Abstract
Purpose
As triple-negative breast cancers are associated with earlier recurrences and poorer survival, optimal treatment of early-stage breast cancer is essential. Several retrospective studies in triple-negative breast cancer have reported conflicting results in overall survival in patients receiving neoadjuvant or adjuvant systemic therapy. This study aims to analyze outcomes of adjuvant versus neoadjuvant in patients with early-stage triple-negative breast cancer with and without BRCA germline mutations.
Methods
Patients with stage I or II triple-negative breast cancer who had BRCA testing were identified from a prospective cohort study of 4027 patients. Clinical, demographic, genetic test results, chemotherapy, recurrence, and survival data were analyzed. Overall survival and disease-free survival were estimated using the Kaplan–Meier method.
Results
319 patients with stage I and II triple-negative breast cancer who met eligibility criteria were included in the analysis. 187 received adjuvant chemotherapy (58.6%) and 132 received neoadjuvant chemotherapy (41.4%). 135 were BRCA positive (42.3%) and 184 were BRCA negative (57.7%). There was no significant association between overall survival or disease-free survival and treatment with neoadjuvant versus adjuvant in the overall cohort. Furthermore, there were no significant differences between patient subgroups (neoadjuvant BRCA positive, neoadjuvant BRCA negative, adjuvant BRCA positive, and adjuvant BRCA negative) with respect to either overall survival or disease-free survival.
Conclusions
Neoadjuvant versus adjuvant with standard anthracycline- and taxane-containing regimens results in similar disease-free survival and overall survival among patients with stage I and II triple-negative breast cancer regardless of BRCA status. Further studies are needed to evaluate whether similar results are observed with newer agents.
Keywords: Triple-negative breast cancer, BRCA mutation, Neoadjuvant chemotherapy, Adjuvant chemotherapy
Introduction
Breast cancer remains a significant cause of morbidity and mortality in the United States. Breast cancer is the second leading cause of cancer deaths following lung cancer. An estimated 252,710 new cases of breast cancer were diagnosed in 2017 in the United States, with 40,610 estimated deaths [1]. Of the breast cancer subtypes, triple-negative breast cancers are associated with earlier recurrences and poorer survival compared to other subtypes of breast cancer [2]. A significant proportion of patients with triple-negative breast cancer are BRCA1/2 mutation carriers, ranging from 10 to 30.8%, with the mutation rate being higher in women less than 50 years old [3–7]. The prevalence also varies by race, with higher frequency of BRCA1/2 mutations in breast cancer patients who are Ashkenazi Jewish (50%), followed by Caucasian (33.3%), Asian (28.5%), African American (20.4%), and Hispanic (20%) [6].
As the treatment options for metastatic triple-negative breast cancer, including those with BRCA mutations, are limited, optimal treatment of those diagnosed with early-stage triple-negative breast cancer is important. One treatment consideration for early-stage triple-negative breast cancer is the initiation of neoadjuvant versus adjuvant chemotherapy. The National Surgical Adjuvant Breast and Bowel Project B-18 (NSABP) was a randomized trial to compare neoadjuvant and adjuvant chemotherapy in patients with operable breast cancer [8]. This study found no statistically significant difference in overall survival between the two groups. With long follow-up, NSABP Protocol B-18 reported a trend in favor of neoadjuvant chemotherapy in women younger than age 50 [9]. However, breast cancer subtypes were not identified in this early study.
There have since been several published retrospective reviews comparing neoadjuvant with adjuvant chemotherapy in patients with triple-negative breast cancer which resulted in conflicting outcomes [10, 11]. One retrospective analysis of 493 patients found that patients who received adjuvant chemotherapy had improved survival after controlling for age, tumor size, nodal status, and stage [10]. Another retrospective review of 385 patients with stage I–III breast cancer found that patients receiving adjuvant chemotherapy had improved overall survival compared to patients undergoing neoadjuvant chemotherapy who had residual disease but not significantly different for those receiving neoadjuvant therapy with pathological complete response [11]. There was a trend towards improvement in survival in neoadjuvant group that achieved a pathological complete response compared to those who received adjuvant chemotherapy.
Furthermore, while these prior studies examined triple-negative breast cancer, there have been no studies comparing neoadjuvant to adjuvant chemotherapy in a cohort with a significant population of BRCA-positive patients. The aim of this study was to analyze outcomes, including overall survival and disease-free survival, of neoadjuvant systemic therapy and adjuvant systemic therapy in patients with early-stage triple-negative breast cancer with and without BRCA germline mutations.
