Abstract
Background and purpose
Telangiectatic osteosarcoma (TOS), a rare variant of osteosarcoma, may be easily misdiagnosed as aneurysmal bone cyst (ABC). The aims of this study were to investigate the diagnostic and prognostic factors of TOS by reviewing our experience with TOS and to develop a diagnostic model that may distinguish TOS from ABC.
Materials and methods
We identified 51 cases of TOS treated at the First Affiliated Hospital of Sun Yat-Sen University from March 2001 to January 2016 and reviewed their records, imaging information and pathological studies. A diagnostic model was developed to differentiate TOS and ABC by Bayes discriminant analysis and was evaluated. The log-rank test was used to analyze the prognostic factors of TOS and to compare the outcome differences between TOS and other high-grade osteosarcoma subtypes.
Results
The multi-disciplinary diagnostic method employed that combined clinical, imaging, and pathological studies enhanced the diagnostic accuracy. Age 18 years or younger and pathologic fracture were more common among the TOS patients than among the ABC patients (P = .004 and .005, respectively). The average white blood cell (WBC), platelet, lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) values of the TOS patients were higher than those of the ABC patients (P = .002, .003, .007, and .007, respectively). Our diagnostic model, including the aforementioned factors, accurately predicted 62% and 78% of the TOS patients in the training and validation sets, respectively. The 5-year estimates of event-free survival and overall survival of the TOS patients were 52.5 ± 9.4% and 54.9 ± 8.8%, respectively, which were similar to those of patients with other osteosarcoma subtypes (P = .950 and .615, respectively). Tumor volume and the LDH level were predictive prognostic factors (P = .040 and .044) but not the presence of pathologic fracture or misdiagnosis (P = .424 and .632, all respectively).
Conclusions
The multi-disciplinary diagnostic method and diagnostic model based on predictive factors, i.e., age, the presence of pathologic fracture, and platelet, LDH, ALP and WBC levels, aided the differentiation of TOS and ABC. Smaller tumors and normal LDH levels were associated with better outcomes.
Abbreviations: ABC, Aneurysmal bone cyst; ALP, Alkaline phosphatase; EFS, Event-free survival; LDH, Lactate dehydrogenase; MR, Magnetic resonance; MTX, Methotrexate; OS, Osteosarcoma; SD, Standard deviations; TOS, Telangiectatic osteosarcoma; WBC, White blood cell
Keywords: Telangiectatic osteosarcoma, Aneurysmal bone cyst, Discriminant analysis, Diagnostic model, Prognostic factor
1. Introduction
Telangiectatic osteosarcoma (TOS) is a rare osteosarcoma subtype with an incidence of 2–12% among all cases of osteosarcoma (OS) [1], [2], [3], [4], [5], first described by Paget [6]. The metaphyses of long tubular bones are common sites for this tumor, and similar to conventional OS, the femur is the most frequent site, followed by the humerus and tibia [7]. Histologically, TOS is described as being composed of multiple aneurysmally dilated cavities filled with blood, and high-grade sarcomatous cells may be observed at the peripheral rim and within septae [8].
TOS has been easily misdiagnosed as a type of benign tumor, particularly aneurysmal bone cyst (ABC) [9], [10], [11]. Although many investigators have described the radiographic and histologic features of TOS [8], [12], [13], the differentiation of TOS from ABC remains a challenging task for radiologists and pathologists. The magnetic resonance (MR) imaging features of TOS include large, lucent lesions and fluid levels simulating those of ABC [14]. Because TOS is sometimes not adequately sampled by core-needle biopsy due to its lytic and cystic nature, cellular atypia and osteoid formation may be absent, which may lead to misdiagnosis [10], [12]. Consequently, misdiagnoses often delay accurate diagnoses and appropriate treatments, which may negatively influence patient prognosis [9].
In addition to the difficulty in diagnosis, TOS is well known for controversy regarding patient prognosis. In 1976, Matsuno et al. proposed that the outcome of TOS was worse than that of conventional OS [2]. However, subsequent reports indicated that the overall survival rate of TOS patients was similar to that of other OS subtypes [4], [15], [16]. Regarding prognostic predictive factors, Scully et al. reported that pathologic fracture correlated with a higher local recurrence rate and a lower survival rate [17]. However, Weiss et al. reported on 22 TOS patients and concluded that the presence of pathologic fracture did not affect the outcome and that chemotherapy with more than 3 active agents led to a better patient outcome [9]. Discrepancies between the previous studies exist, mainly due to the small sample sizes they reported and because some suboptimal case reports lacked an adequate prognostic analysis.
In this study, we reviewed a relatively large sample of 51 cases of TOS treated at the First Affiliated Hospital of Sun Yat-Sen University and characterized the clinical case features with the aim of developing a diagnostic model capable of distinguishing TOS from ABC. Additionally, we evaluated the prognostic factors of TOS and investigated whether a significant prognostic difference existed between TOS and other high-grade OS subtypes.
2. Materials and methods
2.1. Patients
We identified 51 TOS and 162 ABC patients whose diagnoses were confirmed by post-operative pathology and who were treated at our hospital from March 2001 to January 2016 and from January 2003 to May 2014, respectively. We reviewed their medical records, including their clinical characteristics, imaging information, pathological studies, laboratory results, and treatments. The Medical Ethical Committee of our hospital approved this study.
