Abstract
Background
Recent studies have shown that complete blood count (CBC) parameters can effectively predict long-term mortality and re-infarction rates in acute coronary syndrome (ACS). However, the role of these parameters in predicting short term mortality has not been studied extensively. The main objective of this study was to determine whether CBC parameters can predict 30-days mortality and the incidence of major adverse cardiac event (MACE) in ACS patients.
Methodology
A total of 297 patients with ACS were recruited in this prospective study. The relationship of baseline white blood cell (WBC) to mean platelet volume ratio (WMR) with MACE and mortality was assessed during a 30-days follow up. The patients were divided into two groups: Group A [WMR < 1000] and Group B [WMR > 1000]. Multivariate COX regression was performed to calculate hazard ratios (HR).
Results
WMR had the highest area under receiver operating characteristics curve and highest discriminative ability amongst all CBC parameters in predicting mortality. Patients in Group B had a higher mortality rate (p < 0.001) than patients in Group A. WBC count (p = 0.02), platelet count (p = 0.04), WMR (p = 0.008), platelet to lymphocyte ratio (p < 0.001) and neutrophil to lymphocyte ratio (p = 0.03) were significantly higher in the MACE-positive group as compared to MACE-negative. In multivariate cox regression analysis, WMR > 1000 (HR = 2.9, 95% confidence interval 1.3–6.5, p = 0.01) was found to be strongest biochemical marker in predicting mortality.
Conclusion
WMR is an easily accessible and an inexpensive indicator, which may be used as a prognostic marker in patients with ACS.
Keywords: Acute coronary syndrome, Complete blood count, Mean platelet volume, White blood cell
1. Introduction
Although mortality rates of acute coronary syndrome (ACS) have declined over the past four decades, it remains the leading cause of mortality and morbidity worldwide.1 There is a 10% to 20% mortality rate in ACS patients within the first six months of diagnosis; with about half of all deaths occurring within the first 30 days.2
The pathophysiology of ACS is extremely heterogeneous; however, in majority of the cases it is associated with rupture of an atherosclerotic plaque and partial or complete thrombosis of the infarct-related artery.3 Ruptured plaques contribute to thrombus formation by inflammatory mechanisms, which include the role of activated and inactivated platelets and platelet-leukocyte adhesions, leading to the development of ACS.4, 5, 6 Therefore, inflammatory biomarkers related to platelets and leukocytes can be used as prognostic tools and for risk stratification of ACS patients.
An elevated level of mean platelet volume (MPV), a platelet activation marker, has been proven to be essential in detecting a cardiovascular event (CVE). The meta-analysis conducted by S.G.Chu et al. demonstrated that elevated MPV can be used to predict occurrence of acute myocardial infarction (MI), mortality following MI, and re-stenosis following coronary angioplasty.7 Apart from MPV, other potentially useful complete blood count indices include leukocyte count, platelet count, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), red cell distribution width (RDW) and white blood cell (WBC) to MPV ratio (WMR).8, 9, 10 Previous literature has mainly focused on the role of these biomarkers in predicting long term mortality and complications in ACS patients.
Recent studies have shown that the MPV and MPV/platelet count ratio can predict long-term mortality in patients with ischemic cardiovascular disease.11, 12 Similarly, in an analysis of 900 patients in the Stent Primary Angioplasty in Myocardial Infarction (Stent PAMI) trial, investigators found that an elevated WBC count upon hospital admission had a strong independent correlation with re-infarction and death at 1 year.13 However, little is known about the suggested role of the aforementioned indicators, both individually and in combination, in predicting short term outcome in patients admitted for ACS.
Hence, in this prospective study, we sought to determine the efficacy of CBC parameters in estimating short-term mortality (30 days) and the incidence of major adverse cardiac events (MACE) in patients with ACS.
2. Materials and methods
This prospective cohort study was carried out at the Cardiology Department of the Civil Hospital, Karachi, Pakistan after approval from the Institutional Review Board (IRB) of Dow University of Health Sciences. From October 2015 to October 2016; all patients who presented with chief complaint of acute chest pain or were admitted at our institution with ACS were considered.
Adult patients diagnosed with ACS undergoing primary percutaneous intervention (PCI) were eligible for our study. ACS patients were identified by using the following criteria: non-ST elevation myocardial infarction (NSTEMI) was confirmed if patients had raised cardiac enzymes without detectable ST-segment elevation in the electrocardiogram (ECG). ST elevation myocardial infarction (STEMI) was confirmed if patient complained of typical chest pain lasting more than 20 min along with any one of the following characteristics: ST-segment elevation of at least 1 mm, formation of new Q wave, left bundle branch block formation in two or more contiguous leads, and/or two times increase in the cardiac enzymes. Unstable angina (UA) was confirmed if there were detectable ischemic changes on an ECG with no increase in cardiac enzymes.8
Patients were excluded if they were younger than 18 years, had received anticoagulant therapy or an immunosuppressant, had conditions which put them at high risk of serious bleeding, were diagnosed with cancer, active infectious diseases or inflammatory diseases, or severe liver disease.
A total of 350 patients were included in the study. Thirty-five patients refused to become part of the study, which narrowed the sample down to 315 patients. Seventeen patients were lost to follow up which yielded a final sample size of 297.
On receiver operating characteristics (ROC) curve analysis, WMR had highest area under the curve (AUC) and highest discriminative ability amongst all CBC parameters in predicting mortality. Therefore, study population was divided into two groups according to their median values of WMR, namely: Group A [WMR ≤ 1000] and Group B [WMR > 1000].
Once selected, an interviewer-based, pilot tested questionnaire was administered to each patient. Interviewer bias was eliminated by employing similarly qualified individuals, by training them and by keeping them unaware of the outcome of the study. Moreover, the questionnaire was translated into the national language, Urdu, for ease of understanding. Written and oral informed consent was obtained from all patients prior to administering the questionnaire.