Methods
Patient population
Patients were identified from a prospective cohort study of 4027 patients followed at UT MD Anderson Cancer Center (MDACC) who underwent BRCA1/2 genetic testing between 1997 and 2016. Patients over the age of 18 with clinical diagnosis of stage IA to stage IIB triple-negative breast cancer were included in the study. Patients who received the majority of treatment, including chemotherapy and surgery, at an outside institution or without documentation of therapy received were excluded. Patients who received adjuvant endocrine therapy for a second primary hormone receptor-positive breast cancer were excluded. Initial staging was reviewed and based on the seventh edition of the American Joint Committee on Cancer (AJCC) TMN Staging System for Breast Cancer [12]. Patients without initial staging or with tumors greater than 5 cm (T3) were excluded as practice within the institution is to proceed with neoadjuvant chemotherapy. Patients with variants of uncertain significance were analyzed as BRCA non-carriers. Patients receiving both neoadjuvant and adjuvant or no chemotherapy were excluded from the overall survival and disease-free survival analysis. Approval was obtained from the Institutional Review Board at MDACC prior to initiation of this study.
The analysis of the data included clinical and demographic data as well as genetic test results, chemotherapy, recurrence, and survival data.
Pathologic assessment
Pathologic specimens were reviewed by designated breast pathologists at MDACC. Estrogen receptor and progesterone receptor status was determined by immunohistochemical analysis. Nuclear staining > 10% for estrogen receptor and/or progesterone receptor was considered positive. HER2 positivity was defined as gene amplification on fluorescence in situ hybridization with a ratio of 2.0 or 3+ receptor expression on immunohistochemical analysis.
Treatment
Chemotherapy regimens were reviewed and were grouped as anthracycline-based regimens and anthracycline-based regimens with a taxane or other regimen. Anthracycline-based regimens consisted of fluorouracil, epirubicin, and cyclophosphamide; fluorouracil, doxorubicin, and cyclophosphamide; or doxorubicin and cyclophosphamide. Anthracycline-based regimens with a taxane included these regimens with paclitaxel or docetaxel. Patients receiving chemotherapy outside these regimens were classified as other. Number of cycles of chemotherapy was reviewed and documented if patients completed at least 75% of intended chemotherapy.
Surgical therapy was reviewed and classified as either partial or total mastectomy. History of adjuvant radiation therapy was also reviewed and recorded.
Outcome measures and statistical analysis
The primary endpoints of the study were overall survival, computed from date of breast cancer diagnosis to last follow-up date or death date and disease-free survival, computed from date of breast cancer diagnosis to date of local recurrence, metastasis or death, whichever occurred first. Patients who remained alive and without recurrence and/or metastasis were administratively censored at last follow-up date. New primary or contralateral breast cancers were not included as local recurrences. Overall survival and disease-free survival were estimated using the Kaplan–Meier method and log-rank tests were used to compare groups. Cox proportional hazards regression analysis was used to model the association between overall survival and disease-free survival and covariates of interest. Fisher’s exact test was used to compare the distribution of patient characteristics between the subgroups neoadjuvant BRCA positive, neoadjuvant BRCA negative, adjuvant BRCA positive, and adjuvant BRCA negative. The nearest neighbor matching method was used to select pairs of patients treated with neoadjuvant or adjuvant regimens. All statistical analysis were performed using R version 3.3.1. All statistical tests used a significance level of 5%. No adjustments were made for multiple testing.
Results
Three-hundred and nineteen patients with stage I and II triple-negative breast cancer who met eligibility criteria were included in the analysis. Patient characteristics are presented in Table 1. The majority of the patients were less than 50 years old (N = 246, 77.1%) and premenopausal (N = 226, 72.7%) at diagnosis. Most patients were white (N = 198, 62.3%) and had stage II disease (N = 219, 68.7%). Of the 319 patients, 187 received adjuvant chemotherapy (58.6%) and 132 received neoadjuvant chemotherapy (41.4%). 135 were BRCA positive (42.3%) and 184 were BRCA negative (57.7%). The majority of the patients received an anthracycline and taxane regimen (N = 235, 73.7%). Of the 319 patients, 309 (96.9%) completed more than 75% of the standard chemotherapy regimen. 179 (56.5%) received adjuvant radiation therapy. Approximately half of the patients, 162 (50.8%), underwent total mastectomy, while the remainder had a partial mastectomy.
Table 1.