The TOS patients met the diagnostic criteria established by the World Health Organization Classification [18] for the presence of the following: (1) a radiographically lytic and destructive lesion without significant sclerosis; (2) a grossly hemorrhagic multicystic lesion without fleshy or sclerotic areas; and (3) histologically confluent osteoid formation and blood-filled or empty cystic cavities separated by septae containing and/or lined by malignant tumor cells with prominent nuclear atypia. The ABC patients fulfilled the diagnostic criteria [18] for the presence of the following: (1) a radiographically lytic, eccentric and expansile mass with well-defined margins; (2) grossly well-defined and blood-filled cystic spaces separated by tan-white and gritty septae; and (3) multicystic spaces separated by fibrous septae composed of fibroblasts, osteoclastic giant cells and reactive woven bone by histology.
Blood samples were collected from the patients before they underwent initial neoadjuvant chemotherapy or, for those who did not receive neoadjuvant chemotherapy, surgery. The white blood cell (WBC), platelet, serum lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) levels of the samples were measured. Because physiological growth exerts an influence on ALP expression [19], the upper serum ALP limit for patients younger than 18 years was set as 150 U/L, while the upper limit for those 18 years or older was 110 U/L [20].
2.2. Imaging evaluations
Radiographic and MR images were obtained prior to therapy. The specific bone affected, the extent of the lesion and the presence of pathologic fracture were evaluated. The disease stage was determined by chest CT and bone scan. The tumor size was measured in the largest dimension by the MR imaging modality that best represented the lesion range [13], and the tumor volume was calculated according to the formula for the volume of an ellipsoid mass = [π*length*width*depth/6].
2.3. Pathological study
All patients underwent core-needle biopsy or open biopsy before preoperative chemotherapy. The dimensions of the tumor samples were 10–20 mm in length and 3–5 mm in width [13]. The tract of the biopsy depended on the clinical data and imaging.
2.4. Treatment protocol
The treatment protocol for all patients diagnosed with osteosarcoma included chemotherapy and surgery. Standard chemotherapy consisted of at least one cycle of preoperative chemotherapy, which included two courses of methotrexate (MTX) and one course of adriamycin combined with cisplatin, and no less than three cycles of post-operative chemotherapy, each of which was composed of two courses of MTX and one course of adriamycin combined with cisplatin [21]. The interval between each round of chemotherapy was two to three weeks and adjuvant chemotherapy was administered two to three weeks after surgery [22]. Limb-sparing surgery and amputation surgery were performed, and the former was based on the principle of tumor eradication through a wide resection margin [21].
2.5. Post-operative follow-up
During the post-operative chemotherapy period, the patients underwent a chest CT scan and an X-ray of the surgical site every 3 months to facilitate the early detection of lung metastasis and local recurrence. The chest CT scan and X-ray of the surgical site were performed every 3 months for the first 2 years, every 6 months during the 3rd and 4th years and annually for the 5th through 10th years after completion of post-operative chemotherapy.
2.6. Statistical analyses
We used SPSS (version 22.0, Chicago, IL, USA) to analyze the data. Categorical variables were presented as numbers and percentages, and for continuous variables, the means and standard deviations (SD) were used. Differences were considered statistically significant when the P-value was less than .05. The Chi-square test and t-test were employed to determine the diagnostic clinical characteristic variables between the ABC patients and the TOS patients. Bayes discriminant analysis was applied to these variables to develop a diagnostic model composed of two discriminant functions for TOS and ABC. Then, the model was examined in the training set retrospectively and in the validation set prospectively.
The diagnostic study included the 51 TOS cases and the 162 ABC cases to investigate the differences in the clinical characteristics. To develop and validate the diagnostic model, 69 ABC and 13 TOS cases with missing discriminant variable values were excluded. Among the 93 ABC and 38 TOS cases included in this study, 75% of them (70 ABC cases and 29 TOS cases) were randomly chosen for inclusion in the training set, upon which the diagnostic model was based. The remaining 25% of the included cases (23 ABC cases and 9 TOS cases) served as the validation set to examine the accuracy of the diagnostic model.
For the survival analysis, we included 39 patients who were followed up by phone for at least two years after diagnosis unless they had expired. Survival was defined as the time interval from the date of diagnosis to the date of the last follow-up or the date of death from any cause. Event-free survival (EFS) was defined as the time interval from the date of diagnosis to the date of the first event or the date of the last follow-up for patients who had no events. An event included recurrent or progressive disease and death from any cause. Regarding the estimates of EFS and overall survival, Kaplan-Meier survival analysis was performed. Then, log-rank tests were employed to identify those factors that correlated with the prognosis of patients. Due to the small sample size, a multivariate analysis was not performed.
3. Results
3.1. Clinical characteristics
Among the 1128 OS patients treated at our hospital from March 2001 to January 2016, 51 patients had TOS (4.52%). Their ages ranged from 5 to 39 years (median: 16 years). The clinical characteristics are presented in Table 1. Thirty-four patients (67%) were male, and 17 patients (33%) were female. The male/female gender ratio was 2:1. The tumors were located around the knee in 32 cases (63%), and the femur was the most commonly affected site (26 cases, 51%). At presentation, 5 patients (10%) had metastatic disease, and the disease had metastasized to the lung in 4 of these patients, while 46 patients (90%) had localized disease. Seventeen patients (33%) had pathologic fractures.
Table 1.
