Abstract
Objectives
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease, subtyped according to clinical manifestations and autoantibodies. Evidence concerning cigarette smoking and SLE risk has been conflicting. We investigated smoking and SLE risk, overall and by anti-double stranded DNA (dsDNA) presence, in two prospective cohort studies.
Methods
The Nurses’ Health Study (NHS) enrolled 121,701 U.S. female nurses in 1976; NHSII enrolled 116,430 in 1989. Lifestyle, environmental, and medical data were collected through biennial questionnaires. Incident SLE was confirmed by medical record review. Cox regression models estimated hazard ratios (HRs) of SLE, overall and by dsDNA subtype, in association with time-varying smoking status and cumulative smoking pack-years through the 2-year cycle prior to diagnosis, controlling for potential confounders.
Results
Among 286 SLE cases identified (159 in NHS [1978–2012] and 127 in NHSII [1991–2013]), mean age was 49.2 (10.3) years and 42% were dsDNA+ at SLE diagnosis. At baseline, 45% of women had ever smoked, 51% of whom currently smoked. Compared to never smokers, current smokers had increased dsDNA+ SLE risk (HR 1.86 [1.14–3.04]), whereas past smokers did not (HR 1.31 [0.85–2.00]). Women who smoked >10 pack-years (vs. never) had an elevated dsDNA+ SLE risk (HR 1.60 [95%CI 1.04–2.45]) compared to never smokers. No associations were observed between smoking status or pack-years and overall SLE or dsDNA− SLE.
Conclusion
Strong and specific associations of current smoking and >10 pack-years of smoking with dsDNA+ SLE were observed. This novel finding suggests smoking is involved in dsDNA+ SLE pathogenesis.
Keywords: smoking, systemic lupus erythematosus, health services research
INTRODUCTION
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease with subtypes defined by autoantibodies and clinical manifestations. Anti-double stranded DNA (dsDNA) antibodies are specific for SLE diagnosis, are involved in lupus nephritis pathogenesis, and are biomarkers of disease activity(1–4). SLE patients with the anti-dsDNA positive (dsDNA+) subtype have increased risk for a more aggressive disease course, particularly with lupus nephritis and vasculitis.
SLE is associated with genetic and environmental factors (5). Past studies suggest smoking may be a potentially modifiable risk factor for SLE, although case-control studies have demonstrated conflicting results (6–8), and the two prior prospective cohort studies have not demonstrated this association to date (9, 10). In a retrospective SLE case-only study, current smokers were significantly more likely than never smokers to have dsDNA antibodies (OR 4.0 [95% confidence interval {95% CI} 1.6 –10.4])(11).
We aimed to investigate an association between smoking and risk of developing SLE, and risk of SLE subtypes according to dsDNA status, among women. We hypothesized that current smokers, compared to never smokers, have an increased risk of overall and dsDNA+ SLE. To our knowledge, no prior study has investigated the association of smoking with risk of incident SLE, stratified by anti-dsDNA status.
PATIENTS AND METHODS
Study Population
The Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII) are prospective cohorts consisting of registered female nurses who completed a baseline questionnaire and are followed biennially to update risk factors, lifestyle, health practices, and disease diagnoses. NHS, established in 1976, enrolled 121,700 nurses aged 30 to 55 years residing in 11 large U.S. states. NHSII, started in 1989, enrolled 116,670 nurses aged 25 to 42 years in 14 states. Both cohorts are predominantly White (>90%), with >90% response rates to follow-up questionnaires and only 5.0% of person-time lost to follow-up (12). Deaths are reported by participants’ family members and ascertained via National Death Index searches, with cause of death validated by medical record review.
To define an SLE-free cohort, we excluded participants who reported prevalent SLE or other connective tissue diseases (CTD) at study baseline. We also excluded participants who did not provide smoking information on baseline questionnaires. After exclusions, 117,157 women in NHS and 113,527 women in NHSII were included in the analysis.
Identification of Incident SLE
SLE diagnosis was the primary outcome. SLE self-reports are confirmed using the CTD screening questionnaire and medical record review by two independent rheumatologists (13, 14). SLE cases were those fulfilling at least four American College of Rheumatology (ACR) 1997 SLE classification criteria and confirmed by medical record review (15, 16). Anti-dsDNA status at SLE diagnosis was determined by medical record review. Secondary outcomes were dsDNA+ SLE and dsDNA− SLE subtypes.
Smoking Exposure
Smoking was self-reported at baseline and every 2 years. At baseline, participants reported smoking status (never/past/current) and age of smoking initiation. Current smokers provided number of cigarettes smoked per day, whereas past smokers reported age at quitting smoking and number of cigarettes smoked per day before quitting. On subsequent questionnaires, participants reported smoking status and smoking intensity (pre-defined categories: 1–4, 5–14, 15–24, 25–34 or 35–44 cigarettes/day). Smoking duration and time since quitting were calculated from these reports. Pack-years of smoking were derived by multiplying packs per day (20 cigarettes per pack) with years during which that quantity was smoked. All smoking variables used in this analysis were time-varying, with updated information every two years, as smokers often stop and re-start smoking.