At the time of inclusion, all patients were evaluated using a full physical examination and a detailed medical history. In addition, patients were further evaluated according to Killip clinical examination classification,14 New York Heart Association (NYHA) classification15 and thrombolysis in myocardial infarction (TIMI) scores.16
One month following discharge, every patient was followed up by administering an interviewer-based, pilot tested questionnaire to record the incidence of major adverse cardiovascular events (MACE). MACE was considered positive if patients had any one of the following events: non-fatal MI, re-hospitalization, cardiac arrhythmias and death. In addition, other adverse events like cardiogenic shock, kidney dialysis, gastrointestinal (GI) bleeding, coronary artery bypass grafting (CABG) and access site complication were also recorded.
2.1. Laboratory analysis
At base line, venous blood samples were obtained within 30 min of admission to measure hematological indices and biochemical markers. An automated hematology analyzer SYSMEX XN-1000 was used to measure hematological indices. In addition, detailed liver function tests (LFTS), electrolytes, blood urea nitrogen (BUN) and creatinine (Cr) were measured with Roche Cobas c501 chemistry analyzer (Roche Diagnostics). Fasting lipid panels were measured by standard enzymatic methods. Patients were also evaluated for cardiac ischemia markers, echocardiography and a 12-lead ECG. Troponin I was measured by ChemiluminescenceMicroparticle Immune-Assay-CMIA (Cobas c601), while other cardiac enzymes were measured by Roche Cobas c 501. Left ventricular ejection fraction (LVEF) was assessed by two-dimensional echocardiography. Number of diseased vessel(s) (NODV) was evaluated after patients underwent coronary angiography.
2.2. Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or as median (interquartile range), and analyzed for normality by the Shapiro-Wilk test. Categorical variables were compared using the Chi-square or Fisher’s exact test while continuous variables were compared using Independent t-test or Mann-Whitney U tests. ROC curve analysis was conducted to determine prognostic accuracy of biochemical markers in predicting short-term mortality. Survival curve was generated by means of the Kaplan-Meier analysis. Those variables which had a p < 0.25 in univariate analysis were included in Multivariate COX regression analysis. The backward stepwise likelihood ratio method was used to identify the independent predictors of 30-days mortality. All tests were two-tailed and a p-value of less than 0.05 was considered significant. No imputation methods were used to replace missing variables. All analyses were performed with SPSS Statistics, version 17.0 (IBM SPSS Inc., Chicago, IL).
3. Results
The average age of the population was 55.4 ± 10.8 years while more than half (n = 188, 63.2%) of the patients were males. More than half (n = 210; 70%) of the patients were hypertensive, while 108 (36.4%) were known diabetics. Majority of the patients presented with either UA (n = 100, 33.7%) or with STEMI (n = 118, 39.7%). During the mean follow up period of 29.5 days, 41 (13.8%) patients died due to cardiovascular events. Detailed comparisons of the baseline characteristics, clinical features, and biomarkers of the groups are shown in Table 1.
Table 1.
Comparisons of the baseline characteristics, clinical features, and biomarkers between the two groups, A (WMR ≤ 1000) and B (WMR > 1000).
| Variables | WMR ≤ 1000; low | WMR > 1000; high | ap-value |
|---|---|---|---|
| n = 160 | n = 137 | ||
| Age [years] | 55.87 ± 10.48 | 54.77 + 11.21 | d0.382 |
| Male, n (%) | 96 (60.0) | 92 (67.2) | b0.202 |
| Rural area, n (%) | 50 (31.3) | 34 (24.8) | b0.220 |
| Previous History, n (%) | |||
| Diabetic | 54 (34) | 54 (39) | b0.312 |
| Smokers | 45 (28) | 47 (34) | b0.251 |
| HTN | 115 (72) | 95 (69) | b0.633 |
| Familial CAD/MI | 64 (40.0) | 53 (38.7) | b0.817 |
| Prior CABG | 6 (3.8) | 1 (0.7) | c0.128 |
| Prior MI | 90 (56.3) | 96 (70.1) | b0.014 |
| PCI | 28 (17.5) | 18 (13.1) | b0.300 |
| On admission vitals | |||
| Heart rate [bpm] | 81.49 ± 13.99 | 84.97 ± 17.69 | d0.064 |
| Systolic BP [mm Hg] | 129.64 ± 25.17 | 124.41 ± 28.87 | d0.096 |
| Diastolic BP [mm Hg] | 80.15 ± 14.11 | 79.21 ± 18.50 | d0.627 |
| NYHA classification, n (%): | b<0.001 | ||
| Class 1 | 39 (24.4) | 24 (17.5) | |
| Class 2 | 74 (46.3) | 51 (37.2) | |
| Class 3 | 33 (20.6) | 22 (16.1) | |
| Class 4 | 14 (8.8) | 40 (29.2) | |
| KILLIP CLASS, n (%) | b0.221 | ||
| Class 1 | 103 (64.4) | 74 (54) | |
| Class 2 | 41 (25.6) | 40 (29.2) | |