Clinical characteristics of 51 patients with telangiectatic osteosarcoma.
| Characteristic | No. of the patients (%) |
|---|---|
| Age at diagnosis, y | |
| Median | 16 |
| Range | 5–39 |
| <18 | 29 (57) |
| ≥18 | 22 (43) |
| Gender | |
| Male | 34 (67) |
| Female | 17 (33) |
| Primary tumor site | |
| Femur | 26 (51) |
| Tibia | 11 (22) |
| Humerus | 8 (16) |
| Fibula | 3 (6) |
| Ilium | 2 (4) |
| Scapula | 1 (2) |
| Disease stage at diagnosis | |
| Localized | 46 (90) |
| Metastatic | 5 (10) |
| Pathologic fracture | |
| Yes | 17 (33) |
| No | 34 (67) |
3.2. Differential diagnosis from ABC
Of the 51 TOS patients, 40 (78%) were diagnosed with osteosarcoma or invasive/malignant bone tumor by imaging (Table 2), and 11 patients (22%) were misdiagnosed with aneurysmal bone cysts. Preoperatively, the 51 patients underwent a total of 54 core-needle biopsies and 5 open biopsies. Regardless, 11 patients (22%) were misdiagnosed with benign tumors, i.e., ABC (9 cases, 18%) and osteoblastoma (2 cases, 4%), with negative findings in three patients (6%). Considering the clinical, imaging, and pathological studies, the multi-disciplinary diagnoses were TOS (12 cases, 24%), OS (30 cases, 59%), Ewing's sarcoma (1 case, 2%), ABC (7 cases, 14%), and osteoblastoma (1 case, 2%).
Table 2.
Preoperative diagnoses of 51 TOS patients.
| Characteristic | No. of the patients (%) |
|---|---|
| Diagnoses by imaging | |
| Osteosarcoma | 20 (39) |
| Invasive/Malignant bone tumor | 20 (39) |
| Aneurysmal bone cyst | 11 (22) |
| Diagnoses by biopsy | |
| Telangiectatic osteosarcoma | 11 (22) |
| Osteosarcoma/Malignant bone tumor | 26 (51) |
| Aneurysmal bone cyst | 9 (18) |
| Osteoblastoma | 2 (4) |
| Negative findings | 3 (6) |
| Multi-disciplinary diagnoses | |
| Telangiectatic osteosarcoma | 12 (24) |
| Osteosarcoma/Malignant bone tumor | 30 (59) |
| Aneurysmal bone cyst | 7 (14) |
| Ewing's sarcoma | 1 (2) |
| Osteoblastoma | 1 (2) |
The abovementioned data indicate that TOS was easily misdiagnosed as ABC despite the availability of imaging studies and biopsy results. With the aim of improving the diagnostic accuracy, we analyzed the difference in the clinical characteristics between the TOS and the ABC patients (Table 3). The percentage of TOS patients aged 18 years or younger and the percentage of TOS patients who sustained pathologic fracture were higher than those of the ABC patients (P = .004 and .005, respectively). The average WBC, platelet, LDH, and ALP levels of the TOS patients were higher than those of the ABC patients (P = .002, .003, .007, and .007, respectively).
Table 3.
Clinical characteristics of TOS and ABC patients.
| Characteristic | TOS patients (%) | ABC patients (%) | χ2/t | P-value |
|---|---|---|---|---|
| Age | ||||
| <18 | 29 (57) | 55 (34) | 8.53 | .004 |
| ≥18 | 22 (43) | 107 (66) | ||
| Gender | ||||
| Male | 34 (67) | 103 (64) | .16 | .688 |
| Female | 17 (33) | 59 (36) | ||
| Pathologic Fracture | ||||
| Yes | 17 (33) | 25 (15) | 7.85 | .005 |
| No | 34 (67) | 137 (85) | ||
| Primary Tumor Site | ||||
| Femur | 26 (51) | 62 (39) | 3.54 | .617 |
| Tibia | 11 (22) | 21 (13) | ||
| Fibula | 3 (6) | 3 (2) | ||
| Humerus | 8 (16) | 31 (20) | ||
| Ilium | 1 (2) | 4 (2) | ||
| Scapula | 1 (2) | 1 (1) | ||
| Platelet (mean ± SD) ×109, n = 49*, 152 | 291.7 ± 96.2 | 246.2 ± 65.0 | 3.087 | .003 |
| WBC (mean ± SD) ×109, n = 49*, 158 | 8.3 ± 2.7 | 7.1 ± 2.1 | 3.139 | .002 |
| LDH (mean ± SD), n = 38*, 95 | 255.1 ± 183.1 | 168.9 ± 44.0 | 2.871 | .007 |
| ALP (mean ± SD), n = 49*, 150 | 244.9 ± 272.3 | 132.8 ± 119.8 | 2.795 | .007 |
* Platelet, WBC, ALP level for 2 patients were missing in the medical record and LDH wasn’t measured for all patients before neoadjuvant chemotherapy.
No significant differences between the training set and validation set were observed regarding the abovementioned factors (Table 4). These factors were selected and analyzed by the Bayes discriminant method to generate two discriminant functions based on the training set (Table 5). We then applied the functions to both the training set and validation set to evaluate their accuracy (Table 6). For prospective examination, 7 TOS patients (78%) and 16 ABC patients (70%) from the validation set were predicted correctly, and the overall percentage of correct prediction was 71.9%. Additionally, correct prediction retrospectively accounted for 62% of the TOS patients and 81% of the ABC patients in the training set.
Table 4.
Characteristics of TOS and ABC patients in the training set and validation set.
| Characteristics | TOS |
ABC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Training set | Validation set | χ2 | P-value | Training set | Validation set | χ2 | P-value | ||
| Age | |||||||||
| <18 | 15 | 6 | .620 | .431 | 22 | 8 | .089 | .765 | |
| ≥18 | 14 | 3 | 48 | 15 | |||||
| Pathologic Fracture | |||||||||
| Yes | 9 | 4 | .549 | .459 | 12 | 3 | .215 | .643 | |
| No | 20 | 5 | 58 | 20 | |||||
| Platelet (mean ± SD) ×109 | 312.3 ± 111.4 | 297.6 ± 47.4 | .703 | 249.3 ± 57.5 | 252.1 ± 82.8 | .882 | |||
| WBC (mean ± SD) ×109 | 8.4 ± 2.9 | 8.9 ± 2.7 | .630 | 6.9 ± 2.3 | 7.0 ± 1.6 | .900 | |||
| LDH (mean ± SD) | 269.7 ± 205.5 | 208.1 ± 62.6 | .385 | 169.2 ± 44.9 | 164.5 ± 40.3 | .658 | |||
| ALP (mean ± SD) | 235.4 ± 225.9 | 371.4 ± 483.6 | .435 | 137.4 ± 147.0 | 161.4 ± 102.5 | .469 | |||
Table 5.
