Abstract
Background
Little is known regarding barriers to hepatitis C virus (HCV) treatment among people who inject drugs (PWID) in low-resource settings, particularly in the era of direct-acting antiviral therapies.
Methods
Between March, 2015–August, 2016, a cross-sectional survey was administered to community-based PWID in Chennai, India to examine the HCV care continuum and associated barriers. Adjusted prevalence ratios (APR) were estimated by multivariable Poisson regression with robust variance.
Results
All participants were male (n=541); 152 participants had HCV mono-infection and 61 participants had HIV/HCV co-infection. Only one HCV mono-infected and one HIV/HCV co-infected participant was linked to HCV care. Overall, there was moderate knowledge of HCV disease but poor knowledge of HCV treatment. Higher total knowledge scores were negatively associated with HIV/HCV co-infection (vs. HCV mono-infection), though this was not statistically significant in adjusted analysis (APR=0.71[95%CI=0.47–1.06]). Participants ≥45 years (APR=0.73[95%CI=0.58–0.92]) and participants with HIV/HCV co-infection (APR=0.64[95%CI=0.47–0.87]) were less willing to take weekly interferon injections for 12 weeks. Willingness to undergo HCV treatment improved with decreasing duration of therapy, higher perceived efficacy, and use of pills vs. interferon, though willingness to use interferon improved with decreasing duration of therapy. Most participants preferred daily visits to a clinic for HCV treatment versus receiving a month’s supply. Participants ≥45 years (vs. <45 years; APR=0.70[95%CI=0.56–0.88] and participants with HIV/HCV co-infection (APR=0.75[95%CI=0.57–0.98]) were less likely to intend on seeking HCV care. Common reasons for not having already seen a provider for HCV treatment differed by HIV status, and included low perceived need for treatment (HCV-mono-infected), competing money/health priorities and costs/fears about treatment (HIV/HCV-co-infected).
Conclusion
Residual gaps in HCV knowledge and continuing negative perceptions related to interferon-based therapy highlight the need to scale-up educational initiatives. Readiness for HCV treatment was particularly low among HIV/HCV co-infected and older PWID, emphasizing the importance of tailored treatment strategies.
Keywords: people who inject drugs, hepatitis C, HIV, treatment, direct acting antivirals, India
INTRODUCTION
Of the estimated 15.6 million people who inject drugs (PWID) worldwide, approximately 8.2 million have been infected with hepatitis C virus (HCV) (Degenhardt, et al., 2017). Chronic HCV infection is a leading cause of cirrhosis, hepatocellular carcinoma, and premature death (Cepeda et al., 2017; Greub et al., 2000; Kirk et al., 2013; Mehta et al., 2016). Prior to 2014, HCV treatment required weekly injections of pegylated interferon-α and daily doses of ribavirin for 24–48 weeks. These long-duration, interferon-based regimens were associated with suboptimal cure rates (~50%) and severe side effects, leading to high rates of treatment discontinuation. However, with the advent of oral, pan-genotypic direct acting antivirals (DAAs), it is now possible to cure chronic HCV infection in nearly all patients who have access to treatment within 8–12 weeks (>95% efficacy) (Falade-Nwulia et al., 2017; Feld et al., 2015; Kwo et al., 2017). DAA-based regimens are also well-tolerated and have limited contraindications. Consequently, the previous medical barriers to HCV treatment are diminishing (Grebely et al., 2017). At the population level, mathematical models suggest scale-up of HCV treatment is cost-effective and can substantially reduce HCV morbidity and transmission, if coupled with direct prevention (Gountas et al., 2017; Martin et al., 2016; Stone et al., 2017).
Accordingly, in 2016, the World Health Organization (WHO) set a global target to eliminate viral hepatitis as a major public health threat by 2030 (WHO, 2016a). While direct prevention and increased screening initiatives will be key components of public health campaigns, the feasibility of HCV elimination is ultimately contingent upon massively expanding treatment coverage (WHO, 2016a, 2016b). The high cost of DAAs is undeniably a key barrier to this endeavor (WHO, 2016c). However, preferential pharmaceutical pricing contracts and generic production of relatively low-cost DAAs have permitted some countries to roll-out HCV elimination programs (e.g., Egypt and Georgia) (El-Akel et al., 2017; Gvinjilia, 2016). With increased and sustained political will and support, therapy costs for HCV infection are projected to continually decline—similar to what was previously seen with generic antiretroviral therapy (ART) for human immunodeficiency virus (HIV) infection (Hill, Simmons, Gotham, & Fortunak, 2016). Even so, challenges in achieving HCV elimination via ‘treatment as prevention’ will extend far beyond cost in marginalized populations such as PWID.
Globally, PWID have had poor uptake of HCV treatment (Alavi et al., 2015; Grebely et al., 2007; Iversen et al., 2017; Mehta et al., 2008), even in the DAA era (Spradling et al., 2017; Tsui et al., 2016; van Santen et al., 2017). In high-income countries, the high attrition from HCV diagnosis to initiation of HCV treatment among PWID can be explained by a multifactorial network of individual-, provider-, and system- and structural-level barriers (Alavi et al., 2015; Doab, Treloar, & Dore, 2005; Fischer, Vasdev, Haydon, Baliunas, & Rehm, 2005; Grebely et al., 2011; Grebely et al., 2008; Heimer et al., 2002; Kwiatkowski, Fortuin Corsi, & Booth, 2002; Mehta et al., 2008; Mehta et al., 2005; Scheft & Fontenette, 2005; Sulkowski & Thomas, 2005; Treloar et al., 2011; Treloar, Hull, Dore, & Grebely, 2012; Wansom et al., 2017), some of which have not changed despite the availability of DAAs (Asher et al., 2016; Cope, Glowa, Faulds, McMahon, & Prasad, 2016; Falade-Nwulia et al., 2016; Mah et al., 2017; Socias et al., 2017; Valerio, et al., 2018). There is a paucity of data on HCV treatment uptake and associated barriers among PWID in low-and-middle income countries (LMIC) (Alam-Mehrjerdi et al., 2016; Chu et al., 2016; Loewinger et al., 2016; Mukherjee et al., 2017; Souliotis, Agapidaki, Papageorgiou, Voudouri, & Contiades, 2017), particularly from Southern and South-eastern Asia (Wait et al., 2016). Preliminary evidence from this region suggests there is poor knowledge of HCV disease and treatment among PWID attending methadone clinics, needle-exchange programs, and rehabilitation centers (Chu et al., 2016; Loewinger et al., 2016; Mukherjee et al., 2017), which may be indicative of structural barriers related to treatment availability and cost, as well as of low patient readiness for HCV treatment (i.e., low awareness and perceived need for treatment). Individual-level indicators of HCV treatment readiness, such as treatment willingness and intentions, have not been fully examined among PWID in LMIC. Given that readiness at the individual level is a key factor in successful engagement in care and treatment for HCV (Alavi et al., 2015; Grebely, et al., 2011), a lack of HCV treatment readiness among PWID in LMIC could undermine efforts to expand coverage of HCV treatment.
India is home to an estimated 164,820 to 1.1 million (predominantly male) PWID (Aceijas & Rhodes, 2007; Mathers et al., 2008), with recent estimates of HCV mono-infection and HIV/HCV co-infection among PWID of 25.6% and 14.4%, respectively (Solomon et al., 2015). Although India has provided free government-sponsored ART programs for HIV infection since 2004, recent data suggest PWID in India lag behind other populations in linkage to HIV care and ART uptake, which is related to logistical barriers, stigma, and a lack of interest/readiness to initiate ART (McFall et al., 2016; Mehta et al., 2015). Comparable data on the HCV care continuum and associated barriers among PWID in India are limited. India has made strides to remove structural barriers to HCV treatment including the availability of low cost generic DAA medications. Specifically, in March 2015, sofosbuvir, a pan-genotypic DAA, was introduced into the Indian market (Puri et al., 2016). India subsequently leveraged a license from Gilead to produce 11 generic versions of sofosbuvir (Hill et al., 2016), and currently, four generic DAAs are available in India, including the pangenotypic fixed dose combination of sofosbuvir/velpatasvir and sofosbuvir combined with daclatasvir as individual tablets—all for approximately $150 US dollars per course. However, additional structural barriers to HCV treatment access remain. Outside of Punjab, where the state government has launched a free treatment program (Dhiman, Satsangi, Grover, & Puri, 2016), most patients must pay for HCV treatment out-of-pocket (Puri et al., 2016), which includes not only the drug but the monitoring costs (e.g., HCV RNA testing). Additionally, to receive HCV treatment, patients must visit a medical gastroenterologist in settings that may not be favorable to PWID (Puri, et al., 2016). Data on residual individual-level barriers to HCV treatment among PWID in India are needed.
In this study, we aimed to characterize the HCV care continuum, examine perceived barriers to HCV care, and identify factors associated with HCV knowledge, treatment willingness, and intent for specialist assessment among community-based PWID in Chennai, India. Of note, when data were collected, sofosbuvir was the only DAA available in India and the only available pan-genotypic regimens included 12 weeks of pegylated interferon, sofosbuvir and ribavirin, or 24 weeks of sofosbuvir and ribavirin. Given the ongoing challenges HIV-infected PWID face related to HIV care in this setting, we hypothesized that there may be HIV-related differences in barriers to HCV care and treatment.