Patient clinical characteristics
| Variable | N (%) |
|---|---|
| Age | |
| < 50 | 246 (77.1) |
| > 50 | 73 (22.9) |
| Race | |
| Black | 46 (14.5) |
| Hispanic | 54 (17.0) |
| Other | 20 (6.3) |
| White | 198 (62.3) |
| Prevalence of other cancers | |
| No | 285 (89.3) |
| Yes | 34 (10.7) |
| Premenopausal at diagnosis | |
| No | 85 (27.3) |
| Yes | 226 (72.7) |
| Second primary breast cancer | |
| No | 285 (89.3) |
| Yes | 34 (10.7) |
| T stage | |
| T1 | 140 (43.9) |
| T2 | 179 (56.1) |
| N stage | |
| N0 | 210 (65.8) |
| N1 | 109 (34.2) |
| Clinical stage | |
| Stage IA | 100 (31.4) |
| Stage IIA | 150 (47.0) |
| Stage IIB | 69 (21.6) |
| Histology | |
| IDC | 310 (97.2) |
| IDC-ILC | 5 (1.6) |
| Other | 4 (1.2) |
| Nuclear grade | |
| Grade 1 | 5 (1.6) |
| Grade 2 | 15 (4.9) |
| Grade 3 | 284 (93.4) |
| Type of surgical therapy | |
| Partial mastectomy | 157 (49.2) |
| Total mastectomy | 162 (50.8) |
| BRCA status | |
| Negative | 184 (57.7) |
| Positive | 135 (42.3) |
| Chemotherapy agent | |
| Anthracycline | 65 (20.4) |
| Anthracycline + Taxane | 235 (73.7) |
| Other | 19 (6.0) |
| Total chemotherapy completed | |
| < 75% | 10 (3.1) |
| > 75% | 309 (96.9) |
| Radiation | |
| No | 138 (43.5) |
| Yes | 179 (56.5) |
| Chemotherapy | |
| Adjuvant | 187 (58.6) |
| Neoadjuvant | 132 (41.4) |
Associations between BRCA mutation status, treatment type, and tumor characteristics are presented in Table 2. Patients with negative BRCA status and treated with neoadjuvant regimes were less likely to have second breast cancer (0.0%, p values < 0.001) or other cancer (3.6%, p value < 0.008). Patients were more likely to be treated with neoadjuvant regimes if they underwent a total mastectomy (79.2 and 54.8% for BRCA-positive and BRCA-negative status, respectively) or with tumor stage of T2 (81.2 and 82.1% for BRCA-positive and BRCA-negative status, respectively, p values < 0.001).
Table 2.
Associations between BRCA mutation status, treatment type, and tumor characteristics
| Variable | Neoadjuvant BRCA positive |
Neoadjuvant BRCA negative |
Adjuvant BRCA positive |
Adjuvant BRCA negative |
p |
|---|---|---|---|---|---|
| Age | |||||
| < 50 | 41 (85.4) | 61 (72.6) | 69 (79.3) | 75 (75.0) | 0.352 |
| > 50 | 7 (14.6) | 23 (27.4) | 18 (20.7) | 25 (25.0) | |
| Second primary breast cancer | < 0.001 | ||||
| No | 42 (87.5) | 84 (100) | 65 (74.7) | 94 (94.0) | |
| Yes | 6 (12.5) | 0 (0) | 22 (25.3) | 6 (6.0) | |
| T stage | < 0.001 | ||||
| T1 | 9 (18.8) | 15 (17.9) | 53 (60.9) | 63 (63.0) | |
| T2 | 39 (81.2) | 69 (82.1) | 34 (39.1) | 37 (37.0) | |
| N stage | 0.161 | ||||
| N0 | 30 (62.5) | 48 (57.1) | 63 (72.4) | 69 (69.0) | |
| N1 | 18 (37.5) | 36 (42.9) | 24 (27.6) | 31 (31.0) | |
| Type of surgery | < 0.001 | ||||
| Partial mastectomy | 10 (20.8) | 38 (45.2) | 46 (52.9) | 63 (63.0) | |
| Total mastectomy | 38 (79.2) | 46 (54.8) | 41 (47.1) | 37 (37.0) | |
| Chemotherapy agent | < 0.001 | ||||
| Anthracycline | 7 (14.6) | 2 (2.4) | 28 (32.2) | 28 (28.0) | |
| Anthracycline + Taxane | 40 (83.3) | 80 (95.2) | 52 (59.8) | 63 (63.0) | |
| Other | 1 (2.1) | 2 (2.4) | 7 (8.0) | 9 (9.0) |
The pathological complete response rate for 132 patients receiving neoadjuvant chemotherapy was 53.8% (71 patients). Of the patients achieving a pathological complete response, 43 (60.6%) were BRCA negative and 28 (39.4%) were BRCA positive. The 5-year overall survival and disease-free probabilities contingent on pathological complete response are presented in Tables 3 and 4.
Table 3.
The 5-year (60-month) overall survival probabilities (Pr) for the neoadjuvant patients
| Measure | Pr (T > 60) | 95% CI |
|---|---|---|
| Pathological complete response | 0.964 | 0.915–1.000 |
| No pathological complete response | 0.798 | 0.686–0.927 |
Table 4.
