Abstract
Recently we determined that the Transient Receptor Potential Vanilloid 4 ion channel (TRPV4) has a crucial signaling role in a pathway that regulates various aspects of lens epithelium function. Here, we report on a different TRPV channel, TRPV1, in porcine lens. The presence of TRPV1 in the lens was evident from RT-PCR studies and Western blot analysis of MAPK signaling pathway activation caused by the TRPV1 agonist capsaicin. TRPV1 mRNA was detected in the epithelium of porcine as well as human lens. Transient ERK1/2 and p38 MAPK phosphorylation was detected within 1 min in the epithelium isolated from intact porcine lenses exposed to capsaicin (100 nM), a selective TRPV1 agonist, and the response was significantly inhibited by A889245 (1.0 μM), a TRPV1 antagonist. A similar ERK 1/2 and p38 response in the epithelium, also inhibitable by A889245, was evident in lenses treated with hyperosmotic solution (350 vs 300 mOsm). Lenses pre-treated with either the cytosolic Ca2+ chelator BAPTA-AM or the PKC inhibitor sotrastaurin (1.0 μM) had a diminished ERK1/2 activation response to capsaicin and hyperosmotic solution. Taken together the findings support the notion that TRPV1 functions as a plasma membrane ion channel that, when activated, permits the entry of extracellular calcium into the lens epithelium, leading to activation of PKC, ERK1/2 and p38 MAPK. It is significant that the findings confirm earlier proposals that hyperosmotic stress is linked to TRPV1 channel activation in the mouse lens. Further studies are ongoing to determine what functional changes are triggered by the TRPV1-linked signaling pathways and how they might relate to lens volume homeostasis.
Keywords: Lens epithelium, TRPV1, ERK1/2, P38 MAPK
1. Introduction
The lens has two cell types, densely packed fibers that make up most of the structure and a monolayer of epithelial cells that covers the anterior face. Mature fiber cells, which lack organelles, are differentiated to the extent that homeostasis of the fiber mass depends on the epithelium. Functional integration between the two cell types is made possible by the unique architecture of the lens and by efficient coupling between neighboring fibers (Goodenough, Dick et al. 1980, Rae and Kuszak 1983, Lo and Harding 1986, Bassnett, Kuszak et al. 1994, Kuszak, Novak et al. 1995, Mathias, White et al. 2010). Because mature fiber cells have scant Na,K-ATPase activity (Delamere and Dean 1993), the lens must rely to a large extent on the epithelium for sodium-potassium homeostasis of the fiber mass (Gao, Sun et al. 2000). Epithelial cells have robust Na,K-ATPase activity (Tamiya, Dean et al. 2003) (Delamere and Dean 1993).
Recent studies showed evidence for a TRPV4 channel-dependent signaling mechanism in the epithelium that responds in lenses treated with low osmotic strength solution (Shahidullah, Mandal et al. 2012) or damage to the fiber mass (Shahidullah, Mandal et al. 2015). TRPV4 activation initiates signaling pathway responses that lead to changes in epithelium function. TRPV4-dependent responses include hemichannel opening, ATP release and an increase in the activity of Na,K-ATPase in the epithelium (Shahidullah, Mandal et al. 2012, Mandal, Shahidullah et al. 2015). Consistent with TRPV4 activation having a role in the swelling response to hyposmotic solution, a study in mouse lens pointed to activation of TRPV4 by increased cellular hydrostatic pressure. Interestingly, the contrasting response of the mouse lens to hyperosmotic solution was insensitive to TRPV4 antagonists but sensitive to ruthenium red, leading to the proposal it is TRPV1-dependent. Moreover, the mouse lens was found to display a transient hydrostatic pressure response to the TRPV1 agonist capsaicin (Gao, Sun et al. 2015). These findings suggest that the lens might express functional TRPV1 channels in addition to TRPV4. Studies here were carried out to determine whether TRPV1 responses are detectable in the porcine lens.
2. Methods
2.1. Materials
A889425 was purchased from Alomone Labs (Hadassah Ein Kerem, Jerusalem BioPark, Israel). Capsaicin, BAPTA-AM and U0126 were purchased from Sigma (St. Louis, MO, USA). The following antibodies were sourced from Cell Signaling Technology (Danvers, MA, USA): mouse monoclonal anti phospho-P44/42 MAP kinase (Thr-202/Tyr-204); rabbit polyclonal anti P44/42 MAP kinase; rabbit polyclonal anti-phospho-P38 MAPK (Tyr-180/Tyr-182); rabbit polyclonal anti SAPK/JNK; mouse monoclonal anti phosphor-SAPK/JNK (Thr-183/Tyr-185). Other antibodies were: mouse monoclonal p38 (Thermo Fisher Scientific, Waltham, MA); mouse monoclonal anti-AKT (GenWay Biotechnology, San Diego, CA); rabbit polyclonal anti-phospho-AKT (Ser-473) (Santa Cruz Biotechnology, Dallas, TX ). Secondary antibodies (LI-COR Biosciences Lincoln, NE, USA) were: goat anti-rabbit conjugated with IRDye 680 and goat anti-mouse conjugated with IR Dye 800. The Micro BCA Protein Assay Kit was from Thermo Fisher Scientific. Sense and anti-sense primers for human and porcine TRPV1 were obtained from Integrated DNA Technologies, Inc. (Coralville, Iowa).
