Abstract
This study was performed to determine whether cells in the posterior stroma undergo apoptosis in response to endothelial cell injury and to determine whether basement membrane component nidogen-1 was present in the cornea. New Zealand White rabbits had an olive tip cannula inserted into the anterior chamber to mechanically injure corneal endothelial cells over an 8mm diameter area of central cornea with minimal injury to Descemet’s membrane. At one hour (6 rabbits) and four hours (6 rabbits) after injury, three corneas at each time point were cryopreserved in OCT for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and immunohistochemistry (IHC) for vimentin and nidogen-1, and three corneas at each time point were fixed for transmission electron microscopy (TEM). Uninjured corneas were controls. Stromal cells over approximately the posterior 25% of the stroma overlying to the site of corneal endothelial injury underwent apoptosis detected by the TUNEL assay. Many of these apoptotic cells were vimentin+, suggesting they were likely keratocytes or corneal fibroblasts. Stromal cells peripheral to the site of endothelial injury and more anterior stromal cells overlying the site of endothelial injury did not undergo apoptosis. Stromal cell death was confirmed to be apoptosis by TEM. No apoptosis of stromal cells was detected in control, uninjured corneas. Nidogen-1 was detected in the stroma of unwounded corneas, with higher nidogen-1 in the posterior stroma than the anterior stroma. After endothelial scrape injury, concentrations of nidogen-1 appeared to be in the extracellular matrix of the posterior stroma and, possibly, within apoptotic bodies of stromal cells. Thus, posterior stromal cells, likely including keratocytes, undergo apoptosis in response to corneal endothelial injury, analogous to anterior keratocytes undergoing apoptosis in response to epithelial injury.
Keywords: stroma, keratocytes, corneal fibroblasts, apoptosis, corneal endothelial injury, nidogen-1, cornea wound healing, endotheliitis, viral defense mechanisms
1. Introduction
Studies performed over twenty years ago demonstrated that anterior stromal keratocytes undergo apoptosis in response to corneal epithelial scrape injury (Wilson, et al., 1996) and thereby initiate the subsequent complex stromal wound healing response. This finding had an enormous impact on research on the corneal wound healing response and stromal changes that ensue following injuries, surgeries, infections and diseases of the cornea that include damage to the corneal epithelium (Wilson and Kim, 1998; Wilson, et al., 2001; Ljubimov and Saghizadeh, 2015; Torricelli, et al., 2016). Subsequent studies demonstrated this is likely a system that evolved as an immediate cellular response to retard the extension of viral infections, such as herpes simplex virus keratitis, to the stroma and into the eye prior to mobilization of the immune system to fight the threatening infection (Wilson, et al., 1997). Subsequent work also demonstrated this programmed cell death is likely modulated by the Fas/Fas ligand system triggered by interleukin (IL)-1, and possibly other cytokines, released from the injured corneal epithelium (Inoue, 2014).
In recent studies that included injury to the corneal endothelium (Wilson SE, unpublished data, 2015), a decrease in stromal cell density was noted in the posterior stroma in corneas stained with DAPI (4′,6-diamidino-2-phenylindole). The present study was initiated to determine if the endothelial injury triggered posterior stromal cell apoptosis, analogous to anterior keratocyte apoptosis after mechanical epithelial injury, and whether posterior stromal cells produce and up-regulate a basement membrane component, nidogen-1, after endothelial injury—analogous to increases in stromal cell basement membrane components in anterior stromal cells after epithelial injury in rabbits (Santhanam, et al., 2017) and humans (Torricelli, et al., 2015).
2. Materials and methods
2.1 Animals
The Institutional Animal Care and Use Committees (IACUC)at the Cleveland Clinic, Cleveland, OH, USA approved this study. All animals were treated in accordance with the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Twelve, female New Zealand white rabbits, 12 to 15 weeksold, weighing 2.5 to 3.0 kg each, were included in this study. The animals were placed under general anesthesia with 30mg/kg ketamine hydrochloride and xylazine 5mg/kg by IM injection. In addition, topical proparacaine hydrochloride 1% (Alcon, Ft Worth, TX, USA) was applied to each eye prior the surgery. Euthanasia was performed using an intravenous injection of 100 mg/kg Beuthanasia( Shering-Plough, Kenilworth, NJ) while the animals were under general anesthesia.
