Abstract
Cardiotoxicity is the most serious side effect of anthracyclines (doxorubicin, daunorubicin or epirubicin). The incidence of anthracycline induced late cardiac toxicity (AIC) that is overt clinically is 3–5% in the Indian population. Polymorphism in intron 32 (deletion of 25 bp) of MYBPC3 has been shown to be present exclusively in Asians and more so in South India (3–8%). The frequency of the polymorphism is significantly higher (13%) in patients with cardiomyopathy in India. Fifteen patients were identified to have cardiac dysfunction following treatment for malignant lymphoma with doxorubicin containing regimens. Peripheral blood DNA from control, amplified by polymerase chain reaction yielded a 467 bp fragment while in the presence of the 25 bp deletion only a 442 bp fragment was detected. To confirm the presence or absence of the polymorphism, amplified DNA was restricted using Bgl1 in all samples. Bgl1 restricted amplified DNA only if the 25 bp deletion was absent. A 467 base pair band was observed in all the 15 samples, which suggested the absence of polymorphism in MYBPC3. In a sample of DNA from a patient with a deletion in exon 33 (confirmed by sequencing) a 442 bp fragment was detected. Amplified DNA from this patient was not restricted with Bgl1. Wild type MYBPC3 when amplified gave a distinct restriction banding pattern consisting of two bands of 401 bp and 66 bp. Amplified DNA from all peripheral blood samples restricted with Bgl1 suggesting the absence of the polymorphism. In this preliminary report, MYBPC3 does not seem to play a role in anthracycline induced cardiotoxicity.
Keywords: MYBPC3, Polymorphism, Anthracycline, Cardiotoxcity
1. Introduction
Anthracyclines represent one of the most commonly used drugs in the treatment of cancer. The major side effects associated with anthracyclines are bone marrow suppression and cardiac toxicity. Cardiac toxicity can be acute and reversible on cessation of the drug.1 In some patients the toxicity is delayed and presents as heart failure due to cardiomyopathy.2 It is also clear that there is a dose related cardiac toxicity. This is variable depending on the drug. The dose at which there is a linear increase in cardiac toxicity for doxorubicin is 450 mg/m2, for daunorubicin at 550 mg/m2 and for Epirubicin at 900 mg/m2. Currently, there are no biomarkers available to predict cardiotoxicity and the usual cardiac enzymes like CK-Mb and Troponin-I were found to be ineffective.3 The antitumor effects of anthracyclines are mediated by inhibition of DNA synthesis through direct interactions with DNA (intercalation) and inhibition of topoisomerase II (Top II).4 Top II has two isoenzymes in mammalian cells, Top IIα and Top IIβ. Doxorubicin or daunorubicin binds both DNA and Top IIα to form the topoisomerase IIα-doxorubicin-DNA ternary cleavage complex, which triggers cell death. These tumoricidal mechanisms are not likely to contribute to cardiac toxicity because Top IIα is overexpressed in tumor cells and is not detectable in quiescent cells.5 Cardiomyocytes are terminally differentiated cells with very low rates of proliferation. However, a recent study showed that cardiomyocyte-specific deletion of Top IIb (encoding Top IIβ) protects cardiomyocytes from doxorubicin-induced DNA double-strand breaks and transcriptome changes that are responsible for defective mitochondrial biogenesis and reactive oxygen species formation.4 Furthermore, cardiomyocyte-specific deletion of Top IIβ protects mice from the development of doxorubicin-induced progressive heart failure.4
Although the specific mechanisms of action are not completely understood, these agents exert their toxic effects by causing apoptosis, decreased expression of cardiomyocyte-specific genes, and/or altered molecular signaling in cardiomyocytes. However, there have been a few reports to identify genetic factors that predispose to anthracycline related cardiotoxicity.6 These may be particularly important in India where incidence of diabetes mellitus and coronary artery disease is higher compared to the West after adjustment of common risk factors. Further, cardiac toxicity at lower cumulative doses of anthracyclines in the absence of pre-existing cardiac disease is poorly understood. In this context, a polymorphism that is exclusively identified in the Indian population and is associated with cardiomyopathy needs to be investigated further.
