Abstract
Background
Eosinophilic airway inflammation in COPD is associated with exacerbations and responsivity to steroids, suggesting potential shared mechanisms with eosinophilic asthma. However there is no consistent blood eosinophil level that has been used to define the increased exacerbation risk.
Objective
To investigate blood eosinophil levels associated with exacerbation risk in COPD
Methods
Blood eosinophil counts and exacerbation risk were analyzed in moderate-to-severe COPD subjects, using two independent studies of former and current smokers with longitudinal data. The COPDGene study was analyzed for discovery (n=1553) and the ECLIPSE study was analyzed for validation (n=1895). A subset of the ECLIPSE subjects were used to assess the stability of blood eosinophil counts over time.
Results
COPD exacerbation risk increased with higher eosinophil counts. An eosinophil threshold of ≥300 cells/μL showed adjusted incidence rate ratios (IRR) for exacerbations of 1.32 in COPDGene (95% confidence interval (CI) 1.10–1.63). The cutoff of ≥300 cells/μL was validated for prospective risk of exacerbation in ECLIPSE with adjusted IRR of 1.22 (95% CI 1.06–1.41) using 3 year follow up data. Stratified analysis confirmed that the increased exacerbation risk associated with an eosinophil count ≥300 cells/μL was driven by subjects with a history of frequent exacerbations in both COPDGene and ECLIPSE.
Conclusions
Patients with moderate to severe COPD and blood eosinophil count ≥ 300 cells/μL had an increased risk exacerbations in the COPDGene Study which was prospectively validated in the ECLIPSE Study.
Keywords: pulmonary disease, chronic obstructive, asthma, eosinophil, exacerbation
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible airflow limitation and has limited therapeutic options. Patients with COPD are prone to develop exacerbations, which are associated with decline in lung function and increased morbidity and mortality. In COPD, respiratory tract inflammation is thought to be driven by lymphocytes and neutrophils1, compared to inflammation in asthma which is mediated by Th2 cells and eosinophils. 2 In patients with asthma, eosinophilic inflammation is associated with an increased exacerbation risk and loss of disease control upon inhaled corticosteroid (ICS) withdrawal.3 Similarly, increasing evidence suggests that 20–40% of patients with stable COPD have eosinophilic airway inflammation measured by sputum eosinophils4 that is associated with exacerbations5,6 and response to steroids.7–9 Since blood eosinophils are more easily assessed than sputum eosinophils, associations between blood eosinophils and COPD phenotypes have been investigated. Prior studies have reported that a blood eosinophil count of 2% can serve as a surrogate for sputum eosinophilia during an exacerbation of COPD,10 while other studies have reported lack of correlation between sputum and blood eosinophil levels.11–14
Furthermore, there is conflicting evidence about whether elevated blood eosinophil levels are associated with increased exacerbations. Some studies reported increased COPD exacerbation risk with varying levels of blood eosinophils in general and clinical populations15–17 and post hoc analyses of clinical trials,18–21 while other studies have reported lack of associations between blood eosinophils and COPD exacerbations.11–13,22,23 Recently, a clinical trial showed that mepolizumab, an anti-interleukin 5 monoclonal antibody, reduced moderate or severe exacerbations in COPD patients with high blood eosinophil counts,24 demonstrating that blood eosinophils are a useful biomarker to identify eosinophilic inflammation that can be targeted for therapy.
In this study, we aimed to evaluate the relation between blood eosinophil counts and COPD exacerbation risk in two well-phenotyped COPD cohorts, COPDGene25 and ECLIPSE.26 We determined blood eosinophil levels associated with COPD exacerbations in COPDGene, and validated the finding prospectively in ECLIPSE.
METHODS
Study populations
The COPDGene (Genetic Epidemiology of COPD) and ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) studies have been described previously.25,26 Briefly, COPDGene is a multicenter observational study which enrolled 10,192 smokers with and without COPD in Phase 1. Complete blood counts (CBC) were measured at the Phase 2 visit, approximately five years later. Subjects were clinically stable, with at least 30 days since their last exacerbation. The current analysis included 1,553 Phase 2 subjects with GOLD spirometry grade 2–4 COPD (post-bronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) <0.7 with FEV1 < 80% predicted)27 who had available CBC and clinical data. Of these subjects, 1,113 (71%) participated in a longitudinal follow up program to prospectively assess exacerbations following the Phase 2 visit.
