Abstract
Purpose: Cancer survivors are at increased risk for the early development of age-related chronic medical conditions compared with peers without a history of cancer; however, little is known regarding the burden of these conditions among survivors of adolescent and young adult (AYA) cancers. In response, we sought to determine the prevalence of specific comorbidities and frailty among AYAs (15–39 years old at diagnosis) enrolled in a cancer survivorship cohort.
Methods: Using a cross-sectional survey of a tertiary medical center-based cancer survivorship cohort, we determined the prevalence of specific comorbidities and frailty using the survey-based FRAIL assessment. In separate models adjusting for age, we estimated prevalence ratios (PRs) for the associations between patient characteristics and (1) any comorbidity and (2) frailty or prefrailty using log-binomial models.
Results: We identified 271 AYA cancer survivors, most of whom were 30–39 years old at survey (57%). A majority of survivors (n = 163, 60%) reported having at least one comorbidity with the most common being depression (28%), anxiety (27%), asthma (17%), high cholesterol (15%), and hypertension (15%). Of the 184 AYA survivors at least 1 year from cancer diagnosis, 19 (10%) were classified as frail and 39 (21%) as prefrail. Survivors who were smokers (PR 2.0, 95% confidence interval [CI]: 1.16–3.56); obese (PR 1.7, 95% CI: 1.10–2.55); uninsured (PR 2.7, 95% CI: 1.63–4.59); or who reported comorbid depression or anxiety (PR 2.4, 95% CI: 1.51–3.67) were more likely to be frail or prefrail.
Conclusions: The prevalence of frailty and comorbidities is high among AYA cancer survivors suggestive of accelerated aging.
Keywords: : survivorship, frailty, comorbidities
Introduction
Annually in the United States, more than 70,000 adolescents and young adults (AYAs) are diagnosed with cancer, most of whom will become long-term survivors.1,2 Many survivors, however, will experience the early onset of chronic conditions. Among childhood cancer survivors (diagnosed at ages 0–18 years), the increased burden of chronic conditions and an early aging phenotype have been well described. Less is known about potential acceleration of age-related conditions among survivors of AYA cancers.
Frailty, a state of diminished physiological capacity commonly related to aging, often precedes the development of chronic conditions and predicts increased risk for mortality among older adults.3,4 Data regarding frailty among younger adults are limited, but early evidence suggests that frailty may be a better indicator of impending disability and mortality than chronological age in this population.5 With data from the St. Jude Lifetime Cohort, Ness et al. observed that young adult survivors of childhood cancers experienced frailty at rates equivalent to that among individuals ≥60 years old without a history of cancer and that frailty was a reliable predictor of early mortality.6 Nearly 10% of childhood cancer survivors who are young adults at the time of evaluation exhibit a frailty phenotype defined by ≥3 of the following: low muscle mass, exhaustion, low energy expenditure, slowness, or weakness. Over 20% exhibit prefrailty, defined as having two of these factors. This limited evidence suggests that a measure of frailty may be a useful tool in identifying young adult cancer survivors at higher risk of chronic morbidities and early mortality.
The prevalence of frailty among survivors of AYA cancers has not been well described. With data from a clinic-based, cross-sectional survey of AYA cancer survivors in North Carolina, we sought to determine the prevalence of frailty and non-cancer comorbidities in this patient population.
Methods
Population
The University of North Carolina (UNC) Cancer Survivorship Cohort is a large, tertiary medical center-based observational incident-prevalent cohort that integrates clinical and epidemiological data and biospecimens for patients seen at North Carolina Cancer Hospital outpatient oncology clinics between 2010 and 2016. Cohort eligibility requirements include age ≥18 years at enrollment, a North Carolina residence, and English or Spanish language. Potential participants were approached in the UNC outpatient oncology clinics, and 52% of approached individuals were successfully enrolled. All participants provided informed consent. This analysis was limited to participants diagnosed with cancer between the ages of 15 and 39, based on the National Cancer Institute's definition,1 and was approved by the UNC Institutional Review Board (IRB No. 15–2899).
Outcomes
For all Cancer Survivorship Cohort participants, a single cross-sectional baseline survey was completed after enrollment by trained staff using computer-assisted telephone-based interviews. The baseline survey included assessment of patient demographics, health history (including cancer-specific topics), lifestyle, health-related quality of life (National Institutes of Health's Patient-Reported Outcomes Measurement Information System® [PROMIS®] global health short form, RAND 12 Item Health Survey [SF-12], Functional Assessment of Cancer Therapy–General [FACT-G], and Patient-Generated–Subjective Global Assessment [PG-SGA]), and comorbidities. Comorbidity questions were adapted from various sources, including the National Health Interview Survey, the North Carolina-Louisiana Prostate Cancer Project (PCaP), the University of Texas MD Anderson Cancer Center Patient History Database, and the Roswell Park Cancer Institute Data Bank and BioRepository Epidemiologic Questionnaire.7–11 Specific items used in our study and the source instruments are noted in Supplementary Table S1 (Supplementary Data available online at www.libertpub.com/jayao). Participants were asked about common comorbidities, including depression, anxiety, bipolar disorder, schizophrenia, dementia, dyslipidemia, cerebral vascular accident, venous thromboembolism, arthritis, asthma, chronic obstructive pulmonary disorder, congestive heart failure, angina, myocardial infarction, diabetes, HIV infection, hypertension, osteoporosis, inflammatory bowel disease, gastrointestinal ulcers, kidney disorders, and liver disorders. Comorbidity questions included age at diagnosis, limitations on regular activities, and use of prescription medications for reported comorbidities.
