Abstract
Background
Anomalous origin of left coronary artery from pulmonary artery (ALCAPA) is a very rare congenital heart defect characterized by myocardial ischemia and ultimately scaring. The scar burden will determine eventual recovery of left ventricular function after corrective surgery.
Material method
All patients with proven diagnosis of ALCAPA and who underwent treatment at present centre were included. Detail echocardiography and cardiac magnetic resonance imaging (CMR) (delayed Gadolinium enhancement) was performed before and after surgery.
Results
There were 4 patients (3 females, age group 3 months to 3 yr, follow up 6 months to 20 months.) There was no peri operative mortality. All patients had significant improvement in symptom class and LVEF (increase of more than 10%) when evaluated at last follow up. Three patients had pre operative CMR and 3 post operative CMR. All patients had improvement in post operative LVEF, but >50% was observed only in one patient who had less than half thickness delayed gadolinium enhancement. The right coronary flow pattern were unique to disease. The left coronary flow pattern were had significant variation and could predict extent of scared myocardium.
Conclusion
Ischemia in ALCAPA can lead to myocardial scarring even in early infancy. The recovery in left ventricular function is a closely related to scar burden. Coronary flow patterns are unique and give useful insight into disease process and natural history.
1. MNUSCRIPT
Anomalous origin of left coronary artery from pulmonary artery (ALCAPA) leads to ongoing myocardial ischemia and scarring. Extent of pre operative myocardial scar will determine recovery in left ventricular function after surgical correction and long term prognosis. Cardiac magnetic resonance imaging with delayed gadolinium enhancement (CMR) is a well established diagnostic modality in adults to evaluate viability of myocardium and scar burden. To best of our knowledge there is a little work done in pediatric substrate in this respect particularly in ALCAPA.1, 2, 3, 4, 5, 6, 7, 8, 9
Changes in right coronary artery (RCA) pulse wave coronary flow pattern (PWCFP) are diagnostic for ALCAPA.1, 2, 3, 10, 11, 12 However, there is very little understanding about change in PWCFP of left coronary artery (LCA) as well as its prognostic significance. The present study tries to establish the extent of myocardial scar with CMR and correlate it with PWCFP of LCA.
2. Material and method
This is a single center observational study. Informed consent was obtained from all patients. All consecutive patients who underwent corrective surgery for ALCAPA were included. Baseline echocardiography was performed with Philips Epiq 7. Coronaries were assessed in modified short axis, parasternal long axis view and apical 4 chamber view. PWCFP was recorded at lowest Naquest limit with wall filter kept at low. CMR was performed with Philips 3T (Ingenia, Erlangen, Netherlands). Baseline and post Gadolinium enhanced CMR images were obtained. Pre surgical CMR was done on the day of surgery. Post surgical CMRs were done at interval of 7 days to 9 months. Improvement in LVEF more than 10% and functional class by I was defined as successful outcome
Off the 4 patients, 3 (age <1 yr) underwent left subclavian to LAD anastomosis. A 3 yr old girl underwent left internal mammary artery to LAD anastomosis. All surgeries were performed off pump. (without use of heart lung machine). The anastomotic site was fashioned close to left main coronary artery (LMCA) bifurcation so as to allow adequate left circumflex perfusion. LMCA was legated close to its origin from pulmonary artery. Post surgical CMR was performed using same protocol. The study has been approved by ethics committee of hospital.
3. Results
Patients characteristics, baseline and post operative echocardiography CMR findings and surgical details are described in Table 1. Coronary pulse wave Doppler are shown in Image 1. There were no perioperative deaths and none patient required extracorporal membrane oxygenation support. Mean time of ventilation was 45 hrs days (18 hrs day to 90 hrs days). Inotropic support was required for mean 67 hrs (27 hrs to 117 hrs,) There were no significant perioperative complications. None patient required ECMO support. There was improvement in left ventricular ejection fraction, grade of MR and functional class for all the patients. Except one patient all had residual scar on follow up. However, it reduced on follow up (Fig. 2, Fig. 3, Fig. 4).
Table 1.