Coefficients of discriminant functions based on the training set.
| Patients | Variables |
||||||
|---|---|---|---|---|---|---|---|
| Agea | Pathologic Fracturea | WBC ×109 | Platelet ×109 | ALP | LDH | (Constant) | |
| TOS | 6.25 | 1.315 | 1.154 | .042 | .010 | .004 | −15.505 |
| ABC | 6.373 | 1.203 | .992 | .038 | .007 | −.002 | −11.557 |
Age and the presence of pathologic fracture were binary variables in the functions. “<18 years” and “without pathologic fracture” were defined as “0”, while the opposites were defined as “1”.
Table 6.
Retrospective and prospective examination of discriminant function accuracy.
| Group | Predicted ABC (%) | Predicted TOS (%) | |
|---|---|---|---|
| Traininga | ABC | 57 (81) | 13 (19) |
| TOS | 11 (38) | 18 (62) | |
| Validationb | ABC | 16 (70) | 7 (30) |
| TOS | 2 (22) | 7 (78) | |
75.8% of training set cases were correctly classified.
71.9% of validation set cases were correctly classified.
3.3. Patient treatments and outcomes
One patient did not undergo surgery at our hospital. Among the 50 TOS patients who had surgery, fifteen patients (30%) underwent amputation, while the others (35 patients, 70%) underwent a limb-salvage procedure as the first surgery. Eight patients (16%) were diagnosed with benign tumors. Two of them underwent amputation, while the rest received limb-salvage treatment. The amputation rate of the patients misdiagnosed with benign entities was 25%, while that of the patients with correct diagnoses was 29%.
Thirty-nine patients were followed up for 8–167 months (median: 32 months). Twenty-three TOS patients (59.0%) survived with a median follow-up of 59 months (range: 24–167 months), while the other 16 patients (41.0%) died of their diseases. The median time from diagnosis to death was 20 months (range: 8–54 months).
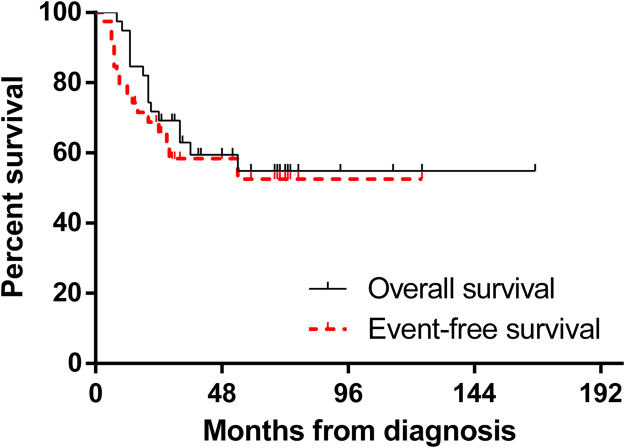
The five-year estimates of EFS and overall survival of the 39 patients were 52.5 ± 9.4% and 54.9 ± 8.8%, respectively (Fig. 1). Additionally, the mean EFS and overall survival estimates were 74 and 102 months, respectively.
Fig. 1.
Kaplan-Meier curves for overall survival and event-free survival of the 39 TOS patients.
The first events of 39 patients were metastases (14 cases), recurrence (6 cases), and second malignancy (1 case). The local recurrence rate for the 39 TOS patients was 15.4%. Nine patients (23%) underwent second surgeries, which included amputation (4 cases), resection of local recurrence (3 cases) and resection of lung metastases (2 cases).
3.4. Potential prognostic factors of TOS
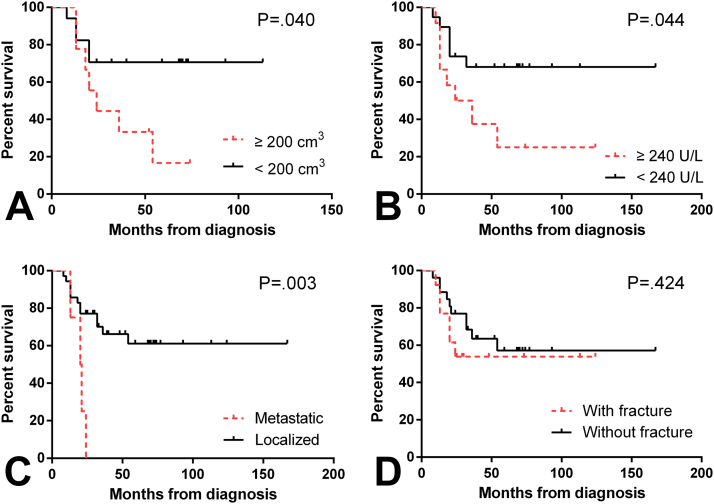
To identify the prognostic factors for EFS and overall survival, we used log-rank tests with univariate analyses (Table 7). As shown in Fig. 2, tumor volume was predictive of patient outcome. Patients whose tumors were larger than 200 cm3 had worse EFS and overall survival estimates than those with smaller tumors (P = .041 and .040, respectively). The patients whose serum LDH levels were less than 240 U/L had better overall survival estimates than those with elevated serum LDH levels (P = .044), with a trend towards significance for EFS (P = .067). Additionally, the TOS outcome correlated with the disease stage at diagnosis (P = .003). Notably, the patients who sustained pathologic fractures had survival estimates similar to those patients without such fractures (P = .489 and .424, respectively). Although six patients were misdiagnosed with benign tumors, their prognoses were no poorer than those of the other patients (P = .781 and .632, respectively). While no significant difference was observed, the EFS and overall survival estimates of the patients receiving standard chemotherapy were comparatively better (P = .059 and .065, respectively).