Frailty assessment was performed among participants who completed the survey at least 1 year following their cancer diagnosis. This was done to limit the possible influence of active therapy on the frailty measure. A sensitivity analysis comparing the proportion of participants meeting the criteria for frailty and prefrailty between those at least 1 year from diagnosis and those at least 2 years from diagnosis was performed. Frailty was assessed using the FRAIL Questionnaire, a five-question scale that reliably predicts declining health function and mortality12 without requiring a face-to-face evaluation. The scale includes items assessing self-reported fatigue, weight loss, morbidities, difficulty with ambulation, and ability to overcome resistance (Supplementary Table S1). The version of the FRAIL measure used in our study is consistent with that originally described.13 The number of positive responses for these components was summed to create the FRAIL index (range 0–5). A value of three or greater was considered frail, and a value of two was considered prefrail. Those with values <2 were considered robust.
Statistical analysis
We categorized participants as having any comorbidity if they reported any of the 23 non-cancer comorbidities included in the baseline survey. Data were missing for <5% of participants for survey items used to assess four of the FRAIL components: fatigue, ambulation, morbidities, and weight loss. Although both SF-12 and PROMIS measures of quality of life were initially part of the baseline survey, SF-12 was removed to reduce redundancy and participant burden. Therefore, responses to the item used to assess ability to overcome resistance (only on SF-12) were missing for 57% of participants included in analyses of frailty. We considered these responses to be missing at random and used a multiple imputation by fully conditional specification approach to impute the missing values for components of the FRAIL index. The imputation was performed 10 times, and the FRAIL index was calculated within each imputation dataset. We then averaged each of the individual components and the FRAIL index score across the 10 imputation datasets. Averaged FRAIL component scores that were non-integers were rounded to the nearest integer. A sensitivity analysis determining the prevalence of frailty and prefrailty was also performed, including only participants with no missing data.
Multivariable models
Two separate log-binomial regression models were used to estimate prevalence ratios (PR) and 95% confidence intervals (CIs) for the independent association between patient characteristics and (1) having reported any comorbidity or (2) being either prefrail or frail, with adjustment for age at the time of survey. Often interpreted similar to risk ratios, PRs are an appropriate measure of effect when the analysis is cross-sectional.14 Survivor demographic factors that were included in the models were sex, race/ethnicity, marital status, education, employment status, and the presence of a child at home. Survivor medical characteristics included smoking status and body mass index. Disease characteristics included the cancer type, cancer stage, time since diagnosis, delayed care due to lack of health insurance, and modalities of treatment (any surgery, any chemotherapy, and any radiation therapy). As depression and anxiety were not included in the criteria for the FRAIL index, the presence of anxiety or depression was included in the model estimating PRs for frailty and prefrailty. Unadjusted PRs were calculated for age at diagnosis and age at survey. All analyses were performed using SAS 9.4 (Cary, North Carolina).
Results