Pre and post operative details.
| No | Age sex | Pre operative Echocardiography/Cardiac MR | Coronary flow on pulse wave | Operative details (off pump) | Post operative |
|---|---|---|---|---|---|
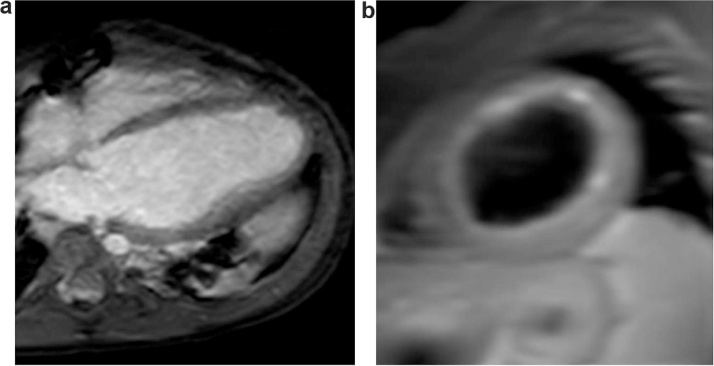
| 1 | 3 Yr F | LCA from facing sinus, no collaterals in IVS, LVEF 11%, moderate mitral regurgitation, Positive longitudinal strain of apical segments | Predominant systolic flow reversal and mild diastolic flow. (Fig. 1a)8 | Left internal mammary artery to LAD anastomosis and LMCA ligation | CMR (9 month) LVEF: 31%, trans mural enhancement of entire apex, anterior segments and mid and distal lateral wall. Mild MR (Fig. 4) |
| LDESV/LVEDV:173/194 ml/m2 | LDESV/LVEDV: 96/139 ml/m2 | ||||
| 2 | 4 mth F | LCA from non facing sinus. collaterals in IVS,Moderate MR, | Prominent diastolic (inverted U) and mild systolic flow reversal (Fig. 1b) | LMCA ligation and left subclavian artery to LAD anastomosis | Cardiac MR (4 weeks): LVEF 39%, more than 50% contrast enhancement of entire apex, mid anterior and apical lateral wall. No contrast enhancement of Basal and mid lateral segments and improvement in contractility. Mild MR |
| Cardiac MR: LVEF 16%, >50% contrast enhancement of apex, anterior and lateral wall | ECHO (15 months): LVEF 43%, apical segments hypokinetic, mild MR | ||||
| LDESV/LVEDV:150/179 ml/m2 | LDESV/LVEDV: 74/129 ml/m2 | ||||
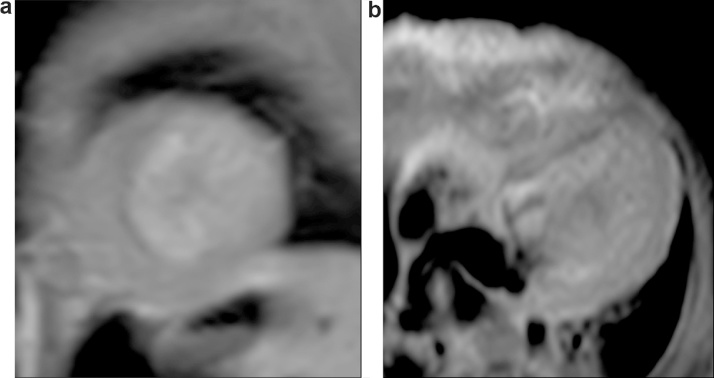
| 3 | 4 mth M | LCA from facing sinus., collaterals in IVS, severe MR, LVEF 28% | Prominent diastolic flow reversal (late diastolic peak). Insignificant systolic component (Fig. 1c) | LMCA ligation and Left Subclavian artery to LAD anastomosis | Cardiac MR: LVEF: 51%, no regional wall motion abnormality, moderate MR, subendocardial enhancement of apex and apical lateral wall. (1 week), (Fig. 3) |
| LDESV/LVEDV:131/182 ml/m2 | ECHO(16 month): LVEF 62%, good contractility of all segments, no MR | ||||
| LDESV/LVEDV: 61/123 ml/m2 | |||||
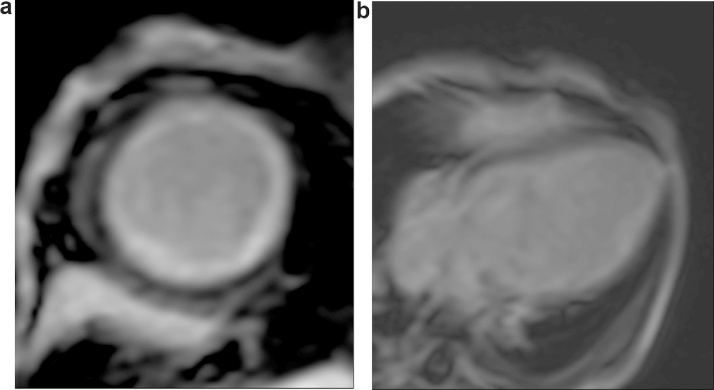
| 4 | 3 mth M | LCA form facing sinus, Collaterals in IVS, severe MR, LVEF 13% | Prominent diastolic flow reversal and significant systolic flow reversal (findings similar to Pt 2) | LMCA ligation and Left Subclavian artery to LAD anastomosis | ECHO (18 months): LVEF 43%, mid anterior anteroseptal and aplical segments severely hypokinetic. No MR (Fig. 2) |
