Abstract
Targeted temperature management is standard of care for cardiac arrest and is in clinical trials for stroke. N6-cyclohexyladenosine (CHA), an A1 adenosine receptor (A1AR) agonist, inhibits thermogenesis and induces onset of hibernation in hibernating species. Despite promising thermolytic efficacy of CHA, prior work has failed to achieve and maintain a prescribed target core body temperature (Tb) between 32°C and 34°C for 24 hours. We instrumented Sprague–Dawley rats (n = 19) with indwelling arterial and venous cannulae and a transmitter for monitoring Tb and ECG, then administered CHA via continuous IV infusion or intraperitoneal (IP) injection. In the first experiment (n = 11), we modulated ambient temperature and increased the dose of CHA in an attempt to manage Tb. In the second experiment (n = 8), we administered CHA (0.25 mg/[kg·h]) via continuous IV infusion and modulated cage surface temperature to control Tb. We rewarmed animals by increasing surface temperature at 1°C h−1 and discontinued CHA after Tb reached 36.5°C. Tb, brain temperature (Tbrain), heart rate, blood gas, and electrolytes were also monitored. Results show that titrating dose to adjust for individual variation in response to CHA led to tolerance and failed to manage a prescribed Tb. Starting with a dose (0.25 mg/[kg·h]) and modulating surface temperature to prevent overcooling proved to be an effective means to achieve and maintain Tb between 32°C and 34°C for 24 hours. Increasing surface temperature to 37°C during CHA administration brought Tb back to normothermic levels. All animals treated in this way rewarmed without incident. During the initiation of cooling, we observed bradycardia within 30 minutes of the start of IV infusion, transient hyperglycemia, and a mild hypercapnia; the latter normalized via metabolic compensation. In conclusion, we describe an intravenous delivery protocol for CHA at 0.25 mg/(kg·h) that, when coupled with conductive cooling, achieves and maintains a prescribed and consistent target Tb between 32°C and 34°C for 24 hours.
Keywords: : adenosine, thermogenesis, thermolytic, surface cooling, cyclohexyladenosine, A1AR
Introduction
Targeted temperature management (TTM) is a lifesaving and neuroprotective intervention for cardiac arrest (CA) and neonatal hypoxic ischemic encephalopathy (Bernard et al., 2002; HACA, 2002; Shankaran et al., 2005). Current evidence of care for post-CA patients recommends lowering core body temperature (Tb) to 32–36°C for up to 24 hours (Callaway et al., 2015; Nolan et al., 2015).
Shivering remains the most common complication of TTM and may negate therapeutic benefits (Choi et al., 2011). Neuromuscular blockers, sedatives, analgesics, and anxiolytic drugs are the most common medications used to eliminate shivering. Side effects of these antishivering drugs include increased risk of respiratory depression, masking of seizure, and increased risk of pneumonia in stroke patients (Lyden et al., 2016). The feasibility of cooling conscious patients was demonstrated in the Intravascular Cooling in Treatment of Stroke clinical trials; however, excessive antishivering medication may have led to a twofold increase in pneumonia rates (Lyden et al., 2016). There is a clear clinical need for safer and more precise control of Tb, especially for conscious stroke patients.
N6-Cyclohexyladenosine (CHA) is an A1 adenosine receptor (A1AR) agonist that belongs to a new generation of thermolytic agents or drugs that inhibit thermogenesis. Administration of this class of drug is expected to suppress the counterproductive cold defense response of shivering and thus mitigate Tb variation during TTM (Tupone et al., 2016). The benefits of TTM, increased survival and neuroprotection, have been documented with use of intraperitoneal (IP)-administered CHA after CA in rats (Jinka et al., 2015). More recently, we found individual variation and risk of overcooling with CHA and provided proof of principle that surface heating prevented overcooling. Still lacking is the understanding of how to achieve precise control of target temperature during CHA administration.
Using implantable telemetry devices to acquire ECG and a conductive surface cooling/heating system, we describe an IV CHA delivery protocol to achieve and maintain a prescribed and consistent target Tb for up to 24 hours.
Methods
Ethical statement
Animal care and experiments were conducted and followed under the guidelines set forth by Guide and Use of Laboratory Animals, eighth Edition (Council, 2011). All procedures and protocols complied with the Animal Welfare Act and were approved by the Institutional Animal Care and Use Committee (IACUC).
Sixteen male and three female Sprague–Dawley rats with an average age of 212.8 ± 9.6 days and weight of 386.1 ± 17.5 g (mean ± standard deviation) were obtained from the breeding colony of the University of Alaska Fairbanks Biological Research and Diagnostic Facility. This colony is derived from S/A Simonsen albino rats (Simonsen Laboratories, Gilroy, CA). Animals were housed in groups of two or more in clear polycarbonate cages (8.5′W·17′D·8.5′H) with white spruce or pine chip bedding. Food and water were available ad libitum. The photoperiod was kept at a cycle of 12 hours light to 12 hours dark. Ambient temperature in the habitat was set to 21°C.
Surgical procedures
Stereotaxic cannula implantation
Rats were administered prophylactic antibiotics (Baytril 8.88 mg/kg) 12 hours before surgery and twice daily (BID) for 3 days postop. Analgesics (Ketoprofen 1.0 mg/kg intramuscular (IM) daily (QD) and Buprenorphine 0.03 mg/kg IM BID) were administered before surgery and for 3 days after surgery.