METHODS
Study Population
Participants were recruited from an ongoing community-based cohort of current and former PWID in Chennai, India (The Chennai HIV, HCV and Eeral study [CHHEERS]), which has been previously described (Solomon et al., 2016). In brief, between February 2012 and July 2015, the study enrolled 1,042 individuals through community outreach, of which 355 (35.6%) were HCV-infected and 148 (14.8%) were HIV-infected. All participants provided informed consent, were ≥18 years of age, and self-reported injection drug use in the five years prior to enrollment. A convenience sample of 860 (83%) individuals were enrolled in longitudinal follow-up. At enrollment and at semi-annual visits, participants completed a structured electronic interviewer-administered questionnaire that collected information on sociodemographics, past and current substance use, and past medical care. Alcohol use was assessed using the Alcohol Use Disorders Identification Test (AUDIT) questionnaire (Babor, Higgins-Biddle, Saunders, Monteiro, & Organization, 2001; Saunders, Aasland, Babor, De la Fuente, & Grant, 1993). A measurement of liver stiffness was ascertained by transient elastography using a FibroScan machine at each visit (EchoSens, Paris, France) (Sandrin et al., 2003). Participants also underwent a blood draw at each visit. The study was approved by the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health and YR Gaitonde Centre for AIDS Research and Education.
Laboratory Testing
We refer to HCV antibody positive and HIV antibody negative participants as HCV mono-infected, and HCV and HIV antibody positive participants as HIV/HCV co-infected. HCV and HIV antibody testing was performed at enrollment and subsequent follow-up visits using the Genedia HCV ELISA 3.0 (Green Cross Medical Science, Chungbuk, Korea) and by double HIV ELISA testing (Murex HIV-1.2.O, Abbott Murex, United Kingdom, and Vironostika® HIV Uniform II Ag/Ab, Biomerieux, The Netherlands), respectively. Among samples from HCV antibody positive participants, chronic HCV infection was determined by the presence of HCV RNA using the RealTime HCV assay (Abbott Molecular Inc., Des Plaines, Illinois). Chronic hepatitis B infection was defined by the presence of hepatitis B surface antigen at enrollment (Hepanostika HBsAg Uniform II; Biomerieux, Zaltbommel, The Netherlands).
Referrals for HCV and HIV clinical care
All participants received standard pre- and post-test counseling for HIV and HCV. HIV-infected participants were referred to local government ART centers, where ARVs are provided free of cost. All participants who tested HCV antibody positive, regardless of chronic HCV infection status, were referred to local government hospitals to consult an HCV specialist (gastroenterologist or hepatologist) to confirm their HCV infection status and be considered for HCV treatment. While care in government hospitals is partially subsidized, there is no provision in Tamil Nadu for government hospitals to pay the costs of HCV testing, treatment or monitoring. Thus, participants who opted to visit the government hospital would have to pay out-of-pocket for all additional HCV testing and treatment.
Data Collection
From March 2015 to August 2016, 541 participants in follow-up were recruited to complete a one-time questionnaire on knowledge, attitudes (willingness), behavioral intentions and perceived barriers regarding HCV care and treatment. The instrument was adapted from a US-based survey to reflect barriers that would be unique to the Indian context (Mehta et al., 2008), and was administered in the local language (Tamil) by trained study staff.
All participants were aware of their HCV infection status prior to data collection, and this was confirmed by self-report as a part of the survey. HCV-infected participants were asked to self-report their HCV care and treatment history. Linkage to care was defined as seeing a specialist (gastroenterologist/hepatologist) who could treat HCV infection. Treatment initiation and completion were self-reported; however, for a subset of participants that received treatment through a CHHEERS-related study, treatment status was confirmed via medical records (Solomon et al., 2017).
All participants completed a knowledge section of the survey. HCV knowledge was assessed by 12 true/false questions (Centers for Disease Control and Prevention, 2003); seven items were related to general HCV prevention and disease course, and five items were related to HCV treatment. An overall HCV knowledge index score was calculated by summing the total correct responses (theoretical range: 0–12). Separate knowledge sub-scores were also calculated for the 7 general HCV disease items (theoretical range: 0–7) and 5 treatment-related items (theoretical range: 0–5).
HCV-infected participants without an HCV treatment history were asked to report their willingness to receive HCV treatment via different formulations, durations and delivery strategies using a 5-point likert scale (definitely willing, somewhat willing, neither willing or unwilling, somewhat unwilling, and definitely unwilling). For example, they were asked to report their willingness to receive weekly interferon injections across different durations. Participants were then presented with comparative scenarios of treatment delivery (i.e., oral pills vs. injections) to identify factors associated with changes in willingness to receive HCV treatment (5-point likert response scale: definitely more willing, somewhat more willing, does not change willingness, somewhat less willing, definitely less willing). To ascertain intent for HCV care and treatment, HCV-infected participants without a history of HCV care or treatment were asked if they wanted/planned to see a provider/specialist who could treat HCV infection in the next year. Finally, to identify perceived barriers to HCV care, HCV-infected participants without a history of HCV care or treatment were asked to provide reason(s) why they had not already seen a provider/specialist who can treat HCV infection.
Statistical Analysis
Participant characteristics were ascertained from the study visit closest to the date the HCV questionnaire was administered. Hereinafter, “recent” refers to the 6-month period preceding the semi-annual study visit. Descriptive statistics (chi-squared or Fisher’s exact tests for categorical variables and Kruskal-Wallis or Mann-Whitney U tests for continuous variables, as appropriate) were used to compare characteristics and responses between HCV uninfected, HCV mono-infected, and HIV/HCV co-infected participants.
Among HCV-infected participants, we examined factors associated with: (1) a higher overall HCV knowledge score (>6 of 12 correct responses), (2) HCV treatment willingness (somewhat or definitely willing to receive weekly interferon injections for 12 weeks), and (3) intent for HCV specialist assessment (wanting/planning to see a specialist who can treat HCV infection within the next year). The analysis of HCV treatment willingness excluded participants with any history of HCV treatment and the analysis of intent for HCV specialist assessment excluded participants with any history of HCV care and/or treatment. For each analysis, prevalence ratios (PR) were estimated using Poisson regression with robust variance estimation. Sociodemographic, behavioral, and clinical variables associated with each outcome in previous studies and/or hypothesized to be associated with each outcome were examined in univariable models; however, the primary predictor of interest was HIV/HCV co-infection status. Variables associated with the outcome in univariable models (P<0.10) were considered for multivariable analysis. The final multivariable models retained statistically significant predictors (P<0.05), except for age and HIV status, which were retained regardless of statistical significance.
Several sensitivity analyses were performed among HCV-infected participants without a history of HCV treatment. First, given the limited variability in the general HCV knowledge sub-score, we examined correlates of higher HCV treatment knowledge (>1 of 5 items). Second, we reexamined correlates of HCV treatment willingness and intent for HCV specialist assessment restricted to participants with chronic HCV infection, as being HCV RNA-positive is a requirement for HCV treatment eligibility. Similarly, we re-assessed perceived barriers to HCV care limited to participants with chronic HCV infection.
Two-tailed P values less than 0.05 indicated statistical significance. Data were analyzed using STATA SE, version 14.2 (College Station, Texas, USA).
RESULTS
Participant Characteristics
All 541 participants were male and the median age was 41 years (Table 1). The majority of participants attained a primary school education or less (57.3%) and were daily wage earners (61.7%). The median monthly income was 90 U.S. dollars. Most participants were married or living with a partner (59.0%). Although recent injection drug use was low (4.3%), 54.7% of participants reported recent non-injection drug use, 36.2% of participants reported harmful/hazardous alcohol use, and 13.5% of participants were alcohol-dependent. There were 328 (60.6%) HCV uninfected, 152 (28.1%) HCV mono-infected, and 61 (11.3%) HIV/HCV co-infected participants (Table 1). 92% of HIV mono-infected and 42.6% of HIV/HCV co-infected participants were on ART (P<0.001).
Table 1.