2.2 Mechanical endothelial injury
After general and topical anesthesia, a lid speculum was placed into one eye and an 8mm diameter circle concentric with the limbus was marked on the epithelial surface with gentian violet. A limbal entry incision was created with a 5mm blade (Bausch & Lomb, Rochester, NY, USA) and 0.3 ml Healon OVD (Abbott Medical Optics Inc. Santa Ana, CA, USA) was injected to maintain the anterior chamber. Subsequently, a smooth olive tip cannula (Beaver-Visitec, Becton-Dickinson, Franklin Lake, NJ, USA) was inserted into the anterior chamber and used to gently damage the endothelium by systematically moving the cannula back and forth across the posterior surface of the cornea underlying the previously marked 8 mm diameter area. The remaining Healon OVD was removed with Simcoe irrigation and aspiration cannula (Bausch & Lomb, Storz, Rochester, NY, USA) and exchanged for balanced salt solution (BSS). The incision site was hydrated to prevent leakage from the anterior chamber. The opposite eye was included as an unwounded control and had a limbal incision, olive tip cannula insertion, Healon OVD injection and exchange for BSS, but no endothelial injury.
2.3 TUNEL assay and immunohistochemistry
Six rabbits were examined at each time point—one hour and four hours after endothelial injury. These time points following injury were selected based on the apoptosis response after injury to the epithelium that were found to be optimal in previous studies (Wilson, et al., 1996; Helena, et al., 1998).
After euthanasia, the corneo-scleral rims of the wounded and unwounded eyes were removed with 0.12 forceps and sharp Westcott scissors (Fairfield, CT, USA) without touching the cornea. Three corneo-scleral rims were included at each time point for the terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay and immunohistochemistry (IHC) for nidogen-1 or vimentin. These corneas were embedded in liquid OCT compound (Sakura Finetek, Torrance, CA, USA) within 24 × 24 × 5 mm mold (Fisher Scientific, Pittsburgh, PA, USA). Tissue specimens were placed in the center of the mold so that block could be bisected and transverse sections cut from the central cornea within the previous endothelial injury.
The cornea-scleral rim within the mold was rapidly frozen on dry ice and the blocks were stored at −80°C until sectioned. Central corneal sections (7μm) were cut with a cryostat (HM 505M; Micron GmbH, Walldorf, Germany) using 25mm × 75mm × 1mm Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA, USA). Slides were maintained at −80°C until the TUNEL assay or IHC for nidogen-1 or vimentin.
To detect DNA fragmentation associated with apoptosis, tissue sections were fixed in acetone at −20°C for 2 min and dried at room temperature for 5 min. Slides were then placed in BSS and a fluorescent-based TUNEL assay was performed according to manufacturer’s protocol (ApopTag, Cat No S7165; Millipore Sigma, St. Louis, MO) as previously described (Helena, et al., 1998).
IHC for nidogen-1 or vimentin was performed as previously described (Netto, et al., 2006). The primary antibody for nidogen-1 was goat anti-human nidogen-1/entactin antigen affinity-purified polyclonal antibody (catalog #AF 2570, R&D Systems, Minneapolis, MN, USA). The primary antibody for vimentin was mouse anti-vimentin antibody M7020 (Dako, Carpinteria, CA, USA). The slides were washed three times with phosphate buffered saline (PBS) and incubated for 1 hour at room temperature with the anti-nidogen-1 primary antibody at 1:50 dilution in 1% bovine serum albumin (BSA) or anti-vimentin primary antibody at 1:100 dilution in 1% bovine serum albumin (BSA). Slides were then incubated for 1 hour with the secondary antibody: for nidogen-1 Alexa 488 donkey anti-goat IgG (Invitrogen, Carlsbad, CA, USA) was used at a dilution of 1:250 at room temperature and for vimentin Alexa 488 donkey anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) was used at a dilution of 1:200 at room temperature. When the TUNEL assay was combined with IHC, the TUNEL assay was performed first and the slides rinsed in PBS before performing IHC.
Positive controls (−9.0D photorefractive keratectomy corneas) at one day after surgery, where cells in the anterior stroma undergo apoptosis were included in each TUNEL assay. Negative controls of wounded and unwounded corneas were included in each assay without primary antibody and without terminal deoxynucleotidyl transferase.
Tissue sections were analyzed and photographed with a Leica DM5000 microscope (Leica, Buffalo Grove, IL, USA) equipped with a Q-image Retiga 4000RV (Surrey, BC, Canada) camera and ImagePro software (Leica). TUNEL cell counts per 200X field were performed by placing the field so it was bisected by the posterior surface of the cornea. Distances were measured with an eyepiece reticle in the microscope.