Rats were placed in a stereotaxic frame. Cannulae (CMA/12; Harvard Apparatus, Holliston, MA) were implanted into the striatum using stereotaxic coordinates relative to bregma (−0.26 mmAP, +3.2 mmL, −0.6 mmD) with the nose bar set at −3.3 mm. The calvarium was exposed and painted with copalite (Temrex Corporation, Freeport, NY), four anchoring screws were placed, and a hole for the guide cannula was drilled. The dura was punctured, and the cannula was lowered to its final depth and secured with dental cement (Stoelting, Wood Dale, IL). After surgery, rats were housed individually with an absorbent underpad in place of wood shavings. Animals were monitored and allowed to recover for 10 days before their next surgical procedure. Headstages were cleaned daily for 2 weeks with a 3% povidone-iodine solution.
Abdominal transmitter implantation and femoral artery and vein cannulation
Telemetry transmitters (CTA-F40; Data Sciences International, Saint Paul, MN) were implanted in the abdominal cavity, and biopotential leads were sutured to the right pectoralis and left intercostal muscle to form a lead II ECG configuration. The femoral artery was exposed and cannulated with a 12 cm 3Fr polyurethane catheter (C30PU-RECA1302; Instech, Plymouth Meeting, PA), and the femoral vein was cannulated with a 10 cm 3Fr polyurethane catheter (C30PU-RJV1420; Instech). A plastic trocar was used to feed the cannulae out through an interscapular incision. The cannulae were attached to a two-channel vascular access harness (VAD115AB; Instech).
Baytril (8.88 mg/kg) was administered 12 hours before surgery and BID for 3 days postop. Pain was managed with buprenorphine 0.003 mg/kg IM BID and ketoprofen (1.0 mg/kg QD) or buprenorphine with an extended release formula (Buprenorphine SR™) 1.0 mg/kg (single injection lasts 72 hours). Sutures and wound clips were removed 7–10 days postop. To maintain catheter patency, lines were flushed every 5 days with saline and filled with a heparin/glycerol locking solution (final concentration of 500 IU/mL of autoclaved USP glycerol).
Study design
Experiment 1 (Titrating dose to achieve desired Tb)
To determine if dose of CHA could be titrated to manage a constant core and brain temperature, CHA or Vehicle was delivered at various doses and rates with the goal of titrating the dose to maintain Tb between 30°C and 32°C or 32–34°C for 24–48 hours. If Tb dropped below 30°C, rats were placed in a room with an ambient temperature of 21°C; if Tb continued to fall, CHA was discontinued. Thermocouple probes (HYPO-33-1-T-G-60-SMPW-M; Omega, Norwalk, CT) inserted through guide cannulae for measurement of brain temperature were calibrated by placing probes in sealed vials containing sterile saline. The sealed vials were placed in circulating water baths set to 33°C and 37°C.
Twenty-four hours before start of drug administration, rats with and without head stages for measuring temperature were placed in a 16°C room. A calibrated thermocouple probe was then inserted through the guide cannula using a custom made adapter and taped in place in the rats with head stages. Drugs were administered intravenously and/or intraperitoneally, depending on the patency of the venous line. If animals did not cool to target Tb, additional CHA was administered at the same or higher dose. Brain temperature was measured in 6 of 11 rats.
Experiment 2 (Modulation of surface temperature)
Results from Experiment 1 showed that titrating the dose of CHA did not allow for precise control of Tb. We next asked if modulating surface temperature could control Tb with a defined target of 32–34°C over 24 hours of continuous IV administration. Furthermore, we asked if we could rewarm rats with surface temperature before CHA was discontinued.
Using a balanced crossover design, rats (n = 8) received continuous IV infusion of either CHA or vehicle over a 24 hours period. We adjusted surface temperature to promote cooling, avoid overcooling, and rewarm the animals after 24 hours at the desired target Tb. The order of treatment with CHA or vehicle alternated between animals, and a 48 hours washout period separated treatments. We placed rats in the experimental cage 24 hours before start of drug delivery to acclimatize to the new environment. Cage surface was neither cooled nor heated during this period. Ambient temperature was maintained at 21°C throughout the experiment. At the start of drug delivery, cage surface temperature was set to 17°C. If Tb dropped below 32°C, cage surface temperature was ramped up to 32°C to maintain Tb at ∼32°C for 24 hours.
CHA administration was continued at the same dose and rate of infusion, and cage temperature was increased 1°C h−1 until Tb reached 36.5°C. We stopped CHA infusion in all rats after Tb reached 36.5°C. Total time to rewarm varied between animals, but the total duration of cooling and rewarming was ∼30 hours. Animals treated with vehicle infusion did not cool, and the infusion was stopped after 24 hours. Blood was sampled from the arterial line using a three-way stopcock during the experimental procedure. Depending on arterial line patency and availability of personnel, blood was sampled approximately every 4 hours, and heparinized saline (30 IU/mL) was infused at 5 μL/min using a syringe pump (CMA/100; Harvard Apparatus) to maintain arterial line patency. ECG and Tb were acquired as described below.
Drugs
CHA was purchased from Sigma-Aldrich (Saint Louis, MO) and dissolved in 25% (w/v) hydroxypropyl-beta-cyclodextrin (Tokyo Chemical Industry CO., Nihonbashi-honcho, Chuo-Ku, Tokyo, Japan) in sterile water. A stock solution of 10 mg/mL CHA was diluted to 1.0 mg/mL in 0.9% sterile NaCl. Vehicle was a 1:10 ratio of 25% (w/v) hydroxypropyl-beta-cyclodextrin in sterile water and 0.9% sterile NaCl. Solutions for injection were sterilized by 0.2 μm filtration (Acrodisc syringe filter; Sigma-Aldrich, Saint Louis).
