Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease for which we currently lack effective treatments or a cure. The pancreatic peptide hormone amylin has recently garnered interest as a potential pharmacological target for the treatment of AD. A number of studies have demonstrated that amylin and amylin analogs like the FDA-approved diabetes drug pramlintide can reduce amyloid burden in the brain and improve cognitive symptoms of AD. However, other data suggest that amylin may have pathological effects in AD due to its propensity to misfold and aggregate under certain conditions. Here, the literature supporting a beneficial versus harmful role of amylin in AD is reviewed. Additionally, several critical gaps in the literature are discussed, such as our limited understanding of the amylin system during aging and in disease states, as well as complexities of amylin receptor signaling and of changing pathophysiology during AD progression that might underlie the seemingly conflicting or contradictory results in the amylin / AD literature.
Keywords: IAPP, obesity, amyloid beta, diabetes, CGRP, pramlintide
1. Introduction
Alzheimer’s disease (AD) is a devastating neurodegenerative disease that directly affects over 5 million patients in the United States and is associated with billions of dollars in care costs (Alzheimer's, 2016). Symptoms and signs of AD include memory loss, cognitive decline, neuroinflammation, and perhaps most strikingly, the presence of amyloid plaques and neurofibrillary tangles in the central nervous system (CNS) (Alzheimer's, 2016; Bature et al., 2017; Heneka et al., 2015). Despite numerous efforts to combat the development of AD and to treat its symptoms, we still lack effective strategies to mitigate or cure AD (Cummings et al., 2016; Cummings et al., 2014), and researchers continue to seek new pharmacotherapeutic targets that may generate novel treatments for this disease.
Most recent efforts to identify and develop new AD pharmacotherapies have focused on reduction of CNS amyloid deposition (Cummings et al., 2016). Amyloid plaques in the brain of patients with AD are deposits of misfolded, aggregated proteins that consist largely of amyloid beta (Aβ), a protein formed from cleavage of amyloid precursor protein (APP) (Muresan and Ladescu Muresan, 2015; Roher et al., 2017; Zhang and Song, 2013). A number of other amyloidogenic diseases also involve aggregation of proteins in similar conformation to the Aβ amyloid plaques in AD (Eisenberg and Jucker, 2012; Koo et al., 1999). This has generated interest in identifying common links between AD and other amyloidogenic diseases (Guerrero-Munoz et al., 2014) which may help to identify new potential treatments for AD (Davies and Koppel, 2009).
Type 2 diabetes mellitus (T2DM) is a major risk factor for the development of AD (Qiu and Folstein, 2006; Zhang et al., 2017a) suggesting that there may be common mechanisms underlying the two diseases (Baram et al., 2016; Gotz et al., 2013; Wijesekara et al., 2017). Indeed, similar to the misfolding and aggregation of Aβ in plaques in the brain during AD, the presence of amyloid deposits in the pancreas is a hallmark of T2DM (Westermark, 1977). In contrast to Aβ-based amyloid in AD, pancreatic amyloid in T2DM consists largely of a pancreatic peptide hormone called amylin that has become a rapidly growing focus of AD research in recent years [see for review (Despa and Decarli, 2013; Lutz and Meyer, 2015; Mietlicki-Baase, 2016; Zhang and Song, 2017)]. Numerous lines of evidence support the notion that the amylin system plays a role in AD, but its effects remain unclear. In vitro studies show that amylin can potentiate neurotoxicity (Jhamandas et al., 2011; Jhamandas and Mactavish, 2012), and that the detrimental effects of Aβ are mediated through activation of amylin receptors (Fu et al., 2012; Jhamandas et al., 2011). Furthermore, aggregated amylin is found in the brains of patients with AD (Jackson et al., 2013). However, the literature is mixed, as other data suggest that amylin is actually beneficial in alleviating symptoms of AD, reducing amyloid burden in the brain and improving cognition (Adler et al., 2014; Wang et al., 2017; Zhu et al., 2015). These newer findings support the potential clinical relevance for amylin-based pharmacotherapy in the treatment of AD, but resolving these data with the literature describing harmful effects of amylin in AD has been challenging, in part due to gaps in the scientific literature on the physiology of the amylin system and its interactions with Aβ.
This review will first describe a few of the physiological roles of amylin, as well as how the misfolding of amylin can contribute to diseases like T2DM and AD. Next, the available evidence supporting a beneficial or harmful role of amylin in AD will be reviewed. Finally, some open areas of research that could greatly clarify our understanding of amylin’s role in AD will be discussed.
2. Amylin – physiology and pathophysiology
2.1 Physiological roles of amylin in glycemic control and energy balance
Amylin, also known as islet amyloid polypeptide (IAPP), is a 37-amino acid peptide that is produced primarily in the pancreas (Ogawa et al., 1990) but also within the brain (Li et al., 2015). Pancreatic amylin is co-secreted with insulin in response to the ingestion of food (Lutz, 2010a) and, under normal post-prandial physiological conditions, amylin is present in the plasma in the picomolar range (Bronsky and Prusa, 2004). As would be expected given its association with insulin signaling, amylin has been widely studied for its role in glycemic control [see for review (Hay et al., 2015; Mietlicki-Baase, 2016)]. Under normal physiological conditions, amylin has a number of key functions relevant to glycemia. For example, amylin improves postprandial glycemic control by slowing gastric emptying (Clementi et al., 1996) and reducing glucagon release (Fehmann et al., 1990). These beneficial glycemic effects have led to the development of an amylin analog, pramlintide, that is FDA-approved for the treatment of diabetes (Chawla and Kochar, 2006; Tran et al., 2015; Younk et al., 2011).
Amylin also has potent effects on energy balance control. Amylin release in response to nutrient ingestion suppresses food intake by acting within the brain to promote satiation (Lutz, 2010b; Lutz et al., 1995a; Lutz et al., 1995b; Mietlicki-Baase et al., 2013). Amylin can penetrate the blood-brain barrier (Banks and Kastin, 1998; Banks et al., 1995) and binds throughout the CNS (Beaumont et al., 1993; Hilton et al., 1995; Paxinos et al., 2004), suggesting that the intake-suppressive effects of amylin may be mediated by distributed nuclei in the brain (Baisley and Baldo, 2014; Dunn-Meynell et al., 2016; Lutz et al., 2001; Mietlicki-Baase et al., 2013; Mollet et al., 2004). Importantly, amylin and amylin analogs such as pramlintide reduce food intake as well as body weight gain in humans as well as non-human animal models (Adler et al., 2014; Mack et al., 2007; Mack et al., 2010; Ravussin et al., 2009; Roth et al., 2008). These body weight-suppressive effects have led to the consideration of the amylin system as a leading candidate for the development of new treatments for obesity (Hay et al., 2015; Jorsal et al., 2016; Mietlicki-Baase and Hayes, 2014; Sadry and Drucker, 2013; Valsamakis et al., 2017).
Amylin binds to and activates its receptor, a complex consisting of a calcitonin receptor (CTR) heterodimerized with a receptor activity modifying protein (RAMP) [see for review (Bower and Hay, 2016; Mietlicki-Baase and Hayes, 2014; Poyner et al., 2002)]. The CTR serves as the G-protein-coupled signaling component of the complex, with the RAMP increasing binding specificity for amylin (Bailey et al., 2012; Morfis et al., 2008; Tilakaratne et al., 2000). There are several subtypes of RAMP (RAMP1–3) and two variants of CTR (CTR-A and CTR-B). Evaluation of the expression of amylin receptor complex components within the CNS suggests higher expression of CTR-A compared to CTR-B (Barth et al., 2004; Mietlicki-Baase et al., 2013; Reiner et al., 2017), and expression of all three RAMPs (Liberini et al., 2016; Mietlicki-Baase et al., 2013; Oliver et al., 2001; Reiner et al., 2017) although expression of RAMP1 and RAMP3 tends to be higher than that of RAMP2 (Ueda et al., 2001). Any combination of a CTR and a RAMP can associate to form a functional amylin receptor, but the AMY1A (CTR-A / RAMP1) and AMY3A (CTR-A / RAMP3) are most well characterized (Bailey et al., 2012; Bower and Hay, 2016).
It should be noted that the physiological roles of amylin are not limited to glycemia and energy balance, although these are arguably its most well-studied functions. Amylin also has effects on a number of other peripheral tissues, including bone, kidney, and the cardiovascular system. These will not be discussed here, but the reader is directed to several excellent reviews on these topics (Hay et al., 2015; Naot and Cornish, 2014; Wookey and Cooper, 1998; Wookey et al., 2006; Young, 2005a, b, c).
2.2 Amylin misfolding and aggregation
Under normal physiological conditions, pancreatic amylin is prevented from misfolding. This is due in part to stabilization of the peptide conformation by association with insulin (Cui et al., 2009; Westermark et al., 1996) and is also influenced by other factors such as absolute levels of amylin (Jordan et al., 1990). However, as mentioned briefly in the introduction, amylin has come to the attention of AD researchers due to its propensity to misfold and aggregate. The amino acid sequence of amylin in several species, including humans and cats, contains amyloidogenic “hot spots” (Andreetto et al., 2010; Pillay and Govender, 2013). Several studies suggest that an amyloidogenic sequence toward the C-terminus of the peptide is prone to fold into beta-pleated sheets (Andreetto et al., 2010; Goldsbury et al., 2000; Pillay and Govender, 2013; Westermark et al., 1990). When levels of amylin are abnormally elevated, such as in a disease state like the early stages of T2DM (Johnson et al., 1989), amylin is more likely to misfold and form oligomers and fibrils (Jordan et al., 1990). These protein aggregates can induce cellular toxicity and apoptosis (Jhamandas and Mactavish, 2012; Konarkowska et al., 2006; Lorenzo et al., 1994; Tucker et al., 1998).