2.4 Transmission electron microscopy
TEM samples were prepared as previously described (Fantes, et al., 1990). Briefly, the excised block of central corneas was rinsed with 0.2M cacodylate buffer three times for 5 min each, post-fixed in 1 % osmium tetroxide for 60 min at 4°C, and dehydrated in increasing concentrations of ethanol from 30% to 95% for 5 min each at 4°C. Finally, dehydration was performed using three 10 min rinses in 100% ethanol and three 15 min rinses with propylene oxide at room temperature. Specimens were then embedded in epoxy resin medium. One-micrometer-thick sections were stained with toluidine blue for a light microscope. Ultrathin 85nm thick sections were cut with a diamond knife and stained with 5% uranyl acetate and lead citrate. Sections were analyzed and photographed using a Philips CM12 transmission electron microscope (TEM) operated at 60 kV (FEI, Hillsboro, OR, USA).
3. Results
Nidogen-1 IHC in unwounded corneas (Fig. 1A) showed, as expected, nidogen-1 in the epithelial basement membrane and Descemet’s basement membrane. In addition, nidogen-1 was detected in the stroma, and there was lower nidogen-1 in the anterior stroma compared to the posterior stroma. At higher magnification (Fig. 1B), the endothelium and the posterior stroma are better seen. In the posterior stroma nidogen-1 may be present in both stromal cells and the surrounding extracellular matrix, but no definite conclusion can be made from the results of this study on stromal cell production.
Fig. 1.
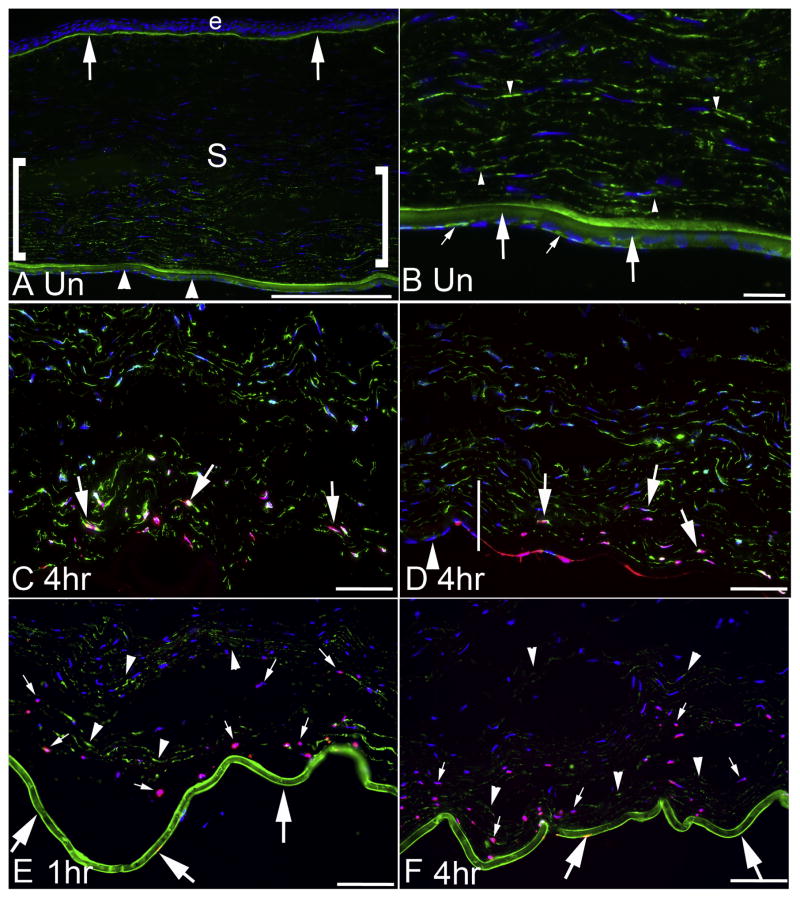
IHC for nidogen-1, IHC for vimentin and TUNEL assay in unwounded corneas and corneas at one hour and four hours after mechanical endothelial injury. A. In an unwounded cornea, nidogen-1 (green) is detected in the epithelial basement membrane (arrows) and Descemet’s basement membrane (arrowheads). Nidogen-1 is also present in the stroma (S), and at higher levels in the posterior stroma (area within the brackets). The bar is 175 μm. B. A higher magnification view of the posterior cornea of an unwounded cornea after IHC for nidogen-1 (green) shows more clearly the staining in Descemet’s basement membrane (large arrows) that is most highly concentrated in the anterior Descemet’s basement membrane—that may correspond to the interfacial matrix zone and/or the anterior banded layer of Descemet’s basement membrane. Also, nidogen-1 is present in corneal endothelial cells (small arrows). At this higher magnification, the nidogen-1 is noted in the posterior stroma and may be present in stromal cells (arrowheads). The bar is 20 μm. C. Vimentin IHC (green) and TUNEL assay (pink) in the central cornea at 4 hours after endothelial injury. At least some TUNEL+ stromal cells (arrows) in the posterior stroma also express vimentin, suggesting they are keratocytes or corneal fibroblasts. DAPI stained nuclei of cells, many of which are also vimentin+, reveal more anterior stromal cells that are not undergoing apoptosis. D. Vimentin IHC (green) and TUNEL assay (pink) in the peripheral cornea at four hours after endothelial injury. The vertical line indicates the approximate transition between the central zone of mechanical endothelial injury (on the right) and the peripheral zone of uninjured endothelium (on the left). The arrowhead indicates intact endothelial cells in the periphery. Posterior stromal cells were detected undergoing apoptosis and some of these cells express vimentin (arrows). DAPI stained nuclei reveal more anterior stromal cells within the injured zone and stromal cells overlying the intact endothelial cells in the peripheral cornea that are not undergoing apoptosis. E and F. Corneas that underwent duplex