Experimental housing
Experiment 1
Rats were placed inside a clear acrylic cylinder cage (29 cm diameter) containing wood shavings at an ambient temperature of 17°C. Food and water were provided ad libitum. To allow for free movement, the cage was placed on top of a turntable (Raturn; BASi, West Lafayette, IN) and rats were fitted with a collar (CMA; Harvard Apparatus) connected to a counter balance arm and movement detector (BASi). To detect transmitter signal, a receiver was placed underneath the cage setup.
Experiment 2
Rats were placed inside a 12′·12′ clear acrylic box without bedding, sitting on top of a custom built, aluminum hydronic surface. The aluminum surface was part of a cooling and heating system controlled by a thermostat, in which circulating water was heated or cooled via thermoelectric Peltier plates and pumped through the aluminum cage floor. This device uses circulating water as a conduction medium, similar to other commercial cooling devices. Heat conductivity of this device may be greater than the water-cooled blankets used clinically due to the high heat conductivity of aluminum.
Food and water were provided ad libitum. The vascular access harness was attached to a tether and swivel (VAHD115T, 375/D/22; Instech) connected to a counter balance arm to provide freedom of movement. To detect transmitter signal, four receivers (RPC1; Data Sciences International) were paired to the implanted transmitter and mounted on each side of the cage with Velcro®.
Data acquisition
Heart rate, ECG tracing, and Tb were measured with CTA-F40 transmitters and RPC1 receivers, and data were acquired with either Dataquest ART or Ponemah software (Data Sciences International). Cage surface temperature was measured using a thermocouple sandwiched between an insulating foam pad and the bottom of the cage feeding to LabVIEW software (National Instruments, Austin, TX). Ambient temperature was acquired with an iButton and corresponding 1-WIRE software (Maxim Integrated, San Jose, CA) and/or with a thermocouple feeding data to LabVIEW software.
Arterial blood was analyzed for the following analytes and derived parameters: pH, PaCO2, PaO2, Na+, K+, Ca2+, Glucose, Lactate, Hct, HCO3−, and SaO2 using a GEM Premier 3000 analyzer and cartridge (Instrument Laboratories, Bedford, MA). Data from the brain thermocouple probe was acquired with an IX-400 data recorder, ETH-401 transducer interface, and LabScribe software (iWorx, Dover, NH). Tbrain was sampled continuously at 10 Hz and averaged over one minute periods. ECG was sampled at 1000 Hz and was used to derive heart rate (HR).
Statistics
Statistical analysis of repeated arterial blood gases and electrolyte samples was accomplished using a linear mixed effects approach (IBM SPSS 19) to rectify missing data due to inconsistent arterial line patency, account for within-rat correlations, and to model time trajectories after treatment. We allowed different intercepts and slopes for individual regression lines across time. Linear regression was used to model Tbrain with Tb using R (version 3.3.1) by averaging results from individual linear models. The significance criterion was set α < 0.05 for all analyses. We report data as mean ± SEM.
Results
Tolerance and variation in response challenge ability to achieve and maintain targeted temperature
Variable thermolytic efficacy of CHA was noted in prior work in our laboratory (Bailey et al., 2017). In this experiment, our goal was to manage Tb within a prescribed Tb range by titrating the dose of CHA until the desired decrease in Tb was achieved. We increased the dose of CHA through IV infusion and/or with bolus injection on a case-by-case basis in an attempt to optimize the dose of CHA to maintain a mild hypothermic state (30–32°C) at an ambient temperature of 17°C.
We found that starting at a low dose of CHA and repeatedly doubling the dose resulted in the development of tolerance. We observed tolerance in all 11 animals with intermittent injections or when dose was increased during continuous IV administration. Our results show that titrating the dose of CHA fails to produce a consistent or controlled decrease in Tb (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ther).
Core body temperature predicts brain temperature during CHA-induced cooling
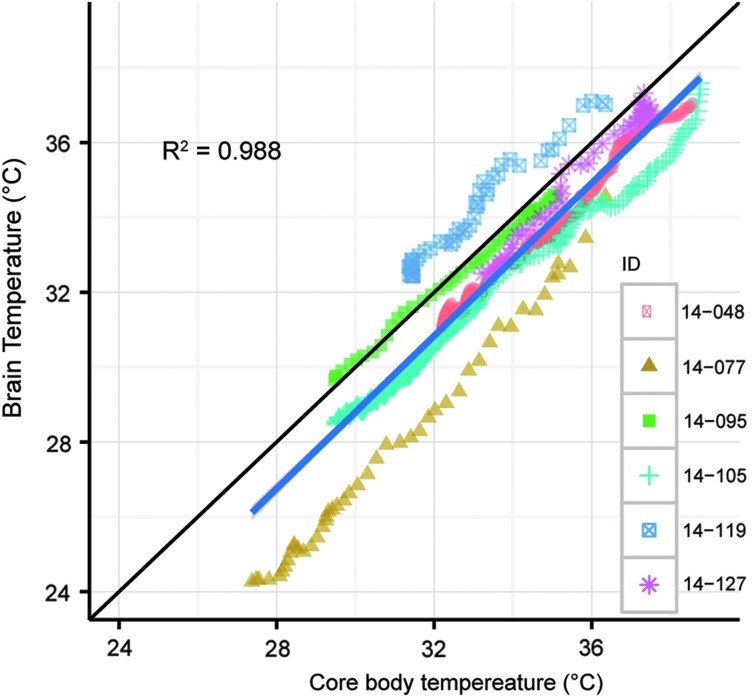
Although dose titration failed to be an effective means to manage target Tb, the wide range of Tb in animals instrumented with IP transmitters and brain thermocouples showed that Tbrain tracked core Tb with a small offset of less than 1°C. Tbrain and core Tb (Fig. 1) were obtained in a total of six experiments. In each case, core Tb and Tbrain were similar. Core Tb and Tbrain analyzed during the first four hours of CHA administration showed a linear relationship with a significant within-subject correlation of 0.98, p < 0.001 (Bland and Altman, 1995). The linear regression yielded an equation of Tbrain = (−0.44 ± 0.68) + (0.98 ± 0.02) Tb (mean ± SEM) showing that on average, Tbrain tracks core Tb. In these experiments, CHA was administered via IP (n = 3), IV (n = 2) and both IV and IP (n = 1) at doses ranging from 0.5–1.0 mg/kg IP and 0.025–0.8 mg/kg IV.