We identified 281 AYAs with a cancer diagnosis enrolled in the UNC Cancer Survivorship Cohort. Ten individuals were excluded because their survey date was before their cancer diagnosis, without having multiple primary cancers. The majority of participants (74%) were women (Table 1). Most participants were 30–39 years old at diagnosis (74%) and at the time of survey (57%). Seventy-three participants (27%) were >40 years old at the time of survey. Approximately two-thirds were married or living with a partner (66%), employed (62%), and/or reported non-Hispanic white race/ethnicity (71%). Thirty-three survivors (12%) reported having delayed care due to lack of health insurance in the previous year. More than half (56%) had a child living at home. The most common diagnoses observed were breast (31%), cervical (11%), testicular (9%), and skin cancer (9%).
Table 1.
Demographic, Lifestyle, and Disease Characteristics of All Adolescent and Young Adult Participants in the UNC Cancer Survivorship Cohort and of Those Completing the Survey At Least 1 Year After Cancer Diagnosis
| All AYAs (n = 271) | AYAs ≥1 year from diagnosis (n = 184) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Age at diagnosis | ||||
| 15–19 | 6 | 2 | 6 | 3 |
| 20–24 | 19 | 7 | 14 | 8 |
| 25–29 | 46 | 17 | 35 | 19 |
| 30–34 | 67 | 25 | 40 | 22 |
| 35–39 | 133 | 49 | 89 | 48 |
| Age at survey | ||||
| <30 | 44 | 16 | 28 | 15 |
| 30–39 | 154 | 57 | 83 | 45 |
| 40–49 | 54 | 20 | 54 | 29 |
| ≥50 | 19 | 7 | 19 | 10 |
| Time since diagnosis | ||||
| <1 | 87 | 32 | — | — |
| 1–5 | 114 | 42 | 114 | 62 |
| >5 | 70 | 26 | 70 | 38 |
| Sex | ||||
| Male | 71 | 26 | 54 | 29 |
| Female | 200 | 74 | 130 | 71 |
| Race/ethnicity | ||||
| Non-Hispanic white | 192 | 71 | 131 | 71 |
| Hispanic white | 17 | 6 | 11 | 6 |
| Black | 42 | 15 | 30 | 16 |
| Other | 20 | 7 | 12 | 7 |
| Marital status | ||||
| Single | 60 | 22 | 41 | 22 |
| Living with partner/married | 179 | 66 | 122 | 67 |
| Separated, divorced, or widowed | 31 | 11 | 20 | 11 |
| Child living at home | ||||
| Yes | 153 | 56 | 94 | 56 |
| Currently employed | ||||
| Yes | 167 | 62 | 110 | 60 |
| Delayed care in past year due to no health insurance | ||||
| Yes | 33 | 12 | 18 | 10 |
| Education | ||||
| ≤High school | 66 | 24 | 46 | 25 |
| Some college | 78 | 29 | 42 | 23 |
| College graduate | 83 | 31 | 63 | 34 |
| Postgraduate/professional degree | 44 | 16 | 33 | 18 |
| Current smoker | ||||
| Yes | 20 | 7 | 10 | 6 |
| BMI at survey | ||||
| <25.0 | 97 | 37 | 64 | 36 |
| 25.0–29.9 | 65 | 25 | 50 | 28 |
| 30.0+ | 100 | 38 | 66 | 37 |
| Cancer site | ||||
| Thyroid | 12 | 4 | 7 | 4 |
| Testis | 24 | 9 | 16 | 9 |
| Skin | 25 | 9 | 12 | 7 |
| Brain | 11 | 4 | 11 | 6 |
| Breast | 83 | 31 | 68 | 37 |
| Cervix | 31 | 11 | 9 | 5 |
| Uterine | 11 | 4 | 3 | 2 |
| Colorectal | 20 | 7 | 17 | 9 |
| Other | 54 | 20 | 41 | 22 |
| Multiple primary cancers | ||||
| Yes | 31 | 11 | 20 | 11 |
| Stage of cancer | ||||
| 0 | 9 | 3 | 7 | 4 |
| 1 | 78 | 29 | 41 | 22 |
| 2 | 43 | 16 | 28 | 15 |
| 3 | 39 | 14 | 26 | 14 |
| 4 | 14 | 5 | 12 | 7 |
| Missing | 88 | 33 | 70 | 38 |
| Treatment | ||||
| Any chemotherapy or radiation | 152 | 56 | 128 | 70 |
| None | 111 | 41 | 48 | 26 |
| Missing | 8 | 3 | 8 | 4 |
AYA, adolescent and young adult; BMI, body mass index.
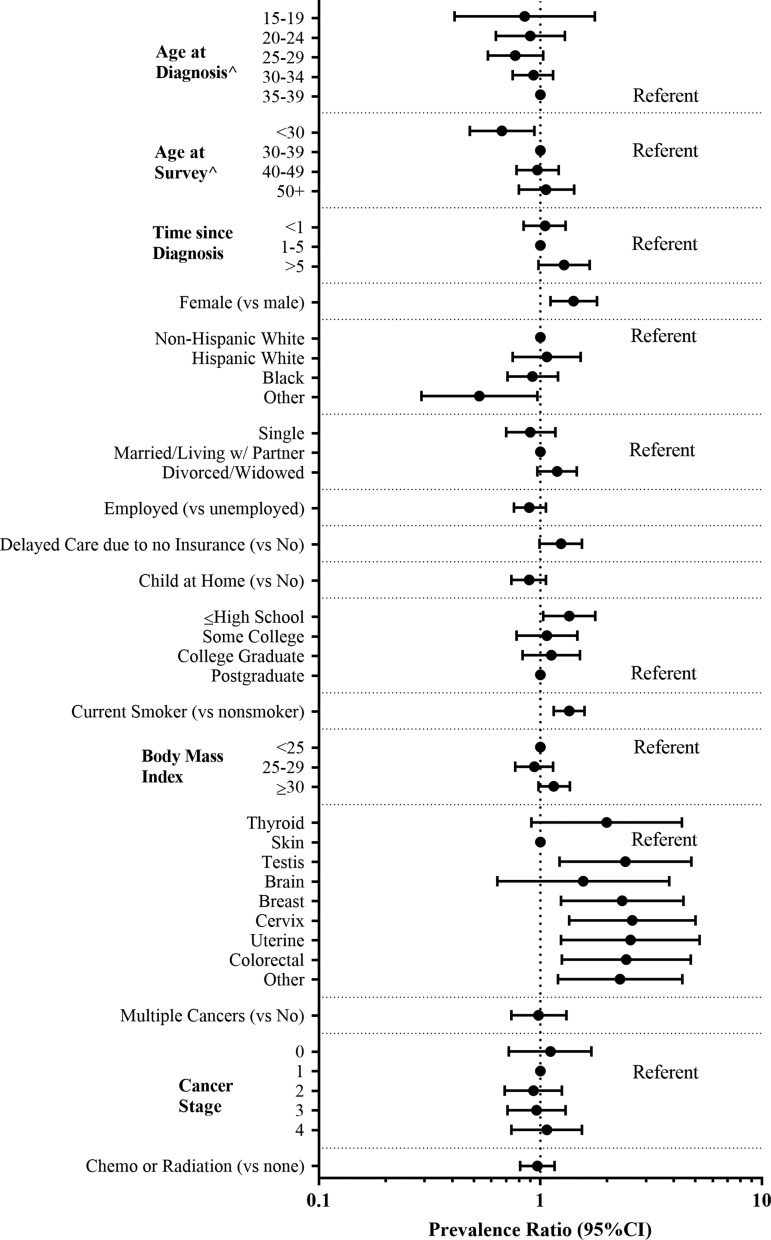
Of the 23 assessed comorbidities, one or more were reported by 163 (60%) participants with an average of 2 comorbidities per survivor (range 1–6) (Table 2). The use of medications for comorbidity management was reported by 102 (38%) of the survivors, and 48 (18%) reported having a comorbidity that limited their usual activities. Depression (28%), anxiety (27%), asthma (17%), high cholesterol (15%), and hypertension (15%) were the most commonly reported comorbidities. With adjustment for age at time of survey, survivors surveyed more than 5 years from diagnosis compared to those with a more recent diagnosis were more likely to report any comorbidity (PR 1.3, 95% CI: 0.98–1.67).
