Abstract
The neuronal ceroid lipofuscinoses (NCLs) are inherited lysosomal storage disorders characterized by general neurodegeneration and premature death. Sight loss is also a major symptom in NCLs, severely affecting the quality of life of patients, but it is not targeted effectively by brain-directed therapies. Here we set out to explore the therapeutic potential of an ocular gene therapy to treat sight loss in NCL due to a deficiency in the transmembrane protein CLN6. We found that, although Cln6nclf mice presented mainly with photoreceptor degeneration, supplementation of CLN6 in photoreceptors was not beneficial. Because the level of CLN6 is low in photoreceptors but high in bipolar cells (retinal interneurons that are only lost in Cln6-deficient mice at late disease stages), we explored the therapeutic effects of delivering CLN6 to bipolar cells using adeno-associated virus (AAV) serotype 7m8. Bipolar cell-specific expression of CLN6 slowed significantly the loss of photoreceptor function and photoreceptor cells. This study shows that the deficiency of a gene normally expressed in bipolar cells can cause the loss of photoreceptors and that this can be prevented by bipolar cell-directed treatment.
Keywords: AAV gene therapy, retina, Batten disease, neuronal ceroid lipofuscinoses, photoreceptors, bipolar cells, lysosomal storage disorders
Holthaus and colleagues show the successful treatment of retinal degeneration in Batten disease. The deficiency in Cln6 causes photoreceptor loss, yet photoreceptor-directed gene therapy is ineffective. Notably, CLN6 gene transfer to bipolar cells, retinal interneurons that are only lost late in the disease, rescues photoreceptors.
Introduction
The neuronal ceroid lipofuscinoses (NCLs) are rare, inherited lysosomal storage disorders that present with severe neurodegeneration and sight loss. This group of diseases is more commonly referred to as Batten disease, and at least 13 genes have been linked to the development of this condition with a combined incidence in different countries of between 1:12,500 and 1:100,000.1 The affected genes code either for a soluble lysosomal protein or a transmembrane protein.2 The development of therapies for forms of NCL caused by defects in lysosomal enzymes is aided by lysosomal cross-correction, which allows the therapeutic enzyme to be taken up by cells from the circulation and other cells via the mannose-6-phosphate pathway.3 This phenomenon has helped to pave the way toward clinical trials for CLN2 disease, a common form of NCL arising from the deficiency in the lysosomal enzyme TPP1 (Clinical Trials.gov: NCT01907087). Cross-correction, however, does not occur for transmembrane proteins, limiting functional restoration to individual cells and increasing the challenge to achieve a therapeutic effect.
CLN3 disease with juvenile onset is the most common form of NCL that is caused by a defect in a membrane-bound protein.4 Other rarer forms caused by transmembrane protein deficiencies include CLN6, CLN7, and CLN8 disease, all variant forms with typically a late-infantile onset and a more rapid progression than CLN3 disease. NCLs usually manifest with seizures, visual decline resulting in blindness, and progressive psychomotor and cognitive deteriorations. The order of the symptoms varies, yet these conditions collectively lead to premature death.5 A major obstacle to developing brain-directed therapies for NCLs arising from transmembrane protein defects is the challenge to deliver agents throughout the brain. Recently, the partial rescue of the brain phenotype in a CLN3 disease mouse model was described following brain-directed adeno-associated virus (AAV)-mediated gene therapies.6, 7 No correction of the disease pathology was reported outside of the brain, underlining the need for treatments not only targeting the brain but also other affected organs like the eye. Moreover, ocular therapies may not only increase the quality of life of patients but also may help with the development of brain-directed treatments for NCLs.
Over the last decade, AAV-mediated gene therapies have been used for several animal models of monogenic retinal degenerations to restore the expression of soluble or transmembrane proteins and to treat vision loss.8, 9, 10 These encouraging studies led to the commencement of clinical trials for inherited retinal degenerative conditions, including Leber congenital amaurosis11, 12, 13 and retinitis pigmentosa.14 Here we explored the therapeutic potential of an ocular AAV-mediated gene therapy in the Cln6nclf mouse, a naturally occurring model of CLN6 disease, variant late infantile. The mice carry a 1-bp insertion mutation in the Cln6 gene, which encodes a membrane-bound endoplasmic reticulum (ER) protein of unknown function.15 The 1-bp insertion results in a frameshift and a truncated short-lived protein product, and it is also found in CLN6 patients of Pakistani origin.16, 17 Cln6nclf mice present with a loss of vision preceding severe neuropathological and behavioral abnormalities.18, 19
In this study, we show that, although retinal disease in Cln6nclf mice is predominantly characterized by photoreceptor degeneration, supplementation of CLN6 in photoreceptors is not therapeutic. We also establish that, in unaffected retinas, CLN6 is expressed in photoreceptors and bipolar cells but the level is much higher in bipolar cells. Although bipolar cells are only lost late in disease, AAV-mediated gene delivery of CLN6 to bipolar cells leads to significantly increased retinal function and long-term preservation of photoreceptors. This study shows that photoreceptor degeneration can be prevented by treating bipolar cells.
Results
CLN6 Is More Highly Expressed in Bipolar Cells Than in Photoreceptors
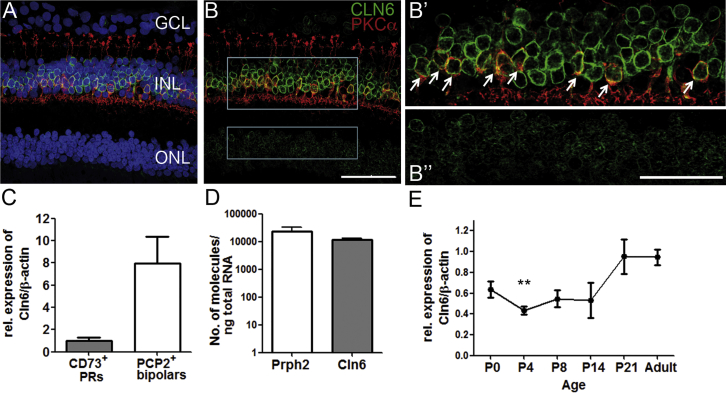
To determine the potential target cells for gene therapy, we investigated the endogenous expression of the CLN6 gene in human and mouse retina. Due to the lack of antibodies against the murine protein, we performed immunohistochemical stainings on human retina from non-diseased donor eyes using a previously reported antiserum against human CLN6.15 We detected a weak fluorescent signal in the outer nuclear layer (ONL) and a strong fluorescent signal in the inner nuclear layer (INL) (Figures 1A, 1B, and 1B”). In the INL, the staining for CLN6 co-localized with staining for PKCα, demonstrating that rod bipolar cells express CLN6 (Figures 1B and 1B’; Figures S2A–S2B’). Co-staining with an antibody against CRALBP, a marker for Mueller glia cells, did not reveal any co-localization (Figures S2A and S2A’). In the absence of antibodies that could be used for co-localization with CLN6 staining, we were unable to determine what other cell types in the INL expressed the gene. Microarray data on the murine retina suggest that the remaining CLN6+ cells are most likely to be other bipolar cell types, as rod bipolar cells only form a minor fraction of the retinal bipolar cell population.20
Figure 1.