The 5-year (60-month) disease-free survival probabilities (Pr) for the neoadjuvant patients
| Measure | Pr (T > 60) | 95% CI |
|---|---|---|
| Pathological complete response | 0.896 | 0.819–0.980 |
| No pathological complete response | 0.699 | 0.575–0.849 |
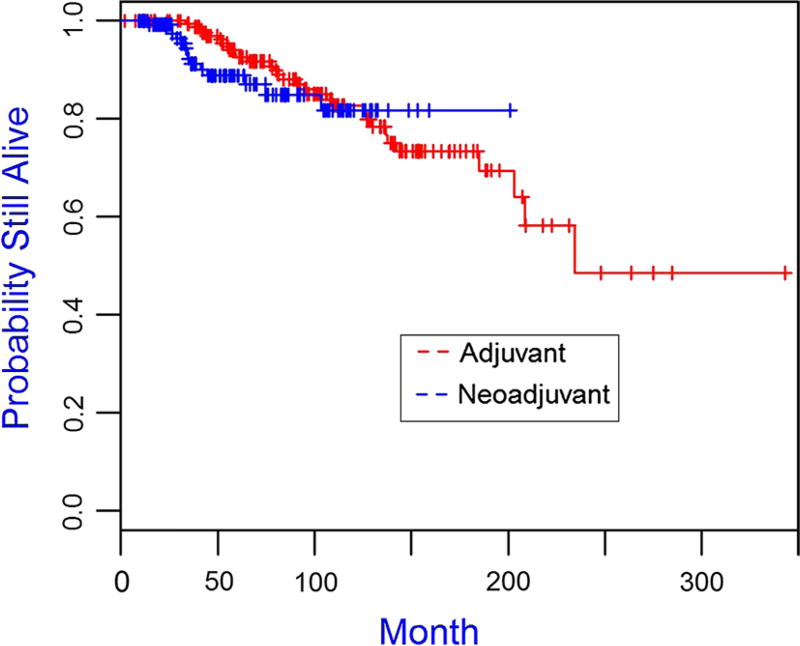
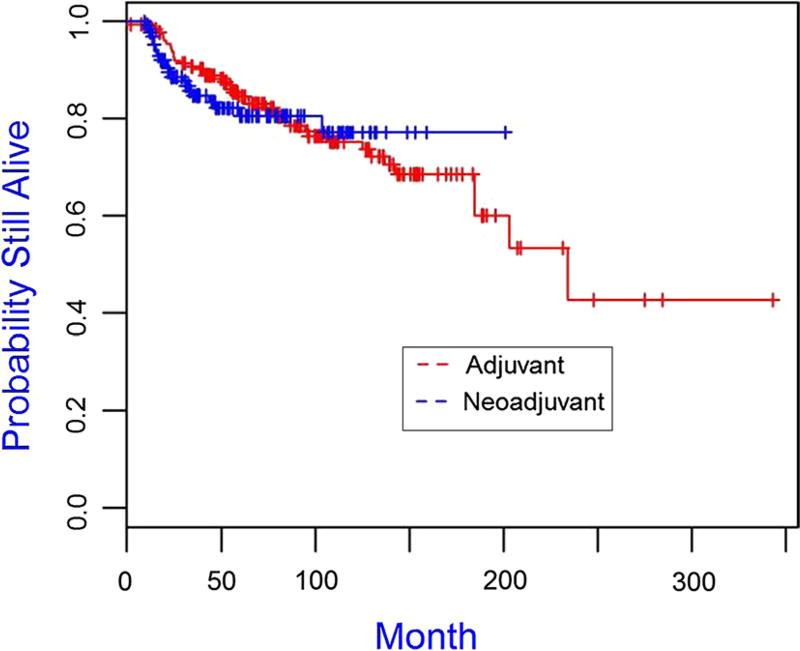
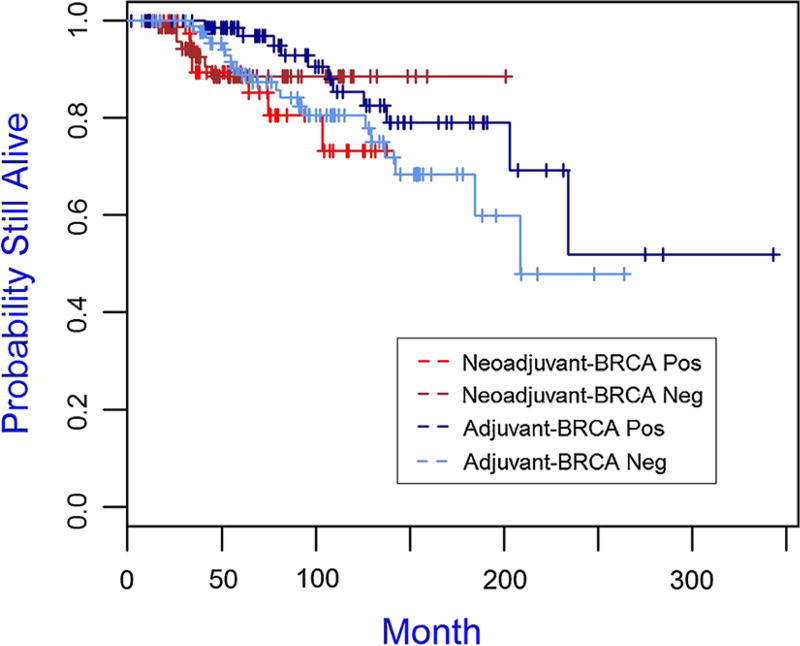
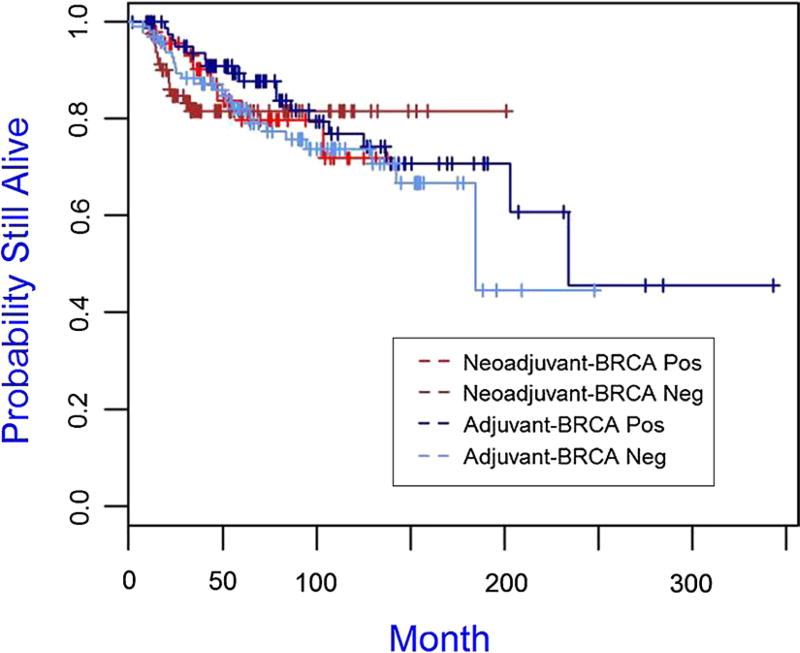
The median follow-up time for the cohort was 75.96 months. The Kaplan–Meier plots for overall survival and disease-free survival are presented in Figs. 1 and 2, 3, 4 and the 5-year overall survival rates and disease-free survival rates are presented in Tables 5 and 6. There was no significant association between overall survival or disease-free survival and treatment with neoadjuvant versus adjuvant in the overall cohort (Figs. 1, 2). Furthermore, there were no significant differences between patient subgroups (neoadjuvant BRCA positive, neoadjuvant BRCA negative, adjuvant BRCA positive, and adjuvant BRCA negative) with respect to either overall survival or disease-free survival (Figs. 3, 4).
Fig. 1.
Kaplan–Meier plot of overall survival for triple-negative breast cancer patients receiving adjuvant (N = 187) and neoadjuvant (N = 132), p value is 0.404
Fig. 2.
Kaplan–Meier plot of disease-free survival for triple-negative breast cancer patients receiving adjuvant (N = 187) and neoadjuvant (N = 132), p value is 0.735
Fig. 3.
Kaplan–Meier plot of overall survival for patient subgroups of neoadjuvant BRCA positive (N = 48), neoadjuvant BRCA negative (N = 84), adjuvant BRCA positive (N = 86), and adjuvant BRCA negative (N = 100), p value is 0.287