Table 2.
Prevalence of Commonly Reported Comorbidities Among Adolescent and Young Adult Survivors by Time Since Diagnosis and Sex
| All AYAs (n = 271) | ≤1 Year since Dx (n = 141) | >1–5 Years since Dx (n = 70) | >5 Years since Dx (n = 60) | Women (n = 200) | Men (n = 71) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Depression | 75 | 28% | 37 | 26% | 23 | 33% | 15 | 25% | 57 | 29% | 18 | 25% |
| Anxiety | 74 | 27% | 35 | 25% | 20 | 29% | 19 | 32% | 62 | 31% | 12 | 17% |
| Asthma | 46 | 17% | 23 | 16% | 12 | 17% | 11 | 18% | 35 | 18% | 11 | 15% |
| High cholesterol | 41 | 15% | 15 | 11% | 11 | 16% | 15 | 25% | 35 | 18% | 6 | 8% |
| Hypertension | 40 | 15% | 13 | 9% | 10 | 14% | 17 | 28% | 35 | 18% | 5 | 7% |
| Arthritis | 28 | 10% | 12 | 9% | 5 | 7% | 11 | 18% | 23 | 12% | 5 | 7% |
| Diabetes | 26 | 10% | 8 | 6% | 8 | 11% | 10 | 17% | 23 | 12% | 3 | 4% |
| ≥1 comorbidity | 163 | 60% | 83 | 59% | 37 | 53% | 43 | 72% | 130 | 65% | 33 | 46% |
| Average number of comorbidities (out of 7) | 2 | Range 1–6 | — | — | — | — | — | — | — | — | — | — |
| ≥1 comorbidity requiring medication managementa | 102 | 38% | 46 | 33% | 22 | 31% | 34 | 57% | 84 | 42% | 18 | 25% |
| Average number of medications (out of 7) | 1.6 | Range 1–4 | — | — | — | — | — | — | — | — | — | — |
| ≥1 activity-limiting comorbidityb | 48 | 18% | 21 | 15% | 13 | 19% | 14 | 23% | 37 | 19% | 11 | 15% |
| Average number of limiting comorbidities (out of 7) | 1.5 | Range 1–5 | — | — | — | — | — | — | — | — | — | — |
Assessed by asking participants “Currently are you taking any prescription medication for condition XX?” for each comorbidity examined.
Assessed by asking participants “Is you activity limited by condition XX?” for each comorbidity examined.
Compared to men, a higher prevalence of comorbidities was reported among women (65% vs. 47%, PR 1.4, 95% CI: 1.11–1.80), and more women reported medication use for comorbidity management (42% vs. 25%). In addition to participant sex and time from diagnosis, smoking (PR 1.4, 95% CI: 1.15–1.58), obesity (PR 1.2, 95% CI: 0.98–1.36), and delayed care due to lack of health insurance (PR 1.2, 95% CI: 0.99–1.54) were also associated with a higher prevalence of comorbidities (Fig. 1). Being employed at the time of survey was associated with a lower prevalence of comorbidities (PR 0.8, 95% CI: 0.69–1.01).
FIG. 1.
PRs and 95% CIs for any comorbidity adjusted for age at time of survey among all 271 AYA survivors. ^PRs unadjusted for age at survey. AYA, adolescent and young adult; CI, confidence interval; PRs, prevalence ratios.
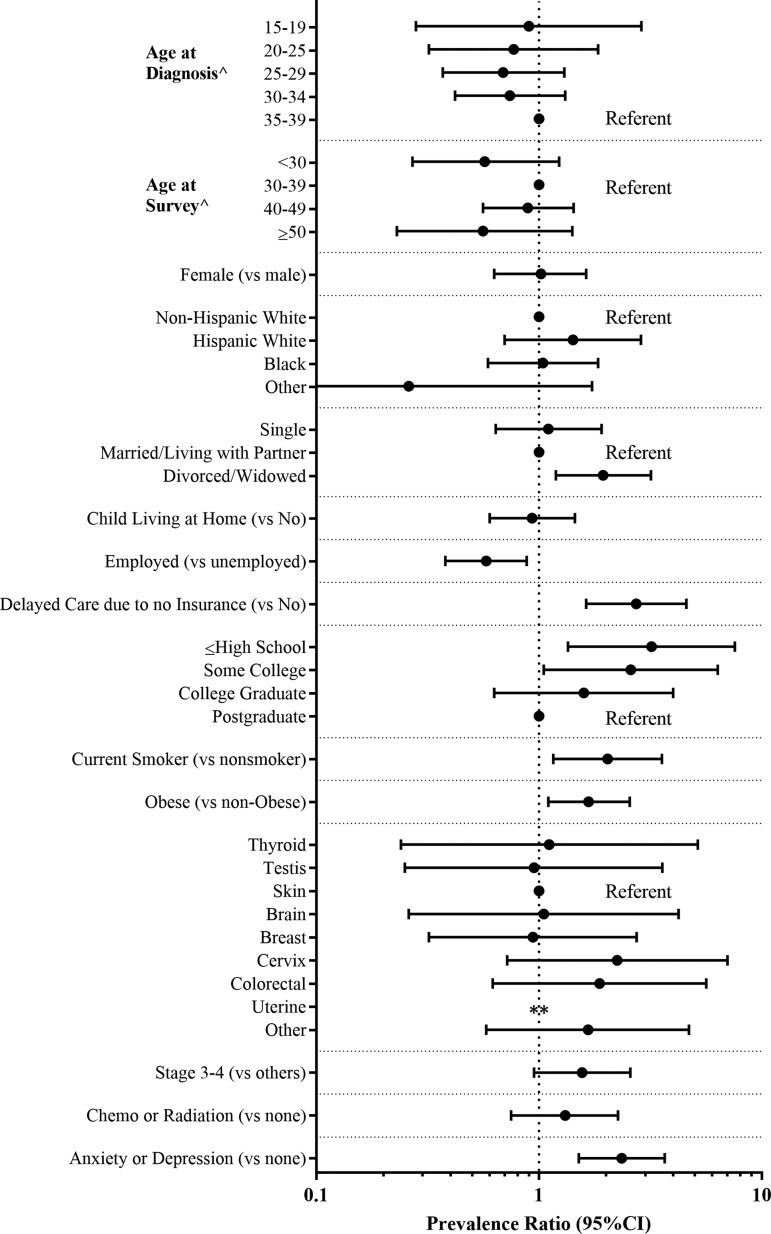
Of the 184 AYA survivors at least 1 year from cancer diagnosis, 19 (10%) were classified as frail according to the FRAIL index. Thirty-nine (21%) were classified as prefrail (Table 3). Between men and women, prevalence of frailty (women: 10% vs. men: 11%) and prefrailty (women: 22% vs. men: 20%) was similar. Difficulty with overcoming resistance (climbing stairs), impaired ambulation, and weight loss were the most common FRAIL components reported by AYA survivors. These three factors were similarly observed among men and women.