The ECLIPSE study was a multicenter multinational 3-year longitudinal study that enrolled 3,291 subjects. We analyzed 1,895 subjects with GOLD spirometry grade 2–4 with complete CBC and 3 year follow up data. Subjects with an exacerbation within four weeks of enrollment were excluded. We also analyzed 243 smoker controls with repeated CBC measurements to measure eosinophil stability. Subjects taking oral corticosteroids were excluded from both COPDGene and ECLIPSE analyses (Supplemental Figure 1).
Exacerbation ascertainment
In COPDGene and ECLIPSE, exacerbations were self-reported, and defined as an episode of increased dyspnea, cough and/or sputum production that required antibiotic and/or systemic steroid treatment. Severe exacerbations were defined as exacerbations requiring Emergency Department visits or hospitalization.14,28 In the longitudinal follow up program for COPDGene participants, exacerbation information was collected by telephone call or web-based survey every 6 months.
Statistical analysis
Descriptive analysis was performed with t test, Fisher’s exact test and Kruskal Wallis test as appropriate. Negative binomial multivariate regression was performed for exacerbation analysis and incidence rate ratios (IRRs) were calculated with 95% confidence intervals. Nested models with and without eosinophils were compared using a likelihood ratio test. Logistic regression was performed for binary variables (frequent exacerbations and severe exacerbations). Linear regression was performed for continuous variables, and the residuals were plotted to check normality assumption. Receiver operating characteristic (ROC) curves for multivariate models with different eosinophil counts were plotted for no exacerbations versus 1 or more exacerbations to evaluate eosinophil cutoffs. Statistical analyses were performed using R version 3.3.1.
RESULTS
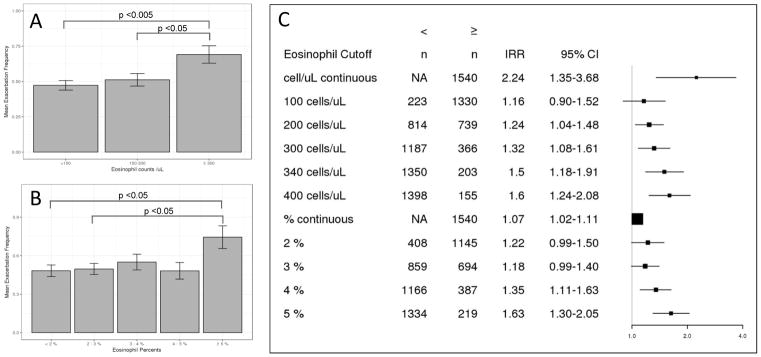
Eosinophil thresholds and exacerbation risk in cross sectional analysis of COPDGene Of the 1765 COPDGene subjects with GOLD grade 2–4 COPD with CBC data, subjects taking chronic oral steroids or with incomplete information were excluded, leaving 1553 subjects for analysis (Supplemental Figure 1). As there is no consensus definition for eosinophilic COPD, we evaluated a range of thresholds ranging from 2–5% and from 100–400 cells/μL for association with exacerbations. We also included eosinophil cutoff of 340 cells/μL, based on a recent publication.15 In negative binomial regression analysis adjusting for known COPD exacerbation risk factors including gastroesophageal reflux (GERD), Saint George’s Respiratory Questionnaire total score (SGRQ), baseline smoking status, post bronchodilator FEV1 percent predicted and white blood cell count (WBC), we observed increasing incidence rate ratios (IRR) for exacerbation frequency as the eosinophil cutoff increased (Figure 1). Eosinophil counts showed a consistent linear relationship with exacerbation risk, which was not clearly seen with eosinophil percentages. Multivariable logistic regression models using different eosinophil cutoffs found that a threshold of 300 cells/μL produced the maximal area under the ROC curve, with the highest sensitivity and specificity (Supplementary Figure 2). Compared to the nested model without eosinophils, inclusion of an eosinophil threshold of 300 cells/μL was statistically significant (likelihood ratio test p = 0.006).
Figure 1.
Risk of COPD exacerbations with increasing blood eosinophil levels in COPDGene. (A) Mean 1 year exacerbation frequency reported at phase 2 visit per blood eosinophil count range in moderate to severe COPD subjects. Error bars indicate standard errors. Difference between group means determined by one-way ANOVA and Tukey honestly significant difference test. (B) Mean 1 year exacerbation frequency reported at phase 2 visit per blood eosinophil percent range in moderate to severe COPD subjects. Error bars indicate standard errors. Difference between group means determined by one-way ANOVA and Tukey honestly significant difference test. (C) Adjusted incidence rate ratios for COPD exacerbations with different eosinophil cutoff values. Risk estimates are derived from negative binomial regression models adjusted for age, sex, race, gastroesophageal reflux, Saint George’s Respiratory Questionnaire score, post bronchodilator forced expiratory volume at 1 second percent predicted, and white blood cell count. IRR: incidence rate ratio. CI: confidence interval.