Fig. 4.
Kaplan–Meier plot of disease-free survival for patient subgroups of neoadjuvant BRCA positive (N = 48), neoadjuvant BRCA negative (N = 84), adjuvant BRCA positive (N = 87), and adjuvant BRCA negative (N = 100), p value is 0.731
Table 5.
The 5-year (60-month) overall survival probabilities (Pr)
| Measure | Pr (T > 60) | 95% CI |
|---|---|---|
| Overall survival | 0.909 | 0.873–0.946 |
| BRAC+ with neoadjuvant | 0.894 | 0.801–0.998 |
| BRAC− with neoadjuvant | 0.885 | 0.807–0.971 |
| BRAC+ with adjuvant | 0.969 | 0.927–1.000 |
| BRAC− with adjuvant | 0.889 | 0.823–0.960 |
Table 6.
The 5-year (60-month) disease-free survival probabilities (Pr)
| Measure | Pr (T > 60) | 95% CI |
|---|---|---|
| Overall survival | 0.829 | 0.784–0.876 |
| BRAC+ with neoadjuvant | 0.798 | 0.671–0.949 |
| BRAC− with neoadjuvant | 0.816 | 0.733–0.909 |
| BRAC+ with adjuvant | 0.877 | 0.804–0.956 |
| BRAC− with adjuvant | 0.821 | 0.745–0.905 |
Using the nearest neighbor matching method, the balance for all covariates for both matched and unmatched samples was checked. The baseline characteristics for the matched sample are presented in Table 7. The absolute standardized mean differences were less than 0.25, which indicated covariate balance between adjuvant and neoadjuvant patients. Univariate Cox models that stratified the matched pairs were fit for both overall survival and disease-free survival, with neoadjuvant and adjuvant as the treatment variable. There was no statistically significant difference for overall survival (HR 1.25, 95% CI 0.336–4.655, p value = 0.739) or disease-free survival (HR 1.25, 95% CI 0.493–3.167, p value = 0.637).
Table 7.