Characteristics of the study population by hepatitis C virus infection status (n = 541).
| Characteristic | Total (n = 541) | HCV Uninfected(n = 328) | HCV Mono-infected(n = 152) | HIV/HCV Co-infected(n = 61) | P value * |
|---|---|---|---|---|---|
| Median age (IQR) | 41 (34 – 46) | 39 (31 – 46) | 45 (40 – 49) | 43 (38 – 45) | <0.001 |
| Male | 541 (100.0) | 328 (100.0) | 152 (100) | 61 (100) | – |
| Education | <0.001 | ||||
| Primary school or less | 310 (57.3) | 197 (60.0) | 74 (48.7) | 39 (63.9) | |
| Secondary school | 86 (15.9) | 63 (19.2) | 19 (12.5) | 4 (6.6) | |
| High school or more | 97 (17.9) | 43 (13.1) | 39 (25.7) | 15 (24.6) | |
| Vocational/University/Graduate | 48 (8.9) | 25 (7.6) | 20 (13.2) | 3 (4.9) | |
| Employment | 0.087 | ||||
| Monthly or weekly wages | 163 (30.1) | 86 (26.2) | 56 (36.8) | 21 (34.4) | |
| Daily wages | 334 (61.7) | 217 (66.2) | 81 (53.3) | 36 (59.0) | |
| Unemployed | 44 (8.1) | 25 (7.6) | 15 (9.9) | 4 (6.6) | |
| Median monthly income, U.S. $ (IQR) | 90 (60 – 120) | 90 (67 – 131) | 90 (60 – 150) | 75 (60 – 90) | 0.029 |
| Residence | 0.218 | ||||
| Own home | 167 (30.9) | 98 (29.9) | 55 (36.2) | 14 (23.0) | |
| Rental property | 334 (61.7) | 209 (63.7) | 84 (55.3) | 41 (67.2) | |
| Other (e.g., homeless) | 40 (7.4) | 21 (6.4) | 13 (8.6) | 6 (9.8) | |
| Marital status | 0.003 | ||||
| Married or living with partner | 319 (59.0) | 184 (56.1) | 107 (70.4) | 28 (45.9) | |
| Divorced, separated, or widowed | 70 (12.9) | 44 (13.4) | 14 (9.2) | 12 (19.7) | |
| Never married | 151 (27.9) | 100 (30.5) | 31 (20.4) | 20 (32.8) | |
| Ever had sex with another man | 69 (12.8) | 40 (12.2) | 22 (14.5) | 7 (11.5) | 0.753 |
| Median duration of injection, years (IQR) | 16 (9 – 22) | 13 (7 – 20) | 20 (15 – 25) | 17 (15–22) | <0.001 |
| Lifetime injection drug use | |||||
| Heroin | 507 (93.7) | 300 (91.5) | 149 (98.0) | 58 (95.1) | 0.012 |
| Buprenorphine | 315 (58.2) | 129 (39.3) | 131 (86.2) | 55 (90.2) | <0.001 |
| Pharmaceutical opiates | 125 (23.1) | 41 (12.5) | 56 (36.8) | 28 (45.9) | <0.001 |
| Stimulants | 17 (3.1) | 5 (1.5) | 9 (5.9) | 3 (4.9) | 0.023 |
| Allergy medications | 305 (56.4) | 129 (39.3) | 125 (82.2) | 51 (83.6) | <0.001 |
| Sedatives | 254 (47.0) | 113 (34.5) | 99 (65.1) | 42 (68.9) | <0.001 |
| Recent injection drug use † | 23 (4.3) | 12 (3.7) | 11 (7.2) | 0 (0.0) | 0.100 |
| Recent non-injection drug use † | 296 (54.7) | 198 (60.4) | 73 (48.0) | 25 (41.0) | 0.005 |
| Recent drug use † # | |||||
| Marijuana | 206 (38.1) | 131 (39.9) | 58 (38.2) | 17 (27.9) | 0.359 |
| Heroin | 6 (1.1) | 4 (1.2) | 2 (1.3) | 0 (0.0) | 1.000 |
| Buprenorphine | 18 (3.3) | 5 (1.5) | 12 (7.9) | 1 (1.6) | 0.002 |
| Pharmaceutical opiates | 6 (1.1) | 6 (1.8) | 0 (0.0) | 0 (0.0) | 0.242 |
| Stimulants | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Allergy medications (injection) | 8 (1.5) | 3 (0.9) | 5 (3.3) | 0 (0.0) | 0.141 |
| Sedatives | 27 (5.0) | 22 (6.7) | 3 (2.0) | 2 (3.3) | 0.063 |
| Chew intoxicating tobacco | 156 (28.8) | 115 (35.1) | 29 (19.1) | 12 (19.7) | 0.001 |
| Median no. cigarettes smoked per day (IQR) | 10 (1 – 15) | 8 (1 – 15) | 10 (5 – 15) | 5 (0 – 10) | <0.001 |
| AUDIT | <0.001 | ||||
| No/mild alcohol use | 272 (50.3) | 138 (42.0) | 86 (56.6) | 48 (78.7) | |
| Harmful/hazardous alcohol use | 196 (36.2) | 140 (42.7) | 46 (30.3) | 10 (16.4) | |
| Alcohol dependence | 73 (13.5) | 50 (15.2) | 20 (13.2) | 3 (4.9) | |
| Ever shared needle/syringe | 282 (52.1) | 122 (37.2) | 109 (71.7) | 51 (83.6) | <0.001 |
| Ever visited SNEP | 128 (23.7) | 34 (10.4) | 66 (43.4) | 28 (45.9) | <0.001 |
| Ever received OST | 61 (11.3) | 8 (2.4) | 40 (26.3) | 13 (21.3) | <0.001 |
| HIV and ART status | <0.001† | ||||
| Negative | 455 (84.1) | 303 (92.4) | 152 (100.0) | 0 (0.0) | |
| Positive and on ART | 58 (10.7) | 23 (7.0) | 0 (0.0) | 35 (57.4) | |
| Positive and no ART use | 28 (5.2) | 2 (0.6) | 2 (0.6) | 26 (42.6) | |
| Chronic HBV infection (HBsAg+) | 35 (6.5) | 20 (6.1) | 12 (7.9) | 3 (4.9) | 0.837 |
| Chronic HCV infection (RNA+)^ | 151 (27.9) | – | 105 (69.1) | 46 (75.4) | 0.416 |
| Liver stiffness | <0.001 | ||||
| Low | 399 (73.8) | 275 (83.8) | 88 (57.9) | 36 (59.0) | |
| Moderate | 64 (11.8) | 29 (8.8) | 25 (16.5) | 10 (16.4) | |
| High | 63 (11.7) | 13 (4.0) | 37 (24.3) | 13 (21.3) | |
| History of NCD‡ | 127 (23.5) | 60 (18.3) | 42 (27.6) | 25 (41.0) | <0.001 |
| History of psychiatric disorder | 8 (1.5) | 3 (0.9) | 3 (2.0) | 2 (3.3) | 0.203 |
| History of tuberculosis | 90 (16.6) | 38 (11.6) | 26 (17.1) | 26 (42.6) | <0.001 |
Note: Data are no. (%) unless specified otherwise.
P values were calculated by Pearson’s χ2 or Fisher’s exact tests for categorical variables, where appropriate, and Kruskal-wallis tests for continuous variables.
Refers to 6-month period prior to study visit.
Includes injection and non-injection routes of drug use.
9 HCV mono-infected participants and 1 HCV/HIV co-infected participant were missing HCV RNA data.
Refers to a self-reported history of diabetes, high cholesterol, high blood pressure, heart disease, lung disease, kidney disease, or cancer.
Abbreviations: ART, antiretroviral therapy; AUDIT, Alcohol Use Disorders Identification Test; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; NCD, non-communicable disease; OST, opioid substitution therapy; SNEP, syringe/needle exchange program.
HCV Care Continuum
Of the 152 HCV mono-infected participants, only one participant reported being linked to a specialist (gastroenterologist) who could treat HCV infection. This participant also reported initiating and completing HCV treatment. There were another 10 HCV mono-infected participants co-enrolled in an on-site clinical trial of HCV treatment (Solomon et al., 2017), 9 of whom had completed treatment at the time the present questionnaire was administered. Of the 61 HIV/HCV co-infected participants, only one participant was linked to a specialist (hepatologist) who could treat HCV infection, and this participant did not initiate HCV treatment.
Knowledge of HCV Infection and Treatment
Among all participants in the study (n=541), there was moderate general knowledge of HCV infection and its consequences (median, 5 of 7 items (IQR=5–5), but poor knowledge of HCV treatment (median, 1 of 5 items [IQR=1–2]), yielding a median overall knowledge score of 6 of 12 items (IQR=6–7; Table 2). While the general knowledge subscore did not significantly vary by HCV or HIV/HCV co-infection status (Table 2), HIV/HCV co-infected participants had poorer treatment knowledge subscores compared to participants with HCV mono-infection and HCV-uninfected participants (P<.01 for both comparisons; Table 2). Just over half of all study participants were aware that HCV infection was curable (53.4%); and HIV/HCV co-infected participants were the least likely to know this (P<0.001; Table 2).
Table 2.