Subjects with eosinophilic COPD were more likely to be male and non-Hispanic white, and had a greater number of exacerbations per year. Subjects with eosinophil counts ≥ 300 cells/μL had higher WBC counts with lower neutrophil percentages. There was no difference in reported inhaled corticosteroid usage between patients with eosinophil counts above or below 300 cells/μL. (Table 1). In multivariable analysis, eosinophilic COPD was associated with higher SGRQ score (total, impact and activity) and less emphysema measured by quantitative analysis of chest CT scans (low attenuation area at −950HU and the 15th percentile of the lung density histogram) (Supplementary Table 1). There was no difference in spirometric measures (FEV1, FVC, FEV1/FVC) or 6 minute walk distance.
Table 1.
Baseline characteristics of subjects with high vs. low blood eosinophils.
| COPDGene | ECLIPSE | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Eosinophil < 300 cells/μL (n=1187) | Eosinophil ≥ 300 cells/μL (n=366) | p value | Eosinophil < 300 cells/μL (n=1477) | Eosinophil ≥ 300 cells/μL (n=418) | p value | |
|
| ||||||
| Age (mean(SD)) | 67.88 (8.19) | 68.27 (8.29) | 0.43 | 63.23 (7.1) | 63.86(6.8) | 0.11 |
|
| ||||||
| Gender (Female) (%) | 567 (47.8) | 130 (35.5) | <0.001 | 542 (36.7) | 114 (27.3) | <0.001 |
|
| ||||||
| Race (White) (%) | 911 (76.7) | 305 (83.3) | 0.009 | 1446 (97.9) | 407 (97.4) | 0.64 |
|
| ||||||
| post-bronchodilator FEV1 % predicted(mean(SD)) | 53.11 (17.0) | 51.43 (17.2) | 0.10 | 47.96 (15.5) | 48.72 (15.9) | 0.38 |
|
| ||||||
| GOLD grade (%) | 0.55 | 0.77 | ||||
| 2 | 695 (58.6) | 204 (55.7) | 644 (43.6) | 179 (42.8) | ||
| 3 | 360 (30.3) | 115 (31.4) | 631 (42.7) | 186 (44.5) | ||
| 4 | 132 (11.1) | 47 (12.8) | 202 (13.7) | 53 (12.7) | ||
|
| ||||||
| FEV1 decline ml/year (mean(SD)) | NA | NA | NA | 34.12 (45.0) | 30.0 (50.0) | 0.18 |
|
| ||||||
| DLCO (ml/min/mmHg )¶ * | 14.70 (6.4) | 15.17 (6.4) | 0.26 | NA | NA | NA |
|
| ||||||
| CT Emphysema % at −950 HU* (mean(SD)) | 12.59 (12.8) | 11.69 (11.7) | 0.31 | 17.77 (12.4) | 17.43 (11.8) | 0.65 |
|
| ||||||
| CT lung density 15th Percentile* (mean(SD)) | −936.30 (27.3) | −933.32 (29.9) | 0.13 | −948.37 (27.07) | −949.35(25.41) | 0.54 |
|
| ||||||
| SGRQ total score (mean(SD)) | 35.21(20.3) | 37.41 (21.8) | 0.07 | 49.97 (20.26) | 47.98 (20.23) | 0.08 |
|
| ||||||
| 6 min walk distance, meters (mean(SD))* | 345.9 (128) | 340.5(138) | 0.50 | 370.9 (120) | 372.2 (122.8) | 0.85 |
|
| ||||||
| Any asthma history (%) | 299 (25.2) | 101 (27.6) | 0.51 | 326 (24.0) | 102 (26.3) | 0.39 |
|
| ||||||
| Asthma-COPD overlap (%) | 130 (11.3) | 54 (15.3) | 0.06 | 120 ( 9.4) | 47 (12.7) | 0.09 |
|
| ||||||
| Bronchodilator reversibility† (%) | 189 (15.9) | 65 (17.8) | 0.46 | 318 (21.5) | 120 (28.7) | 0.003 |
|
| ||||||
| Exacerbations/year‡ (mean(SD)) | 0.49 (0.9) | 0.69 (1.2) | <0.001 | 1.15 (1.3) | 1.37 (1.6) | 0.004 |