FIG. 1.
Tb predicts Tbrain during the first 4 hours of CHA-induced cooling. Tbrain = −(0.44 ± 0.68) + (0.99 ± 0.02) Tb (mean ± SEM). The linear model represents the mean slope and y-intercept from these experiments (n = 6). CHA, N6-cyclohexyladenosine; HR, heart rate; Tb, body temperature; Tbrain, brain temperature.
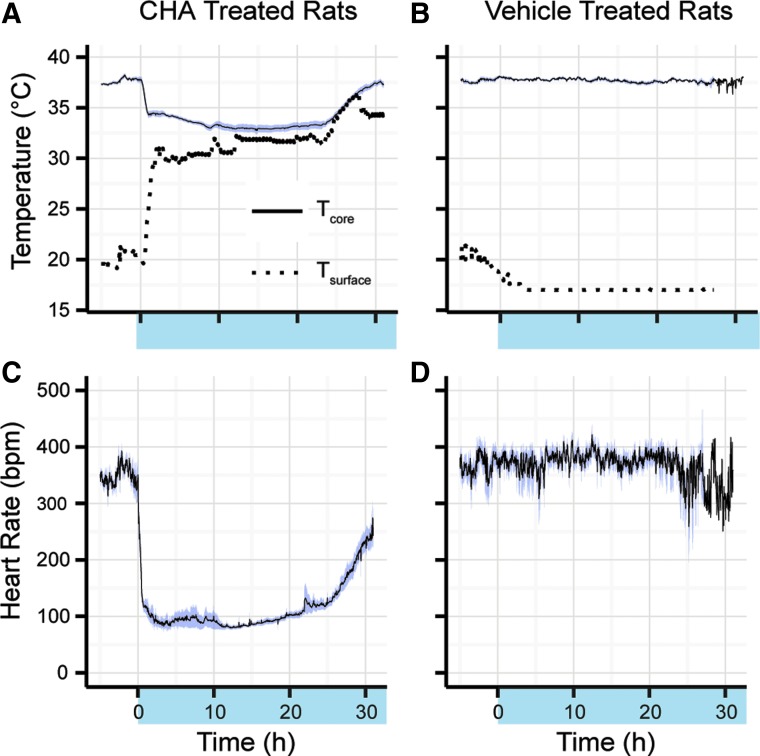
Precise control of core body temperature during cooling and rewarming can be managed with surface temperature modulation and continuous IV administration of CHA
To overcome variation in response, which carries risk of overcooling, we adjusted “dialed-in” core Tb to target Tb using the dynamically cooled and heated surface (Fig. 2). Continuous infusion of CHA 0.25 mg/(kg·h) and modulation of surface cage temperature over 24 hours to 31.6°C ± 0.5°C with an ambient temperature of 21.1°C ± 0.3°C resulted in the lowering of Tb to 33.2°C ± 0.3°C (Fig. 3A). HR decreased to an average of 89 ± 4 bpm over 24 hours from a baseline 24 hours HR of 362 ± 9 bpm (Fig. 2C). Vehicle administration over 24 hours with the surface cage temperature at 17.1°C ± 0.1°C and an ambient temperature of 19.5°C ± 0.5°C resulted in no change in Tb (Fig. 2B) or HR (Fig. 2D). Tb and HR during vehicle delivery over 24 hours were 37.7°C ± 0.1°C and 380 ± 12 bpm.
FIG. 2.
Targeted temperature is maintained with continuous IV infusion of CHA (0.25 mg/[kg·h]), indicated by shaded x-axis region; results are shown as mean ± SEM over 24 hours (n = 8). (A) Surface temperature was set to 17°C at start of CHA administration and increased to prevent overcooling; Tb over 24 hours was 33.2°C ± 0.3°C. (B) Surface temperature lowered to 17.1°C ± 0.1°C with vehicle administration resulted in a Tb of 37.7°C ± 0.1°C. (C) HR decreased to an average of 89 ± 4 bpm over 24 hours. (D) HR during vehicle administration remained within normal limits for the duration of the experiment.
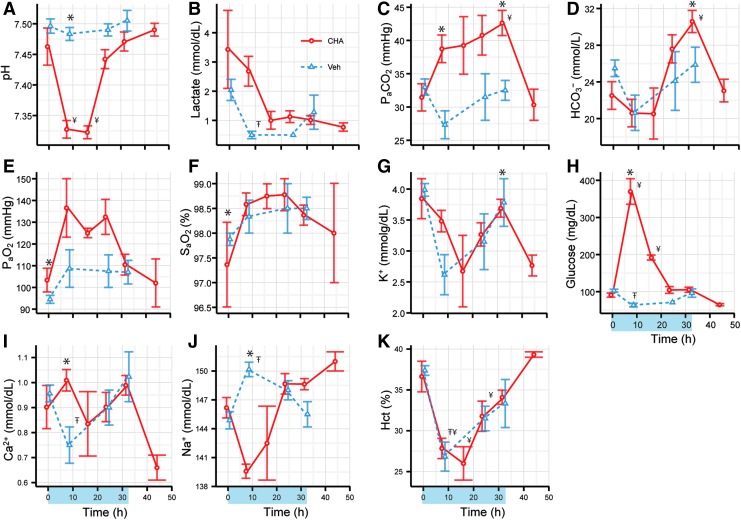
FIG. 3.