CLN6 Is More Highly Expressed in Bipolar Cells Than in Photoreceptors in Human and Mouse Retina
Staining for CLN6 (green) and PKCα (red) on human retinal section showing high levels of CLN6 in the INL and lower levels in the ONL with (A) and without (B) DAPI nuclear counterstaining in blue. Gray boxes show areas of higher-magnification images. Scale bar, 50 μm. Single confocal image reveals (B’) CLN6+ rod bipolar cells (arrows) and (B”) CLN6+ cells in the ONL. Scale bar, 25 μm. (C) Cln6 expression level in adult mouse bipolar cells relative to photoreceptors after normalization for β-actin (mean ± SEM; n = 3 samples of 3–5 pooled retinas each; not significant, Mann-Whitney U test). (D) Absolute expression levels of Cln6 and Peripherin2 in adult wild-type mouse retinas (means ± SEM). Peripherin2, n = 3 mice; Cln6nclf, n = 4 mice. (E) Time course of the expression level of Cln6 in wild-type mouse retinas relative to adult levels, normalized for β-actin (mean ± SEM; Kruskall-Wallis, **p < 0.01 relative to P21; n = 2–4 mice per time point).
Cln6nclf mice have been reported to present with a predominant loss of photoreceptor function and photoreceptor cells.18, 19 We found that, from post-natal day 21 (P21), retinal function in Cln6-deficient mice was progressively compromised under scotopic light conditions, as measured by electroretinography (ERG) recordings (Figures S1A, S1C, and S1D). The photopic ERG was unchanged (Figure S1B). Histological assessment and quantitative analyses of the ONL revealed no severe histological changes at P14 (Figures S1E and S1F). However, Cln6nclf mice have a significantly reduced number of photoreceptor nuclei at 1 and 6 months compared with wild-type controls (Figures S1G and S1H). The number of rod bipolar cells in the INL was only reduced in mutant mice at 6 months (p < 0.001) (Figures S1I and S1J). In line with previous reports, these data show that Cln6nclf mice suffer from an early but mildly progressing rod-mediated retinal degeneration, while loss of rod bipolar cells became only evident at late disease stages.
In the murine retina, we assessed the expression of Cln6 by real-time qPCR. To confirm the expression of Cln6 in murine photoreceptors and bipolar cells, we performed real-time qPCRs on mRNA obtained from photoreceptors, sorted via fluorescence-activated cell sorting (FACS) and labeled with the cell surface marker CD73, and rod bipolar cells, labeled with td.tomato in a Pcp2.Cre/td.tomato mouse line (Figures S2B and S2C). In line with the staining on human retina, we found a trend with a higher expression level of Cln6 in rod bipolar cells than in photoreceptors (Figure 1C). A direct comparison of the absolute number of mRNA molecules by real-time qPCR showed that Cln6 was expressed to similarly high levels in whole mouse retina as the photoreceptor-specific gene Peripherin2, encoding a highly abundant retinal protein21 (Figure 1D). We studied the relative expression level of Cln6 in the retina over time using real-time qPCR, and we found that Cln6 was expressed from birth to adulthood. From P0 to P14, a period when retinal cells undergo post-natal maturation leading to initiation of the phototransduction cascade and eye opening, the expression level of Cln6 was relatively stable at around 50% of adult levels. At P21 the expression level of Cln6 increased to the level that was detected in adults (Figure 1E), which may indicate that Cln6 could play a more important role during vision than during the ocular development.
AAV2/8-Mediated Supplementation of CLN6 in Photoreceptors Is Not Therapeutic in Cln6nclf Mice
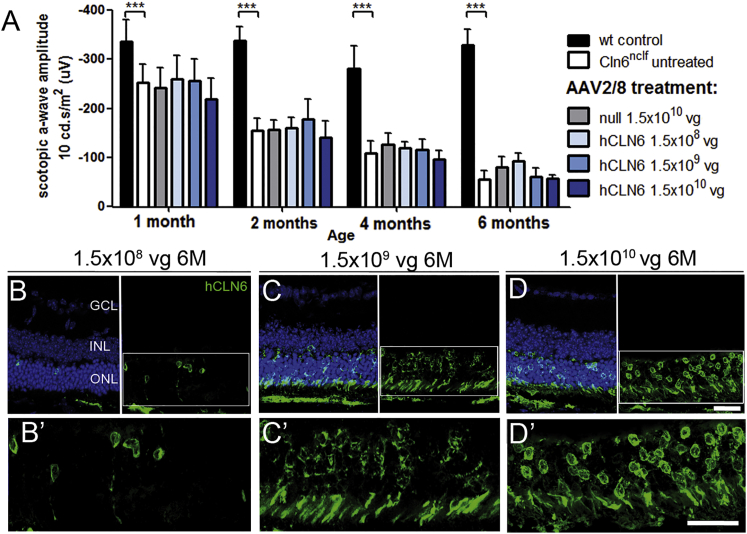
We established that CLN6 was expressed in photoreceptors and that Cln6 deficiency led to severe loss of photoreceptors in mice, while no early death of bipolar cells was detected in Cln6nclf mice despite the high endogenous expression level of CLN6 in bipolar cells. Consequently, we set out to develop a gene supplementation therapy targeting photoreceptors, and we treated pre-symptomatic P9–P10 Cln6nclf mice subretinally with an AAV2/8 vector carrying the ubiquitous cytomegalovirus (CMV) promoter and the human CLN6 (hCLN6) transgene (Figure S3). We administered three different doses ranging from 1.5 × 108 vector genomes (vg)/eye to 1.5 × 1010 vg/eye, and we performed ERG recordings at 1, 2, 4, and 6 months of age. As untreated Cln6nclf mice presented with a progressive reduction in rod photoreceptor function, the main functional readout to assess whether the treatment was successful was the scotopic a-wave. None of the treated animals had significantly higher a-wave amplitudes than the untreated or AAV2/8.null vector-treated mutant animals at any time point (Figure 2A). Immunostaining confirmed the presence of the transgene at 6 months. In line with the ERG recordings, the ONL did not appear to be preserved in any of the treated eyes (Figures 2B–2D’; Figure S1G). Further experiments targeting the photoreceptor cells, including earlier treatment at P5 and use of a weak photoreceptor-specific mouse opsin promoter,22 did not preserve photoreceptor function or improve photoreceptor survival. Since the human and mouse CLN6 protein share about 90% amino acid identity, we also assessed the efficacy of AAV8 carrying mouse Cln6 and a CMV promoter to exclude that slight differences in the amino acid sequence between human and mouse CLN6 could affect the outcome of the treatment. No treatment effect was observed (Table S1). These experiments demonstrated that an AAV-mediated gene supplementation therapy targeting photoreceptor cells was not therapeutic in Cln6-deficient mice.
Figure 2.
Subretinal Delivery of AAV2/8.CMV.hCLN6 Did Not Rescue Photoreceptors in Cln6nclf Mice
(A) Scotopic ERG a-wave amplitudes of Cln6nclf mice that received subretinal injections with AAV2/8.CMV.hCLN6 at P9–P11. Wild-type: n = 5–7 eyes, N = 2; Cln6nclf untreated: n = 14–17 eyes, N = 5; null: n = 3–4 eyes, N = 1; 1.5 × 108 vg/eye: n = 6 eyes, N = 1; 1.5 × 109 vg/eye: n = 7 eyes, N = 2; 1.5 × 1010 vg/eye: n = 6–8 eyes, N = 2. ***p < 0.001 (2-way ANOVA with Bonferroni post-test). (B–D) hCLN6 staining confirmed transgene expression in photoreceptors at 6 months after transduction with 1.5 × 108 vg/eye (B) and high magnification (B’), 1.5 × 109 vg/eye (C) and high magnification (C’), and 1.5 × 1010 vg/eye (D) and high magnification (D’). Scale bar, 25 μm.