|
| ||||||
| Severe exacerbations§ ((%), mean(SD)) | 190 (16) | 69 (18.9) | 0.23 | 0.27 (0.6) | 0.29 (0.7) | 0.55 |
|
| ||||||
| BMI, kg/m2 (mean(SD)) | 28.06 (6.4) | 28.52(6.4) | 0.22 | 26.42 (5.7) | 26.65 (5.5) | 0.45 |
|
| ||||||
| Pack-Years of smoking (mean(SD)) | 51.96 (24.3) | 51.43 (25.8) | 0.72 | 47.91 (26.6) | 50.53 (28.0) | 0.08 |
|
| ||||||
| Current smokers (%) | 404 (34.0) | 116 (31.7) | 0.44 | 926 (62.7) | 266 (63.6) | 0.77 |
|
| ||||||
| Inhaled corticosteroid use (%)* | 568 (48.4) | 187 (51.1) | 0.40 | 1047 (70.9) | 310 (74.2) | 0.21 |
|
| ||||||
| WBC (mean(SD)) | 7.32 (2.2) | 8.32 (1.9) | <0.001 | 7.70 (2.3) | 8.76 (2.4) | <0.001 |
|
| ||||||
| eosinophil % (mean(SD)) | 2.01 (1.1) | 5.15 (2.3) | <0.001 | 2.10 (1.2) | 5.56 (2.7) | <0.001 |
|
| ||||||
| neutrophil % (mean(SD)) | 62.06 (10.2) | 59.61 (9.7) | <0.001 | 65.29 (8.9) | 62.99 (8.4) | <0.001 |
|
| ||||||
| lymphocyte % (mean(SD)) | 27.08 (9.3) | 26.30 (8.9) | 0.16 | 26.06 (8.1) | 24.91 (7.3) | 0.009 |
|
| ||||||
| monocyte % (mean(SD)) | 8.26 (2.5) | 8.26 (2.2) | 1.00 | 6.22 (2.3) | 6.15 (2.2) | 0.57 |
|
| ||||||
| basophil % (mean(SD)) | 0.60 (0.6) | 0.68 (0.6) | 0.02 | 0.33 (0.2) | 0.38 (0.2) | <0.001 |
Diffusion capacity of the lung for carbon monoxide (DLCO), adjusted for altitude and hemoglobin.
subsets of subjects with complete information were used. DLCO: Eosinophil < 300 cells/μL n = 1003, Eosinophil ≥ 300 cells/μL n= 304, Chest CT data: COPDGene (Eosinophil < 300 cells/μL n = 863, Eosinophil ≥ 300 cells/μL n= 256), ECLIPSE ( Eosinophil < 300 cells/μL n= 1263, Eosinophil ≥ 300 cells/μL n= 347). 6 minute walk distance: COPDGene (Eosinophil < 300 cells/μL n = 1166, Eosinophil ≥ 300 cells/μL n= 355), ECLIPSE(Eosinophil < 300 cells/μL n = 1457, Eosinophil ≥ 300 cells/μL n= 414). Asthma: COPDGene (Eosinophil <300 cells/μL 1322, Eosinophil ≥ 300 cells/μL n= 184), ECLIPSE (Eosinophil <300 cells/μL 1170, Eosinophil ≥ 300 cells/μL n= 370).)
Defined as change in FEV1 at least 200ml and 12% after bronchodilator. 2 subjects from COPDGene had missing data.
Number of exacerbations in the prior year to Phase 2 study visit is reported for COPDGene and exacerbation rate during the overall study period is reported for ECLIPSE.
Severe exacerbations defined by Emergency Room visits or hospitalization was a binary variable in COPDGene. In ECLIPSE, severe exacerbation rate during the overall study period is reported.
In a multivariable model, exacerbation frequency was significantly associated with female sex, white race, GERD, higher SGRQ total score, lower post bronchodilator FEV1 percent predicted and eosinophil counts ≥ 300 cells/μL (Table 2). Eosinophil counts ≥ 300 cells/μL had IRR of 1.32 (95% CI 1.10–1.61) (Table 2). Furthermore, eosinophilic COPD was associated frequent exacerbations (≥ 2/year) in a logistic regression analysis (OR 1.58, 95% CI 1.07–2.30) (Table 2).
Table 2.
