Abstract
Background
Among the estimated 340,000 people who inject drugs (PWID) in Ukraine, HCV prevalence is approximately 70%. As HCV treatment availability increases, an assessment of the HCV treatment cascade is needed to guide HCV prevention and treatment strategies.
Methods
Opioid dependent PWID were interviewed and tested for HIV and HCV in five Ukrainian cities from January 2014 to March 2015. Logistic regression was used to examine the independent correlates of two cascade steps: a) anti-HCV positive status awareness; b) chronic HCV confirmation; and of c) annual HCV testing for PWID.
Results
Among 1,613 PWID, 1,002 (62.1%) had anti-HCV positive test result, of which 568 (56.7%) were aware of it before the study and 346 (34.5%) reported previous confirmatory testing for chronic HCV. Independent correlates of being aware they had anti-HCV positivity included: current [AOR: 3.08; 95%CI: 2.16-4.40] or prior [AOR: 1.85; 95%CI: 1.27-2.68] opioid agonistic treatment (OAT) experience, relative to no prior OAT, living in Lviv [AOR: 0.50; 95%CI: 0.31-0.81] or Odesa [AOR: 2.73; 95%CI: 1.51-4.93] relative to Kyiv and being aware of having HIV [AOR: 4.10; 95%CI: 2.99-5.62]. Independent correlates of confirming HCV infection among those who were aware of their anti-HCV positive status included: current OAT [AOR: 2.00; 95%CI: 1.24-3.23], relative to prior OAT, the middle income category [AOR: 1.74, 95%CI: 1.15-2.63], relative to the lowest, and receiving ART [AOR: 4.54; 95%CI: 2.85-7.23]. Among 1,613 PWID, 918 (56.9%) were either HCV negative or not aware of their HCV positive status, of which 198 (21.6%) reported recent anti-HCV test (during last 12 month). Recent anti-HCV test in this group was associated with current [AOR: 7.17; 95%CI: 4.63-11.13] or prior [AOR: 2.24; 95%CI: 1.32-3.81] OAT experience, relative to no prior OAT.
Conclusion
Encouraging PWID to participate in OAT may be an effective strategy to diagnose and link PWID who are HCV positive to care. Among HIV negative participants, regular HCV testing may be ensured by participation in OAT. More studies are needed to assess HCV treatment utilization among PWID in Ukraine and OAT as a possible way to retain them in treatment.
Keywords: HCV, Ukraine, people who inject drugs (PWID), HCV testing, cascade of care Declaration of interest: none
Background
Globally, an estimated 71 million people have viraemic hepatitis C virus (HCV) infection (“Polaris Observatory, H. C. V. Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study,” 2017). Over the past 25 years, HCV-associated disability-adjusted life years have more than doubled and HCV-related mortality is increasing (Stanaway et al., 2016). People who inject drugs (PWID) account for most HCV cases in Eastern Europe and Central Asia with global prevalence of HCV among PWID exceeding 52%. (Degenhardt et al., 2017). Ukraine is home to Europe's most devastating drug injection epidemic, with 1.2% of the population injecting opioids (United Nations Office on Drugs and Crime (UNODC), 2016) The national estimate of HCV prevalence among PWID in Ukraine is approximately 70% (Hope, Eramova, Capurro, & Donoghoe, 2014), with more than 240,000 individuals infected with HCV and needing treatment. Despite evidence that HCV testing and treatment are increasing globally (Milne et al., 2015; Smith, Combellick, Jordan, & Hagan, 2015), PWID continue to lack access to effective treatment. Getting tested for antibodies to HCV (anti-HCV)and confirming chronic HCV infection are the necessary steps in the treatment cascade (Meyer et al., 2015). For most patients, the diagnosis is a two-step process: testing for anti-HCV using serological tests followed by confirmation of viremia with polymerase chain reaction (PCR) test that detects HCV RNA in the blood of the patient. Since 14-26% of infected patients clear their HCV and do not become chronically infected, the confirmation of viremia is needed to decide if treatment is necessary (Lauer & Walker 2001).
A 2013 cross-sectional survey of PWID in Ukraine reported that 50% of PWIDs were aware of their anti-HCV positive status (Salyuk & Sazonova, 2015). In this convenience sample, 9.4% of PWID with HCV reported receiving some treatment (Salyuk & Sazonova, 2015). Suboptimal awareness and treatment availability coupled with high financial costs of treatment are among the main barriers to universal treatment of chronic HCV. In fact, pegylated-interferon and ribavirin treatment was introduced in Ukraine only in 2013 with some state funding which excluded PWID and funding by international donors for 100 PWID on opioid agonist therapy (Luhmann et al., 2015). Treatment with direct acting antiviral (DAA) medications was piloted in a group of 1,500 patients starting in 2015 (A. Mazhnaya et al., 2017). This scale-up project of HCV treatment with DAA for PWID was implemented in community-based settings and was shown to be feasible even for actively injecting participants (A. Mazhnaya et al., 2017). While opportunities for better treatment coverage in Ukraine are being explored, it is critical for program planners to better understand factors related to the hepatitis C testing and treatment cascade in PWID.
Opioid agonist therapy (OAT) with methadone or buprenorphine not only effectively reduces opioid use, but also reduces HIV and HCV transmission (Gowing, Farrell, Bornemann, Sullivan, & Ali, 2011; Roux et al., 2008), and improves engagement in treatment in the HIV cascade of care (Low et al., 2016; Roux et al., 2008). In Ukraine, buprenorphine maintenance treatment became available to a limited number of patients in 2004 and OAT using methadone was introduced in 2008 (Bruce, Dvoryak, Sylla, & Altice, 2007; Schaub, Chtenguelov, Subata, Weiler, & Uchtenhagen, 2010). Despite its documented benefits, OAT is under-scaled due to individual, structural and policy barriers (Bojko et al., 2015; Mazhnaya et al., 2016), resulting in only 2.7% of the estimated 340,000 opioid injectors in Ukraine being on treatment. OAT has demonstrated benefits on the HIV (A Mazhnaya et al., 2017) and TB (Morozova, Dvoryak, & Altice, 2013) treatment cascades in Ukraine, but has yet to be studied for HCV.