The AAV2/2 Variant 7m8 Transduces Bipolar Cells Efficiently in Wild-Type Mice
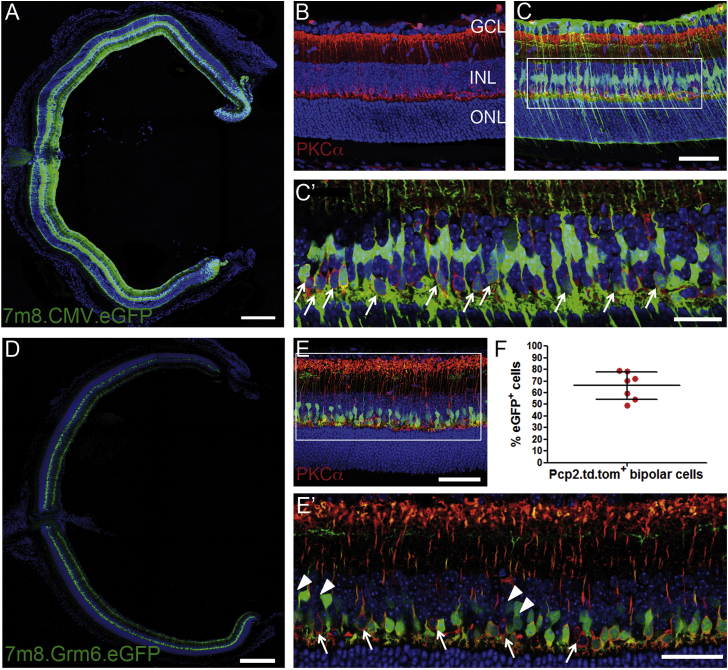
Conventional AAV serotypes poorly transduce cells in the INL, and bipolar cells are among the cell types least amenable to viral transduction.23 Several studies have been undertaken to engineer AAV vectors to enhance their transduction efficiency or to increase cell type-specific transduction.24, 25 The recently developed AAV2/2 variant 7m8 was reported to penetrate the retina and transduce cells in all retinal layers following intravitreal delivery in adult mice.26 To investigate the transduction efficiency of bipolar cells, we produced a 7m8 vector carrying EGFP under the CMV promoter. Wild-type mice received intravitreal injections of the vector at P5, an age before the inner limiting membrane (ILM) is fully established. The ILM can act as a natural barrier in adult animals when AAVs are delivered intravitreally, preventing efficient penetration of viral particles into deep layers of the rodent retina.27, 28 At 3 weeks post-injection, we observed a widespread pan-retinal expression of EGFP (Figure 3A). Higher-magnification images showed strongly transduced ganglion and Mueller glia cells as well as more sparsely transduced photoreceptors (Figure 3C). Immunostaining for PKCα and single confocal images revealed that several rod bipolar cells expressed EGFP (Figures 3B and 3C’, white arrows).
Figure 3.
AAV 7m8 Vector Transduced Mouse Bipolar Cells Efficiently
(A) Section of wild-type mouse eye 3 weeks post-intravitreal injection of 1 × 1010 vg 7m8.CMV.EGFP at P5. Scale bar, 250 μm. (B and C) Higher-magnification images of wild-type retinas treated with 7m8.CMV.EGFP and immunostained for PKCα (red) without (B) and with (C) the green channel shown. Scale bar, 50 μm. (C’) Single merged image of 7m8.CMV.EGFP-treated wild-type retina showing transduced PKCα+ rod bipolar cells. Scale bar, 25 μm. (D) Section of wild-type mouse eye 3 weeks post-intravitreal injection of 1 × 1010 vg 7m8.Grm6.EGFP at P5. Scale bar, 250 μm. (E) Higher-magnification image of 7m8.Grm6.EGFP-treated retina stained for PKCα (red). Scale bar, 50 μm. (E’) Single image demonstrating transduction of the majority of PKCα+ rod bipolar cells (arrows indicate non-transduced PKCα+ cells) and some cone ON bipolar cells (arrowheads), identified by the location of their cell bodies in the INL and synaptic termini in the inner plexiform layer (IPL). Scale bar, 25 μm. (F) Quantification by flow cytometry of the proportion of rod bipolar cells transduced by 7m8 at P5–P6 (mean ± SD; n = 7 eyes).
To visualize better the transduction of bipolar cells, we injected wild-type animals with a 7m8 vector carrying EGFP under the weak Purkinje cell protein 2 (PCP2) promoter29 (Figures S4A and S4A’) or the 4× enhanced Grm6 (Grm6) promoter,25 driving expression in rod bipolar cells or all ON bipolar cells, respectively. Magnified single images showed that the majority of PKCα+ bipolar cells and several cone ON bipolar cells (arrowheads) contained EGFP (Figures 3D and 3E). We quantified the proportion of transduced bipolar cells following the administration of 7m8 by flow cytometry. PCP2.Cre/td.tomato mice received intravitreal injections with 7m8.CMV.EGFP at P5 or P6, and we found that approximately 65% of the td.tomato+ rod bipolar cells were positive for EGFP (Figure 3F). We further analyzed the number of transduced photoreceptors, and we established that approximately 25% of the CD73+ photoreceptors expressed EGFP (Figures S4B and S4C). Due to the lack of specific markers labeling all cone bipolar cells, we did not quantify the transduction of other types of bipolar cells.
Intravitreal Delivery of 7m8.CMV.hCLN6 Slowed the Loss of Photoreceptor Function and Photoreceptor Cells in Cln6nclf Mice
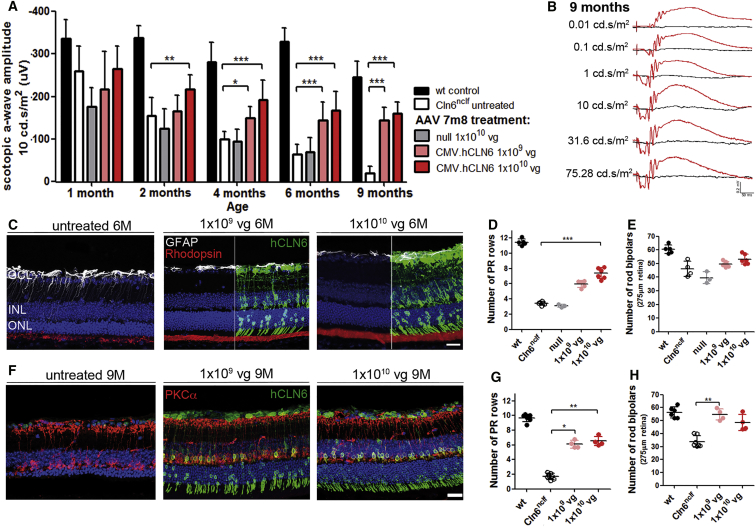
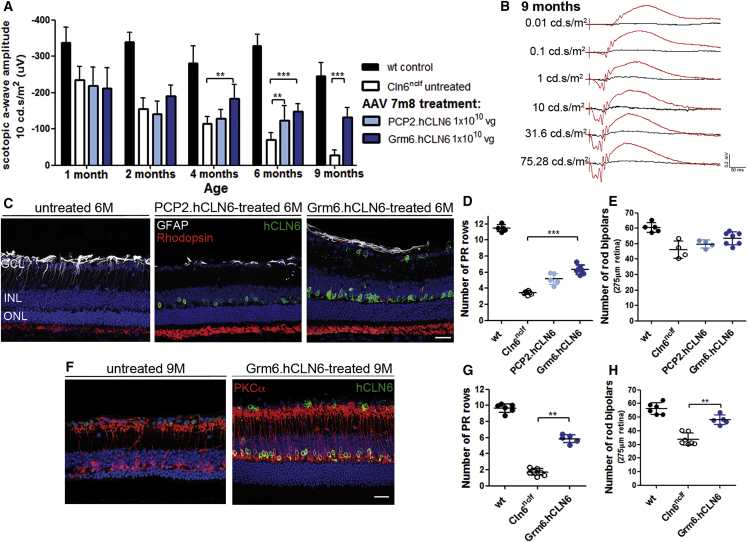
ERG recordings, at 1 month following intravitreal injections of 7m8.CMV.hCLN6 in Cln6nclf mice at P5–P6, demonstrated that mutant eyes that received a vector dose of 1 × 1010 vg had significantly higher scotopic a-wave amplitudes than untreated eyes or eyes that received 7m8.null control vector. The ERG amplitudes decreased slightly but remained significantly higher in treated than in untreated or 7m8.null-treated eyes at 4, 6, and 9 months. Representative scotopic ERG traces of a mutant mouse that received 1 × 109 vg of 7m8.CMV.hCLN6 in one eye and no treatment in the contralateral eye are shown in Figure 4B, demonstrating the striking difference in retinal function between treated (red traces) and untreated (black traces) eyes at 9 months (see also Figure S5A and Table S2). Histological assessment of the treated eyes at 6 months confirmed the expression of human CLN6 in cells of all retinal layers. Consistent with the ERG recordings, treated retinas had a thicker ONL, a better structure of photoreceptor outer segment (OS), and reduced activation of Mueller glia cells, as indicated by DAPI, rhodopsin, and glial fibrillary acidic protein (GFAP) staining, respectively (Figure 4C). Counts of DAPI+ photoreceptor nuclei showed that the number of nuclei was higher, with an average of 6 and 7 rows of nuclei in the low-titer and high-titer groups compared with 4 rows in untreated mutant eyes (Figure 4D).