Baseline characteristics between adjuvant and neoadjuvant treatments for matched patients (85 matched pairs and n = 170 patients)
| Variable | Overall (%) | Adjuvant (n = 85) | Neodjuvant (n = 85) | Standard mean difference |
|---|---|---|---|---|
| Age | ||||
| < 50 | 129 (75.9) | 64 (75.3) | 65 (76.5) | 0.028 |
| > 50 | 41 (24.1) | 21 (24.7) | 20 (23.5) | |
| Race | ||||
| Black | 26 (15.3) | 15 (17.6) | 11 (12.9) | |
| Hispanic | 29 (17.1) | 13 (15.3) | 16 (18.8) | 0.099 |
| Other | 12 (7.1) | 6 (7.1) | 6 (7.1) | 0.000 |
| White | 103 (60.6) | 51 (60.0) | 52 (61.2) | 0.024 |
| Prevalence of other cancers | ||||
| No | 153 (90.0) | 75 (88.2) | 78 (91.8) | − 0.154 |
| Yes | 17 (10.0) | 10 (11.8) | 7 (8.2) | |
| Premenopausal at diagnosis | ||||
| No | 49 (28.8) | 24 (28.2) | 25 (29.4) | − 0.027 |
| Yes | 121 (71.2) | 61 (71.8) | 60 (70.6) | |
| Second primary breast cancer | ||||
| No | 158 (92.9) | 79 (92.9) | 79 (92.9) | 0.000 |
| Yes | 12 (7.1) | 6 (7.1) | 6 (7.1) | |
| T stage | ||||
| T1 | 53 (31.2) | 29 (34.1) | 24 (28.2) | 0.150 |
| T2 | 117 (68.8) | 56 (65.9) | 61 (71.8) | |
| N stage | ||||
| N0 | 107 (62.9) | 58 (68.2) | 49 (57.6) | 0.215 |
| N1 | 63 (37.1) | 27 (31.8) | 36 (42.4) | |
| Clinical stage | ||||
| Stage IA | 35 (20.6) | 20 (23.5) | 15 (17.6) | |
| Stage IIA | 90 (52.9) | 47 (55.3) | 43 (50.6) | − 0.094 |
| Stage IIB | 45 (26.5) | 18 (21.2) | 27 (31.8) | 0.223 |
| Histology | ||||
| IDC | 167 (98.2) | 83 (97.6) | 84 (98.8) | |
| IDC-ILC | 0 (0) | 0 (0) | 0 (0) | |
| Other | 3 (1.8) | 2 (2.4) | 1 (1.2) | − 0.094 |
| Nuclear grade | ||||
| Grade 1 | 2 (1.2) | 2 (2.4) | 0 (0) | |
| Grade 2 | 5 (3.0) | 1 (1.2) | 4 (4.7) | − 0.164 |
| Grade 3 | 160 (95.8) | 79 (96.3) | 81 (95.3) | 0.049 |
| Type of surgical therapy | ||||
| Partial mastectomy | 77 (45.3) | 41 (48.2) | 36 (42.4) | 0.121 |
| Total mastectomy | 93 (54.7) | 44 (51.8) | 49 (57.6) | |
| BRCA status | ||||
| Negative | 102 (60.0) | 51 (60.0) | 51 (60.0) | 0.000 |
| Positive | 68 (40.0) | 34 (40.0) | 34 (40.0) | |
| Chemotherapy agent | ||||
| Anthracycline | 24 (14.1) | 15 (17.6) | 9 (10.6) | |
| Anthracycline + Taxane | 140 (82.4) | 67 (78.8) | 73 (85.9) | 0.240 |
| Other | 6 (3.5) | 3 (3.5) | 3 (3.5) | 0.000 |
| Total chemotherapy completed | ||||
| < 75% | 5 (2.9) | 3 (3.5) | 2 (2.4) | 0.094 |
| > 75% | 165 (97.1) | 82 (96.5) | 83 (97.6) | |
| Radiation | ||||
| No | 73 (42.9) | 40 (47.1) | 33 (38.8) | 0.169 |
| Yes | 97 (57.1) | 45 (52.9) | 52 (61.2) |
In the multivariate analysis, patients who received more than 75% of total chemotherapy were found to have significantly better overall survival (p = 0.047, HR 0.222, 95% CI 0.063–0.786) and disease-free survival (p = 0.018, HR 0.209, 95% CI 0.070–0.624) compared to those patients that received less than 75% of total chemotherapy, regardless of BRCA status. There was no statistically significant difference between overall survival or disease-free survival and age, race, stage, chemotherapy regimen, type of surgical therapy, or BRCA status.
Discussion
This study analyzed outcomes, including overall survival and disease-free survival, of neoadjuvant versus adjuvant in patients with early-stage triple-negative breast cancer with and without BRCA germline mutations. Similar to NSABP B-18, there was no difference between overall survival and disease-free survival among patients receiving neoadjuvant compared to adjuvant when examining this specific subset of patients.
Prior retrospective studies have reported conflicting results when analyzing triple-negative breast cancer. An initial study showed a significant overall survival benefit with adjuvant chemotherapy [10]. This study, however, included stage III patients and reported that patients receiving neoadjuvant chemotherapy were more likely to be younger, have increased tumor size, positive nodes, and an advanced stage. There may have been a selection bias towards patients with more aggressive disease receiving neoadjuvant chemotherapy and therefore having a worse outcome based on the biology of disease rather than treatment regimen.