TUNEL assay (pink) and nidogen-1 IHC (green) showed heavy nidogen-1 staining in Descemet’s basement membrane (large arrows) at both one hour and four hours, respectively, after mechanical endothelial injury. A posterior band of apoptotic stromal cells (small arrows in D and E) was noted in all corneas at one hour and four hours after endothelial injury that extended approximately 100 to 200 μm anterior to the posterior corneal surface, depending on the individual cornea and the injured site examined in an individual cornea. Nidogen-1 was also detected (arrowheads) in extracellular matrix and/or stromal cells that were not undergoing apoptosis. Blue stain is DAPI in all panels. The bars in C, D, E and F are 50 μm. Note there is more edema in the stroma after endothelial injury in panels C, D, E and F. No primary antibody controls for each panel are shown in Supplement Fig. 1.
When TUNEL assay was performed with IHC for vimentin at one hour (not shown) and 4 hrs (Fig. 1C and 1D) after endothelial injury nearly 100% of stromal cells in the posterior 25% of the stroma were undergoing apoptosis in each cornea evaluated (Table). Many cells undergoing apoptosis were vimentin+, suggesting they were keratocytes or corneal fibroblasts (Fig 1C and 1D). The area of overlying stromal cell apoptosis corresponded with the area of endothelial mechanical injury (Fig. 1D). Thus, stromal cells peripheral to the endothelial injury did not undergo apoptosis.
Table.
| Group 1 | hr TUNEL+ cells/200X field* | 1 hr Extension of TUNEL+ cells from posterior corneal surface (μm)† | 1 hr Total Thickness of cornea (μm) | 4 hr TUNEL+ cells/200X field* | 4 hr Extension of TUNEL+ cells from posterior corneal surface (μm)† | 4 hr Total Thickness of cornea (μm) |
|---|---|---|---|---|---|---|
| Unwounded central control cornea | 0 ± 0 | — | 439 ± 20 | 0 ± 0 | — | 439 ± 20 |
| Wounded central cornea | 132 ± 25 | 222 ± 4 | 833 ± 27 | 124 ± 16 | 218 ± 5 | 900 ± 20 |
| Wounded peripheral cornea with intact endothelium | 0 ± 0 | — | 542 ± 8 | 0 ± 0 | — | 554 ± 6 |
Values represent means ± standard deviations from the three corneas used for the TUNEL assay at each time point.
Columns represent the number of TUNEL+ cells per 200× field bisected by the posterior corneal surface.
Columns are the distance of the most anterior apoptotic cell from the Descemet’s membrane in wounded corneas. Statistical comparison between the groups was performed using a two-way ANOVA test. The wounded central corneas at 1 hour and 4 hours had significantly more TUNEL+ stromal cells per 200X field than unwounded control corneas (p<0.001) or the peripheral cornea with intact endothelial cells in corneas with a central 8mm endothelial injury (p<0.001). No TUNEL+ cells were observed in the unwounded control corneas or in the periphery where the endothelium was intact in corneas with central endothelial injuries. Distance measurements were made on corneal sections at the microscope and are limited by the tissue sectioning and swelling of the stroma in the central 8mm area where the endothelium was injured.
Combined TUNEL and nidogen-1 staining showed heavy nidogen-1 in Descemet’s basement membrane at one hour after mechanical endothelial injury (Fig. 1E). Nidogen-1 continued to be detectible in the stroma overlying the site of endothelial injury and a portion of the nidogen-1 appeared to possibly be in the apoptotic bodies derived from stromal cells undergoing apoptosis. At four hours after endothelial injury (Fig. 1F), combined TUNEL assay and nidogen-1 IHC showed posterior stromal cells undergoing apoptosis anterior to Descemet’s basement membrane. Nidogen-1 was present in the posterior stroma.