| Cardiac MR: >50% contrast enhancement of apical segments, mid anterior and lateral wall | LDEDV/LVESV: 58/109 ml/m2 | ||||
| LDESV/LVEDV: 171/197 ml/m2 |
Fig. 2.
Pre-operative CMR (post gadolinium enhancement) a) short axis and b) apical 4 chamber, Patient no 4.
Fig. 3.
Post-operative CMR (post gadolinium enhancement) a) short axis and b) apical 4 chamber view of patient 3.
Fig. 4.
Post-operative CMR (post gadolinium enhancement) a) short axis and b) apical 4 chamber view patient 1.
4. Discussion
In a new born with ALCAPA, pulmonary artery pressure is the perfusion pressure of LV myocardium. Without any significant intracardiac shunt(ventricular septal defect, aortopulmonary window, patent ductus arteriosus etc) pulmonary artery pressure would drop over a period of weeks as a natural process. The resultant impairment in tissue perfusion leads to ischemia and triggers development of epicardial and endomyocardial collaterals between RCA and LCA. Former collateral are responsible for systolic flow reversal in LCA and characteristic higher systolic flow in RCA. The latter feed left coronary mainly during diastole as they are compressed by contracting myocardium. In spite of extensive collaterals development, communication between LCA and PA decompresses coronaries, worsens tissue perfusion and aggravates ischemia. Long standing ischemia ultimately leads to myocardial infarction and fibrosis. Poor diastolic flow reversal may be result of fibrotic obliteration of intra myocardial collaterals as a result of scarring. In natural history, with progressive scarring, not only baseline LVEF will be low but also diastolic component of LAD flow will be lost. Also, the recovery in myocardial function after surgery will be suboptimal. Thus loss of diastolic component and increase of systolic component suggest scarred myocardium, low baseline LVEF and sub optimal recovery after surgery.10
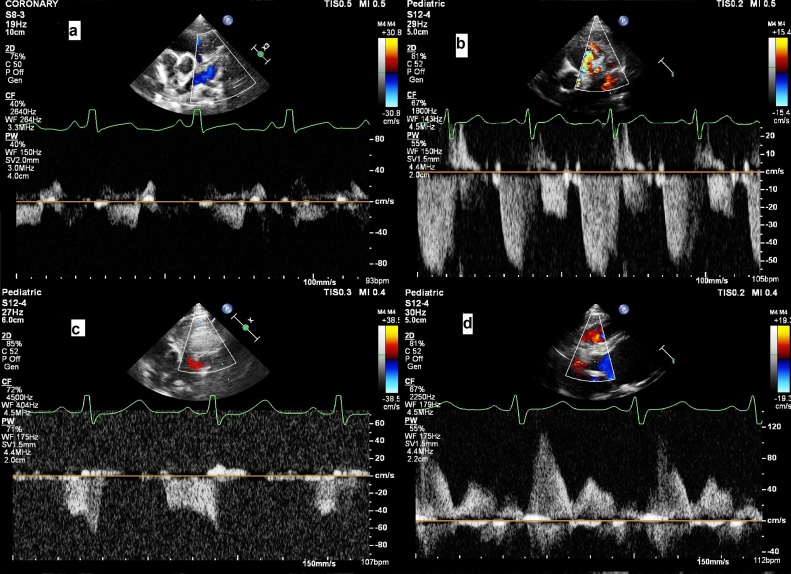
The peculiar right coronary flow pattern is diagnostic of ALCAPA in adults.1, 2, 3 To the best of our knowledge it has been demonstrated in early infancy for the first time (Fig. 1d). It definitely adds to diagnostic utility of same and should be specifically sought for in every patient with depressed left ventricular systolic dysfunction irrespective of age and even in early infancy. It will of interest to know when this pattern develops after birth.