Although a significant portion of patients with triple-negative breast cancer may harbor a BRCA mutation, this patient population has not been reported in previous studies. This is the first study to include a large cohort of BRCA-positive patients to our knowledge. We report that neoadjuvant and adjuvant lead to similar outcomes among early-stage BRCA mutation-positive breast cancer patients and we conclude that these patients may be offered either neoadjuvant or adjuvant.
It is important to recognize this conclusion is relevant to treatments with chemotherapy regimens including anthracyclines with or without taxanes. Future studies are needed to evaluate if these conclusions will remain valid with newer agents. PARP inhibitors are promising new treatment agents for patients with BRCA mutations. These agents inhibit the enzyme poly(ADP-ribose) polymerase which results in multiple double-stranded DNA breaks that cannot be efficiently repaired in tumors harboring BRCA1/2 mutations. Several phase I and II studies demonstrated efficacy of PARP inhibitors in patients with BRCA mutations in the metastatic setting [13, 14]. More recently, metastatic BRCA-positive patients receiving olaparib were found to have increased progression-free survival as compared to single-agent chemotherapy in a phase III study [15]. There are ongoing trials with PARP inhibitors in the metastatic stage in combination with chemotherapy [16].
Further studies are ongoing to investigate the use of PARP inhibitors in early-stage breast cancer as well. Adding rucaparib to cisplatin in the adjuvant setting in patients with triple-negative breast cancer did not improve 2-year disease-free survival [17]. However, a phase II study with veliparib and carboplatin in triple-negative breast cancer in the neoadjuvant setting estimates a 51% pCR compared to a 26% response in the control group [18]. There are multiple ongoing trials to further investigate the benefits of adding PARP inhibitors neoadjuvant stage. The PARTNER trial is a randomized phase II/III trial using neoadjuvant platinum-based chemotherapy with PARP inhibitors in patients with triple-negative breast cancer (ClinicalTrials.gov Identifier: NCT03150576). Another ongoing phase II neoadjuvant trial examines single-agent talazoparib in patients with BRCA mutations (ClinicalTrials.gov Identifier: NCT02282345). PARP inhibitors are also being studied in the adjuvant setting, including the OlympiA trial which is a large phase III adjuvant trial (ClinicalTrials.gov Identifier: NCT02032823).
There have also been favorable results in treating BRCA1/2 patients with platinum chemotherapy agents. Patients with BRCA mutations have deficiencies in DNA repair which may be more sensitive to platinum agents which result in crosslinking of DNA. In the metastatic setting, patients with BRCA1/2 mutations receiving carboplatin had greater response and improved progression-free survival compared to patients treated with docetaxel [19]. One neoadjuvant study found a pathological complete response rate of 61% in patients with BRCA1 mutation who were treated with neoadjuvant cisplatin [20]. However, in a recently published secondary analysis of the GeparSixto trial, there was no additive benefit to adding carboplatin to neoadjuvant chemotherapy in patients with a BRCA1/2 mutation [21]. In this subgroup analysis, BRCA1/2-mutated patients had a high pathological complete response rate after receiving neoadjuvant anthracycline [16 of 24 (66.7%)] which was not significantly increased by adding carboplatin [17 of 26 (65.4%)] [21]. A previous study reported also reported a high pathological complete response rate of 45.6% in BRCA1 mutation carriers receiving neoadjuvant anthracyclines and taxanes alone [22]. More studies are needed to determine the efficacy of carboplatin in patients with BRCA1/2 mutations. One ongoing trial randomizes BRCA1/2-positive patients to receive cisplatin versus standard of care doxorubicin and cyclophosphamide in the neoadjuvant setting (ClinicalTrials.gov Identifier: NCT01670500).
There are several strengths of this current study. Patients were limited to early-stage disease, and patients with stage III disease were excluded. This reduces any potential confounding for patients with more aggressive disease who may receive neoadjuvant chemotherapy to downsize disease prior to surgery. The study is also, to the best of our knowledge, the first to include a large cohort of BRCA-positive patients.
There are some limitations of the current study as well. As patients were not prospectively randomized to treatment arms, there may be an underlying selection bias. Although the majority of the patients received an anthracycline- and taxane-based regimen, there were variations in chemotherapy regimens delivered. However, there was no statistically significant difference between overall survival and disease-free survival when controlling for age, race, stage, type of chemotherapy regimen, or type of surgical therapy.
Another limitation is the relatively small sample size, even though this is the largest cohort of BRCA1/2 mutation carriers analyzed for this comparison. In matched analyses, we observed a hazard ratio of 1.25 (95% CI 0.336–4.655) for patients treated with neoadjuvant compared to those treated with adjuvant regimes. With the sample size and the observed proportions of deaths for both treatment groups, a two-sided test of whether the hazard ratio was one would achieve around 80% power at a 0.05 significance level when the study population hazard ratio is actually 3.89 (PASS 13 Power Analysis and Sample Size Software). It is anticipated that the power will decrease if the study population hazard ratio is smaller, which might not be detected due to the relatively small sample size and is a limitation of the study [23]. The matched analysis, however, found no statistically significant difference for overall survival or disease-free survival between the two groups of patients.