2.2. Krebs solution
Lenses were incubated at 37°C in Krebs solution, control osmolarity 300 mOsm, that was equilibrated with 5% CO2 and adjusted to pH 7.4 prior to use. Krebs solution composition was (in mM): 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 1 MgCl2, and 5.5 glucose. In specified experiments lenses were incubated in hyperosmotic Krebs solution in which osmolarity was increased to 350 mOsm by addition of mannitol. Osmolarity was checked, and adjusted is necessary, by using a 5004 Micro-Osmette osmometer (Precision Systems Inc., MA).
2.3. Lenses
Pig eyes were purchased from the Meat Science Laboratory at the University of Arizona, or from Hatfield Quality Meats (Philadelphia, PA), or from West Valley Processing (Buckeye AZ) and the use of animal tissue for research was approved by the Institutional Animal Care and Use Committee at the University of Arizona. Human donor eyes were purchased from the National Disease Research Interchange (Philadelphia, PA) and their use was approved by the Institutional Review Board at the University of Arizona. To obtain the lens, the rear of the globe was dissected open and the suspensory ligaments were cut so the lens could be removed intact and undamaged, then placed in Krebs solution. Lenses were incubated at 37°C for at least 3 h in control Krebs solution before experiments commenced. In specified experiments the capsule-epithelium was removed from the intact lens by using fine forceps to create a small tear, then peeling it from the fiber mass.
2.4.1. RNA isolation
Total RNA was isolated with an RNeasy Mini kit (Qiagen, CA, USA). Freshly isolated lens capsule-epithelium was lysed in RLT buffer containing 1% β-mercaptoethanol and homogenized using a battery operated hand-held Kimble Kontes tissue homogenizer (DWK Life Sciences, NJ). The tissue lysate was placed onto a QIAshredder column and centrifuged for 2 min at 14 000 rpm. The eluent was placed onto an RNeasy mini column in order to bind RNA. After washing, the RNA was eluted by 50 μl of RNase-free water and then quantified at 260/280nm using a spectrophotometer (NanoDrop technologies, Inc., Wilmington, DE).
2.4.2. First-Strand cDNA Synthesis and Polymerase Chain Reaction
RT-PCR was carried out as described earlier (Pelis, Shahidullah et al. 2009). Briefly, 0.5 μg total RNA was reverse transcribed to cDNA by using SuperScript III Reverse Transcriptase (Thermo Scientific, USA) following manufacturer’s protocol on an Applied Biosystem Gene Amp PCR System (Model 9700; Thermo Scientific, USA). Complementary DNA (5μl) was used for PCR reaction for gene amplification using Platinum Pfx DNA Polymerase kit (Thermo Scientific, USA) following manufacturer recommended protocol. The studies used custom-designed TRPV1 primers (Integrated DNA Technologies, Inc., IA, USA) for pig (forward: 5′-GGACAAGCTGTGGGAATCAT-3′, reverse: 5′-TGGGATTCGCTACCTTTCAG-3′) and for human (forward: 5′-AAGCCCAGGAAAACACCTTT-3′, reverse: 5′-CTGCTGCAACAGCTTGATTC-3′). We used a 2-min hold at 94°C and then 35 30sec cycles of denaturing at 94°C, 30 sec annealing at 55°C, and an extension at 72°C for 1 min. At the end of the reaction PCR product was analyzed by electrophoresis on an agarose gel (2%) containing ethidium bromide (0.2μg/ml). ϕX174 DNA Marker Hae III Digest was used as base pair standards. Signals were visualized by UV exposure employing a benchtop UV transilluminator (UVP Inc., USA). Images were captured using a high resolution camera.
2.5. Western blot
The capsule-epithelium was homogenized in ice-cold lysis buffer (pH 7.5) with the composition (in mM): 50 HEPES, 150 NaCl, 1 EDTA, 10 sodium fluoride, 10 sodium pyrophosphate, 2 sodium orthovanadate, 10% glycerol, 1% sodium deoxycholate and 1% Triton X-100 as well as a protease inhibitor cocktail (Thermos Fisher Scientific, IL) and phosphatase inhibitor 1 and 2 cocktails (EMD Millipore, MA). Using a Misonix S3000 sonicator (Misonix, New York, USA), the homogenization protocol was four cycles of 15 sec at a 6W power setting, each cycle followed by a 5 sec interval. The homogenate was placed in a centrifuge at 13,000g for 30 min at 4°C to pellet mitochondria, nuclei and debris. After determining supernatant protein content by BCA (bicinchoninic acid) assay, the supernatant was used for Western blot or frozen in liquid nitrogen and stored at −80°C for later analysis. Using standard procedures, proteins were separated on a 7.5% SDS-PAGE gel and then transferred to nitrocellulose membrane that was placed overnight in blocking buffer at 4°C (AquaBlock, East Coast Biologics Inc., ME, USA). After this, the membrane was incubated at 4°C overnight with primary antibody diluted as follows: rabbit polyclonal anti p44/42 MAP kinase at 1:1000 (ERK1/2); mouse monoclonal anti phospho-p44/42 MAP kinase (Thr-202/Tyr-204) at 1:2000 (phospho-ERK1/2); mouse monoclonal anti-p38 at 1:1000; rabbit polyclonal anti-phospho-p38 MAPK (Tyr-180/Tyr-182) at 1:1000; rabbit polyclonal anti SAPK/JNK at 1:1000; mouse monoclonal anti phospho-SAPK/JNK (Thr-183/Tyr-185) at 1:1000; mouse monoclonal anti-AKT at 1:1000); rabbit polyclonal anti-phospho-AKT (Ser-473) at 1:500. After washing the membrane (3 x 5-min) with TBST (Tris-buffered saline and Tween 200), it was immersed for 90 min (room temperature) in secondary antibody solution: goat anti-rabbit conjugated with IRDye 680 or goat anti-mouse conjugated with IR Dye 800 at 1:20,000 dilution. Then, the membrane was again washed three times with TBST and finally three times with PBS. Western blot bands were detected, recorded and quantified by using an Odyssey infrared scanner (LI-COR, Lincoln, NE). The strategy of using secondary antibodies detectable at different infra-red wavelengths (680 and 800 nm) permitted simultaneous detection of the total protein and its phosphorylated form (e.g. ERK1/2 and phospho-ERK1/2).
