Abstract
Background
Malaria is increasing in some recently urbanized areas that historically were considered lower risk. Understanding what drives urban transmission is hampered by inconsistencies in how “urban” contexts are defined. A dichotomized “urban–rural” approach, based on political boundaries may misclassify environments or fail to capture local drivers of risk. Small-scale agriculture in urban or peri-urban settings has been shown to be a major risk determinant.
Methods
Household-level Anopheles abundance patterns in and around Malawi’s commercial capital of Blantyre (~ 1.9 M pop.) were analysed. Clusters (N = 64) of five houses each located at 2.5 km intervals along eight transects radiating out from Blantyre city centre were sampled during rainy and dry seasons of 2015 and 2016. Mosquito densities were measured inside houses using aspirators to sample resting mosquitoes, and un-baited CDC light traps to sample host seeking mosquitoes.
Results
Of 38,895 mosquitoes captured, 91% were female and 87% were Culex spp. Anopheles females (N = 5058) were primarily captured in light traps (97%). Anopheles abundance was greater during rainy seasons. Anopheles funestus was more abundant than Anopheles arabiensis, but both were found on all transects, and had similar associations with environmental risk factors. Anopheles funestus and An. arabiensis females significantly increased with distance from the urban centre, but this trend was not consistent across all transects. Presence of small-scale agriculture was predictive of greater Anopheles spp. abundance, even after controlling for urbanicity, number of nets per person, number of under-5-year olds, years of education, and season.
Conclusions
This study revealed how small-scale agriculture along a rural-to-urban transition was associated with An. arabiensis and An. funestus indoor abundances, and that indoor Anopheles density can be high within Blantyre city limits, particularly where agriculture is present. Typical rural areas with lower house density and greater distance from urban centres reflected landscapes more suitable for Anopheles reproduction and house invasion. However, similar characteristics and elevated Anopheles abundances were also found around some houses within the city limits. Thus, dichotomous designations of “urban” or “rural” can obscure important heterogeneity in the landscape of Plasmodium transmission, suggesting the need for more nuanced assessment of urban malaria risk and prevention efforts.
Electronic supplementary material
The online version of this article (10.1186/s12936-018-2375-5) contains supplementary material, which is available to authorized users.
Keywords: Urban–rural, Urban malaria, Small-scale agriculture, Vector ecology, Anopheles, Malawi
Background
Malaria continues to take the lives of nearly half a million people every year, with 90% of deaths occurring in sub-Saharan Africa (SSA). The World Health Organization (WHO) estimates 216 million cases and 445,000 deaths due to malaria in 2016, an increase of approximately 5 million cases during 2015 [1]. Malaria is endemic throughout most of SSA and is the leading cause of death in Malawi among children under five years of age [2].
In 2017, approximately 3.2 million people in Malawi (17% of the population) lived in an urban setting [3, 4]. With an annual urban growth rate of 4%, Malawi has one of the highest rates of urbanization of any African country [3, 4]. Although malaria in SSA has been widely studied, most research has been carried out in rural contexts, and little is known about how increasing urbanicity may be affecting Plasmodium transmission and malaria risk.
Considerable evidence suggests that people living in urban settings in SSA have improved health, including decreased infant mortality, better nutritional status, increased vaccine coverage, and increased access to care [5]. Specific to malaria, studies have shown that long-lasting insecticidal net (LLIN) use is higher in urban compared to rural settings, and overall parasite prevalence in children living in large cities in SSA is less than half that of children living in rural communities within the same zone of malaria endemicity [6–8]. Urban areas generally experience lower incidence of malaria compared to rural settings, as greater human population densities may reduce individual-level exposure [9–11, 13, 14]. Less vegetation and polluted water sources may reduce the number of suitable breeding sites for Anopheles mosquito vectors and limit opportunities for vector dispersal from breeding sites [10–15].
Nonetheless, knowledge about local Plasmodium transmission in highly heterogeneous urban areas remains limited. Conditions of urban poverty, poor quality infrastructure, and small-scale crop production may enhance anopheline breeding habitats, particularly for adaptable species such as Anopheles funestus [12, 14, 16, 18, 19]. In addition, urban land use is often poorly monitored, especially in areas regarded as peri-urban “sprawl” [14]. Urban small-scale agriculture gardens may also provide more suitable breeding and resting sites for Anopheles mosquitoes, thereby contributing to local, urban Plasmodium transmission [17–19].
Definitions of “urban” vary widely among countries, have changed over time, and are often incomplete or not useful for studying disease [20, 21]. Traditionally, a dichotomized approach has been used to differentiate “rural” from “urban”. In developing countries where urbanization is highly variable, this approach may misclassify or fail to capture the fine-scale heterogeneity within these broad classifications, which is essential to understanding underlying drivers of various disease-causing processes [22, 23]. Understanding specific urban or rural factors that are protective against or risky for malaria should improve disease surveillance and help target control efforts [24].
This aim of this study was to characterize the diversity and abundance of Anopheles species along an urban–rural continuum in Blantyre, Malawi, and to assess which household-level and surrounding peri-domestic environmental characteristics are associated with increased vector abundance.
Methods
Study design
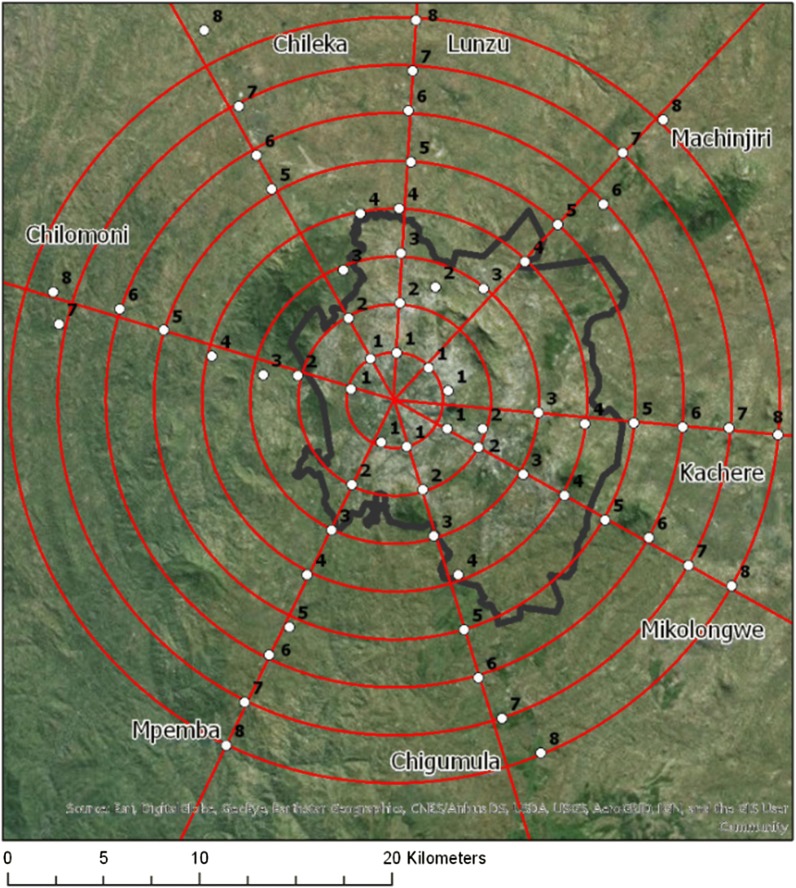
A total of 320 houses were identified for study, comprised of five houses at each of eight locations situated 2.5 km apart along each of eight transects radiating out from Blantyre city centre (Fig. 1). Houses were sampled during five, 6-week periods in both rainy and dry seasons between February 2015 and August 2016, for a possible total of 1600 house-samples. The study protocol was approved by the University of Malawi College of Medicine Research and Ethics Committee, as well as the Institutional Review Boards at Michigan State University and the University of Michigan [25].