This study aimed to examine the first steps in the HCV treatment cascade among PWID in Ukraine and look for the independent correlates associated with: 1) being aware of anti-HCV positive status; 2) receipt of confirmatory HCV testing; and 3) among those with negative or unknown status, the proportion who adhered to HCV testing recommendations (annual testing) (“AASLD and IDSA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C,” 2014-2017). Specifically, we hypothesize that, among other factors, OAT is related to higher level of anti-HCV positive status awareness, confirmatory HCV testing and annual anti-HCV testing among HCV negative participants or with unknown status.
Methods
Data collection
The methods for the cross-sectional biobehavioral study involving 1,613 PWID in 5 cities in Ukraine (Kiev, Odesa, Mykolaiv, Dnipro, Lviv) conducted from January 2014 to March 2015 have been previously described (Makarenko et al., 2016). Eligibility included age 18 years or older, ICD-10 criteria for opioid dependence, lived/worked in the city where the survey was conducted, provided informed consent, and agreed to undergo rapid HIV and HCV testing. Three groups of opioid dependent PWID were included: (1) never on OAT; (2) previously on OAT; (3) currently on OAT. For the first group, respondent-driven sampling (RDS) was used, and random selection from the OAT registry was used for the second and the third group. “Seeds” for RDS were recruited at community outreach sites in each city and included at least one: female, individual aged 18-25, and an individual who had injected less than two years. After completing, a computer-assisted, self-administered instrument (CASI) using Qualtrics®, point-of-care HIV and HCV (CITO TEST HIV 1/2/0, Pharmasco and CITO TEST HCV, Pharmasco) testing was performed along with pre- and post-test counseling by trained staff. The study was approved by the institutional review boards at Yale University and the Gromashevsky Institute at the National Academy of Medical Sciences, Kyiv, Ukraine.
Measures
The dependent variables were assessed: 1) being aware of having positive anti-HCV status (and confirmed using rapid onsite testing); 2) self-report of confirmatory testing for the subsample already aware of their anti-HCV positive status; and 3) compliance with recommendations for annual HCV testing (“AASLD and IDSA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C,” 2014-2017) for PWID who are unaware of their HCV status before the study or believe themselves to not have HCV.
Definitions of independent variables: Key among our hypotheses is that patients on OAT would have higher engagement in the HCV treatment cascade. Being on OAT was confirmed by chart review of patients randomly sampled at OAT sites. Participants who were previously on OAT were confirmed by chart review and could not have taken OAT in the previous 10 days. Based on the review of the previous studies of OAT implementation in Ukraine (Makarenko et al., 2017; Makarenko et al., 2016) in addition to standard socio-demographic characteristics we measured duration of injection drug use, rapid HIV test results and HIV status self-report, and current antiretroviral treatment based on self-report. Cohabitation with sexual partner, presence of children in the household, and importance of religion (measured in Likert scale) were characteristics indicating the presence of social support which was found to be an important correlate in previous studies of PWID (Artenie et al., 2015; Solomon et al., 2015; Ti et al., 2013). Income level was categorized into: <1200 UAH (150 USD), 1200-3500 (150-437 USD) and >3500 (437USD) based on minimum poverty level and average monthly wage for Ukraine in 2014. Educational attainment was categorized as (1) high school drop-out, (2) completion of high school (including vocational school), (3) some college or higher education. Alcohol use was assessed using the AUDIT, with scores ≥8 for men and ≥4 for women defining an harmful or hazardous drinking (Saunders, Aasland, Babor, De la Fuente, & Grant, 1993). Addiction severity was measured using the DAST-10 (Yudko, Lozhkina, & Fouts, 2007). Scores ≤5 were indicative of low to moderate addiction, whereas scores >5 indicated substantial to severe addiction. Depression was assessed using the CES-D-10, with scores >10 classified as moderate to severe depression (Zhang et al., 2012).
Statistical analysis
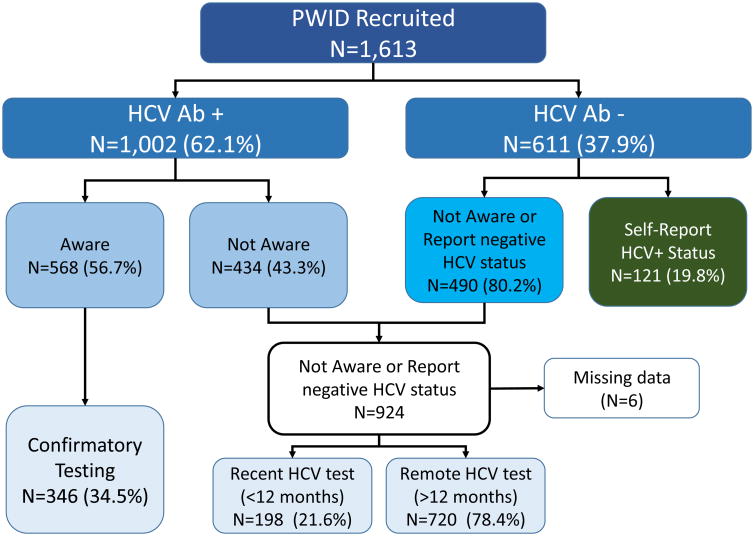
A diagram of the subject disposition for the analytical sample is displayed in Figure 1. First, we examined the frequency distributions of categorical variables and mean (or median) of continuous variables. Then, we conducted bivariate analyses of the associations between the independent variables and study outcomes. Pearson's Chi-square tests were used to assess the statistical significance of associations between two categorical variables, while t-tests were used to compare mean values of continuous variables.