2.6. Statistical analysis
The data are presented as mean ± SE of results from a specified number of independent measurements, and were subjected to one way analysis of variance and the Bonferroni post hoc multiple comparison test. p <0.05 was considered significant.
3. Results
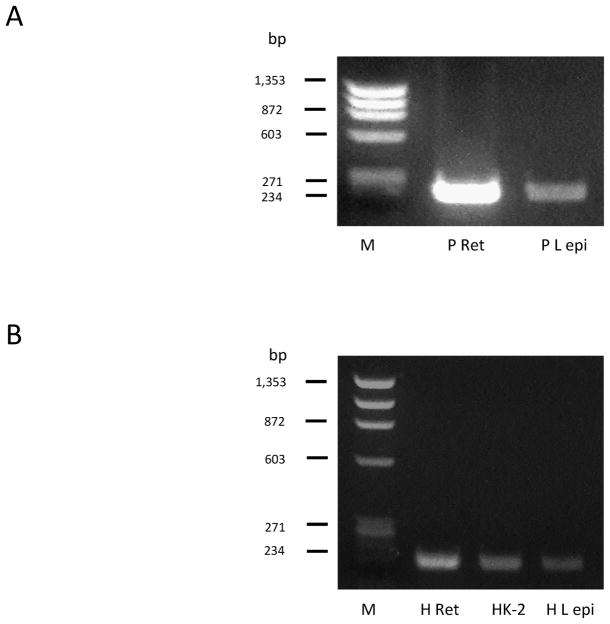
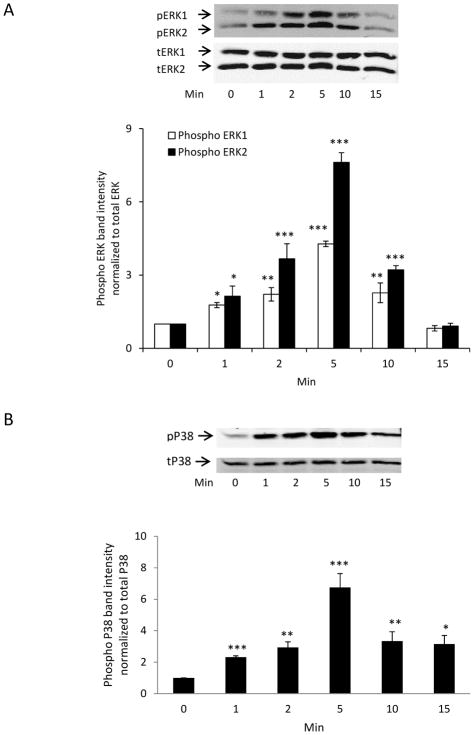
TRPV1 expression in the lens was examined by means of RT-PCR. TRPV1 mRNA was detected in porcine lens epithelium (Fig. 1A). Porcine retina was used a positive control. In the human, TRPV1 mRNA also was observed in the lens epithelium as well as in retina and kidney, which were used as positive controls (Fig. 1B). The functionality of TRPV1 was gauged by exposing intact porcine lenses to the TRPV1 agonist capsaicin then examining signaling pathway activation. Activation (phosphorylation) of two major MAPKs, namely the extracellular signal-regulated kinases (ERK1/2) (Fig. 2A) and the P38 MAPK (Fig. 2B), was observed in the epithelium isolated from lenses exposed to 100 nM capsaicin for as little as 1 min. The ERK1/2 and P38 phosphorylation responses were transient. Phosphorylation was highest after 5 min capsaicin exposure and returned to baseline by 15 min. No detectable increase in phosphorylation of either Akt (protein kinase B) (Fig. 2C) or c-Jun NH2-terminal Kinases (JNK) (Fig. 2D) was observed in the epithelium of capsaicin-treated lenses.
Figure 1.
RT-PCR detection of the TRPV1 message in porcine and human lens epithelium. Human or porcine retina and human kidney were included as positive controls. M = molecular marker; P Ret = Pig retina; P L epi = Pig lens epithelium; H Ret = Human retina; HK-2 = Human Kidney-2 cells; H L epi = Human lens epithelium.
Figure 2.
Time course on signaling pathway activation responses to capsaicin. Intact lenses were exposed to Krebs solution that contained capsaicin (100 nM) for 0–15 min, then the epithelium was removed for Western blot analysis. Panel A: ERK1/2 phosphorylation. Panel B: p38 MAPK phosphorylation. Panel C: AKT phosphorylation. Panel D: JNK phosphorylation. The bar graph in each panel shows mean ± SEM of 3 independent experiments. * (p<.05), ** (p<.01) and *** (p<.001) indicate significant differences from control.