Fig. 1.
Sampling design (black boundary denotes Blantyre city administrative boundary). The eight transects were aligned with major roads leading outwards from Blantyre city centre towards rural Blantyre. Clusters of five households each within a distance of 1.5 km of the road were chosen at random from within a 500 m × 500 m area at each of the 64 sampling points. Each 500 m × 500 m area was divided into a grid of 25 subunits, each 100 m × 100 m, and five houses were chosen at random from five of the 25 subunits. If more than one household was in a 100 m × 100 m subunit, only one was selected. If fewer than five houses were located within the total 500 m × 500 m area, houses nearest to the grid were selected progressively until five households total were identified [25]
Informed consent to administer a questionnaire and collect mosquitoes was obtained from the head of household or another adult resident of the household at the time of the first survey visit. Socio-demographic and environmental data were collected for each household and its surrounding area. Household questionnaires were administered by trained surveyors to obtain demographic and malaria risk or prevention information. Data on housing construction and peri-domestic land use/land-cover (LULC) were collected by direct observation of the house, and within a ~ 50 m radius surrounding the dwelling. House construction variables included windows (open or partially open vs. closed), eaves (open or partially open vs. closed) and roofing material (iron sheets, thatch, and tile). The presence or absence of various LULC types including any type of agriculture (maize, millet, cassava, tomato, potato, green peas and/or cocoa), fruit trees, forest, and grazing land were documented. All questionnaire and observational data were recorded on tablets using OpenDataKit collect software.
Mosquito collection
To measure malaria vector species abundance and distribution, indoor resting adult mosquitoes were sampled using Prokopack™ aspirators, and foraging adult mosquitoes were collected using CDC miniature light traps [26, 27]. During each household visit, survey team members spent ~ 10 min. aspirating walls and ceilings of sleeping and living spaces, beneath furniture, behind curtains, and around clothing. Light traps without chemical attractants were turned on at dusk by a household member and were removed the following morning by a study team member. All mosquitoes collected by light trap or aspiration were returned the same day to the entomology lab for morphological species identification, sexing, and determination of blood-feeding by microscopy [28]. Species identification of all Anopheles females was later confirmed by Polymerase Chain Reaction (PCR) at the International Center of Excellence for Malaria Research (ICEMR) Molecular Core facilities at Malawi College of Medicine. Details of laboratory methods for mosquito speciation are presented in Additional file 1.
Satellite-derived variables
Global positioning system (GPS) coordinates for each sampled household were recorded on tablets with a mean accuracy of ± 4.9 m. Multiple GPS-derived locations at the same house were averaged. Composite Google Earth imagery was extracted and analysed with ArcMap 10.2.1 (ESRI, Redlands, CA); dates ranged from January 2015 to December 2016 depending on the highest resolution image with minimal cloud cover available for each region. All households in a 50 m buffer around each observation were digitized and density was computed. Study sites were classified as “within Blantyre city limits” (urban) or “outside Blantyre city limits” (rural) in ArcMap 10.2.1 based on official governmental administrative boundary limits. Publicly available, spatially referenced data were downloaded and values at the household-level were extracted in ArcMap 10.2.1 or QGIS 2.18 (Open Source) for elevation [digital elevation model (DEM)], normalized difference vegetation index (NDVI), and percentage of cropland within a 50 m radius around each dwelling [29–31]. NDVI was calculated in QGIS 2.18 using bands 4 (Red) and 5 (near infrared (NIR)) from Landsat 8 OLI/TIRS C1 Level-1 30 m resolution satellite imagery for two time points, March 21, 2016, and July 27, 2016, corresponding to one rainy and one dry season within the study period respectively [32–34]. NDVI ranges from − 1 to + 1.
Statistical analyses
Descriptive statistics were summarized for all characteristics of households with non-missing exposure, outcome, and covariate data. Mosquito abundance data were summarized for rainy and dry seasons. All statistical analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC).
Negative binomial regression models were used to evaluate possible associations of explanatory variables with counts of Anopheles funestus and Anopheles arabiensis, separately. Explanatory variables included those involving household demographics (number of rooms per house, number of household members, number of children under 5 years old, education status, and sex of household head), and anti-malaria behaviours (bed net ownership and use). In addition, associations between Anopheles abundances and household environmental and peri-domestic characteristics were evaluated, including season, elevation, urban or rural status, distance from city centre, surrounding house density, NDVI, presence of various LULC types (e.g. agriculture, forest, and grazing), livestock ownership, windows, and eaves (open or partially open vs. closed), and roof type.
Abundances of An. funestus and An. arabiensis were analysed as count data with an equal observation time of one trap-night considered for each household. Counts of Anopheles spp. (arabiensis and funestus) mosquitoes caught by light trap exhibited significant over-dispersion. Zero An. arabiensis and An. funestus mosquitoes were captured in 85.2 and 79.7% of households, respectively, one mosquito was caught in 5.6 and 7.1% of households, respectively, and two or more mosquitoes were caught in 9.2 and 13.2% of households, respectively.
Variances of mosquito counts exceeded means, thus Poisson and negative binomial models were compared to determine best fit. Zero-inflated models were not considered despite overdispersion of zero mosquito counts, because zero-inflated models assume that the zero outcome is due to two different processes, one process with zero being the only possible outcome. Since exposure to malaria vectors is generally ubiquitous in this study area, other types of statistical models were compared. Both Poisson and negative binomial model types gave equivalent effect estimates, but negative binomial models for both An. funestus and An. arabiensis fitted the data better based on Akaike information criterion (AIC) and the likelihood ratio test. Logistic regression models were also explored using presence or absence of An. funestus and An. arabiensis separately as the outcome variable. Observations were assumed to be independent due to the cross-sectional nature of the sampling design.
Results
Descriptive statistics
A total of 1548 household surveys were completed during five sample periods in 2015 and 2016. During these surveys, 1472 successful light-trap-nights captured a total of 38,895 mosquitoes (Table 1). Because aspiration capture was inconsistent and produced few adult Anopheles mosquitoes (of 7246 total aspiration captures, only 217 were Anopheles and the remainder Culex), no further analyses of these data were undertaken. Most mosquitoes (87%) collected by light trap were Culex spp., of which the majority (90%) were female Culex. For this report, no analyses of Culex mosquitoes were done as Culex do not contribute to the transmission of human malaria. A total of 4935 Anopheles spp. mosquitoes were collected using CDC light traps; female Anopheles made up 99% of the Anopheles captured (N = 4888) (Table 1).
Table 1.