Table 7.
Univariate analyses of event-free survival and overall survival of the 39 TOS patients.a
| Factor | Event-free survival |
Overall survival |
|||
|---|---|---|---|---|---|
| No. of the patients | 5-Year Estimate, % | P-value | 5-Year Estimate, % | P-value | |
| Age | |||||
| <18 | 23 | 51.6 ± 11.5 | .682 | 53.8 ± 11.0 | .717 |
| ≥18 | 16 | 56.3 ± 14.4 | 57.9 ± 13.7 | ||
| Gender | |||||
| Male | 25 | 56.8 ± 10.7 | .879 | 57.5 ± 10.4 | .855 |
| Female | 14 | 48.0 ± 15.5 | 52.1 ± 14.7 | ||
| Primary Tumor Site | |||||
| Femur | 20 | 41.3 ± 12.9 | .222 | 48.8 ± 11.4 | .375 |
| Other Bones | 19 | 58.4 ± 15.5 | 58.9 ± 15.5 | ||
| Tumor Volume (cm3), n = 26b | |||||
| <200 | 17 | 69.7 ± 11.4 | .041 | 70.6 ± 11.1 | .040 |
| ≥200 | 9 | 16.7 ± 14.2 | 16.7 ± 14.2 | ||
| Disease stage at diagnosis | |||||
| Localized | 35 | 58.7 ± 10.0 | .003 | 61.1 ± 9.2 | .003 |
| Metastatic | 4 | 0 ± 0 | 0 ± 0 | ||
| Pathologic Fracture | |||||
| Yes | 13 | 53.8 ± 13.8 | .489 | 53.8 ± 13.8 | .424 |
| No | 26 | 53.1 ± 11.6 | 57.1 ± 10.7 | ||
| Platelet×109, n = 38b | |||||
| <300 | 23 | 58.0 ± 10.9 | .716 | 59.8 ± 10.5 | .785 |
| ≥300 | 15 | 40.7 ± 16.0 | 46.6 ± 14.4 | ||
| LDH, n = 31b | |||||
| <240 | 19 | 66.0 ± 11.5 | .067 | 68.0 ± 10.8 | .044 |
| ≥240 | 12 | 25.0 ± 14.4 | 25.0 ± 14.4 | ||
| ALP, n = 38b | |||||
| <110/150 | 14 | 69.3 ± 12.9 | .182 | 70.7 ± 12.4 | .166 |
| ≥110/150 | 24 | 38.6 ± 12.7 | 43.3 ± 11.6 | ||
| WBC×109, n = 38b | |||||
| <10 | 32 | 60.4 ± 9.1 | .225 | 60.7 ± 9.0 | .412 |
| ≥10 | 6 | 0 ± 0 | 33.3 ± 19.2 | ||
| Standard Chemotherapy | |||||
| Yes | 17 | 70.8 ± 12.9 | .059 | 70.6 ± 12.8 | .065 |
| No | 22 | 40.7 ± 11.4 | 43.4 ± 11.0 | ||
| Type of Surgery | |||||
| Limb Salvage | 26 | 66.1 ± 10.0 | .093 | 67.9 ± 9.4 | .053 |
| Amputation | 13 | 28.7 ± 15.3 | 26.9 ± 15.1 | ||
| Misdiagnosed with benign tumors | |||||
| Yes | 6 | 50.0 ± 20.4 | .781 | 50.0 ± 20.4 | .632 |
| No | 33 | 52.1 ± 10.8 | 55.8 ± 9.7 | ||
39 patients were included for a minimum of 2-year follow-up.
Tumor volume, platelet, LDH, ALP, and WBC level were measured for 26, 38, 31, 38, 38 patients respectively.
Fig. 2.
Kaplan-Meier curves for the overall survival of TOS patients according to tumor volume (A), serum LDH level (B), disease stage at diagnosis (C), and the presence of pathologic fracture (D).