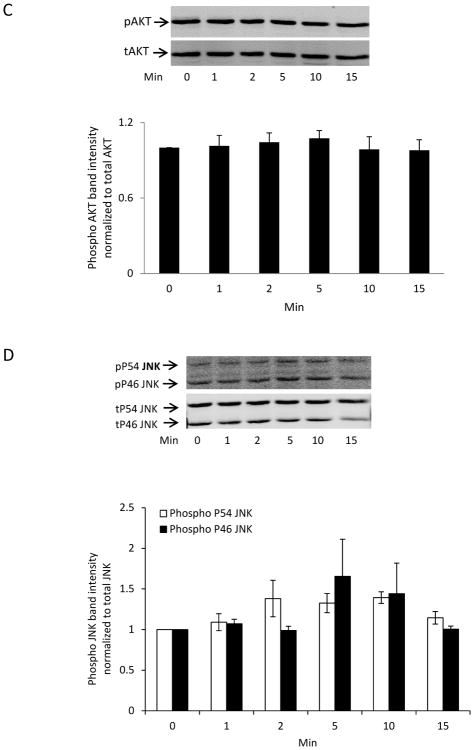
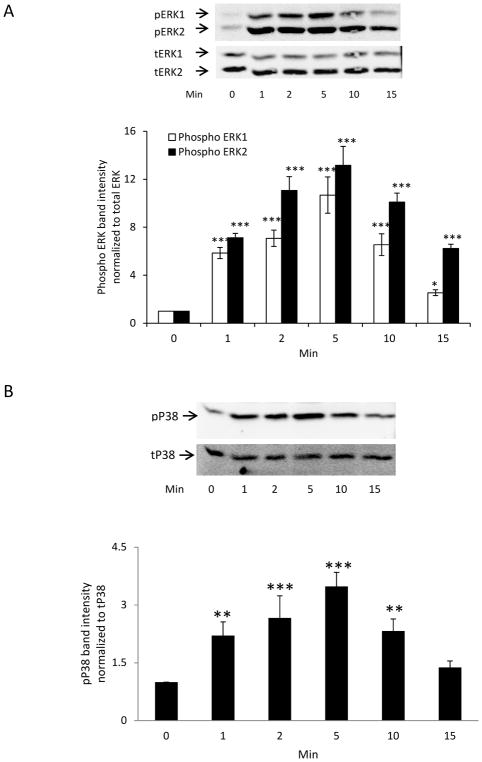
ERK1/2 and the P38 MAPK were activated in the epithelium of lenses subjected to a mild hyperosmotic challenge (Fig. 3). In lenses exposed to 350 mOsm solution (vs 300 mOsm control), the time course of the ERK1/2 and P38 MAPK phosphorylation response in the epithelium was similar to the pattern observed in capsaicin-treated lenses. In contrast, neither AKT nor JNK was activated (Supplement Fig. 1).
Figure 3.
Time course of ERK and p38 MAPK activation in the epithelium of intact porcine lenses exposed to hyperosmotic solution. Intact lenses were exposed to hyperosmotic solution (350 mOsm) for 0–15 min, then the epithelium was removed for Western blot analysis of ERK1/2 (A) or p38 (B) phosphorylation. Bar graph in each panel shows mean ± SEM of 3 independent experiments. * (p<0.05), ** (p<.01) and *** (p<.001) indicate significant differences from control.
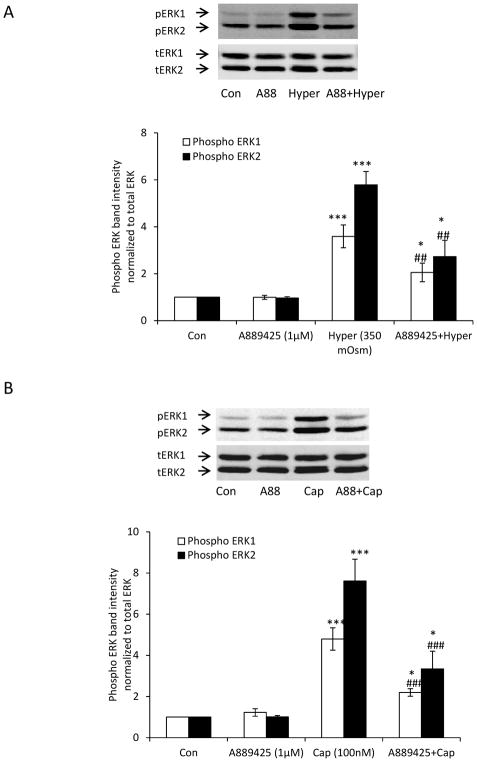
ERK1/2 activation in the epithelium was used as readout in studies to test for TRPV1 involvement in the lens response to hyperosmotic solution. When intact lenses were exposed to hyperosmotic solution (350 mOsm) for 5 min in the presence of a TRPV1 antagonist A889425 (1 μM), the magnitude of the increase in ERK1/2 phosphorylation in the epithelium was significantly reduced (Fig. 4A). The capsaicin-induced ERK1/2 phosphorylation response also was reduced by A889425 (Fig. 4B).
Figure 4.