Fig. 1.
The diagram of subject disposition for the analytical sample of PWID. The anti-HCV test result was provided to the participant after he comleted the questionnaire regarding his previous anti-HCV tests results and HCV status confirmation.
Univariate logistic regression models were used to estimate unadjusted odds ratios (OR) for the association between OAT (currently on OAT/ previously on OAT / never on OAT) and outcomes: aware of positive anti-HCV status, ever undergone confirmatory testing for HCV in a medical institution, and been tested for hepatitis C during last 12 months preceding the interview. A multivariate logistic regression model was fit for each of the outcomes to compute the adjusted OR (AOR). Variables with associations at p-value<0.2 in bivariate analyses were initially put into multivariate regression models. A backward elimination approach was used to determine the final set of variables in each model. A significance level of p<0.05 based on Wald test results was chosen as a cut-off to keep each variable in the model. To further verify that no important covariates were missing, variables that were discarded with p>0.05 were put back into the model and retained if the exposure effect estimate changed by ≥10%. Hosmer and Lemeshow and Pearson goodness of fit tests were used to verify the model choice. All analyses were conducted using SAS software, Version 9.4 of the SAS System for Windows.
Results
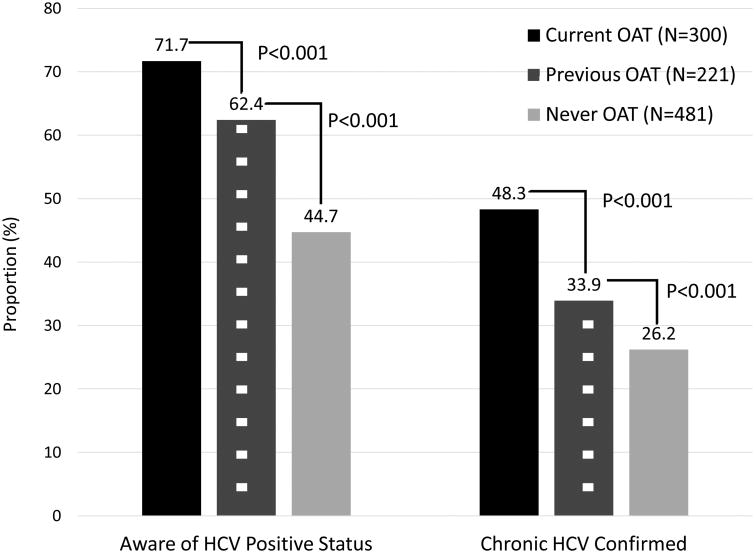
Among the 1613 participants tested for HCV, 1002 (62.1%) were anti-HCV positive. Among this 1002 person sub-sample, 568 (56.7%) were aware of their positive anti-HCV status and only 346 (34.5%) had reported previous confirmatory testing. A stratified analysis based on their OAT status (Figure 2) suggested significantly higher levels for those on OAT, relative to those previously and never on OAT, for being both aware of their anti-HCV positive status, but also in terms of confirmatory HCV testing. Among 1,613 PWID, 918 (56.9%) were either HCV negative or were not aware of their anti-HCV positive status prior to the study. In this subsample who should be tested annually, 198 (21.6%) reported recent HCV testing (during the last 12 months).
Fig. 2. The HCV Treatment Cascade for PWID infected with HCV in Ukraine (N=1,002), stratified by their prior experience with opioid agonist therapies.
The characteristics of the total sample of PWID, along with those with and without reactive anti-HCV subsamples are summarized in Table 1. Most participants were in their mid-thirties (mean: 36 years), male (76.4%) had completed high school (63.1%) and injected on average 17 years. A substantial proportion of the sample was not employed (37.6%), lived with a spouse or partner (35.6%), and had previously (55.8%) or currently (26.9%) received OAT. Almost half of the sample (46.9%) met criteria for harmful or hazardous drinking and had moderate to severe addiction. Overall, 668 (41.4%) tested positive for HIV, of which 573 (85.8%) were previously diagnosed and aware of their HIV positive status.
Table 1. Comparison of HCV status among people who inject drugs (N=1613).