While these limitations may account for the lack of difference in outcomes between neoadjuvant and adjuvant, the most likely reason is that there is too little difference between timing of chemotherapy between the two approaches, especially when viewed in the context of the overall clinical course of primary breast cancer.
In conclusion, neoadjuvant versus adjuvant resulted in similar disease-free survival and overall survival among patients with stage I and II triple-negative breast cancer regardless of BRCA status, similar to the prior larger randomized controlled study. At this time, both adjuvant and neoadjuvant are viable options for this patient population. Further studies are needed to evaluate whether similar results are observed with newer agents, such as platinums, PARP inhibitors, and other targeted agents in patients with triple-negative breast cancer and BRCA germline mutations.
Acknowledgments
We would like to acknowledge that this work was funded by the IBC foundation and the Andrew and Lillian A. Posey Foundation.
Dr. Jennifer Litton reports contracted research from Pfizer, Novartis, EMD-Serono, AstraZeneca, GlaxoSmithKline, and Genentech. Advisory Boards for Astra Zeneca and Pfizer, both uncompensated.
Footnotes
Conflicts of interest The remaining authors declare that they have no conflicts of interest.
References
- 1.American Cancer Society. [Accessed 4 Dec 2017];Cancer Facts & Figures 2017 Supplemental Data. 2017 https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf.
- 2.Stover D, Bell CF, Tolaney SM. Neoadjuvant and adjuvant chemotherapy considerations for triple negative breast cancer. Am J Hematol Oncol. 2016;12(3):6–12. [Google Scholar]
- 3.Hartman AR, Kaldate RR, Sailer LM, et al. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer. 2012;118:2787–2795. doi: 10.1002/cncr.26576. [DOI] [PubMed] [Google Scholar]
- 4.Meyer P, Landgraf K, Hogel B, et al. BRCA2 mutations and triple-negative breast cancer. [Accessed 26 Sept 2017];PLoS ONE. 2012 7(5):e38361. doi: 10.1371/journal.pone.0038361. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0038361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Klemp JR, Kimler BF, et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat. 2014;145:707–714. doi: 10.1007/s10549-014-2980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenup R, Buchanan A, Lorizio W, et al. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol. 2013;20:3254–3258. doi: 10.1245/s10434-013-3205-1. [DOI] [PubMed] [Google Scholar]
- 7.Kwon JS, Gutierrez-Barrera AM, Young D, et al. Expanding the criteria for BRCA mutation testing in breast cancer survivors. J Clin Oncol. 2010;28:4214–4220. doi: 10.1200/JCO.2010.28.0719. [DOI] [PubMed] [Google Scholar]
- 8.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. Monogr Natl Cancer Inst. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 9.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy CR, Gao F, Margenthaler JA. Neoadjuvant versus Adjuvant chemotherapy for triple negative breast cancer. J Surg Res. 2010;163(1):52–57. doi: 10.1016/j.jss.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Fisher CS, Ma CX, Gillanders WE, et al. Neoadjuvant chemotherapy is associated with improved survival compared with adjuvant chemotherapy in patients with triple-negative breast cancer only after complete pathologic response. Ann Surg Oncol. 2011;19(1):253–258. doi: 10.1245/s10434-011-1877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7. Springer; New York: 2010. [Google Scholar]
- 13.Somlo G, Frankel PH, Arun BK, et al. Efficacy of the PARP inhibitor veliparib with carboplatin or as a single agent in patients with germline BRCA1- or BRCA2-associated metastatic breast cancer: California cancer consortium trial NCT01149083. Clin Cancer Res. 2017;23(15):4066–4076. doi: 10.1158/1078-0432.CCR-16-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 15.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 16.Isakoff SJ, Puhalla S, Domchek SM, et al. A randomized phase II study of veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in BRCA1/2 metastatic breast cancer: design and rationale. Future Oncol. 2017;13(4):307–320. doi: 10.2217/fon-2016-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller K, Tong Y, Jones D, et al. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple negative breast cancer: final efficacy results of Hoosier Oncology Group BRE09-146. J Clin Oncol. 2015;33(15 suppl):1082. [Google Scholar]
- 18.Rugo HS, Olopade OI, DeMichele A, et al. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375(1):23–34. doi: 10.1056/NEJMoa1513749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tutt A. The TNT trial; Presented at 2014 San Antonio Breast Cancer Symposium; Dec 2014; San Antonio, TX. [Google Scholar]
- 20.Byrski T, Huzarski T, Dent R, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401–405. doi: 10.1007/s10549-014-3100-x. [DOI] [PubMed] [Google Scholar]
- 21.Hahnen E, Lederer B, Hauke J, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.1007. https://doi.org/10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed]
- 22.Arun B, Bayraktar S, Liu DD, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol. 2011;29(28):3739–3746. doi: 10.1200/JCO.2011.35.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Keefe DJ. Brief report: post hoc power, observed power, a priori power, retrospective power, prospective power, achieved power: sorting out appropriate uses of statistical power analyses. Commun Methods Meas. 2007;1(4):291–299. [Google Scholar]