The inhibitory influence of a TRPV1 antagonist A889425 on the ERK activation response to hyperosmotic solution and also the response to capsaicin. Intact lenses were pre-incubated for 20 min in the presence of absence of A889425 (1 μM) and then treated for 5 min with either hyperosmotic Krebs solution (350 mOsm) (Panel A) or capsaicin (100 nM) (Panel B) in the continued presence/absence of A889425. The epithelium was then removed for analysis of ERK1/2 phosphorylation. The bar graph in each panel shows mean ± SEM of 3 independent experiments. * (p<0.05), **(p<0.01) and *** (p<.001) indicate significant differences from control. ## (p<0.01) and ### (p<.001) indicate significant differences from hyperosmotic treatment. Con = Control; Hyper = Hyperosmotic Krebs solution; A88 = A889425; Cap = Capsaicin.
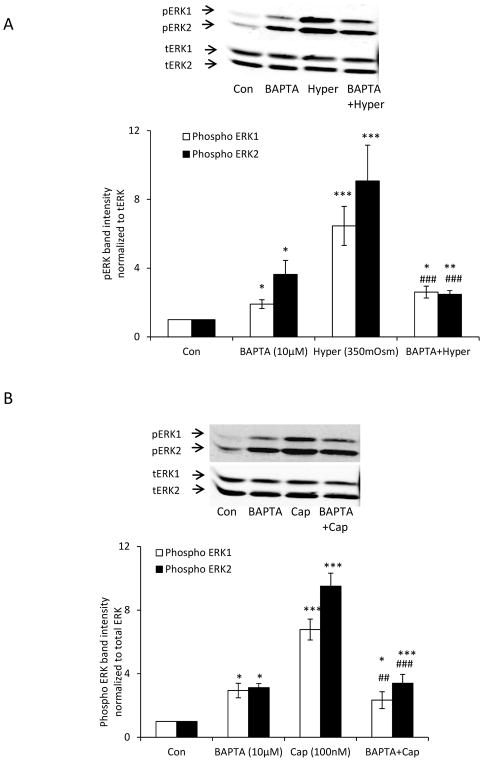
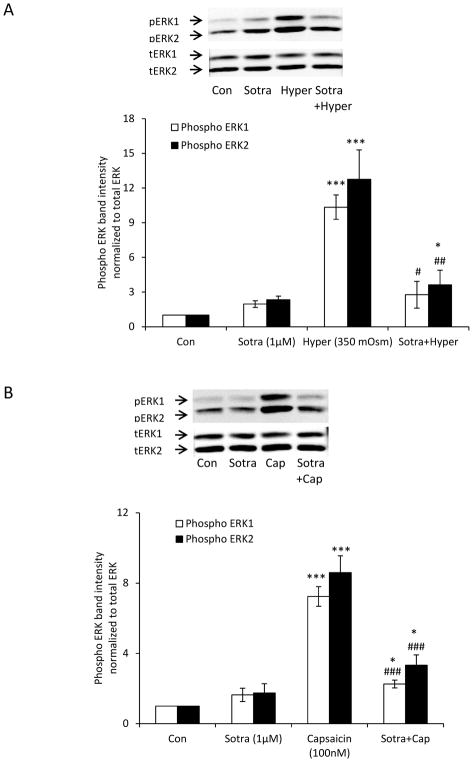
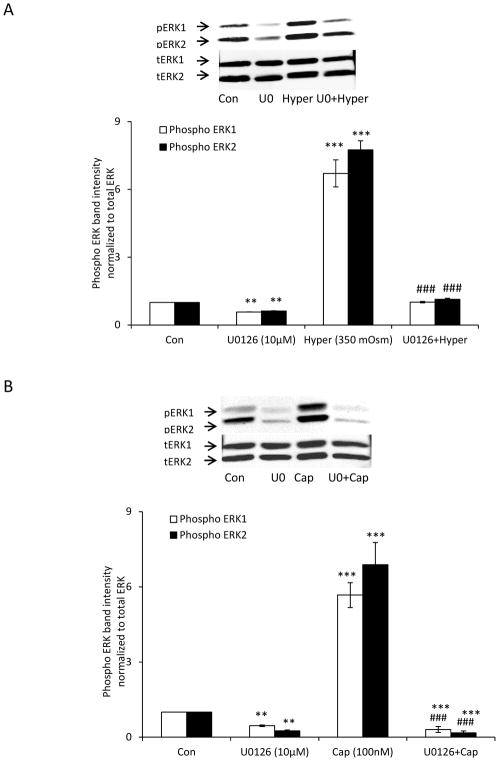
The ability of A889425 to suppress the responses in lenses exposed to capsaicin and hyperosmotic solution suggests that ERK1/2 activation is TRPV1-dependent. Reasoning that TRPV1 is a non-selective cation channel with significant permeability to calcium, we tested whether the ERK1/2 response is calcium-dependent. Intact lenses were preincubated with the BAPTA-AM, a cytosolic calcium chelator, in isosmotic Krebs solution (300 mOsm), before being exposed to hyperosmotic solution (350 mOsm). Under these conditions, the magnitude of the ERK1/2 phosphorylation response in the epithelium was markedly reduced (Fig. 5A). The ERK1/2 phosphorylation response to TRPV1 agonist capsaicin also was reduced in lenses subjected to BAPTA-AM pretreatment (Fig. 5B). It should be recognized, however, that some degree of ERK1/2 activation was observed in the epithelium of lenses subjected to BAPTA-AM treatment alone. To ascertain whether the ERK1/2 phosphorylation response is mediated by protein kinase C (PKC), we tested whether a selective pan PKC inhibitor, sotrastaurin, alters the lens responses to hyperosmotic solution and capsaicin. When intact lenses were exposed to hyperosmotic solution in the presence of sotrastaurin (1.0 μM), the increase in ERK1/2 phosphorylation in the epithelium was almost abolished (Fig. 6A). The ERK1/2 phosphorylation response to capsaicin was similarly suppressed by sotrastaurin (Fig. 6B). Consistent with expectations, the selective MEK inhibitor, U0126, completely prevented the responses to hyperosmotic solution (Fig. 7A) and capsaicin (Fig. 7B).
