Abstract
Objectives
To evaluate the role of periodontitis in viridans group streptococci (VGS) bacteremia and infective endocarditis (IE).
Methods
A total of 200 subjects including two groups. Group A- 34 subjects undergoing tooth extraction with periodontitis, 46 subjects undergoing tooth extraction without periodontitis and 40 healthy controls. Group B: 40 confirmed cases of IE (17 with and 23 without periodontitis) and 40 healthy controls. Subgingival plaque and blood samples were obtained and processed by standard procedures.
Results
A total of 53 blood samples (66.25%) yielded positive cultures after tooth extraction. The relationship between the presence of periodontitis and a positive blood culture was significantly higher (p = 0.05) for tooth extraction cases with periodontitis (79.40%) than tooth extraction cases without periodontitis (56.50%). Periodontitis was observed in 42.5% of IE cases. Out of the 40 patients of IE, the blood samples yielded 40 different isolates, majority were viridans streptococci 15 (37.5%) and staphylococci nine (22.5%). No statistically significant difference was observed between the subgingival plaque and blood isolates of periodontitis in both the groups, indicating similarity of biotypes of viridans streptococci isolated from the blood and the subgingival plaque. Similarity was also observed between the antibiogram profiles of viridans streptococci from both the groups.
Conclusions
Periodontitis enhances viridans streptococcal bacteremia and may be a potential risk factor for IE.
Keywords: Infective endocarditis, Periodontitis, Viridans group streptococci
1. Introduction
Several studies have established that periodontitis is a risk factor for infective endocarditis (IE).1 Gingivitis and periodontitis are among the most common human infections. Gingivitis can develop within days and includes inflammatory changes of the gingiva most commonly induced by accumulation of dental plaque. Periodontitis results from a complex interplay between chronic bacterial infection and the inflammatory host response leading to irreversible destruction of tooth-supporting tissues, with tooth loss as a common end point.2 Periodontitis is associated with elevated inflammation that may contribute to bacteremia associated with IE risk.
It has been reported that patients with periodontitis have inflamed and ulcerated crevicular or pocket epithelium around the teeth, which can act as a portal of entry for bacteria from oral cavity to the blood stream.3 Lockhart et al. showed that the generalized presence of gingival bleeding after tooth brushing was associated with an almost eightfold increase in bacteremia risk.4 This is consistent with another study reporting the incidence and the magnitude of bacteremia induced by chewing, tooth brushing and invasive dental procedures to be associated with gingival inflammation induced by periodontitis.5
Periodontitis is a potential risk factor for translocation of bacteria from oral cavity into the blood stream via ulcerated inflamed crevice and pocket epithelium and the adjacent gingival microcirculation. This may occur following invasive dental procedures and also during normal daily activities.4, 5, 6, 7, 8, 9 Bacteremia and low-grade systemic inflammation induced by periodontal infections may be a risk for systemic conditions like cardiovascular diseases including IE, stroke, premature low birth weight delivery and diabetes mellitus.10
The earlier study shows that most of the IE cases occur as a result of microorganisms reaching the heart through the blood stream.11 It is this explanation that has attracted the attention of medical and dental specialists towards this heart condition. The access into the bloodstream and thereby to the heart resulting into cardiovascular ailment is promoted by periodontitis induced inflammation. The present study was carried out to determine the association of periodontitis and viridians group streptococci (VGS) bacteremia in patients of tooth extraction and IE and to compare the biotypes and antimicrobial profiles of VGS in subgingival plaque and blood of these patients.
2. Material and methods
This study was approved by the ethical committee of the Ashwini Rural Medical College, Hospital & Research Centre, Solapur, Maharashtra, India. The study included a total of 200 subjects including 80 healthy controls distributed in two groups as follows:
Group A subjects: In this group, 80 patients (34 with periodontitis and 46 without periodontitis) undergoing tooth extraction and 40 healthy controls were enrolled. Demographic information and medical histories from the participants were obtained and thorough clinical and radiographic examinations of their teeth were conducted after written informed consent. Patients with fewer than 10 teeth; an active viral infection, poorly controlled systemic disease, penicillin allergy, antimicrobial usage within three months prior dental treatment, temperature greater than 100.5 °F or facial cellulitis; or immune-compromised by virtue of disease or medications were excluded from the study.
Group B subjects: In this group, 40 (17 with periodontitis and 23 without periodontitis) confirmed cases of IE above the age of 18 years and fulfilling Duke diagnostic criteria12 and 40 controls were enrolled. Informed consent was obtained from each patient. Patients who proved to have any source of infection other than IE were excluded from the study. Patients in whom blood cultures for bacteria turned negative and later on showed fungal growth were also excluded. Pregnant women, patients unable to give informed consent or non-cooperative in the dental examination and known conditions requiring prophylactic antibiotic treatment before dental examinations were also excluded from the study.
2.1. Dental examination (Group A and B subjects)
Assessment of periodontal status was performed by means of clinical attachment loss (CAL), probing pocket depths (PPD), which was measured to the nearest whole millimetre at six sites per tooth by using a William’s periodontal probe. Oral hygiene indices such as- papillary bleeding index (PBI),13 plaque index (PI)14 and gingival index (GI)15 were also assessed. All assessments were done by a single trained examiner.
2.2. Sample collection
Subgingival plaque samples of the tooth were collected from the gingival area of buccal and lingual tooth surfaces of affected tooth using sterile curettes into sterile transport media (group A and B).
Clinical samples of blood were obtained from healthy controls and patients undergoing tooth extraction. Blood for culture was collected from the site in the antecubital fossa with standard precautions.16 For each subject 5 ml of venous blood was drawn before and after 3 min of dental extraction (group A).17, 18, 19, 20
Three blood samples were collected aseptically from healthy controls and patients of IE for aerobic culture from three different sites of the body (right cubital fossa, left cubital fossa and left wrist) at intervals over 24 h (group B).16
2.3. Microbiological analysis
Subgingival plaque specimens (group A and B) were inoculated onto special media, tryptone soya blood agar supplemented with strepto supplement (nalidixic acid 3.750 mg, nemomycin sulphate 1.060 mg and polymixin B sulphate 8500 units for 500 ml media) and mutans sanguis agar (Himedia laboratories, Mumbai). Cultures with VGS growth were further subjected to standard biochemical identification using automated Vitek 2 (bioMérieux) system to complete the strain identification. Antimicrobial susceptibilities were measured in MIC by automated Vitek 2 (bioMérieux) system in accordance with CLSI standards.21
All the blood samples (group A and B) were screened using automated BD Bactec™ 9050 automated system. Five ml of aseptically collected venous blood was inoculated directly into BACTEC culture media (BactecPlus; Becton Dickinson and Company, Sparks, MD, USA) and processed. The blood culture vials were tested on days one, three, five and seven. Cultures with VGS growth were further subjected to standard biochemical identification using automated Vitek 2 (bioMérieux) system to complete the strain identification. Antimicrobial susceptibilities were measured in MIC by automated Vitek 2 (bioMérieux) system in accordance with CLSI standards.21
During our investigation on subgingival microbial communities of the oral cavity and its possible role in bacteremia, with focus on viridans streptococci, automated Vitek 2 (bioM_erieux) system revealed identification of six uncommon isolates of Streptococci, five from subgingival plaque samples and one from blood sample, respectively. All the six strains were selected for 16S rRNA gene analysis for more accurate identification. The 16S rRNA gene sequences from strains obtained in this study have been deposited in the GenBank under accession numbers KJ575555–KJ575560.