Summary of mosquitoes collected by CDC light traps
| Sex | Genus | Trap nights | Total | Average per light-trap-night | Standard deviation |
|---|---|---|---|---|---|
| Female | Culex | 1472 | 30,237 | 20.5 | 47.1 |
| Anopheles | 1472 | 4888 | 3.3 | 17.6 | |
| Aedes | 1472 | 156 | 0.1 | 0.9 | |
| Male | Culex | 1472 | 3526 | 2.4 | 9.7 |
| Anopheles | 1472 | 47 | 0.0 | 0.3 | |
| Aedes | 1472 | 41 | 0.0 | 0.3 |
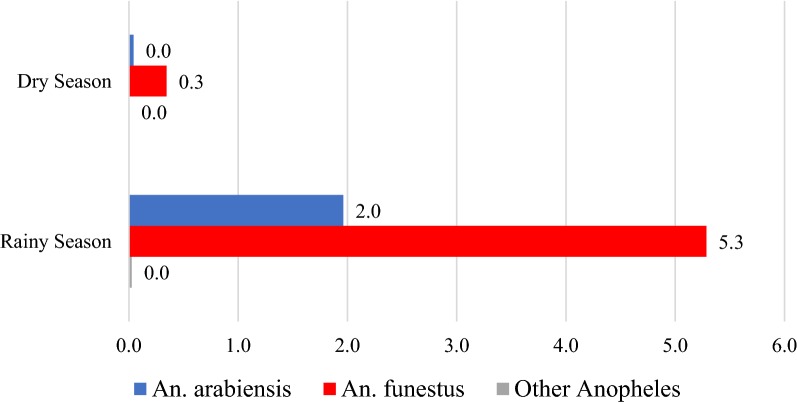
After identification of sex and genus by microscopy, a total of 4550 Anopheles spp. mosquitoes were tested by PCR to determine sibling species; An. arabiensis and An. funestus species were identified. Both An. arabiensis and An. funestus species were captured in the rainy and dry seasons with female An. funestus being much more abundant overall. During the rainy season, the average number of female An. funestus was 5.3 per light-trap-night compared to an average of 2.0 female An. arabiensis. During the dry season, an average of 0.3 female An. funestus were captured per light-trap-night compared to an average of 0 female An. arabiensis (Fig. 2, Table 2). While An. funestus was more abundant than An. arabiensis, both species were found on all transects, including within Blantyre city limits (Fig. 3c, d).
Fig. 2.
Average number of female An. arabiensis and An. funestus per light-trap-night, by season
Table 2.
Summary of Anopheles spp. mosquitoes collected by CDC light traps
| Sex | Species | Trap nights | Total | Average per light-trap-night | Standard deviation |
|---|---|---|---|---|---|
| Dry season | |||||
| Female | Anopheles arabiensis | 845 | 36 | 0.0 | 0.4 |
| Anopheles funestus | 845 | 291 | 0.3 | 2.1 | |
| Other Anopheles | 845 | 2 | 0.0 | 0.1 | |
| Male | Anopheles arabiensis | 861 | 0 | 0.0 | 0.0 |
| Anopheles funestus | 861 | 4 | 0.0 | 0.1 | |
| Other Anopheles | 861 | 0 | 0.0 | 0.0 | |
| Rainy season | |||||
| Female | Anopheles arabiensis | 578 | 1134 | 2.0 | 6.5 |
| Anopheles funestus | 578 | 3055 | 5.3 | 22.0 | |
| Other Anopheles | 578 | 15 | 0.0 | 0.4 | |
| Male | Anopheles arabiensis | 611 | 4 | 0.0 | 0.1 |
| Anopheles funestus | 611 | 9 | 0.0 | 0.2 | |
| Other Anopheles | 611 | 0 | 0.0 | 0.0 | |
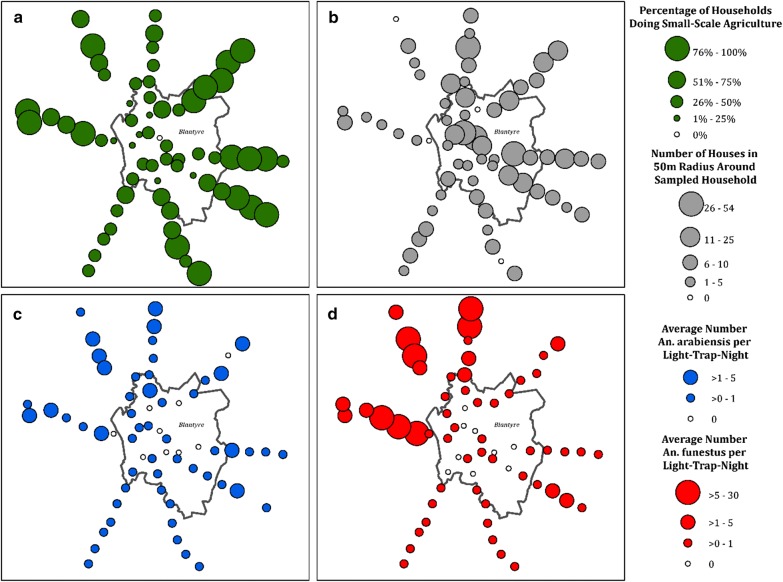
Fig. 3.
Distribution of a peri-domestic agriculture, b urban house density, c Female An. arabiensis, and d Female An. funestus for household clusters along an urban–rural continuum in Blantyre, Malawi
Household-level data were summarized for all 1548 households with non-missing data, and included demographic characteristics, malaria risk or prevention information, and environmental data (including peri-domestic LULC and housing structure characteristics) (Table 4). Almost two-thirds (65.6%) of study households were located within “rural” Blantyre. Households had an average of 3.6 rooms, 0.6 children under 5 years of age, and a head of the household of age 40.5 years on average. About half (52.5%) of all household heads had at least some primary education, and another third (32.3%) had some secondary education. Households owned an average of 1.6 total nets, with less than one net (0.5 nets) per person on average. Nearly two-thirds (63.7%) of respondents reported that they had slept under a bed net the night prior to the survey, and 59.2% reported that other family members had slept under a bed net the night prior to the survey (Table 4).
Table 4.