MYBPC3 is a gene which has been shown to be causally associated with a type of heritable cardiomyopathy in Indian patients.7 It maps to chromosomal arm 11p11.2 and encodes for Cardiac myosin binding protein C (cMyBP-C), a key constituent of the thick filaments localized to doublets in the C-zone of the A- band of the sarcomere, the basic unit of muscle contraction.8 More than 30 polymorphisms have been described in MYBPC3, but a specific 25 bp deletion in the intron 32 is associated with increased risk of heart failure due to cardiomyopathy in Indian patients. This deletion occurs at a frequency of 13.8% (13% heterozygotes and 0.8% homozygotes) in Indian patients with hypertrophic cardiomyopathy and only in 2.9% (heterozygotes) of healthy control individuals.7 Interestingly, the frequency of this 25 bp deletion in MYBPC3 gene was higher in the Southern states in this study (4% vs. 0% in Central and North India).7 The frequency of this polymorphism in normal individuals was variable between states in India (2–8%) and has not been identified so far in the Western hemisphere.
Deletion of 25 bp in intron 32 at the branch point causes skipping of Exon 33. The loss of Exon 33 is associated with a shift in the open reading frame and an unscheduled translational stop in the 3′ non-translated sequence. The consequence of MYBP-C exon 33 skipping (due to the 25 bp deletion) is the substitution in the domain C10 of 62 amino acids in the wild type with a different 55 amino acids in the mutant.7 This common polymorphism in MYBPC3 is associated with chronic risk of heart failure. The delayed symptoms, mild hypertrophy and influence of secondary risk factors pose a lifelong threat to carriers.7 We wished to evaluate the frequency of this polymorphism in patients with anthracycline related cardiotoxicity, to assess whether it could be a predisposing factor.
2. Materials and methods
The Institutional Ethics committee of Cancer Institute, Adyar, approved the present study. Informed consent was taken from each patient. In the present study, a total of 15 patients with anthracycline related cardiotoxicity were evaluated. All patients were treated for malignant lymphoma with regimens that contained doxorubicin and had completed therapy and were on follow up. To exclude other confounding factors patients with preexisting heart disease were excluded. Only patients who had a drop in ejection fraction of approximately 10% during or after treatment with or without overt signs of cardiac failure were selected. A sample of peripheral blood was obtained from all patients. Genomic DNA was extracted from the peripheral blood of all subjects using standard protocol with phenol and chloroform.9 Genotyping for the MYBPC3 25 bp deletion in the Intron 32 was carried out using Polymerase Chain reaction (PCR) followed by restriction digestion. A total of 100 ng genomic DNA was PCR amplified in a 25 μl volume reaction as follows: 0.5 μl of Taq polymerase enzyme (5000U/ml, NEB), 0.25 μl of 10 mM dNTP’s (SIGMA), 2.5 μl of 1X standard Taq buffer and 1 μl of 10 μM of each primer Forward (5′-CACCTATGAGCCACCCAACT-3′) and reverse (5′-GCCATTCTTGAACCAGGAAA-3′) (SIGMA) in a total volume of 25 μl. For each experiment a control without template was used to exclude any non-specific amplification.
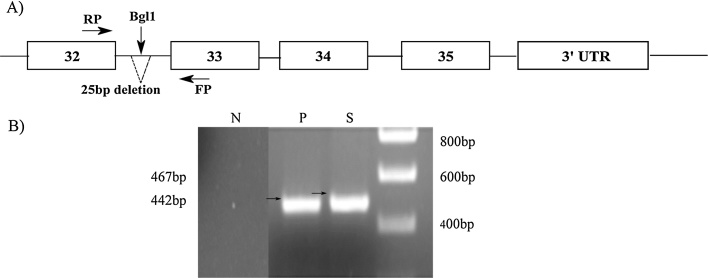
The amplifications were performed after initial denaturation and Taq polymerase activation at 94 °C for 5 min followed by 35 cycles with 94 °C for 45 s, 60 °C for 45 s and 72 °C for 45 s, with an additional step of final extension at 72 °C for 10 min. PCR amplicons were restricted using Bgl1 enzyme to confirm the presence of 25 bp deletion. Since the enzyme sites are located in the regions flanking the deletion, restriction occurs only if the gene is wild type, giving two bands of 401 bp and 66 bp, respectively. If there is a 25 bp deletion in the intron 32 of MYBPC3 gene (Fig. 1), the enzyme cannot restrict the amplified fragment. The PCR fragments and restriction products were resolved on 2% agarose gel. A sample of control DNA from a patient who had a deletion in intron 32 confirmed by sequencing was obtained from Medgenome.10
Fig. 1.
MYBPC3 gene amplification using PCR.