Figure 5.
The influence of BAPTA-AM on the ERK1/2 activation response to hyperosmotic solution and also the response to capsaicin. Intact lenses were pre-incubated for 60 min in isosmotic (300 mOsm) Krebs solution that contained BAPTA-AM (10 μM) and then exposed to hyperosmotic Krebs solution (350 mOsm) (Panel A) or capsaicin (100 nM) (Panel B) for 5 min in the continued presence/absence of BAPTA-AM. The epithelium was then isolated for analysis of ERK1/2 phosphorylation. The bar graph in each panel shows mean ± SEM of 3 independent experiments. * (p<.05), ** (p<.01) and *** (p<0.001) indicate significant differences from control. # (p<0.05), ## (p<0.01) and ### (p<0.001) indicate significant differences from hyperosmotic treatment. Con = Control; Hyper = Hyperosmotic Krebs solution; Cap = Capsaicin.
Figure 6.
The influence of the protein kinase C inhibitor sotrastaurin on the ERK1/2 activation response to hyperosmotic solution and also the response to capsaicin. Intact lenses were pre-incubated for 60 min in isosmotic (300 mOsm) Krebs solution that contained sotrastaurin (1.0 μM) and then exposed to hyperosmotic Krebs solution (350 mOsm) (Panel A) or capsaicin (100 nM) (Panel B) for 5 min in the continued presence/absence of sotrastaurin. The epithelium was then isolated for analysis of ERK1/2 phosphorylation. The bar graph in each panel shows mean ± SEM of 3 independent experiments. * (p<0.05), ** (p<.01) and *** (p<.001) indicate significant differences from control. # (p<0.05), ## (p<0.01) and ### (p<0.001) indicate significant differences from hyperosmotic solution and capsaicin treatment, respectively. Con = Control; Sotra = Sotrastaurin; Hyper = Hyperosmotic Krebs solution; Cap = Capsaicin.
Figure 7.
The influence of U0126 on the ERK1/2 activation response to hyperosmotic solution and also the response to capsaicin. Intact lenses were pre-incubated for 20 min in isosmotic (300 mOsm) Krebs solution that contained U0126 (10 μM) and then exposed to hyperosmotic Krebs solution (350 mOsm) (Panel A) or capsaicin (100 nM) (Panel B) for 5 min in the continued presence/absence of U0126. The epithelium was then isolated for analysis of ERK1/2 phosphorylation. The bar graph in each panel shows mean ± SEM of 3 independent experiments. ** (p<.01), and *** (p<.001) indicate significant differences from control. ### (p<0.001) indicates significant difference from capsaicin or hyperosmotic treatment. Con = Control; Hyper = Hyperosmotic Krebs solution; U0 = U0126; Cap = Capsaicin.
4. Discussion
Members of the TRPV, vanilloid, family of cation channels are found in a broad assortment of tissues. In the lens, TRPV4 attracted attention because the channels are activated by hyposmotic stimuli. Subjecting the lens to hyposmotic solutions causes a number of functional responses that are qualitatively similar to responses elicited by TRPV4 agonists. Moreover, the hyposmotic responses are prevented by TRPV4 antagonists (Shahidullah, Mandal et al. 2012). In mouse lens, a capsaicin response, in the form of a transient change of intracellular hydrostatic pressure, provided a clue pointing to the possible functional significance a different TRPV channel subtype, TRPV1. Interestingly, TRPV1 and TRPV4 hydrostatic pressure responses were opposite. TRPV1 appeared to have a role in the mouse lens shrinkage response to hyperosmotic solution while TRPV4 was linked to the swelling response (Gao, Sun et al. 2015). In our present study we confirmed TRPV1 mRNA expression in the epithelium of porcine lens as well as human lens by using an RT-PCR approach. This aligns with earlier reports that used antibodies to detect TRPV1 protein expression by Western blot analysis (Delamere, Mandal et al. 2016) and immunohistochemistry (Martinez-Garcia, Martinez et al. 2013). In rabbit as well as in human, immunolocalization showed TRPV1 restricted to the lens epithelium. That study showed TRPV1 distributed rather diffusely in the lens epithelial cells, causing us to wonder about its functional role as an ion channel in the plasma membrane. Here, our goal was to demonstrate functionality of TRPV1 in the lens, which we did by measuring signaling responses to a relatively low concentration of capsaicin, a TRPV1 agonist, and confirming inhibition of the response by A889425, a selective TRPV1 antagonist.
Intact lenses exposed to the TRPV1 agonist capsaicin for as little as 1 min displayed a signaling response in the epithelium. Both ERK1/2 and P38 MAPK were transiently activated as evidenced by increased phosphorylation. In contrast, AKT and JNK activation was not detected in capsaicin-treated lenses. Capsaicin-dependent responses have previously been observed in pain sensing neuronal pathways and attributed at least in part to TRPV1 activation (Chen, Willcockson et al. 2009). Involvement of MAPK pathways also have been reported in neuropathic pain (Lin, Hsieh et al. 2015, Qu, Jia et al. 2016). The absence of AKT activation in porcine lens by capsaicin was unexpected because several lines of evidence link it to the AKT pathway in mouse lens (Sellitto, Li et al. 2013, Gao, Sun et al. 2015). This could reflect species differences.