Arterial acid-base, electrolyte, and blood gas analysis indicated acute hyperglycemia and resolution of a mild respiratory acidosis by metabolic compensation (n = 8). A significant difference between CHA and vehicle are indicated by asterisks (*). A significant difference between baseline and time during CHA treatment is indicated by the symbol ¥. A significant difference between baseline and time during vehicle treatment is indicated by the symbol Ŧ. (A) arterial pH (B) lactate (C) arterial PaCO2 (D) bicarbonate (E) PaO2 (F) hemoglobin oxygen saturation (G) potassium (H) glucose (I) calcium (J) sodium (K) hematocrit.
Acid-base, electrolyte, and blood gas analysis indicate acute hyperglycemia
Clinical protocols for TTM in CA patients require frequent monitoring of arterial blood gases and electrolytes. Similarly, we monitored blood gases and electrolytes throughout CHA-assisted cooling. Arterial pH dropped from 7.46 ± 0.03 to 7.33 ± 0.01 during the first eight hours of cooling with CHA and continued to remain low for the next eight hours (Fig. 3A, p = 0.002, main effect of drug). We observed a minimum pH of 7.32 ± 0.01 16 hours after onset of CHA administration; pH began to increase and returned to baseline values at 24 hours.
The initial fall in pH was not associated with a rise in lactate (Fig. 3B). Although PaCO2 tended to increase in CHA treated animals, the increase was not statistically significant (Fig. 3C). Bicarbonate levels began to rise at 16 hours in CHA-treated animals and reached a maximum of 30.6 ± 1.2 mmol/L at 32 hours (Fig. 3D, p = 0.004, drug × time). Blood glucose surged to a maximum of 370.0 ± 34.6 mg/dL after 8 hours of cooling and returned to a baseline of 104.5 ± 9.2 mg/dL after 24 hours (Fig. 3H, p = 0.03, main effect of drug). Arterial PaO2 increased slightly during the 24 hours of cooling with CHA compared to vehicle-treated animals (p = 0.03, main effect of drug). No other analyte measured showed a statistically significant influence of treatment.
Discussion
The purpose of the present study was to optimize a CHA delivery protocol to achieve and maintain a prescribed and consistent target Tb for 24 hours. In our first approach, we used IV drug delivery and doubled the dose of CHA in an attempt to reach a stable target Tb. We found evidence of acute tolerance to CHA, when we increased doses with or without breaks in drug administration (Supplementary Data). Overall, we found that short duration of time between injections and increasing the dose during continuous IV infusion determines tolerance. Tolerance following intermittent injections is consistent with prior literature (Roman et al., 2008) showing that doses of an A1AR agonist repeated at 12–24 hours intervals lead to tolerance. To our knowledge, this is the first report of tolerance during IV titration of CHA.
Development of tolerance is consistent with in vitro studies showing downregulation and desensitization of A1AR after chronic treatment with CHA (Hettinger et al., 1998; von Lubitz, 1999). Moreover, repeated daily administration of N6-cyclopentyladenosine (another A1AR agonist) was shown previously to decrease receptor density in hippocampus and somatosensory cortex and attenuate the drop in Tb (Roman et al., 2008). Desensitization is thought to be mediated by internalization and degradation of receptors or the uncoupling of receptors from their G-proteins (Jacobson et al., 1996).
In these same experiments, Tb proved to be a reliable proxy for Tbrain. After averaging results from individual linear models, the average offset of 0.44°C between Tbrain and Tb and a slope not different from one suggests that Tbrain closely mirrors Tb. This close relationship between Tb and Tbrain holds over a wide range of Tb and is consistent with an inhibition of thermogenesis. We interpret the variation in the difference between Tb and Tbrain observed in individual experiments as error in temperature measurements. This error should be minimized in future studies by more frequent calibration of thermocouples and transmitters. We only report the first four hours of Tbrain and Tb during cooling in experiment one: some animals overcooled and did not rewarm or technical complications prevented analysis during rewarming.
The y-intercept of 0.44°C should not be overinterpreted as the predicted difference between brain and body temperature since the line crosses the y-intercept at body temperature values that are outside of the range of the observed values. Tbrain was not measured in experiment two. We expect the relationship between Tb and Tbrain would have been similar to what was described in experiment one, although additional studies are needed to confirm this explanation. Sampling resolution was high enough to capture a one minute lag time between Tbrain and Tb.
Neuroprotective benefits of TTM require that Tbrain be decreased and maintained using core Tb to predict Tbrain. Other studies have shown that Tb predicts Tbrain, but that Tbrain lags behind Tb (Coppler et al., 2016). Still others have shown that Tb does not predict Tbrain (Childs and Lunn, 2013). The method of cooling affects the relationship between Tb and Tbrain as well as size of the animal; size impacts surface area to volume ratio and thus rates of heat loss.