Assessment of Time-Varying Covariates
Potential covariates were chosen based on prior studies in NHS cohorts or medical literature demonstrating an association with smoking or SLE. Time-varying data on potential confounders were assessed by self-report on biennial questionnaires. Sociodemographic data included age, race/ethnicity (by self-report), questionnaire cycle, body mass index (BMI), and U.S. Census tract-based median household income as a measure of area socioeconomic status. Given the strong correlation of smoking with alcohol intake, we adjusted for time-varying alcohol consumption in three categories (never, >0 to <5 grams/day, ≥5 grams/day) as in a previous analysis (17). Reproductive covariates shown to be associated with incident SLE, including oral contraceptive (OCP) use, menarche onset age, menopausal status and postmenopausal hormone use, were examined as potential confounders (18). Missing covariate data were carried forward one cycle and if missing beyond one cycle, we included a variable category for missing data.
Statistical Analysis
In our primary analyses, we assessed the association between time-varying smoking status and SLE risk, overall and by dsDNA subtype, through the 2-year cycle prior to SLE onset. Person-years of follow-up accrued from return of baseline questionnaire until the 2-year cycle prior to SLE diagnosis, end of follow-up, death, or date of censor, whichever came first. Participants were censored for self-reported CTD (SLE, rheumatoid arthritis, scleroderma, Sjögren’s syndrome, mixed connective tissue disease or inflammatory myositis) not subsequently validated as SLE. To address missing smoking status and smoking duration, we carried forward the last observation up to two questionnaire cycles.
We examined baseline characteristics across categories of smoking status in each cohort. We used Cox proportional hazards models to assess the hazard ratios (HRs) and 95% CI for smoking status and all SLE, dsDNA+ SLE, and dsDNA− SLE in separate models, controlling for time-varying covariates. We constructed three models for each endpoint: 1) age- and questionnaire period adjusted; 2) additionally adjusted for alcohol; and 3) additionally adjusted for race, socioeconomic status, and reproductive factors. Based on the generalized Wald test for a joint hypothesis on all covariate-time interactions in the models, the proportional hazards assumption was not violated. All analyses were performed separately in NHS and NHSII and then, as estimates were similar, data were pooled. In a sensitivity analysis to evaluate the robustness of pooling the data, hazard ratio estimates from the two cohorts were meta-analyzed using DerSimonian and Laird random effects models(19).
We conducted several secondary analyses. First, we investigated the association of cumulative smoking in pack-years and risk of SLE and anti-dsDNA subtypes. Second, we cross-classified smoking status and pack-years and examined SLE risk overall and by dsDNA. Third, we separately evaluated the associations of smoking intensity (collapsed for ease of interpretability to >0 to <15 or ≥15 cigarettes/day) and duration (≥ 20 years or <20 years) with SLE risk. Fourth, we conducted a “lagged analysis” in which the exposure window ended two questionnaire cycles (four years, versus one cycle or two years in the primary analysis) prior to the outcome window, as SLE may develop insidiously pre-diagnosis and new illness could change smoking behavior. Lastly, we examined whether quitting smoking was associated with reduced SLE risk over time. We determined cut-points for categories of continuous exposure variables non-parametrically with restricted cubic splines (20).
Data analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, North Carolina, USA) with a two-sided alpha of 0.05 as statistical significance. All aspects of this study were approved by the Partners’ HealthCare Institutional Review Board.
RESULTS
Among 230,672 women with 5.6 million person-years of follow-up, we identified 286 incident SLE cases: 159 SLE cases in NHS and 127 in NHSII. The average annual SLE incidence rate in each cohort was 4.9 per 100,000 person-years for NHS and 5.3 per 100,000 person-years for NHSII. This incidence rate is as expected for predominantly White women who were all age ≥25 years at cohort entry. At baseline, 45% of women in both cohorts were ever smokers, of whom 51% were current smokers. Age-adjusted baseline characteristics of study participants categorized by smoking status are shown in Table 1. Age, race, caloric intake, BMI, postmenopausal status, postmenopausal hormone use, and early menarche were similar across smoking status categories within each cohort. Alcohol consumption was higher among current and past smokers compared to never smokers in both cohorts. Most current smokers in both cohorts had smoked >10 pack-years, although women in NHS were heavier smokers than those in NHSII at baseline.
Table 1.