Knowledge of hepatitis C virus infection among community-based people who inject drugs in Chennai, India (n = 541).
| No. with Correct Response (%) | Correct Answer | Total(n = 541) | HCV Uninfected(n = 328) | HCV Mono-infected(n = 152) | HIV/HCV Co-infected(n = 61) | P value * |
|---|---|---|---|---|---|---|
| General HCV Knowledge Items | ||||||
| 1. Someone with hepatitis C can look and feel fine | True | 517 (95.6) | 310 (94.5) | 149 (98.0) | 58 (95.1) | 0.219 |
| 2. Infection with the hepatitis C virus can cause the liver to stop working | True | 476 (88.0) | 279 (85.1) | 140 (92.1) | 57 (93.4) | 0.037 |
| 3. Someone with a positive hepatitis C antibody result today can test negative in the future | False | 440 (81.3) | 266 (81.1) | 117 (77.0) | 57 (93.4) | 0.014 |
| 4. People who inject drugs should get the hepatitis A and B vaccine whether or not they have hepatitis C | True | 134 (24.8) | 84 (25.6) | 38 (25.0) | 12 (19.7) | 0.644 |
| 5. Everyone with chronic hepatitis C infection will develop liver failure or liver cancer in the future | False | 117 (21.6) | 69 (21.0) | 33 (21.7) | 15 (24.6) | 0.830 |
| 6. A vaccine is available for hepatitis C virus | False | 492 (90.9) | 298 (90.9) | 140 (92.1) | 54 (88.5) | 0.682 |
| 7. People with hepatitis C should avoiding drinking alcohol | True | 535 (98.9) | 324 (98.8) | 151 (99.3) | 60 (98.4) | 0.702 |
| HCV Treatment-related Knowledge Items | ||||||
| 8. Hepatitis C infection can be cured | True | 289 (53.4) | 185 (56.4) | 86 (56.6) | 18 (29.5) | <0.001 |
| 9. Hepatitis C can be cured with just a couple of pills a day taken for 12 weeks | True | 97 (17.9) | 54 (16.5) | 34 (22.4) | 8 (13.1) | 0.183 |
| 10. Hepatitis C treatment causes bad side effects in a lot of people who take them | False | 96 (17.7) | 135 (41.2) | 25 (16.5) | 3 (4.9) | <0.001 |
| 11. Some people’s bodies can naturally remove (clear) hepatitis C without taking medication or undergoing treatment | True | 63 (11.7) | 36 (11.0) | 22 (14.5) | 5 (8.2) | 0.373 |
| 12. Once somebody has completely been treated and cleared of hepatitis C, they cannot get re-infected with hepatitis C | False | 226 (41.8) | 103 (31.4) | 87 (57.2) | 36 (59.0) | <0.001 |
| Median Knowledge Index Scores (IQR) | ||||||
| General knowledge subscore (theoretical range: 0–7) | 5 (5 – 5) | 5 (5 – 6) | 5 (5 – 5) | 5 (5 – 6) | 0.418 | |
| Treatment knowledge subscore (theoretical range: 0–5) | 1 (1 – 2) | 1 (1 – 2) | 1 (1 – 3) | 1 (1 – 1) | 0.013 | |
| Overall knowledge score (theoretical range: 0–12) | 6 (6 – 7) | 7 (6 – 7) | 7 (6 – 8) | 6 (6 – 7) | 0.069 |
P values were calculated by Pearson’s χ2 or Fisher’s exact tests for categorical variables, where appropriate, and Kruskal-wallis tests for continuous variables.
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range
Among HCV-infected participants (n=213), correlates of a higher overall HCV knowledge score (>6 of 12 items) are shown in Table S1. Although HIV/HCV co-infection was negatively associated with a higher overall HCV knowledge score (PR=0.57 [95%CI=0.38–0.85]), this association was no longer statistically significant in the multivariable model (adjusted prevalence ratio (APR)=0.71 [95%CI=0.47–1.06]). In multivariable analysis, older age (≥45 years; APR=1.50 [95%CI=1.14–1.97]), higher educational attainment (≥high school: APR=1.34 [95%CI=1.02–1.78]), recent injection drug use (APR=1.40 [95%CI=1.02–1.93]), alcohol dependence (vs. no/mild alcohol use; APR=1.81 [95%CI=1.39–2.37]), and history of HCV treatment (APR=1.70 [95%CI=1.18–2.44]) were positively associated with a higher overall HCV knowledge score (Table S1).
Among HCV-infected participants without a history of HCV treatment (n=202), older age (≥45 years; APR=1.42 [95%CI=1.00–2.01]), higher educational attainment (≥high school: APR=1.47 [95%CI=1.02–2.10]), alcohol dependence (vs. no/mild alcohol use; APR=2.52 [95%CI=1.73–3.67]) were positively associated with a higher HCV treatment knowledge score (>1 of 5 items), whereas HIV/HCV co-infection was negatively associated with a higher HCV treatment knowledge score (APR=0.52 [95%CI=0.30–0.90]; Table S2).
HCV Treatment Willingness
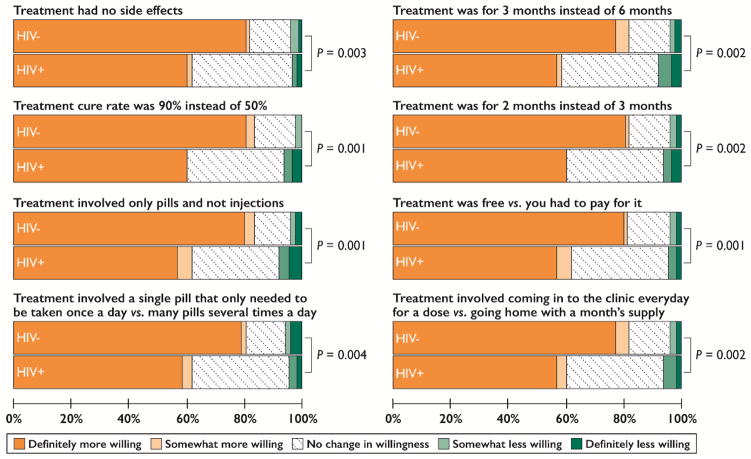
Of HCV-infected participants without a history of HCV treatment and with complete data on HCV treatment willingness (n=201), willingness to receive weekly interferon injections improved with decreasing duration of therapy among both HCV mono-infected and HIV/HCV co-infected participants (Figure 1). In addition to decreasing duration of therapy, other factors that improved willingness to receive HCV treatment were higher perceived efficacy, use of pills vs. interferon, simpler oral regimens, treatment without side effects, and low treatment costs (Figure 2). When asked how much they would be willing to pay monthly for treatment, 93.6% (132/141) and 95.0% (57/60) of HCV mono-infected and HIV/HCV co-infected participants, respectively, said $0. Lastly, 82.3% (116/141) and 60.0% (36/60) of HCV mono-infected and HIV/HCV co-infected participants, respectively, were somewhat or definitely more willing to undergo HCV treatment if they had to come in to the clinic every day to receive a treatment dose rather than going home with a month’s supply (P=0.001; Figure 2).
Figure 1.
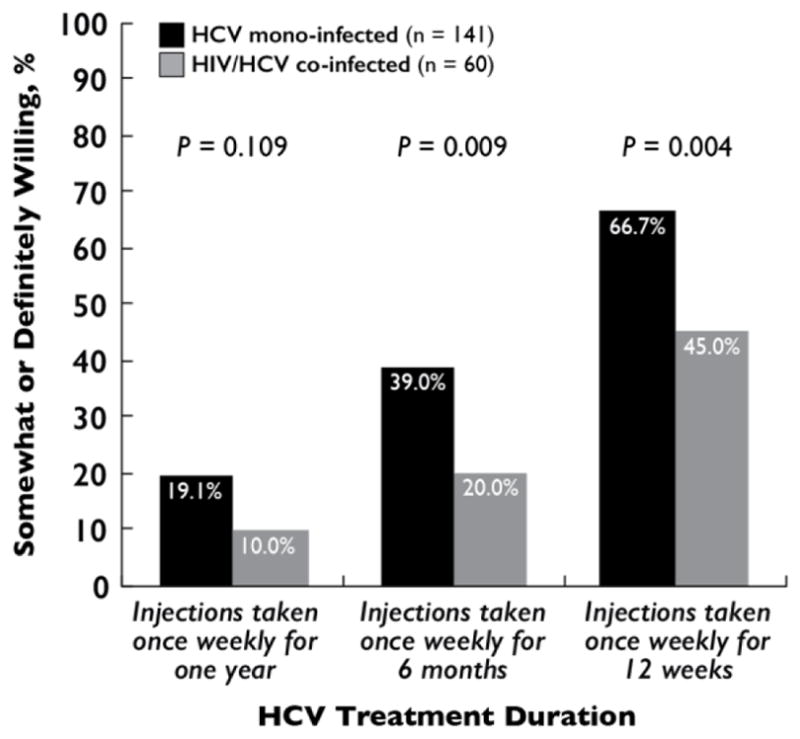
Willingness to undergo treatment for hepatitis C virus infection among treatment-naïve, community-based people who inject drugs in Chennai, India (n = 201).
Note: This analysis was limited to HCV-infected participants without a history of HCV treatment and complete data on the outcome. P values were calculated by Pearson’s χ2 tests.
Figure 2.
Factors associated with improved willingness to undergo treatment for hepatitis C virus infection among treatment-naïve, community-based people who inject drugs in Chennai, India (n=201).
Note: Data are among 141 HCV mono-infected participants and 60 HIV/HCV co-infected participants without a history of HCV treatment and complete data on the outcomes. P values were calculated by Mann-Whitney U tests.