2.4. Biotyping and resistogram studies
Biotyping of viridans streptococci isolates and categorization into different 5 groups, as per Facklam, 200222 along with antibiogram studies as per CLSI guidelines21 were performed by using automated Vitek 2 system. These biotypes and antibiograms of subgingival plaque and blood isolates of Group A and B subjects with periodontitis were analyzed statistically to find an association between oral and blood isolates.
2.5. Statistical analysis
Descriptive statistics such as mean, SD and percentage were used. Comparison between three groups for categorical variable was done by using Chi-square test. Comparison between three groups for continuous variable was done by using ANOVA test followed by post hoc Bonferroni Multiple Comparisons Test for normally distributed data or Kruskal-Wallis Test followed by post hoc Dunn's Multiple Comparisons Test for non-normally distributed data.
3. Results
3.1. Group A
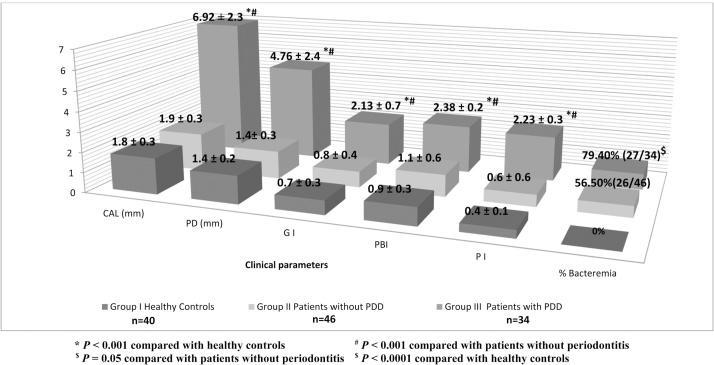
Fifty three of the blood samples (66.25%) yielded positive cultures after tooth extraction; whereas the blood cultures before tooth extraction were negative in all the three groups. The mean age of the subjects in all the three groups was 50 years and the male to female ratio was 1.1:1. The incidence of bacteremia was significantly higher (p = 0.05) in subjects with periodontitis 27 out of 34 (79.40%) than patients without periodontitis 26 out of 46 (56.50%) (Chart 1).
Chart 1.
Incidence of post extraction bacteremia related to clinical attachment loss (CAL), periodontal pocket depth (PD), gingival index (GI), papillary bleeding index (PBI) and plaque index (PI). All values are presented in (Mean ± standard deviation).
The bacteriological analysis of subgingival plaque samples of 80 patients undergoing tooth extraction with and without periodontitis showed 100% positivity yielding 370 different isolates of which 260 (70.27%) were viridans streptococci and 110 (29.72%) were other isolates. A total of 67 VGS isolates were isolated from 46 patients without periodontitis; however, 134 VGS isolates were isolated from 34 patients with periodontitis. Streptococcus mitis, Steptococcus oralis, Streptococcus mutans and Streptococcus sanguinis were the most common VGS isolated from patients with and without periodontitis.
Out of the 80 blood samples collected from patients undergoing tooth extraction with and without periodontitis, 53 (66.25%) yielded positive cultures, producing 122 different isolates of which 76 (62.30%) were viridans streptococci and 46 (37.30%) were other bacteria. Subjects with periodontitis showed high rates of isolation 58 (47.54%) of VGS, than subjects without periodontitis 18 (14.75%) (Table 1).
Table 1.
Microorganisms isolated in aerobic blood cultures following tooth extraction in patients with and without periodontitis (n = 80).
| Microorganism isolated | Bacteremia in patients without periodontitis Group I (26/46) | Bacteremia in patients with periodontitis Group II (27/34) | Total isolates |
|---|---|---|---|
| Bacterial isolates | No. of isolates | ||
| Acinetobacter lwoffii | 0 | 1 | 1 |
| Actinomycetes israelii | 0 | 4 | 4 |
| Aeromonas hydrophila | 0 | 1 | 1 |
| Aeromonas salmonicida | 0 | 1 | 1 |
| Alliococcus otitis | 0 | 1 | 1 |
| CONS | 3 | 4 | 7 |
| Enterobacter cloacae | 0 | 1 | 1 |
| Enterococcus faecium | 1 | 1 | 2 |
| Enterococcus spp. | 0 | 1 | 1 |
| Erysipelothrix rhusiopathiae | 1 | 1 | 2 |
| Escherischia coli | 0 | 1 | 1 |
| Klebiella pneumonia | 0 | 1 | 1 |
| Kocuria kristinae | 1 | 2 | 3 |
| Kocuria rosea | 2 | 6 | 8 |
| Lactococcus gravieae | 1 | 1 | 2 |
| Micrococcus species | 1 | 2 | 3 |
| Neisseriae spp. | 0 | 2 | 2 |
| Rothia mucilaginosa | 0 | 1 | 1 |
| Staphlyococcus aureus | 1 | 2 | 3 |
| Pseudomonas aeruginosa | 1 | 0 | 1 |
| Sub-total 1 | 12 | 34 | 46 |
| Viridans group streptococci | |||
| Streptococcus anginosus | 0 | 1 | 1 |
| Streptococcus constellatus | 1 | 1 | 2 |
| Streptococcus gordonii | 0 | 2 | 2 |
| Streptococcus mitis | 4 | 13 | 17 |
| Streptococcus mutans | 3 | 7 | 10 |
| Streptococcus oralis | 3 | 10 | 13 |
| Streptococcus parasanguinis | 1 | 2 | 3 |
| Streptococcus sanguinis | 2 | 9 | 11 |
| Streptococcus sinensis | 0 | 1 | 1 |
| Granulicatella adiacens | 2 | 4 | 6 |
| Granulicatella elegans | 2 | 8 | 10 |
| Sub-total 2 | 18 | 58 | 76 |
| Total isolates (1 + 2) | 30 | 92 | 122 |
Bacteremia after tooth extraction was multi-bacillary and VGS bacteremia was observed in all 27 patients with periodontitis, whereas only 16 patients had VGS bacteremia out of 26 bacteremia positive subjects without periodontitis.