Figure 4.
Intravitreal Administration of 7m8.CMV.hCLN6 Slowed Loss of Photoreceptor Function and Photoreceptor Cells in Cln6nclf Mice
(A) Scotopic ERG a-wave amplitudes of Cln6nclf mice after injection of 7m8.CMV.hCLN6 at P5–P6. Wild-type: n = 5–7 eyes, N = 2; Cln6nclf untreated: n = 10–11 eyes (1–6 months), N = 4, n = 5 (9 months), N = 2; null: n = 3–6 eyes, N = 2; 1 × 109 vg/eye: n = 11–13 eyes (1–6 months), N = 4, n = 5 (9 months), N = 2; 1 × 1010 vg/eye: n = 12 eyes (1–6 months), N = 4, n = 4 (9 months), N = 2. **p < 0.01 and ***p < 0.001 (2-way ANOVA with Bonferroni post-test). (B) Scotopic ERG traces of a Cln6nclf mouse 9 months after the administration of 1 × 109 vg 7m8.CMV.hCLN6 in one eye (red). Contralateral uninjected eye is in black. (C) hCLN6 staining (green) on treated and untreated eyes at 6 months. GFAP (white) and Rhodopsin (red) showed better morphology in treated eyes. (D) Quantification of photoreceptor rows and (E) PKCα+ cells at 6 months. ***p < 0.001 (Kruskal-Wallis test). (F) hCLN6 (green) and PKCα (red) staining on treated and untreated eyes at 9 months. (G) Quantification of photoreceptor rows and (H) PKCα+ cells at 9 months post-treatment. **p < 0.01 and *p < 0.05 (Kruskal-Wallis test). Scale bars, 25 μm.
At 6 months, counts of PKCα+ cells did not reveal any significant changes between treated and untreated eyes (Figure 4E). Histological assessment at 9 months confirmed the long-term expression of the therapeutic transgene in treated retinas. It also was apparent that, while the retinal degeneration continued to progress from 6 to 9 months resulting in 2 remaining rows of photoreceptor nuclei in Cln6-deficient mice, the number of photoreceptor nuclei was maintained following the 7m8 treatment with 6 and 7 rows of nuclei in the two treatment groups (Figures 4F and 4G). Similarly, the quantification of rod bipolar cells showed that untreated eyes continued to lose PKCα+ cells from 6 to 9 months, whereas treated retinas had significantly higher numbers of rod bipolar cells at 9 months, as highlighted by immunostaining for PKCα (Figures 4F and 4G). We did not maintain any mice for longer than 9 months since motor and behavioral skills, body weight, and survival deteriorated progressively as part of the natural disease progression in Cln6-deficient mice.16
7m8-Mediated Supplementation of CLN6 in Bipolar Cells Specifically Rescued the Photoreceptor Degeneration in Cln6nclf Mice
To investigate whether the transduction of bipolar cells alone was sufficient to treat the photoreceptor degeneration in Cln6-deficient mice, we produced two 7m8.hCLN6 vectors, one carrying the weak PCP2 promoter to drive expression in rod bipolar cells and one carrying the 4× enhanced Grm6 promoter to drive expression in all ON bipolar cells. Cln6nclf mice at P5 or P6 received intravitreal injections with either vector at a dose of 1 × 1010 vg/eye. At 4 months, the scotopic a-wave amplitudes were significantly higher in Grm6.hCLN6-treated retinas, reduced slightly over time, but remained significantly higher up to 9 months. Retinas treated with the PCP2.hCLN6 construct showed a weaker therapeutic effect and had significantly increased scotopic a-wave amplitudes only at 6 months (Figure 5A). Figure 5B shows representative scotopic ERG traces of a 9-month-old Cln6nclf mouse that received 7m8.Grm6.hCLN6 treatment in one eye and no treatment in the contralateral eye (see also Figure S5B and Table S3). We did not follow up on mice that received injections with 7m8.PCP2.hCLN6 for longer than 6 months in view of the limited beneficial effect.
Figure 5.
hCLN6 Delivery to Bipolar Cells Slowed the Loss of Photoreceptor Function and Photoreceptor Cells in Cln6nclf Mice
(A) Scotopic ERG a-wave amplitudes of Cln6nclf mice after injection of 7m8.PCP2.hCLN6 or 7m8.Grm6.hCLN6 (1 × 1010 vg/eye) at P5–P6. **p < 0.01 and ***p < 0.001 (2-way ANOVA with Bonferroni post-test). Wild-type: n = 5–7 eyes, N = 2; Cln6nclf untreated: n = 7–9 eyes (1–6 months), N = 3, n = 5 (9 months), N = 2; PCP2.hCLN6: n = 10–11 eyes, N = 4; Grm6.hCLN6: n = 14 eyes (1–6 months), N = 5, n = 7 (9 months), N = 3. (B) Scotopic ERG traces of a Cln6nclf mouse 9 months after the administration of 1 × 1010 vg 7m8.Grm6.hCLN6 in one eye (red). Contralateral uninjected eye is in black. (C) hCLN6 staining (green) on treated and untreated eyes at 6 months confirmed transgene expression in bipolar cells. GFAP (white) and Rhodopsin (red) show better morphology in treated eyes. (D) Quantification of photoreceptor rows and (E) PKCα+ cells, 6 months post-treatment. (F) hCLN6 (green) and PKCα (red) staining on treated and untreated eyes at 9 months. (G) Quantification of photoreceptor rows and (H) PKCα+ cells at 9 months post-treatment. **p < 0.01 and ***p < 0.001 (Kruskal-Wallis test). Scale bars, 25 μm.