The amino acid sequence of amylin is highly conserved, but not completely identical among different species (Betsholtz et al., 1989; Nishi et al., 1992; Ohagi et al., 1991). Indeed, the amino acid sequence is key to understanding the amyloidogenic characteristics of amylin. Although human amylin is amyloidogenic and can aggregate into plaques, species that are commonly used as rodent models of disease such as rats and mice have nonamyloidogenic amylin due to proline substitutions in a key region of the amino acid sequence that stabilize the conformation of the peptide and prevent the formation of amylin fibrils (Westermark et al., 1990). This becomes a critical consideration in the study of amylin-based amyloid formation in vivo, and different strategies must be used to evaluate amylin aggregation in species like rats and mice, as their native amylin is not prone to amyloidogenesis.
3. The unresolved role of amylin in AD
3.1 Amylin and Aβ: amyloidogenic proteins in AD
Amylin can clearly act as an amyloidogenic peptide in disease. The pathological effects of amyloidogenic amylin in T2DM are relatively well-studied, as the existence of amylin-based amyloid plaques in the pancreas of patients with T2DM has been known for over a century (Opie, 1901). Numerous studies have established that T2DM is associated with cognitive deficits and is an important risk factor for the development of AD (Li and Huang, 2016; Li et al., 2016b; Riederer et al., 2017; Umegaki, 2014; Yuan and Wang, 2017). Amylin may be a crucial link between these diseases. For example, increased amylin levels have been suggested to be an important factor in T2DM-associated cognitive deficits (Ly and Despa, 2015). As hyperamylinemia sets the stage for misfolding and aggregation of the peptide (Jordan et al., 1990; Lutz and Meyer, 2015), this suggests that amylin aggregation and plaque formation may be relevant to both T2DM and AD, and in fact may be a mechanism by which the risk for AD is elevated in T2DM patients. Therefore, it is important to understand how amylin might interact with Aβ, the major component of amyloid plaques in the brain of individuals with AD (Jackson et al., 2013; Roher et al., 1993; Wong et al., 1985; Yamaguchi et al., 2000), to contribute to the development and/or progression of AD.
Aβ is a normal product of cleavage of APP. Aβ is toxic and can induce apoptosis in neurons (Dore et al., 1997; Jhamandas et al., 2011), but is usually cleared from the brain in healthy individuals (Tarasoff-Conway et al., 2015). When this process fails, Aβ levels in the brain increase and, much like amylin, the protein misfolds and accumulates into oligomers and amyloid plaques (Biere et al., 1995; Motter et al., 1995; Roberts et al., 2017). These oligomers and plaques are hallmarks of AD and are associated with neurotoxicity and cognitive decline [see for review (Echeverria and Cuello, 2002; Solomon, 2008; Takahashi et al., 2017)].
Although Aβ is the major component of amyloid plaques in AD, recent work has revealed that amylin is also colocalized with Aβ in CNS amyloid plaques in patients with AD (Jackson et al., 2013). Although the pancreas is the primary source of amylin in the body, amylin can cross the blood-brain barrier (Banks and Kastin, 1998; Banks et al., 1995). This suggests that circulating amylin may penetrate the CNS where, in a pathophysiological state, it can accumulate with or alongside Aβ to form plaques. Both mechanisms appear to be possible; while misfolded amylin can deposit and incorporate into existing Aβ aggregates, amylin can also act as a “seed” for plaque formation, around which additional Aβ and/or amylin can aggregate to generate larger plaques (Berhanu et al., 2013; Ono et al., 2014). The process of aggregation itself does not require the presence of the amylin receptor, as misfolding and aggregation of Aβ and/or amylin can occur in vitro (Tiiman et al., 2013; Yan et al., 2014), but amylin receptors may mediate some of the effects of Aβ and amylin on AD symptoms. This is discussed in more detail in the following sections.
3.2 Pathological effects of the amylin system in AD
Several studies over the past two decades suggest that amylin contributes to the pathophysiology of AD. Numerous in vitro studies have shown that human amylin can be neuroinflammatory and toxic to cells, including neuronal [e.g., primary cultures of human fetal neurons (Jhamandas et al., 2011; Jhamandas and Mactavish, 2012) or rat fetal neurons (Lorenzo and Yankner, 1996; Tucker et al., 1998)] and glial cells [e.g., human astrocytoma cells (Gitter et al., 2000), primary cultures of hamster microglia (Colton et al., 2000)], particularly at high concentrations or when “aged” to enhance fibrillation and aggregation of the peptide. Given the similarities between these data and the neurotoxic effects of Aβ, this may suggest that amylin accumulation within the CNS of individuals with AD has detrimental effects similar to those produced by Aβ.
These findings lead to the question of the mechanisms by which amylin and Aβ exert neurotoxic effects in the CNS. Interestingly, it appears that amylin receptor signaling may mediate the effects of both peptides. In particular, the AMY3A receptor (CTR-A / RAMP3) appears to be relevant for AD (Fu et al., 2012; Jhamandas et al., 2011). It is thought that activation of AMY3A by Aβ or amylin exerts toxicity by altering potassium conductance (Jhamandas et al., 2011) and calcium concentrations (Fu et al., 2012; Kawahara et al., 2000) in the cell, followed by disruption of the cell membrane and initiation of apoptosis (Jhamandas and Mactavish, 2012). As the components of the AMY3A receptor are expressed in the brain (see Section 2.1), it is possible that these in vitro findings are recapitulated in vivo, and increased CNS AMY3A receptor signaling due to elevated amylin and/or Aβ may mediate some of the neurotoxic events of AD. Pharmacological antagonists of the amylin receptor can attenuate or block the neurotoxic effects of either amylin or Aβ (Jhamandas et al., 2011), suggesting the potential physiological relevance of this effect. Despite these findings, it should be noted that other groups have found that Aβ does not increase cAMP in HEK293S or Cos7 cells expressing AMY1A, AMY2A, or AMY3A, suggesting that Aβ may not activate amylin receptors (Gingell et al., 2014). There are several metholodogical differences between this study and others that may underlie the discrepant findings, including the use of cells transiently transfected with the amylin receptor (Gingell et al., 2014) versus the use of stably transfected cells (Fu et al., 2012) or human fetal neurons (Jhamandas et al., 2011; Jhamandas and Mactavish, 2012) as the model. Although this study brings into question whether the toxic effects of Aβ are fully amylin receptor-mediated, the majority of currently available data support the hypothesis that Aβ and amylin produce deleterious effects on cell function and survival via amylin receptors.
Recent data also indicate that increased levels of amylin can lead to its accumulation in the microvasculature of the brain, damaging both blood vessels and white matter in the HIP rat, a model in which human amylin is overexpressed; these rats are also hyperglycemic and exhibit some cognitive and behavioral deficits (Ly et al., 2017). However, in the same paper, amylin knockout rats given daily injections of aggregated human amylin for 1 week showed a slightly different pattern of effects; while amylin was still detected in the CNS microvasculature, white matter was not damaged (Ly et al., 2017). There are several differences in these animal models that might explain these different results; for example, chronically elevated amylin levels in the HIP rat could elicit compensatory changes not observed with the comparatively acute administration of amylin in the amylin knockout animal. Nevertheless, these results are consistent with human data indicating amylin accumulation in the CNS vasculature of patients with AD (Jackson et al., 2013) and may suggest another way in which amyloidogenic or aggregated amylin can potentially contribute to CNS damage.
3.3 Beneficial effects of amylin in AD
Based on the data discussed thus far, it would appear that amylin is strictly harmful or detrimental in AD. However, newer evidence suggests that the role of amylin in AD is not as straightforward as it may seem. Amylin or amylin-based pharmacotherapies have recently been shown to decrease levels of CNS amyloid in rodent models of AD. Indeed, systemic administration of amylin or the amylin analog pramlintide reduces the Aβ burden in areas of the brain that are heavily impacted by AD, such as the hippocampus and cortex, in the 5XFAD mouse model of AD (Wang et al., 2017; Zhu et al., 2015; Zhu et al., 2017b). Intriguingly, these reductions in central Aβ are thought to be driven by an efflux of Aβ from the brain to the blood. Intraperitoneal injection of amylin increased circulating levels of Aβ in the Tg2576 mouse (increases in both Aβ1–40 and Aβ1–42 in serum), the 5XFAD mouse (increased serum Aβ1–42), and the Dutch APP mouse (increased serum Aβ1–40) (Mohamed et al., 2017; Zhu et al., 2015). Of potential clinical relevance, similar effects were observed after subcutaneous pramlintide administration in human patients with AD, who displayed increased plasma Aβ1–40 after acute pramlintide treatment (Zhu et al., 2017a). The mechanisms by which amylin-induced efflux of Aβ occurs are not completely understood, but may involve LRP1, a protein involved in Aβ transport. In vitro studies in mouse brain endothelial cells, used as a model of the blood-brain barrier, show that amylin upregulates trafficking of LRP1 to the cell membrane (Mohamed et al., 2017) suggesting a potential mechanism by which amylin increases transport of Aβ from the CNS into the blood. In addition, although much of the research on amylin’s effects in AD has focused on reductions in CNS amyloid burden, there are a number of other neuropathological changes that occur in the brain in AD. Interestingly, amylin and amylin receptor agonists also appear to attenuate some of these other AD-related pathophysiologies, such as tauopathy and neuroinflammation, in various mouse models of AD including SAMP8 (Adler et al., 2014), 5XFAD (Wang et al., 2017; Zhu et al., 2017b), and 3xTgAD (Zhu et al., 2017b) mice.