| Class 3 | 6 (3.8) | 10 (7.3) | |
| Class 4 | 10 (6.3) | 13 (9.5) | |
| LVEF [%] | 48.38 ± 11.64 | 43.84 ± 11.02 | d0.001 |
| NODV, n (%): | b0.004 | ||
| 1 vessel, n (%) | 45 (28.1) | 35 (25.5) | |
| > 1 vessel, n (%) | 94 (58.8) | 98 (71.5) | |
| Cardiac enzymes | |||
| Troponin-I [ng/mL] | 2.40 (3.26) | 3.50 (3.00) | e<0.001 |
| CK[U/L] | 112.00 (173.3) | 207.00 (226.50) | e0.006 |
| CKMB [U/L] | 41.33 ± 21.50 | 45.78 ± 20.29 | d0.069 |
| LDH[U/L] | 296.00 (179.80) | 357.00 (136.50) | e0.003 |
| AST[U/L] | 27.50 (18.70) | 35.44 (31.50) | e0.001 |
| Biochemical markers | |||
| Cholesterol [mg/dl] | 162.63 ± 43.39 | 155.50 ± 42.66 | d0.156 |
| HDL [mg/dl] | 33.32 ± 9.66 | 34.49 ± 8.61 | d0.275 |
| LDL [mg/dl] | 95.09 ± 38.26 | 88.26 ± 40.04 | d0.134 |
| Triglycerides [mg/dl] | 153.23 ± 83.57 | 165.43 ± 86.56 | d0.218 |
| Total Bilirubin [mg/dl] | 0.46 ± 0.18 | 0.49 ± 0.19 | d0.267 |
| ALT [U/L] | 20.00 (16.80) | 26.00 (30.0) | e0.016 |
| ALP [U/L] | 90.82 ± 25.50 | 93.39 ± 29.73 | d0.428 |
| Sodium [mEq/L] | 137.59 ± 5.73 | 136.95 ± 5.98 | d0.349 |
| BUN [mg/dL] | 13.73 ± 5.73 | 15.58 ± 6.28 | d0.009 |
| Creatinine [mg/dL] | 1.02 ± 0.31 | 1.15 ± 0.37 | d0.001 |
| WBC count[×103/μL] | 8.58 ± 1.65 | 13.74 ± 2.66 | d<0.001 |
| Neutrophil [×103/μL] | 6.38 ± 1.02 | 7.31 ± 1.19 | d<0.001 |
| Lymphocyte [×103/μL] | 2.63 ± 0.91 | 1.97 ± 0.98 | d<0.001 |
| Hemoglobin [gm/dl] | 12.48 ± 2.09 | 12.51 ± 2.35 | d0.888 |
| RBC count[×106/μL] | 4.69 ± 0.70 | 4.54 ± 0.88 | d0.109 |
| Hematocrit [%] | 38.13 ± 5.78 | 37.32 ± 6.69 | d0.263 |
| Platelet count [×103/uL] | 270.77 ± 84.22 | 312.58 ± 119.16 | d0.001 |
| MPV [fL] | 10.63 ± 1.12 | 10.15 ± 1.34 | d0.001 |
| RDW [%] | 15.02 ± 2.07 | 15.04 ± 1.61 | d0.902 |
| WMR | 831.80 (253.90) | 1330.90 (352.00) | e<0.001 |
| PLR | 101.25 (65.02) | 148.70 (108.65) | e<0.001 |
| NLR | 2.31 (2.10) | 3.55 (3.50) | e<0.001 |
| Follow up features, n (%) | |||
| Re hospitalization | 26 (16.3) | 33 (24.1) | b0.091 |
| MI | 26 (16.3) | 31 (22.6) | b0.164 |
| Cardiogenic shock | 13 (8.1) | 18 (13.1) | b0.159 |
| Cardiac arrhythmia | 7 (4.4) | 14 (10.2) | b0.050 |
| GI Bleeding | 13 (8.1) | 8 (5.8) | b0.444 |
| Dialysis | 2 (1.3) | 4 (2.9) | c0.420 |
| Transfusion | 17 (10.6) | 17 (12.4) | b0.630 |
| CABG | 13 (8.1) | 18 (13.1) | b0.159 |
| Stent placement | 44 (27.5) | 44 (32.1) | b0.385 |
| Access site complication | 1 (0.6) | 2 (1.5) | c0.597 |
| Mortality | 10 (6.3) | 31 (22.6) | b<0.001 |
| TIMI score STEMI | 3.58 ± 1.03 | 5.04 ± 0.84 | d<0.001 |
| TIMI score NSTEMI/UA | 3.45 ± 1.59 | 4.69 ± 1.52 | d<0.001 |
| Duration of hospital stay [days] | 7.00 (5.00) | 7.00 (5.50) | e0.123 |
| Diagnosis, n (%): | b<0.001 | ||
| NSTEMI | 33 (20.6) | 46 (33.6) | |
| STEMI | 54 (33.8) | 64 (46.7) | |
| UA | 73 (45.6) | 27 (19.7) | |
All values are presented as mean ± standard deviation, median (±IQR) and frequency (percentages). WMR value is categorized according to its median; BP—blood pressure; CK-MB—creatine kinase MB isoenzyme; HTN—Hypertension; CAD—coronary artery disease; CABG—coronary artery bypass grafting; PCI— percutaneous coronary intervention; HDL—high density lipoprotein; LDL—low density lipoprotein; MI—myocardial infarction; NYHA—New York Heart Association; LDH—Lactate dehydrogenase; AST—Aspartate Aminotransferase; ALT—Alanine transaminase; ALP—Alkaline phosphatase; MPV—mean platelet volume; RDW—Red Cell Distribution Width; WMR—; PLR— platelets to lymphocytes ratio; NLR— neutrophils to lymphocytes ratio; NSTEMI—non ST elevation myocardial infarction;STEMI—ST elevation myocardial infarction; UA—unstable angina; BUN—blood urea nitrogen; Cr—creatinine; TIMI—thrombolysis in myocardial infarction.
p values <0.05 were considered statistically significant.
chi-square test.
Fisher-exact test.
Independent t-test.
Mann-Whitney test was used to compare quantitative data without normal distribution.
On comparison between Group A (WMR < 1000, n = 160) and Group B [WMR > 1000, n = 137], there was no significant difference in prevalence of co-morbid conditions such as diabetes (p = 0.312), hypertension (p = 0.63) and familial coronary artery disease (CAD) or MI (p = 0.82). Similarly there was no significant difference in re-hospitalization rates (p = 0.09), non-fatal MI (p = 0.16) and cardiac arrhythmias (p = 0.05). However rates of multi-vessel disease (58.8% vs 71.5%, p = 0.004), the incidence of mortality (6.3% vs 22.6%, p < 0.001) and TIMI scores (p < 0.001) were significantly lower in group A as compared to group B. Furthermore, patients in group B had a greater incidence of STEMI and NSTEMI while UA was more common in the group A (p < 0.001).