Analyses of the retinal histology showed that, at 6 months, hCLN6 was present in bipolar cells, rod OS had a better morphology, and Mueller glia cell activation was reduced in PCP2.hCLN6- and Grm6.hCLN6-treated retinas (Figure 5C). Some ganglion cells appeared to express human CLN6 following the administration of 7m8.Grm6.hCLN6. This was surprising because no expression of EGFP was detected in the ganglion cell layer (GCL) when 7m8.Grm6.EGFP was administered to wild-type mice (see Figures 3D and 3E). Grm6 promoter-driven expression in retinal ganglion cells (RGCs) has been described previously.30 A possible explanation for the preferential expression of CLN6 over GFP in the GCL may be that the sequence of the CLN6 transgene could contain elements that promote the leaky activity of the Grm6 promoter. In agreement with the functional restoration, the thickness of the ONL was increased in both treatment groups, however, this only reached significance in 7m8.Grm6.hCLN6-treated retinas with 6 rows of photoreceptor nuclei compared with 4 rows in untreated mutant retinas at 6 months (Figure 5D). No significant difference in the number of rod bipolar cells was detected following the treatment at 6 months (Figure 5E), but at 9 months the number of PKCα+ cells was significantly increased (p = 0.0057) (Figure 5H), as highlighted by representative images in Figure 5F. From 6 to 9 months, the rows of photoreceptor nuclei remained higher in the treated eyes with 6 rows compared with 2 rows in untreated retinas (p = 0.0025) (Figures 5F and 5G).
Discussion
AAV-mediated gene therapies have proven a successful treatment strategy to combat photoreceptor degenerations in animal models carrying mutations in various genes.8, 9, 10 Here we described the development of a successful treatment for the retinal degeneration in Cln6-deficient mice, a model of NCL that carries a mutation in the Cln6 gene, encoding a transmembrane ER protein of unknown function. We demonstrated that the photoreceptor degeneration in Cln6nclf mice was amenable to treatment when the therapeutic CLN6 transgene was delivered specifically to bipolar cells, a retinal cell type downstream of photoreceptors. The bipolar cells themselves were only lost at late disease stages after the majority of photoreceptors had died. The treatment with the ubiquitous promoter had the biggest beneficial effect, most likely because expression was achieved in all transduced bipolar cell subtypes. The administration of bipolar cell subtype-specific promoter constructs resulted in a slightly lower therapeutic effect. CLN6 deficiency in photoreceptors did not play a central role in the development or progression of the photoreceptor degeneration.
For the successful treatment of the retinal phenotype in Cln6nclf mice, it was vital to understand the endogenous expression pattern of CLN6 in the retina. It was surprising that CLN6 is more highly expressed in bipolar cells than in photoreceptors, since bipolars are not lost early in disease. Based on our data, a scenario could be envisaged where gene supplementation therapies targeting only the degenerating brain regions or the cell types that are lost early in the disease could be ineffective. Hence, for successful brain-directed gene therapies for CLN6 disease, variant late infantile, it may be necessary to conduct a detailed analysis of the endogenous CLN6 expression in the different cell types and regions of the brain. While brain-directed gene therapy for CLN6 deficiency using a ubiquitous promoter may be successful and may not require the targeting of specific cells, there is, however, a risk that widespread high-level non-specific expression of CLN6 may result in toxicity. The use of a specific promoter targeting the appropriate cells would decrease that risk.
Retinal disorders arising from defects in bipolar cells are rare. One such disease is complete congenital stationary night blindness (cCSNB), which is caused by mutations in genes expressed in bipolar cells. These lead to a complete loss of the ERG b-wave that is accurately recapitulated in mouse models harboring mutations in genes such as Grm6 or Nyctalopin (Nyx).31, 32 AAV-mediated gene transfer to the bipolar cells of a Nyx-deficient mouse has previously been shown to partially restore bipolar cell function in this stationary disease.33 In contrast to the phenotype observed in Cln6nclf mice, cCSNB does not result in a severe loss of photoreceptors. To our knowledge, this is the first report to describe a retinal dystrophy in which a gene defect in bipolar cells mediates the loss of photoreceptors while leaving the bipolar cells largely unaffected. The ERG recordings performed in our study did not resolve whether the decrease in the scotopic b-wave in untreated Cln6nclf mice was a result of the reduced rod photoreceptor function, an intrinsic reduction of rod bipolar cell function, or a combination of both. Since the b-wave is dependent on the presence of the a-wave, it reflects photoreceptor function, synaptic transmission from photoreceptors to bipolar cells, and bipolar cell function.34 Therefore, it remains unclear whether bipolar cells, which are not lost at early disease stages in Cln6-deficient mice, function normally. It could be speculated that abnormal bipolar cell function could affect the function and viability of photoreceptors as these cells are metabolically very active and sensitive to intra- or extracellular changes,35 but further studies are required to determine the disease mechanism in Cln6-deficient mice.
We have confirmed that in mice the intravitreal administration of the recently developed 7m8 vector in the first post-natal week led to a widespread transgene expression in all retinal layers, including bipolar cells. However, larger animals, such as non-human primates, have a significantly thicker ILM, posing a greater challenge for retinal transduction after intravitreal AAV administration. Following intravitreal injection in non-human primates, transduction by the 7m8 vector appears to be limited to the fovea and retinal areas in close proximity to blood vessels,26, 36 although the efficiency of bipolar cell transduction was not assessed in these studies. It thus remains to be determined whether other vectors and/or approaches are required for efficient targeting of bipolar cells in large animals and for human applications. Alternative vectors able to transduce the bipolar cells in the murine retina have been described, including AAV2/8BP2 and tyrosine-mutated AAVs.25, 37 However, as for AAV 7m8, the efficiency with which these vectors can transduce bipolar cells in larger animals has proved insufficient, making them less attractive for clinical use.36, 37
The ultimate aim of this study was to investigate whether gene therapy is a potential strategy to treat the retinal degeneration in CLN6 disease, variant late infantile. As lysosomal cross-correction does not occur for CLN6, an ocular AAV-mediated treatment of Cln6nclf mice presents a very similar challenge to deficiencies in CLN3 or other transmembrane defects of NCL. This study therefore also provides a proof of concept of gene therapy for CLN3 disease, juvenile, the most common form of NCL caused by a transmembrane protein defect. Of note is that, once CLN3 disease patients have lost their vision, it can take several years before they develop severe neurological symptoms, creating a time window for therapeutic interventions to treat the visual failure.38 Recently, mutations in CLN3 were also linked to cases of non-syndromic retinal degeneration in five unrelated patient families, highlighting further the need for ocular treatments for this condition.39
Here we established that endogenous CLN6 is more highly expressed in bipolar cells than in photoreceptors in mouse and human retinas, consistent with results from a microarray study on the mouse retina.20 The same microarray study indicates that Cln3 is expressed in a variety of retinal cell types with a striking abundance only in microglia.20 In a Cln3 knockin reporter mouse (Cln3lacZ/lacZ), X-gal staining was present in bipolar cells as early as P14 and expression in photoreceptors became evident from P21.40 ERG recordings in Cln3-deficient mice showed electronegative retinal responses with a preserved a-wave, an approximately 30% decrease in the b-wave, and no significant cell loss at 12 months.41 In vivo assessment of Cln3-deficient mice also revealed that the GCL (including the inner plexiform layer [IPL] and the nerve fiber layer) and the INL were significantly thinner at 18 months than in wild-type mice, while the outer retina measured from the outer plexiform layer (OPL) to OS and from the RPE to the choroid did not show any differences,42 which may indicate that morphological alterations occur only in the inner retina. In line with these findings, CLN3 disease patients present with an electronegative maximal response and only mild a-wave disturbances at the onset of the disease, pointing to an early inner retinal dysfunction.38 Collectively, these data indicate that bipolar cells could play an important role, not only in CLN6 disease but also in CLN3 disease. Although CLN6 and CLN3 encode for proteins involved in different cellular processes with different subcellular localization,2 the same strategy to deliver the therapeutic transgene to bipolar cells may be the key to an effective ocular treatment for both diseases.