Collectively, these data suggest that amylin treatment may actually be beneficial to treat AD-related pathophysiology. Given that one of the major symptoms of AD is cognitive decline, it is also critical to understand whether amylin influences memory and cognitive ability in AD, as amelioration of these symptoms would help to improve the quality of life for patients with AD. The majority of the available literature suggests that in general, plasma amylin is positively associated with cognitive ability. In elderly individuals, higher levels of amylin are associated with better performance on verbal and visuospatial memory tasks (Qiu et al., 2014), although this study did not account for AD diagnosis of the participants. Other studies have categorized participants by status of AD and/or mild cognitive impairment and show that individuals with AD or mild cognitive impairment have lower non-fasting plasma amylin levels compared to a cognitively normal control group (Adler et al., 2014), consistent with a positive correlation between amylin and cognition. However, another report shows that fasted amylin levels are higher in patients with mild cognitive impairment or AD compared to individuals without dementia (Morris et al., 2016). These discordant findings may be a result of fasting status at the time of blood draw, or could be due to the exclusion of individuals with moderate or severe AD in the latter study (Morris et al., 2016). Beneficial effects of amylin and amylin receptor agonists on cognitive ability are also revealed in animal models of AD. It has been consistently demonstrated that peripheral administration of amylin or pramlintide improves performance in tasks of learning and memory in various animal models of AD including SAMP8, 5XFAD, and 3xTgAD mice (Adler et al., 2014; Zhu et al., 2015; Zhu et al., 2017b). Importantly, the improvements in AD symptoms in 5XFAD mice produced by chronic amylin administration, including reduced amyloid burden and improved performance on learning and memory tasks (e.g., Y-maze) are attenuated by co-administration of AC253, suggesting that these benefits are mediated by amylin receptor activation (Zhu et al., 2017b). Collectively, these data indicate a beneficial effect of exogenous amylin / pramlintide administration on cognition and memory in humans with AD as well as in animal models of the disease.
Some studies suggest that amylin levels are lower in individuals with AD [(Adler et al., 2014; Qiu and Zhu, 2014); but also see (Fawver et al., 2014)]. If amylin is decreased in AD, it follows that at least some AD symptoms might be improved by restoring amylin signaling via administration of amylin or amylin analogs like pramlintide. Given the ability of amylin to cross-seed or associate with Aβ (Berhanu et al., 2013; Ono et al., 2014), one might expect that it would be critical to use nonamyloidogenic amylin to prevent further aggregation or plaque formation. However, human amylin, which is amyloidogenic and prone to aggregation, can produce reductions in central Aβ that are associated with improved cognitive ability in 5XFAD mice, as assessed by performance on the Y-maze and Morris water maze (Zhu et al., 2017b). The mechanism by which administration of exogenous amylin ligands – amyloidogenic or not – improves AD symptoms remains an open empirical question.
As noted throughout this section, the available in vivo results to date have been generated from a variety of animal models of AD. There are a number of key differences among these animal models, including the mutation(s) targeted as well as the particular physiological and behavioral symptoms of AD exhibited by each model [for review, see (Lee and Han, 2013; Platt et al., 2013; Webster et al., 2014)]. On one hand, the variety of AD models used to evaluate the effects of amylin and amylin analogs on the disease could be viewed as a limitation in the field, as it may reduce the ability to directly compare findings between studies. However, this could also be viewed as evidence in favor of the potential utility of amylin-based compounds to treat AD. Given that AD involves a constellation of pathophysiological and behavioral changes, and that in vivo studies generally support the idea that amylin and pramlintide produce beneficial effects on symptoms in several different mouse models of AD (Adler et al., 2014; Zhu et al., 2015; Zhu et al., 2017b), this may suggest broader applicability of amylin-based pharmacotherapies for treatment of this complex disease.
3.4 Amylin may improve AD symptoms via indirect effects on other systems
The beneficial effects of amylin in AD described above are presumed to be direct effects of the peptide or its analogs. However, many of these experiments involve chronic treatment with amylin or pramlintide [e.g., (Adler et al., 2014; Wang et al., 2017; Zhu et al., 2015; Zhu et al., 2017b)], and the long-term administration of amylin-based pharmacotherapies can have potent beneficial effects on risk factors associated with AD development and progression. For example, pramlintide is used clinically in the treatment of diabetes, and administration of pramlintide over several weeks or months would be expected to improve glycemic control (Kolterman et al., 1996; McQueen, 2005; Pullman et al., 2006). Reducing blood glucose levels should diminish the increased risk for AD in individuals with hyperglycemia and T2DM (Barbagallo and Dominguez, 2014; Bosco et al., 2011; Roriz-Filho et al., 2009; Sridhar et al., 2015; Zhang et al., 2017a). Similarly, chronic amylin or pramlintide treatment reduces food intake and body weight (Arnelo et al., 1996; Olsson et al., 2007; Pullman et al., 2006; Ravussin et al., 2009), which might mitigate the increased risk for AD that is conferred by obesity or overweight (Gustafson et al., 2003; Kivipelto et al., 2005; Moser and Pike, 2016).
A number of studies evaluating the ability of amylin-based treatment to improve outcomes in animal models of AD do not report body weight or glycemic outcomes in the treatment versus control groups, which could be helpful in disentangling these issues. One animal study in which SAMP8 mice were given chronic pramlintide treatment showed that pramlintide-mediated improvements in cognition and reductions in expression of inflammatory markers such as cyclooxygenase 2 (COX-2) in the hippocampus were accompanied by weight loss (Adler et al., 2014). Importantly though, this does not disentangle whether the weight loss was in any way causal to the improvements in AD-related pathophysiology. Other studies using chronic amylin-based treatments have reported body weight matching of experimental groups (Zhu et al., 2015; Zhu et al., 2017b), but presumably this was done before amylin or pramlintide treatment, and final body weights at the time of testing are not typically reported. Additionally, amylin-mediated changes in glycemic control are rarely reported in this literature, yet this represents a key factor that may impact AD symptoms. Despite these limitations, it is important to reiterate that the available data clearly demonstrate the ability of acute amylin to induce beneficial effects relevant to AD, such as the induction of Aβ efflux from the CNS after amylin treatment (Mohamed et al., 2017; Zhu et al., 2015). This underscores the notion that, although chronic amylin administration may influence other variables related to AD, it also likely has direct effects on AD pathophysiology that cannot be attributed to long-term changes in glycemic control or body weight.
4. Unresolved issues in understanding amylin’s role in AD
The data described in Section 3 present conflicting views of the role of amylin in AD and its possible contribution to the progression of the disease. Some data point to endogenous amylin as potentially problematic or detrimental in AD, but other findings indicate that exogenous amylin and amylin analogs may be therapeutically useful. There are several unresolved issues that may help us to gain a better understanding of the effects of amylin in AD. These include [1] complexities of the amylin receptor system and amylin signaling in general; [2] changes in the amylin system during healthy aging and during AD; and [3] how the pathophysiological profile of AD changes as the disease progresses. In this section, these areas of research are discussed in the context of what is known and what might be gained from further scientific investigation.
4.1 Amylin and its receptor: a complex system
As described in Section 3.3, administration of amylin receptor agonists (amylin, pramlintide) can attenuate symptoms of AD in animal models. However, a recent study suggests that treatment with an amylin receptor antagonist can also improve AD symptoms. Jhamandas’s group showed that central infusion of the amylin receptor antagonist AC253, or peripheral administration of cyclic AC253 (a modified form of AC253 with better CNS penetrance), improved performance on the Morris water maze in TgCRND8 mice (Soudy et al., 2017). This result is consistent with in vitro studies suggesting that amylin receptor antagonists block the toxic effects of amylin and Aβ (Fu et al., 2012; Jhamandas et al., 2011; Jhamandas and Mactavish, 2012), but also raises the question of how both agonists and antagonists of the amylin receptor can have beneficial effects on AD symptoms in animal models. This may relate to the complexities of amylin signaling, dosing of amylin receptor ligands, and the receptors to which amylin binds.
As described in Section 2.1, there are 6 possible amylin receptors that can be formed from the various combinations of CTRs and RAMPs. Amylin and amylin receptor agonists can bind to any of these receptors and engage downstream signaling events, but typically, research on amylin signaling in AD focuses on only one or a subset of the different amylin receptor subtypes. For example, Soudy et al. report that the amylin receptor antagonist cyclic AC253 is expected to potently inhibit AMY3A based on in vitro data, but its effects on other amylin receptors are not reported (Soudy et al., 2017). It has also been shown that AC253 may act as a partial agonist at the amylin receptor (Zhu et al., 2017b). Although this is untested for cyclic AC253, this could represent another mechanism by which amylin receptor activation could be differentially impacted by various ligands and perhaps provide some resolution to these discrepancies in the literature.
A related consideration is the dosing of the amylin receptor ligands in these and other studies. For example, several in vivo studies suggesting that pramlintide administration is beneficial in improving the symptoms of AD use chronic administration of pramlintide, with doses in the µg/kg range given daily for several weeks (Adler et al., 2014; Zhu et al., 2015). However, recently published in vitro electrophysiological analyses in mouse brain hippocampal tissue suggest that nanomolar concentrations of pramlintide can attenuate baseline long-term potentiation (LTP) deficits in the hippocampus of TgCRND8 mice as well as the reduction in LTP induced by application of amylin or Aβ, raising the possibility that pramlintide might act as an antagonist at the amylin receptor at low concentrations (Kimura et al., 2017). In addition, human amylin-induced changes in intracellular calcium in rat hippocampal neurons in vitro were mediated by amylin receptors at low concentrations of amylin, but at higher concentrations the effects were independent of amylin receptors, possibly due to aggregation of the ligand at higher concentrations (Zhang et al., 2017b). These findings suggest that dosing may be a critical factor in teasing apart the beneficial versus detrimental effects of amylin receptor ligands on features of AD. Unfortunately, much of the available in vivo work does not include thorough physiological and behavioral dose-response analyses that would be required to determine whether the differential effects of various doses of amylin receptor ligands in vitro are recapitulated in vivo. This is especially important given the interest in amylin-based compounds as potential pharmacotherapies for treating AD; although the literature clearly shows that certain doses of amylin and pramlintide can improve AD symptoms, it is unclear what the effective ranges may be. This is a critical area of investigation that needs to be addressed in the literature. Furthermore, it is also important to gain a better understanding of the dose-response effects of these ligands not only on their own, but also to evaluate whether their function may be different in the presence of other amylin receptor ligands, which may shed light onto some of the existing discrepancies between current in vivo and in vitro data.