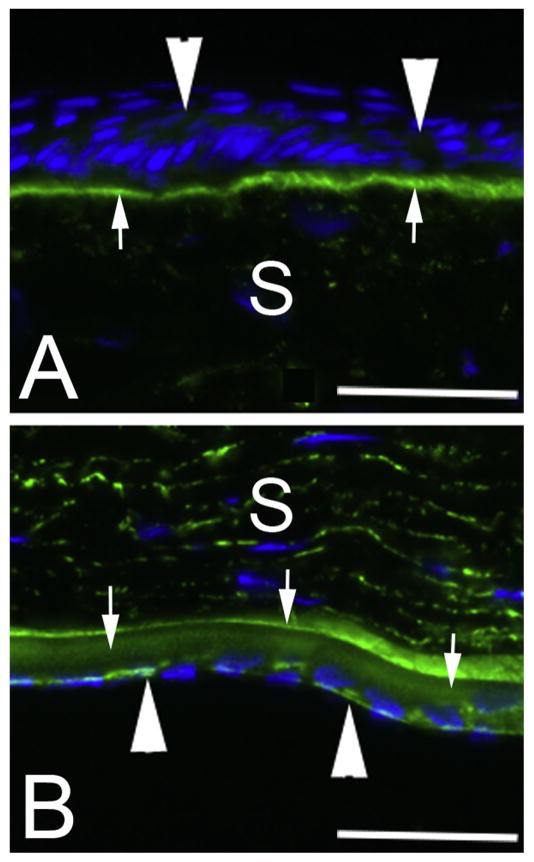
Higher magnification images of nidogen-1 IHC in unwounded corneas shows relatively low expression of nidogen-1 in the uninjured corneal epithelium (Fig. 2A) despite the epithelial basement membrane staining heavily for the basement membrane component. Epithelial nidogen-1 expression did not increase after endothelial injury (not shown). Nidogen-1 is also present at low levels in the anterior stroma but it is not possible to determine conclusively whether this nidogen-1 is in extracellular matrix, stromal cells, or both. Nidogen-1 is expressed at high levels in uninjured corneal endothelial cells (Fig. 2B). Nidogen-1 is also detected throughout Descemet’s basement membrane but is especially concentrated anteriorly in what may correspond the anterior banded layer of Descemet’s membrane. Nidogen-1 was present at higher levels in the posterior stroma of the uninjured corneas (Fig. 1A and Fig. 2B).
Fig. 2.
Nidogen-1 IHC in unwounded rabbit corneas at higher magnification. Nidogen-1 is detected in epithelial cells (arrowheads in A). It is also detected in endothelial cells (arrowheads in B). Nidogen-1 is present at lower levels in the anterior stroma (A) than in the posterior stroma (B). S is stroma. Nidogen-1 is present at high levels in the epithelial basement membrane (A, arrows) and Descemet’s basement membrane (B arrows) with larger amounts in the anterior Descemet’s basement membrane, possibly corresponding to the anterior banded layer. The magnification bars are 40 μm. Negative controls of wounded and unwounded corneas without primary antibody and without terminal deoxynucleotidyl transferase are shown in Supplement Fig. 2.
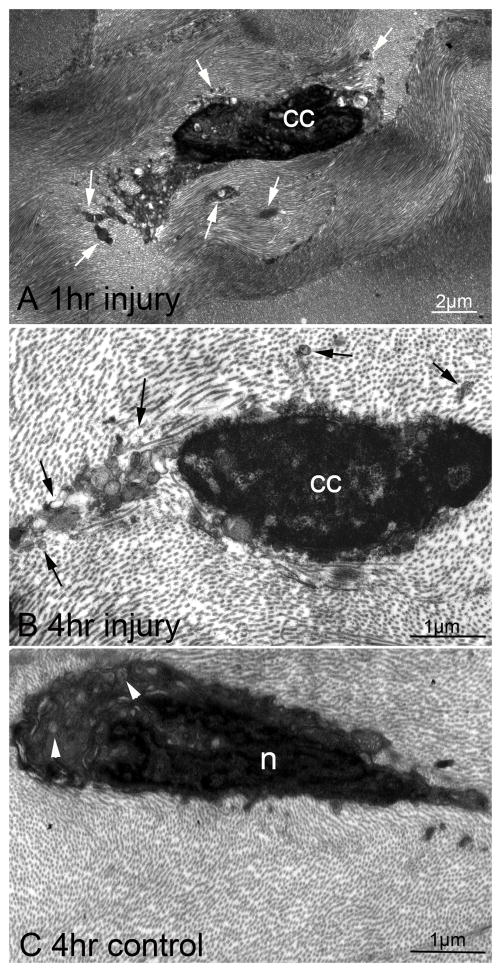
TEM of central corneal posterior stromal cells at 1 hour (Fig. 3A) or 4 hours (Fig. 3B) after mechanical endothelial injury showed posterior stromal cells with ultrastructural changes of apoptosis, including chromatin condensation and the formation of apoptotic bodies containing cytoplasmic contents of the cells. Only normal stromal cells without signs of apoptosis were detected in control corneas that did not have mechanical endothelial injury (Fig. 3C).