Baseline age-standardized characteristics of participants in the Nurses’ Health Study in 1976 and Nurses’ Health Study II in 1989 categorized by smoking status
| Characteristics | NHS (N=117,145) | NHSII (N=113,527) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Never | Past | Current | Never | Past | Current | |
| Number of participants (%) | 51,655 (44.1) | 26,889 (23.0) | 38,601 (33.0) | 74,166 (65.3) | 24,152 (21.3) | 15,209 (13.4) |
|
| ||||||
| Mean age in years(SD)a | 42.4 (7.4) | 42.6 (7.1) | 42.4 (7.1) | 34.0 (4.7) | 35.2 (4.5) | 34.8 (4.6) |
| White race (%) | 92 | 94 | 94 | 91 | 94 | 93 |
| Median income ≥$60K (%)b | 46 | 53 | 49 | 43 | 50 | 40 |
| Mean calorie intake (Kcal/day, SD) | 1588 (502) | 1553 (488) | 1546 (510) | 1799 (547) | 1783 (542) | 1753 (559) |
|
| ||||||
| Mean body mass index (kg/m2, SD) | 24.1 (4.3) | 23.9 (4.3) | 23.2 (3.9) | 24.1 (5.1) | 24.1 (5.0) | 24.1 (5.0) |
|
| ||||||
| Smoking in pack-year categories | ||||||
| 0 (%) | 100 | 0 | 0 | 100 | 0 | 0 |
| >0 to ≤10 (%) | 0 | 58 | 20 | 0 | 69 | 36 |
| >10 (%) | 0 | 42 | 80 | 0 | 31 | 64 |
|
| ||||||
| Oral contraceptive use, ever (%) | 45 | 49 | 49 | 81 | 89 | 89 |
|
| ||||||
| Postmenopausal (%) | 31 | 30 | 34 | 6 | 6 | 8 |
| Any postmenopausal hormone use (%) | 13 | 14 | 15 | 3 | 3 | 4 |
|
| ||||||
| Early menarche (≤10 years), (%) | 6 | 6 | 6 | 8 | 8 | 9 |
|
| ||||||
| Alcohol use in categories (g/d), (%)c | ||||||
| None | 33 | 19 | 19 | 43 | 28 | 28 |
| >0 to <5 | 27 | 27 | 24 | 42 | 43 | 40 |
| ≥5 | 19 | 34 | 32 | 15 | 28 | 32 |
Means (SD) or percentages, age-standardized to distribution of study population
Not age-standardized
Zip code-level median household income from the U.S. Census
Cumulative average daily alcohol consumption
The presenting manifestations at SLE diagnosis, overall and by dsDNA subtype, are shown in Table 2. Of the 286 incident SLE cases, 42% were dsDNA+ at diagnosis. Mean age at SLE diagnosis was 49.2 years (SD 10.3). There were more non-Whites in the dsDNA+ (12.6%) versus dsDNA− (6.1%) subgroup. Among women with dsDNA+ SLE, there were lower rates of arthritis (65.3% vs. 79.4%), higher rates of hematologic involvement (65.3% vs. 53.3%), and similar rates of renal involvement (16.5% vs. 16.4%) compared to dsDNA− SLE in records reviewed around the time of SLE diagnosis.
Table 2.
Characteristics of participants at SLE diagnosis in Nurses’ Health Study and Nurses’ Health Study II by anti-double stranded DNA antibody (anti-dsDNA) status
| Characteristics at SLE diagnosis | Overall SLE(N=286) | dsDNA+ SLE(N=121) | dsDNA− SLE (N=165) |
|---|---|---|---|
| Mean age at diagnosis, years(SD) | 49.2 (10.3) | 49.9 (9.6) | 48.7 (10.8) |
| White race (%) | 91.6 | 88.4 | 93.9 |
| Anti-nuclear antibody positive (%) | 97.6 | 98.4 | 97.0 |
| Arthritis (%) | 73.4 | 65.3 | 79.4 |
| Hematologic involvement (%) | 58.4 | 65.3 | 53.3 |
| Renal involvement (%) | 16.4 | 16.5 | 16.4 |
| Mean number of ACR SLE criteria met (SD) | 4.9 (1.1) | 5.2 (1.2) | 4.7 (0.9) |
| Diagnosed by ACR member rheumatologist (%) | 79.0 | 76.0 | 81.2 |
SD= standard deviation dsDNA+ = double stranded DNA positive, dsDNA− = double stranded DNA negative
Among SLE cases, the largest proportion of past and current smokers smoked 15–24 cigarettes/day (34.4% and 37.5%). Mean smoking duration among SLE cases was greater for current than past smokers (26.4 [SD 8.9] vs. 16.1 [SD 10.8] years). Among SLE cases, mean time since quitting among past smokers was 16.8 (SD 12.8) years.
No significant risk was observed among past or current smokers (vs. never smokers) for SLE overall or dsDNA− SLE risk (Table 3). However, current smoking was associated with a strongly increased risk of dsDNA+ SLE (HR 1.77 [95%CI 1.09–2.88] after age- and sex-adjustment, and increased further after adjustment for alcohol use (HR 1.91 [95%CI 1.17–3.12]). This risk remained significant in the multivariable model (HR 1.86 [95%CI 1.14–3.04]). In the sensitivity analysis meta-analyzing HRs from the two cohorts, we found similar results for current compared to never smoking (e.g. MV-adjusted HR for dsDNA+ SLE 1.81 [95%CI 1.10–2.96]), but no association with overall SLE or dsDNA− SLE. Furthermore, in a “lagged” analysis allowing 4 years before SLE diagnosis, the risk of dsDNA+ SLE was potentially even more elevated among current compared to never smokers (MV-adjusted HR 1.93 [95%CI 1.17–3.18]).
Table 3.