It is worth noting that amylin or pramlintide may also change the activity of receptors other than amylin receptors. Amylin belongs to a family of peptides including calcitonin, calcitonin gene-related peptide (CGRP), and adrenomedullin that are highly promiscuous and can bind to several types of receptors with differing binding affinity (Hay et al., 2006; Poyner et al., 2002; Wimalawansa, 1997). In addition to binding to amylin receptors, amylin can bind to the naked CTR (e.g., without an associated RAMP) as well as the calcitonin receptor-like receptor (CLR) with or without RAMP (Hay et al., 2006). Therefore, amylin receptor ligands are capable of altering signaling at a variety of receptors which could potentially have different consequences for AD. “Off-target” (e.g. non-amylin receptor- / non-AMY3A-mediated) binding of an amylin receptor agonist or antagonist could change the net effects of amylin signaling in the brain in a way that improves AD symptoms, highlighting the need to carefully and systematically evaluate the role of various amylin and CGRP-family receptors in the effects of amylin-based ligands on AD.
4.2 Age-related changes in the amylin system
In thinking about the potential utility of amylin-based pharmacotherapies for the treatment of AD in humans, the effect of aging on the amylin system has often been overlooked. Our understanding of changes to the amylin system during healthy aging or in a pathophysiological state such as AD is extremely limited. A few reports describe age-related changes to the amylin system, and of those, most have examined plasma amylin levels. While some studies suggest that there is no significant association between age and plasma amylin levels in older adults (Li et al., 2016a), other reports comparing younger and older adults have revealed that amylin release is decreased in older adults (Dechenes et al., 1998) or in middle age but not older adulthood (Edwards et al., 1996). The reason for these discrepant results is unknown but may be related to methodological differences among these studies.
Another important question is whether the physiological effects of amylin are the same throughout the lifespan or whether they differ in aging. Food intake studies in rats demonstrate that food-deprived older rats display an anorectic response to amylin at a dose that does not impact feeding in young rats that have been deprived of food for the same amount of time (Lutz et al., 1994). Although this difference could be due to changes in baseline energy balance control in older rats (Lutz et al., 1994), this still suggests that aging may directly or indirectly impact the efficacy of amylin signaling and/or function. In order to critically evaluate the potential clinical relevance of amylin-based pharmacotherapies for AD, it is important to understand not only what happens to the amylin system during normal aging but also whether AD causes further changes in expression or function of the amylin system. Potential mechanisms underlying age-and/or AD-related changes in amylin function may include alterations in amylin receptor expression, intracellular response to receptor agonists, amylin release (as described above), or another aspect of amylin physiology. An age-related increase in CTR and RAMP3 expression has been observed in discrete brain areas of the TgCRND8 mouse model of AD (Jhamandas et al., 2011), supporting the idea that AD may change amylin signaling and highlighting the need to examine this more broadly. Yet in general, these possibilities are extremely underinvestigated.
Understanding the amylin system in aging and deciphering how it may relate to AD is further complicated by needing to understand how expression of amylin and its receptors is altered not only during normal healthy aging versus AD, but also in AD-related comorbidities such as T2DM and obesity. These represent important areas of investigation that will provide a more comprehensive understanding of amylin physiology required to fully understand the effects of the peptide in AD. Clearly there are several key gaps in our current knowledge of how the amylin system changes over the lifespan, even within the context of normal healthy aging. Moreover, diseases such as T2DM and obesity are often comorbid with AD and are known to influence the physiology of the amylin system (Johnson et al., 1989; Pieber et al., 1994; Reinehr et al., 2007). More research is undoubtedly required to understand the basic physiological changes that occur in the amylin system during aging, as well as how AD and related comorbidities may change the system and potentially alter the efficacy of amylin-based pharmacotherapies.
4.3 Changes in AD pathophysiology during disease progression
Given that AD is a progressive disease, a major challenge in understanding the role of any physiological system in the context of AD and its potential utility as an AD pharmacotherapy is to consider its role at different stages of the disease. The signs and symptoms of AD change and evolve as the disease becomes more advanced. This section focuses on changes in the processes of protein misfolding and aggregation throughout the course of AD, and how this may be relevant to understanding the role of amylin in AD pathophysiology as well as the ability of amylin-based pharmacotherapies to improve AD symptoms.
Amyloid plaque formation in AD begins with misfolding of monomeric peptides, which aggregate into oligomers. Some oligomers appear to seed formation of fibrils which can then grow by further accumulation of misfolded protein to form mature amyloid plaques, while other oligomers remain soluble (Ma and Nussinov, 2010; Morgado and Fändrich, 2011; Necula et al., 2007). The particular mechanisms by which this occurs are not fully understood. Importantly, the prevalent form(s) of misfolded and/or aggregated protein differ as AD progresses. Earlier stages of the disease are characterized by higher levels of soluble oligomers and minimal presence of amyloid plaques; in contrast, plaques predominate in more advanced stages of AD (Kawarabayashi et al., 2001). It should be noted that there are several types of Aβ oligomers (dimers, trimers, etc.) and even the levels of these different Aβ oligomers change over time (Takeda et al., 2013).
Although amyloid plaques were considered for many years to be the relevant cytotoxic species in AD [for review see (Goure et al., 2014)], the presence or amount of amyloid plaque does not necessarily correlate well with other symptoms of AD, particularly the cognitive decline associated with the disease (Terry et al., 1991). This highlighted the idea that “pre-plaque” forms of misfolded protein might be more detrimental in the course of the disease. Indeed, the notion that solubilized oligomeric Aβ is more toxic than Aβ plaques has become increasingly accepted in the literature [see for review (Ono, 2017; Viola and Klein, 2015)]. Neuropathology and cognitive deficits occur in several different mouse models of AD independent of plaque accumulation (DaRocha-Souto et al., 2011; Hsia et al., 1999; Lesne et al., 2008; Meilandt et al., 2009), and the presence of oligomeric Aβ is associated with AD in humans (Mc Donald et al., 2010).
In contrast to the literature exploring how various aggregated forms of Aβ contribute to AD pathology, very few papers have addressed the differences between amylin-based oligomers and fibrils. Little is known about the relative toxicity of various aggregated forms of amylin, representing an important gap in the literature. In the periphery, oligomeric amylin appears to be more toxic than fibrils. Under experimental conditions promoting formation of amylin-based oligomers and fibrils in vitro, specifically inhibiting fibril formation had no effect on β-cell apoptosis, suggesting that oligomers were responsible for the apoptotic effects of amylin (Meier et al., 2006). Only a few reports specifically address the presence and/or aggregation of oligomeric amylin in the brain during AD. Jackson et al. demonstrated that several oligomeric forms of amylin are expressed in brain tissue from AD patients (Jackson et al., 2013). It is unclear whether one or more of these oligomers are associated with the neurotoxicity or cognitive decline observed in individuals with AD. In APP-transgenic mice, exogenous soluble oligomeric Aβ was shown to accumulate in CNS plaques while exogenous soluble oligomeric amylin did not (Gaspar et al., 2010), but any potential impact of these soluble oligomers on the behavioral symptoms of AD was not reported. Intriguingly, it has been suggested that some conformations of oligomeric amylin may actually help to disrupt further aggregation of Aβ (Baram et al., 2016), highlighting the need to better understand the interactions of oligomeric amylin with other aggregating / amyloidogenic peptides in AD.
Differences in the presence and relative levels of particular types of protein aggregates at different stages of AD also raises the possibility that a treatment may be more or less effective at particular times during the course of disease progression. This may be especially critical in understanding whether amylin is helpful in the treatment of AD or whether it is pathological. As noted in Section 3.3, several studies have demonstrated that exogenous application of amylin or pramlintide increases brain-to-blood efflux of Aβ, reduces amyloid plaque burden in the CNS, and improves performance on tests of cognitive ability and memory in animal models of AD. Interestingly, the outcomes of these studies have been derived from mice of a range of ages. Whereas some experiments initiate administration of amylin-based pharmacotherapy in mice at ~3 months of age (Soudy et al., 2017; Zhu et al., 2015; Zhu et al., 2017b), others begin several months later (Zhu et al., 2015). These converging data are encouraging and could be viewed as evidence of the generalizability of the beneficial effects of amylin over the course of AD, supporting the possible relevance of amylin-based treatments for AD. Nevertheless, this also underscores the fact that the effects of amylin in AD rodent models have not been systematically examined and compared at different ages. This could provide insight into whether and when amylin may be helpful versus harmful in AD. Further, this may also be useful to tease apart the ideal timing of amylin-based treatments, e.g., whether amylin or pramlintide might be more effective to ameliorate AD symptoms when treatment is initiated at a specific age or at a particular point during the progression of the disease pathology.