Fig. 3.
Transmission electron microscopy of posterior stromal cells in corneas with mechanical endothelial injury and control unwounded corneas. A. At one hour after mechanical endothelial injury, an overlying posterior stromal cell showed the morphologic changes of apoptosis, including chromatin condensation (cc) and the formation of apoptotic bodies (arrows) that contained cytoplasm and organelles from the dying cells. These apoptotic bodies dispersed into the surrounding stromal lamellae (mag. 22,000X). These TEM morphologic changes characteristic of apoptosis were noted in most stromal cells in the posterior 10% to 20% of the corneas that had endothelial injury (not shown). The apoptotic cells could include keratocytes, resident immune cells, resident mesenchymal stem cells, non-myelinating Schwann cells, myelinating Schwann cells, and even bone marrow-derived cells. B. Similarly, at four hours after mechanical endothelial injury, chromatin condensation (cc) and formation of apoptotic bodies (arrows) was noted in a posterior stromal cell (mag 41,000X). These ultrastructural changes were noted in most posterior stromal cells extending 10 to 20% depth anterior to the posterior stromal surface in all injured corneas (not shown). C. In a control cornea without mechanical endothelial injury, a posterior stromal cell had normal ultrastructure with the heterogeneous density of the nucleus (n) and the presence of organelles (arrows) in the cytoplasm, without apoptotic bodies (mag. 41,000X). No stromal cells with ultrastructural changes suggestive of apoptosis was detected in unwounded corneas (not shown).
4. Discussion
This study demonstrates that mechanical corneal endothelial injury triggers apoptosis of posterior stromal cells extending over 200 micrometers into the posterior stroma overlying injured corneal endothelial cells in corneas swollen to a total thickness of 800 to 950 micrometers by the endothelial injury. Apoptosis of stromal cells was detected with the TUNEL assay (Fig. 1) and confirmed by ultrastructural changes in the affected cells, such as chromatin condensation and the formation of apoptotic bodies containing the intracellular contents of the dying cells (Fig. 2). Vimentin expression in some apoptotic stromal cells suggests they are keratocytes or corneal fibroblasts but does not exclude the possibility that resident immune cells, Schwann cells or even bone marrow-derived cells also undergoing apoptosis in response to corneal endothelial injury.
Care was taken in this study to limit the injury to the corneal endothelial cells within a central zone 8 mm in diameter while preserving Descemet’s membrane (basement membrane) in this central 8 mm of cornea, as well as endothelial cells and Descemet’s membrane in the more peripheral cornea. No apoptosis of stromal cells was noted overlying the uninjured peripheral corneal endothelium with either the TUNEL assay (Fig. 1D) or TEM (not shown).
The endothelial injury-posterior stromal cell apoptosis response is analogous to the epithelial injury-keratocyte apoptosis response that is present in the anterior stroma (Wilson, et al., 1996). The authors hypothesize that the function of this response, similar to the epithelial injury-keratocyte apoptosis response (Wilson, et al., 1997), is to serve as an immediate defense mechanism to limit the spread of viral infections by organisms such as herpes simplex virus (HSV), cytomegalovirus (CMV) and the varicella zoster virus (VZV), that may infect the corneal endothelium and spread to adjacent cells in the stroma, prior to the immune system response to the infection (Inoue, 2014; Zheng, et al., 2000).