Table 3.
Prevalence of Frailty and Prefrailty Among 184 Adolescent and Young Adult Survivors Surveyed At Least 1 Year Following Cancer Diagnosis
| Total | Female | Male | ||||
|---|---|---|---|---|---|---|
| Frailty constituent | n | % | n | % | n | % |
| Fatigue | 20 | 11 | 16 | 12 | 4 | 7 |
| Resistance | 88 | 48 | 62 | 48 | 26 | 48 |
| Ambulation | 42 | 23 | 29 | 22 | 13 | 24 |
| Illness | 3 | 2 | 3 | 2 | 0 | 0 |
| Weight loss | 41 | 22 | 27 | 21 | 14 | 26 |
| Prefrail (2 factors) | 39 | 21 | 28 | 22 | 11 | 20 |
| Frail (≥3 factors) | 19 | 10 | 13 | 10 | 6 | 11 |
As a sensitivity analysis, the prevalence of (pre)frailty was assessed among participants with no missing data. Seventy-two individuals had no missing data, nine of whom (13%) were classified as frail and 17 (24%) were prefrail. These prevalence estimates were similar to those observed within the full sample using imputed data. The prevalence of a positive response to the “Resistance” item of the FRAIL Index, the most commonly missing item, was also similar between the participants missing no data (51%) and the full sample using imputed data (48%). A second sensitivity analysis was performed examining the proportion of participants greater than 2 years from diagnosis meeting criteria for prefrailty and frailty. These proportions (20% prefrail and 8% frail) were similar to those among participants one or more years from diagnosis.
While survivors classified as frail or prefrail were most often 30–39 years old at the time of diagnosis (frail: 16/19, 84%, and prefrail: 28/39, 72%) (Table 4), the prevalence of either frailty or prefrailty was not statistically higher among those diagnosed in their 30s compared to other ages (Fig. 2). Survivors who were divorced, widowed, or separated (vs. married) or smokers (vs. non-smokers) were more likely to be frail or prefrail (vs. robust). In addition, participants with advanced stage cancer (stages 3/4 vs. 1/2: PR 1.6, 95% CI: 0.94–2.55), who were obese (PR 1.7, 95% CI: 1.09–2.54), who delayed care due to lack of health insurance (PR 2.7, 95% CI: 1.63–4.59), or who reported comorbid depression or anxiety (PR 2.3, 95% CI: 1.48–3.62) were more likely to be frail or prefrail (vs. robust).
Table 4.
Prevalence of Frailty and Prefrailty by Demographic, Disease, and Lifestyle Characteristics Among 184 Adolescent and Young Adult Survivors Surveyed At Least 1 Year After Cancer Diagnosis
| Frail, n = 19 (10%) | Prefrail, n = 39 (21%) | Robust, n = 12 (69%) | |||||
|---|---|---|---|---|---|---|---|
| Total n = 184 | n | % | n | % | n | % | |
| Age at diagnosis | |||||||
| 15–19 | 6 | 0 | 0 | 2 | 33 | 4 | 67 |
| 20–24 | 14 | 1 | 7 | 3 | 21 | 10 | 71 |
| 25–29 | 35 | 2 | 6 | 6 | 17 | 27 | 77 |
| 30–34 | 40 | 4 | 10 | 7 | 18 | 29 | 73 |
| 35–39 | 89 | 12 | 13 | 21 | 24 | 56 | 63 |
| Age at survey | |||||||
| <30 | 28 | 2 | 7 | 4 | 14 | 22 | 79 |
| 30–39 | 83 | 11 | 13 | 20 | 24 | 52 | 63 |
| 40–49 | 54 | 5 | 9 | 12 | 22 | 37 | 69 |
| ≥50 | 19 | 1 | 5 | 3 | 16 | 15 | 79 |
| Time since diagnosis | |||||||
| 1–5 | 114 | 15 | 13 | 24 | 21 | 75 | 66 |
| >5 | 70 | 4 | 6 | 15 | 21 | 51 | 73 |
| Sex | |||||||