5. Conclusion
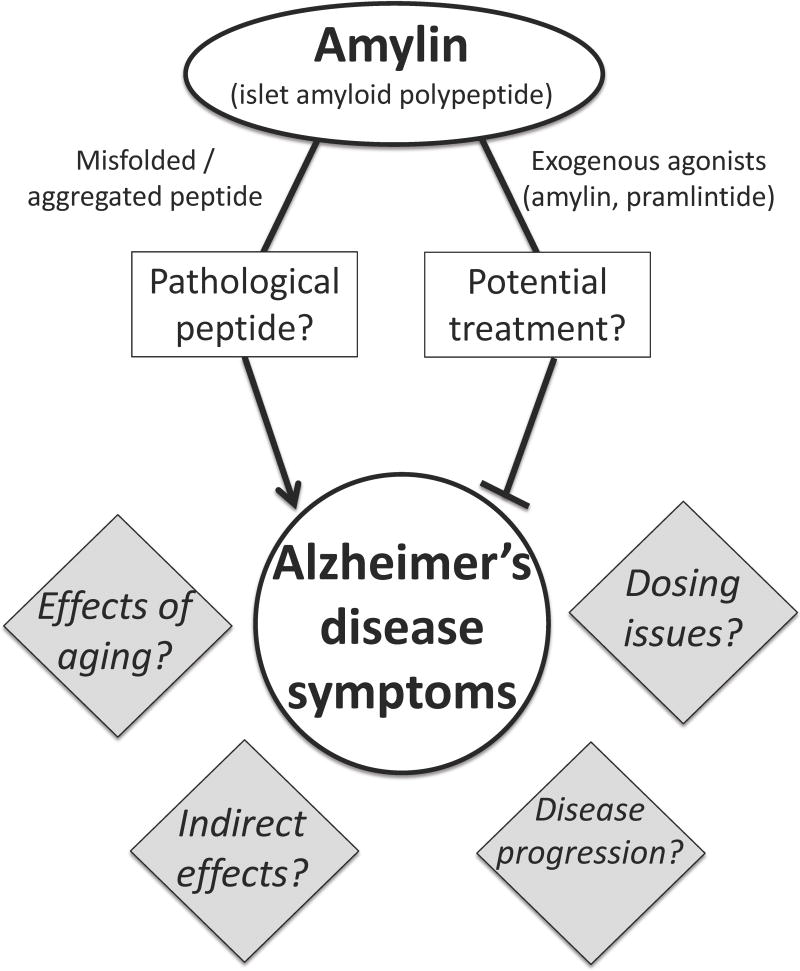
AD directly affects millions of adults each year with health care costs in the billions. Despite many advances in our understanding of the biological underpinnings of this disease, we lack effective treatments, and there is an urgent need to identify new potential pharmacological strategies to treat or cure AD. Amylin shows promise as a possible candidate for AD treatment, as recent data suggest that amylin and amylin analogs can reduce CNS amyloid burden and improve the cognitive symptoms of AD. However, other data have suggested that amylin contributes to AD symptoms and pathophysiology. Some of these key points discussed here are summarized in Figure 1. Going forward, it is critical to reconcile these conflicting data in order to fully understand how amylin acts in AD and whether it is beneficial or detrimental in treating this disease. More research is undoubtedly needed to understand the physiology of the amylin system in aged individuals and to identify how its effects in AD may change as the disease progresses. Filling in these critical gaps in the literature may shed light onto how seemingly discrepant reports of amylin’s function in AD may actually relate or be compatible, resulting in a better understanding of AD and hopefully leading to effective pharmacological treatments or a cure.
Figure 1.
The role of amylin in Alzheimer’s disease remains an open question. As reviewed in this paper, numerous studies show that amylin can misfold and aggregate, and can cause or exacerbate pathophysiological changes associated with AD [e.g., (Fu et al., 2012; Jhamandas et al., 2011; Jhamandas and Mactavish, 2012)]. However, other data indicate that exogenous administration of amylin receptor agonists can ameliorate AD symptoms [e.g., (Adler et al., 2014; Zhu et al., 2015; Zhu et al., 2017b)], suggesting that amylin-based pharmacotherapies may be useful in the treatment of AD. There are currently several gaps in the literature that limit our understanding of the effects of amylin in AD, a few of which are represented in the gray diamonds.
Highlights.
-
-
Amylin is a peptide hormone with established roles in glycemia and energy balance
-
-
Amylin is prone to misfolding and aggregation under certain conditions
-
-
Recent research has highlighted a role for amylin in Alzheimer’s disease
-
-
Mixed findings show positive and negative effects of amylin in Alzheimer’s disease
-
-
Amylin may be a novel pharmacological target to treat Alzheimer’s disease
Acknowledgments
The author thanks Katherine Balantekin for her helpful editorial suggestions.
Funding
This work was supported by the National Institutes of Health [grant numbers DK103804, DK114211].
The author receives funding from Zealand Pharma that was not used in support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The author receives funding from Zealand Pharma that was not used in support of this work. The author declares no other competing financial interests.
References
- Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R, Smith MA, Lee HG, Arnold SE, Casadesus G. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiol Aging. 2014;35:793–801. doi: 10.1016/j.neurobiolaging.2013.10.076. [DOI] [PubMed] [Google Scholar]
- Alzheimer's A. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Andreetto E, Yan LM, Tatarek-Nossol M, Velkova A, Frank R, Kapurniotu A. Identification of hot regions of the Abeta-IAPP interaction interface as high-affinity binding sites in both cross- and self-association. Angew Chem Int Ed Engl. 2010;49:3081–3085. doi: 10.1002/anie.200904902. [DOI] [PubMed] [Google Scholar]
- Arnelo U, Permert J, Adrian TE, Larsson J, Westermark P, Reidelberger RD. Chronic infusion of islet amyloid polypeptide causes anorexia in rats. Am J Physiol. 1996;271:R1654–1659. doi: 10.1152/ajpregu.1996.271.6.R1654. [DOI] [PubMed] [Google Scholar]
- Bailey RJ, Walker CS, Ferner AH, Loomes KM, Prijic G, Halim A, Whiting L, Phillips AR, Hay DL. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br J Pharmacol. 2012;166:151–167. doi: 10.1111/j.1476-5381.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisley SK, Baldo BA. Amylin receptor signaling in the nucleus accumbens negatively modulates mu-opioid-driven feeding. Neuropsychopharmacology. 2014;39:3009–3017. doi: 10.1038/npp.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Maness LM, Huang W, Jaspan JB. Permeability of the blood-brain barrier to amylin. Life Sci. 1995;57:1993–2001. doi: 10.1016/0024-3205(95)02197-q. [DOI] [PubMed] [Google Scholar]
- Baram M, Atsmon-Raz Y, Ma B, Nussinov R, Miller Y. Amylin-Abeta oligomers at atomic resolution using molecular dynamics simulations: a link between Type 2 diabetes and Alzheimer's disease. Phys Chem Chem Phys. 2016;18:2330–2338. doi: 10.1039/c5cp03338a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo M, Dominguez LJ. Type 2 diabetes mellitus and Alzheimer's disease. World J Diabetes. 2014;5:889–893. doi: 10.4239/wjd.v5.i6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth SW, Riediger T, Lutz TA, Rechkemmer G. Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res. 2004;997:97–102. doi: 10.1016/j.brainres.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Bature F, Guinn BA, Pang D, Pappas Y. Signs and symptoms preceding the diagnosis of Alzheimer's disease: a systematic scoping review of literature from 1937 to 2016. BMJ Open. 2017;7:e015746. doi: 10.1136/bmjopen-2016-015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont K, Kenney MA, Young AA, Rink TJ. High affinity amylin binding sites in rat brain. Mol Pharmacol. 1993;44:493–497. [PubMed] [Google Scholar]
- Berhanu WM, Yasar F, Hansmann UH. In silico cross seeding of Abeta and amylin fibril-like oligomers. ACS Chem Neurosci. 2013;4:1488–1500. doi: 10.1021/cn400141x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C, Christmansson L, Engstrom U, Rorsman F, Svensson V, Johnson KH, Westermark P. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989;251:261–264. doi: 10.1016/0014-5793(89)81467-x. [DOI] [PubMed] [Google Scholar]
- Biere AL, Ostaszewski B, Zhao H, Gillespie S, Younkin SG, Selkoe DJ. Co-expression of beta-amyloid precursor protein (betaAPP) and apolipoprotein E in cell culture: analysis of betaAPP processing. Neurobiol Dis. 1995;2:177–187. doi: 10.1006/nbdi.1995.0019. [DOI] [PubMed] [Google Scholar]
- Bosco D, Fava A, Plastino M, Montalcini T, Pujia A. Possible implications of insulin resistance and glucose metabolism in Alzheimer's disease pathogenesis. J Cell Mol Med. 2011;15:1807–1821. doi: 10.1111/j.1582-4934.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower RL, Hay DL. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br J Pharmacol. 2016;173:1883–1898. doi: 10.1111/bph.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronsky J, Prusa R. Amylin fasting plasma levels are decreased in patients with osteoporosis. Osteoporos Int. 2004;15:243–247. doi: 10.1007/s00198-003-1538-5. [DOI] [PubMed] [Google Scholar]
- Chawla PS, Kochar MS. What's new in clinical pharmacology and therapeutics. WMJ. 2006;105:24–29. [PubMed] [Google Scholar]
- Clementi G, Caruso A, Cutuli VM, de Bernardis E, Prato A, Amico-Roxas M. Amylin given by central or peripheral routes decreases gastric emptying and intestinal transit in the rat. Experientia. 1996;52:677–679. doi: 10.1007/BF01925572. [DOI] [PubMed] [Google Scholar]
- Colton CA, Chernyshev ON, Gilbert DL, Vitek MP. Microglial contribution to oxidative stress in Alzheimer's disease. Ann N Y Acad Sci. 2000;899:292–307. doi: 10.1111/j.1749-6632.2000.tb06195.x. [DOI] [PubMed] [Google Scholar]
- Cui W, Ma JW, Lei P, Wu WH, Yu YP, Xiang Y, Tong AJ, Zhao YF, Li YM. Insulin is a kinetic but not a thermodynamic inhibitor of amylin aggregation. FEBS J. 2009;276:3365–3371. doi: 10.1111/j.1742-4658.2009.07061.x. [DOI] [PubMed] [Google Scholar]
- Cummings J, Aisen PS, DuBois B, Frolich L, Jack CR, Jr, Jones RW, Morris JC, Raskin J, Dowsett SA, Scheltens P. Drug development in Alzheimer's disease: the path to 2025. Alzheimers Res Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaRocha-Souto B, Scotton TC, Coma M, Serrano-Pozo A, Hashimoto T, Sereno L, Rodriguez M, Sanchez B, Hyman BT, Gomez-Isla T. Brain oligomeric beta-amyloid but not total amyloid plaque burden correlates with neuronal loss and astrocyte inflammatory response in amyloid precursor protein/tau transgenic mice. J Neuropathol Exp Neurol. 2011;70:360–376. doi: 10.1097/NEN.0b013e318217a118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, Koppel J. Mechanism-based treatments for Alzheimer's disease. Dialogues Clin Neurosci. 2009;11:159–169. doi: 10.31887/DCNS.2009.11.2/pdavies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechenes CJ, Verchere CB, Andrikopoulos S, Kahn SE. Human aging is associated with parallel reductions in insulin and amylin release. Am J Physiol. 1998;275:E785–791. doi: 10.1152/ajpendo.1998.275.5.E785. [DOI] [PubMed] [Google Scholar]