Fig. 1.
PWCFP of LAD in a) Patient 1, b) Patient 2, c) Patient 3, d) PWCFP of RCA.
Though of similar age group, the flow patterns, CMR and improvement in LVEF of 3 vs 2/4 were strikingly different. Late diastolic peaking of flow reversal in LAD may be result of delayed relaxation of ischemic but viable myocardium (Patient 3). While inverted ‘U’ may suggest component of fixed obstruction along with dynamic. It may also suggest that, intra myocardial collaterals develop earlier than epicardial. Presence of significant flow from epicardial collaterals may also be an indicator of diminished flow in myocardial collaterals and scarred tissue. Very minimal diastolic flow in Patient 1 was result of extensive scar because she presented late. The probable reasons for early onset of scarring would be 1) run off from LMCA or 2) lesser collateral development or 3) altered tissue response to ischemia. Lange et al. has also reported that post operative outcome has been influenced by baseline LVEF, age, height and weight at presentation.12, 14
Secinaro et al., found that in post ALCAPA repair patients, presence of late gadolinium enhancement was suggestive of coronary occlusion. Post operative LVEF of all study patients was more than 60%.8 While in a pre and postoperative comparative CMR study by Latus et al, only 2 of 8 patients had baseline scar (10 to 12% of myocardium). It was s/o involvement of 2 myocardial segments. ECMO was required in 2 patients. Post operative LVEF improved to more than 50% in all of them. As number of patient with preoperative scar was a small proportion, they could not make a definitive conclusion on effect of same on post operative recovery. Also number of scarred segments were less than those observed in present study.9 Brown et al. studied pre operative cardiac MR of 2 patients who had significant pre operative scar burden. These patients did not have significant improvement following surgery and ultimately required heart transplant. He suggest role of cardiac MR, to screen patient who will not benefit from corrective surgery and directly referred to transplant.13
There are a few indication to evaluate myocardial viability in pediatric population as reasons for the same are spars like ALCAPA, post coronary transfer, etc. Though of comparable sensitivity and specificity, there are distinct advantages of CMR over positron emission tomography scan like no exposure to ionizing radiation.8, 9 As the procedure is time consuming, we used general anesthesia in all patients to prevent motion artifact. We used same criteria for myocardial viability as for adult population. In spite of small size, spatial and temporal resolution as well as image quality was satisfactory. The results were very close to expected disease process.
At a instance, though there was more than 50% gadolinium enhancement at baseline (lateral segment Patient 2), the tissue appeared normal after surgery. This may be related to very diminished perfusion in said area. Patient 1 underwent CMR before surgery, but the myocardium was excessively thinned out hence it was not possible to perform viability study. For the patients who had pre and post studies available, there was no new scar thus ruling out surgery related myocardial injury.
CMR findings gives a valuable insight into disease process and explains changes in PWCFP. The later can be used as a marker to assess scar burden and predict recovery after corrective surgery.
5. Study limitations
Our study is limited by the small patient size. Use of CMR for viability is not standardized in paediatric population. Why a small substrate of patients with ALCAPA do not develop LV dysfunction, remains a mystery. All the patients in present study received coronary bypass graft. Not all patients are having pre or post surgery CMR.
Based on our results, we advocate further research in the pre- and postoperative CMR evaluation of patients with ALCAPA with the hopeful objective to establish criteria that include the impact of pre-existing myocardial scars on early postoperative course, overall recovery of ventricular function and long-term prognosis.