The present study will need to be performed in larger animals and humans to confirm this close relationship between Tb and Tbrain; however, we expect that inhibition of thermogenesis will favor a tight relationship between Tb and Tbrain because inhibition of thermogenesis eliminates the source of heat to the core and thus to the brain. One benefit of inhibiting thermogenesis with CHA during cooling may be rapid cooling of the brain and reduced lag time between Tb and Tbrain.
To avoid overcooling, we utilized a hydronic surface, heated and cooled via thermoelectric Peltier plates, to precisely modulate Tb. Although Bailey provided proof of concept for this device to prevent overcooling caused by individual variation in response to CHA (Bailey et al., 2017), we show in this study that external control of conductive cooling and heating lowers and maintains Tb at a prescribed target Tb between 32°C and 34°C over a 24 hours period with continuous IV CHA infusion.
This duration of cooling and target temperature is similar to current medical protocols and the duration of cooling exceeds prior preclinical studies (Feketa and Marrelli, 2015; Lee et al., 2016; Wyse and McNett, 2016). The choice of targeted temperature is controversial due to a recent study which showed equivalent survival rates in patients cooled to 36°C vs. 33°C (Nielsen et al., 2013). The current findings suggest that CHA may be used to inhibit shivering and facilitate precise control of a target Tb of 36°C or 33°C.
Our experiment is novel because it is the first to administer CHA via continuous IV infusion, a realistic method of long-term administration in a hospital intensive care unit (ICU) setting. The use of dynamic surface cooling also mimics current medical devices (e.g., Arctic Sun®) utilized in TTM (Lundbye, 2012). Also, this experiment is the first to actively rewarm by increasing surface temperature to the normothermic target of 37°C during continuous CHA infusion. Prior studies rewarmed passively after CHA was discontinued by using the temperature gradient between Tb and Ta to reestablish baseline Tb (Jinka et al., 2015). In this study, we show that the rate of rewarming, during the third and final phase of TTM, can be precisely and dynamically controlled with surface temperature modulation and IV CHA inhibition of thermogenesis.
One complication of uncontrolled and rapid rewarming is a shift in electrolyte balance, hypotension, and life threatening arrhythmias (Mirzoyev et al., 2010). This makes precise control of the rewarming process all the more necessary. Counterproductive heat generated during shivering in all phases of TTM and subsequent increase in cerebral metabolism may negate neuroprotective benefits of cooling (Saigal et al., 2015). It is expected that the protocol described in this study will improve outcome after CA, since we previously demonstrated a neuroprotective benefit of CHA-assisted cooling after CA (Jinka et al., 2015). This expectation requires further testing in preclinical cerebral ischemia/reperfusion models.
The current study is also the first to monitor changes in HR during IV CHA-assisted cooling and rewarming over a 24 hours time period using telemetry in nonanesthetized and undisturbed freely moving rats. We interpret the increase in HR during the rewarming phase with CHA still on board as reversal of bradycardia caused by lowered Tb. The ∼150 bpm increase in HR after rewarming, but before discontinuation of CHA, illustrates that both CHA and lowered Tb contribute to bradycardia. Bradycardia at 37°C Tb may result from direct effects of CHA on cardiac pacemaker cells (Lerman, 2003) and from increased vagal tone due to drug action within the central nervous system (CNS) (Tupone et al., 2013).
Interestingly, bradycardia during TTM is a clinical prognosticator of favorable outcomes after CA (Staer-Jensen et al., 2014). Decreased metabolic demand, indicated by a reduction in HR, may explain why bradycardia predicts favorable outcome. Studies show that the rate of oxygen consumption decreases before the drop in Tb during onset of hibernation and that HR is a proxy for metabolic rate (Currie et al., 2014). Rapid onset of bradycardia in the current study is consistent with metabolic suppression secondary to the inhibition of thermogenesis (Kozyreva et al., 2015).
Given our systemic route of CHA administration, it is not possible to decipher between direct cardiac effects and indirect effects related to inhibition of metabolism during the cooling phase or to increased parasympathetic influence. However, we have shown in a previous study that administration of a peripherally acting adenosine receptor antagonist, which does not cross the blood–brain barrier, reverses bradycardia (Jinka et al., 2015).
Hyperglycemia is a known complication of TTM (Saigal et al., 2015). We observed an early spike in glucose during the initiation of cooling which resolved by the end of the experiment. Two mechanisms likely explain this rise in blood glucose: cold induced insulin resistance and stress hyperglycemia (Lundbye, 2012; Deane and Horowitz, 2013). The latter is the more plausible explanation. If hyperglycemia was mediated directly by the cold, blood glucose should have remained elevated throughout the cooling phase; instead, hyperglycemia was only present during cooling initiation. Stress may have been induced by the sudden decrease in cardiovascular parameters or changes in blood gas and pH.
Ettleson et al. (2014) found that hypothermia during TTM in humans had no effect on blood glucose and determined that hyperglycemia was likely due to the severity of stress after CA. Another factor associated with insulin resistance is enhanced lipolysis due to obesity, but CHA should actually decrease insulin resistance by an A1AR-mediated reduction in lipolysis (Morigny et al., 2016). Other preclinical studies have found that hyperglycemia during cooling compromises the neuroprotective efficacy of cooling (Zhang et al., 2009).
Importantly, hyperglycemia and hypotension may counter neuroprotective benefit of hypothermia as best illustrated in early studies of high doses of adenine nucleotides. Inspired by torpor-like effects of adenosine monophosphate (AMP) at doses up to 3.5 g/kg in mice (Zhang et al., 2006), others asked if hypothermia induced by high doses of adenine nucleotides would be neuroprotective after stroke. Doses of 2.0 g/kg adenosine triphosphate (ATP) or 1.4 g/kg AMP in rats produced hypotension (40–55 mmHg lasting 6 hours) and worsened stroke outcomes (Zhang et al., 2009, 2013). In the ATP study, increased infarct size was attributed to a combination of hypotension and hyperglycemia, whereas increased seizure activity was thought to be due to hypocalcemia.