Materials and Methods
Mice
Cln6nclf mice were kindly provided by Professor Thomas Braulke (UKE, Hamburg). C57BL/6J and PCP2.Cre mice were purchased from Harlan Laboratories (UK). PCP2.Cre mice were paired with td.tomato (flox.STOP.flox.td.tomato) mice, and a Cre+ litter was confirmed by genotyping. All mice were maintained under cyclic light conditions (12-hr light/dark). If not further specified, the ages of adult mice ranged from 8 to 12 weeks. Animal experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the UK Home Office license (PPL 70/8120).
Plasmid Construct
The hCLN6 cDNA was cloned into a pD10 vector containing the CMV promoter. To drive expression in rod bipolar cells, the CMV promoter was replaced with the PCP2 promoter kindly provided by Professor John Oberdick (OSU, Ohio). To drive expression in all ON bipolar cells, CLN6 cDNA was cloned into the pD10 vector carrying the 4×Grm6 (4× enhanced mGluR6) promoter kindly provided by Botond Roska (FMI, Basel). To produce a null vector, the pD10 backbone was used carrying an unrelated transgene without promoter and start codon.
Production of rAAV and Intraocular Administration
Recombinant AAV2/8 and 7m8 vector were produced through a triple-transient transfection method and purified by affinity-based AVB Sepharose column (GE Healthcare, UK), as described previously.43 Viral particle titers were determined by real-time qPCR using primers aligning to the SV40 poly A tail of the genomic plasmid and a specific probe carrying a FAM dye label. Viral vector administration was performed under general anesthesia using an operating microscope (Carl Zeiss) and a 34G needle (Hamilton) as described.10 A total volume of 1.5 μL or 1 μL recombinant AAV (rAAV) was injected into juvenile (P9–P10) and early post-natal (P5–P6) animals, respectively. At least two eyes were left uninjected in every mutant cohort, and no animal received the same treatment in left and right eyes.
ERG
ERG recordings were obtained from both eyes in mutant and wild-type animals using commercially available equipment (Espion ERG Diagnosys System), as described previously.10 After dark adaptation overnight, scotopic examinations were performed under single-flash recording using increasing light intensities. Photopic single-flash recordings were obtained following 5 min of light adaptation at a background light intensity of 30 cd/m2. N represents the number of independent animal cohorts and n represents the number of eyes in independent animals. ERG data from the same wild-type cohorts are presented in Figures 2, 4, and 5 to provide a reference.
Tissue Preparation
Human paraffin sections of the mid-retina were provided by Dr. Michael Powner (UCL, London). Institutional Review Board (IRB)/Ethics Committee approval was obtained from Dr. Marcus Fruttiger (UCL, London). The tissue was fixed in 2% paraformaldehyde (PFA) and obtained as described previously.44 6-μm-thick retinal sections were deparaffinized in xylene substitute (Sigma-Aldrich) and rehydrated in alcohol gradients of 100%, 75%, 50%, and 30%, followed by a final PBS wash. For antibody staining, the slides were heated to 120°C in 90% glyercol and 10% citrate buffer (pH 6.0). For mouse retinal sections, the murine eyes were enucleated following cervical dislocation, and cornea, iris, and lens were removed. The eye cups were fixed in 4% PFA for 1–2 hr, cryoprotected in 20% sucrose, and flash-frozen in embedding matrix OCT.
Immunohistochemistry
The slides were briefly washed in PBS and blocking buffer was employed containing 5% normal goat serum (NGS), 1% BSA, and 0.1% Triton X-100. The samples were incubated in primary antibody diluted in blocking buffer at 4°C overnight. Sections were washed in PBS and incubated in secondary antibody diluted in blocking buffer. Subsequently sections were washed in PBS, washed with 600 nM DAPI for 5 min, and mounted in DAKO mounting media. The slides were stored in the dark at 4°C until imaging by confocal microscopy (Leica DM5500Q).
Quantification of Retinal Cross Sections
Eyes were sectioned in a sagittal orientation (18 μm) and collected on glass slides. Confocal images (275 × 275 μm) were taken from the superior and inferior midcentral retina of three sagittal cross sections on each slide. To assess the ONL thickness, three vertical columns of DAPI+ photoreceptor nuclei were counted in each of the six single confocal images. The counts of PKCα+ cells were performed on the corresponding projection images. PKCα counts in Figure S1 were performed on stained sections using mouse anti-PKCα antibody (Sigma-Aldrich). For all other PKCα counts, staining was performed using mouse anti-PKCα antibody (Santa Cruz Biotechnology). Counts included age-matched wild-type controls. Researchers performing counts were masked to the genotype of the animals and treatment received.
Real-Time qRT-PCR
Total RNA was extracted from mouse retinas or cell samples sorted via FACS using the RNeasy Mini Kit (QIAGEN). The QuantiTect Reverse Transcription Kit (QIAGEN) was utilized to generate cDNA. qRT-PCRs were performed in 96-well plates in a PCR thermal cycler (Applied Biosciences 7900HT) using a 2× FastStart TagMan Probe Mastermix assay (Roche) with a probe concentration of 100 nm and a primer concentration of 200 nm with the following primers:
forward β-actin (5′-AAGGCCAACCGTGAAAAGAT-3′),
reverse β-actin (5′-GTGGTACGACCAGAGGCATAC-3′),
forward Cln6 (5′-AGAGCCACATGCCAGGAC-3′),
reverse Cln6 (5′-GGCGAAGAAGGTGAAGATGA-3′),
forward Peripherin2 (5′-TGGATCAGCAATCGCTACCT-3′), and
reverse Peripherin2 (5′-CCATCCACGTTGCTCTTGA-3′).
Triplicate reactions were carried out for each sample. The Ct values were calculated using the computer program SDS 2.2.2 (Applied Biosciences). To determine relative expression levels, the Ct values of the triplicate reactions for every gene were averaged and normalized to the averaged β-actin Ct values of each corresponding sample (ΔCt). For absolute quantification, a dilution series of plasmid DNA ranging from 1.5 × 103 to 1.5 × 107 molecules was used to produce a standard curve. A dilution series of wild-type mouse retina cDNA was performed in the ratios of 1:10:100. The obtained Ct values were averaged per triplicate reaction, and the number of specific cDNA molecules per nanogram total mRNA was calculated by standard curve intrapolation.
Retinal Semithin Sections
Eyes were enucleated and the cornea and lens removed. The eyecups were fixed in Karnovsky fixative for at least 30 hr at 4°C. The eyes were dehydrated by passage through ascending ethanol series (50%–100%) and propylene oxide, and they were infiltrated overnight with a 1:1 mixture of propylene oxide: Epoxy resin. After a further 8 hr in full resin, eyes were embedded in fresh resin and incubated overnight at 60°C. Semithin (0.7-μm) sections were cut in the inferior-superior axis passing through the optic nerve head using a Leica ultracut S microtome. Retinal semithin sections were stained with a 1% mixture of toluidine blue-borax in 50% ethanol. Images were captured of the inferior and superior mid-retina from three sections per eye (in total six images per eye) using a Qimaging camera (MicroPublisher 5.0 RT) mounted on a light microscope (Leica DM IRB). Images were exported as .tif files and measurements were performed in ImageJ.