A) Schematic representation of MYBPC3 gene showing the 25 bp deletion and primers used for PCR amplification.
B) P represents positive control DNA.
S represents wild type DNA amplicon without deletion and N represents negative control.
3. Results
3.1. Patients
All the patients were treated for malignant lymphoma and had cardiotoxicity during or after treatment. The clinical details including type of treatment and cardiac morbidity is shown (Table 1a, Table 1b). All the patients had completed treatment and were on follow up.
Table 1a.
Clinical features of patients.
| S.No | Age | SEX | COMORBIDITIES | DIAGNOSIS | STAGE | TYPE OF CHEMO-THERAPY | MED RT | DOSE RT | STATUS | LFUP | RECURRENCE | SALVAGE CHEMO | DOD | CAUSE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | M | NIL | FL | 4 | CHOP | NIL | NIL | Dead | 29.09.12 | 07.06.12 | 0 | 29.9.12 | Cancer |
| 2 | 51 | M | " | FL | 4 | " | " | " | CR | 28.09.14 | " | Alive | ||
| 3 | 53 | F | " | DLBCL | 3 | " | " | " | " | 31.12.15 | " | Alive | ||
| 4 | 54 | F | " | " | 1 | " | " | " | " | 24.10.14 | " | Alive | ||
| 5 | 21 | M | " | " | 3 | " | " | " | " | 13.10.15 | " | Alive | ||
| 6 | 67 | M | " | " | 1 | " | " | " | " | 21.10.14 | " | Alive | ||
| 7 | 44 | M | " | " | 4 | " | " | " | " | 23.12.15 | " | Alive | ||
| 8 | 55 | M | " | " | 1 | " | " | " | " | 02.12.14 | " | Alive | ||
| 9 | 50 | M | " | " | 2 | " | Yes | 30 | " | 03.02.15 | " | Alive | ||
| 10 | 43 | F | Diabetes | FL | 3 | " | NIL | NIL | " | 02.04.15 | " | Alive | ||
| 11 | 66 | M | HIV | DLBCL | 3 | " | " | " | " | 04.11.15 | " | Alive | ||
| 12 | 16 | M | NIL | HL | 3 | ABVD | " | " | " | 11.3.14 | " | Alive | ||
| 13 | 12 | M | " | " | 4 | Hybrid | " | " | " | 11.12.15 | " | Alive | ||
| 14 | 40 | F | " | " | 4 | ABVD | " | " | " | 24.12.14 | " | Alive | ||
| 15 | 14 | F | " | " | 2 | ABVD | " | " | " | 18.04.15 | " | Alive |
FL: Follicular.
DLBCL: Diffuse Large B-cell Lymphoma.
ABVD: Adriamycin, Bleomycin, Vinblastine, Dacarbazine.
CHOP: Cyclophosphamide, Hydroxydaunorubicin, Oncovin, Prednisone.
Med RT: Mediastinal radiation therapy.
DOD: Date of death.
CR: Complete remission.
Table 1b.
Details of cardiac toxicity.
| S. No. | TYPE OF CHEMO-THERAPY | DATE OF CHEMO-THERAPY | CYCLES | ADRIAMYCIN TOTAL DOSE | ECG AT DYSFUNCTION | EJECTION FRACTION |
STATUS | DOSE ADJUSTMENT | PHARMACOLOGICAL INTERVENTION | RESPONSE | SYMPTOMS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre(%) | Post(%) | |||||||||||
| 1 | CHOP | 19.9.07 | 6 | 300 | Normal | 74 | 48 | ACUTE | NIL | Yes | Improved | Symptomatic |
| 2 | " | 16.09.05 | 6 | 300 | ST-T changes | 71 | 55 | " | " | No | " | " |
| 3 | " | 21.12.07 | 8 | 400 | " | 65 | 32 | " | " | Yes | " | " |
| 4 | " | 16.9.06 | 6 | 300 | Normal | 75 | 63 | " | " | No | " | Asymptomatic |
| 5 | " | 25.12.07 | 8 | 400 | " | 73 | 64 | " | " | No | No improvement | " |
| 6 | " | 9.10.07 | 6 | 300 | " | 76 | 67 | " | " | No | Improved | " |
| 7 | " | 16.07.11 | 8 | 400 | ST-T changes | 73 | 63 | CHRONIC | " | No | " | " |
| 8 | " | 6.4.10 | 8 | 400 | " | 70 | 57 | ACUTE | " | Yes | " | Symptomatic |
| 9 | " | 11.12.12 | 8 | 400 | Normal | 68 | 48 | " | " | Yes | " | " |
| 10 | " | 6.2.15 | 3 | 150 | " | 61 | 42 | " | Yes | Yes | No improvement | " |
| 11 | " | 8.5.14 | 6 | 300 | ST-T changes | 78 | 32 | " | NIL | Yes | " | Asymptomatic |
| 12 | ABVD | 30.9.10 | 8 | 350 | Normal | 80 | 69 | " | " | No | Improved | Asymptomatic |
| 13 | Hybrid | 12.7.11 | 8 | 210 | " | 69 | 60 | " | " | No | " | " |
| 14 | ABVD | 20.12.10 | 8 | 400 | ST-T changes | 60 | 40 | " | " | Yes | No improvement | Symptomatic |
| 15 | ABVD | 19.3.06 | 8 | 400 | Normal | 65 | 53 | " | " | No | Improved | Asymptomatic |
ABVD: Adriamycin, Bleomycin, Vinblastine, Dacarbazine.