Bivariate analysis of household demographics, behavioural factors, and peri-domestic environmental characteristics by presence/absence of agriculture
| All households | Agriculture absent | Agriculture present | P value (T test or Chi square) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean or Freq | SD or % | N | Mean or Freq | SD or % | N | Mean or Freq | SD or % | ||
| Demographics | ||||||||||
| Number of rooms | 1546 | 3.7 | 1.9 | 726 | 3.8 | 2.1 | 820 | 3.6 | 1.6 | 0.14 |
| Number of children under age 5 | 1520 | 0.6 | 0.8 | 700 | 0.5 | 0.7 | 820 | 0.6 | 0.8 | < 0.01 |
| Number slept in the house | 1520 | 3.8 | 1.9 | 700 | 3.7 | 2.0 | 820 | 3.9 | 1.7 | 0.13 |
| Age of household head (years) | 1520 | 40.5 | 16.8 | 700 | 40.7 | 16.4 | 820 | 40.3 | 17.1 | 0.64 |
| Male head of household | 1520 | 621 | 40.9% | 700 | 290 | 41.4% | 820 | 331 | 40.4% | 0.67 |
| Highest educational attainment of head of household | 1509 | 692 | 817 | < .0001 | ||||||
| No formal education | 95 | 6.3% | 35 | 5.1% | 60 | 7.3% | ||||
| Some primary education | 792 | 52.5% | 315 | 45.5% | 477 | 58.4% | ||||
| Some secondary education | 487 | 32.3% | 250 | 36.1% | 237 | 29.0% | ||||
| Some college | 135 | 8.9% | 92 | 13.3% | 43 | 5.3% | ||||
| Malaria prevention practices | ||||||||||
| Total number of nets per household | 1546 | 1.6 | 1.3 | 726 | 1.7 | 1.4 | 820 | 1.5 | 1.2 | 0.0001 |
| Respondent used a net night prior to study | 1548 | 986 | 63.7% | 728 | 463 | 63.6% | 820 | 523 | 63.8% | 0.94 |
| Other family members used a net night prior to study | 1548 | 917 | 59.2% | 728 | 414 | 56.9% | 820 | 503 | 61.3% | 0.07 |
| Ave. number of nets per person | 1520 | 0.5 | 0.4 | 700 | 0.5 | 0.4 | 820 | 0.4 | 0.4 | < .0001 |
| Household environmental characteristics (50 m buffer) | ||||||||||
| Within Blantyre city limits | 1548 | 532 | 34.4% | 728 | 352 | 48.4% | 820 | 180 | 22.0% | < .0001 |
| Increasing distance from city centre (2.5 km intervals) | 1548 | 728 | 820 | < .0001 | ||||||
| 2.5 km | 190 | 12.3% | 143 | 19.6% | 47 | 5.7% | ||||
| 5 km | 193 | 12.5% | 132 | 18.1% | 61 | 7.4% | ||||
| 7.5 km | 196 | 12.7% | 115 | 15.8% | 81 | 9.9% | ||||
| 10 km | 193 | 12.5% | 82 | 11.3% | 111 | 13.5% | ||||
| 12.5 km | 193 | 12.5% | 81 | 11.1% | 112 | 13.7% | ||||
| 15 km | 196 | 12.7% | 61 | 8.4% | 135 | 16.5% | ||||
| 17.5 km | 194 | 12.5% | 51 | 7.0% | 143 | 17.4% | ||||
| 20 km | 193 | 12.5% | 63 | 8.7% | 130 | 15.9% | ||||
| Section/region | 1548 | 728 | 820 | < .0001 | ||||||
| Lunzu (1) | 190 | 12.3% | 113 | 15.5% | 74 | 9.0% | ||||
| Chileka (2) | 193 | 12.5% | 106 | 14.6% | 84 | 10.2% | ||||
| Chilomoni (3) | 196 | 12.7% | 86 | 11.8% | 104 | 12.7% | ||||
| Mpemba (4) | 193 | 12.5% | 120 | 16.5% | 74 | 9.0% | ||||
| Chigumula (5) | 193 | 12.5% | 77 | 10.6% | 122 | 14.9% | ||||
| Mikolongwe (6) | 196 | 12.7% | 82 | 11.3% | 113 | 13.8% | ||||
| Kachere (7) | 194 | 12.5% | 84 | 11.5% | 115 | 14.0% | ||||
| Machinjiri (8) | 193 | 12.5% | 60 | 8.2% | 134 | 16.3% | ||||
| Rainy season (vs. dry) | 1548 | 618 | 39.9% | 728 | 124 | 17.0% | 820 | 494 | 60.2% | < .0001 |
| Elevation (m) | 1548 | 945.0 | 175.9 | 728 | 947.2 | 165.3 | 820 | 943.0 | 184.9 | 0.64 |
| Number nearby households | 1548 | 8.3 | 10.4 | 728 | 9.6 | 13.0 | 820 | 7.2 | 7.2 | < .0001 |
| Amount of land used for growing crops (%) | 1548 | 25.8 | 40.4 | 728 | 21.4 | 38.7 | 820 | 29.6 | 41.5 | < .0001 |
| NDVI (rainy season) | 1548 | 0.3 | 0.1 | 728 | 0.3 | 0.1 | 820 | 0.3 | 0.1 | < .0001 |
| NDVI category (rainy season) | 1548 | 728 | 820 | 0.20 | ||||||
| ≤ 0.1 (barren: rock/sand/urban) | 4 | 0.3% | 2 | 0.3% | 6 | 0.7% | ||||
| > 0.1 and ≤ 0.4 (shrub/grassland) | 1291 | 83.4% | 617 | 84.8% | 674 | 82.2% | ||||
| > 0.4 and ≤ 1 (temperate/tropical rainforest) | 251 | 16.2% | 107 | 14.7% | 144 | 17.6% | ||||
| NDVI (dry season) | 1548 | 0.2 | 0.0 | 728 | 0.2 | 0.0 | 820 | 0.2 | 0.0 | < .0001 |
| NDVI category (dry season) | 1548 | 728 | 820 | < .0001 | ||||||
| ≤ 0.1 (barren: rock/sand/urban) | 92 | 5.9% | 73 | 10.0% | 19 | 2.3% | ||||
| > 0.1 and ≤ 0.4 (shrub/grassland) | 1456 | 94.1% | 655 | 90.0% | 801 | 97.7% | ||||
| > 0.4 and ≤ 1 (temperate/tropical rainforest) | 0 | 0% | 0 | 0% | 0 | 0% | ||||
| Fruit trees | 1548 | 1134 | 73.3% | 728 | 439 | 60.3% | 820 | 695 | 84.8% | < .0001 |
| Grazing | 1548 | 275 | 17.8% | 728 | 217 | 8.7% | 820 | 212 | 25.9% | < .0001 |
| Forest | 1548 | 108 | 7.0% | 728 | 65 | 8.9% | 820 | 43 | 5.2% | < 0.01 |
| Ownership of goats | 1546 | 221 | 14.3% | 726 | 76 | 10.5% | 820 | 145 | 17.7% | < .0001 |
| Ownership of chickens | 1546 | 582 | 37.6% | 726 | 248 | 34.2% | 820 | 334 | 40.7% | 0.01 |
| Housing construction | ||||||||||
| Closed (vs. fully/partially open) windows | 1527 | 175 | 11.5% | 719 | 93 | 12.9% | 808 | 82 | 10.2% | 0.09 |
| Closed (vs. fully/partially open) eaves | 1537 | 146 | 9.5% | 720 | 76 | 10.6% | 817 | 70 | 8.6% | 0.19 |
| Roof type | 1512 | 694 | 818 | < .0001 | ||||||
| Iron sheets | 1129 | 74.7% | 565 | 81.4% | 564 | 68.9% | ||||
| Thatched | 379 | 25.1% | 126 | 18.2% | 253 | 30.9% | ||||
| Tile | 4 | 0.3% | 3 | 0.4% | 1 | 0.1% | ||||
Italic values indicate significance of p value (p < 0.05)
Households were located, on average, at 945 m above sea level, with those in urban Blantyre averaging higher elevation (1057 m) compared to rural Blantyre (886 m). Forty percent (39.9%) of household-samples occurred during the rainy season and 60.1% during the dry season. Slightly over half (53.0%) of all households were growing crops at the time of sampling (direct observation), and three quarters (73.3%) had cultivated fruit trees nearby. Approximately one-fifth (17.8%) of households were raising animals for nearby grazing, with 14.3 and 37.6% reporting ownership of goats and chickens respectively. Just 7.0% of households were located near forested areas. Most houses (88.5%) had open or partially open windows, and 90.5% had open or partially open eaves. Approximately three-quarters (74.7%) of roofs were constructed with iron sheets and most of the remainder were thatch (25.1%), with < 1% tile (Table 4).
House density within 50 m of sampled households averaged 8.3 other dwellings (satellite-derived). Households dedicated an average of 25.8% of surrounding land (within a 50 m radius) to crop production (satellite-derived). The average NDVI during the rainy season was 0.3, and 0.2 during the dry season (satellite-derived) (Table 4).
Measures of small-scale agriculture and nearby house density were heterogeneous along the urban–rural continuum in Blantyre, Malawi. Although the proportion of households producing small-scale agriculture tended to increase with distance from the city centre, there were households within Blantyre city limits engaged in small-scale crop production. Likewise, there were clusters within Blantyre city limits with low nearby-house density and clusters outside of Blantyre city limits situated in high house density (Fig. 3a, b).
Bivariate analysis
Negative binomial regression models were used to quantify the associations of explanatory variables with the number of female Anopheles mosquitoes in each household, using separate analyses for An. funestus and An. arabiensis.