Although TRPV1 is classed as a non-selective cation channel, it is frequently the case that the functional impact of channel opening results from calcium entry into the cytoplasm from outside the cell. This aligns with the observation that lenses pre-treated with the cytosolic Ca2+ chelator BAPTA-AM had a diminished ERK1/2 activation response to capsaicin. We also examined PKC signaling because conventional PKC (cPKC) activation is calcium-dependent. The ability of the pan PKC inhibitor sotrastaurin to blunt the response also fits the notion that capsaicin-stimulated ERK1/2 activation follows calcium entry via TRPV1 channels. Taken together the findings support the notion that TRPV1 functions as a plasma membrane ion channel that, when activated, permits the entry of extracellular calcium into the lens epithelium, leading to PKC activation and then PKC-dependent ERK1/2 activation. In the renal medullary thick ascending limb, hyperosmotic stress also has been reported to increase ERK1/2 and p38 MAP kinase activity but had no effect on JNK activity (Watts, Mari et al. 1998). Hyperosmotic solution-induced increased in the activity of PKC-delta and ERK1/2 also has previously been shown in human tracheal epithelium (Liedtke and Cole 2002). Similar to the present findings in the intact porcine lens, in NIH/3T3 cells it was shown that hyperosmotic induced PKC activation is required for ERK1/2 activation (Zhuang, Hirai et al. 2000).
While capsaicin is a selective TRPV1 activator, the channels in various tissues also are reported to open in response to a range of perturbations including temperature, pH and mechanical and hyperosmotic stimuli (Caterina, Schumacher et al. 1997, Nishihara, Hiyama et al. 2011, Cao, Cordero-Morales et al. 2013). Here we show that the ERK1/2 and p38 signaling response in the epithelium of lenses treated with hyperosmotic solution had similar time course to the capsaicin response and was inhibited by the TRPV1 antagonist A88924. When we focused on ERK1/2 activation in the epithelium, it was apparent that the calcium chelator BAPTA-AM and the PKC inhibitor sotrastaurin had similar inhibitory effects on the lens response to hyperosmotic solution and the response to TRPV1 agonist capsaicin. In other words, the ERK1/2 signaling response in lenses subjected to hyperosmotic stimulus was consistent with TRPV1 channel activation.
5. Conclusion
The functional contribution of TRPV1 is evident from ERK1/2 and p38 MAPK activation in the epithelium of lenses exposed to capsaicin, a selective TRPV1 agonist. Moreover, the findings suggest TRPV1 activation is critical for the ERK1/2 and p38 MAPK activation that occurs in the epithelium of lenses exposed to hyperosmotic solution. The significance of the study lies in the confirmation of earlier proposals that hyperosmotic stress is linked to TRPV1 channel activation in the lens epithelium (Gao, Sun et al. 2015) and this, in turn is linked to multiple downstream signaling pathways, including PKC, ERK and p38 MAPK. Further studies are ongoing to determine what functional changes are triggered by the signaling pathways and how they relate to lens volume homeostasis.
Supplementary Material
AKT and JNK phosphorylation observed in the epithelium of intact porcine lenses exposed to hyperosmotic solution (350 mOsm). Intact lenses were exposed to hyperosmotic solution (350 mOsm) for 0–15 min, then the epithelium was removed for analysis of (A) AKT and (B) JNK phosphorylation. The bar graph shows mean ± SEM of 3 independent experiments.
Highlights.
TRPV1 is expressed in lens epithelium.
Exposing the lens to TRPV1 agonist capsaicin activates ERK1/2 and p38 in the epithelium.
Capsaicin and hyperosmotic solution activate a similar signaling responses in the lens.