3.2. Group B
A total of 40 confirmed IE patients and 40 healthy controls fulfilling inclusion criteria were investigated. Twenty three (58%) were male and 17 (42%) were female. The mean age of the patients in the study was 48 ± 20 years (range: 17–83 years).
Out of the 40 infective endocarditis cases, underlying cardiac disease was found in 30 (75%) cases, majority of which were valvular heart disease 25 (62.5%). Congenital heart disease was found in three (7.5%) cases and cardiomyopathy was found in two (5%) cases. Mitral valve (47.5%) was predominantly involved in the process of colonization by the bacteria followed by aortic (35%), tricuspid (15%) and pulmonary valve (2.5%). Pulmonary vegetations were diagnosed in a single patient (Table 2).
Table 2.
Valve involvement in cases of IE.
| Valve affected | Number of IE cases |
|||
|---|---|---|---|---|
| Native valves | Prosthetic valves | Total | % | |
| Mitral | 18 | 1 | 19 | (47.5) |
| Aortic | 13 | 1 | 14 | (35) |
| Tricuspid | 6 | – | 6 | (15) |
| Pulmonary | 1 | – | 1 | (2.5) |
| Total | 38 | 2 | 40 | (100) |
The bacteriological analysis of subgingival plaque samples of 40 patients of IE showed (100%) positive results; yielding 199 different isolates, of which majority were 111 (55.78%) VGS and 88 (44.22%) were other bacterial isolates. Isolation of VGS was highest among the subjects with periodontitis 69 (34.67%), when compared with subjects without periodontitis 42 (21.10%).
Among the 40 IE patients, the most predominant blood culture isolates were VGS 15 (37.5%), followed by Staphylococcus aureus 9 (22.5%), Coagulase negative staphylococci 3 (7.5%) and Enterococci 2 (5%). Amongst the viridans streptococci the most common species isolated were Streptococcus sangunis (33%), Streptococcus mitis (33%) and Streptococcus oralis (20%). VGS were the most common isolates (14) among the IE patients with periodontitis (n = 17), however, only one VGS was isolated from the IE patients without periodontitis (n = 23).
3.3. Results of statistical comparison between biotypes and antibiograms of the oral and blood isolates of viridans streptococci from Group A and B patients with periodontitis
Comparison of VGS isolated from subgingival plaque and blood of periodontitis patients undergoing tooth extraction is given in Table 3,4 . In both the sites, Streptococcus mitis was the predominant species, comprising 16.89% of total isolates from subgingival plaque and 14.39% from blood. Streptococcus oralis was the second most commonly isolated species representing 14.67% from the oral cavity and 10.87% from blood, similarly Streptococcus sanguinis (10.22% in the subgingival plaque and 9.78% in the blood). Other species of viridans streptococci were present in less numbers.
Table 3.
Comparison of species of viridans streptococci isolated from the subgingival plaque and blood of patients of periodontitis after undergoing tooth extraction (n = 34).
| Sr. No. | Viridans streptococci strains isolated | Subgingival plaque isolates (167/225) |
Blood isolates (58/92) |
χ2 value | p value | ||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||||
| 1. | Streptococcus mitis | 38 | (16.89) | 13 | (14.13) | 0.19 | 0.66 |
| 2. | Steptococcus oralis | 33 | (14.67) | 10 | (10.87) | 0.51 | 0.47 |
| 3. | Streptococcus sanguinis | 23 | (10.22) | 9 | (9.78) | 0.014 | 1.0 |
| 4. | Granulicatella elegans | 21 | (9.33) | 8 | (8.70) | 0.032 | 1.0 |
| 5. | Streptococcus mutans | 16 | (7.11) | 7 | (7.61) | 0.02 | 1.0 |
| 6. | Granulicatella adiacens | 12 | (5.33) | 4 | (4.35) | 0.006 | 1.0 |
| 7. | Streptococcus parasanguinis | 7 | (3.11) | 2 | (2.17) | 0.007 | 1.0 |
| 8. | Streptococcus constellatus | 5 | (2.22) | 1 | (1.09) | 0.048 | 1.0 |
| 9. | Streptococcus anginosus | 4 | (1.78) | 1 | (1.09) | 0.20 | 1.0 |
| 10. | Streptococcus gordonii | 3 | (1.33) | 2 | (2.17) | 0.002 | 1.0 |
| 11. | Streptococcus sinensis | 1 | (0.44) | 1 | (1.09) | 0.43 | 0.49 |
| 12. | Streptococcus hyointestinalis | 1 | (0.44) | 0 | (0) | – | – |
| 13. | Streptococcus pluranimalium | 1 | (0.44) | 0 | (0) | – | – |
| 14. | Streptococcus thoraltensis | 1 | (0.44) | 0 | (0) | – | – |
| 15. | Streptococcus tigurinus | 1 | (0.44) | 0 | (0) | – | – |
| Total isolates | 167 | (74.22) | 58 | (63.04) | |||
There was no statistically significant difference between subgingival plaque and blood isolates of viridans streptococci isolated from periodontitis patients undergoing tooth extraction (p > 0.05).
If the mouth were the source of VGS causing bacteremia, the predominance of Streptococcus mitis in the oral cavity of these patients corresponds well to its predominance amongst blood culture isolates (p = 0.66). Streptococcus oralis being the second most common species also showed a similar distribution of oral and blood isolates (p = 0.47). Similarly all the others isolates of VGS which included Streptococcus sanguinis, Granulicatella elegans, Streptococcus mutans, Granulicatella adiacens, Streptococcus parasanguinis, Streptococcus constellatus, Streptococcus anginosus, Streptococcus gordonii and Streptococcus sinensis also showed a high rate of similarity (p > 0.5) of isolation and distribution among the subgingival plaque and blood samples.
Comparison of resistogram profiles of 167 VGS isolated from subgingival plaque and 58 VGS from blood of patients of periodontitis undergoing tooth extraction (n = 34) is given in Table 5. The isolates from the subgingival plaque and as well as blood sample of patients with periodontitis undergoing tooth extraction were similar in their patterns of the antibiograms and isolates from both these sites were almost indistinguishable.
Table 5.