CHOP: Cyclophosphamide, Hydroxydaunorubicin, Oncovin, Prednisone.
3.2. Analysis of MYBPC3
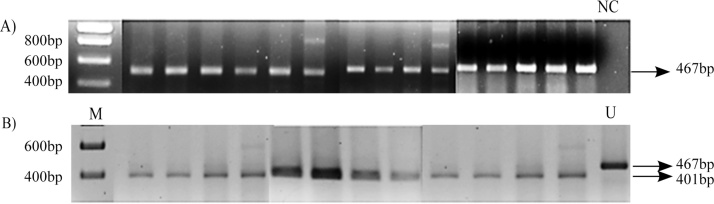
The DNA from the control and patients was amplified by PCR with appropriate primers around the deletion in intron 32 (Fig. 1). The PCR products were resolved on an agarose gel (2%). The DNA from a patient with a 25 bp deletion7 and a normal healthy control is shown (Fig. 2). This shows that there is a slight decrease in size of the product when there is a deletion in MYPBPC3. We next amplified samples from all patients and resolved products on an agarose gel. The DNA from all patients showed the correct size of 467 bp. As the difference in size when there is a deletion is quite small we digested all PCR products with Bgl1. All the products from patients were restricted with Bgl1 confirming the absence of any deletion (Fig. 2). The size of amplicon of wild type allele is 467 bp whereas the mutant allele is 442 bp. The wild type allele is cut by the restriction enzyme BglI and yields a 401 bp and 66 bp fragment product. The mutant allele on the contrary, does not have BglI restriction site producing the same 442 bp product.
Fig. 2.
MYBPC3 gene amplification and restriction digestion.
A) Gel electrophoresis of PCR products.
B) Restriction digestion of PCR product using Bgl1 enzyme.
M = Marker.
NC = Negative Control.
U = Undigested amplicon.
A 467 base pair band was observed in all the samples, which suggested the absence of polymorphism in MYBPC3 (25 bp deletion). This was further confirmed by restriction digestion analysis using BglI enzyme which gave a distinct restriction banding pattern if MYBPC3 was wild type (401 bp and 66 bp). In this group of patients, polymorphism in intron 32 of the MYBPC3 gene was not detected.