| Male | 54 | 6 | 11 | 11 | 20 | 37 | 69 |
| Female | 130 | 13 | 10 | 28 | 22 | 89 | 68 |
| Race/ethnicity | |||||||
| Non-Hispanic white | 131 | 14 | 11 | 28 | 21 | 89 | 68 |
| Hispanic white | 11 | 3 | 27 | 2 | 18 | 6 | 55 |
| Black | 30 | 1 | 3 | 9 | 30 | 20 | 67 |
| Other | 12 | 1 | 8 | 0 | 0 | 11 | 92 |
| Marital status | |||||||
| Single | 41 | 6 | 15 | 7 | 17 | 28 | 68 |
| Living with partner/married | 122 | 8 | 7 | 26 | 21 | 88 | 72 |
| Separated, divorced, or widowed | 20 | 5 | 25 | 5 | 25 | 10 | 50 |
| Child living at home | |||||||
| Yes | 94 | 9 | 10 | 20 | 21 | 65 | 69 |
| No | 75 | 9 | 12 | 16 | 21 | 50 | 67 |
| Currently employed | |||||||
| Yes | 110 | 10 | 9 | 17 | 15 | 83 | 75 |
| No | 73 | 9 | 12 | 22 | 30 | 42 | 58 |
| Delayed care in past year due to no insurance | |||||||
| Yes | 18 | 5 | 28 | 6 | 33 | 7 | 39 |
| No | 160 | 12 | 8 | 31 | 19 | 117 | 73 |
| Education | |||||||
| ≤High school | 46 | 8 | 17 | 14 | 30 | 24 | 52 |
| Some college | 42 | 5 | 12 | 11 | 26 | 26 | 62 |
| College graduate | 63 | 5 | 8 | 10 | 16 | 48 | 76 |
| Postgraduate/professional degree | 33 | 1 | 3 | 4 | 12 | 28 | 85 |
| Current smoker | |||||||
| Yes | 10 | 3 | 30 | 3 | 30 | 4 | 40 |
| No | 167 | 14 | 8 | 34 | 20 | 119 | 71 |
| BMI at survey | |||||||
| <25.0 | 64 | 6 | 9 | 9 | 14 | 49 | 77 |
| 25.0–29.9 | 50 | 3 | 6 | 10 | 20 | 37 | 74 |
| 30.0+ | 66 | 9 | 14 | 19 | 29 | 38 | 58 |
| Cancer site | |||||||
| Thyroid | 7 | 0 | 0 | 2 | 29 | 5 | 71 |
| Testis | 16 | 2 | 13 | 2 | 13 | 12 | 75 |
| Skin | 12 | 0 | 0 | 3 | 25 | 9 | 75 |
| Brain | 11 | 2 | 18 | 1 | 9 | 8 | 73 |
| Breast | 68 | 4 | 6 | 12 | 18 | 52 | 76 |
| Cervix | 9 | 3 | 33 | 2 | 22 | 4 | 44 |
| Uterine | 3 | 0 | 0 | 0 | 0 | 3 | 100 |
| Colorectal | 17 | 3 | 18 | 5 | 29 | 9 | 53 |
| Other | 41 | 5 | 12 | 12 | 29 | 24 | 59 |
| Multiple primary cancers | |||||||
| Yes | 20 | 4 | 20 | 4 | 20 | 12 | 60 |
| No | 164 | 15 | 9 | 35 | 21 | 114 | 70 |
| Cancer stage | |||||||
| 0 | 7 | 0 | 0 | 3 | 43 | 4 | 57 |
| 1 | 41 | 3 | 7 | 8 | 20 | 30 | 73 |
| 2 | 28 | 4 | 14 | 3 | 11 | 21 | 75 |
| 3 | 26 | 3 | 12 | 6 | 23 | 17 | 65 |
| 4 | 12 | 2 | 17 | 6 | 50 | 4 | 33 |
| Unknown | 70 | 7 | 10 | 13 | 19 | 50 | 71 |
| Treatment | |||||||
| Any surgery | 157 | 13 | 8 | 30 | 19 | 114 | 73 |
| Any radiation | 92 | 9 | 10 | 17 | 18 | 66 | 72 |
| Any chemotherapy | 109 | 13 | 12 | 23 | 21 | 73 | 67 |
| Depression | |||||||
| Yes | 58 | 11 | 19 | 16 | 28 | 31 | 53 |
| No | 122 | 7 | 6 | 21 | 17 | 94 | 77 |
| Anxiety | |||||||
| Yes | 54 | 12 | 22 | 16 | 30 | 26 | 48 |
| No | 126 | 6 | 5 | 21 | 17 | 99 | 79 |
| Asthmaa | |||||||
| Yes | 32 | 4 | 13 | 10 | 31 | 18 | 56 |
| No | 148 | 14 | 9 | 27 | 18 | 107 | 72 |
| Hypertensiona | |||||||
| Yes | 33 | 7 | 21 | 9 | 27 | 17 | 52 |
| No | 147 | 11 | 7 | 28 | 19 | 108 | 73 |
| Dyslipidemiaa | |||||||
| Yes | 31 | 1 | 3 | 13 | 42 | 17 | 55 |
| No | 149 | 17 | 11 | 24 | 16 | 108 | 72 |
| Arthritisa | |||||||
| Yes | 18 | 2 | 11 | 7 | 39 | 9 | 50 |
| No | 161 | 16 | 10 | 29 | 18 | 116 | 72 |
| Diabetesa | |||||||
| Yes | 20 | 2 | 10 | 6 | 30 | 12 | 60 |
| No | 160 | 16 | 10 | 31 | 19 | 113 | 71 |
Constituents of the FRAIL index.
FIG. 2.