Among laboratory values, MPV (10.63 vs 10.15 fL, p < 0.001) was significantly higher in group A, in contrast, the WBC count (8.58 vs 13.74 × 103/μL, p < 0.001) was much lower for the same group of patients. Moreover, values of LFTs including alanine transaminase (ALT) (p = 0.02) and aspartate transaminase (AST) (p = 0.001), serum BUN (p = 0.009), serum Cr (p = 0.001), CK (p = 0.006) and Troponin-I (p < 0.001) were significantly higher in group B as compared to group A. Values of other biochemical markers are shown in Table 1.
In comparison to MACE-negative patients, patients in the MACE-positive group had significantly lower values of LVEF (p = 0.003) and increased incidence of cardiogenic shock (p = 0.003), GI bleeding (p < 0.001) and need for blood transfusion (p = 0.02). Similarly, adverse events including incidence of a bypass surgery (p = 0.03) and multi-vessel disease (p = 0.03) also occurred more frequently in this group. Analysis of biomarkers revealed that MACE-positive patients had significantly higher values of WBC count (p = 0.02), lymphocyte count (p = 0.01), platelet count (p = 0.04), WMR (p = 0.008), PLR (p < 0.001) and NLR (p = 0.03) (Table 2).
Table 2.
Comparison of the baseline characteristics, clinical features and biochemical markers between major adverse cardiovascular events (MACE) positive and MACE negative group.
| Variables | MACE-positive | MACE-negative | ap-value |
|---|---|---|---|
| n = 102 | n = 195 | ||
| Age [years] | 54.49 ± 11.41 | 55.82 ± 10.50 | d0.317 |
| Male, n (%) | 68 (66.7) | 120 (61.5) | b0.384 |
| Rural area, n (%) | 36 (35.3) | 48 (24.6) | b0.052 |
| Previous Hx, n (%) | |||
| Diabetic | 37 (36.3) | 71 (36.4) | b0.982 |
| Smokers | 29 (28.4) | 63 (32.3) | b0.493 |
| HTN | 66 (64.7) | 144 (73.8) | b0.100 |
| Familial Hx of CAD/MI | 37 (36.3) | 80 (41.0) | b0.426 |
| Prior CABG | 3 (2.9) | 4 (2.1) | c0.695 |
| Prior MI | 39 (38.2) | 72 (36.9) | b0.824 |
| PCI | 19 (18.6) | 27 (13.8) | b0.279 |
| On admission vitals | |||
| Heart rate [bpm] | 83.34 ± 15.34 | 82.97 ± 16.18 | d0.851 |
| Systolic BP [mm Hg] | 125.43 ± 26.71 | 128.16 ± 27.20 | d0.409 |
| Diastolic BP [mm Hg] | 79.65 ± 14.93 | 79.75 ± 16.95 | d0.958 |
| NYHA classification, n (%): | b0.018 | ||
| Class 1 | 14 (13.7) | 49 (25.1) | |
| Class 2 | 43 (42.2) | 82 (42.1) | |
| Class 3 | 18 (17.6) | 37 (19.0) | |
| Class 4 | 27(26.5) | 27 (13.8) | |
| KILLIP CLASS, n (%): | b0.414 | ||
| Class 1 | 58 (56.9) | 119 (61) | |
| Class 2 | 26 (25.5) | 55 (28.2) | |
| Class 3 | 7 (6.9) | 9 (4.6) | |
| Class 4 | 11 (10.8) | 12 (6.2) | |
| LVEF [%} | 43.57 ± 11.89 | 47.71 ± 11.16 | d0.003 |
| Number of diseased vessels, n (%): | b0.031 | ||
| 1 vessel | 21 (20.6) | 59 (30.3) | |
| >1 vessel | 76 (74.5) | 116 (59.5) | |
| Cardiac enzymes | |||
| Troponin-I [ng/mL] | 2.90 (3.21) | 2.90 (3.86) | e0.775 |
| CK [U/L] | 147.50 (203.00) | 126.00 (182.00) | e0.555 |
| CKMB [U/L] | 43.53 ± 21.78 | 43.30 ± 20.69 | d0.928 |
| LDH [U/L] | 340.23 (159.5) | 312.00 (180.00) | e0.599 |
| AST [U/L] | 33.50 (24.0) | 31.00 (20.0) | e0.780 |
| Biochemical markers | |||
| Cholesterol[mg/dL] | 167.72 ± 47.20 | 154.96 ± 40.28 | d0.021 |
| HDL [mg/dL] | 33.82 ± 8.22 | 33.88 ± 9.69 | d0.962 |
| LDL [mg/dL] | 99.35 ± 42.66 | 88.07 ± 36.75 | d0.018 |
| Triglycerides [mg/dL] | 166.66 ± 82.07 | 154.77 ± 86.47 | d0.253 |
| Total Bilirubin [mg/dl] | 0.50 ± 0.19 | 0.46 ± 0.19 | d0.181 |
| ALT [U/L] | 21.00 (23.5) | 23.00 (21.0) | e0.806 |