Association between cigarette smoking status and risk of incident SLE among participants in Nurses’ Health Study and Nurses’ Health Study II, overall and by anti-double stranded DNA (dsDNA) antibody status
| Cigarette Smoking Status | |||
|---|---|---|---|
|
| |||
| Never | Past | Current | |
| Overall SLE | |||
| Cases/Person-Years | 148/3,074,178 | 90/1,759,984 | 48/808,162 |
| Age-Adjusted HR (95%CI)a | 1.00 (ref) | 1.12 (0.86–1.47) | 1.07 (0.77–1.50) |
| Alcohol-Adjusted HR (95%CI)b | 1.00 (ref) | 1.22 (0.93–1.60) | 1.17 (0.83–1.65) |
| Multivariable-Adjusted HR (95%CI)c | 1.00 (ref) | 1.18 (0.89–1.55) | 1.14 (0.81–1.61) |
|
| |||
| dsDNA+ SLE | |||
| Cases/Person-Years | 56/3,073,263 | 39/1,759,395 | 26/807,828 |
| Age-Adjusted HR (95%CI)a | 1.00 (ref) | 1.29 (0.85–1.95) | 1.77 (1.09–2.88) |
| Alcohol-Adjusted HR (95%CI)b | 1.00 (ref) | 1.37 (0.89–2.09) | 1.91 (1.17–3.12) |
| Multivariable-Adjusted HR (95%CI)c | 1.00 (ref) | 1.31 (0.85–2.00) | 1.86 (1.14–3.04) |
|
| |||
| dsDNA− SLE | |||
| Cases/Person-Years | 92/3,073,468 | 51/1,759,406 | 22/807,827 |
| Age-Adjusted HR (95%CI)a | 1.00 (ref) | 1.02 (0.72–1.45) | 0.72 (0.44–1.16) |
| Alcohol-Adjusted HR (95%CI)b | 1.00 (ref) | 1.13 (0.79–1.61) | 0.79 (0.49–1.29) |
| Multivariable-Adjusted HR (95%CI)c | 1.00 (ref) | 1.09 (0.76–1.56) | 0.76 (0.47–1.24) |
p for heterogeneity between the cohorts >0.05 for all analyses
Adjusted for age (months), questionnaire cycle, cohort
Additionally adjusted for alcohol intake
Additionally adjusted for race, early menarche, oral contraceptive use body mass index, zip code-level median household income from U.S. census.
HR = hazard ratio; CI = confidence interval, g/day=grams per day, dsDNA+ = double stranded DNA positive, dsDNA− = double stranded DNA negative
In secondary analyses, we examined smoking in pack-years (Table 4). Based on the results of the restricted cubic splines, we defined pack-years using an ordinal variable (0 pack-years, >0 to ≤10 pack-years, >10 pack-years). Although no significant association for smoking in pack-years and risk of overall SLE or dsDNA− SLE was demonstrated, women who smoked >10 pack-years had a significantly elevated risk of dsDNA+ SLE (HR 1.60 [95%CI 1.04–2.45], p-trend 0.04) compared to never smokers. To further investigate this finding, in an analysis cross-classifying smoking status with pack-years, current smokers who smoked >10 pack-years had a 67% increased risk of dsDNA+ SLE (HR 1.67 [95%CI 0.98–2.85, p-trend 0.07 across pack-year categories], but no increased risk of SLE overall (HR 1.05 [95%CI 0.72–1.51, p-trend 0.81]. However, among past smokers, no similar association was demonstrated between increased pack-years and all SLE or dsDNA+ SLE.
Table 4.
Association between cigarette smoking in pack-years and risk of incident SLE among participants in Nurses’ Health Study and Nurses’ Health Study II, overall and by anti-double stranded DNA (dsDNA) antibody status
| Pack-years | ||||
|---|---|---|---|---|
|
| ||||
| Never smoker | >0 to ≤10 | >10 | p-trend | |
| Overall SLE | ||||
| Cases/Person-Years | 148/3,074,178 | 52/1,032,876 | 86/1,535,233 | |
| Age-Adjusted HR (95%CI)a | 1.00 (ref) | 1.03 (0.75–1.41) | 1.16 (0.88–1.54) | 0.28 |
| Alcohol-Adjusted HR (95%CI)b | 1.00 (ref) | 1.11 (0.81–1.54) | 1.27 (0.96–1.68) | 0.10 |
| Multivariable-Adjusted HR (95%CI)c | 1.00 (ref) | 1.09 (0.79–1.51) | 1.22 (0.92–1.61) | 0.18 |
|
| ||||
| dsDNA+ SLE | ||||
| Cases/Person-Years | 56/3,073,263 | 24/1,032,491 | 41/1,534,731 | |
| Age-Adjusted HR (95%CI)a | 1.00 (ref) | 1.27 (0.78–2.05) | 1.57 (1.04–2.39) | 0.04 |
| Alcohol-Adjusted HR (95%CI)b | 1.00 (ref) | 1.35 (0.83–2.20) | 1.68 (1.10–2.58) | 0.02 |
| Multivariable-Adjusted HR (95%CI)c | 1.00 (ref) | 1.32 (0.81–2.16) | 1.60 (1.04–2.45) | 0.04 |
|
| ||||
| dsDNA− SLE | ||||
| Cases/Person-Years | 92/3,073,468 | 28/1,032,494 | 45/1,534,739 | |
| Age-Adjusted HR (95%CI)a | 1.00 (ref) | 0.88 (0.57–1.35) | 0.93 (0.64–1.35) | 0.75 |
| Alcohol-Adjusted HR (95%CI)b | 1.00 (ref) | 0.97 (0.63–1.49) | 1.03 (0.70–1.50) | 0.87 |
| Multivariable-Adjusted HR (95%CI)c | 1.00 (ref) | 0.94 (0.61–1.46) | 0.98 (0.67–1.44) | 0.96 |
p for heterogeneity between the cohorts >0.05 for all analyses
Adjusted for age (months), questionnaire cycle, cohort
Additionally adjusted for alcohol intake
Additionally adjusted for race, early menarche, oral contraceptive use body mass index, zip code-level median household income from U.S. census.