4. Discussion
The large variation in the development of anthracycline related cardiotoxicity suggests there could be genetic predisposing factors. The molecular analysis of mutations that is observed in cardiomyopathies due to anthracyclines has been pursued worldwide6. Among them are the identification of new mutations, the modes of transmission, the relative frequencies of genes involved, disease mechanisms or the role of genetic modifiers, which may vary depending on ethnic (or regional) background.11 Briefly, genetic variants that increase the development of cardiac toxicity include genes that encode for proteins affecting anthracycline pharmacokinetics, mechanisms of free radical deactivation, or of repair of the myocardial damage induced by anthracyclines. Polymorphisms in genes involved in intra-cellular transport of drug such as NADPH oxidase and MRP1 and 2 have been shown to confer higher risk of cardiotoxicity.12 Similarly polymorphisms in carbonyl anhydrase, HFE gene which is responsible for hemochromatosis, have also been shown to confer an increased risk.11
In 2003, four different mutations (two in MYH7 and 2 in MYBPC3) that is associated with heritable cardiomyopathy were identified.13 The mutations identified in MYH7 were a single base exchange in codon 712 (Arg–Leu) and a 3 bp deletion in position 927. Both mutations in MYBPC3 were deletions. One was a 2 bp deletion in exon 16 and the other was 25 bp deletion in the Intron 32. It was observed that the 25 bp deletion in the Intron 32 of MYBPC3 seemed to be the bonafide cause of Hypertrophic cardiomyopathy (HCM) in south Indian families13. Several population based studies were performed to associate the polymorphism in MYBPC3 with heritable cardiomyopathies. A large scale case control study of 6273 individuals belonging to 107 ethnic populations across 35 states representing all of India for the presence of this particular polymorphism (25 bp deletion in the Intron 32) was performed. The deletion was found in major Indian populations with a frequency ranging from 2 to 8%.7
The polymorphism (25 bp deletion), a common MYBPC3 variant is associated with chronic risk of heart failure. The delayed symptoms, mild hypertrophy and influence of secondary risk factors pose a lifelong threat to carriers. The high prevalence of this polymorphism in Indian populations offers an excellent opportunity to investigate the role of this deletion further in other diseases. This study failed to reveal any association between the polymorphism in MYBPC3 with anthracycline induced cardiotoxicity. However, this was not a case control study but examined the prevalence of this polymorphism in patients who had documented cardiotoxicity. This is a preliminary report and requires a larger number of patients before definite conclusions can be drawn. Polymorphisms of other myosin binding protein genes such as MYH7 located on chromosome 14 should also be studied as a risk factor along with MYBPC3. As anthracycline does not cause cardiotoxicity in all patients who receive it, host factors should be considered. This has been recently explored using genome wide association using a cohort study involving 385 patients exposed to anthracycline. This identified a novel locus near the gene PRDM2 that conferred increased risk for cardiotoxicity.14 Similarly, genes that are important in normal cardiac function and in causing cardiac muscle disease have to be systematically explored in patients with toxicity for identification of genetic factors. Ideally such a genetic variant has to be confirmed in a separate cohort. This preliminary report is a step in that direction.
Acknowledgements
We thank all the patients who helped with this study. We acknowledge staff in the tumor registry who helped us with collecting clinical data.
References
- 1.Visscher H., Ross C.J., Rassekh S.R. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M., Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Kilickap S., Barista I., Akgul E. TnT can be a useful marker for early detection of anthracycline cardiotoxicity. Ann Oncol. 2005;16:798–804. doi: 10.1093/annonc/mdi152. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S., Liu X., Bawa-Khalfe T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 5.Tewey K.M., Rowe T.C., Yang L. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226:466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- 6.Visscher H., Rassekh S.R., Sandor G.S. Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children. Pharmacogenomics. 2015;16:1065–1076. doi: 10.2217/pgs.15.61. [DOI] [PubMed] [Google Scholar]
- 7.Dhandapany P.S., Sadayappan S., Xue Y. A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat Genet. 2009;41:187–191. doi: 10.1038/ng.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niimura H., Bachinski L.L., Sangwatanaroj S. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N Engl J Med. 1998;338:1248–1257. doi: 10.1056/NEJM199804303381802. [DOI] [PubMed] [Google Scholar]
- 9.Moore D. Purification and concentration of DNA from aqueous solutions. Curr Protoc Immunol. 2001;1 doi: 10.1002/0471142735.im1001s8. [Chapter 10, Unit 10] [DOI] [PubMed] [Google Scholar]
- 10.Mahadevan L., Yesudas A., Sajesh P.K. Prevalence of genetic variants associated with cardiovascular disease risk and drug response in the Southern Indian population of Kerala. Indian J Hum Genet. 2014;20:175–184. doi: 10.4103/0971-6866.142896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mele D., Nardozza M., Spallarossa P. Current views on anthracycline cardiotoxicity. Heart Fail Rev. 2016;21:621–634. doi: 10.1007/s10741-016-9564-5. [DOI] [PubMed] [Google Scholar]
- 12.Wojnowski L., Kulle B., Schirmer M. AD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 13.Waldmuller S., Sakthivel S., Saadi A.V. Novel deletions in MYH7 and MYBPC3 identified in Indian families with familial hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2003;35:623–636. doi: 10.1016/s0022-2828(03)00050-6. [DOI] [PubMed] [Google Scholar]
- 14.Wells Q.S., Veatch O.J., Fessel J.P. Genome-wide association and pathway analysis of left ventricular function after anthracycline exposure in adults. Pharmacogenet Genomics. 2017;27:247–254. doi: 10.1097/FPC.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]