None declared.
References
- 1.Charlie Ma CM, Lomax T, editors . Proton and carbon ion therapy. Florida: CRC Press; 2012. p. 256. [Google Scholar]
- 2. Particle therapy facilities in operation [Internet] Information about technical equipment. Log in to get newest data on patient statistics. c2015. Available From: [https://www.ptcog.ch/index.php/facilities-in-operation. ]
- 3.Lankarani KB, Khosravizadegan Z, Rezaianzadeh A, Honarvar B, Moghadami M, Faramarzi H, et al. Data coverage of a cancer registry in southern Iran before and after implementation of a population-based reporting system: a 10-year trend study. BMC Health Serv Res. 2013;13:169. doi: 10.1186/1472-6963-13-169. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health and, Treatment Deputy, Ministry of, Health and, Medical Education. Iranian annual of national cancer registration reports 2008-2009. Tehran: Center for Disease Control & Prevention; 2011. p. 450. Persian. [Google Scholar]
- 5. Statistical Centre of Iran. Population Estimation. Tehran: Plan & Budget Organization; 2015. Available from: [https://www.amar.org.ir/english/Statistics-by-Topic/Population#2224493-releases. ]
- 6.Mayer R, Mock U, Jager R, Potter R, Vutuc C, Eiter H, et al. Epidemiological aspects of hadron therapy: a prospective nationwide study of the Austrian project MedAustron and the Austrian Society of Radiooncology (OEGRO) Radiother Oncol. 2004;73:S24–8. doi: 10.1016/s0167-8140(04)80008-2. [DOI] [PubMed] [Google Scholar]
- 7.Orecchia R, Krengli M. Number of potential patients to be treated with proton therapy in Italy. Tumori. 1998;84:205–8. doi: 10.1177/030089169808400218. [DOI] [PubMed] [Google Scholar]
- 8.Engels H, Wambersie A. Cancer epidemiology and patient recruitment for hadron therapy. Strahlenther Onkol. 1999;175:95–9. doi: 10.1007/BF03038902. [DOI] [PubMed] [Google Scholar]
- 9.Baron MH, Pommier P, Favrel V, Truc G, Balosso J, Rochat J. A “one-day survey”: as a reliable estimation of the potential recruitment for proton- and carbon- ion therapy in France. Radiother Oncol. 2004;73:S15–7. doi: 10.1016/s0167-8140(04)80005-7. [DOI] [PubMed] [Google Scholar]
- 10.Krengli M, Orecchia R. Medical aspects of the National Centre For Oncological Hadrontherapy (CNAO-Centro Nazionale Adroterapia Oncologica) in Italy. Radiother Oncol. 2004;73 Suppl 2:S21–3. doi: 10.1016/s0167-8140(04)80007-0. [DOI] [PubMed] [Google Scholar]
- 11.Patin S, Pommier P, Yi H, Baron MH, Balosso J. Epidemiological study of the incidence of cancers eligible for proton or carbon ions therapy: methodology and results of recruitment estimation. J Cancer Epidemiol. 2013;2013:107646. doi: 10.1155/2013/107646. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Croock R, Lievens Y, Madani I, De Neve W. Feasibility study of a Hadron Therapy Centre in Belgium. Brussel: Belgian Hadron Therapy Centre Foundation; 2013. Available from: [http://bhtc.sckcen.be/~/media/Files/Bhtc/exec_summary_20_05_2013.pdf?la=en. ]
- 13. Rosenblatt E, Meghzifene A, Belyakov O, Abdel-Wahab M. Relevance of Particle Therapy to Developing Countries. Int J Radiat Oncol Biol Phys. 2016;95:25–9. doi: 10.1016/j.ijrobp.2015.12.370. [DOI] [PubMed] [Google Scholar]
- 14. Department of Health and Human Services. NIH/NCI Planning for a National Center for Particle Beam Radiation Therapy Research (P20). Bethesda: National Institutes of Health; 2013. Available from: [http://grants.nih.gov/grants/guide/pa-files/PAR-13-371.html. ]
- 15.Linz U, Alonso J. Laser-driven ion accelerators for tumor therapy revisited. Physical Review Accelerators and Beams. 2016;19:124802. doi: 10.1103/PhysRevAccelBeams.19.124802. [DOI] [Google Scholar]
- 16.Verdu-Andres S, Amaldi U, Faus-Golfe A. CABOTO, a high-gradient linac for hadrontherapy. J Radiat Res. 2013;54 Suppl 1:i155–61. doi: 10.1093/jrr/rrt053. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contreras J, Zhao T, Perkins S, Sun B, Goddu S, Mutic S, et al. The world’s first single-room proton therapy facility: Two-year experience. Pract Radiat Oncol. 2017;7:e71–e6. doi: 10.1016/j.prro.2016.07.003. [DOI] [PubMed] [Google Scholar]