PRs and 95% CIs for frailty or prefrailty adjusted for age at survey among 184 AYA survivors surveyed at least 1 year. ∧PRs unadjusted for age at survey; **Unable to calculate (no frail or prefrail survivors of uterine cancer).
Discussion
In this cross-sectional analysis of a hospital-based cancer survivorship cohort, we observed high prevalence of both frailty (10% frail and 21% prefrail) and comorbidities (60%, with at least one reported comorbidity) among survivors diagnosed as AYAs. Survivors of childhood cancers develop chronic morbidities such as heart disease and cognitive impairment earlier and more frequently than their cancer-free peers.15–18 However, there is little evidence regarding the prevalence of frailty and comorbidities among survivors of AYA cancers. In this report, we begin describing the burden of frailty and comorbidities experienced by AYA cancer survivors to help direct future research.
Frailty, a state of diminished physiological capacity and reduced ability to respond to stress, is a known precursor to morbidity and mortality among older adults3,19–23 and may serve as a better indicator of impending disability and mortality than chronological age among young adults.5 The prevalence of frailty observed in our cohort of AYA cancer survivors (10%) is similar to that among young adult survivors of childhood cancers reported by Ness (8%) and among individuals 60 years and older without a history of cancer (7%–13%).3,24,25 To our knowledge, only one study has previously reported the population-based prevalence of frailty in young adults without cancer. Using both an accumulation of deficits/frailty index model and a clinical-based (Fried) model for frailty determination, Kehler et al. reported a prevalence of frailty among the general Canadian young adult population from 1.8% to 5.3% across frailty definitions for 18–34 year olds and 4.3%–5.7% among 35–49 year olds.26 Our results therefore suggest that survivors of AYA cancers exhibit levels of frailty at least two-fold higher compared with similarly aged peers without a cancer history. The prevalence of frailty observed among AYA cancer survivors in our study was comparable to that among cancer-free individuals 30 years older3,24,25 and may indicate accelerated aging conferred after cancer diagnosis and its treatment.
In our study, several patient demographic and lifestyle characteristics were associated with a higher prevalence of (pre)frailty. Current smokers, obese survivors, and those with either an anxiety or depression disorder were more likely to meet the criteria for being frail. More surprisingly, a significantly higher prevalence of frailty was observed among survivors who delayed care secondary to not having health insurance in the previous year. The significance of this observation is uncertain. Due to the cross-sectional design of this study, we are unable to determine if access to healthcare is protective against the development of frailty or if frail survivors are simply more likely to be unemployed and subsequently have less access to affordable health insurance coverage. Similarly, we are unable to answer whether survivors suffering from anxiety and depression are more likely to become frail or if frail survivors are more prone to developing depression and anxiety. This association has been reported before,27 and significant overlap exists between the symptoms of depression and frailty (fatigue and weight loss), which likely contributes to this observed association. Further prospective studies could help to disentangle these questions.
If frailty is proven to be a reliable predictor for impending disability and early mortality among young adult cancer survivors, this phenotype could serve as a useful means of risk stratification and indicator for those who likely will require more intensive survivorship care. A screening instrument for frailty that is easily administered without the need for a face-to-face evaluation is appealing and could be a potentially useful tool for clinicians.
Individual comorbidities were also highly prevalent among the survivors of AYA cancers in our study with the majority of survivors (60%) reporting at least one. In this study, the prevalence of many comorbidities is higher than that reported among AYAs without a history of cancer, including depression (28% vs. 20%28), hypertension (15% vs. 7%29), arthritis (10% vs. 7%30), and diabetes (10% vs. 2%31). Similarly, a study by Tai et al. using the 2009 Behavioral Risk Factors Surveillance System found that, compared to young adults without a cancer history, survivors of young adult cancers experience higher rates of poor mental health (20% vs. 10%), asthma (15% vs. 8%), and diabetes (12% vs. 9%).32 These findings indicate that AYA cancer survivors may experience a higher burden of comorbidities compared to the general AYA population.
Medication use for comorbidity management was also common among the participants in this study, with nearly 40% of participants reporting the use of at least one medication. Many comorbidities can become chronic in nature, raising concern that long-term medication use may become a significant medical and financial burden for survivors. In our data, AYA survivors surveyed within 1 year of diagnosis frequently reported a comorbidity (59%), suggesting that comorbidities may need to be managed concurrently with treatment and emphasizing the need for multidisciplinary and coordinated care.
Not surprisingly, comorbidities were more prevalent among smokers and obese AYAs. These findings underscore the importance of counseling survivors regarding lifestyle choices (smoking cessation, healthy diet, and routine physical activity) that may mitigate comorbidity development or severity. It is unclear why women in our study were more likely to report comorbidities. Possible explanations could include male reluctance to report medical problems or the types of cancers experienced by males in this study cohort. Many men in the cohort were diagnosed with testicular cancer that may be treated with less intensive therapy, such as surgery alone, compared to more intensive regimens among women with breast or cervical cancers.
Our findings should be considered in light of certain limitations. The cross-sectional design of this study limits our ability to characterize the timing of frailty and comorbidity onset. Sample sizes in subgroups defined by time since diagnosis were small, leading to imprecise estimates. Because the UNC Cancer Survivorship Cohort focused on solid tumor cancers that demonstrated health disparities, the cohort does not reflect the population distribution of all cancer types. In addition, the study cohort included 73 individuals who were >40 years old at the time of survey. We attempted to address the confounding effect of age on comorbidities by controlling for age at the time of survey. Many AYA cancers are underrepresented, including lymphomas, leukemias, and sarcomas. While the frailty measure used in our study, the FRAIL index, has not been validated specifically in AYA cancer survivors, it has been validated in middle-aged adults12 and does not require a face-to-face assessment, making it a feasible measure for survey-based research. It is uncertain whether a frailty measure using self-reported data only is as reliable as more objective measures of assessment. Direct comparisons of frailty measures in the AYA cancer population would be useful to clarify this. In addition, more work is needed to determine whether frailty in young adults, as previously observed in older adults, serves as a predictor for early morbidity and mortality.