FACS and Flow Cytometry Analysis
Cell sorting was performed using a BD Influx Cell Sorter (BD Biosciences, USA) fitted with a 200-mW 488-nm blue laser that was used to excite GFP detected in the 488- to 530/40-nm channel, a 50-mW 561-nm yellow/green laser that was used to excite tdTomato detected in the 561- to 585/29-nm channel, and a 100-mW 640-nm red laser used to excite an anti-mouse CD73-APC antibody (BioLegend, 127209) detected in the 640- to 670/30-nm channel. Sorting of photoreceptors and bipolar cells was performed with a 70-μm nozzle at 50 psi. To determine cell viability, DRAQ7 (Biostatus, DR71000) dead cell stain was added to the samples at a final concentration of 0.6 μM for 5 min at room temperature. All of the samples were analyzed using a BD LSRFortessa™ X-20 flow cytometer (BD Biosciences, USA) fitted with 5 lasers (i.e., 355-, 405-, 488-, 561-, and 640-nm lasers). Results were subsequently analyzed using FlowJo software version (v.)9.9.5.
Statistical Analysis
Data are represented as means ± SD or ± SEM with the appropriate n (number of independent samples) values as indicated. The appropriate tests and p values are provided in the figure legends with *p < 0.05, **p < 0.01, and ***p < 0.001.
Author Contributions
S.-M.k.H., J.R., L.A.-H., R.A.P., Y.D., A.G., R.D.S., and J.H. planned and conducted the experiments. S.-M.K.H., R.D.S., and A.J.S. analyzed the data. R.A.P., Y.D., A.G., R.D.S., M.R., R.M., S.A., U.F.O.L., A.J.S., S.E.M., and R.R.A. provided expertise and feedback. A.J.S., S.E.M., and R.R.A. secured funding and supervised the study. S.-M.k.H., A.J.S., S.E.M., and R.R.A. conceptualized the study and wrote the paper.
Conflicts of Interest
U.F.O.L. is an employee of F. Hoffmann-La Roche Ltd. The remaining authors do not declare any conflicts of interest.
Acknowledgments
We thank Professor Thomas Braulke for the Cln6nclf mouse line and Dr. Michael Powner for providing non-diseased human retinal tissue. This project was supported by the Batten Disease Family Association, charity No 1084908, Beefy’s Charity Foundation, the European Union’s Horizon 2020 research and innovation programme (grant 66691), the European Union Seventh Framework Programme (FP7/2007–2013, grant 281234), RP Fighting Blindness (grant GR576), and Moorfields Eye Charity and Medical Research Council. R.R.A. is partially supported by the NIHR Biomedical Research Centre at Moorfields Eye Hospital and the UCL Institute of Ophthalmology.
Footnotes
Supplemental Information includes five figures and three tables and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.02.027.
Contributor Information
Sara E. Mole, Email: s.mole@ucl.ac.uk.
Robin R. Ali, Email: r.ali@ucl.ac.uk.
Supplemental Information
References
- 1.Mole S., Williams R., Goebel H. Second Edition. Oxford University Press; 2011. The Neuronal Ceroid Lipofuscinoses (Batten Disease) [Google Scholar]
- 2.Kollmann K., Uusi-Rauva K., Scifo E., Tyynelä J., Jalanko A., Braulke T. Cell biology and function of neuronal ceroid lipofuscinosis-related proteins. Biochim. Biophys. Acta. 2013;1832:1866–1881. doi: 10.1016/j.bbadis.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Sands M.S., Davidson B.L. Gene therapy for lysosomal storage diseases. Mol. Ther. 2006;13:839–849. doi: 10.1016/j.ymthe.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Consortium T.I.B.D., The International Batten Disease Consortium Isolation of a novel gene underlying Batten disease, CLN3. Cell. 1995;82:949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- 5.Schulz A., Kohlschütter A., Mink J., Simonati A., Williams R. NCL diseases - clinical perspectives. Biochim. Biophys. Acta. 2013;1832:1801–1806. doi: 10.1016/j.bbadis.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sondhi D., Scott E.C., Chen A., Hackett N.R., Wong A.M.S., Kubiak A., Nelvagal H.R., Pearse Y., Cotman S.L., Cooper J.D., Crystal R.G. Partial correction of the CNS lysosomal storage defect in a mouse model of juvenile neuronal ceroid lipofuscinosis by neonatal CNS administration of an adeno-associated virus serotype rh.10 vector expressing the human CLN3 gene. Hum. Gene Ther. 2014;25:223–239. doi: 10.1089/hum.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch M.E., Aldrich A., Fallet R., Odvody J., Burkovetskaya M., Schuberth K., Fitzgerald J.A., Foust K.D., Kielian T. Self-Complementary AAV9 Gene Delivery Partially Corrects Pathology Associated with Juvenile Neuronal Ceroid Lipofuscinosis (CLN3) J. Neurosci. 2016;36:9669–9682. doi: 10.1523/JNEUROSCI.1635-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komáromy A.M., Alexander J.J., Rowlan J.S., Garcia M.M., Chiodo V.A., Kaya A., Tanaka J.C., Acland G.M., Hauswirth W.W., Aguirre G.D. Gene therapy rescues cone function in congenital achromatopsia. Hum. Mol. Genet. 2010;19:2581–2593. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho L.S., Xu J., Pearson R.A., Smith A.J., Bainbridge J.W., Morris L.M., Fliesler S.J., Ding X.Q., Ali R.R. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum. Mol. Genet. 2011;20:3161–3175. doi: 10.1093/hmg/ddr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiguchi K.M., Carvalho L.S., Rizzi M., Powell K., Holthaus S.-M.K., Azam S.A., Duran Y., Ribeiro J., Luhmann U.F., Bainbridge J.W. Gene therapy restores vision in rd1 mice after removal of a confounding mutation in Gpr179. Nat. Commun. 2015;6:6006. doi: 10.1038/ncomms7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Testa F., Maguire A.M., Rossi S., Pierce E.A., Melillo P., Marshall K., Banfi S., Surace E.M., Sun J., Acerra C. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology. 2013;120:1283–1291. doi: 10.1016/j.ophtha.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bainbridge J.W.B., Mehat M.S., Sundaram V., Robbie S.J., Barker S.E., Ripamonti C., Georgiadis A., Mowat F.M., Beattie S.G., Gardner P.J. Long-term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weleber R.G., Pennesi M.E., Wilson D.J., Kaushal S., Erker L.R., Jensen L., McBride M.T., Flotte T.R., Humphries M., Calcedo R. Results at 2 Years after Gene Therapy for RPE65-Deficient Leber Congenital Amaurosis and Severe Early-Childhood-Onset Retinal Dystrophy. Ophthalmology. 2016;123:1606–1620. doi: 10.1016/j.ophtha.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Ghazi N.G., Abboud E.B., Nowilaty S.R., Alkuraya H., Alhommadi A., Cai H., Hou R., Deng W.T., Boye S.L., Almaghamsi A. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum. Genet. 2016;135:327–343. doi: 10.1007/s00439-016-1637-y. [DOI] [PubMed] [Google Scholar]