| Characteristic | Total sample (N=1613) | Anti-HCV positive (N=1002) | Anti-HCV negative (N=611) | p-value |
|---|---|---|---|---|
|
| ||||
| N (%) | N (%) | N (%) | ||
|
| ||||
| Male gender | 1233 (76.4) | 766 (76.5) | 467 (76.4) | 0.995 |
| Age - mean (SD) | 36 (8.3) | 36.8 (8.0) | 35.4 (8.7) | 0.002 |
| Living with spouse/partner | 574 (35.6) | 370 (36.9) | 204 (33.4) | 0.150 |
| Have dependent children | 840 (52.1) | 542 (54.1) | 298 (48.8) | 0.038 |
| Importance of religion | 0.239 | |||
| Not important | 354 (22) | 207 (20.7) | 147 (24.1) | |
| Fairly important | 783 (48.5) | 499 (49.8) | 284 (46.5) | |
| Extremely important | 476 (29.5) | 296 (29.5) | 180 (29.5) | |
| Education level | 0.840 | |||
| Less than high school | 249 (15.4) | 151 (15.1) | 98 (16.0) | |
| High school (including vocational schools) | 1018 (63.1) | 633 (63.2) | 385 (63.0) | |
| Some university education or higher | 346 (21.5) | 218 (21.8) | 128 (21.0) | |
| Employment | 0.107 | |||
| Full time/part time permanent job | 752 (46.6) | 453 (45.2) | 299 (48.9) | |
| Temporary/ Seasonal/ Day laborer | 254 (15.8) | 152 (15.2) | 102 (16.7) | |
| Not employed | 607 (37.6) | 397 (39.6) | 210 (34.4) | |
| Income | 0.445 | |||
| <1200 UAH | 551 (34.2) | 354 (35.3) | 197 (32.2) | |
| 1200-3499 UAH | 746 (46.3) | 456 (45.5) | 290 (47.5) | |
| >=3500 UAH | 316 (19.6) | 192 (19.2) | 124 (20.3) | |
| City of residence | <0.001 | |||
| Kyiv | 413 (25.6) | 356 (35.5) | 57 (9.3) | |
| Odessa | 215 (13.3) | 93 (9.3) | 122 (20) | |
| Mykolaiv | 344 (21.3) | 222 (22.2) | 122 (20) | |
| Dnipro | 368 (22.8) | 213 (21.3) | 155 (25.4) | |
| Lviv | 273 (16.9) | 118 (11.8) | 155 (25.4) | |
| OAT experience | <0.001 | |||
| Never on OAT | 900 (55.8) | 481 (48.0) | 419 (68.6) | |
| Previously on OAT | 279 (17.3) | 221 (22.1) | 58 (9.5) | |
| Currently on OAT | 434 (26.9) | 300 (29.9) | 134 (21.9) | |
| Years of injection – mean (SD) | 17 (9) | 18.1 (8.6) | 15.5 (9.9) | <0.001 |
| Addiction severity (moderate to severe) | 1376 (85.3) | 872 (87.0) | 504 (82.5) | 0.013 |
| Moderate to severe depression | 968 (60.0) | 602 (60.1) | 366 (59.9) | 0.944 |
| Harmful or hazardous drinking | 756 (46.9) | 481 (48.0) | 275 (45.0) | 0.242 |
| Aware of positive HIV status | 573 (35.5) | 395 (39.4) | 178 (29.1) | <0.001 |
| Positive HIV test result (rapid test) | 668 (41.4) | 441 (44.0) | 227 (37.2) | 0.007 |
| Currently prescribed ART | 314 (19.5) | 218 (21.8) | 96 (15.7) | 0.003 |
| Have ever been tested for hepatitis C and received the result | 1011 (62.7) | 685 (68.4) | 326 (53.4) | <0.001 |
| Aware of positive hepatitis C status | - | 568 (56.7) | - | - |
| Have undergone confirmatory testing for hepatitis C | - | 346 (34.5) | - | - |
ART – antiretroviral therapy, SD – standard deviation.
Independent correlates of anti-HCV positive status awareness and confirmation of chronic HCV among anti-HCV positive PWID
Among the 1002 participants with anti-HCV positive test, 568 (56.7%) were aware of their anti-HCV status. Correlates of being aware of anti-HCV status in those with documented infection are presented in Table 2. Independent correlates of being aware of anti-HCV positive status included: current or prior OAT experience, residing in Lviv or Odesa, being aware of having HIV, co-habitation with a spouse/partner, duration of injection, and harmful or hazardous drinking. Correlates of having confirmed chronic HCV infection in those 568 participants aware of their anti-HCV positive serostatus are presented in Table 2. Independent correlates of confirming chronic HCV infection included: current OAT enrollment, residence in Dnipro, higher income status, having children and receiving ART.
Table 2. Correlates of being aware of anti-HCV positive status and getting confirmatory HCV test, among anti-HCV positive PWID.
| Aware of anti-HCV (N=1,002) | positive status | Have undergone testing for hepatitis | confirmatory C (N=568) | |
|---|---|---|---|---|
|
|
||||
| AOR (95% CI) | P-value | AOR (95% CI) | P-value | |
|
| ||||
| OAT experience | ||||
| Never on OAT | 1.00 | 1.43 (0.89-2.30) | 0.144 | |
| Previously on OAT | 1.85 (1.27-2.68) | <0.001 | 1.00 | |
| Currently on OAT | 3.08 (2.16-4.40) | <0.001 | 2.00 (1.24-3.23) | 0.004 |
| City of residence | ||||
| Kyiv | 1.00 | 1.00 | ||
| Odessa | 2.73 (1.51-4.93) | <.001 | 0.69 (0.37-1.29) | 0.237 |
| Mykolaiv | 0.85 (0.58-1.26) | 0.422 | 0.75 (0.45-1.25) | 0.269 |
| Dnipro | 0.72 (0.47-1.10) | 0.127 | 0.39 (0.22-0.68) | 0.001 |
| Lviv | 0.50 (0.31-0.81) | 0.005 | 1.34 (0.62-2.91) | 0.460 |
| Have dependent children | - | 1.57 (1.08-2.27) | 0.017 | |
| Living with spouse/partner | 1.51 (1.13-2.03) | 0.006 | - | - |
| Income | ||||
| <1200 UAH | - | - | 1.00 | |
| 1200-3499 UAH | - | - | 1.74 (1.15-2.63) | 0.009 |
| >=3500 UAH | - | - | 1.64 (0.98-2.73) | 0.059 |
| Years of injection | 1.02 (1.00-1.04) | 0.08 | 1.03 (1.00-1.05) | 0.066 |
| Harmful or hazardous drinking | 1.44 (1.08-1.93) | 0.015 | - | |
| Importance of religion | ||||
| Not important | 1.00 | - | - | |
| Fairly important | 1.70 (1.18-2.45) | 0.005 | - | - |
| Extremely important | 1.52 (1.02-2.27) | .04 | - | - |
| Aware of positive HIV status | 4.10 (2.99-5.62) | <.001 | ||
| Currently prescribed ART | - | - | 4.54 (2.85-7.23) | <.001 |
PWID currently enrolled into OAT [AOR: 3.08; 95%CI: 2.16-4.40] or having prior experience with OAT [AOR: 1.85; 95%CI: 1.27-2.68] have higher odds of being aware of positive anti-HCV status compared to those who had never been on OAT. Furthermore, PWID who are currently on OAT had 2.00 [95%CI: 1.24-3.23] higher odds of confirming chronic HCV infection compared to those with prior OAT experience.