3.5. Comparison of the outcomes between TOS and other high-grade OS subtypes
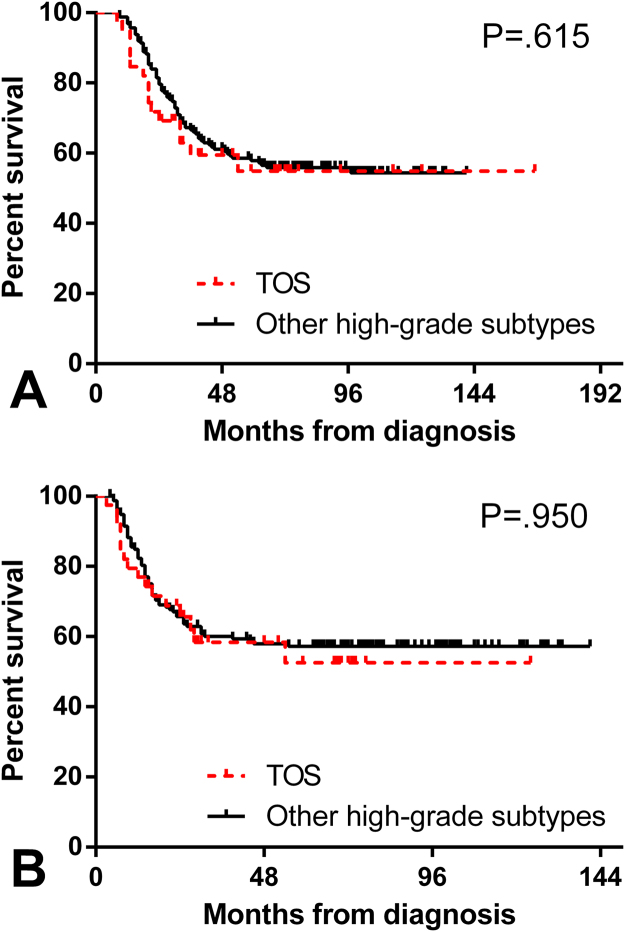
With the aim of investigating additional prognostic characteristics of TOS, we also compared the outcome of TOS to those of other high-grade OS subtypes (162 cases treated at our hospital from April 2003 to October 2010). The five-year overall survival estimates were 54.9 ± 8.8% for TOS patients and 57.9 ± 3.9% for those with other high-grade OS subtypes, while the EFS estimates at 5 years were 52.5 ± 9.4% for the TOS patients and 65.7 ± 3.8% for those with the other high-grade OS subtypes, respectively. No significant differences in overall survival (P = .615) or EFS (P = .950) were observed between the TOS patients and those with other high-grade OS subtypes (Fig. 3). The local recurrence rate (15.4%) of the TOS patients was slightly higher than that (8.0%) of the other OS subtypes (χ2 = 1.99, P = .160).
Fig. 3.
Kaplan-Meier curves for the overall survival (A) and event-free survival (B) of TOS and other high-grade osteosarcoma subtypes.
4. Discussion
As a rare subtype of osteosarcoma, TOS may be easily misdiagnosed as ABC. Although previous reports have fully described the clinical, imaging and pathologic features [8], [12], [13] of TOS, arriving at a correct diagnosis remains demanding, and controversies persist regarding the prognostic factors of TOS. By following up on a relatively large series at our hospital, our retrospective review focused on identifying the diagnostic and prognostic factors of TOS for clinical practice.
The patient characteristics of TOS were summarized in this study. TOS has a higher incidence in males. The percentage of male patients was 54–67% in previous reports [12], [23] and 67% in our study. Pathologic fracture occurred in 33% of our patients (Table 1), which was significantly higher than the fracture rate for conventional OS (6–13%) [11], [17], [24]. The higher rate of pathological fracture may be attributed to the massive bone destruction associated with TOS [18].
Currently, despite advanced imaging and biopsy techniques, the accurate and early diagnosis of TOS remains difficult, particularly regarding the differentiation of TOS from ABC. Although they underwent imaging procedures, 11 patients (22%) were misdiagnosed with ABC (Table 2). Regarding biopsy results, nine patients (18%) were still misdiagnosed with ABC, with negative findings in three patients (6%). By combining clinical, imaging, and pathological studies, the multi-disciplinary diagnoses reduced the number of misdiagnosed patients to seven (14%). The multi-disciplinary diagnosis lowered the risk of misdiagnoses and was crucial to achieve higher diagnostic accuracy.
The need to improve the diagnostic accuracy of TOS is obvious and urgent. Pathological results have been regarded as the gold standard for diagnosing TOS. However, the percentage of misdiagnoses and negative findings by core-needle biopsy was high, mainly due to the limited sample volume and pathological similarities between TOS and ABC. When a definitive biopsy result cannot be obtained, a diagnostic model whose variables are composed of clinical characteristics may aid clinical decision-making.
Our previous research demonstrated that tumor size, Enneking stage, pretreatment platelet and neutrophil counts and pretreatment ALP levels might be useful prognostic factors [22]. Similarly, we found differences in the clinical characteristics between TOS and ABC and developed a diagnostic model. According to our data (Table 3), age, the presence of pathologic fracture, and platelet, LDH, ALP and WBC levels were significant predictive factors (P=.004, .005, .003, .007, .007, and .002, respectively). We used a Bayes discriminant method to generate two discriminant functions (Table 5), which predicted 78% of the TOS patients correctly in the validation set (Table 6). To further validate the model, we intended to apply the functions to 12 patients whose core-needle biopsy results were ABC or negative findings; however, two of them were excluded because serum LDH data were lacking. In the further validation using the remaining ten patients, eight patients (80%) were predicted correctly (Table 8), which demonstrated that our model was relatively accurate.
Table 8.
Validation in ten patients whose core-needle biopsy results were ABC or negative.
| Case | Gender | Agea | Fracturea | WBC | PLT | ALP | LDH | YABCb | YTOSb | Biopsy results | Predictionc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 0 | 1 | 9.45 | 250 | 100 | 204 | 8.81 | 9.03 | ABC | TOS |
| 2 | Female | 0 | 1 | 7.82 | 268 | 239 | 271 | 8.72 | 9.56 | Negative | TOS |
| 3 | Female | 0 | 1 | 9.37 | 312 | 141 | 152 | 11.48 | 11.74 | Negative | TOS |
| 4 | Male | 0 | 0 | 8.33 | 244 | 164 | 210 | 6.71 | 6.84 | ABC | TOS |
| 5 | Male | 0 | 0 | 7.56 | 266 | 124 | 213 | 6.49 | 6.48 | ABC | ABC |
| 6 | Male | 0 | 0 | 2.27 | 323 | 93 | 350 | 2.92 | 3.01 | ABC | TOS |
| 7 | Male | 1 | 0 | 7.51 | 367 | 120 | 298 | 16.46 | 17.22 | ABC | TOS |
| 8 | Male | 1 | 1 | 7.49 | 554 | 65 | 1251 | 22.45 | 29.63 | Negative | TOS |
| 9 | Male | 1 | 1 | 6.85 | 323 | 106 | 151 | 15.53 | 15.19 | ABC | ABC |
| 10 | Male | 1 | 0 | 9.16 | 461 | 107 | 223 | 21.72 | 22.64 | ABC | TOS |
“<18” and “without pathologic fracture” were defined as “0”, while the opposites were defined as “1”.