Single statistically significant predictors of greater household-level abundances of both An. funestus and An. arabiensis included more children under-5 years old, use of a bed net the preceding night, greater distance from the city centre, survey during the rainy season, higher proportion of surrounding land used for cropping (satellite-derived), presence of small-scale agriculture within a 50 m radius around household (direct observation), an NDVI during the dry season of > 0.1 and ≤ 0.4, typically corresponding to shrub/grassland (satellite-derived), ownership of goats, and having a thatched roof (vs. iron sheets) (Table 3). It is of note that similar associations were observed between Anopheles spp. abundances and measures of small-scale agriculture originating from various sources, including direct observation and satellite-derived measures of NDVI and percentage of land used for cropping.
Table 3.
Bivariate negative binomial models of association between species-specific mosquito abundances and various predictors
| An. arabiensis | An. funestus | |||
|---|---|---|---|---|
| 95% CI | P value | 95% CI | P value | |
| Demographics | ||||
| Number of rooms | 0.8 (0.7, 0.9) | < 0.01 | 0.7 (0.6, 0.8) | 0.0001 |
| Number of children under age 5 | 1.9 (1.3, 2.7) | < 0.001 | 1.9 (1.3, 2.8) | < 0.001 |
| Number slept in the house | 1.0 (0.8, 1.1) | 0.75 | 0.9 (0.8, 1.1) | 0.42 |
| Age of household head (years) | 0.99 (0.98, 1.0) | 0.09 | 0.98 (0.97, 0.99) | < 0.01 |
| Male head of household | 1.1 (0.7, 1.7) | 0.78 | 1.3 (0.8, 2.0) | 0.23 |
| Highest educational attainment of head of household | < 0.001 | 0.0001 | ||
| No formal education | Ref | Ref | ||
| Some primary education | 1.3 (0.5, 3.2) | 2.8 (1.2, 6.9) | ||
| Some secondary education | 0.5 (0.2, 1.3) | 1.6 (0.6, 4.0) | ||
| Some college | 0.4 (0.1, 1.2) | 0.2 (0.1, 0.6) | ||
| Malaria prevention practices | ||||
| Total number of nets per household | 0.8 (0.6, 0.9) | 0.01 | 0.7 (0.6, 0.9) | < 0.001 |
| Respondent used a net night prior to study | 2.5 (1.5, 3.9) | 0.0001 | 2.2 (1.4, 3.4) | < 0.001 |
| Other family members used a net night prior to study | 3.2 (2.0, 4.9) | 0.0001 | 2.5 (1.6, 3.9) | 0.0001 |
| Ave. number of nets per person | 0.2 (0.1, 0.5) | 0.0001 | 0.3 (0.2, 0.6) | < 0.001 |
| Household environmental characteristics (50 m buffer) | ||||
| Within Blantyre city limits | 0.1 (0.1, 0.2) | 0.0001 | 0.02 (0.01, 0.03) | 0.0001 |
| Increasing distance from city centre (2.5 km intervals) | 1.5 (1.4, 1.7) | 0.0001 | 1.7 (1.5, 1.9) | 0.0001 |
| Section/region | ||||
| Lunzu (1) | Ref | Ref | Ref | Ref |
| Chileka (2) | 1.1 (0.5, 2.6) | 0.75 | 1.8 (0.9, 3.6) | 0.08 |
| Chilomoni (3) | 1.0 (0.4, 2.4) | 0.97 | 1.1 (0.5, 2.1) | 0.87 |
| Mpemba (4) | 0.1 (0, 0.3) | 0.0001 | 0.1 (0, 0.1) | 0.0001 |
| Chigumula (5) | 0.2 (0.1, 0.4) | 0.0001 | 0.1 (0, 0.1) | 0.0001 |
| Mikolongwe (6) | 0.9 (0.4, 2.0) | 0.75 | 0.1 (0.1, 0.2) | 0.0001 |
| Kachere (7) | 0.3 (0.1, 0.7) | < 0.01 | 0 (0, 0.1) | 0.0001 |
| Machinjiri (8) | 0.5 (0.2, 1.2) | 0.11 | 0.1 (0, 0.2) | 0.0001 |
| Rainy (vs. dry) season | 46.1 (29.6, 71.6) | 0.0001 | 15.3 (10.5, 22.4) | 0.0001 |
| Elevation (m) | 0.996 (0.995, 0.997) | 0.0001 | 0.992 (0.991, 0.993) | 0.0001 |
| Number nearby households | 0.9 (0.9, 1.0) | 0.0001 | 0.9 (0.8, 0.9) | 0.0001 |
| Amount of land used for growing crops (%) | 2.3 (1.2, 4.2) | < 0.01 | 1.8 (1.0, 3.1) | 0.04 |
| NDVI (rainy season) | 0.4 (0, 6.5) | 0.50 | 2.6 (0.2, 30.4) | 0.44 |
| NDVI category (rainy season) | ||||
| ≤ 0.1 (barren: rock/sand/urban) | Ref | Ref | Ref | Ref |
| > 0.1 and ≤ 0.4 (shrub/grassland) | 2.6 (0.1, 94.7) | 0.60 | 0.6 (0, 17.6) | 0.80 |
| > 0.4 and ≤ 1 (temperate/tropical rainforest) | 1.8 (0, 69.9) | 0.74 | 0.8 (0, 22.3) | 0.89 |
| NDVI (dry season) | 96.5 (0, 212,362.4) | 0.25 | 0.1 (0, 304.7) | 0.53 |
| NDVI category (dry season) | ||||
| ≤ 0.1 (barren: rock/sand/urban) | Ref | Ref | Ref | Ref |
| > 0.1 and ≤ 0.4 (shrub/grassland) | 19.4 (5.2, 72.1) | < .0001 | 111.6 (22, 566.7) | < .0001 |
| > 0.4 and ≤ 1 (temperate/tropical rainforest) | NA | NA | NA | NA |
| Agriculture | 5.8 (3.8, 8.8) | 0.0001 | 4.0 (2.6, 6.1) | 0.0001 |
| Fruit trees | 0.9 (0.6, 1.6) | 0.82 | 0.8 (0.5, 1.2) | 0.27 |
| Grazing | 0.6 (0.3, 1.0) | 0.06 | 0.5 (0.3, 0.9) | 0.02 |
| Forest | 0.4 (0.2, 1.1) | 0.08 | 0.2 (0.1, 0.4) | 0.0001 |
| Ownership of goats | 3.1 (1.7, 5.8) | < 0.001 | 3.9 (2.1, 7.0) | 0.0001 |
| Ownership of chickens | 1.3 (0.8, 2.1) | 0.25 | 1.7 (1.1, 2.6) | 0.03 |
| Housing construction | ||||
| Closed (vs. fully/partially open) windows | 0.9 (0.4, 1.8) | 0.72 | 0.5 (0.2, 0.9) | 0.03 |
| Closed (vs. fully/partially open) eaves | 2.0 (1.0, 4.3) | 0.06 | 2.2 (1.1, 4.5) | 0.03 |
| Roof type | ||||
| Iron sheets | Ref | Ref | Ref | Ref |
| Thatched | 3.0 (1.8, 4.9) | 0.0001 | 4.1 (2.5, 6.6) | 0.0001 |
| Tile | 0.5 (0, 37.0) | 0.74 | 0.6 (0, 31.2) | 0.80 |
Italic values indicate significance of p value (p < 0.05)
Fewer mosquitoes of both species were independently associated with more rooms in the house, higher educational attainment of the household head, greater total number of bed nets, greater average number of bed nets per person, location within Blantyre city limits, location within certain sections of the study area (Mpemba, Chigumula, and Kachere), and greater nearby house density (Table 3).