HR = hazard ratio; CI = confidence interval, g/day=grams per day, dsDNA+ = double stranded DNA positive, dsDNA− = double stranded DNA negative
Among current smokers, increasing smoking intensity (≥15 versus >0 to <15 cigarettes per day) was not associated with higher dsDNA+ SLE risk after multivariable adjustment (p-value 0.38). However, among current smokers, increasing smoking duration was related to increased dsDNA + SLE risk (multivariable HR 1.85 [95%CI 1.09–3.13]) for those continuing to smoke for ≥20 years compared to never smokers. No association was demonstrated for increasing smoking duration and overall or dsDNA− SLE, or among past smokers.
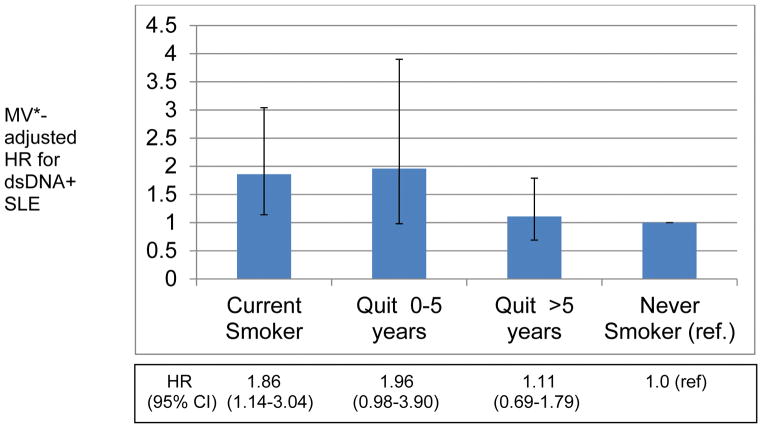
Among past smokers, no association between time since quitting and risk of SLE or dsDNA− SLE was found. However, after quitting smoking for >5 years, the risk of dsDNA+ SLE was no longer significantly elevated (HR 1.11 [95%CI 0.69–1.79] vs. never smokers), demonstrating a significant threshold in risk reduction at >5 years (Figure 1).
Figure 1.
Association of Smoking Cessation and risk of anti- double stranded DNA Positive (dsDNA+) SLE among participants in Nurses’ Health Study and Nurses’ Health Study II
*Adjusted for: age, questionnaire cycle, cohort, alcohol intake, race, early menarche, oral contraceptive use, body mass index, zip code-level median household income from US census
DISCUSSION
In these large prospective cohorts of women followed for many years prior to SLE onset, we found a strong and specific association between smoking and dsDNA+ SLE. While these analyses revealed no association between smoking and risk of overall SLE, dsDNA+ SLE risk was increased nearly two-fold among current smokers and by 60% among women who smoked >10 pack-years, compared to never smokers. Additionally, among current smokers, dsDNA+ SLE risk was nearly doubled by smoking ≥20 years. Finally, we demonstrated a significant reduction in dsDNA+ SLE risk among past smokers after quitting smoking for >5 years. Thus, we found positive short-term risk using time-varying updated smoking status and long-term risk using cumulative cigarette smoking in pack-years over a time period as long as up to 37 years. We also demonstrated that risk of dsDNA+ SLE decreases with smoking cessation. This is the largest and longest prospective study to investigate SLE risk using repeated measures of smoking exposure. Furthermore, these studies newly describe a specific association between current smoking and the subtype of SLE characterized by anti-dsDNA antibodies.
Our findings are consistent with and extend prior studies. Although epidemiologic studies of smoking and SLE risk have been somewhat conflicting (8, 21–23), our earlier meta-analysis of 7 case-control and 2 cohort studies, demonstrated elevated SLE risk among current smokers (OR 1.5, 95% CI 1.09–2.08) compared to non-smokers, but not past smokers (OR 0.98, 95%CI 0.75–1.27)(24). Since then, additional case-control studies demonstrated an elevated risk of SLE for both current and past smokers compared to never smokers (7, 8, 25, 26). However, an updated meta-analysis incorporating these additional case-control studies also demonstrated an elevated risk of SLE among current smokers (OR 1.56 [95%CI 1.26–1.95]) but not past smokers (OR 1.23 [95% CI 0.93–1.63])(27). Furthermore, current smoking was associated with presence of ≥1 autoantibody (adjusted OR 1.53, 95% CI 1.04–2.24) among SLE patients, but was not significantly associated with anti-dsDNA alone (26).