Comparison of antibiogram (%R) of 167 viridans streptococci isolated from subgingival plaque and 58 viridans streptococci from blood of patients of periodontitis undergoing tooth extraction (n = 34).
| Viridans streptococci species | PEN | AMP | FEP | CTX | CRO | VAN | ERY | AZM | CLR | TCY | LVX | OFX | CLI | QDA | LNZ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %R |
% R |
% R |
% R |
% R |
% R |
% R |
% R |
% R |
% R |
% R |
% R |
% R |
% R |
% R |
||||||||||||||||
| Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | |
| S. mitis | 18 | 8 | 13 | 8 | 16 | 8 | 34 | 15 | 29 | 8 | 0 | 0 | 63 | 46 | 66 | 69 | 24 | 23 | 32 | 8 | 26 | 8 | 11 | 8 | 47 | 31 | 26 | 8 | 0 | 0 |
| S. oralis | 18 | 10 | 9 | 10 | 15 | 10 | 24 | 20 | 15 | 10 | 0 | 0 | 58 | 60 | 70 | 60 | 21 | 20 | 24 | 20 | 15 | 30 | 9 | 20 | 48 | 40 | 21 | 10 | 0 | 0 |
| S. sanguinis | 13 | 11 | 13 | 11 | 9 | 22 | 26 | 33 | 9 | 22 | 0 | 0 | 70 | 56 | 22 | 67 | 17 | 11 | 13 | 11 | 17 | 22 | 13 | 11 | 30 | 33 | 13 | 11 | 0 | 0 |
| S. parasanguinis | 0 | 0 | 0 | 0 | 14 | 50 | 14 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 29 | 100 | 14 | 0 | 14 | 0 | 14 | 0 | 14 | 0 | 14 | 50 | 0 | 50 | 0 | 0 |
| S. gordonii | 0 | 0 | 0 | 0 | 33 | 0 | 33 | 0 | 0 | 0 | 0 | 0 | 67 | 0 | 33 | 50 | 33 | 0 | 33 | 0 | 33 | 0 | 33 | 50 | 33 | 0 | 0 | 0 | 0 | 0 |
| S. anginosus | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 25 | 100 | 25 | 0 | 0 | 0 | 50 | 0 | 25 | 0 | 25 | 0 | 0 | 0 | 0 | 0 |
| S. constellatus | 0 | 0 | 0 | 0 | 20 | 0 | 20 | 0 | 20 | 0 | 0 | 0 | 20 | 0 | 20 | 100 | 0 | 0 | 20 | 0 | 0 | 0 | 20 | 0 | 20 | 100 | 0 | 0 | 0 | 0 |
| S. mutans | 6 | 14 | 0 | 14 | 13 | 29 | 6 | 14 | 6 | 14 | 0 | 0 | 31 | 71 | 31 | 57 | 13 | 14 | 13 | 14 | 13 | 14 | 13 | 14 | 38 | 57 | 6 | 0 | 0 | 0 |
| S. hyointestinalis | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – |
| S. sinensis | 0 | 0 | 0 | 0 | 100 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 100 | 100 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. pluranimalium | 0 | – | 100 | – | 0 | – | 0 | – | 0 | – | 0 | – | 100 | – | 100 | – | 100 | – | 0 | – | 100 | – | 100 | – | 100 | – | 0 | – | 0 | – |
| S. thoraltensis | 0 | – | 100 | – | 100 | – | 100 | – | 100 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – |
| S. tigurinus | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – |
| G. adiacens | 5 | 0 | 0 | 0 | 5 | 0 | 5 | 25 | 5 | 25 | 0 | 0 | 14 | 50 | 14 | 25 | 14 | 0 | 14 | 25 | 14 | 25 | 10 | 0 | 24 | 25 | 0 | 25 | 0 | 0 |
| G. elegans | 8 | 13 | 0 | 13 | 17 | 13 | 25 | 13 | 8 | 13 | 0 | 0 | 42 | 50 | 50 | 38 | 17 | 25 | 17 | 25 | 17 | 13 | 8 | 13 | 42 | 25 | 25 | 13 | 0 | 0 |
VGS—viridans group streptococci, Pq—plaque, Bd—blood, AMP—ampicillin, AZM—azithromycin, CLI—clindamycin, CLR—clarithromycin, CRO—cefitriaxone, CTX—cefotaxime, ERY—erythromycin, FEP—cefepime, LNZ—linezolid, LVX—levofloxacin, OFX—ofloxacin, PEN—penicillin, QDA—quinupristin/dalfopristin, TCY—tetracycline, VAN—vancomycin.
There was no statistically significance difference (p > 0.05) between antimicrobial pattern of resistance of subgingival plaque and blood isolates of periodontitis patients undergoing tooth extraction except S. sanguinis to azithromycin (Fisher's exact test was used).
Comparison of biotypes of VGS from subgingival plaque and blood of patients of IE with periodontitis is given in Table 6,7. At both the sites, VGS were the predominant species, comprising 62.73% of total isolates from subgingival plaque and 76.47% from blood. Staphylococcus aureus was the second most commonly isolated organism representing 6.36% from the oral cavity and 11.76% from blood. Other organisms were present in less numbers.
Table 6.
Comparison of species of bacteria isolated from the subgingival plaque and blood of patients of infective endocarditis with periodontitis (n = 17).
| Sr. No. | Bacterial species isolated | subgingival plaque isolates (110) |
blood isolates (17) |
χ2-value | P value | ||
|---|---|---|---|---|---|---|---|
| No | (%) | No | (%) | ||||
| 1. | Viridans streptococci | 69 | (62.73) | 13 | (76.47) | 0.69 | 0.40 |
| 2. | Staphylococcus aureus | 7 | (6.36) | 2 | (11.76) | 0.09 | 1.0 |
| 3. | Enterococcus spp. | 8 | (7.27) | 1 | (5.88) | 0.04 | 1.0 |
| 4. | Corynebacterium spp. | 4 | (3.64) | 0 | (0) | – | – |
| 5. | Gemella morbillorum | 4 | (3.64) | 0 | (0) | – | – |
| 6. | CONS | 4 | (3.64) | 0 | (0) | – | – |
| 7. | Kocuria rosea | 2 | (1.82) | 0 | (0) | – | – |
| 8. | Neisseriae spp. | 2 | (1.82) | 0 | (0) | – | – |
| 9. | Micrococcus species | 2 | (1.82) | 0 | (0) | – | – |
| 10. | Kocuria cristinae | 1 | (0.91) | 0 | (0) | – | – |
| 11. | Pseudomonas aeruginosa | 1 | (0.91) | 0 | (0) | – | – |
| 12. | Bacillus species | 1 | (0.91) | 0 | (0) | – | – |
| 13. | Rothia spp. | 4 | (3.64) | 0 | (0) | – | – |
| 14. | Acinetobacter lwoffi | 0 | (0.00) | 0 | (0) | – | – |
| 15. | Eikenella spp. | 1 | (0.91) | 0 | (0) | – | – |
| 16. | Erysipelothrix rhusiopathiae | 0 | (0) | 1 | (5.88) | – | – |
| Total aerobic isolates | 110 | (100) | 17 | (100) | |||
There was no statistically significant difference between bacterial isolates of subgingival plaque and blood of patients of infective endocarditis with periodontitis (P > 0.05).