YABC and YTOS were values calculated by two discriminant functions stated in Table 5.
If YTOS was higher than YABC, the prediction would be TOS, and vice versa.
The abovementioned predictive factors and the diagnostic model may serve as an auxiliary diagnostic method along with the imaging and biopsy results, which will assist clinicians to rule out the possibility of ABC in TOS patients when radiologists and pathologists cannot arrive at definitive diagnoses. The main advantages of this model include its easily obtained variables, ease of application, and relatively high accuracy. However, the accuracy may be further improved by the addition of more cases and the consideration of additional diagnostic factors.
Despite the difficulty in diagnosis, the 5-year estimates of EFS and the overall survival of the 39 patients were 52.5 ± 9.4% and 54.9 ± 8.8%, respectively (Fig. 1). The outcome of the TOS patients was similar to that of patients with other high-grade OS subtypes (Fig. 3), in agreement with previously reported findings [5], [9]. No significant difference was observed in the local recurrence rate between TOS and the other OS subtypes (12.5% vs 8.0%, P=.39). Moreover, the amputation rate was not significantly higher than that of the other OS subtypes (30% vs 25%, P=.49).
We also attempted to determine the potential predictive factors for patient outcome (Table 7, Fig. 2). Tumor volume was associated with the five-year EFS and overall survival estimates (P=.041 and .040, respectively). LDH levels also correlated with overall survival (P=.044). Angelini et al. confirmed the correlation between smaller tumor volume and better outcome in a univariate analysis; however, its prognostic significance was not borne out by multivariate analysis in a review of 87 cases [23]. The predictive significance of tumor volume and LDH level warrant further investigation and examination.
However, the presence of pathologic fracture, which was reported as a risk factor for worse outcome [17], [24], was not significant in our study. Among the 17 patients who sustained pathologic fractures, only four (24%) had amputations, and the remaining 13 patients underwent limb-salvage surgery. Additionally, the misdiagnoses did not result in a higher probability of amputation or a poorer outcome (Table 7). The presence of pathologic fracture or misdiagnosis did not appear to correlate with amputation or a worse outcome likely because of the high chemo-sensitivity of TOS [5], [9], [25] and advances in neoadjuvant chemotherapy. For those patients with a small tumor volume and a satisfactory response to chemotherapy, limb-salvage surgery would be a more favorable choice, regardless of the presence of pathologic fracture.
This study had several limitations. First, values were missing for some patients in the Chi-square test and t-test; thus, the sample size was smaller when we excluded 69 ABC patients and 13 TOS patients to ensure the accuracy of our diagnostic model. Second, due to the low incidence of TOS, only 39 patients were followed up for at least two years. The sample size was too small for multivariate analysis.
5. Conclusion
The use of a multi-disciplinary diagnostic method and a diagnostic model composed of the variables of age, the presence of pathologic fracture, and platelet, LDH, ALP, and WBC levels may aid the differentiation of TOS from ABC. TOS shared a similar prognosis with other OS subtypes. Additionally, the tumor volume and LDH level were prognostic factors, while the presence of pathologic fracture did not correlate with either the type of surgery or the outcome.
Acknowledgments
Not applicable.
Acknowledgments
Competing interests
The authors declare that there are no conflicts of interest to disclose.
Funding
The authors declare that no funding was received for the study.
Contributor Information
Jun-qiang Yin, Email: yinjunqiang77@163.com.
Yi-wei Fu, Email: fungaiwai@163.com.
Xian-biao Xie, Email: biao_zairen@163.com.
Xiao-yu Cheng, Email: chengxy9@mail2.sysu.edu.cn.
Xiao-yu Yang, Email: yangxy46@mail2.sysu.edu.cn.
Wei-hai Liu, Email: liuweihai91@163.com.
Jian Tu, Email: jiantu@foxmail.com.
Zhen-hua Gao, Email: zhenhua_gao@163.com.
Jing-nan Shen, Email: shenjingnan@126.com.
References
- 1.Rosen G., Huvos A.G., Marcove R., Nirenberg A. Telangiectatic osteogenic sarcoma. Improved survival with combination chemotherapy. Clin. Orthop. Relat. Res. 1986;207:164–173. [PubMed] [Google Scholar]
- 2.Matsuno T., Unni K.K., McLeod R.A., Dahlin D.C. Telangiectatic osteogenic sarcoma. Cancer. 1976;38(6):2538–2547. doi: 10.1002/1097-0142(197612)38:6<2538::aid-cncr2820380643>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari S., Smeland S., Mercuri M., Bertoni F., Longhi A., Ruggieri P., Alvegard T.A., Picci P., Capanna R., Bernini G., Muller C., Tienghi A., Wiebe T., Comandone A., Bohling T., Del Prever A.B., Brosjo O., Bacci G., Italian G. Saeter, Sarcoma G. Scandinavian. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian sarcoma groups. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2005;23(34):8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 4.Farr G.H., Huvos A.G., Marcove R.C., Higinbotham N.L., Foote F.W., Jr. Telangiectatic osteogenic sarcoma. A review of twenty-eight cases. Cancer. 1974;34(4):1150–1158. doi: 10.1002/1097-0142(197410)34:4<1150::aid-cncr2820340426>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G., Ferrari S., Ruggieri P., Biagini R., Fabbri N., Campanacci L., Bacchini P., Longhi A., Forni C., Bertoni F. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop. Scand. 2001;72(2):167–172. doi: 10.1080/000164701317323426. [DOI] [PubMed] [Google Scholar]
- 6.J. Paget, Lectures on Surgical Pathology, Lindsay & Blakiston, 1854.