| ALP [U/L] | 92.98 ± 27.27 | 91.50 ± 27.70 | d0.659 |
| Sodium [mEq/L] | 136.70 ± 6.27 | 137.61 ± 5.60 | d0.204 |
| BUN [mg/dL] | 16.13 ± 7.01 | 13.78 ± 5.33 | d0.003 |
| Creatinine [mg/dL] | 1.09 ± 0.33 | 1.08 ± 0.35 | d0.802 |
| WBC count [×103/μL] | 11.20 ± 5.5 | 10.10 ± 4.0 | d0.021 |
| Neutrophil [×103/μL] | 6.94 ± 1.33 | 6.74 ± 1.12 | d0.170 |
| Lymphocyte [×103/μL] | 2.12 ± 1.01 | 2.44 ± 0.98 | d0.010 |
| Hemoglobin [gm/dl] | 12.40 ± 2.07 | 12.54 ± 2.28 | d0.630 |
| RBC count [×106/μL] | 4.58 ± 0.85 | 4.64 ± 0.76 | d0.550 |
| Hematocrit [%] | 37.41 ± 6.28 | 37.94 ± 6.20 | d0.491 |
| Platelet count [×103/μL] | 307.47 ± 104.12 | 280.94 ± 102.69 | d0.036 |
| MPV [fL] | 10.41 ± 1.26 | 10.41 ± 1.25 | d0.965 |
| RDW [%] | 15.12 ± 1.78 | 14.98 ± 1.92 | d0.566 |
| WMR | 1056.95 (542.80) | 967.40 (448.40) | e0.008 |
| PLR | 147.68 (122.10) | 109.50 (68.7) | e<0.001 |
| NLR | 3.55 (3.10) | 2.70 (2.13) | e0.034 |
| Follow up features, n (%) | |||
| Cardiogenic shock | 18 (17.6) | 13 (6.7) | b0.003 |
| GI Bleeding | 14 (13.7) | 7 (3.6) | b0.001 |
| Dialysis | 2 (2.0) | 4 (2.1) | b0.661 |
| Transfusion | 18 (17.6) | 16 (8.2) | b0.015 |
| CABG | 16 (15.7) | 15 (7.7) | b0.032 |
| Stent placement, | 36 (35.3) | 52 (26.7) | b0.122 |
| Access site complication | 0 (0.0) | 3 (1.5) | c0.554 |
| Duration of hospital stay [days] | 7.00 (5.3) | 7.00 (5.0) | e0.496 |
| TIMI scoreSTEMI | 4.56 ± 1.58 | 4.53 ± 1.88 | d0.928 |
| TIMI scoreNSTEMI/UA | 3.69 ± 1.31 | 3.86 ± 1.37 | d0.428 |
| Diagnosis, n (%): | b0.985 | ||
| NSTEMI | 27 (26.5) | 52 (26.7) | |
| STEMI | 40 (39.2) | 78 (40.0) | |
| UA | 35 (34.3) | 65 (33.3) | |
All values are presented as mean ± standard deviation, median (IQR) and frequency (percentages). BP—blood pressure; CK-MB—creatine kinase MB isoenzyme; HTN— Hyper tension; CAD— coronary artery disease; CABG— coronary artery bypass grafting; PCI— percutaneous coronary intervention; HDL—high density lipoprotein; LDL—low density lipoprotein; MI—myocardial infarction; NYHA—New York Heart Association; LDH—Lactate dehydrogenase; AST—Aspartate Aminotransferase; ALT—Alanine transaminase; ALP—Alkaline phosphatase; MPV—mean platelet volume; RDW—Red Cell Distribution Width; WMR—; PLR— platelets to lymphocytes ratio; NLR— neutrophils to lymphocytes ratio; NSTEMI—non ST elevation myocardial infarction;STEMI— ST elevation myocardial infarction; UA—unstable angina; BUN— blood urea nitrogen; Cr—creatinine; TIMI— thrombolysis in myocardial infarction.
p values <0.05 were considered statistically significant.
chi-square test.
Fisher-exact test.
Independent t-test.
Mann-Whitney test was used to compare quantitative data without normal distribution.
3.1. Diagnostic and survival analysis
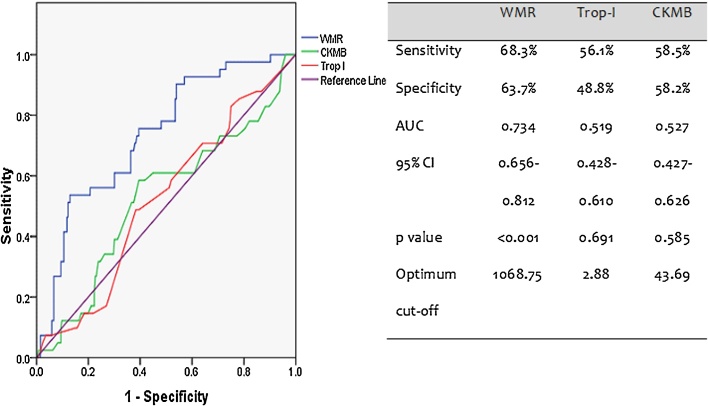
According to the ROC curve analysis, the AUC of WMR was 0.7 (95% confidence interval (CI) 0.7–0.8, p < 0.001) which was greater than those of creatine kinase MB (CK-MB) and troponin I. WMR had the highest discriminative ability to predict short term mortality than two of the most specific cardiac enzymes (Fig. 1).
Fig. 1.
Receiver operating characteristics curve predicting prognostic ability of the biochemical markers.
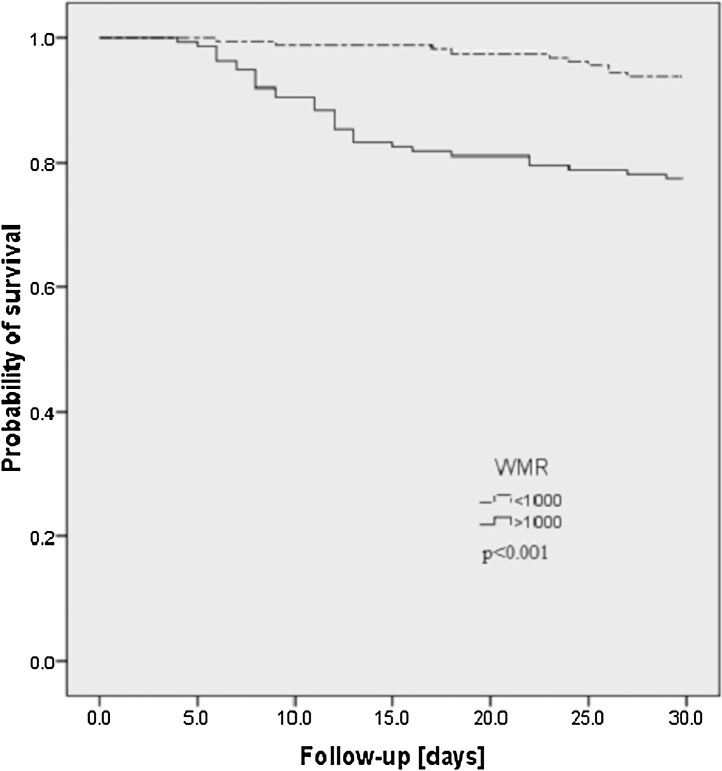
Kaplan-Meier survival curve showed that the mortality rate was significantly higher in patients with WMR > 1000 than those with WMR ≤ 1000 (22.6% [31/137] vs 6.3% [10/160]) (log-rank test, p value < 0.001) (Fig. 2).