If the mouth were the source of viridans streptococci causing bacteremia, the predominance of VGS in the oral cavity of these patients corresponds well to its predominance amongst blood culture isolates (p > 0.05). All the isolates of VGS showed a high rate of similarity of isolation and distribution among the subgingival plaque and blood samples.
Comparison of resistogram profiles of 69 VGS strains isolated from subgingival plaque and 13 VGS isolated from blood of IE patients (n = 17) are given in Table 8. The results indicate that isolates from the subgingival plaque and as well as blood sample of patients with periodontitis undergoing tooth extraction were similar in there patterns of the antibiograms, that isolates from both these sites were almost indistinguishable.
Table 8.
Comparison of antibiogram (% R) of 69 viridans streptococci strains isolated from subgingival plaque and 13 viridans streptococci isolated from blood of infective endocarditis patients (n = 17).
| Viridans streptococci Species | PEN | AMP | FEP | CTX | CRO | VAN | ERY | AZM | CLR | TCY | LVX | OFX | CLI | QDA | LNZ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %R |
%R |
%R |
%R |
%R |
%R |
%R |
%R |
%R |
%R |
%R |
%R |
%R |
%R |
%R |
||||||||||||||||
| Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | Pq | Bd | |
| S. mitis | 42 | 60 | 25 | 80 | 25 | 60 | 42 | 40 | 25 | 40 | 0 | 0 | 67 | 80 | 50 | 80 | 33 | 40 | 25 | 60 | 17 | 80 | 33 | 40 | 58 | 60 | 33 | 40 | 0 | 0 |
| S. oralis | 21 | 67 | 14 | 33 | 21 | 33 | 21 | 33 | 14 | 33 | 0 | 0 | 50 | 67 | 71 | 33 | 14 | 33 | 14 | 33 | 21 | 67 | 14 | 67 | 36 | 33 | 21 | 33 | 0 | 0 |
| S. sanguinis | 0 | 40 | 10 | 40 | 20 | 20 | 30 | 40 | 20 | 0 | 0 | 0 | 50 | 60 | 40 | 40 | 20 | 40 | 10 | 60 | 20 | 40 | 10 | 60 | 30 | 40 | 10 | 0 | 0 | 0 |
| S. parasanguinis | 0 | – | 0 | – | 100 | – | 100 | – | 0 | – | 0 | – | 0 | – | 100 | – | 0 | – | 0 | – | 0 | – | 0 | – | 100 | – | 0 | – | 0 | – |
| S. gordonii | 0 | – | 0 | – | 100 | – | 0 | – | 100 | – | 0 | – | 100 | – | 100 | – | 0 | – | 0 | – | 0 | – | 100 | – | 0 | – | 0 | – | 0 | – |
| S. anginosus | 0 | – | 0 | – | 100 | – | 0 | – | 0 | – | 0 | – | 100 | – | 100 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – |
| S. constellatus | 0 | – | 0 | – | 0 | – | 20 | – | 0 | – | 0 | – | 0 | – | 60 | – | 20 | – | 0 | – | 0 | – | 20 | – | 40 | – | 0 | – | 0 | – |
| S. mutans | 0 | – | 0 | – | 29 | – | 14 | – | 14 | – | 0 | – | 43 | – | 57 | – | 43 | – | 29 | – | 43 | – | 29 | – | 43 | – | 0 | – | 0 | – |
| S. sinensis | 0 | – | 0 | – | 100 | – | 0 | – | 0 | – | 0 | – | 0 | – | 100 | – | 0 | – | 0 | – | 0 | – | 0 | – | 100 | – | 0 | – | 0 | – |
| G. adiacens | 0 | – | 0 | – | 20 | – | 20 | – | 0 | – | 0 | – | 20 | – | 40 | – | 20 | – | 20 | – | 0 | – | 0 | – | 20 | – | 0 | – | 0 | – |
| G. elegans | 17 | – | 17 | – | 17 | – | 25 | – | 17 | – | 0 | – | 42 | – | 50 | – | 17 | – | 17 | – | 25 | – | 8 | – | 25 | – | 17 | – | 0 | – |
VGS—viridans group streptococci, Pq—plaque, Bd—blood, AMP—ampicillin, AZM—azithromycin, CLI—clindamycin, CLR—clarithromycin, CRO—cefitriaxone, CTX—cefotaxime, ERY—erythromycin, FEP—cefepime, LNZ—linezolid, LVX—levofloxacin, OFX—ofloxacin, PEN—penicillin, QDA—quinupristin/dalfopristin, TCY—tetracycline, VAN—vancomycin.
There was no statistically significance difference (p > 0.05) between antimicrobial pattern of resistance of subgingival plaque and blood isolates of infective endocarditis with periodontitis except S. mitis to levofloxacin (Fisher's exact test was used).
4. Discussion
This study shows that among all the patients undergoing tooth extraction, periodontitis was prevalent among a considerable number of patients (42.5%), whereas the remaining patients (57.5%) were without periodontitis. Periodontal diseases are a second major cause after dental caries for tooth extraction and our findings are in agreement with other similar studies.23, 24
Isolation of VGS from the subgingival plaque was highest among the subjects with periodontitis 167 (45.30%), when compared to subjects without periodontitis 93 (25.13%). Overall rate of bacterial isolation was higher in subjects with periodontitis 225 (60.81%) than subjects without periodontitis 145 (39.18%). These findings suggest that periodontitis plays a very important contributory role in promoting viridans streptococcal colonization, especially in the dental biofilm that grows above the gingival crest and predominance of streptococci in the oral niche could be a factor in determining their entry into the bloodstream.25, 26, 27, 28, 29, 30
A high rate of VGS bacteremia (47.54%) was observed in patients with periodontitis undergoing tooth extraction, than patients without periodontitis (14.75%), indicating the contributory role of periodontitis in promoting bacteremia through the already inflamed gingiva serving as an additional route for oral bacterial species to gain access into the bloodstream and cause a more severe bacteremia.6, 31
Parahitiyawa et al.31 reported that the incidence of bacteremia varies from 13 to 96% and the bacteremic incidence appears to be influenced positively by the presence of periodontitis and other odontogenic infections.32 Our results show significantly higher incidence of bacteremia in periodontitis patients than the patients without periodontitis and are consistent with findings of earlier studies.6, 31
In the present study out of 40 IE patients, the incidence of periodontitis was observed in 17 (42.5%) whereas 23 (57.5%) were without periodontitis. These findings indicate that the incidence of periodontitis in IE patients was observed in considerable number of patients and appears to be an important IE risk factor. Furthermore, periodontitis is positively influenced by poor oral hygiene. These results are similar to that in previous studies where the investigators have found a positive association between periodontitis and stroke, atherosclerosis.1, 33 These studies also support our findings of a specific link between periodontal disease and IE risk. Nevertheless, a causal relationship cannot be inferred from our data, and large intervention studies are required to test for causality.