- Despa F, Decarli C. Amylin: what might be its role in Alzheimer's disease and how could this affect therapy? Expert Rev Proteomics. 2013;10:403–405. doi: 10.1586/14789450.2013.841549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A. 1997;94:4772–4777. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Le Foll C, Johnson MD, Lutz TA, Hayes MR, Levin BE. Endogenous VMH amylin signaling is required for full leptin signaling and protection from diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2016;310:R355–365. doi: 10.1152/ajpregu.00462.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Cuello AC. Intracellular A-beta amyloid, a sign for worse things to come? Mol Neurobiol. 2002;26:299–316. doi: 10.1385/MN:26:2-3:299. [DOI] [PubMed] [Google Scholar]
- Edwards BJ, Perry HM, Kaiser FE, Morley JE, Kraenzle D, Kreutter DK, Stevenson RW. Age-related changes in amylin secretion. Mech Ageing Dev. 1996;86:39–51. doi: 10.1016/0047-6374(95)01664-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawver JN, Ghiwot Y, Koola C, Carrera W, Rodriguez-Rivera J, Hernandez C, Dineley KT, Kong Y, Li J, Jhamandas J, Perry G, Murray IV. Islet amyloid polypeptide (IAPP): a second amyloid in Alzheimer's disease. Curr Alzheimer Res. 2014;11:928–940. doi: 10.2174/1567205011666141107124538. [DOI] [PubMed] [Google Scholar]
- Fehmann HC, Weber V, Goke R, Goke B, Eissele R, Arnold R. Islet amyloid polypeptide (IAPP;amylin) influences the endocrine but not the exocrine rat pancreas. Biochem Biophys Res Commun. 1990;167:1102–1108. doi: 10.1016/0006-291x(90)90636-2. [DOI] [PubMed] [Google Scholar]
- Fu W, Ruangkittisakul A, MacTavish D, Shi JY, Ballanyi K, Jhamandas JH. Amyloid beta (Abeta) peptide directly activates amylin-3 receptor subtype by triggering multiple intracellular signaling pathways. J Biol Chem. 2012;287:18820–18830. doi: 10.1074/jbc.M111.331181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar RC, Villarreal SA, Bowles N, Hepler RW, Joyce JG, Shughrue PJ. Oligomers of beta-amyloid are sequestered into and seed new plaques in the brains of an AD mouse model. Exp Neurol. 2010;223:394–400. doi: 10.1016/j.expneurol.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Gingell JJ, Burns ER, Hay DL. Activity of pramlintide, rat and human amylin but not Abeta1–42 at human amylin receptors. Endocrinology. 2014;155:21–26. doi: 10.1210/en.2013-1658. [DOI] [PubMed] [Google Scholar]
- Gitter BD, Cox LM, Carlson CD, May PC. Human amylin stimulates inflammatory cytokine secretion from human glioma cells. Neuroimmunomodulation. 2000;7:147–152. doi: 10.1159/000026432. [DOI] [PubMed] [Google Scholar]
- Goldsbury C, Goldie K, Pellaud J, Seelig J, Frey P, Muller SA, Kistler J, Cooper GJ, Aebi U. Amyloid fibril formation from full-length and fragments of amylin. J Struct Biol. 2000;130:352–362. doi: 10.1006/jsbi.2000.4268. [DOI] [PubMed] [Google Scholar]
- Gotz J, Lim YA, Eckert A. Lessons from two prevalent amyloidoses-what amylin and Abeta have in common. Front Aging Neurosci. 2013;5:38. doi: 10.3389/fnagi.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goure WF, Krafft GA, Jerecic J, Hefti F. Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer's disease immunotherapeutics. Alzheimers Res Ther. 2014;6:42. doi: 10.1186/alzrt272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Munoz MJ, Castillo-Carranza DL, Kayed R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem Pharmacol. 2014;88:468–478. doi: 10.1016/j.bcp.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015;67:564–600. doi: 10.1124/pr.115.010629. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton JM, Chai SY, Sexton PM. In vitro autoradiographic localization of the calcitonin receptor isoforms, C1a and C1b, in rat brain. Neuroscience. 1995;69:1223–1237. doi: 10.1016/0306-4522(95)00322-a. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K, Barisone GA, Diaz E, Jin LW, DeCarli C, Despa F. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol. 2013;74:517–526. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas JH, Li Z, Westaway D, Yang J, Jassar S, MacTavish D. Actions of beta-amyloid protein on human neurons are expressed through the amylin receptor. Am J Pathol. 2011;178:140–149. doi: 10.1016/j.ajpath.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas JH, Mactavish D. beta-Amyloid protein (Abeta) and human amylin regulation of apoptotic genes occurs through the amylin receptor. Apoptosis. 2012;17:37–47. doi: 10.1007/s10495-011-0656-3. [DOI] [PubMed] [Google Scholar]
- Johnson KH, O'Brien TD, Jordan K, Westermark P. Impaired glucose tolerance is associated with increased islet amyloid polypeptide (IAPP) immunoreactivity in pancreatic beta cells. Am J Pathol. 1989;135:245–250. [PMC free article] [PubMed] [Google Scholar]
- Jordan K, Murtaugh MP, O'Brien TD, Westermark P, Betsholtz C, Johnson KH. Canine IAPP cDNA sequence provides important clues regarding diabetogenesis and amyloidogenesis in type 2 diabetes. Biochem Biophys Res Commun. 1990;169:502–508. doi: 10.1016/0006-291x(90)90359-u. [DOI] [PubMed] [Google Scholar]
- Jorsal T, Rungby J, Knop FK, Vilsboll T. GLP-1 and Amylin in the Treatment of Obesity. Curr Diab Rep. 2016;16:1. doi: 10.1007/s11892-015-0693-3. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Kuroda Y, Arispe N, Rojas E. Alzheimer's beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J Biol Chem. 2000;275:14077–14083. doi: 10.1074/jbc.275.19.14077. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R, MacTavish D, Yang J, Westaway D, Jhamandas JH. Pramlintide Antagonizes Beta Amyloid (Abeta)- and Human Amylin-Induced Depression of Hippocampal Long-Term Potentiation. Mol Neurobiol. 2017;54:748–754. doi: 10.1007/s12035-016-9684-x. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Schwartz S, Corder C, Levy B, Klaff L, Peterson J, Gottlieb A. Effect of 14 days' subcutaneous administration of the human amylin analogue, pramlintide (AC137), on an intravenous insulin challenge and response to a standard liquid meal in patients with IDDM. Diabetologia. 1996;39:492–499. doi: 10.1007/BF00400683. [DOI] [PubMed] [Google Scholar]
- Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J. 2006;273:3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- Koo EH, Lansbury PT, Jr, Kelly JW. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci U S A. 1999;96:9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Han PL. An update of animal models of Alzheimer disease with a reevaluation of plaque depositions. Exp Neurobiol. 2013;22:84–95. doi: 10.5607/en.2013.22.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Kotilinek L, Ashe KH. Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience. 2008;151:745–749. doi: 10.1016/j.neuroscience.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Wallack M, Mwamburi M, Abdul-Hay SO, Leissring MA, Qiu WQ. Age and its association with low insulin and high amyloid-beta peptides in blood. J Alzheimers Dis. 2016a;49:129–137. doi: 10.3233/JAD-150428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Huang E. An Update on Type 2 Diabetes Mellitus as a Risk Factor for Dementia. J Alzheimers Dis. 2016;53:393–402. doi: 10.3233/JAD-160114. [DOI] [PubMed] [Google Scholar]
- Li W, Wang T, Xiao S. Type 2 diabetes mellitus might be a risk factor for mild cognitive impairment progressing to Alzheimer's disease. Neuropsychiatr Dis Treat. 2016b;12:2489–2495. doi: 10.2147/NDT.S111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Kelly L, Heiman M, Greengard P, Friedman JM. Hypothalamic Amylin Acts in Concert with Leptin to Regulate Food Intake. Cell Metab. 2015;22:1059–1067. doi: 10.1016/j.cmet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Liberini CG, Boyle CN, Cifani C, Venniro M, Hope BT, Lutz TA. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur J Neurosci. 2016;43:653–661. doi: 10.1111/ejn.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Lorenzo A, Yankner BA. Amyloid fibril toxicity in Alzheimer's disease and diabetes. Ann N Y Acad Sci. 1996;777:89–95. doi: 10.1111/j.1749-6632.1996.tb34406.x. [DOI] [PubMed] [Google Scholar]
- Lutz TA. The role of amylin in the control of energy homeostasis. Am J Physiol Regul Integr Comp Physiol. 2010a;298:R1475–1484. doi: 10.1152/ajpregu.00703.2009. [DOI] [PubMed] [Google Scholar]
- Lutz TA. Roles of amylin in satiation, adiposity and brain development. Forum Nutr. 2010b;63:64–74. doi: 10.1159/000264394. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Del Prete E, Scharrer E. Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol Behav. 1994;55:891–895. doi: 10.1016/0031-9384(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Del Prete E, Scharrer E. Subdiaphragmatic vagotomy does not influence the anorectic effect of amylin. Peptides. 1995a;16:457–462. doi: 10.1016/0196-9781(94)00203-i. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol Behav. 1995b;58:1197–1202. doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Meyer U. Amylin at the interface between metabolic and neurodegenerative disorders. Front Neurosci. 2015;9:216. doi: 10.3389/fnins.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord. 2001;25:1005–1011. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- Ly H, Despa F. Hyperamylinemia as a risk factor for accelerated cognitive decline in diabetes. Expert Rev Proteomics. 2015;12:575–577. doi: 10.1586/14789450.2015.1104251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly H, Verma N, Wu F, Liu M, Saatman KE, Nelson PT, Slevin JT, Goldstein LB, Biessels GJ, Despa F. Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann Neurol. 2017;82:208–222. doi: 10.1002/ana.24992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Nussinov R. Polymorphic C-terminal beta-sheet interactions determine the formation of fibril or amyloid beta-derived diffusible ligand-like globulomer for the Alzheimer Abeta42 dodecamer. J Biol Chem. 2010;285:37102–37110. doi: 10.1074/jbc.M110.133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack C, Wilson J, Athanacio J, Reynolds J, Laugero K, Guss S, Vu C, Roth J, Parkes D. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1855–1863. doi: 10.1152/ajpregu.00297.2007. [DOI] [PubMed] [Google Scholar]