- 7.Turel M.K., Joseph V., Singh V., Moses V., Rajshekhar V. Primary telangiectatic osteosarcoma of the cervical spine. J. Neurosurg. Spine. 2012;16(4):373–378. doi: 10.3171/2011.12.SPINE111037. [DOI] [PubMed] [Google Scholar]
- 8.Murphey M.D., Jaovisidha S. wan, Temple H.T., Gannon F.H., Jelinek J.S., Malawer M.M. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545–553. doi: 10.1148/radiol.2292021130. [DOI] [PubMed] [Google Scholar]
- 9.Weiss A., Khoury J.D., Hoffer F.A., Wu J., Billups C.A., Heck R.K., Quintana J., Poe D., Rao B.N., Daw N.C. Telangiectatic osteosarcoma: the St. Jude Children's Research Hospital's experience. Cancer. 2007;109(8):1627–1637. doi: 10.1002/cncr.22574. [DOI] [PubMed] [Google Scholar]
- 10.Moore D.D., Luu H.H. Osteosarcoma. Cancer Treat. Res. 2014;162:65–92. doi: 10.1007/978-3-319-07323-1_4. [DOI] [PubMed] [Google Scholar]
- 11.Liu J.J., Liu S., Wang J.G., Zhu W., Hua Y.Q., Sun W., Cai Z.D. Telangiectatic osteosarcoma: a review of literature. OncoTargets Ther. 2013;6:593–602. doi: 10.2147/OTT.S41351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangle N.A., Layfield L.J. Telangiectatic osteosarcoma. Arch. Pathol. Lab. Med. 2012;136(5):572–576. doi: 10.5858/arpa.2011-0204-RS. [DOI] [PubMed] [Google Scholar]
- 13.Gao Z.H., Yin J.Q., Liu D.W., Meng Q.F., Li J.P. Preoperative easily misdiagnosed telangiectatic osteosarcoma: clinical-radiologic-pathologic correlations. Cancer Imaging. 2013;13(4):520–526. doi: 10.1102/1470-7330.2013.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarmish G., Klein M.J., Landa J., Lefkowitz R.A., Hwang S. Imaging characteristics of primary osteosarcoma: nonconventional subtypes. Radiogr. A Rev. Publ. Radiol. Soc. N. Am. Inc. 2010;30(6):1653–1672. doi: 10.1148/rg.306105524. [DOI] [PubMed] [Google Scholar]
- 15.Huvos A.G., Rosen G., Bretsky S.S., Butler A. Telangiectatic osteogenic sarcoma: a clinicopathologic study of 124 patients. Cancer. 1982;49(8):1679–1689. doi: 10.1002/1097-0142(19820415)49:8<1679::aid-cncr2820490824>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Mervak T.R., Unni K.K., Pritchard D.J., McLeod R.A. Telangiectatic osteosarcoma. Clin. Orthop. Relat. Res. 1991;270(270):135–139. [PubMed] [Google Scholar]
- 17.Scully S., Ghert M., Zurakowski D., Thompson R., Gebhardt M. Pathologic Fracture in Osteosarcoma. Progn. Import. Treat. Implic. 2002 [PubMed] [Google Scholar]
- 18.Fletcher C.D.M., B. J.A, Hogendoorn P.C.W., Mertens F. IARC Press; Lyon: 2013. World Health Organization Classification of Tumours of Soft Tissue and Bone. [Google Scholar]
- 19.Szulc P., Seeman E., Delmas P.D. Biochemical measurements of bone turnover in children and adolescents. Osteoporos. Int.: J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2000;11(4):281–294. doi: 10.1007/s001980070116. [DOI] [PubMed] [Google Scholar]
- 20.Han J., Yong B., Luo C., Tan P., Peng T., Shen J. High serum alkaline phosphatase cooperating with MMP-9 predicts metastasis and poor prognosis in patients with primary osteosarcoma in Southern China. World J. Surg. Oncol. 2012;10:37. doi: 10.1186/1477-7819-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan P.X., Yong B.C., Wang J., Huang G., Yin J.Q., Zou C.Y., Xie X.B., Tang Q.L., Shen J.N. Analysis of the efficacy and prognosis of limb-salvage surgery for osteosarcoma around the knee. Eur. J. Surg. Oncol.: J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2012;38(12):1171–1177. doi: 10.1016/j.ejso.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Wang B., Tu J., Yin J., Zou C., Wang J., Huang G., Xie X., Shen J. Development and validation of a pretreatment prognostic index to predict death and lung metastases in extremity osteosarcoma. Oncotarget. 2015;6(35):38348–38359. doi: 10.18632/oncotarget.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angelini A., Mavrogenis A.F., Trovarelli G., Ferrari S., Picci P., Ruggieri P. Telangiectatic osteosarcoma: a review of 87 cases. J. Cancer Res Clin. Oncol. 2016;142(10):2197–2207. doi: 10.1007/s00432-016-2210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scully S.P., Temple H.T., O'Keefe R.J., Mankin H.J., Gebhardt M. The surgical treatment of patients with osteosarcoma who sustain a pathologic fracture. Clin. Orthop. Relat. Res. 1996;324:227–232. doi: 10.1097/00003086-199603000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Colomina J., Peiro A., Trullols L., Garcia I. Telangiectatic osteosarcoma. J. Orthop. Surg. 2013;21(1):96–99. doi: 10.1177/230949901302100124. [DOI] [PubMed] [Google Scholar]