In the present study, the frequency of isolation of VGS was highest (37.5%), followed by Staphylococcus aureus (22.5%). Our results were in accordance with the several other studies which have also reported higher incidence of VGS in IE cases.34, 35, 36, 37, 38 Some studies have reported increasing frequency of Staphylococcus aureus isolation followed by VGS.39, 40 These variations in frequency of isolation could be explained by differences in geographical regions as well as differences in patient populations, however, rapid identification of the etiological agent is vital for successful management of the patient.
VGS are among the most common causes of IE.41 They are also present in the dental plaque surrounding the teeth. Our results are in agreement with previous studies, in which a strong association between the incidence of bacteremia and oral hygiene and gingival disease has been demonstrated; moreover, these associations strengthen as the indices increase in severity.42 These data strongly suggest that the gingival sulcus is the main source and portal to the bloodstream for oral bacterial species such as VGS, detected in the blood.6, 31, 43, 44, 45
In the present study, we have made comparisons between biotypes of the oral and blood isolates of VGS from Group A and B patients with periodontitis. The species of VGS isolated from the subgingival plaque and blood of patients of periodontitis undergoing tooth extraction (group A) as well as patients of IE with periodontitis (group B), were compared statistically (Table 3, Table 6), there was no statistically significant difference (p > 0.05) between the species distribution and rates of isolation of VGS isolated from both the groups. Similarly, when we compared patient wise distribution of microorganisms isolated from subgingival plaque and blood of patients from both the groups (A and B) (Table 4, Table 7), we found that, all the VGS strains showed a uniform similarity between the biotypes and rates of isolation. If the mouth were the source of VGS causing bacteremia, this pattern of similarity of the VGS from both the sites reflects that periodontitis enhances the ability of these organisms to access the blood-stream and also correlates well to their association with bacteremia, which may eventually lead to IE.6, 31, 43, 44, 45
Table 4.
Comparison between blood and subgingival plaque isolates in patients undergoing tooth extraction with periodontitis.
| Patient ID No. | Microorganisms isolated from Blood and subgingival plaque of patients undergoing tooth extraction with Periodontitis (n = 34) | Microorganisms Present/Absent in blood of patients with periodontitis after tooth extraction | Same microorganisms Present/Absent from subgingival plaque of patients undergoing tooth extraction with Periodontitis | % similarity of Blood and plaque isolates |
|---|---|---|---|---|
| TE 1 | Staphlyococcus aureus | Present | Present | 91.17 |
| TE 2 | Streptococcus sanguinius | Present | Present | |
| TE 3 | Enterococcus spp. | Present | Present | |
| TE 5 | Escherischia coli | Present | Absent | |
| TE 7 | Streptococcus gordonii | Present | Present | |
| TE 8 | Streptococcus mitis | Present | Present | |
| TE 10 | Rothia mucilaginosa | Present | Present | |
| TE 12 | Streptococcus oralis | Present | Present | |
| TE 14 | Granulicatella elegans | Present | Present | |
| TE 15 | Kocuria rosea | Present | Present | |
| TE 17 | Streptococcus sanguinius | Present | Present | |
| TE 19 | Aeromonas salmonicida | Present | Absent | |
| TE 20 | Streptococcus mitis | Present | Present | |
| TE 22 | Kocuria rosea | Present | Present | |
| TE 24 | Neisseriae spp. | Present | Present | |
| TE 25 | Streptococcus sanguinius | Present | Present | |
| TE 27 | Streptococcus sanguinius | Present | Present | |
| TE 29 | Granulicatella elegans | Present | Present | |
| TE 30 | Kocuria rosea | Present | Present | |
| TE 34 | Streptococcus oralis | Present | Present | |
| TE 36 | Streptococcus sanguinius | Present | Present | |
| TE 38 | Kocuria rosea | Present | Present | |
| TE 40 | Streptococcus oralis | Present | Present | |
| TE 42 | Granulicatella elegans | Present | Present | |
| TE 45 | Kocuria kristinae | Present | Present | |
| TE 47 | Streptococcus mitis | Present | Present | |
| TE 48 | Aeromonas hydrophila | Present | Absent | |
| TE 50 | Enterococcus faecium | Present | Present | |
| TE 54 | Streptococcus mutans | Present | Present | |
| TE 56 | Streptococcus oralis | Present | Present | |
| TE 60 | Streptococcus sanguinius | Present | Present | |
| TE 65 | Kocuria rosea | Present | Present | |
| TE 66 | Streptococcus mitis | Present | Present | |
| TE 71 | Granulicatella adiacens | Present | Present | |
| Total | 34 | 31 | ||
Table 7.
Comparison between blood and subgingival plaque isolates in infective endocarditis patients with periodontitis.