Compared to HCV mono-infected participants (66.7%[94/141]), HIV/HCV co-infected participants (45%[27/60]) were less likely to be willing to receive weekly interferon injections for 12 weeks (PR=0.68 [95%CI=0.50–0.91]; Table S3). In multivariable analysis, HIV/HCV co-infected participants (APR=0.63 [95%CI=0.47–0.84]) and older participants (≥45 years; APR=0.74 [95%CI=0.59–0.94]) were significantly less likely to be willing to receive weekly interferon injections for 12 weeks (Table S3). Having attended a needle/syringe exchange program was also significantly associated with willingness to take interferon injections for 12 weeks (APR=1.40 [95%CI=1.13–1.73]; Table S3). In a sensitivity analysis further restricted to participants with chronic HCV infection (n=140), willingness to receive weekly interferon injections for 12 weeks remained positively associated with having ever attended a needle/syringe exchange program (APR=1.50 [95%CI=1.13–1.98]) and negatively associated with HIV/HCV co-infection (APR=0.64 [95%CI=0.45–0.91]; Table S4). The negative association between older age and willingness to receive weekly interferon injections for 12 weeks, however, was no longer statistically significant in the sensitivity analysis (≥45 years; APR=0.79 [95%CI=0.59–1.04]; Table S4).
Intent for HCV Specialist Assessment
Of participants naïve to HCV treatment, not linked to specialist care, and with complete data on behavioral intentions (n=200), HIV/HCV co-infected participants (50.9% [30/59]) were less likely than HCV mono-infected participants (68.1% [96/141]) to report wanting or planning to see a specialist who could treat HCV infection in the next year (PR=0.75 [95%CI=0.57–0.98]; Table S5). This association was also statistically significant in the multivariable model (APR=0.75 [95%CI=0.57–0.98]; Table S5). Older participants (≥45 years; APR=0.73 [95%CI=0.58–0.92]) were also significantly less likely to report wanting or planning to see a specialist who could treat HCV infection in the next year (Table S5). Neither severity of liver stiffness nor presence of other comorbidities was significantly associated with wanting or planning to seek specialist care in the next year (Table S5). In a sensitivity analysis further restricted to participants with chronic HCV infection (n=139), HIV/HCV co-infection (APR=0.64 [95%CI=0.46–0.89]) and older age (APR=0.70 [95%CI=0.53–0.93]) remained negatively associated with wanting or planning to see a specialist who could treat HCV infection in the next year (Table S6).
Perceived Barriers to HCV Care
Of participants naïve to HCV treatment, not linked to specialist care, and with complete data on perceived barriers (n=200), reasons for not having already seen a specialist were indicative of barriers at the patient-, provider-, and systems-level (Table 3). Perceived barriers to HCV care also varied by HIV/HCV co-infection status (Table 3). HIV/HCV co-infected participants were significantly more likely than HCV mono-infected participants to report fearing side effects of HCV treatment, not wanting to take interferon injections, hearing that others were treated poorly when undergoing HCV treatment, not being able to afford care or treatment, and being worried about/busy with money problems and other health conditions (P<0.05). Participants with HCV mono-infection were significantly more likely than HIV/HCV co-infected participants to report not feeling sick and wanting to avoid going to a government hospital for care (P<0.05). Regardless of infection status, the majority reported that they needed more information about HCV treatment (Table 3). Similar findings were observed in a sensitivity analysis limited to participants with chronic HCV infection (n=139; Table S7).
Table 3.
Perceived barriers to linkage to care for hepatitis C virus infection among treatment-naïve, community-based people who inject drugs in Chennai, India (n = 200).
| Reason for Not Seeking Specialist Care | HCV mono-infected (n = 141) | HIV/HCV co-infected (n = 59) | P value * |
|---|---|---|---|
| Low Perceived Need for Treatment | |||
| Does not feel sick | 92 (65.3) | 20 (33.9) | <0.001 |
| Believes severe liver outcomes are unlikely | 33 (23.4) | 10 (17.0) | 0.311 |
| Poor Perceptions of Treatment | |||
| Needs more information about treatment | 106 (75.2) | 50 (84.8) | 0.136 |
| Afraid of side effects from treatment | 91 (64.5) | 51 (86.4) | 0.002 |
| Wants to avoid interferon injections | 83 (58.9) | 47 (79.7) | 0.005 |
| Competing Interests | |||
| Worried about /busy with money problems | 9 (6.4) | 48 (81.4) | <0.001 |
| Worried about /busy with other health conditions (e.g., HIV) | 0 (0.0) | 56 (94.9) | <0.001 |
| Worried about /busy with reducing drug/alcohol use | 11 (7.8) | 5 (8.5) | 0.873 |
| Provider-level Barriers | |||
| Previously treated badly by providers due to injection drug use | 8 (5.7) | 2 (3.4) | 0.726 |
| Heard about others being treated badly when they went for HCV treatment | 71 (50.4) | 41 (69.5) | 0.013 |
| Systems-level Barriers | |||
| Wants to avoid going to a government hospital for care | 96 (68.1) | 16 (27.1) | <0.001 |
| Can’t afford to pay for care or treatment | 94 (66.7) | 48 (81.4) | 0.037 |
Data are no. (%). This analysis was limited to HCV-infected participants without a history of HCV care or treatment and complete data on perceived barriers.
P values were calculated by Pearson’s χ2 tests.
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range.
DISCUSSION
Increasing HCV treatment coverage among PWID has significant implications for HCV elimination at the population-level and HCV eradication at the individual-level. Although India has increasing availability of generic DAAs, we observed limited uptake of HCV treatment among community-based PWID in Chennai. There were residual gaps in HCV knowledge, especially regarding treatment even in this population that has been repeatedly counseled on their HCV infection. Older and HIV/HCV co-infected participants had lower treatment knowledge, lower willingness to undergo HCV treatment, and were less likely to intend on seeking HCV care in the near future, suggesting that these populations are less ready to initiate HCV treatment. Our study also provides insight on the multifaceted perceived barriers to engagement in care, which differed between HCV mono-infected and HIV/HCV co-infected participants.
The literature on HCV treatment barriers among PWID in LMIC is sparse and even fewer studies have been conducted in the DAA era. Our findings are comparable to other data from the region including studies from Nepal, Malaysia, Iran and China (Alam-Mehrjerdi et al., 2016; Chu et al., 2016; Loewinger et al., 2016; Mukherjee et al., 2017), all of which demonstrate that a key barrier to engaging in HCV care and/or treatment is limited knowledge of HCV among PWID, including those accessing opioid and needle exchange services. Of these clinic-based studies, only one includes data from the DAA era (Mukherjee et al., 2017). Low HCV treatment knowledge remains a common issue among PWID in high-income settings in the DAA era as well (Mah et al., 2017; Valerio et al., 2018). Collectively, these data highlight the need to develop and scale-up educational initiatives related to HCV infection and treatment, as adequate HCV literacy among PWID could facilitate improvements in HCV care and treatment uptake.
To the best of our knowledge, this is the first study to examine barriers to HCV care and treatment by HIV/HCV co-infection status among PWID in a resource-limited setting. HIV/HCV co-infected participants had lower willingness to undergo HCV therapy, and a lower likelihood to intend on seeking HCV care in comparison to HCV mono-infected participants. Moreover, the perceived barriers to seeking HCV care differed among the two groups with HIV/HCV co-infected participants more likely to report competing interests, misperceptions about treatment, and poor perceptions of the patient-provider relationship. These findings are not surprising in the context of what we know regarding barriers to HIV care and ART uptake among PWID in India (McFall et al., 2016; Mehta et al., 2015; Solomon et al., 2008). In addition, the HIV-related differences in HCV treatment willingness and perceived barriers to HCV care observed in this study are reflective of what was previously observed in the pre-DAA era in high-income countries (Grebely, Oser, Taylor, & Dore, 2013; Mehta et al., 2005). However, these data are in stark contrast to what is now being observed in the DAA era in some high-income countries, where high HCV treatment willingness has been documented among PWID regardless of HIV/HCV co-infection status (Mah et al. 2017; Socias et al., 2017), and HCV treatment uptake is increasing in HCV mono-infected and HIV/HCV co-infected populations (Falade-Nwulia et al., 2017; Roberson, Lagasca, & Kan, 2017). It is imperative that we develop tailored strategies to similarly increase HCV treatment uptake among PWID in India, and particularly PWID living with HIV/HCV co-infection, given the high levels of liver disease and mortality that we have previously documented in this group (Mehta et al., 2016; Solomon et al., 2009).
This study was conducted when the only pan-genotypic regimens available in India included 12 weeks of pegylated interferon, sofosbuvir and ribavirin or 24 weeks of sofosbuvir and ribavirin, and thus included questions on acceptability of shortened durations of interferon injections. While interferon is no longer considered a viable option for HCV treatment in many settings, a recent clinical trial within this same population demonstrated the superiority of sofosbuvir and pegylated interferon for 12 weeks among persons with a history of substance use in the setting of high-levels of non-adherence (Solomon et al., 2017). Moreover, recent data have demonstrated the effectiveness of an even shorter duration of treatment such as 4 weeks of pegylated interferon, sofosbuvir, ledipasvir, and ribavirin (Ovrehus et al., 2017). Our data support that there may still be a role for interferon in some settings, particularly in populations that are receptive to injections. In this study, two-thirds of HCV mono-infected participants and nearly half of HIV/HCV co-infected participants reported willingness to take weekly interferon injections for 12 weeks. As expected, both HCV mono-infected and HIV/HCV co-infected participants preferred shorter regimens. The use of interferon in combination with oral DAAs requires further study, as shortening the duration of therapy beyond 12 weeks could facilitate increases in HCV treatment uptake.