PWID who were aware of their HIV positive status had 4.10 [95%CI: 2.99-5.62] higher odds of knowing that they are anti-HCV positive. Duration of injection was associated with being aware of positive anti-HCV status and confirmatory testing [AOR: 1.02; 95%CI: 1.00-1.04 and AOR: 1.03; 95% CI: 1.0-1.05, respectively].
PWID in Odessa had 2.73 [95%CI: 1.51-4.93] higher odds and PWID in Lviv had 0.50 [95%CI: 0.31-0.81] lower odds of HCV status awareness compared to PWID in Kyiv. PWID living in Dnipro had lower odds to confirm their HCV status compared to PWID in Kyiv [AOR: 0.39; 95%CI: 0.22-0.68].
Living with a spouse or partner and harmful or hazardous drinking were each associated with slightly higher odds of being aware of anti-HCV status [AOR: 1.51; 95%CI: 1.13-2.03 and AOR: 1.44; 95%CI: 1.08-1.93, respectively]. PWID ranking religion as fairly or extremely important had ∼70% higher odds of being aware of their positive anti-HCV status [AOR: 1.70; 95%CI: 1.18-2.45 and AOR: 1.52; 95%CI: 1.02-2.27, respectively], compared to those stating that religion is not important.
PWID with dependent children had a higher odds of confirmatory HCV testing [AOR: 1.57; 95%CI: 1.08-2.27], compared to childless participants. Income was also associated with confirmatory testing: AOR: 1.74 [95%CI: 1.15-2.63], comparing middle to low income. Current prescribed ART was associated with confirmatory HCV testing [AOR: 4.54; 95%CI: 2.85-7.23].
Independent correlates of recent HCV testing among PWID who are either HCV negative or not aware of their anti-HCV positive status prior to the study
Independent correlates of recent anti-HCV testing (during the 12 month prior to the interview), as recommended by guidelines for hepatitis C testing (“AASLD and IDSA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C,” 2014-2017), analyzed among PWID who were either HCV negative or were not aware of their HCV positive status included: current or prior OAT experience, residence in Odesa, longer injection duration, harmful or hazardous drinking, and female gender. Having previously (AOR: 2.24; 95%CI: 1.32-3.81] and presently (AOR: 7.17; 95%CI: 4.63-11.13) being on OAT was associated with recent anti-HCV testing while residing in Odessa was associated with 3.45 higher odds of recent anti-HCV testing (95%CI: 1.99-5.99] compared to Kyiv. Other independent correlates of recent anti-HCV testing included harmful or hazardous drinking [AOR: 1.56; (95%CI: 1.09-2.23], shorter injection duration [AOR: 0.98; 95%CI: 0.96-1.00], and female gender [AOR: 1.61; 95%CI: 1.08-2.41].
Discussion
Several key lessons emerged from this study where the HCV treatment cascade was used to as a framework for determining gaps that are crucial for curbing the HCV epidemic. First, similar to other studies (Hope et al., 2014; Salyuk & Sazonova, 2015), anti-HCV prevalence in randomly selected PWID is 62%, with most of these (56.7%) being aware of being infected. Important among these findings is that being prescribed OAT was independently the strongest correlate of both anti-HCV testing, but also HCV confirmation. PWID currently on OAT were significantly more likely to be aware and complete confirmatory testing. To our knowledge, this is the first study examining the early elements of the HCV treatment (and prevention) cascade in Ukraine and has important implications for future intervention. This study, combined with a recent study of treatment completion rates in PWID treated for HCV as part of a pilot study (A. Mazhnaya et al., 2017) begins to provide a glimpse into the future HCV landscape.
The HCV prevalence assessment, limited to five cities but recruited using random selection, is similar to a nationwide but unpublished study of HCV prevalence in PWID where anti-HCV prevalence was 55% and anti-HCV awareness was 50% (Salyuk & Sazonova, 2015). The slightly higher prevalence and awareness status may be explained in part by the current study being restricted to five regions and ones most impacted by HIV. The high prevalence estimates of HIV (41.4%) in our sample may be explained by choice of regions for study as well as preferential acceptance of HIV positive individuals to OAT programs in Ukraine.
The association of OAT and HCV continuum of care is similar to the case of HIV where being on OAT in Ukraine was associated with each step of the HIV continuum of care, including HIV testing (A Mazhnaya et al., 2017). Though HCV treatment has been introduced solely as a pilot study, a systematic review of the influence of OAT on the distal part of the HIV cascade are similarly supportive of OAT's role at increasing access to and utilization of clinical care (Low et al., 2016).
Concerning in this study is the finding that while HCV prevalence itself was high, only half of those with HCV had completed confirmatory testing. This is an especially important step since only those with chronic HCV infection, verified through confirmatory testing, would be eligible for treatment as new therapies become available. One explanation for low confirmatory testing is that since HCV treatments are uncommon, there is no urgency for further assessment. The geographic differences in HCV confirmatory testing need further examination, though, they may be explained by case management teams that facilitate the confirmatory testing for HCV/HIV positive individual by referring him to private laboratories for testing, supporting with the information about HCV, and subsidizing the cost of testing. The finding that participants with the higher income are more likely to complete confirmatory testing suggests that the major barrier to this step is the need for resources to cover this testing. One of the key implementation findings from the pilot HCV treatment project in Ukraine found that subsidizing HCV testing costs was a key factor associated with treatment scale-up (A. Mazhnaya et al., 2017).