Others found that hypothermia induced by high-dose 500 mg/kg AMP persisted in A1AR deficient mice showing that hypothermia induced by high doses is not mediated by A1AR (Tao et al., 2011). A more recent study targeted A1AR with AMP at a lower dose of 50 mg/kg, avoided hypotension and demonstrated enhanced survival and neuroprotection in a middle cerebral artery model of stroke (Muzzi et al., 2013). Similarly, our prior work demonstrated neuroprotective efficacy of CHA-assisted cooling after CA (Jinka et al., 2015). Moreover, hypocalcemia was not observed in the present study.
Development of CHA or other A1AR agonists as thermolytic agents for TTM will nonetheless require better understanding and management of hyperglycemia and hypotension before these drugs can be translated for clinical applications. In the clinical setting, the transient hyperglycemia might be managed with a tight insulin protocol with sufficient monitoring to avoid hyperglycemia. In the current study, glycemic levels tended to normalize without intervention. Hyperglycemia and hypoglycemia exacerbate cerebral ischemia/reperfusion injury (Dave et al., 2011) such that early management and control of initial hyperglycemia is critical to maximize neuroprotective benefits of cooling.
The present study suggests that respiratory depression is not a significant concern, given that PaO2 and SaO2 remained within normal ranges. Minimal CO2 retention with a small but statistically significant decrease in pH was not indicative of respiratory depression since SaO2 remained over 97%. Blood gas analysis revealed a small decrease in pH that corresponded with a trend toward a rise in PaCO2 after CHA administration, although this change in pH was within normal physiological values in rats (Subramanian et al., 2013). The tendency for CO2 to increase could have been mediated by A1AR agonist-induced bronchoconstriction or hypoventilation (Lerman, 2003). Importantly, this change in CO2 was not statistically significant or sufficient to drive arterial pH outside of normal values.
Blood oxygen saturation remained normal throughout the experiment indicating that oxygen supply was not compromised during treatment. These parameters along with a decrease in lactate also indicate that organ perfusion was adequate. The fall in lactate may indicate increased clearance or decreased anaerobic metabolism. Another possible mechanism to explain the fall in lactate is the conversion of lactate to glucose via the Cori cycle in the liver. This latter mechanism could also account for the elevated glucose during the beginning of the cooling phase.
The initial fall in hematocrit seen in both CHA and vehicle groups likely represents a hemodilutional effect due to IV saline flushes at the start of the experiment to flush arterial lines and continuous infusion of saline used to keep lines open for the duration of the experiment. Levels returned to baseline consistent with hemodilution seen with bolus IV therapy (Grathwohl et al., 1996).
The findings of this study contribute to our understanding of the efficacy of A1AR agonists as novel thermolytic agents. If safety can be optimized, inhibition of thermogenesis with A1AR agonists has potential to transfer precise control of core Tb from the patient to the clinician.
Supplementary Material
Acknowledgments
We thank Chris Terzi, Katrina Dowell, and Carl Murphy for technical assistance; and Katrina McCandless for animal handling. This work was supported by Sloan Scholar Alfred P. Sloan Foundation's Indigenous Graduate Partnership (SIGP) Program (B.L. and I.R.B.); National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM115371 (B.L.), American Heart Association postdoctoral fellowship (to Z.B.), National Institutes of Health National Institute of Neurologic Disorders and Stroke [Grant R15NS070779]; Alaska Space Grant Program pilot project; and Alaska INBRE P20GM103395. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
Dr. Drew is the Scientific Director of Be Cool Pharmaceutics. All other authors claim no conflict of interest in this investigation.