Greater abundances of An. funestus alone were associated with owning chickens and having closed (vs. open or partially open) eaves. On the other hand, fewer An. funestus were associated with certain sections of the study area (Mikolongwe and Machinjiri), presence of animals for nearby grazing, location near forested areas, and having closed (vs. open or partially open) windows (Table 3). These findings may be suggestive of differences in vector behaviour.
To consider potential confounding, variables that were significantly associated with both An. funestus and An. arabiensis were further evaluated for significant relationships with the presence of small-scale agriculture. Potential confounders were determined to be the number of under 5-year-olds, educational attainment of the head of household, total number of bed nets per household, the household-average of bed nets, location within Blantyre city limits, increasing distance from city centre, rainy season, nearby house density, percentage of cropped land within a 50 m radius of the household, NDVI category during the dry season, and ownership of goats (Tables 3 and 4). Not all variables identified as potential confounders were included in the final models, as percent crop and NDVI category during the dry season were both highly correlated with the main predictor of interest, small-scale agriculture.
Multivariate analysis
Multivariate negative binomial models were used to quantify the association of small-scale agriculture and various urbanity measures with the number of female Anopheles mosquitoes in each household, adjusting for confounding. Anopheles funestus and An. arabiensis were analysed separately for a total of 1387 household-visits after excluding those with missing outcome, exposure, or risk factor information.
Small-scale agriculture and increasing distance from city centre (in 2.5 km intervals) were significantly associated with increased abundances of An. funestus and An. arabiensis, while location within Blantyre city limits and greater nearby house density were significantly associated with decreased abundances of both Anopheles species (Table 5). These relationships remained similar after adjusting for the number of bed nets per person, number of children under 5 years old, education level, and rainy/dry season; however, the effect size of small-scale agriculture on Anopheles spp. abundances generally decreased after adjustment becoming non-significant (Table 6). As expected, season was a strong predictor of Anopheles abundances; inclusion of rainy/dry season in the models attenuated the effect of agriculture on An. arabiensis and reversed the direction of the association between agriculture and An. funestus. The effects of various urbanicity measures on Anopheles spp. abundances remained stable and significant after adjusting for confounding.
Table 5.
Unadjusted multivariate negative binomial models of associations between Anopheles abundances, presence of small-scale agriculture, and urbanicity measures
| An. arabiensis | An. funestus | |||
|---|---|---|---|---|
| 95% CI | P value | 95% CI | P value | |
| Model 1a | ||||
| Agriculture | 5.2 (3.4, 7.9) | < .0001 | 2.7 (1.8, 4.0) | < .0001 |
| Within city limits | 0.2 (0.1, 0.3) | < .0001 | 0.02 (0.01, 0.04) | < .0001 |
| Model 2a | ||||
| Agriculture | 5.6 (3.7, 8.6) | < .0001 | 3.4 (2.3, 5.1) | < .0001 |
| Increasing distance | 1.4 (1.3, 1.6) | < .0001 | 1.6 (1.4, 1.7) | < .0001 |
| Model 3a | ||||
| Agriculture | 5.5 (3.6, 8.4) | < .0001 | 3.7 (2.4, 5.6) | < .0001 |
| House densitya | 0.5 (0.4, 0.8) | 0.001 | 0.3 (0.2, 0.4) | < .0001 |
Italic values indicate significance of p value (p < 0.05)
aUnits are an additional 10 households within a 50 m radius of the sampled household
Table 6.
Multivariate negative binomial models of associations between Anopheles abundances, presence of small-scale agriculture, and urbanicity measures, adjusted for number of nets per person, number of children under 5 years old, education level, and rainy season
| An. arabiensis | An. funestus | |||
|---|---|---|---|---|
| 95% CI | P value | 95% CI | P value | |
| Model 1b | ||||
| Agriculture | 1.4 (0.8, 2.2) | 0.21 | 0.6 (0.4, 0.9) | 0.01 |
| Within city limits | 0.2 (0.1, 0.2) | < .0001 | 0.02 (0.01, 0.04) | < .0001 |
| Model 2b | ||||
| Agriculture | 1.6 (1.0, 2.5) | 0.07 | 0.7 (0.4, 1.2) | 0.19 |
| Increasing distance | 1.3 (1.2, 1.5) | < .0001 | 1.4 (1.3, 1.6) | < .0001 |
| Model 3b | ||||
| Agriculture | 1.5 (1.0, 2.5) | 0.08 | 0.7 (0.5, 1.2) | 0.21 |
| House densitya | 0.5 (0.4, 0.7) | < 0.001 | 0.2 (0.1, 0.3) | < .0001 |
Italic values indicate significance of p value (p < 0.05)
aUnits are an additional 10 households within a 50 m radius of the sampled household
Interactions were assessed between the main effect, presence of small-scale agriculture, and various urbanicity measures. A significant positive interaction was observed between agriculture and “urban” (within Blantyre city limits), while a significant negative interaction was observed between agriculture and increasing distance from city centre (increasingly rural) for An. arabiensis only (Table 7). These findings imply that the presence of small-scale agriculture is more predictive of An. arabiensis abundance at houses within Blantyre city limits and for houses increasingly close to Blantyre city centre. There was no significant interaction found between small-scale agriculture and nearby house density for either An. arabiensis or An. funestus.
Table 7.
Multivariate negative binomial models of associations and interactions between Anopheles abundances, presence of small-scale agriculture, and urbanicity measures
| An. arabiensis | An. funestus | |||
|---|---|---|---|---|
| 95% CI | P value | 95% CI | P value | |
| Model 1c | ||||
| Agriculture | 3.8 (2.3, 6.2) | < .0001 | 2.7 (1.7, 4.2) | < .0001 |
| Within city limits | 0.1 (0, 0.2) | < .0001 | 0.02 (0.01, 0.05) | < .0001 |
| Agriculturea within city limits | 3.4 (1.2, 9.6) | 0.02 | 1.0 (0.4, 2.8) | 1.00 |
| Model 2c | ||||
| Agriculture | 15.6 (5.4, 45.2) | < .0001 | 8.2 (2.9, 23.8) | < .0001 |
| Increasing distance | 1.6 (1.4, 1.8) | < .0001 | 1.7 (1.5, 2.0) | < .0001 |
| Agriculturea increasing distance | 0.8 (0.7, 1.0) | 0.04 | 0.8 (0.7, 1.0) | 0.07 |
| Model 3c | ||||
| Agriculture | 5.3 (2.7, 10.5) | <.0001 | 4.2 (2.0, 8.9) | 0.0001 |
| Housing density | 0.5 (0.3, 0.9) | 0.02 | 0.3 (0.2, 0.6) | < 0.001 |
| Agriculturea house densitya | 1.0 (0.5, 2.2) | 0.91 | 0.8 (0.3, 2.0) | 0.65 |
Italic values indicate significance of p value (p < 0.05)
aUnits are an additional 10 households within a 50 m radius of the sampled household
Discussion
The reasons why malaria persists in many urbanizing areas of sub-Saharan Africa are multifaceted and not well understood. One key question is whether incident cases in urban settings are resulting from transmission there, or from infection acquired during travel to more rural settings, which then is transported back to urban residences. Understanding such drivers of malaria risk is critical in contexts experiencing rapid urbanization, such as Malawi, where the urban growth rate is 4% per annum [4]. Malaria prevention among the ~ 3.2 million (17%) of Malawi’s population living in an urban setting is limited by inadequate knowledge of what determines risk [4]. One challenge is the diverse and imprecise definitions of “urban” or “rural”, which may be misleading and can obscure local heterogeneity across the risk landscape. To understand what constitutes risk may be further complicated by uneven urbanization, making it difficult to prioritize where resources and interventions should be directed. Results from this study demonstrate that small-scale crop production and other peri-domestic environmental factors are major influences on the local abundance of malaria vectors, even in high-density urban areas.