The WHO has called for the implementation of public health approaches to HCV treatment delivery (WHO, 2016a, 2016b, 2016c). Nearly all participants in this study were unwilling to pay for HCV treatment, which is not surprising given that for most, their monthly income was well below current costs of treatment. Without universal access to government-sponsored HCV treatment, treatment uptake will likely remain unacceptably low among PWID in resource-limited settings. Building on existing public health infrastructure could be a key strategy to facilitate treatment delivery. In India, directly observed therapy (DOT) is the standard mode of care for TB (Bayer & Wilkinson, 1995; Iseman, Cohn, & Sbarbaro, 1993). Surprisingly, a higher proportion of participants in this study preferred making daily visits to a clinic to receive therapy in comparison to receiving a month’s supply, suggesting that a DOT-based approach to HCV treatment may be acceptable in this population. The efficacy of clinic-based DOT approaches for HCV treatment has been demonstrated in PWID using both interferon-containing and interferon-free regimens (Grebely et al., 2017; Radley, Tait, & Dillon, 2017; Schitz, Moser, Marchart, Haltmayer, & Gschwantler, 2016). While field-based DOT can be resource-intensive (Solomon et al., 2017), it may be a useful strategy for PWID with significant barriers that impede treatment adherence and completion (e.g., HCV/HIV co-infected PWID)—particularly if duration of treatment can be shortened. Given the simplicity and safety of current regimens, a clinic- or field-based DOT approach also provides an opportunity to task-shift HCV treatment to nonspecialist providers as is being done in other settings (Kattakuzhy et al., 2017). It should also be noted that integration of HCV treatment delivery with harm reduction and addiction treatment could increase the overall well-being of PWID, and reduce the risk of reinfection (Vickerman, Martin, Turner, & Hickman, 2012).
This study has limitations that require consideration. First, as a cross-sectional study, we cannot infer causality from reported associations. Second, participant characteristics including drug use and treatment history were ascertained by self-report. In addition, the results of this study may not be generalizable to other drug-using populations, such as PWID in resource-rich settings, women who inject drugs, and PWID actively injecting drugs. It is a strength, however, that participants in this study were recruited through community outreach, as opposed to previous studies of clinic-based populations that typically exhibit greater health-seeking behaviors. Since participants in this study were already aware of their HCV infection status and previously received HCV counseling, true HCV knowledge is likely to be even lower in the general population of PWID. Furthermore, while HCV knowledge, treatment willingness, and treatment intent are key measures of treatment readiness, and have been shown to be predictive of treatment uptake in other PWID populations (Alavi et al., 2015; Grebely et al., 2011), treatment readiness may not necessarily result in successful engagement in care in this setting. Finally, as HCV treatment regimens continue to evolve, it will be important to re-examine willingness to undergo HCV treatment using interferon-containing and/or interferon-free DAA-based regimens of shorter durations than assessed in this study.
Much work is needed to increase HCV treatment coverage among PWID and maximize the control of HCV infection. Here, we demonstrate that HCV treatment remains out-of-reach among PWID in Chennai, India. The differential attitudes of and barriers to HCV treatment described by HIV co-infection status in this study, and in comparison to other settings, clearly supports the notion that a “one-size-fits-all” approach to HCV treatment delivery is inappropriate for PWID (Bruggmann & Litwin, 2013). Recognition of these differential barriers in the design and implementation of public health HCV elimination programs may yield more equitable uptake of HCV treatment.
Supplementary Material
HIGHLIGHTS.
PWID had poor linkage to HCV care and uptake of HCV treatment.
PWID had inadequate knowledge of HCV treatment.
HCV treatment willingness improved with decreasing duration of therapy.
PWID preferred daily visits to a clinic for HCV treatment.
Barriers to HCV care differed by HIV/HCV co-infection status.
Acknowledgments
Sources of Support: The work presented in this manuscript was supported by the Johns Hopkins Center for AIDS Research (grant P30 AI094189), the National Institutes of Health (grants R01DA026727, DP2DA040244, K24 DA034621 & T32AI102623), and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases.
We thank our study staff and participants who made this research possible. This work was supported by the National Institutes of Health [R01DA026727, DP2DA040244, K24 DA034621 and T32AI102623] and the Johns Hopkins Center for AIDS Research [P30 AI094189]. The study was also supported in-part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases.
Footnotes
Previous Presentation: Data were presented in-part at the Conference on Retroviruses and Opportunistic Infections (Abstract No. 558), February 13–16, 2017, Seattle, Washington, USA.
Declaration of Competing Interests: All authors: no reported conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (WHO), W. H. O. Combating hepatitis B and C to reach elimination by 2030. Retrieved 03/27/17 from http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf?ua=1.
- (WHO), W. H. O. Global health sector strategy on viral hepatitis, 2016–2021. Retrieved 03/27/2017 from http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1.
- (WHO), W. H. O. Global report on access to hepatitis C treatment. Focus on overcoming barriers. Retrieved 03/27/2017 from http://www.who.int/hepatitis/publications/hep-c-access-report/en/
- Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug Policy. 2007;18:352–358. doi: 10.1016/j.drugpo.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Alam-Mehrjerdi Z, Moradi A, Xu F, Zarghami M, Salehi-Fadardi J, Dolan K. Willingness to Receive Treatment for Hepatitis C among Injecting Drug Users on Methadone Program: Implications for Education and Treatment. Addict Health. 2016;8:90–97. [PMC free article] [PubMed] [Google Scholar]
- Alavi M, Micallef M, Fortier E, Dunlop A, Balcomb A, Day C, Treloar C, Bath N, Haber P, Dore G. Effect of treatment willingness on specialist assessment and treatment uptake for hepatitis C virus infection among people who use drugs: The ETHOS study. Journal of viral hepatitis. 2015;22:914–925. doi: 10.1111/jvh.12415. [DOI] [PubMed] [Google Scholar]
- Asher AK, Portillo CJ, Cooper BA, Dawson-Rose C, Vlahov D, Page KA. Clinicians' Views of Hepatitis C Virus Treatment Candidacy With Direct-Acting Antiviral Regimens for People Who Inject Drugs. Subst Use Misuse. 2016;51:1218–1223. doi: 10.3109/10826084.2016.1161054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG Organization WH. AUDIT: The alcohol use disorders identification test: Guidelines for use in primary health care 2001 [Google Scholar]
- Bayer R, Wilkinson D. Directly observed therapy for tuberculosis: history of an idea. Lancet. 1995;345:1545–1548. doi: 10.1016/s0140-6736(95)91090-5. [DOI] [PubMed] [Google Scholar]
- Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(Suppl 2):S56–61. doi: 10.1093/cid/cit271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention; National Center for Health Statistics, editor. National Health and Examination Survey Questionnaire. Hyattsville, MD: US Department of Helath and Human Services; 2003 pp. [Google Scholar]
- Cepeda JA, Thomas DL, Astemborski J, Sulkowski MS, Kirk GD, Mehta SH. Increased mortality among persons with chronic hepatitis C with moderate or severe liver disease: a cohort study. Clin Infect Dis. 2017 doi: 10.1093/cid/cix207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CE, Wu F, He X, Zhou K, Cheng Y, Cai W, Geng E, Volberding P, Tucker JD. Hepatitis C Virus Treatment Access Among Human Immunodeficiency Virus and Hepatitis C Virus (HCV)-Coinfected People Who Inject Drugs in Guangzhou, China: Implications for HCV Treatment Expansion. Open Forum Infect Dis. 2016;3:ofw065. doi: 10.1093/ofid/ofw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope R, Glowa T, Faulds S, McMahon D, Prasad R. Treating Hepatitis C in a Ryan White-Funded HIV Clinic: Has the Treatment Uptake Improved in the Interferon-Free Directly Active Antiviral Era? AIDS Patient Care STDS. 2016;30:51–55. doi: 10.1089/apc.2015.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, Stone J, Cunningham EB, Trickey A, Dumchev K, Lynskey M, Griffiths P, Mattick RP, Hickman M, Larney S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman RK, Satsangi S, Grover GS, Puri P. Tackling the Hepatitis C Disease Burden in Punjab, India. J Clin Exp Hepatol. 2016;6:224–232. doi: 10.1016/j.jceh.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doab A, Treloar C, Dore GJ. Knowledge and attitudes about treatment for hepatitis C virus infection and barriers to treatment among current injection drug users in Australia. Clinical Infectious Diseases. 2005;40:S313–S320. doi: 10.1086/427446. [DOI] [PubMed] [Google Scholar]