The apoptosis response in stromal cells adjacent to injured endothelial cells could be triggered by at least two mechanisms in the dying cells that are keratocytes. First, corneal endothelial cells constitutively express both Fas and Fas ligand, but normal quiescent keratocytes only express Fas (Wilson, et al., 1996b; Mohan, et al., 1997). When corneal endothelial cells are injured, transmembrane Fas ligand may undergo cleavage and processing through a metalloprotease-mediated process to become soluble Fas ligand that could diffuse into the stroma and bind to Fas on keratocytes, and initiate apoptosis (Huang, et a., 1999; Schneider, et al., 1998). This mechanism could also apply to bone marrow-derived cells, resident immune cells, and/or Schwann cells in the stroma but this has not been studied. Alternatively, corneal endothelial cells also constitutively produce interleukin (IL)-1α (Wilson and Lloyd, 1991; Wilson, et al., 1994)and IL -1β(Weng, et al., 1997). Both IL -1αand IL -1βlack signal sequences for extracellular transportbut are released from the corneal endothelium by injury or death of the cells (Dinarello CA, 1997; Wilson and Lloyd, 1991; Wilson, et al., 1994; Weng, et al., 1997). When IL-1α or IL-1βbinds to keratocyte IL -1 receptors, they induce the production of Fas ligand, likely depending on the cytokine concentration and the overall cellular milieu, and trigger autocrine suicide of keratocytes that normally don’t produce Fas ligand (Mohan, et al., 1997). Other unknown mechanisms (likely involving soluble mediatorssince there is extension of the apoptosis well anterior tothe endothelium )could also trigger the stromal cell apoptosis response following corneal endothelial cell injury or death, including in cells other than keratocytes.
It is unknown whether the stromal swelling that occurs after endothelial injury could have a role in triggering stromal cell apoptosis. However, the mechanical effects of swelling on cell viability, if any, would likely be associated with necrosis, and necrosis was not detected with TEM in stromal cells in this study.
This study also demonstrates, as expected, that nidogen-1 is present in the EBM and Descemet’s basement membrane in both corneas with and without endothelial injury. Interestingly, even in unwounded corneas (Fig. 1A), nidogen-1 was detected at higher levels in the posterior half of the stroma than the anterior half of the stroma. It is impossible to discern in this study whether this nidogen-1 is within both the extracellular matrix and the stromal cells, although nidogen-1, nidogen-2, and perlecan messenger RNAs have been detected in stromal cells in the anterior stroma of unwounded and epithelial -wounded rabbit corneas (Santhanam, et al., 2017). Thus, it remains possible that all of the nidogen-1 detected in the posterior stroma was produced by endothelial cells and only associated with the extracellular matrix, not the stromal cells.
Endothelial cells in unwounded corneas (Fig. 2B) and in the periphery of wounded corneas (not shown) expressed large amounts of nidogen-1. Descemet’s membrane in normal corneas, in contrast to the epithelial basement membrane, thickens throughout life (Johnson, Bourne and Campbell, 1982). We hypothesize this nidogen-1 in the posterior stroma and endothelium may contribute to this progressive thickening.
Investigation of other BM components in the corneas of rabbits is limited by the availability of antibodies effective in immunohistochemistry for rabbit BM antigens. In the event of more extensive injuries that significantly damage Descemet’s BM, corneal endothelial cell-produced, and possibly stromal cell-produced, BM components could contribute to repair of Descemet’s BM or production of a Descemet’s BM -like layer if the endothelial cells repopulate the posterior surface of the cornea. This would be of critical importance because the intact, functional Descemet’s BM-endothelial complex likely limits the passage of transforming growth factor beta from the aqueous humor into the corneal stroma and thereby modulates the development of myofibroblasts that could trigger posterior corneal fibrosis ( Marino, et al., 2017), similar to the likely role of functional BMs limiting fibrosis in other organs( Wilson, et al., 2017).
The observation that corneal endothelial injury triggers apoptosis of the overlying stromal cells has important implications regarding stromal wound healing responses to corneal injuries, infections and endothelial replacement surgeries (such as penetrating keratoplasty, Descemet’s stripping automated endothelial keratoplasty [DSAEK], Descemet’s membrane endothelial keratoplasty[ DMEK]). Thus, not only does a posterior stromal wound healing response ensue but, depending on the type and extent of injury, myofibroblasts may develop from precursor cells, leading to fibrosis in the posterior stroma( Marino, et al., 2017)and potentially on the posterior surface of the stroma. This is likely another mechanism for development of posterior corneal fibrosis (or haze) commonly associated with these types of injuries, infections and surgeries, in addition to the transformation of residual endothelial cells that have been studied by other investigators (Kay et al., 1982; Silbert and Baum, 1979).
5. Conclusions
Mechanical corneal endothelial injury triggers apoptosis of stromal cells anterior to the injury in rabbits. The basement membrane protein nidogen-1 is differentially localized in the stroma of the unwounded rabbit corneas, with higher levels in the posterior stroma than the anterior stroma. Nidogen-1 is also expressed at high levels in corneal endothelial cells in unwounded rabbit corneas.
Supplementary Material
Highlights.