While An. funestus and An. arabiensis were often associated with similar risk factors, several species-specific risk factors were also identified, implying that different strategies may need to be utilized to address species-specific malaria risk. Greater An. funestus and An. arabiensis abundances inside households were predicted by the presence of more under 5-year-olds, greater distance from the city centre, rainy season, more peri-domestic land used for crop production, an NDVI during the dry season of > 0.1 and ≤ 0.4, typically corresponding to shrub/grassland, but which could also reflect the presence of small-scale agriculture in this setting, goat ownership, and having a thatched vs. iron or tile roof. These associations are generally consistent with what has been seen in other similar SSA high-transmission settings and have been explained by various biological and behavioural pathways [35–38]. More An. funestus alone were predicted by chicken ownership and having closed (vs. open or partially open) eaves, suggestive of differences in species behaviour and species-specific risk.
Fewer mosquitoes of both species were independently predicted in households with more rooms, a higher level of educational attainment of the household head, location within Blantyre city limits, location in certain sections of the study area, and higher nearby house density. Plausible mechanisms for these associations have also been proposed in other studies, and mostly involve physical or knowledge-based relationships to mosquito breeding or household access [36, 37]. Fewer An. funestus only were associated with other sections of the study area, presence of animals for nearby grazing, location near forested areas, and having closed (vs. open or partially open) windows [39].
The number of bed nets per household and their reported use were associated differently with vector abundance. More An. funestus and An. arabiensis were observed in households with greater bed net use the night preceding the survey; however, fewer mosquitoes of both species were observed in households where more total bed nets were present, and with a higher number of bed nets per person. These findings are provocative and suggest that people more readily use nets when mosquitoes are more obvious or annoying, while the presence of more nets inside the dwelling, regardless of their nighttime use, may reduce survival or repel mosquitoes from these indoor settings. McCann et al. found that indoor Anopheles density decreased with increasing LLIN use when analysed categorically, which is contrary to the findings from this study [40]. One possible explanation is the cross-sectional nature of our study design. In areas where mosquitoes are more abundant, people may tend to use mosquito nets more frequently or consistently; however, it is not possible to definitely assess the direction of the association from this study alone.
As expected, the effect of seasonality on Anopheles abundances was large and significant, and impacted the other observed effects. Season attenuated the model effect size of small-scale agriculture on An. arabiensis and reversed the direction of the association between small-scale agriculture and An. funestus. Seasonality is a well-known predictor of Plasmodium transmission, as heavy rains in these settings can often allow for Anopheles breeding habitats to expand [35].
Finally, small-scale subsistence agriculture was found to be associated with greater Anopheles spp. abundance in urban and peri-urban Blantyre, even after adjusting for degree of urbanicity and other confounders. This suggests that small-scale agriculture is an important risk factor for greater malaria vector abundance, even in urbanized areas. Furthermore, results demonstrated that small-scale agriculture was more important to Anopheles spp. abundance in “urban” households located within city limits, as evidenced by significant interaction terms between small-scale agriculture and urbanicity measures. In other words, small-scale agriculture is more predictive of Anopheles spp. presence in households located within city limits and at distances closer to the city centre, but less predicative of Anopheles spp. presence in households located outside of Blantyre city limits and at distances further from the city centre. This observation implies there are additional factors at play in more rural area households which were not adequately captured in this study.
Conclusion
The role of environmental characteristics, particularly small-scale agriculture, in the reproduction and survival of malaria vectors in urban habitats is critical, yet still enigmatic. Findings from this study indicate that poverty, poor quality housing, and small-scale agriculture in urban settings contribute to conditions that amplify anopheline mosquito abundance, particularly for adaptable species such as An. funestus, and may thereby augment the risk of urban transmission. Household-level and peri-domestic environmental characteristics found to be associated with malaria vector abundance were identified by characterizing the presence of Anopheles species along an urban–rural continuum in this highly endemic transmission setting. These insights contribute to a better understanding of heterogeneous risk along the urban–rural continuum that impact on local Plasmodium transmission, and that require elucidation for malaria-prevention efforts to become more effective.
Additional file
Additional file 1. Details of laboratory methods for mosquito speciation.
Authors’ contributions
ND assisted with data and sample collection, completed data cleaning and all statistical and spatial analyses, and drafted the manuscript. TM contributed to the design and execution of the transect study, as well as providing critical review of the manuscript. CK managed the Surveillance of Wild Anopheles Transmitters (SWAT) field team and the data collection process, as well as the processing of all mosquito samples and data entry. AB managed the database for the study as well as ensuring quality control of the data. DPM assisted with conception of study hypotheses and designed and managed the study. CD performed the molecular testing, drafted the laboratory methods section, and provided critical review of the manuscript. EDW contributed to the design of the study, the IRB approval processes, the interpretation of results, and provided critical review of the manuscript. MLW assisted with conception of study hypotheses, statistical analyses, and interpretations, and provided critical review of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the many Malawian families who allowed us to disrupt their lives to study them and their households. The careful efforts of the Surveillance of Wild Anopheles Transmitters (SWAT) field team, the Entomology Laboratory assistants from the Malaria Alert Centre, and Dr. Terrie Taylor from the College of Osteopathic Medicine at Michigan State University are gratefully acknowledged. In addition, the efforts of Malawi International Center of Excellence for Malaria Research (ICEMR) Molecular Core laboratory technicians in collecting and processing mosquito samples, along with those of Dr. Karl Seydel who supervises the Molecular Core, are most appreciated.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the independent Institutional Review Boards (IRB) of the University of Malawi College of Medicine, Michigan State University, and the College of Medicine at the University of Malawi. Informed consent was obtained by an adult from each household included in this study.
Funding
Funding was provided by 4U19AI089683 for the Malawi ICEMR. Additional support for NFD came from the Office of Global Public Health (School of Public Health) and the International Institute, both at the University of Michigan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AIC
Akaike information criterion
- CDC
Centers for Disease Control and Prevention
- DEM
digital elevation model
- DNA
deoxyribonucleic acid
- GIS
geographic information systems
- GPS
global positioning system
- ICEMR
International Center of Excellence for Malaria Research
- IRB
Institutional Review Board
- LLIN
long-lasting insecticidal net
- NIR
near infrared
- NDVI
normalized difference vegetation index
- PCR
polymerase chain reaction
- SAS
Statistical Analysis System
- SSA
Sub-Saharan Africa
- WHO
World Health Organization
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12936-018-2375-5) contains supplementary material, which is available to authorized users.
References
- 1.WHO . World malaria report. Geneva: World Health Organization; 2017. [Google Scholar]
- 2.Demographic and Health Surveys . Malawi malaria indicator survey. Rockville: Demographic and Health Surveys; 2017. [Google Scholar]
- 3.The World factbook: Malawi (Washington, DC: Central Intelligence Agency). https://www.cia.gov/library/publications/the-world-factbook/index (2018). Accessed 12 May 2018.