References
- Bailey IR, Laughlin B, Moore LA, Bogren LK, Barati Z, Drew KL. Optimization of thermolytic response to A1 adenosine receptor agonists in rats. J Pharmacol Exp Ther 2017;362:424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563 [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1—correlation within subjects. BMJ 1995;310:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S465–S482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs C, Lunn KW. Clinical review: brain-body temperature differences in adults with severe traumatic brain injury. Crit Care 2013;17:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care 2011;14:389–394 [DOI] [PubMed] [Google Scholar]
- Coppler PJ, Marill KA, Okonkwo DO, Shutter LA, Dezfulian C, Rittenberger JC, et al. Concordance of brain and core temperature in comatose patients after cardiac arrest. Ther Hypothermia Temp Manag 2016;6:194–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press; 2011 [Google Scholar]
- Currie SE, Kortner G, Geiser F. Heart rate as a predictor of metabolic rate in heterothermic bats. J Exp Biol 2014;217:1519–1524 [DOI] [PubMed] [Google Scholar]
- Dave KR, Tamariz J, Desai KM, Brand FJ, Liu A, Saul I, et al. Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin-induced diabetic rats. Stroke 2011;42:1404–1411 [DOI] [PubMed] [Google Scholar]
- Deane AM, Horowitz M. Dysglycaemia in the critically ill—significance and management. Diabetes Obes Metab 2013;15:792–801 [DOI] [PubMed] [Google Scholar]
- Ettleson MD, Arguello V, Wallia A, Arguelles L, Bernstein RA, Molitch ME. Hyperglycemia and insulin resistance in cardiac arrest patients treated with moderate hypothermia. J Clin Endocrinol Metab 2014;99:E2010–E2014 [DOI] [PubMed] [Google Scholar]
- Feketa VV, Marrelli SP. Systemic administration of the TRPV3 ion channel agonist carvacrol induces hypothermia in conscious rodents. PLoS One 2015;10:e0141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grathwohl KW, Bruns BJ, LeBrun CJ, Ohno AK, Dillard TA, Cushner HM. Does hemodilution exist? Effects of saline infusion on hematologic parameters in euvolemic subjects. South Med J 1996;89:51–55 [DOI] [PubMed] [Google Scholar]
- HACA. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–556 [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Leid M, Murray TF. Cyclopentyladenosine-induced homologous down-regulation of A1 adenosine receptors (A1AR) in intact neurons is accompanied by receptor sequestration but not a reduction in A1AR mRNA expression or G protein α-subunit content. J Neurochem 1998;71:221–230 [DOI] [PubMed] [Google Scholar]
- Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci 1996;17:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Combs VM, Drew KL. Translating drug-induced hibernation to therapeutic hypothermia. ACS Chem Neurosci 2015;6:899–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozyreva TV, Meyta ES, Khramova GM. Effect of the sympathetic nervous system co-transmitters ATP and norepinephrine on thermoregulatory response to cooling. Temperature (Austin) 2015;2:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Wei L, Gu X, Won S, Wei ZZ, Dix TA, et al. Improved therapeutic benefits by combining physical cooling with pharmacological hypothermia after severe stroke in rats. Stroke 2016;47:1907–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JW, Lerman BB. CVT-510: a selective A1 adenosine receptor agonist. Cardiovasc Drug Rev 2003;21:277–292 [DOI] [PubMed] [Google Scholar]
- Lundbye JB. Therapeutic Hypothermia After Cardiac Arrest: Clinical Application and Management. London: Springer-Verlag, 2012 [Google Scholar]
- Lyden P, Hemmen T, Grotta J, Rapp K, Ernstrom K, Rzesiewicz T, et al. Results of the ICTuS 2 trial (intravascular cooling in the treatment of stroke 2). Stroke 2016;47:2888–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzoyev SA, McLeod CJ, Bunch TJ, Bell MR, White RD. Hypokalemia during the cooling phase of therapeutic hypothermia and its impact on arrhythmogenesis. Resuscitation 2010;81:1632–1636 [DOI] [PubMed] [Google Scholar]
- Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie 2016;125:259–266 [DOI] [PubMed] [Google Scholar]
- Muzzi M, Blasi F, Chiarugi A. AMP-dependent hypothermia affords protection from ischemic brain injury. J Cereb Blood Flow Metab 2013;33:171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013;369:2197–2206 [DOI] [PubMed] [Google Scholar]
- Nolan JP, Hazinski MF, Aickin R, Bhanji F, Billi JE, Callaway CW, et al. Part 1: executive summary: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2015;95:e1–e31 [DOI] [PubMed] [Google Scholar]
- Roman V, Keijser JN, Luiten PG, Meerlo P. Repetitive stimulation of adenosine A1 receptors in vivo: changes in receptor numbers, G-proteins and A1 receptor agonist-induced hypothermia. Brain Res 2008;1191:69–74 [DOI] [PubMed] [Google Scholar]
- Saigal S, Sharma JP, Dhurwe R, Kumar S, Gurjar M. Targeted temperature management: current evidence and practices in critical care. Indian J Crit Care Med 2015;19:537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N Engl J Med 2005;353:1574–1584 [DOI] [PubMed] [Google Scholar]
- Staer-Jensen H, Sunde K, Olasveengen TM, Jacobsen D, Draegni T, Nakstad ER, et al. Bradycardia during therapeutic hypothermia is associated with good neurologic outcome in comatose survivors of out-of-hospital cardiac arrest. Crit Care Med 2014;42:2401–2408 [DOI] [PubMed] [Google Scholar]
- Subramanian RK, Sidharthan A, Maneksh D, Ramalingam L, Manickam AS, Kanthakumar P, et al. Normative data for arterial blood gas and electrolytes in anesthetized rats. Indian J Pharmacol 2013;45:103–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Zhao Z, Lee C. C 5′- Adenosine monophosphate induced hypothermia reduces early stage myocardial ischemia/reperfusion injury in a mouse model. Am J Transl Res 2011;3:351–361 [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Cetas JS, Morrison SF. Hibernation, hypothermia and a possible therapeutic “Shifted Homeostasis” induced by central activation of A1 adenosine receptor (A1AR). Nihon Shinkei Seishin Yakurigaku Zasshi 2016;36:51–54 [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci 2013;33:14512–14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lubitz DK. Adenosine and cerebral ischemia: therapeutic future or death of a brave concept? Eur J Pharmacol 1999;371:85–102 [DOI] [PubMed] [Google Scholar]
- Wyse J, McNett M. Targeted temperature management: effects of initial protocol implementation on patient outcomes. Dimens Crit Care Nurs 2016;35:229–234 [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Luo Y, Ji X, Nemoto EM, Chen J. When hypothermia meets hypotension and hyperglycemia: the diverse effects of adenosine 5′-monophosphate on cerebral ischemia in rats. J Cereb Blood Flow Metab 2009;29:1022–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature 2006;439:340–343 [DOI] [PubMed] [Google Scholar]
- Zhang M, Li W, Niu G, Leak RK, Chen J, Zhang F. ATP induces mild hypothermia in rats but has a strikingly detrimental impact on focal cerebral ischemia. J Cereb Blood Flow Metab 2013;33:e1–e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.