- Mack CM, Soares CJ, Wilson JK, Athanacio JR, Turek VF, Trevaskis JL, Roth JD, Smith PA, Gedulin B, Jodka CM, Roland BL, Adams SH, Lwin A, Herich J, Laugero KD, Vu C, Pittner R, Paterniti JR, Jr, Hanley M, Ghosh S, Parkes DG. Davalintide (AC2307), a novel amylin-mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int J Obes (Lond) 2010;34:385–395. doi: 10.1038/ijo.2009.238. [DOI] [PubMed] [Google Scholar]
- Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, Selkoe DJ, Ince PG, Walsh DM, Medical Research Council Cognitive, F., Ageing, S The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–1341. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen J. Pramlintide acetate. Am J Health Syst Pharm. 2005;62:2363–2372. doi: 10.2146/ajhp050341. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Kayed R, Lin CY, Gurlo T, Haataja L, Jayasinghe S, Langen R, Glabe CG, Butler PC. Inhibition of human IAPP fibril formation does not prevent beta-cell death: evidence for distinct actions of oligomers and fibrils of human IAPP. Am J Physiol Endocrinol Metab. 2006;291:E1317–1324. doi: 10.1152/ajpendo.00082.2006. [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Cisse M, Ho K, Wu T, Esposito LA, Scearce-Levie K, Cheng IH, Yu GQ, Mucke L. Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci. 2009;29:1977–1986. doi: 10.1523/JNEUROSCI.2984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki-Baase EG. Amylin-mediated control of glycemia, energy balance, and cognition. Physiol Behav. 2016;162:130–140. doi: 10.1016/j.physbeh.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Hayes MR. Amylin activates distributed CNS nuclei to control energy balance. Physiol Behav. 2014;136:39–46. doi: 10.1016/j.physbeh.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Rupprecht LE, Olivos DR, Zimmer DJ, Alter MD, Pierce RC, Schmidt HD, Hayes MR. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology. 2013;38:1685–1697. doi: 10.1038/npp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed LA, Zhu H, Mousa YM, Wang E, Qiu WQ, Kaddoumi A. Amylin Enhances Amyloid-beta Peptide Brain to Blood Efflux Across the Blood-Brain Barrier. J Alzheimers Dis. 2017;56:1087–1099. doi: 10.3233/JAD-160800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet A, Gilg S, Riediger T, Lutz TA. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol Behav. 2004;81:149–155. doi: 10.1016/j.physbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Morfis M, Tilakaratne N, Furness SG, Christopoulos G, Werry TD, Christopoulos A, Sexton PM. Receptor activity-modifying proteins differentially modulate the G protein-coupling efficiency of amylin receptors. Endocrinology. 2008;149:5423–5431. doi: 10.1210/en.2007-1735. [DOI] [PubMed] [Google Scholar]
- Morgado I, Fändrich M. Assembly of Alzheimer's Aβ peptide into nanostructured amyloid fibrils. Current Opinion in Colloid & Interface Science. 2011;16:508–514. [Google Scholar]
- Morris JK, Vidoni ED, Mahnken JD, Montgomery RN, Johnson DK, Thyfault JP, Burns JM. Cognitively impaired elderly exhibit insulin resistance and no memory improvement with infused insulin. Neurobiol Aging. 2016;39:19–24. doi: 10.1016/j.neurobiolaging.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VA, Pike CJ. Obesity and sex interact in the regulation of Alzheimer's disease. Neurosci Biobehav Rev. 2016;67:102–118. doi: 10.1016/j.neubiorev.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, Chang L, Miller B, Clark C, Green R, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- Muresan V, Ladescu Muresan Z. Amyloid-beta precursor protein: Multiple fragments, numerous transport routes and mechanisms. Exp Cell Res. 2015;334:45–53. doi: 10.1016/j.yexcr.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naot D, Cornish J. Cytokines and Hormones That Contribute to the Positive Association between Fat and Bone. Front Endocrinol (Lausanne) 2014;5:70. doi: 10.3389/fendo.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- Nishi M, Sanke T, Ohagi S, Ekawa K, Wakasaki H, Nanjo K, Bell GI, Steiner DF. Molecular biology of islet amyloid polypeptide. Diabetes Res Clin Pract. 1992;15:37–44. doi: 10.1016/0168-8227(92)90065-y. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Harris V, McCorkle SK, Unger RH, Luskey KL. Amylin secretion from the rat pancreas and its selective loss after streptozotocin treatment. J Clin Invest. 1990;85:973–976. doi: 10.1172/JCI114528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohagi S, Nishi M, Bell GI, Ensinck JW, Steiner DF. Sequences of islet amyloid polypeptide precursors of an Old World monkey, the pig-tailed macaque (Macaca nemestrina), and the dog (Canis familiaris) Diabetologia. 1991;34:555–558. doi: 10.1007/BF00400272. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Kane SA, Salvatore CA, Mallee JJ, Kinsey AM, Koblan KS, Keyvan-Fouladi N, Heavens RP, Wainwright A, Jacobson M, Dickerson IM, Hill RG. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur J Neurosci. 2001;14:618–628. doi: 10.1046/j.0953-816x.2001.01688.x. [DOI] [PubMed] [Google Scholar]
- Olsson M, Herrington MK, Reidelberger RD, Permert J, Arnelo U. Comparison of the effects of chronic central administration and chronic peripheral administration of islet amyloid polypeptide on food intake and meal pattern in the rat. Peptides. 2007;28:1416–1423. doi: 10.1016/j.peptides.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Ono K. Alzheimer's disease as oligomeropathy. Neurochem Int. 2017 doi: 10.1016/j.neuint.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Ono K, Takahashi R, Ikeda T, Mizuguchi M, Hamaguchi T, Yamada M. Exogenous amyloidogenic proteins function as seeds in amyloid beta-protein aggregation. Biochim Biophys Acta. 2014;1842:646–653. doi: 10.1016/j.bbadis.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Opie EL. On the Relation of Chronic Interstitial Pancreatitis to the Islands of Langerhans and to Diabetes Melutus. J Exp Med. 1901;5:397–428. doi: 10.1084/jem.5.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Chai SY, Christopoulos G, Huang XF, Toga AW, Wang HQ, Sexton PM. In vitro autoradiographic localization of calcitonin and amylin binding sites in monkey brain. J Chem Neuroanat. 2004;27:217–236. doi: 10.1016/j.jchemneu.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Pieber TR, Roitelman J, Lee Y, Luskey KL, Stein DT. Direct plasma radioimmunoassay for rat amylin-(1–37): concentrations with acquired and genetic obesity. Am J Physiol. 1994;267:E156–164. doi: 10.1152/ajpendo.1994.267.1.E156. [DOI] [PubMed] [Google Scholar]
- Pillay K, Govender P. Amylin uncovered: a review on the polypeptide responsible for type II diabetes. Biomed Res Int. 2013;2013:826706. doi: 10.1155/2013/826706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt TL, Reeves VL, Murphy MP. Transgenic models of Alzheimer's disease: better utilization of existing models through viral transgenesis. Biochim Biophys Acta. 2013;1832:1437–1448. doi: 10.1016/j.bbadis.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Pullman J, Darsow T, Frias JP. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vasc Health Risk Manag. 2006;2:203–212. doi: 10.2147/vhrm.2006.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WQ, Au R, Zhu H, Wallack M, Liebson E, Li H, Rosenzweig J, Mwamburi M, Stern RA. Positive association between plasma amylin and cognition in a homebound elderly population. J Alzheimers Dis. 2014;42:555–563. doi: 10.3233/JAD-140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Zhu H. Amylin and its analogs: a friend or foe for the treatment of Alzheimer's disease? Front Aging Neurosci. 2014;6:186. doi: 10.3389/fnagi.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T, de Sousa G, Niklowitz P, Roth CL. Amylin and its relation to insulin and lipids in obese children before and after weight loss. Obesity (Silver Spring) 2007;15:2006–2011. doi: 10.1038/oby.2007.239. [DOI] [PubMed] [Google Scholar]
- Reiner DJ, Mietlicki-Baase EG, Olivos DR, McGrath LE, Zimmer DJ, Koch-Laskowski K, Krawczyk J, Turner CA, Noble EE, Hahn JD, Schmidt HD, Kanoski SE, Hayes MR. Amylin Acts in the Lateral Dorsal Tegmental Nucleus to Regulate Energy Balance Through Gamma-Aminobutyric Acid Signaling. Biol Psychiatry. 2017;82:828–838. doi: 10.1016/j.biopsych.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P, Korczyn AD, Ali SS, Bajenaru O, Choi MS, Chopp M, Dermanovic-Dobrota V, Grunblatt E, Jellinger KA, Kamal MA, Kamal W, Leszek J, Sheldrick-Michel TM, Mushtaq G, Meglic B, Natovich R, Pirtosek Z, Rakusa M, Salkovic-Petrisic M, Schmidt R, Schmitt A, Sridhar GR, Vecsei L, Wojszel ZB, Yaman H, Zhang ZG, Cukierman-Yaffe T. The diabetic brain and cognition. J Neural Transm (Vienna) 2017;124:1431–1454. doi: 10.1007/s00702-017-1763-2. [DOI] [PubMed] [Google Scholar]