- 4.National Statistical Office . Population and housing census: preliminary, report. Malawi: National Statistical Office; 2008. [Google Scholar]
- 5.Hay S, Guerra C, Tatem A, Atkinson P, Snow R. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holtz T, Marum L, Mkandala C, Chizani N, Roberts J, Macheso A, et al. Insecticide-treated bednet use, anaemia, and malaria parasitaemia in Blantyre District, Malawi. Trop Med Int Health. 2002;7:220–230. doi: 10.1046/j.1365-3156.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- 7.Monasch R, Reinisch A, Steketee R, Korenromp E, Alnwick D, Bergevin Y. Child coverage with mosquito nets and malaria treatment from population-based surveys in African countries: a baseline for monitoring progress in Roll Back Malaria. Am J Trop Med Hyg. 2004;71(Suppl 2):232–238. [PubMed] [Google Scholar]
- 8.Pond B. Malaria indicator surveys demonstrate a markedly lower prevalence of malaria in large cities of sub-Saharan Africa. Malar J. 2013;12:313. doi: 10.1186/1475-2875-12-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousema T, Griffin J, Sauerwein R, Smith D, Churcher T, Takken W, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trape J, Lefebvre-Zante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, et al. Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. Am J Trop Med Hyg. 1992;47:181–189. doi: 10.4269/ajtmh.1992.47.181. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen A, Slooff R. Vector-borne disease problems in rapid urbanization: new approaches to vector control. Bull World Health Organ. 1992;70:1–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Keating J, MacIntyre K, Mbogo C, Githeko A, Regens J, Swalm C, et al. A geographic sampling strategy for studying relationships between human activity and malaria vectors in urban Africa. Am J Trop Med Hyg. 2003;68:357–365. [PubMed] [Google Scholar]
- 13.Qi Q, Guerra C, Moyes C, Elyazar I, Gething P, Hay S, et al. The effects of urbanization on global Plasmodium vivax malaria transmission. Malar J. 2012;11:403. doi: 10.1186/1475-2875-11-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Silva P, Marshall J. Factors contributing to urban malaria transmission in sub-Saharan Africa: a systematic review. J Trop Med. 2012;2012:819563. doi: 10.1155/2012/819563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsay S, Campbell H, Adiamah J, Greenwood A, Bangali J, Greenwood B. Malaria in a peri-urban area of the Gambia. Ann Trop Med Parasitol. 1990;84:553–562. doi: 10.1080/00034983.1990.11812510. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly M, McCall P, Lengeler C, Bates I, D’Alessandro U, Barnish G, et al. Malaria and urbanization in sub-Saharan Africa. Malar J. 2005;4:12. doi: 10.1186/1475-2875-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klinkenberg E, McCall P, Wilson M, Amerasinghe F, Donnelly M. Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar J. 2008;7:151. doi: 10.1186/1475-2875-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afrane Y, Klinkenberg E, Drechsel P, Owusu-Daaku K, Garms R, Kruppa T. Does irrigated urban agriculture influence the transmission of malaria in the city of Kumasi, Ghana? Acta Trop. 2004;89:125–134. doi: 10.1016/j.actatropica.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Matthys B, N’Goran E, Koné M, Koudou B, Vounatsou P, Cissé G, et al. Urban agricultural land use and characterization of mosquito larval habitats in a medium-sized town of Côte d’Ivoire. J Vector Ecol. 2006;31:319–333. doi: 10.3376/1081-1710(2006)31[319:UALUAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.United Nations Population Division . World urbanization prospects: the 2001 revision. New York: United Nations Population Division; 2002. [Google Scholar]
- 21.Vlahov D, Galea S. Urbanization, urbanicity, and health. J Urban Health. 2002;79(Suppl 1):1–12. doi: 10.1093/jurban/79.suppl_1.S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDade T, Adair L. Defining the ‘urban’ in urbanization and health: a factor analysis approach. Soc Sci Med. 2001;53:55–70. doi: 10.1016/S0277-9536(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 23.Dahly D, Adair L. Quantifying the urban environment: a scale measure of urbanicity outperforms the urban-rural dichotomy. Soc Sci Med. 2007;67:1407–1419. doi: 10.1016/j.socscimed.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathanga D, Kapito Tembo A, Mzilahowa T, Bauleni A, Mtimaukenena K, Taylor T, et al. Patterns and determinants of malaria risk in urban and peri-urban areas of Blantyre, Malawi. Malar J. 2016;15:590. doi: 10.1186/s12936-016-1623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker E, Mathanga D, Wilson ML, Mzilahowa T, Taylor T, Kapito-Tembo A. Anopheles mosquito abundance along an urban-to-rural gradient in Blantyre. Protocol. 2014;1–20.
- 26.John W. Hock (Gainesville, Florida). Improved prokopack aspirator. Model 1419. 2009.
- 27.John W. Hock (Gainesville, Florida). CDC miniature light trap. Model 512. 2012.
- 28.Gillies M, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region) South African Inst Med Res. 1987;55:1–143. [Google Scholar]
- 29.ASTER Global Digital Elevation Model. NASA JPL. 2009. 10.5067/aster/astgtm.002. Accessed 18 May 2018.
- 30.Landsat 8 OLI/TIRS C1 Level-1. USGS. Accessed 18 May 2018.
- 31.Kellndorfer J, Cartus O, Bishop J, Walker W, Holecz F. Large scale mapping of forests and land cover with synthetic aperture radar data. In: Holecz F, Pasquali P, Milisavljevic N, Closson D, editors. Land applications of radar remote sensing. Rijeka: InTech; 2014. pp. 59–94. [Google Scholar]
- 32.Ke Y, Im J, Lee J, Gong H, Ryu Y. Characteristics of Landsat 8 OLI-derived NDVI by comparison with multiple satellite sensors and in situ observations. Rem Sens Environ. 2015;164:298–313. doi: 10.1016/j.rse.2015.04.004. [DOI] [Google Scholar]
- 33.Roy D, Wulder M, Loveland T, Woodcock C, Allen R, Anderson M, et al. Landsat-8: science and product vision for terrestrial global change research. Rem Sens Environ. 2014;145:154–172. doi: 10.1016/j.rse.2014.02.001. [DOI] [Google Scholar]
- 34.ARSET Advanced NDVI Webinar Series. NASA. 2016.
- 35.Kelley-Hope L, Hemingway J, McKenzie F. Environmental factors associated with the malaria vectors Anopheles gambiae and Anopheles funestus in Kenya. Malar J. 2009;8:268. doi: 10.1186/1475-2875-8-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghebreyesus T, Haile M, Witten K, Getachew A, Yohannes M, Lindsay S, et al. Household risk factors for malaria among children in the Ethiopian highlands. Trans R Soc Trop Med Hyg. 2000;94:17–21. doi: 10.1016/S0035-9203(00)90424-3. [DOI] [PubMed] [Google Scholar]
- 37.Kirby M, Green C, Milligan P, Sismanidis C, Jasseh M, Conway D, et al. Risk factors for house-entry by malaria vectors in a rural town and satellite villages in The Gambia. Malar J. 2008;7:2. doi: 10.1186/1475-2875-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson I, Borrell L, El-Sadr W, Teklehaimanot A. Individual and household level factors associated with malaria incidence in a Highland Region of Ethiopia: a multilevel analysis. Am J Trop Med Hyg. 2009;80:103–111. [PubMed] [Google Scholar]
- 39.Mzilahowa T, Luka-Banda M, Uzalili V, Mathanga D, Campbell C, Mukaka M, et al. Risk factors for Anopheles mosquitoes in rural and urban areas of Blantyre District, southern Malawi. Malawi Med J. 2016;28:154–158. doi: 10.4314/mmj.v28i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCann R, Messina J, MacFarlane D, Bayoh M, Gimnig J, Giorgi E, et al. Explaining variation in adult Anopheles indoor resting abundance: the relative effects of larval habitat proximity and insecticide-treated bed net use. Malar J. 2017;16:288. doi: 10.1186/s12936-017-1938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Details of laboratory methods for mosquito speciation.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.