| Patient ID No. | Microorganisms isolated from blood of infective endocarditis patients with Periodontitis (n = 17) | Microorganisms Present/Absent in blood of infective endocarditis patients with Periodontitis | Same microorganisms Present/Absent from subgingival plaque of infective endocarditis patients with Periodontitis | % similarity of Blood and plaque isolates |
|---|---|---|---|---|
| IE3 | Streptococcus sanguinius | Present | Present | 94.1 |
| IE4 | Staphylococcus aureus | Present | Present | |
| IE5 | Streptococcus mitis | Present | Present | |
| IE7 | Staphylococcus aureus | Present | Present | |
| IE8 | Streptococcus sanguinius | Present | Present | |
| IE10 | Streptococcus oralis | Present | Present | |
| IE12 | Streptococcus mitis | Present | Present | |
| IE16 | Streptococcus oralis | Present | Present | |
| IE19 | Streptococcus mitis | Present | Present | |
| IE30 | Streptococcus oralis | Present | Present | |
| IE32 | Erysipelothrix rhusiopathiae | Present | Absent | |
| IE33 | Streptococcus sanguinius | Present | Present | |
| IE34 | Streptococcus sanguinius | Present | Present | |
| IE36 | Streptococcus sanguinius | Present | Present | |
| IE38 | Streptococcus mitis | Present | Present | |
| IE39 | Enterococcous | Present | Present | |
| IE40 | Streptococcus mitis | Present | Present | |
| Total | 17 | 16 | ||
In the present study, we have also made comparisons between antibiograms of the oral and blood isolates of VGS from group A and B patients with periodontitis. The antibiogram profiles of VGS isolated from the subgingival plaque and blood of patients of periodontitis undergoing tooth extraction (group A) as well as patients of IE with periodontitis (group B), were compared statistically (Tables 5,8), there was no statistically significant difference (p > 0.05) between the resistance profiles of VGS isolated from both the groups. All the strains of VGS showed a uniform similarity between the antibiogram profiles. If the mouth were the source of VGS causing bacteremia, this pattern of similarity of the antibiograms of VGS from both the sites reflects that the periodontitis enhances the ability of these organisms to access the blood-stream and the same oral strains are associated with bacteremia, which may eventually lead to IE.6, 31, 43, 44, 45
All these findings (Table 4, Table 5, Table 6) show that biochemical identification profiles supported by antibiograms were useful in demonstrating similarity of strains VGS from subgingival plaque and blood of patients. The subgingival plaque was the most likely source of VGS bacteremia and provided a proof that inflammation induced by periodontitis opens up a channel for the entry of these organisms into blood stream and the same oral strains are associated with bacteremia, which may eventually lead to IE (Table 8).
The finding that VGS from the oral cavity could access the blood stream suggests that other combinations of microorganisms from this source could also cause polymicrobial bacteremia.6, 31, 43, 44, 45
Poor oral hygiene results in plaque and calculus accumulation around teeth that can lead to inflammation and ulceration of the gingival tissues, which precedes periodontitis and eventual tooth loss.46 Our study shows a significant relationship between oral hygiene and periodontal disease parameters and the risk of developing IE. This pattern of similarity of the VGS from both the sites (Table 4, Table 5, Table 6) reflects that periodontitis enhances the ability of these organisms to access the blood stream and also correlates well to their association with bacteremia which may eventually lead to IE. The subgingival plaque was the most likely source of VGS bacteraemia and provided a proof that inflammation induced by periodontitis could be a portal of entry of these organisms.
In conclusion, our study indicates that periodontal disease is significantly associated with IE risk in patients with pre-existing cardiovascular diseases such as valvular heart disease. Gingivitis and periodontitis are treatable and preventable conditions. Therefore, their identification as IE risk factors would have a major impact on IE prevention.
Conflict of interest
None to declare.
Source of funding
None to declare.
References
- 1.Bartova J., Sommerova P., Lyuya-Mi Y. Periodontitis as a risk factor of atherosclerosis. J Immunol Res. 2014;2014 doi: 10.1155/2014/636893. 636893. Epub 2014 Mar 23. Review. PMID:24741613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noack B., Genco R.J., Trevisan M., Grossi S., Zambon J.J., De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 3.Ito H.O. Infective endocarditis and dental procedures: evidence, pathogenesis, and prevention. J Med Invest. 2006;53:189–198. doi: 10.2152/jmi.53.189. [DOI] [PubMed] [Google Scholar]
- 4.Lockhart P.B., Brennan M.T., Thornhill M. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140:1238–1244. doi: 10.14219/jada.archive.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forner L., Larsen T., Kilian M., Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401–407. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 6.Lockhart P.B., Brennan M.T., Sasser H.C., Fox P.C., Paster B.J., Bahrani-Mougeot F.K. Bacteremia associated with tooth brushing and dental extraction. Circulation. 2008;117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinane D.F., Riggio M.P., Walker K.F., MacKenzie D., Shearer B. Bacteraemia following periodontal procedures. J Clin Periodontol. 2005;32:708–713. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 8.Lucas V.S., Gafan G., Dewhurts S., Roberts G.J. Prevalence, intensity and nature of bacteraemia after toothbrushing. J Dent. 2008;36:481–487. doi: 10.1016/j.jdent.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Silver J.G., Martin A.W., McBride B.C. Experimental transient bacteraemias in human subjects with varying degrees of plaque accumulation and gingival inflammation. J Clin Periodontol. 1997;4:92–99. doi: 10.1111/j.1600-051x.1977.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 10.Seymour G.J., Ford P.J., Cullinan M.P., Leishman S., Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl. 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 11.Drangsholt M.T. A new causal model of dental diseases associated with endocarditis. Ann Periodontol. 1998;3:184–196. doi: 10.1902/annals.1998.3.1.184. [DOI] [PubMed] [Google Scholar]
- 12.Li J.S., Sexton D.J., Mick N. Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 13.Lenox J.A., Kopczyk R.A. A clinical system for scoring a patient’s oral hygiene performance. J Am Dent Assoc. 1973;86:849–852. doi: 10.14219/jada.archive.1973.0178. [DOI] [PubMed] [Google Scholar]
- 14.O’leary T.J., Drake R.B., Naylor J.E. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:610. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 16.Cheesbrough M. 2nd ed. Cambridge University Press; Cambridge: 2006. District laboratory practice in tropical countries. Part 2. [Google Scholar]