Several case-control studies have demonstrated a possible dose-response relationship for SLE risk with increasing pack-years (8, 28, 29). However, case-control studies are prone to recall and reverse causation biases. Two past prospective cohort study analyses, the NHS in 1996 and the Black Women’s Health Study (BWHS) in 2003, did not demonstrate significant associations between smoking and SLE risk, although the point estimate among current smokers in BWHS was elevated (OR 1.60 [95% CI 0.80–3.30]) (9, 10). Both cohorts were limited at the time by small sample size, one-time baseline assessment of exposure in BWHS, and short exposure duration.
Epidemiologic evidence suggests that tobacco smoke exposure is associated with other autoimmune diseases such as rheumatoid arthritis, Graves’ disease, and primary biliary cirrhosis (30–34). Notably, our findings parallel RA studies demonstrating an association between smoking and increased risk of seropositive RA (with rheumatoid factor and/or anti-cyclic citrullinated peptide antibodies), but not seronegative RA (33, 35). We have previously demonstrated increased risk of seropositive RA among both current (RR 1.58 [1.21–2.06]) and past smokers (RR 1.60 [1.27–2.02]), and with ≥10 pack-years of smoking, as well as with increased smoking duration and intensity compared to never smokers(33). However, whereas RA risk remained elevated until 20 years after smoking cessation (33), here we find dsDNA+ SLE risk was reduced after >5 years of smoking cessation, suggesting that dsDNA+ SLE risk may decline more rapidly with cessation.
Our results suggest a biologic role for smoking in the development of the anti-dsDNA+ subtype of SLE, although a mechanistic basis is not yet understood. Exposure to toxic components from cigarette smoke (e.g., tars, nicotine, carbon monoxide, polycyclic aromatic hydrocarbons, and free radicals) induce oxidative stress, damage endogenous proteins and DNA, and lead to genetic mutations and gene activation (36). Toxic components in cigarette smoking also induce epigenetic changes, resulting in altered gene expression affecting immune homeostasis(37, 38) and augmented production of pro-inflammatory cytokines including TNF-α and interleukin-6 (39, 40).
As in many tobacco-induced complex diseases, genetic background likely plays a role in whether a smoker will develop anti-dsDNA antibodies and SLE. In a past case-control study, the cytochrome P450 1A1 rs4646903 genotype and glutathione S-transferase M1 deletion genotype, both of which are involved in detoxification pathways, were associated with greatly increased SLE risk among smokers (OR 17.5 [95% CI 3.20–95.9]) with both risk genotypes (41). Smoking also stimulates surface expression of CD95 on B and CD4+ T cells, potentially leading to ineffective clearing of apoptotic neutrophils and dsDNA autoantibody production (42–44). Reactive oxygen species from tobacco damage DNA, forming immunogenic DNA adducts, which may result in dsDNA antibody production (30, 31). While our study was not designed to investigate disease mechanisms, our findings suggest the possibility of a gene-environment interaction, in that smoking may act as a trigger, or a “second hit” in those genetically predisposed to dsDNA+ SLE.
Compared to previous studies, a major strength of this study is the use of two large cohorts with over 5.6 million person-years of prospective follow-up. Detailed exposure data updated every two years allowed for evaluation of updated smoking status, cumulative smoking in pack-years, duration, intensity and time since quitting, enhancing precision and reducing the likelihood of misclassification of exposure, within-subject variation, and recall biases. Anti-dsDNA antibody status was assessed at SLE diagnosis, minimizing the possibility that dsDNA antibodies may have normalized after drug treatment. Furthermore, our “lagged” analysis in which smoking exposures were not updated through any closer than 4 years before the SLE outcome window, demonstrated a potentially even greater risk of current smoking for incident dsDNA+ SLE, suggesting that smokers may quit in the years preceding SLE diagnosis.
Given our strict definition of SLE, we may have excluded possible SLE cases at the time of medical record review that may later have become more clinically apparent. As we assessed dsDNA seropositivity at SLE diagnosis, cases that later developed dsDNA antibodies may have been misclassified as being negative. However, given that dsDNA antibodies become positive years before diagnosis (45), this misclassification was likely uncommon. Finally, given that the NHS cohorts include mostly healthy, White U.S. women working in advanced nursing professions, there is a potential lack of generalizability to younger women, males, and non-Whites. This is particularly relevant given that the association between smoking and dsDNA+ SLE may vary by race/ethnicity (21).