In conclusion, we provide evidence regarding the prevalence of frailty and comorbidities among AYA cancer survivors. The prevalence of frailty among AYAs in our study was equivalent to populations aged 60 and older. These findings suggest that AYA cancer survivors are a vulnerable group at risk of significant morbidity. Further prospective assessment of AYA cancer survivors is needed to determine whether frailty measures are predictive of future morbidity and mortality, as has been observed in childhood cancer patients.
Supplementary Material
Acknowledgments
The authors thank the UNC Health Registry/Cancer Survivorship Cohort (HR/CSC) participants for their important contributions. The HR/CSC is funded, in part, by the UNC Lineberger Comprehensive Cancer Center's University Cancer Research Fund. This project was reviewed and approved by the Human Research Protections Program (IRB No. 09-0605) at the University of North Carolina at Chapel Hill. Dr. Smitherman is supported by the North Carolina Translational and Clinical Sciences Institute (UL1TR001111) and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR001109. This research was supported, in part, by a Faculty Development award from the UNC Office of the Provost.
Author Disclosure Statement
The authors have no conflicts of interest relevant to the (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the article for publication to disclose.
References
- 1.National Institutes of Health. Adolescent young adult oncology progress review group: closing the gap: research and care imperatives for adolescents and young adults with cancer. NIH Publication No. 06-6067. Bethesda, MD; 2006 [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. (Eds). SEER cancer statistics review, 1975–2014. Bethesda, MD: National Cancer Institute; https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017 [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56 [DOI] [PubMed] [Google Scholar]
- 4.Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(3 suppl):1–29 [PubMed] [Google Scholar]
- 5.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183:E487–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness KK, Krull KR, Jones KE, et al. . Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31(36):4496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics. National Health Interview Survey, 2010. National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville, MD; 2010. Accessed September25, 2017 from: www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm [Google Scholar]
- 8.Gammon MD, Schoenberg JB, Ahsan H, et al. . Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89(17):1277–84 [DOI] [PubMed] [Google Scholar]
- 9.Schroeder JC, Bensen JT, Su LJ, et al. . The North Carolina - Louisiana Prostate Cancer Project (PCaP): methods and design of a multidisciplinary population-based cohort study of racial differences in prostate cancer outcomes. Prostate. 2006;66(11):1162–76 [DOI] [PubMed] [Google Scholar]
- 10.The University of Texas MD Anderson Cancer Center. Patient history database. Accessed September25, 2017 from: https://my.mdanderson.org/forms/PHDB.pdf
- 11.Roswell Park Cancer Institute. Data bank and biorepository epidemiologic questionnaire. Accessed September25, 2017 from: www.roswellpark.edu/shared-resources/data-bank-and-biorepository-dbbr/data
- 12.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abellan van Kan G, Rolland YM, Morely JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–2 [DOI] [PubMed] [Google Scholar]
- 14.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemol. 2005;162:199–200 [DOI] [PubMed] [Google Scholar]
- 15.Hudson MM, Ness KK, Gurney JG, et al. . Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. . Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–15 [DOI] [PubMed] [Google Scholar]
- 17.Landier W, Armenian SH, Lee J, et al. . Yield of screening for long-term complications using the children's oncology group long-term follow-up guidelines. J Clin Oncol. 2012;30:4401–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16:1027–33 [DOI] [PubMed] [Google Scholar]
- 20.Sepehri A, Beggs T, Hassan A, et al. . The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg. 2014;148:3110–7 [DOI] [PubMed] [Google Scholar]
- 21.Shamliyan T, Talley KMC, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12:719–36 [DOI] [PubMed] [Google Scholar]
- 22.Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70:716–21 [DOI] [PubMed] [Google Scholar]
- 23.Kojima G. Frailty as a predictor of hospitalisation among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70:722–9 [DOI] [PubMed] [Google Scholar]
- 24.Macklai NS, Spagnoli J, Junod J, Santos-Eggimann B. Prospective association of the SHARE-operationalized frailty phenotype with adverse outcomes: evidence from 60+ community-dwelling Europenas living in 11 countries. BMC Geriatrics. 2013;13:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64A:675–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehler DS, Ferguson T, Stammers AN, et al. . Prevalence of frailty in Canadians 18–79 years old in the Canadian Health Measures Survey. BMC Geriatr. 2017;17:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucharska-Newton AM, Palta P, Burgard S, et al. . Operationalizing frailty in the Atherosclerosis Risk in Communities Study cohort. J Gerantol A Biol Med Sci. 2017;72(3):382–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessler RC, Birnbaum H, Briomet E, et al. . Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R). Psychol Med. 2010;40:225–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon SS, Burt V, Louis T, Carroll MD. Hypertension among adults in the US, 2009–2010. NCHS Data Brief. 2012;107:1–8 [PubMed] [Google Scholar]
- 30.Barbour KE, Helmick CG, Boring M, Brady TJ. Vital Signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation–United States, 2013–2015. MMWR. 2017;66:246–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowie CC, Rust KF, Ford ES, et al. . Full accounting of diabetes and pre-diabetes in the US population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai E, Buchanan N, Townsend J, et al. . Health status of adolescent and young adult cancer survivors. Cancer. 2012;118(19):4884–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.