- Roberts BR, Lind M, Wagen AZ, Rembach A, Frugier T, Li QX, Ryan TM, McLean CA, Doecke JD, Rowe CC, Villemagne VL, Masters CL. Biochemically-defined pools of amyloid-beta in sporadic Alzheimer's disease: correlation with amyloid PET. Brain. 2017;140:1486–1498. doi: 10.1093/brain/awx057. [DOI] [PubMed] [Google Scholar]
- Roher AE, Kokjohn TA, Clarke SG, Sierks MR, Maarouf CL, Serrano GE, Sabbagh MS, Beach TG. APP/Abeta structural diversity and Alzheimer's disease pathogenesis. Neurochem Int. 2017;110:1–13. doi: 10.1016/j.neuint.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ. beta-Amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roriz-Filho JS, Sa-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves MLF, Moriguti JC, Roriz-Cruz M. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792:432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat Rev Endocrinol. 2013;9:425–433. doi: 10.1038/nrendo.2013.47. [DOI] [PubMed] [Google Scholar]
- Solomon B. Immunological approaches for amyloid-beta clearance toward treatment for Alzheimer's disease. Rejuvenation Res. 2008;11:349–357. doi: 10.1089/rej.2008.0689. [DOI] [PubMed] [Google Scholar]
- Soudy R, Patel A, Fu W, Kaur K, MacTavish D, Westaway D, Davey R, Zajac J, Jhamandas J. Cyclic AC253, a novel amylin receptor antagonist, improves cognitive deficits in a mouse model of Alzheimer's disease. Alzheimer's & Dementia: Translational Research & Clinical Interventions. 2017;3:44–56. doi: 10.1016/j.trci.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar GR, Lakshmi G, Nagamani G. Emerging links between type 2 diabetes and Alzheimer's disease. World J Diabetes. 2015;6:744–751. doi: 10.4239/wjd.v6.i5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Nagao T, Gouras GK. Plaque formation and the intraneuronal accumulation of beta-amyloid in Alzheimer's disease. Pathol Int. 2017;67:185–193. doi: 10.1111/pin.12520. [DOI] [PubMed] [Google Scholar]
- Takeda S, Hashimoto T, Roe AD, Hori Y, Spires-Jones TL, Hyman BT. Brain interstitial oligomeric amyloid beta increases with age and is resistant to clearance from brain in a mouse model of Alzheimer's disease. FASEB J. 2013;27:3239–3248. doi: 10.1096/fj.13-229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Menard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tiiman A, Noormagi A, Friedemann M, Krishtal J, Palumaa P, Tougu V. Effect of agitation on the peptide fibrillization: Alzheimer's amyloid-beta peptide 1–42 but not amylin and insulin fibrils can grow under quiescent conditions. J Pept Sci. 2013;19:386–391. doi: 10.1002/psc.2513. [DOI] [PubMed] [Google Scholar]
- Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther. 2000;294:61–72. [PubMed] [Google Scholar]
- Tran L, Zielinski A, Roach AH, Jende JA, Householder AM, Cole EE, Atway SA, Amornyard M, Accursi ML, Shieh SW, Thompson EE. Pharmacologic treatment of type 2 diabetes: injectable medications. Ann Pharmacother. 2015;49:700–714. doi: 10.1177/1060028015573010. [DOI] [PubMed] [Google Scholar]
- Tucker HM, Rydel RE, Wright S, Estus S. Human amylin induces "apoptotic" pattern of gene expression concomitant with cortical neuronal apoptosis. J Neurochem. 1998;71:506–516. doi: 10.1046/j.1471-4159.1998.71020506.x. [DOI] [PubMed] [Google Scholar]
- Ueda T, Ugawa S, Saishin Y, Shimada S. Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Res Mol Brain Res. 2001;93:36–45. doi: 10.1016/s0169-328x(01)00179-6. [DOI] [PubMed] [Google Scholar]
- Umegaki H. Type 2 diabetes as a risk factor for cognitive impairment: current insights. Clin Interv Aging. 2014;9:1011–1019. doi: 10.2147/CIA.S48926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsamakis G, Konstantakou P, Mastorakos G. New Targets for Drug Treatment of Obesity. Annu Rev Pharmacol Toxicol. 2017;57:585–605. doi: 10.1146/annurev-pharmtox-010716-104735. [DOI] [PubMed] [Google Scholar]
- Viola KL, Klein WL. Amyloid beta oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129:183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Zhu H, Wang X, Gower AC, Wallack M, Blusztajn JK, Kowall N, Qiu WQ. Amylin Treatment Reduces Neuroinflammation and Ameliorates Abnormal Patterns of Gene Expression in the Cerebral Cortex of an Alzheimer's Disease Mouse Model. J Alzheimers Dis. 2017;56:47–61. doi: 10.3233/JAD-160677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer's dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet. 2014;5:88. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P. Amyloid of human islets of Langerhans. II. Electron microscopic analysis of isolated amyloid. Virchows Arch A Pathol Anat Histol. 1977;373:161–166. doi: 10.1007/BF00432160. [DOI] [PubMed] [Google Scholar]
- Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P, Li ZC, Westermark GT, Leckstrom A, Steiner DF. Effects of beta cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett. 1996;379:203–206. doi: 10.1016/0014-5793(95)01512-4. [DOI] [PubMed] [Google Scholar]
- Wijesekara N, Ahrens R, Sabale M, Wu L, Ha K, Verdile G, Fraser PE. Amyloid-beta and islet amyloid pathologies link Alzheimer disease and type 2 diabetes in a transgenic model. FASEB J. 2017 doi: 10.1096/fj.201700431R. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit Rev Neurobiol. 1997;11:167–239. doi: 10.1615/critrevneurobiol.v11.i2-3.40. [DOI] [PubMed] [Google Scholar]
- Wong CW, Quaranta V, Glenner GG. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985;82:8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wookey PJ, Cooper ME. Amylin: physiological roles in the kidney and a hypothesis for its role in hypertension. Clin Exp Pharmacol Physiol. 1998;25:653–660. doi: 10.1111/j.1440-1681.1998.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Wookey PJ, Lutz TA, Andrikopoulos S. Amylin in the periphery II: An updated mini-review. ScientificWorldJournal. 2006;6:1642–1655. doi: 10.1100/tsw.2006.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Maat-Schieman ML, van Duinen SG, Prins FA, Neeskens P, Natte R, Roos RA. Amyloid beta protein (Abeta) starts to deposit as plasma membrane-bound form in diffuse plaques of brains from hereditary cerebral hemorrhage with amyloidosis-Dutch type, Alzheimer disease and nondemented aged subjects. J Neuropathol Exp Neurol. 2000;59:723–732. doi: 10.1093/jnen/59.8.723. [DOI] [PubMed] [Google Scholar]
- Yan LM, Velkova A, Kapurniotu A. Molecular characterization of the hetero-assembly of beta-amyloid peptide with islet amyloid polypeptide. Curr Pharm Des. 2014;20:1182–1191. doi: 10.2174/13816128113199990064. [DOI] [PubMed] [Google Scholar]
- Young A. Cardiovascular effects. Adv Pharmacol. 2005a;52:239–250. doi: 10.1016/S1054-3589(05)52014-3. [DOI] [PubMed] [Google Scholar]
- Young A. Effects on bone. Adv Pharmacol. 2005b;52:269–280. doi: 10.1016/S1054-3589(05)52016-7. [DOI] [PubMed] [Google Scholar]
- Young A. Renal effects. Adv Pharmacol. 2005c;52:251–268. doi: 10.1016/S1054-3589(05)52015-5. [DOI] [PubMed] [Google Scholar]
- Younk LM, Mikeladze M, Davis SN. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert Opin Pharmacother. 2011;12:1439–1451. doi: 10.1517/14656566.2011.581663. [DOI] [PubMed] [Google Scholar]
- Yuan XY, Wang XG. Mild cognitive impairment in type 2 diabetes mellitus and related risk factors: a review. Rev Neurosci. 2017 doi: 10.1515/revneuro-2017-0016. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen C, Hua S, Liao H, Wang M, Xiong Y, Cao F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer's disease. Diabetes Res Clin Pract. 2017a;124:41–47. doi: 10.1016/j.diabres.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Zhang N, Yang S, Wang C, Zhang J, Huo L, Cheng Y, Wang C, Jia Z, Ren L, Kang L, Zhang W. Multiple target of hAmylin on rat primary hippocampal neurons. Neuropharmacology. 2017b;113:241–251. doi: 10.1016/j.neuropharm.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Zhang X, Song W. The role of APP and BACE1 trafficking in APP processing and amyloid-beta generation. Alzheimers Res Ther. 2013;5:46. doi: 10.1186/alzrt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Song W. Islet amyloid polypeptide: Another key molecule in Alzheimer's pathogenesis? Prog Neurobiol. 2017;153:100–120. doi: 10.1016/j.pneurobio.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Zhu H, Stern RA, Tao Q, Bourlas A, Essis MD, Chivukula M, Rosenzweig J, Steenkamp D, Xia W, Mercier GA, Tripodis Y, Farlow M, Kowall N, Qiu WQ. An amylin analog used as a challenge test for Alzheimer's disease. Alzheimers Dement (N Y) 2017a;3:33–43. doi: 10.1016/j.trci.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang X, Wallack M, Li H, Carreras I, Dedeoglu A, Hur JY, Zheng H, Li H, Fine R, Mwamburi M, Sun X, Kowall N, Stern RA, Qiu WQ. Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer's disease. Mol Psychiatry. 2015;20:252–262. doi: 10.1038/mp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Xue X, Wang E, Wallack M, Na H, Hooker JM, Kowall N, Tao Q, Stein TD, Wolozin B, Qiu WQ. Amylin receptor ligands reduce the pathological cascade of Alzheimer's disease. Neuropharmacology. 2017b;119:170–181. doi: 10.1016/j.neuropharm.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]