- El-Akel W, El-Sayed MH, El Kassas M, El-Serafy M, Khairy M, Elsaeed K, Kabil K, Hassany M, Shawky A, Yosry A, Shaker MK, ElShazly Y, Waked I, Esmat G, Doss W. National treatment programme of hepatitis C in Egypt: Hepatitis C virus model of care. J Viral Hepat. 2017;24:262–267. doi: 10.1111/jvh.12668. [DOI] [PubMed] [Google Scholar]
- Falade-Nwulia O, McAdams-Mahmoud A, Irvin R, Niculescu A, Page K. Primary Care Providers Knowledge, Attitude and Practices Related to Hepatitis C Screening and Treatment in the Oral Direct Acting Antiviral Agents Era. J Community Med Health Educ. 2016;6 doi: 10.4172/2161-0711.1000481. 2161-0711.1000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017 doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade-Nwulia O, Sutcliffe C, Moon J, Chander G, Wansom T, Keruly J, Katzianer J, Nathanson A, Marks J, Mehta S, Thomas D, Moore R, Sulkowski M. High Hepatitis C cure rates among black and non-black HIV-infected adults in an urban center. Hepatology. 2017 doi: 10.1002/hep.29308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL, Mazzotta F, Moreno C, Yoshida E, Shafran SD, Towner WJ, Tran TT, McNally J, Osinusi A, Svarovskaia E, Zhu Y, Brainard DM, McHutchison JG, Agarwal K, Zeuzem S Investigators A. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- Fischer B, Vasdev S, Haydon E, Baliunas D, Rehm J. Willing to undergo hepatitis C treatment in a sample of injection drug users in Toronto, Canada. Presse Med. 2005;34:1209–1212. doi: 10.1016/s0755-4982(05)84158-2. [DOI] [PubMed] [Google Scholar]
- Gountas I, Sypsa V, Anagnostou O, Martin N, Vickerman P, Kafetzopoulos E, Hatzakis A. Treatment and primary prevention in people who inject drugs for chronic hepatitis C infection: is elimination possible in a high-prevalence setting? Addiction. 2017;112:1290–1299. doi: 10.1111/add.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Bryant J, Hull P, Hopwood M, Lavis Y, Dore G, Treloar C. Factors associated with specialist assessment and treatment for hepatitis C virus infection in New South Wales, Australia. Journal of viral hepatitis. 2011;18:e104–e116. doi: 10.1111/j.1365-2893.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- Grebely J, Dalgard O, Cunningham EB, Hajarizadeh B, Foster GR, Bruggmann P, Conway B, Backmund M, Robaeys G, Swan T, Amin J, Marks PS, Quiene S, Applegate TL, Weltman M, Shaw D, Dunlop A, Hellard M, Bruneau J, Midgard H, Bourgeois S, Staehelin C, Dore GJ, Group AS. Efficacy of response-guided directly observed pegylated interferon and self-administered ribavirin for people who inject drugs with hepatitis C virus genotype 2/3 infection: The ACTIVATE study. Int J Drug Policy. 2017 doi: 10.1016/j.drugpo.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Grebely J, Genoway K, Khara M, Duncan F, Viljoen M, Elliott D, Raffa JD, DeVlaming S, Conway B. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy. 2007;18:437–443. doi: 10.1016/j.drugpo.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Grebely J, Genoway KA, Raffa JD, Dhadwal G, Rajan T, Showler G, Kalousek K, Duncan F, Tyndall MW, Fraser C. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug and Alcohol dependence. 2008;93:141–147. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis. 2013;207(Suppl 1):S19–25. doi: 10.1093/infdis/jis928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Swan T, Hickman M, Bruneau J, Bruggmann P, Dalgard O, Litwin A, Backmund M, Dore GJ International Network for Hepatitis in Substance U. Contradictory advice for people who inject drugs in the 2016 EASL Recommendations on Treatment of Hepatitis C. J Hepatol. 2017 doi: 10.1016/j.jhep.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, Burgisser P, Erb P, Boggian K, Piffaretti JC, Hirschel B, Janin P, Francioli P, Flepp M, Telenti A. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- Gvinjilia L. National progress toward Hepatitis C elimination—Georgia, 2015–2016. MMWR. Morbidity and mortality weekly report. 2016:65. doi: 10.15585/mmwr.mm6541a2. [DOI] [PubMed] [Google Scholar]
- Heimer R, Clair S, Grau LE, Bluthenthal RN, Marshall PA, Singer M. Hepatitis-associated knowledge is low and risks are high among HIV-aware injection drug users in three US cities. Addiction. 2002;97:1277–1287. doi: 10.1046/j.1360-0443.2002.t01-1-00211.x. [DOI] [PubMed] [Google Scholar]
- Hill A, Simmons B, Gotham D, Fortunak J. Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C. J Virus Erad. 2016;2:28–31. [PMC free article] [PubMed] [Google Scholar]
- Iseman MD, Cohn DL, Sbarbaro JA. Directly observed treatment of tuberculosis. We can't afford not to try it. N Engl J Med. 1993;328:576–578. doi: 10.1056/NEJM199302253280811. [DOI] [PubMed] [Google Scholar]
- Iversen J, Grebely J, Catlett B, Cunningham P, Dore GJ, Maher L. Estimating the cascade of hepatitis C testing, care and treatment among people who inject drugs in Australia. Int J Drug Policy. 2017 doi: 10.1016/j.drugpo.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Kattakuzhy S, Gross C, Emmanuel B, Teferi G, Jenkins V, Silk R, Akoth E, Thomas A, Ahmed C, Espinosa M, Price A, Rosenthal E, Tang L, Wilson E, Bentzen S, Masur H, Kottilil S the AP. Expansion of Treatment for Hepatitis C Virus Infection by Task Shifting to Community-Based Nonspecialist Providers: A Nonrandomized Clinical Trial. Ann Intern Med. 2017;167:311–318. doi: 10.7326/M17-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk GD, Mehta SH, Astemborski J, Galai N, Washington J, Higgins Y, Balagopal A, Thomas DL. HIV, age, and the severity of hepatitis C virus-related liver disease: a cohort study. Ann Intern Med. 2013;158:658–666. doi: 10.7326/0003-4819-158-9-201305070-00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski CF, Fortuin Corsi K, Booth RE. The association between knowledge of hepatitis C virus status and risk behaviors in injection drug users. Addiction. 2002;97:1289–1294. doi: 10.1046/j.1360-0443.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, Felizarta F, Sulkowski MS, Gane E, Maliakkal B, Overcash JS, Gordon SC, Muir AJ, Aguilar H, Agarwal K, Dore GJ, Lin CW, Liu R, Lovell SS, Ng TI, Kort J, Mensa FJ. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol. 2017;67:263–271. doi: 10.1016/j.jhep.2017.03.039. [DOI] [PubMed] [Google Scholar]
- Loewinger G, Sharma B, Karki DK, Khatiwoda P, Kainee S, Poudel KC. Low knowledge and perceived Hepatitis C risk despite high risk behaviour among injection drug users in Kathmandu, Nepal. Int J Drug Policy. 2016;33:75–82. doi: 10.1016/j.drugpo.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Mah A, Hull MW, DeBeck K, Milloy MJ, Dobrer S, Nosova E, Wood E, Kerr T, Hayashi K. Knowledge of hepatitis C and treatment willingness amongst people who inject drugs in an era of direct acting antivirals. Int J Drug Policy. 2017 doi: 10.1016/j.drugpo.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Vickerman P, Dore GJ, Grebely J, Miners A, Cairns J, Foster GR, Hutchinson SJ, Goldberg DJ, Martin TC. Prioritization of HCV treatment in the direct-acting antiviral era: an economic evaluation. Journal of hepatology. 2016;65:17–25. doi: 10.1016/j.jhep.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP Reference Group to the U. N. o. H. I. V, Injecting Drug U. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- McFall AM, Mehta SH, Srikrishnan AK, Lucas GM, Vasudevan CK, Celentano DD, Kumar MS, Solomon S, Solomon SS. Getting to 90: linkage to HIV care among men who have sex with men and people who inject drugs in India. AIDS Care. 2016;28:1230–1239. doi: 10.1080/09540121.2016.1168915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, Strathdee SA, Thomas DL. Limited uptake of hepatitis C treatment among injection drug users. Journal of community health. 2008;33:126. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Lucas GM, Solomon S, Srikrishnan AK, McFall AM, Dhingra N, Nandagopal P, Kumar MS, Celentano DD, Solomon SS. HIV care continuum among men who have sex with men and persons who inject drugs in India: barriers to successful engagement. Clinical Infectious Diseases. 2015:civ669. doi: 10.1093/cid/civ669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, McFall AM, Srikrishnan AK, Kumar MS, Nandagopal P, Cepeda J, Thomas DL, Sulkowski MS, Solomon SS. Open Forum Infectious Diseases. Vol. 3. Oxford University Press; 2016. Morbidity and Mortality Among Community-Based People Who Inject Drugs With a High Hepatitis C and Human Immunodeficiency Virus Burden in Chennai, India; p. ofw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Thomas DL, Sulkowski MS, Safaein M, Vlahov D, Strathdee SA. A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users. Aids. 2005;19:S179–S189. doi: 10.1097/01.aids.0000192088.72055.90. [DOI] [PubMed] [Google Scholar]