Fig. 2.
Kaplan-Meier survival curve for patients with short-term (30 days) mortality in patients with WMR > 1000 and low WMR ≤ 1000.
3.2. Multivariate analysis
MI (HR 9.7, 95% CI 4.2–22.5, p < 0.001), GI bleeding (HR 7.4, 95% CI 2.5–22.3, p < 0.001), prior CABG (HR 4.1, 95% CI 1.5–11.4, p = 0.006) and NODV > 1 (HR 3.0, 95% CI 1.1–8.0, p = 0.03) were independent predictors of short-term mortality. Moreover, age (HR 1.0, 95% CI 0.9–1.0, p = 0.02), MPV (HR 0.7, 95% CI 0.5–0.8, p = 0.002), neutrophils (HR 1.4, 95% CI 1.0–1.9, p = 0.03), PLR (HR 1.0, 95% CI 1.001–1.008, p = 0.02) and LVEF (HR 0.9, 95% CI 0.9–1.0, p = 0.001) were also associated with mortality. Furthermore, WMR > 1000 (HR 2.9, 95% CI 1.3–6.5, p = 0.01) was the strongest biochemical marker in predicting mortality (Table 3).
Table 3.
Multivariate Cox regression analysis identifying the independent predictors of short-term mortality.
| Variables | HR | 95% CI | p-value |
|---|---|---|---|
| Age | 0.957 | 0.924–0.991 | +0.015 |
| Area* (Rural) | 4.788 | 2.045–11.209 | +<0.001 |
| MPV | 0.650 | 0.498–0.848 | +0.002 |
| Neutrophils | 1.420 | 1.040–1.938 | +0.027 |
| PLR | 1.004 | 1.001–1.008 | +0.017 |
| LDH | 1.003 | 1.000–1.005 | 0.051 |
| LVEF | 0.944 | 0.911–0.978 | +0.001 |
| Re hospitalization* | 0.357 | 0.145–0.882 | +0.026 |
| MI* | 9.696 | 4.186–22.459 | +<0.001 |
| GIB* | 7.407 | 2.456–22.340 | +<0.001 |
| CABG* | 4.122 | 1.488–11.419 | +0.006 |
| STENT* | 2.050 | 0.976–4.305 | 0.058 |
| NODV>1* | 3.030 | 1.149–7.993 | +0.025 |
| WMR>1000* | 2.910 | 1.293–6.548 | +0.010 |
+p < 0.05 was considered statistically significant.
For discrete variables, 0 = absence, 1 = presence. MPV: mean platelet volume; PLR: platelet to lymphocyte ratio; LDH: lactate dehydrogenase; LVEF: left ventricular ejection fraction; MI: myocardial infarction GIB: gastrointestinal tract bleeding; CABG: coronary artery bypass grafting; NODV: number of diseased vessel WMR: wbc to mean platelet volume ratio.
4. Discussion
This is the first study to assess the prognostic role of CBC, particularly WMR, in determining the short-term mortality and incidence of MACE in patients admitted for ACS in South East Asia. In this study, we found that an elevated WMR on admission, was associated with a substantial rise in the risk of MACE, and was the most accurate marker amongst all CBC components in predicting short-term mortality.
Apart from WMR, other blood count indices such as neutrophil count, RDW, MPV, high NLR and high PLR have been reported to be effective predictors of cardiovascular morbidity and mortality.17, 18, 19, 20, 21 However in our study we found WMR to be a stronger marker than MPV, WBC, NLR, PLR and RDW. Although there are various laboratory investigations to measure platelet activity and other inflammatory markers, they have not been adopted for routine clinical practice. This may be due to insufficient data about the ideal investigation and technique, unknown cut-off values for categorizing patients based on risk, and the uncertainty about the analysis and clinical efficacy of results.22 Our objective was to highlight the value of a simple baseline investigation rather than other, costlier and clinically unavailable options.
Our results illustrate that admission MPV level is not a predictor of cardiovascular events at 30 days of follow up. This is similar to another study which found no relationship between elevated baseline MPV and prognosis and incidence of MACE in ACS patients.8 However, a study done by Estévez-Loureiro et al.23 found that raised MPV is an independent predictor of 30-day mortality in patients with STEMI undergoing primary PCI. MPV is a potentially useful platelet activation marker which is widely available and easily measured hematologic parameter in a CBC test. There has been a wide array of results in previous literature, with some studies proving admission MPV to be a useful prognostic tool23, 24 in patients with ACS, while others completely disproving it.25 Vagdatli et al.26 stated that platelet distribution width (PDW), a measure of variability in platelet size, is a more specific marker of platelet activation than MPV. Therefore, MPV may not be used in the clinical setting for short term outcomes due to its lesser specificity and discriminative ability as compared to other CBC components.
An increased leukocyte count is a prominent indicator of compromised microvascular reperfusion. Prior ACS studies have demonstrated the use of NLR as an admission prognostic marker and a risk stratification tool of adverse outcomes,27, 28 albeit there have been conflicting results in terms of the most appropriate leukocyte subtype.29, 30, 31, 32 He and colleagues33 reported that average NLR was a useful and powerful predictor of mortality and in-hospital cardiovascular events in Chinese patients presenting with STEMI, thus providing additive predictive value to conventional risk factors and commonly used biomarkers e.g. C-reactive protein (CRP). Similarly, Barron et al.34 presented that acute MI patients with a leukocyte count in the highest quintile had a higher 30-day mortality rate than patients with a leukocyte count in the lower quintiles. In our study, we found that WBC count was associated with MACE incidence at 30-day follow up, but it did not predict short term mortality.