In conclusion, this study demonstrates a strong and specific association between current smoking and risk of dsDNA+ SLE, a severe subtype of SLE. In particular, current smoking and smoking >10 pack-years were associated with increased risk of dsDNA+ SLE, which was independent of alcohol intake, sociodemographic, lifestyle and reproductive factors. However, quitting smoking was shown to reduce dsDNA+ SLE risk to that of non-smokers after 5 years, suggesting that dsDNA+ SLE risk may be modifiable. These findings have implications for SLE prevention efforts and risk modification strategies. Furthermore, these results demonstrate the importance of studying specific SLE subtypes and provide insight into potential mechanisms of disease pathogenesis warranting further research.
Acknowledgments
Grant Support: Research reported in this publication was supported by National Institutes of Health (NIH) grants AR049880, AR047782, AR066109, AR066953, AR070514, AR069688, CA186107, CA176726, CA49449, and CA67262. Dr. Barbhaiya and Dr. Sparks are both supported by the Rheumatology Research Foundation Scientist Development Awards. Dr. Tedeschi is supported by the Lupus Foundation of American Career Development Award. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
We thank the participants in the NHS and NHSII cohorts for their dedication and continued participation in these longitudinal studies, as well as NHS staff in the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School for their assistance with this project.
References
- 1.Yung S, Cheung KF, Zhang Q, Chan TM. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. JASN. 2010;21(11):1912–27. doi: 10.1681/ASN.2009080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun KH, Yu CL, Tang SJ, Sun GH. Monoclonal anti-double-stranded DNA autoantibody stimulates the expression and release of IL-1beta, IL-6, IL-8, IL-10 and TNF-alpha from normal human mononuclear cells involving in the lupus pathogenesis. Immunology. 2000;99(3):352–60. doi: 10.1046/j.1365-2567.2000.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Fu R, Guo C, Huang Y, Wang H, Wang S, et al. Anti-dsDNA antibodies bind to TLR4 and activate NLRP3 inflammasome in lupus monocytes/macrophages. J Transl Med. 2016;14(1):156. doi: 10.1186/s12967-016-0911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CY, Tseng HM, Chen LC, Tsao CH, Kuo ML, Ou LS, et al. Use of a new fluorescence immunoassay to detect anti-dsDNA antibodies is more correlated with disease activity and complement than the ELISA method in SLE patients. Lupus. 2003;12(4):266–73. doi: 10.1191/0961203303lu331oa. [DOI] [PubMed] [Google Scholar]
- 5.Barbhaiya M, Costenbader KH. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol. 2016;28(5):497–505. doi: 10.1097/BOR.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Washio M, Horiuchi T, Kiyohara C, Kodama H, Tada Y, Asami T, et al. Smoking, drinking, sleeping habits, and other lifestyle factors and the risk of systemic lupus erythematosus in Japanese females: findings from the KYSS study. Mod Rheumatol. 2006;16(3):143–50. doi: 10.1007/s10165-006-0474-6. [DOI] [PubMed] [Google Scholar]
- 7.Ekblom-Kullberg S, Kautiainen H, Alha P, Leirisalo-Repo M, Julkunen H. Smoking and the risk of systemic lupus erythematosus. Clin Rheumatol. 2013;32(8):1219–22. doi: 10.1007/s10067-013-2224-4. [DOI] [PubMed] [Google Scholar]
- 8.Kiyohara C, Washio M, Horiuchi T, Asami T, Ide S, Atsumi T, et al. Cigarette smoking, alcohol consumption, and risk of systemic lupus erythematosus: a case-control study in a Japanese population. J Rheumatol. 2012;39(7):1363–70. doi: 10.3899/jrheum.111609. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Guerrero J, Karlson EW, Colditz GA, Hunter DJ, Speizer FE, Liang MH. Hair dye use and the risk of developing systemic lupus erythematosus. Arthritis Rheum. 1996;39(4):657–62. doi: 10.1002/art.1780390418. [DOI] [PubMed] [Google Scholar]
- 10.Formica M, Palmer JR, Rosenberg L, McAlindon TE. Smoking, Alcohol Consumption, and Risk of Systemic Lupus Erythematosus in the Black Women’s Health Study. J Rheumatol. 2003;30:1222–6. [PubMed] [Google Scholar]
- 11.Freemer MM, King TE, Jr, Criswell LA. Association of smoking with dsDNA autoantibody production in systemic lupus erythematosus. Ann Rheum Dis. 2006;65:581–4. doi: 10.1136/ard.2005.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–90. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56(4):1251–62. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- 14.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5:297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 17.Barbhaiya M, Lu B, Sparks JA, Malspeis S, Chang SC, Karlson EW, et al. Influence of Alcohol Consumption on the Risk of Systemic Lupus Erythematosus among Women in the Nurses’ Health Study Cohorts. Arthritis Care Res. 2016 doi: 10.1002/acr.22945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and Menopausal Factors and Risk of Systemic Lupus Erythematosus in Women. Arthritis Rheum. 2007;56(4):1251–62. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 21.Ghaussy NO, Sibbitt WL, Qualis CR. Cigarette Smoking, Alcohol Consumption, and the Risk of Systemic Lupus Erythematosus: A Case-Control Study. J Rheumatol. 2001;28:2449–53. [PubMed] [Google Scholar]