- Mukherjee TI, Pillai V, Ali SH, Altice FL, Kamarulzaman A, Wickersham JA. Evaluation of a hepatitis C education intervention with clients enrolled in methadone maintenance and needle/syringe programs in Malaysia. Int J Drug Policy. 2017 doi: 10.1016/j.drugpo.2017.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovrehus ALH, Krarup H, Birkemose I, Holm DK, Mossner B, Ernst A, Christensen PB. Four weeks of ledipasvir/sofosbuvir and ribavirin with or without pegylated interferon for chronic hepatitis C in non-cirrhotic people who inject drugs. A randomized trial. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.11.031. [DOI] [PubMed] [Google Scholar]
- Puri P, Saraswat VA, Dhiman RK, Anand AC, Acharya SK, Singh SP, Chawla YK, Amarapurkar DN, Kumar A, Arora A, Dixit VK, Koshy A, Sood A, Duseja A, Kapoor D, Madan K, Srivastava A, Kumar A, Wadhawan M, Goel A, Verma A, Shalimar, Pandey G, Malik R, Agrawal S. Indian National Association for Study of the Liver (INASL) Guidance for Antiviral Therapy Against HCV Infection: Update 2016. J Clin Exp Hepatol. 2016;6:119–145. doi: 10.1016/j.jceh.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley A, Tait J, Dillon JF. DOT-C: A cluster randomised feasibility trial evaluating directly observed anti-HCV therapy in a population receiving opioid substitute therapy from community pharmacy. Int J Drug Policy. 2017 doi: 10.1016/j.drugpo.2017.05.042. [DOI] [PubMed] [Google Scholar]
- Roberson JL, Lagasca AM, Kan VL. Comparison of the Hepatitis C Continua of Care between HCV/HIV Co-Infected and HCV Mono-Infected Patients in Two Treatment Eras during 2008–2015. AIDS Res Hum Retroviruses. 2017 doi: 10.1089/AID.2017.0092. [DOI] [PubMed] [Google Scholar]
- Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Scheft H, Fontenette DC. Psychiatric barriers to readiness for treatment for hepatitis C Virus (HCV) infection among injection drug users: clinical experience of an addiction psychiatrist in the HIV-HCV coinfection clinic of a public health hospital. Clin Infect Dis. 2005;40(Suppl 5):S292–296. doi: 10.1086/427443. [DOI] [PubMed] [Google Scholar]
- Schitz A, Moser S, Marchart K, Haltmayer H, Gschwantler M. Direct Observed Therapy of Chronic Hepatitis C With Interferon-Free All-Oral Regimens at a Low-Threshold Drug Treatment Facility-a New Concept for Treatment of Patients With Borderline Compliance Receiving Opioid Substitution Therapy. Am J Gastroenterol. 2016;111:903–905. doi: 10.1038/ajg.2016.119. [DOI] [PubMed] [Google Scholar]
- Socias ME, Ti L, Dong H, Shoveller J, Kerr T, Montaner J, Milloy MJ. High prevalence of willingness to use direct-acting antiviral-based regimens for hepatitis C virus (HCV) infection among HIV/HCV coinfected people who use drugs. HIV Med. 2017 doi: 10.1111/hiv.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S, Sulkowski M, Ambrose P, Srikrishnan A, McFall A, Ramasamy B, Kumar M, Anand S, Thomas D, Mehta S. Directly Observed Therapy of Sofosbuvir/Ribavirin +/− Peginterferon with minimal molecular monitoring for the treatment of chronic hepatitis C in people with a history of drug use in Chennai, India (C-DOT) Journal of viral hepatitis. 2017 doi: 10.1111/jvh.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Celentano DD, Srikrishnan AK, Vasudevan CK, Anand S, Kumar MS, Solomon S, Lucas GM, Mehta SH. Mortality among injection drug users in Chennai, India (2005–2008) Aids. 2009;23:997–1004. doi: 10.1097/QAD.0b013e32832a594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Mehta SH, Srikrishnan AK, Solomon S, McFall AM, Laeyendecker O, Celentano DD, Iqbal SH, Anand S, Vasudevan CK. Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study. The Lancet infectious diseases. 2015;15:36–45. doi: 10.1016/S1473-3099(14)71045-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Srikrishnan AK, McFall AM, Kumar MS, Saravanan S, Balakrishnan P, Solomon S, Thomas DL, Sulkowski MS, Mehta SH. Burden of liver disease among community-based people who inject drugs (PWID) in Chennai, India. PloS one. 2016;11:e0147879. doi: 10.1371/journal.pone.0147879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Srikrishnan AK, Mehta SH, Vasudevan CK, Murugavel KG, Thamburaj E, Anand S, Kumar MS, Latkin C, Solomon S. High prevalence of HIV, HIV/hepatitis C virus co-infection and risk behaviors among IDUs in Chennai, India: a cause for concern. Journal of acquired immune deficiency syndromes (1999) 2008;49:327. doi: 10.1097/QAI.0b013e3181831e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souliotis K, Agapidaki E, Papageorgiou M, Voudouri N, Contiades X. Access to treatment for Hepatitis C among injection drug users: results from the cross-sectional HOPE IV study. Int J Equity Health. 2017;16:101. doi: 10.1186/s12939-017-0601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling PR, Xing J, Rupp LB, Moorman AC, Gordon SC, Lu M, Teshale EH, Boscarino JA, Schmidt MA, Daida YG, Holmberg SD Chronic Hepatitis Cohort Study, I. Uptake of and Factors Associated With Direct-acting Antiviral Therapy Among Patients in the Chronic Hepatitis Cohort Study, 2014 to 2015. J Clin Gastroenterol. 2017 doi: 10.1097/MCG.0000000000000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Martin NK, Hickman M, Hutchinson SJ, Aspinall E, Taylor A, Munro A, Dunleavy K, Peters E, Bramley P, Hayes PC, Goldberg DJ, Vickerman P. Modelling the impact of incarceration and prison-based hepatitis C virus (HCV) treatment on HCV transmission among people who inject drugs in Scotland. Addiction. 2017 doi: 10.1111/add.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski MS, Thomas DL. Perspectives on HIV/hepatitis C virus co-infection, illicit drug use and mental illness. Aids. 2005;19(Suppl 3):S8–12. doi: 10.1097/01.aids.0000192064.09281.48. [DOI] [PubMed] [Google Scholar]
- Treloar C, Hull P, Bryant J, Hopwood M, Grebely J, Lavis Y. Factors associated with hepatitis C knowledge among a sample of treatment naive people who inject drugs. Drug and Alcohol dependence. 2011;116:52–56. doi: 10.1016/j.drugalcdep.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Treloar C, Hull P, Dore GJ, Grebely J. Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug and alcohol review. 2012;31:918–924. doi: 10.1111/j.1465-3362.2012.00468.x. [DOI] [PubMed] [Google Scholar]
- Tsui JI, Ko SC, Krupitsky E, Lioznov D, Chaisson CE, Gnatienko N, Samet JH. Insights on the Russian HCV Care Cascade: Minimal HCV Treatment for HIV/HCV Co-infected PWID in St. Petersburg. Hepatol Med Policy. 2016:1. doi: 10.1186/s41124-016-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio H, McAuley A, Innes H, Palmateer N, Goldberg DJ, Munro A, Taylor A, Hutchinson SJ. Determinants of hepatitis C antiviral effectiveness awareness among people who inject drugs in the direct-acting antiviral era. Int J Drug Policy. 2018;52:115–122. doi: 10.1016/j.drugpo.2017.12.014. [DOI] [PubMed] [Google Scholar]
- van Santen DK, van der Helm JJ, Lindenburg K, Schim van der Loeff M, Prins M. HIV and hepatitis C treatment uptake among people who use drugs participating in the Amsterdam Cohort Studies, 1985–2015. Int J Drug Policy. 2017 doi: 10.1016/j.drugpo.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Vickerman P, Martin N, Turner K, Hickman M. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction. 2012;107:1984–1995. doi: 10.1111/j.1360-0443.2012.03932.x. [DOI] [PubMed] [Google Scholar]
- Wait S, Kell E, Hamid S, Muljono DH, Sollano J, Mohamed R, Shah S, Abbas Z, Johnston J, Tanwandee T. Hepatitis B and hepatitis C in southeast and southern Asia: challenges for governments. The Lancet Gastroenterology & Hepatology. 2016;1:248–255. doi: 10.1016/S2468-1253(16)30031-0. [DOI] [PubMed] [Google Scholar]
- Wansom T, Falade-Nwulia O, Sutcliffe CG, Mehta SH, Moore RD, Thomas DL, Sulkowski MS. Barriers to Hepatitis C Virus (HCV) Treatment Initiation in Patients With Human Immunodeficiency Virus/HCV Coinfection: Lessons From the Interferon Era. Open Forum Infect Dis. 2017;4:ofx024. doi: 10.1093/ofid/ofx024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.