Contrary to previous literature, we found that WMR holds greater predictive value over total WBC count and other leukocyte differentials as it incorporates two of the most prominent inflammatory mediators, namely MPV and WBC count, into a single value. MPV is a measure of platelet size, and larger platelets are known to be enzymatically more active and have a greater thrombotic potential thus are more likely to occlude the coronary vessels.35 Whereas leukocytes may cause oxidative and proteolytic damage, induce hypercoagulability and activate tissue factor. These mechanisms may lead to thrombus formation and infarct expansion.36 These findings are consistent with the studies conducted by Dehghani et al.8, 37 who found that WMR is a better predictor of long term mortality and incidence of adverse outcomes in patients with NSTEMI than WBC and MPV. A similar analysis performed by Çiçek G and colleagues38 demonstrated the superiority of elevated WMR levels on admission, in predicting long term mortality and MACE incidence in STEMI patients as compared to other CBC components, such as MPV, RDW, PLR-NLR and WBC-MPV combinations. Our results show that WMR had a higher sensitivity to predict short term outcomes than two of the most specific cardiac enzymes, namely CK-MB and Troponin I. Moreover, cardiac enzymes may take a few hours before appearing in the serum; therefore, calculating WMR is not only sensitive but a faster and a more efficient marker.
Additionally, patients in group B had a greater incidence of STEMI and NSTEMI while UA was more common in the group A. We speculate that group B patients may have had a larger infarct size or multi-vessel infarcts as shown by higher levels of leukocyte count, CK and Troponin I, hence a more severe cardiac manifestation. Our results further illustrate that patients with a higher WMR level were associated with elevated LFTs, BUN and Cr indicating greater hepatic and renal damage and dysfunction.
As the evaluation of WMR is inexpensive, quick, and accessible in nearly every laboratory worldwide, it proves to be a useful tool for rapid categorization of patients into risk groups. It is of utmost importance that high risk patients are identified and segregated from low risk patients for not only mortality and MACE assessment, but also due to economic constraints. Clinical facilities and funds allocated to the healthcare system in many developing countries are limited. With this differentiation, patients with less urgent needs can be discharged quicker, freeing up space and medical attention for high risk patients. Furthermore, patient risk stratification helps healthcare personnel in making early and accurate therapeutic decisions. In patients with high WMR levels, a more vigorous and aggressive approach is used in terms of treatment and in-hospital monitoring of treatment outcome. In this high-risk category, clinicians can also schedule more frequent follow up visits to rule out any late cardiac complications. Moreover, the clinical significance of a simple yet accurate indicator is most prominent in the underdeveloped cities and rural areas, where other conventional markers such as interleukins and CRP are not attainable.
Our study does have several limitations which need to be addressed. Firstly, we conducted a single centre study using a non-randomized sampling technique thus yielding a small sample size. Secondly, we conducted CBC once rather than serial testing at regular time-intervals. Furthermore, we did not measure other, more specific pro-inflammatory markers including P-selectin, high-sensitivity C-reactive protein, interleukins, selectin molecules, adhesion ligands and receptors, and markers of oxidative stress to show any comparison between WMR and such biomarkers. Further studies are required to validate these results and define the exact role of these parameters in the clinical front. The interaction of the TIMI-STEMI risk score with these prognostic measures may also be an area of interest for future investigations.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The author(s) declare(s) that there is no conflict of interest.
Acknowledgement
None.
References
- 1.Sanchis-Gomar F., Perez-Quilis C., Leischik R. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:1–12. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarkees M.L., Bavry A.A. Acute coronary syndrome (unstable angina and non-ST elevation MI) Clin Evid (Online) 2009;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Davies M.J., Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984;310(18):1137–1140. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- 4.Davì G., Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 5.Botto N., Sbrana S., Trianni G. An increased platelet–leukocytes interaction at the culprit site of coronary artery occlusion in acute myocardial infarction: A pathogenic role for no-reflow phenomenon? Int J Cardiol. 2007;117:123–130. doi: 10.1016/j.ijcard.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan Z.S., Jackson S.P. The role of platelets in atherothrombosis. ASH Educ Progr Book. 2011:51–61. doi: 10.1182/asheducation-2011.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Chu S.G., Becker R.C., Berger P.B. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dehghani M.R., Rezaei Y., Taghipour-Sani L. White blood cell count to mean platelet volume ratio as a novel non-invasive marker predicting long-term outcomes in patients with non-ST elevation acute coronary syndrome. Cardiol J. 2015;22:437–445. doi: 10.5603/CJ.a2015.0015. [DOI] [PubMed] [Google Scholar]
- 9.Menon V., Lessard D., Yarzebski J. Leukocytosis and adverse hospital outcomes after acute myocardial infarction. Am J Cardiol. 2003;92:368–372. doi: 10.1016/s0002-9149(03)00651-9. [DOI] [PubMed] [Google Scholar]
- 10.Tonelli M., Sacks F., Arnold M. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 11.Azab B., TorbeyE. Singh J. Mean platelet volume/platelet count ratio as a predictor of long-term mortality after non-ST-elevation myocardial infarction. Platelets. 2011;22:557–566. doi: 10.3109/09537104.2011.584086. [DOI] [PubMed] [Google Scholar]
- 12.Slavka G., Perkmann T., Haslacher H. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol. 2011;31:1215–1218. doi: 10.1161/ATVBAHA.110.221788. [DOI] [PubMed] [Google Scholar]