Overlying keratocytes undergo apoptosis following mechanical injury to corneal endothelial cells in rabbits.
The epithelial basement membrane and Descemet’s basement membrane have high concentrations of nidogen-1 in rabbits.
There is higher nidogen-1 in keratocytes and extracellular matrix in the posterior stroma compared to anterior stroma.
Acknowledgments
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and P30-EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY. Dr. Lassance is supported by NEI training grant T32 EY007157. Research reported in this publication was supported by the Office of the Director, National Institutes of Health under award number S10RR031536 for transmission electron microscopy.
Footnotes
Proprietary interest statement:None of the authors have any commercial or proprietary interest in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–65. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- Fantes FE, Hanna KD, Waring GO, III, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (Photorefractive Keratectomy) in monkeys. Arch Ophthalmol. 1990;108:665–675. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–283. [PubMed] [Google Scholar]
- Huang DCS, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-xL. Proc Natl Acad Sci USA. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y. Review of clinical and basic approaches to corneal endotheliitis. Cornea. 2014;33(Suppl 11):S3–8. doi: 10.1097/ICO.0000000000000228. [DOI] [PubMed] [Google Scholar]
- Kay ED, Cheung CC, Jester JV, Nimni ME, Smith RE. Type I collagen and fibronectin synthesis by retrocorneal fibrous membrane. Invest Ophthalmol Vis Sci. 1982;22:200–12. [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Bose K, Tam KP, Wilson SE. Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp Eye Res. 2017;161:101–105. doi: 10.1016/j.exer.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Liang Q, Kim WJ, Helena MC, Baerveldt F, Wilson SE. Apoptosis in the cornea: further characterization of Fas-Fas ligand system. Exp Eye Res. 1997;65:575–89. doi: 10.1006/exer.1997.0371. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J Refract Surg. 2006;22:562–574. doi: 10.3928/1081-597x-20060601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam A, Marino GK, Torricelli AAM, Wilson SE. Epithelial basement membrane (EBM) regeneration and changes in EBM component mRNA expression in the anterior stroma after corneal injury. Mol Vision. 2017;23:39–51. [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its pro-apoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert AM, Baum JL. Origin of the retrocorneal membrane in the rabbit. Arch Ophthalmol. 1979;97:1141–3. doi: 10.1001/archopht.1979.01020010595019. [DOI] [PubMed] [Google Scholar]
- Torricelli AAM, Marino GK, Santhanam A, Wu J, Singh A, Wilson SE. Epithelial basement membrane proteins perlecan and nidogen-2 are up-regulated in stromal cells after epithelial injury in human corneas. Exp Eye Res. 2015;134:33–8. doi: 10.1016/j.exer.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142:110–8. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Mohan RR, Li Q, Wilson SE. IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: Interleukin-1 beta expression in the cornea. Cornea. 1997;16:465–71. [PubMed] [Google Scholar]
- Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, Chwang EL. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–8. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Kim WJ. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmol Vis Sci. 1998;39:220–6. [PubMed] [Google Scholar]
- Wilson SE, Li Q, Weng J, Barry-Lane PA, Jester JV, Liang Q, Wordinger RJ. The Fas/Fas ligand system and other modulators of apoptosis in the cornea. Invest Ophthalmol Vis Sci. 1996;37:1582–92. [PubMed] [Google Scholar]
- Wilson SE, Lloyd SA. Epidermal growth factor and its receptor, basic fibroblast growth factor, transforming growth factor beta-1, and interleukin-1 alpha messenger RNA production in human corneal endothelial cells. Invest Ophthalmol Vis Sci. 32:2747–56. [PubMed] [Google Scholar]
- Wilson SE, Marino GK, Torricelli AAM, Medeiros CS. Corneal fibrosis: injury and defective regeneration of the epithelial basement membrane. A paradigm for fibrosis in other organs? Matrix Biology. 2017;64:17–26. doi: 10.1016/j.matbio.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–37. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Pedroza L, Beuerman R, Hill JM. Herpes simplex virus type-1 infection of corneal epithelial cells induces apoptosis of the underlying keratocytes. Exp Eye Res. 1997;64:775–9. doi: 10.1006/exer.1996.0266. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Schultz GS, Chegini N, Weng J, He YG. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp Eye Res. 1994;59:63–72. doi: 10.1006/exer.1994.1081. [DOI] [PubMed] [Google Scholar]
- Zheng X, Yamaguchi M, Goto T, Okamoto S, Ohashi Y. Experimental corneal endotheliitis in rabbit. Invest Ophthalmol Vis Sci. 2000;41:377–85. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.