A TRPV1 antagonist suppresses the signaling responses to a hyperosmotic stimulus.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (Grant number EY006915). The funding authority had no role in experimental design and interpretation of data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassnett S, Kuszak JR, Reinisch L, Brown HG, Beebe DC. Intercellular communication between epithelial and fiber cells of the eye lens. Journal of Cell Science. 1994;107(4):799. doi: 10.1242/jcs.107.4.799. [DOI] [PubMed] [Google Scholar]
- Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77(4):667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen Y, Willcockson HH, Valtschanoff JG. Influence of the vanilloid receptor TRPV1 on the activation of spinal cord glia in mouse models of pain. Experimental neurology. 2009;220(2):383–390. doi: 10.1016/j.expneurol.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamere NA, Dean WL. Distribution of lens sodium-potassium-adenosine triphosphatase. Invest Ophthalmol Vis Sci. 1993;34(7):2159–2163. [PubMed] [Google Scholar]
- Delamere NA, Mandal A, Shahidullah M. The Significance of TRPV4 Channels and Hemichannels in the Lens and Ciliary Epithelium. J Ocul Pharmacol Ther. 2016;11:11. doi: 10.1089/jop.2016.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Sun X, White TW, Delamere NA, Mathias RT. Feedback Regulation of Intracellular Hydrostatic Pressure in Surface Cells of the Lens. Biophys J. 2015;109(9):1830–1839. doi: 10.1016/j.bpj.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Sun X, Yatsula V, Wymore RS, Mathias RT. Isoform-specific function and distribution of Na/K pumps in the frog lens epithelium. Journal of Membrane Biology. 2000;178(2):89–101. doi: 10.1007/s002320010017. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Dick JS, Lyons JE. Lens metabolic cooperation: A study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. The Journal of Cell Biology. 1980;86(2):576–589. doi: 10.1083/jcb.86.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszak JR, Novak LA, Brown HG. An ultrastructural analysis of the epithelial-fiber interface (EFI) in primate lenses. Experimental Eye Research. 1995;61(5):579–597. doi: 10.1016/s0014-4835(05)80052-1. [DOI] [PubMed] [Google Scholar]
- Liedtke CM, Cole TS. Activation of NKCC1 by hyperosmotic stress in human tracheal epithelial cells involves PKC-delta and ERK. Biochim Biophys Acta. 2002;13(1):77–88. doi: 10.1016/s0167-4889(01)00189-6. [DOI] [PubMed] [Google Scholar]
- Lin JG, Hsieh CL, Lin YW. Analgesic Effect of Electroacupuncture in a Mouse Fibromyalgia Model: Roles of TRPV1, TRPV4, and pERK. PLoS ONE. 2015;10(6):e0128037. doi: 10.1371/journal.pone.0128037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WK, Harding CV. Structure and distribution of gap junctions in lens epithelium and fiber cells. Cell and Tissue Research. 1986;244(2):253–263. doi: 10.1007/BF00219200. [DOI] [PubMed] [Google Scholar]
- Mandal A, Shahidullah M, Delamere NA. Calcium entry via connexin hemichannels in lens epithelium. Experimental Eye Research. 2015;132(0):52–58. doi: 10.1016/j.exer.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia MC, Martinez T, Paneda C, Gallego P, Jimenez AI, Merayo J. Differential expression and localization of transient receptor potential vanilloid 1 in rabbit and human eyes. Histol Histopathol. 2013;28(11):1507–1516. doi: 10.14670/HH-28.1507. [DOI] [PubMed] [Google Scholar]
- Mathias RT, White TW, Gong X. Lens Gap Junctions in Growth, Differentiation, and Homeostasis. Physiological reviews. 2010;90(1):179–206. doi: 10.1152/physrev.00034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara E, Hiyama TY, Noda M. Osmosensitivity of Transient Receptor Potential Vanilloid 1 Is Synergistically Enhanced by Distinct Activating Stimuli Such as Temperature and Protons. PLoS ONE. 2011;6(7):e22246. doi: 10.1371/journal.pone.0022246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelis RM, Shahidullah M, Ghosh S, Coca-Prados M, Wright SH, Delamere NA. Localization of Multidrug Resistance-Associated Protein 2 in the Nonpigmented Ciliary Epithelium of the Eye. Journal of Pharmacology and Experimental Therapeutics. 2009;329(2):479–485. doi: 10.1124/jpet.108.149625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y-J, Jia L, Zhang X, Wei H, Yue S-W. MAPK Pathways Are Involved in Neuropathic Pain in Rats with Chronic Compression of the Dorsal Root Ganglion. Evidence-based Complementary and Alternative Medicine : eCAM. 2016;2016:6153215. doi: 10.1155/2016/6153215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae JL, Kuszak JR. The electrical coupling of epithelium and fibers in the frog lens. Experimental Eye Research. 1983;36(3):317–326. doi: 10.1016/0014-4835(83)90114-8. [DOI] [PubMed] [Google Scholar]
- Sellitto C, Li L, Gao J, Robinson ML, Lin RZ, Mathias RT, White TW. AKT activation promotes PTEN hamartoma tumor syndrome–associated cataract development. The Journal of Clinical Investigation. 2013;123(12):5401–5409. doi: 10.1172/JCI70437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. American Journal of Physiology - Cell Physiology. 2012;302(12):C1751–1761. doi: 10.1152/ajpcell.00010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA. Damage to lens fiber cells causes TRPV4-dependent Src family kinase activation in the epithelium. Experimental Eye Research. 2015;140:85–93. doi: 10.1016/j.exer.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya S, Dean WL, Paterson CA, Delamere NA. Regional distribution of Na,K-ATPase activity in porcine lens epithelium. Invest Ophthalmol Vis Sci. 2003;44(10):4395–4399. doi: 10.1167/iovs.03-0287. [DOI] [PubMed] [Google Scholar]
- Watts BA, Mari JF, Davis RJ, Good DW. Hypertonicity activates MAP kinases and inhibits HCO-3 absorption via distinct pathways in thick ascending limb. Am J Physiol. 1998;275(4):F478–486. doi: 10.1152/ajprenal.1998.275.4.F478. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Hirai SI, Ohno S. Hyperosmolality induces activation of cPKC and nPKC, a requirement for ERK1/2 activation in NIH/3T3 cells. Am J Physiol Cell Physiol. 2000;278(1):C102–109. doi: 10.1152/ajpcell.2000.278.1.C102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AKT and JNK phosphorylation observed in the epithelium of intact porcine lenses exposed to hyperosmotic solution (350 mOsm). Intact lenses were exposed to hyperosmotic solution (350 mOsm) for 0–15 min, then the epithelium was removed for analysis of (A) AKT and (B) JNK phosphorylation. The bar graph shows mean ± SEM of 3 independent experiments.