Conflict of interest
None.
Industry Funding
None.
References
- 1.Yau J., Singh R., Halpern E., Fischman D. Anomalous origin of the left coronary artery from the pulmonary artery in adults: a comprehensive review of 151 adult cases and a new diagnosis in a 53-year-old woman. Clin Cardiol. 2011;34(4):204–210. doi: 10.1002/clc.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian Z., Fang L., Liu Y., Zhang S. Anomalous origin of the left coronary artery from the pulmonary artery detected by echocardiography in an asymptomatic adult. Intern Med. 2013;52:233–236. doi: 10.2169/internalmedicine.52.7643. [DOI] [PubMed] [Google Scholar]
- 3.Drinković N., Margetic E., Šmalcelj A., Brida V. Echocardiographic diagnosis of anomalous origin of the left coronary artery from the pulmonary artery. Eur J Echocardiogr. 2008;9:309–310. doi: 10.1016/j.euje.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Shan K., Constantine G., Sivananthan M., Flamm S.D. Role of cardiac magnetic resonance imaging in the assessment of myocardial viability. Circulation. 2004;109:1328–1334. doi: 10.1161/01.CIR.0000120294.67948.E3. [DOI] [PubMed] [Google Scholar]
- 5.Menon N. Bland-White-Garland syndrome in a neonate with review of literature. J Res Med Dent Sci. 2015;1(3):94–97. [Google Scholar]
- 6.Pelliccia A. Congenital coronary artery anomalies in young patients, new perspectives for timely identification. JACC. 2001;37(No. 2):598–600. doi: 10.1016/s0735-1097(00)01122-0. [DOI] [PubMed] [Google Scholar]
- 7.Hauser M. Cngenital anomalies of the coronary arteries. Heart. 2005;91:1240–1245. doi: 10.1136/hrt.2004.057299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Secinaro A., Ntsinjana H., Tann O., Schuler P., Muthurangu V., Hughes M., Tsang V., Taylor A. Cardiovascular magnetic resonance findings in repaired anomalous left coronary artery to pulmonary artery connection (ALCAPA) J Cardiovasc Magn Reson. 2011;13:27. doi: 10.1186/1532-429X-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latus H., Gummel K., Rupp S., Mueller M., Jux C., Kerst G., Akintuerk H., Bauer J., Schranz D., Apitz C. Cardiovascular magnetic resonance assessment of ventricular function and myocardial scarring before and early after repair of anomalous left coronary artery from the pulmonary artery. J Cardiovasc Magn Reson. 2014;16:3. doi: 10.1186/1532-429X-16-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhalgat P., Naik A., Salvi P., Joshi S. Colour Doppler and pulse wave assessment of flow in anomalous left coronary artery from pulmonary artery: Pre and Post surgery. Ann Pediatr Card. 2016;9:122–124. doi: 10.4103/0974-2069.180675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesselhoeft H., Fawcett J., Johnson A. Anomalous origin of the left coronary artery from the pulmonary trunk its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation. 1968;XXXVIII(August):403–425. doi: 10.1161/01.cir.38.2.403. [DOI] [PubMed] [Google Scholar]
- 12.Lange R., Vogt M., Hörer J., Cleuziou J., Menzel A., Holper K., Hess J., Schreiber C. Long-term results of repair of anomalous origin of the left coronary artery from the pulmonary artery. Ann Thorac Surg. 2007;83:1463–1471. doi: 10.1016/j.athoracsur.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Browne L., Kearney D., Taylor M., Chung T., Slesnick T., Nutting A., Krishnamurthy R. ALCAPA: the role of myocardial viability studies in determining prognosis. Pediatr Radiol. 2010;40:163–167. doi: 10.1007/s00247-009-1412-5. [DOI] [PubMed] [Google Scholar]
- 14.Heermann P., Heindel W., Schülke C. Coronary artery anomalies: diagnosis and classification based on cardiac ct and mri (cmr)—from alcapa to anomalies of termination. Fortschr Rüntgenstr. 2017;189:29–38. doi: 10.1055/s-0042-119452. [DOI] [PubMed] [Google Scholar]