Several key findings regarding one's awareness of anti-HCV positivity merit further discussion, including the association with HIV status and ART receipt. A likely explanation for this is integrated care sites evolving through Ukraine that integrate OAT, HIV and TB treatment (Bachireddy et al., 2014; Sylla, Bruce, Kamarulzaman, & Altice, 2007). Integrating multiple services for PWID with HIV increases their access to ART compared to those receiving OAT on non-co-located settings (Bachireddy et al., 2014). So, our study indirectly supports the hypothesis that engaging HIV-positive PWID into OAT at integrated care sites also increases their chance to receive anti-HCV test. These results give the additional support to the model of health care delivery that is able to integrate different health care services for the key populations and thereby improve individual and public health. This is especially important since the presence of HIV accelerates progression of HCV to end-stage liver disease (Thein, Yi, Dore, & Krahn, 2008) and HCV treatment for this group should be prioritized (Soto et al., 1997), especially as liver disease has become the leading cause of death among patients with HIV (“Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1–infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies,” 2010; Ioannou et al., 2013; Weber et al., 2013). Since full correction of the adverse effect of HIV infection on HCV prognosis was not observed as a result of ART, HIV/HCV co-infected patients have faster progression of fibrosis and should consider earlier initiation of HCV treatment (Thein et al., 2008). In Ukraine, with estimated HIV prevalence of 26% among PWID with chronic HCV infection (estimated 197,000) (Hope et al., 2014; Salyuk & Sazonova, 2015), an estimated total of 51,200 PWID with HIV/HCV co-infection would need treatment for both conditions.
Higher awareness of HCV positive status among PWID in Lviv and Odessa can be explained by activity of local non-governmental organizations who are more actively engaged into rising awareness of the anti-HCV status among local population of PWID, compared to the other cities (“ICF “Alliance for Public Health” Annual report, 2015,” 2016). The rapid testing for anti-HCV is provided for key populations at the offices of these NGO free of charge due to the support of international donor organizations (“ICF “Alliance for Public Health” Annual report, 2015,” 2016).
Living with a spouse or partner, importance of religion, or harmful or hazardous drinking were all associated with being aware of positive anti-HCV status. The findings of having a spouse or emphasizing the importance of religion are likely associated with the presence of social support. Social support, either intrinsically available or provided through peer interventions, have been associated with high levels of engagement in healthcare (Artenie et al., 2015). Importantly, harmful or hazardous drinking and its association with being aware of anti-HCV status is crucial since it provides opportunities to counsel about the synergistic and negative influences of alcohol use on HCV infection (Tsui et al., 2007). More importantly, these patients should be prioritized, similar to the case of HIV, for early HCV treatment.
The participants with dependent children, longer duration of injection and high relative income were also more likely to confirm chronic HCV infection in this study. Since usually women take care of children in Ukraine, higher uptake of health care by women may explain the association between having children and confirmatory testing (Artenie et al., 2015). The high cost of confirmatory HCV testing, and need for out-of-pocket payments for it may explain the association between higher income and chronic HCV confirmation. Although the constitution of Ukraine guarantees free healthcare for all citizens, out-of-pocket payments are common and often required for gaining access to medical services (Stepurko, Pavlova, Gryga, & Groot, 2013; Stepurko, Pavlova, Gryga, Murauskiene, & Groot, 2015).
Findings associated with the HCV prevention cascade provide insights for future prevention services. Key among them was the finding that current and previous OAT was correlated with recommended annual anti-HCV testing for those who do not know themselves to be HCV-infected. Being on chronic treatment for an opioid use disorder allows patients to more fully engage in HCV prevention and treatment services. Importantly, this study found that those who may be at increased risk for HCV: in particular women (Fitzgerald, Lundgren, & Chassler, 2007), those with shorter duration of injection (Folch et al., 2016) and with drinking problems had higher odds to have been recently tested. This may represent that either these PWID perceived themselves to be at highest risk, or that there was targeted testing for those at higher risk. Women may be more vulnerable to HCV when in the sexual partnerships because of paraphernalia sharing and injecting after their partners (Shulga, 2012). There is the need to account for relationships in couples when designing and implementation of HCV testing and treatment with gender and empowerment components.
Though there are many important findings from this study, it is not without limitations. First, anti-HCV prevalence and other characteristics were based on RDS for those never on OAT and may be not accurate estimates for the all PWID never on OAT in these cities. The estimates obtained in those currently or previously on OAT, however, are truly random and may be useful for program planners, as proportion estimates for that group are generalizable to the five cities where the study was conducted. Second, we relied on self-report for confirmatory HCV testing, which may be overestimated because participants might not fully understand the approach to HCV diagnostics that include two different test types. Therefore, they may perceive the repeated test for anti-HCV as confirmatory test. While this is a common strategy in many studies, having had confirmation of prior HCV testing would have been a less biased method. Studies with longitudinal design, detection of chronic HCV infection and HCV treatment uptake among PWID are warranted.