- 17.Dajani A.S., Taubert K.A., Wilson W. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. J Am MedAssoc. 1997;277:1794–1801. [PubMed] [Google Scholar]
- 18.Roberts G.J., Gardner P., Simmons N.A. Optimum sampling time for detection of odontogenic bacteraemia in children. Int J Cardiol. 1992;35:311–315. doi: 10.1016/0167-5273(92)90228-u. [DOI] [PubMed] [Google Scholar]
- 19.Maharaj B., Coovadia Y., Vayej A.C. An investigation of the frequency of bacteraemia following dental extraction, tooth brushing and chewing. Cardiovasc J Afr. 2012;23(6):340–344. doi: 10.5830/CVJA-2012-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockhart P.B., Brennan M.T., Kent M.L., Norton H.J., Weinrib D.A. Impact of amoxicillin prophylaxis on the incidence, nature, and duration of bacteremia in children after intubation and dental procedures. Circulation. 2004;109(23):2878–2884. doi: 10.1161/01.CIR.0000129303.90488.29. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute . vol. 32. Clinical and Laboratory Standards Institute; 2015. (Performance standards for antimicrobial susceptibility testing; Twenty-Third Informational Supplement). January 2013, CLSI document M100-S23, No. 3. [Google Scholar]
- 22.Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15:613–630. doi: 10.1128/CMR.15.4.613-630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morita M., Kimura T., Kanegae M., Ishikawa A., Watanabe T. Reasons for extraction of permanent teeth in Japan. Commun Dent Oral Epidemiol. 1994;22:303–306. doi: 10.1111/j.1600-0528.1994.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 24.Jovino-Silveira R.C., Caldas Ade F., Jr., de Souza E.H., Gusmão E.S. Primary reason for tooth extraction in a Brazilian adult population. Oral Health Prev Dent. 2005;3(3):151–157. [PubMed] [Google Scholar]
- 25.Igarashi T., Yamamoto A., Goto N. Polymerase chain reaction for identification of oral streptococci: Streptococcus mutans, Streptococcus sobrinus, Streptococcus downei and Streptococcus salivarius. J Microbiol Methods. 1998;34:81–91. [Google Scholar]
- 26.Tomas I., Alvarez M., Limeres J., Potel C., Medina J., Diz P. Prevalence, duration and aetiology of bacteraemia following dental extractions. Oral Dis. 2007;13:56–62. doi: 10.1111/j.1601-0825.2006.01247.x. [DOI] [PubMed] [Google Scholar]
- 27.Takai S., Kuriyama T., Yanagisawa M., Nakagawa K., Karasawa T. Incidence and bacteriology of bacteremia associated with various oral and maxillofacial surgical procedures. Oral Surg Oral Med Oral Pathol Endod. 2005;99:292–298. doi: 10.1016/j.tripleo.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 28.(a) Brennan M.T., Kent M.L., Fox P.C., Norton H.J., Lockhart P.B. The impact of oral disease and nonsurgical treatment on bacteremia in children. J Am Dent Assoc. 2007;138:80–85. doi: 10.14219/jada.archive.2007.0025. [DOI] [PubMed] [Google Scholar]; (b) Cohen J., Worsley A.M., Goldman J.M., Donelly J.P., Catovski D., Galton D.A. Septicaemia caused by viridans streptococci in neutropenic patients with leukaemia. Lancet. 1983;ii:1452–1454. doi: 10.1016/s0140-6736(83)90799-7. [DOI] [PubMed] [Google Scholar]
- 29.Henslee J., Bostrom B., Weisdorf D., Ramsay N., McGlave P., Kersey J. Streptococcal sepsis in bone marrow transplant patients. Lancet. 1984;i:393. doi: 10.1016/s0140-6736(84)90440-9. [DOI] [PubMed] [Google Scholar]
- 30.Bayliss R., Clarke C., Oakley C.M., Somerville W., Whitfield A.G., Young S.E. The microbiology and pathogenesis of infective endocarditis. Br Heart J. 1983;50:513–519. doi: 10.1136/hrt.50.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parahitiyawa N.B., Jin L.J., Leung W.K., Yam W.C., Samaranayake L.P. Microbiology of odontogenic bacteremia: beyond endocarditis. Clin Microbiol Rev. 2009;22(1):46–64. doi: 10.1128/CMR.00028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorelick P.B. Stroke prevention therapy beyond antithrombotics: unifying mechanisms in ischemic stroke pathogenesis and implications for therapy: an invited review. Stroke. 2002;33:862–875. doi: 10.1161/hs0302.103657. [DOI] [PubMed] [Google Scholar]
- 33.Nakatani S., Mitsutake K., Ohara T. Recent picture of infective endocarditis in Japan–lessons from Cardiac Disease Registration (CADRE-IE) Circ J. 2013;77(6):1558–1564. doi: 10.1253/circj.cj-12-1101. [DOI] [PubMed] [Google Scholar]
- 34.Barrau K., Boulamery A., Imbert G. Causative organisms of infective endocarditis according to host status. Clin Microbiol Infect. 2004;10:302–308. doi: 10.1111/j.1198-743X.2004.00776.x. [DOI] [PubMed] [Google Scholar]
- 35.Tariq M., Alam M., Munir G., Khan M.A., Smego R.A., Jr. Infective endocarditis: a five-year experience at a tertiary care hospital in Pakistan. Int J Infect Dis. 2004;8:163–170. doi: 10.1016/j.ijid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Garg N., Kandpal B., Garg N. Characteristics of infective endocarditis in a developing country – clinical profile and outcome in 192 Indian patients, 1992–2001. Int J Cardiol. 2005;15:253–260. doi: 10.1016/j.ijcard.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 37.Kanafani Z.A., Mahfouz T.H., Kanj S.S. Infective endocarditis at a tertiary care centre in Lebanon: predominance of streptococcal infection. J Infect. 2002;45:152–159. doi: 10.1016/s0163-4453(02)91041-8. [DOI] [PubMed] [Google Scholar]
- 38.Loupa C., Mavroidi N., Boutsikakis I. Infective endocarditis in Greece: a changing profile. Epidemiological, microbiological and therapeutic data. Clin Microbiol Infect. 2004;10:556–561. doi: 10.1111/j.1469-0691.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- 39.Tariq M., Siddiqui B.K., Jadoon A. Clinical profile and outcome of infective endocarditis at the Aga Khan University Hospital. Int J Collaborative Res Intern Med Public Health. 2009;1:84–99. [Google Scholar]
- 40.Douglas C.W.I., Heath J., Hampton K.K., Preston F.E. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol. 1993;39:179–182. doi: 10.1099/00222615-39-3-179. [DOI] [PubMed] [Google Scholar]
- 41.Lockhart P.B., Brennan M.T., Thornhill M. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140:1238–1244. doi: 10.14219/jada.archive.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bahrani-Mougeot F.K., Paster B.J., Coleman S., Ashar J., Barbuto S., Lockhart P.B. Diverse and novel oral bacterial species in blood following dental procedures. J Clin Microbiol. 2008;46:2129–2132. doi: 10.1128/JCM.02004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Meer J.T.M., Thompson J., Valkenburg H.A., Michel M.F. Epidemiology of bacterial endocarditis in the Netherlands, I: patient characteristics. Arch Intern Med. 1992;152:1863–1868. doi: 10.1001/archinte.152.9.1863. [DOI] [PubMed] [Google Scholar]
- 44.Strom B.L., Abrutyn E., Berlin J.A. Dental and cardiac risk factors for infective endocarditis: a population-based case-control study. Ann Intern Med. 1998;129:761–769. doi: 10.7326/0003-4819-129-10-199811150-00002. [DOI] [PubMed] [Google Scholar]
- 45.Albandar J.M., Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70(1):30–43. doi: 10.1902/jop.1999.70.1.30. [DOI] [PubMed] [Google Scholar]