- 15.Mole S.E., Michaux G., Codlin S., Wheeler R.B., Sharp J.D., Cutler D.F. CLN6, which is associated with a lysosomal storage disease, is an endoplasmic reticulum protein. Exp. Cell Res. 2004;298:399–406. doi: 10.1016/j.yexcr.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Bronson R.T., Donahue L.R., Johnson K.R., Tanner A., Lane P.W., Faust J.R. Neuronal ceroid lipofuscinosis (nclf), a new disorder of the mouse linked to chromosome 9. Am. J. Med. Genet. 1998;77:289–297. doi: 10.1002/(sici)1096-8628(19980526)77:4<289::aid-ajmg8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Gao H., Boustany R.-M.N., Espinola J.A., Cotman S.L., Srinidhi L., Antonellis K.A., Gillis T., Qin X., Liu S., Donahue L.R. Mutations in a novel CLN6-encoded transmembrane protein cause variant neuronal ceroid lipofuscinosis in man and mouse. Am. J. Hum. Genet. 2002;70:324–335. doi: 10.1086/338190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirza M., Volz C., Karlstetter M., Langiu M., Somogyi A., Ruonala M.O., Tamm E.R., Jägle H., Langmann T. Progressive retinal degeneration and glial activation in the CLN6 (nclf) mouse model of neuronal ceroid lipofuscinosis: a beneficial effect of DHA and curcumin supplementation. PLoS ONE. 2013;8:e75963. doi: 10.1371/journal.pone.0075963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartsch U., Galliciotti G., Jofre G.F., Jankowiak W., Hagel C., Braulke T. Apoptotic photoreceptor loss and altered expression of lysosomal proteins in the nclf mouse model of neuronal ceroid lipofuscinosis. Invest. Ophthalmol. Vis. Sci. 2013;54:6952–6959. doi: 10.1167/iovs.13-12945. [DOI] [PubMed] [Google Scholar]
- 20.Siegert S., Cabuy E., Scherf B.G., Kohler H., Panda S., Le Y.-Z., Fehling H.J., Gaidatzis D., Stadler M.B., Roska B. Transcriptional code and disease map for adult retinal cell types. Nat. Neurosci. 2012;15:487–495. doi: 10.1038/nn.3032. S1–S2. [DOI] [PubMed] [Google Scholar]
- 21.Travis G.H., Sutcliffe J.G., Bok D. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991;6:61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]
- 22.Pawlyk B.S., Smith A.J., Buch P.K., Adamian M., Hong D.-H., Sandberg M.A., Ali R.R., Li T. Gene replacement therapy rescues photoreceptor degeneration in a murine model of Leber congenital amaurosis lacking RPGRIP. Invest. Ophthalmol. Vis. Sci. 2005;46:3039–3045. doi: 10.1167/iovs.05-0371. [DOI] [PubMed] [Google Scholar]
- 23.Dalkara D., Sahel J.-A. Gene therapy for inherited retinal degenerations. C. R. Biol. 2014;337:185–192. doi: 10.1016/j.crvi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Kay C.N., Ryals R.C., Aslanidi G.V., Min S.-H., Ruan Q., Sun J., Dyka F.M., Kasuga D., Ayala A.E., Van Vliet K. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS ONE. 2013;8:e62097. doi: 10.1371/journal.pone.0062097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cronin T., Vandenberghe L.H., Hantz P., Juttner J., Reimann A., Kacsó A.E., Huckfeldt R.M., Busskamp V., Kohler H., Lagali P.S. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol. Med. 2014;6:1175–1190. doi: 10.15252/emmm.201404077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalkara D., Byrne L.C., Klimczak R.R., Visel M., Yin L., Merigan W.H., Flannery J.G., Schaffer D.V. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013;5:189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 27.Harvey A.R., Kamphuis W., Eggers R., Symons N.A., Blits B., Niclou S., Boer G.J., Verhaagen J. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: a comparison with lentiviral vectors. Mol. Cell. Neurosci. 2002;21:141–157. doi: 10.1006/mcne.2002.1168. [DOI] [PubMed] [Google Scholar]
- 28.Dalkara D., Kolstad K.D., Caporale N., Visel M., Klimczak R.R., Schaffer D.V., Flannery J.G. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 2009;17:2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberdick J., Smeyne R.J., Mann J.R., Zackson S., Morgan J.I. A promoter that drives transgene expression in cerebellar Purkinje and retinal bipolar neurons. Science. 1990;248:223–226. doi: 10.1126/science.2109351. [DOI] [PubMed] [Google Scholar]
- 30.van Wyk M., Hulliger E.C., Girod L., Ebneter A., Kleinlogel S. Present Molecular Limitations of ON-Bipolar Cell Targeted Gene Therapy. Front. Neurosci. 2017;11:161. doi: 10.3389/fnins.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masu M., Iwakabe H., Tagawa Y., Miyoshi T., Yamashita M., Fukuda Y., Sasaki H., Hiroi K., Nakamura Y., Shigemoto R. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 32.Pardue M.T., McCall M.A., LaVail M.M., Gregg R.G., Peachey N.S. A naturally occurring mouse model of X-linked congenital stationary night blindness. Invest. Ophthalmol. Vis. Sci. 1998;39:2443–2449. [PubMed] [Google Scholar]
- 33.Scalabrino M.L., Boye S.L., Fransen K.M.H., Noel J.M., Dyka F.M., Min S.-H., Ruan Q., De Leeuw C.N., Simpson E.M., Gregg R.G. Intravitreal delivery of a novel AAV vector targets ON bipolar cells and restores visual function in a mouse model of complete congenital stationary night blindness. Hum. Mol. Genet. 2015;24:6229–6239. doi: 10.1093/hmg/ddv341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockton R.A., Slaughter M.M. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J. Gen. Physiol. 1989;93:101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong-Riley M. Energy metabolism of the visual system. Eye Brain. 2010;2:99–116. doi: 10.2147/EB.S9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramachandran P.S., Lee V., Wei Z., Song J.Y., Casal G., Cronin T., Willett K., Huckfeldt R., Morgan J.I., Aleman T.S. Evaluation of dose and safety of AAV7m8 and AAV8BP2 in the non-human primate retina. Hum. Gene Ther. 2017;28:154–167. doi: 10.1089/hum.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mowat F.M., Gornik K.R., Dinculescu A., Boye S.L., Hauswirth W.W., Petersen-Jones S.M., Bartoe J.T. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther. 2014;21:96–105. doi: 10.1038/gt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins J., Holder G.E., Herbert H., Adams G.G.W. Batten disease: features to facilitate early diagnosis. Br. J. Ophthalmol. 2006;90:1119–1124. doi: 10.1136/bjo.2006.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F., Wang H., Tuan H.-F., Nguyen D.H., Sun V., Keser V., Bowne S.J., Sullivan L.S., Luo H., Zhao L. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum. Genet. 2014;133:331–345. doi: 10.1007/s00439-013-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding S.-L., Tecedor L., Stein C.S., Davidson B.L. A knock-in reporter mouse model for Batten disease reveals predominant expression of Cln3 in visual, limbic and subcortical motor structures. Neurobiol. Dis. 2011;41:237–248. doi: 10.1016/j.nbd.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Staropoli J.F., Haliw L., Biswas S., Garrett L., Hölter S.M., Becker L., Skosyrski S., Da Silva-Buttkus P., Calzada-Wack J., Neff F. Large-scale phenotyping of an accurate genetic mouse model of JNCL identifies novel early pathology outside the central nervous system. PLoS One. 2012;7:e38310. doi: 10.1371/journal.pone.0038310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groh J., Stadler D., Buttmann M., Martini R. Non-invasive assessment of retinal alterations in mouse models of infantile and juvenile neuronal ceroid lipofuscinosis by spectral domain optical coherence tomography. Acta Neuropathol. Commun. 2014;2:54. doi: 10.1186/2051-5960-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidoff A.M., Ng C.Y.C., Sleep S., Gray J., Azam S., Zhao Y., McIntosh J.H., Karimipoor M., Nathwani A.C. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J. Virol. Methods. 2004;121:209–215. doi: 10.1016/j.jviromet.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Powner M.B., Gillies M.C., Tretiach M., Scott A., Guymer R.H., Hageman G.S., Fruttiger M. Perifoveal müller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117:2407–2416. doi: 10.1016/j.ophtha.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.