In conclusion, apart from well establish health benefits that OAT participation brings to an individual, it can improve outcomes related to HCV cascade of care, including awareness of HCV status, confirmation of chronic HCV infection and adherence to recommended HCV testing. Continuing the efforts to expand OAT and ensure the high retention rate for the enrolled participants of OAT programs should pay off not only by controlling the opioid use epidemic and reducing HIV transmission but also by ensuring higher rates of anti-HCV status awareness and confirmation. As international donor organizations will not be able to support the OAT programs in Ukraine in the near future, the commitment of state to maintaining the current infrastructure and funding for OAT is especially important to reduce the burden of diseases associated with injection drug use, including HIV and HCV. As HCV treatment is scaled up, there may become increased recognition of the importance of treatment and increasing proportions of patients with or at risk for HCV will be tested, confirmed and ultimately treated.
Table 3. Correlates of anti-HCV testing during the previous 12 months among PWID that are either HCV negative or not aware of their anti-HCV positive status (N=918).
| HCV test during last 12 | month (N=198) | |
|---|---|---|
|
| ||
| AOR(95% CI) | P-value | |
|
| ||
| OAT experience | ||
| Never on OAT | 1.00 | |
| Previously on OAT | 2.24 (1.32-3.81) | 0.003 |
| Currently on OAT | 7.17 (4.63-11.13) | <0.001 |
| City of residence | ||
| Kyiv | 1.00 | |
| Odessa | 3.45 (1.99-5.99) | <0.001 |
| Mykolaiv | 1.09 (0.65-1.81) | 0.747 |
| Dnipro | 0.97 (0.58-1.64) | 0.922 |
| Lviv | 0.63 (0.35-1.11) | 0.108 |
| Gender (female) | 1.61 (1.08-2.41) | 0.019 |
| Harmful or hazardous drinking | 1.56 (1.09-2.23) | 0.015 |
| Injection duration | 0.98 (0.96-1.00) | 0.025 |
Acknowledgments
The study received research support from the National Institutes on Drug Abuse (FLA: R01 DA033679 and R01 DA043125) (AZ: K01 DA037826) and training support from Fogarty International Center/NIH grants through the AIDS International Training and Research Program by SUNY-Downstate Medical Center (Grant #D43 TW000233).
Footnotes
Conflict of Interest Statement: Olena Iakunchykova, Anna Meteliuk, Alexei Zelenev, Alyona Mazhnaya, Melissa Tracy, Frederick L. Altice declare no conflict of interest regarding the paper “Hepatitis C virus status awareness and test results confirmation among people who inject drugs in Ukraine”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AASLD and IDSA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. 2014-2017 https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance_September_21_2017_f.pdf.
- Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1–infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50(10):1387. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artenie AA, Jutras-Aswad D, Roy E, Zang G, Bamvita JM, Levesque A, Bruneau J. Visits to primary care physicians among persons who inject drugs at high risk of hepatitis C virus infection: room for improvement. J Viral Hepat. 2015;22(10):792–799. doi: 10.1111/jvh.12393. [DOI] [PubMed] [Google Scholar]
- Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 2014;134:106–114. doi: 10.1016/j.drugalcdep.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojko MJ, Mazhnaya A, Makarenko I, Marcus R, Dvoriak S, Islam Z, Altice FL. “Bureaucracy & Beliefs”: Assessing the barriers to accessing opioid substitution therapy by people who inject drugs in Ukraine. Drugs: Education, Prevention and Policy. 2015;22(3):255–262. doi: 10.3109/09687637.2015.1016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine--programme description and policy implications. Int J Drug Policy. 2007;18(4):326–328. doi: 10.1016/j.drugpo.2006.12.011. doi:S0955-3959(06)00254-4[pii]10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Larney S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T, Lundgren L, Chassler D. Factors associated with HIV/AIDS high-risk behaviours among female injection drug users. AIDS Care. 2007;19(1):67–74. doi: 10.1080/09540120600731727. [DOI] [PubMed] [Google Scholar]
- Folch C, Casabona J, Espelt A, Majo X, Merono M, Gonzalez V, et al. Group RS. High Prevalence and Incidence of HIV and HCV Among New Injecting Drug Users With a Large Proportion of Migrants--Is Prevention Failing? Subst Use Misuse. 2016;51(2):250–260. doi: 10.3109/10826084.2015.1092991. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. The Cochrane Library. 2011 doi: 10.1002/14651858.CD004145.pub4. [DOI] [PubMed] [Google Scholar]
- Hope VD, Eramova I, Capurro D, Donoghoe MC. Prevalence and estimation of hepatitis B and C infections in the WHO European Region: a review of data focusing on the countries outside the European Union and the European Free Trade Association. Epidemiology and Infection. 2014;142(2):270–286. doi: 10.1017/S0950268813000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICF. “Alliance for Public Health” Annual report, 2015. 2016 Retrieved 1/19/2018, from http://aph.org.ua/wp-content/uploads/2016/07/ar2015_en.pdf.
- Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57(1):249–257. doi: 10.1002/hep.25800. [DOI] [PubMed] [Google Scholar]
- Lauer GM, Walker BD. Hepatitis C Virus Infection. New England Journal of Medicine. 2001;345(1):41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, et al. Vickerman P. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann N, Champagnat J, Golovin S, Maistat L, Agustian E, Inaridze I, et al. Bouscaillou J. Access to hepatitis C treatment for people who inject drugs in low and middle income settings: Evidence from 5 countries in Eastern Europe and Asia. Int J Drug Policy. 2015;26(11):1081–1087. doi: 10.1016/j.drugpo.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Makarenko I, Mazhnaya A, Marcus R, Bojko MJ, Madden L, Filippovich S, et al. Altice FL. Willingness to pay for opioid agonist treatment among opioid dependent people who inject drugs in Ukraine. Int J Drug Policy. 2017;45:56–63. doi: 10.1016/j.drugpo.2017.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko I, Mazhnaya A, Polonsky M, Marcus R, Bojko MJ, Filippovych S, et al. Altice FL. Determinants of willingness to enroll in opioid agonist treatment among opioid dependent people who inject drugs in Ukraine. Drug Alcohol Depend. 2016;165:213–220. doi: 10.1016/j.drugalcdep.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhnaya A, Bojko MJ, Marcus R, Filippovych S, Islam Z, Dvoriak S, Altice FL. In Their Own Voices: Breaking the Vicious Cycle of Addiction, Treatment and Criminal Justice Among People who Inject Drugs in Ukraine. Drugs (Abingdon Engl) 2016;23(2):163–175. doi: 10.3109/09687637.2015.1127327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhnaya A, Marcus R, Bojko MJ, Zelenev A, Makarencko J, Pykalo I, et al. Altice FL. The Influence of Opioid Agonist Treatments on the HIV Treatment Cascade in People Who Inject Drugs in Ukraine. AIDS. 2017 doi: 10.1097/QAI.0000000000001827. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhnaya A, Meteliuk A, Barnard T, Zelenev A, Filippovych S, Altice FL. Implementing and scaling up HCV treatment services for people who inject drugs and other high risk groups in Ukraine: An evaluation of programmatic and treatment outcomes. Int J Drug Policy. 2017;47:187–195. doi: 10.1016/j.drugpo.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JP, Moghimi Y, Marcus R, Lim JK, Litwin AH, Altice FL. Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic Hepatitis C care continuum. Int J Drug Policy. 2015;26(10):922–935. doi: 10.1016/j.drugpo.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne R, Price M, Wallace B, Drost A, Haigh-Gidora I, Nezil FA, Fraser C. From principles to practice: Description of a novel equity-based HCV primary care treatment model for PWID. International Journal of Drug Policy. 2015;26(10):1020–1027. doi: 10.1016/j.drugpo.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Morozova O, Dvoryak S, Altice FL. Methadone treatment improves tuberculosis treatment among hospitalized opioid dependent patients in Ukraine. Int J Drug Policy. 2013;24(6):e91–98. doi: 10.1016/j.drugpo.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaris Observatory, H. C. V. Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- Roux P, Carrieri MP, Villes V, Dellamonica P, Poizot-Martin I, Ravaux I, Spire B. The impact of methadone or buprenorphine treatment and ongoing injection on highly active antiretroviral therapy (HAART) adherence: evidence from the MANIF2000 cohort study. Addiction. 2008;103(11):1828–1836. doi: 10.1111/j.1360-0443.2008.02323.x. [DOI] [PubMed] [Google Scholar]
- Salyuk T, Sazonova I. Current challenges associated with hepatitis C virus (HVC) among people who inject drugs (PWID) in Ukraine. Journal of Clinical Virology. 2015;69:237. [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schaub M, Chtenguelov V, Subata E, Weiler G, Uchtenhagen A. Feasibility of buprenorphine and methadone maintenance programmes among users of home made opioids in Ukraine. Int J Drug Policy. 2010;21(3):229–233. doi: 10.1016/j.drugpo.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Shulga L. HIV gender-based vulnerabilities of women using drugs in long-term heterosexual relationships: baseline results from a randomized trial in Ukraine. Tobacco Control & Public Health in Eastern Europe. 2012;2 [Google Scholar]
- Smith DJ, Combellick J, Jordan AE, Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): A systematic review and meta-analysis. International Journal of Drug Policy. 2015;26(10):911–921. doi: 10.1016/j.drugpo.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Mehta SH, Srikrishnan AK, Solomon S, McFall AM, Laeyendecker O, et al. Quinn TC. Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study. Lancet Infect Dis. 2015;15(1):36–45. doi: 10.1016/s1473-3099(14)71045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto B, Sanchez-Quijano A, Rodrigo L, del Olmo JA, Garcia-Bengoechea M, Hernandez-Quero J, et al. Lissen E. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26(1):1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. Cooke GS. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepurko T, Pavlova M, Gryga I, Groot W. Informal payments for health care services– Corruption or gratitude? A study on public attitudes, perceptions and opinions in six Central and Eastern European countries. Communist and Post-Communist Studies. 2013;46(4):419–431. [Google Scholar]
- Stepurko T, Pavlova M, Gryga I, Murauskiene L, Groot W. Informal payments for health care services: the case of Lithuania, Poland and Ukraine. Journal of Eurasian Studies. 2015;6(1):46–58. [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 2007;18(4):306–312. doi: 10.1016/j.drugpo.2007.03.001. doi:S0955-3959(07)00085-0[pii]10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22(15):1979–1991. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- Ti L, Kaplan K, Hayashi K, Suwannawong P, Wood E, Kerr T. Low rates of hepatitis C testing among people who inject drugs in Thailand: implications for peer-based interventions. J Public Health (Oxf) 2013;35(4):578–584. doi: 10.1093/pubmed/fds105. [DOI] [PubMed] [Google Scholar]
- Tsui JI, Saitz R, Cheng DM, Nunes D, Libman H, Alperen JK, Samet JH. Awareness of hepatitis C diagnosis is associated with less alcohol use among persons co-infected with HIV. J Gen Intern Med. 2007;22(6):822–825. doi: 10.1007/s11606-007-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) Word Drug Report 2016. Vienna, Austria: 2016. United Nations Publication, Sales No. E.16.XI.7) [Google Scholar]
- Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, et al. Ledergerber B. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14(4):195–207. doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. Journal of substance abuse treatment. 2007;32(2):189–198. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Zhang W, O'Brien N, Forrest JI, Salters KA, Patterson TL, Montaner JS, et al. Lima VD. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7(7):e40793. doi: 10.1371/journal.pone.0040793. [DOI] [PMC free article] [PubMed] [Google Scholar]