- 13.Pellizzon G.G., Dixon S.R., Stone G.W. Relation of admission white blood cell count to long-term outcomes after primary coronary angioplasty for acute myocardial infarction (The Stent PAMI Trial) Am J Cardiol. 2003;91:729–731. doi: 10.1016/s0002-9149(02)03416-1. [DOI] [PubMed] [Google Scholar]
- 14.Mello B.H., Oliveira G.B., Ramos R.F. Validation of the Killip-Kimball classification and late mortality after acute myocardial infarction. Arq Bras Cardiol. 2014;103(2):107–117. doi: 10.5935/abc.20140091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raphael C., Briscoe C., Davies J. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93:476–482. doi: 10.1136/hrt.2006.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antman E.M., Cohen M., Bernink P.J. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 17.Tamura A., Watanabe T., Nasu M. Association between neutrophil counts on admission and left ventricular function in patients successfully treated with primary coronary angioplasty for first anterior wall acute myocardial infarction. Am J Cardiol. 2001;88:678–680. doi: 10.1016/s0002-9149(01)01815-x. [DOI] [PubMed] [Google Scholar]
- 18.Elbasan Z., Gür M., Şahin D.Y. Association of mean platelet volume and pre-and postinterventional flow with infarct-related artery in ST-segment elevation myocardial infarction. Angiology. 2013;64:440–446. doi: 10.1177/0003319712455685. [DOI] [PubMed] [Google Scholar]
- 19.Uyarel H., Ergelen M., Cicek G. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2011;22:138–144. doi: 10.1097/MCA.0b013e328342c77b. [DOI] [PubMed] [Google Scholar]
- 20.Şahin D.Y., Elbasan Z., Gür M. Neutrophil to lymphocyte ratio is associated with the severity of coronary artery disease in patients with ST-segment elevation myocardial infarction. Angiology. 2013;64:423–429. doi: 10.1177/0003319712453305. [DOI] [PubMed] [Google Scholar]
- 21.Yayla Ç, Akboğa M.K., Canpolat U. Platelet to lymphocyte ratio can be a predictor of infarct-related artery patency in patients with ST-segment elevation myocardial infarction. Angiology. 2015;66(9):831–836. doi: 10.1177/0003319715573658. [DOI] [PubMed] [Google Scholar]
- 22.Wurtz M., Lerkevang Grove E. Interindividual variability in the efficacy of oral antiplatelet drugs: definitions, mechanisms and clinical importance. Curr Pharm Des. 2012;18:5344–5361. doi: 10.2174/138161212803251925. [DOI] [PubMed] [Google Scholar]
- 23.Estévez-Loureiro R., Salgado-FernándezJ, Marzoa-Rivas R. Mean platelet volume predicts patency of the infarct-related artery before mechanical reperfusion and short-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Thromb Res. 2009;124:536–540. doi: 10.1016/j.thromres.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Goncalves S.C., Labinaz M., Le May M. Usefulness of mean platelet volume as a biomarker for long-term outcomes after percutaneous coronary intervention. Am J Cardiol. 2011;107:204–209. doi: 10.1016/j.amjcard.2010.08.068. [DOI] [PubMed] [Google Scholar]
- 25.Shah B., Oberweis B., Tummala L. Mean platelet volume and long-term mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2013;111:185–189. doi: 10.1016/j.amjcard.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vagdatli E., Gounari E., Lazaridou E. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14:28–32. [PMC free article] [PubMed] [Google Scholar]
- 27.D’Ascenzo F., Biondi-Zoccai G., Moretti C. TIMI, GRACE and alternative risk scores in Acute Coronary Syndromes: a meta-analysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemp Clin Trials. 2012;33:507–514. doi: 10.1016/j.cct.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Brkovic V., Dobric M., Beleslin B. Additive prognostic value of the SYNTAX score over GRACE, TIMI, ZWOLLE, CADILLAC and PAMI risk scores in patients with acute ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Int J Cardiovasc Imaging. 2013;29:1215–1228. doi: 10.1007/s10554-013-0202-1. [DOI] [PubMed] [Google Scholar]
- 29.Dehghani M.R., Rezaei Y., Taghipour-Sani L. Superiority of totalwhite blood cell count over other leukocyte differentials forpredicting long-term outcomes in patients with non-ST elevation acute coronary syndrome. Biomarkers. 2014;19:378–384. doi: 10.3109/1354750X.2014.915429. [DOI] [PubMed] [Google Scholar]
- 30.Horne B.D., Anderson J.L., John J.M. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 31.Sabatine M.S., Morrow D.A., Cannon C.P. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 substudy. J Am Coll Cardiol. 2002;40:1761–1768. doi: 10.1016/s0735-1097(02)02484-1. [DOI] [PubMed] [Google Scholar]
- 32.Huang G., Zhong X.N., Zhong B. Significance of white blood cell count and its subtypes in patients with acute coronary syndrome. Eur J Clin Invest. 2009;39:348–358. doi: 10.1111/j.1365-2362.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 33.He J., Li J., Wang Y. Neutrophil-to-lymphocyteratio (NLR) predicts mortality and adverse-outcomes afterST-segment elevation myocardial infarction in Chinese people. Int J Clin Exp Pathol. 2014;7:4045–4056. [PMC free article] [PubMed] [Google Scholar]
- 34.Barron H.V., Harr S.D., Radford M.J. The association between white blood cell count and acute myocardial infarction mortality in patients >or ¼ 65 years of age: findings from the cooperative cardiovascular project. J Am Coll Cardiol. 2001;38(6):1654–1661. doi: 10.1016/s0735-1097(01)01613-8. [DOI] [PubMed] [Google Scholar]
- 35.Endler G., Klimesch A., Sunder-Plassmann H. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2002;117:399–404. doi: 10.1046/j.1365-2141.2002.03441.x. [DOI] [PubMed] [Google Scholar]
- 36.Dharma Surya, Hapsari Rosmarini, Bambang B. Blood leukocyte count on admission predicts cardiovascular events in patients with acute non-ST elevation myocardial infarction. Int J Angiol. 2015;24:127–132. doi: 10.1055/s-0035-1544178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dehghani M.R., Rezaei Y., Fakour S. White blood cell count to mean platelet volume ratio is a prognostic factor in patients with non-ST elevation acute coronary syndrome with or without metabolic syndrome. Korean Circ J. 2016;46:229–238. doi: 10.4070/kcj.2016.46.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Çiçek G., Açıkgöz S.K., Yayla Ç. White blood cell count to mean platelet volume ratio: a novel and promising prognostic marker for ST-segment elevation myocardial infarction. Cardiol J. 2016;23(3):225–235. doi: 10.5603/CJ.a2016.0001. [DOI] [PubMed] [Google Scholar]