- 22.Ghaussy NO, Sibbitt WL, Qualls CR. Cigarette smoking, alcohol consumption, and the risk of systemic lupus erythematosus: a case-control study. J Rheumatol. 2001;28(11):2449–53. [PubMed] [Google Scholar]
- 23.Hardy CJ, Palmer BP, Muir KR, Sutton AJ, Powell RJ. Smoking history, alcohol consumption, and systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 1998;57(8):451–5. doi: 10.1136/ard.57.8.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costenbader KH, Kim DJ, Peerzada J, Lockman S, Nobles-Knight D, Petri M, et al. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis Rheum. 2004;50(3):849–57. doi: 10.1002/art.20049. [DOI] [PubMed] [Google Scholar]
- 25.Washio M, Horiuchi T, Kiyohara C, Kodama H, Tada Y, Asami T, et al. Smoking, drinking, sleeping habits, and other lifestyle factors and the risk of systemic lupus erythematosus in Japanese females: findings from the KYSS study. Mod Rheumatol. 2006;(16):143–50. doi: 10.1007/s10165-006-0474-6. [DOI] [PubMed] [Google Scholar]
- 26.Young KA, Terrell DR, Guthridge JM, Kamen DL, Gilkeson GS, Karp DR, et al. Smoking is not associated with autoantibody production in systemic lupus erythematosus patients, unaffected first-degree relatives, nor healthy controls. Lupus. 2014;23(4):360–9. doi: 10.1177/0961203314520838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang F, Li S, Jia C. Smoking and the risk of systemic lupus erythematosus: an updated systematic review and cumulative meta-analysis. Clin Rheum. 2015;34(11):1885–92. doi: 10.1007/s10067-015-3008-9. [DOI] [PubMed] [Google Scholar]
- 28.Hardy CJ, Palmer BP, Muir KR, Sutton AJ, Powell RJ. Smoking history, alcohol consumption, and systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 1998;57:451–5. doi: 10.1136/ard.57.8.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata C, Fujita S, Iwata H, Kurosawa Y, Kobayashi K, Kobayashi M, et al. Systemic lupus erythematosus: a case-control epidemiologic study in Japan. Int J Dermatol. 1995;34(5):333–7. doi: 10.1111/j.1365-4362.1995.tb03614.x. [DOI] [PubMed] [Google Scholar]
- 30.Petruzzelli S, Celi A, Pulera N, Baliva F, Viegi G, Carrozzi L, et al. Serum antibodies to benzo(a)pyrene diol epoxide-DNA adducts in the general population: effects of air pollution, tobacco smoking, and family history of lung diseases. Cancer Res. 1998;58(18):4122–6. [PubMed] [Google Scholar]
- 31.Mooney LA, Perera FP, Van Bennekum AM, Blaner WS, Karkoszka J, Covey L, et al. Gender differences in autoantibodies to oxidative DNA base damage in cigarette smokers. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10(6):641–8. [PubMed] [Google Scholar]
- 32.Prummel MF, Wiersinga WM. Smoking and risk of Graves’ disease. JAMA. 1993;269(4):479–82. [PubMed] [Google Scholar]
- 33.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503 e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 34.Parikh-Patel A, Gold EB, Worman H, Krivy KE, Gershwin ME. Risk factors for primary biliary cirrhosis in a cohort of patients from the united states. Hepatology (Baltimore, Md) 2001;33(1):16–21. doi: 10.1053/jhep.2001.21165. [DOI] [PubMed] [Google Scholar]
- 35.Karlson EW, Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis Rheum. 1999;42(5):910–7. doi: 10.1002/1529-0131(199905)42:5<910::AID-ANR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 36.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 37.Bauer M, Fink B, Thurmann L, Eszlinger M, Herberth G, Lehmann I. Tobacco smoking differently influences cell types of the innate and adaptive immune system-indications from CpG site methylation. Clin Epigenetics. 2015;7:83. doi: 10.1186/s13148-016-0249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dogan MV, Shields B, Cutrona C, Gao L, Gibbons FX, Simons R, et al. The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women. BMC genomics. 2014;15:151. doi: 10.1186/1471-2164-15-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89(9):1117–9. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 40.Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997;17(10):2167–76. doi: 10.1161/01.atv.17.10.2167. [DOI] [PubMed] [Google Scholar]
- 41.Kiyohara C, Washio M, Horiuchi T, Asami T, Ide S, Atsumi T, et al. Risk modification by CYP1A1 and GSTM1 polymorphisms in the association of cigarette smoking and systemic lupus erythematosus in a Japanese population. Scand J Rheumatol. 2012;41(2):103–9. doi: 10.3109/03009742.2011.608194. [DOI] [PubMed] [Google Scholar]
- 42.Bijl M, Horst G, Limburg P, Kallenberg C. Effects of smoking on activation markers, Fas expression and apoptosis of peripheral blood lymphocytes. Eur J Clin Invest. 2001;31:550–3. doi: 10.1046/j.1365-2362.2001.00842.x. [DOI] [PubMed] [Google Scholar]
- 43.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–J65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Kirkham PA, Spooner G, Rahman I, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem Biophys Res Commun. 2004;318(1):32–7. doi: 10.1016/j.bbrc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]