Multivariable models of blood eosinophil count ≥ 300 cells/μL and exacerbation risk in COPDGene.
| COPDGene: Year Prior to Visit 2 (Cross sectional) | COPDGene: Longitudinal Follow Up | |||||
|---|---|---|---|---|---|---|
| Exacerbation Frequency* (n=1553) | Frequent Exacerbations† (n=1281) | Exacerbation Rate* (n=1113) | ||||
| Factors | IRR* (95% CI) | p value | OR† (95% CI) | p value | IRR* (95% CI) | p value |
| Age | 0.98 (0.97–1.00) | 0.007 | 0.96 (0.94–0.99) | 0.003 | 0.99(0.97–1.01) | 0.48 |
| Female | 1.43 (1.20–1.71) | <0.001 | 1.83(1.30–2.58) | <0.001 | 0.87(0.65–1.15) | 0.31 |
| Non White Race | 0.73 (0.57–0.92) | 0.008 | 0.63(0.40–0.99) | 0.05 | 1.73 (1.19–2.55) | 0.005 |
| SGRQ total score‡ | 1.02 (1.02–1.03) | <0.001 | 1.04(1.03–1.05) | <0.001 | 1.02(1.01–1.03) | <0.001 |
| post-bronchodilator FEV1 % predicted § | 0.98 (0.98–0.99) | <0.001 | 0.97(0.96–0.98) | <0.001 | 0.99(0.98–1.00) | 0.003 |
| GERD | 1.33 (1.11–1.59) | 0.002 | 1.35(0.95–1.91) | 0.09 | 1.08(0.81–1.45) | 0.57 |
| Current smoking | 0.71(0.57–0.89) | 0.002 | 0.56(0.37–0.84) | 0.006 | 0.63(0.45–0.90) | 0.01 |
| Previous Exacerbations | NA | NA | NA | NA | 2.51(1.87–3.37) | <0.001 |
| WBC | 1.00 (0.96–1.04) | 0.97 | 1.02(0.94–1.10) | 0.66 | 1.03(0.96–1.10) | 0.44 |
| Eosinophil ≥300 | 1.32 (1.08–1.61) | 0.006 | 1.58(1.07–2.30) | 0.019 | 1.33(0.92–1.95) | 0.13 |
Risk estimate from negative binomial regression.
Odds ratio from logistic regression comparing subjects with 2 or more exacerbations per year to subjects with less than 1 exacerbation per year.
per 1 point increase in score,
per percentage point increase in FEV1
Prospective analysis of COPDGene
Of the 1553 patients in the cross-sectional analysis, 1113 (71.6%) had at least one follow up contact after the COPDGene Phase 2 visit, for a total of 1561 person-years (mean 512 days). There was no difference in follow up duration or proportion lost to follow up between eosinophilic and non-eosinophilic COPD. The same covariates as in the cross sectional multivariable model were used, with the addition of prior exacerbation history. Subjects with eosinophil counts ≥ 300 cells/μL at the phase 2 visit had an increased prospective exacerbation rate, with a similar risk estimate as the cross-sectional analysis (IRR 1.33, 95% CI 0.91–1.95); however eosinophilic COPD was not statistically significant in multivariable regression (Table 2). When we performed stratified analysis based on the exacerbation frequency in the prior year, we found that the predictive ability of eosinophilic COPD for future exacerbations was driven by the subset of subjects with a history of frequent exacerbations (≥ 2 per year, IRR 1.96, 95% CI 1.21–3.21, p = 0.008). In subjects with 2 or more exacerbations in the prior year, the annual exacerbation rate during follow up was 2.39 in those with elevated eosinophils, compared to 1.42 without elevated eosinophils (Supplementary Table 2). High eosinophil count was not significantly associated with future exacerbations in subjects with a history of one exacerbation or fewer in the previous year (Table 4, Supplementary Table 2).
Table 4.
Risk of elevated blood eosinophils on future exacerbations, stratified by prior exacerbation history. (A) Incidence rate ratio (IRR) associated with eosinophil counts ≥ 300 cells/μL derived from multivariable analysis for exacerbation rate in COPDGene Longitudinal Follow-up Study, (B) IRR associated with eosinophil counts ≥ 300 cells/μL derived from multivariable analysis for exacerbation rate in ECLIPSE overall study period. Multivariable model adjusted for age, sex, race, Saint George Respiratory Questionnaire score, gastroesophageal reflux disease, current smoking, and white blood cell count.
| (A) | ||||||
|---|---|---|---|---|---|---|
| COPDGene Longitudinal Follow Up Stratified Analysis | ||||||
| (Non-eosinophilic vs. eosinophilic) | No exacerbations in the prior year (666 vs. 98) | 1 exacerbation in the prior year (165 vs. 28) | ≥2 exacerbations in the prior year (125 vs. 31) | |||
| IRR (95% CI) | p value | IRR (95% CI) | p value | IRR (95% CI) | p value | |
| Eosinophil ≥300 at phase 2 visit | 1.12 (0.57–2.28) | 0.73 | 1.02 (0.48–2.20) | 0.97 | 1.96 (1.21–3.21) | 0.008 |
| (B) | ||||||
|---|---|---|---|---|---|---|
| ECLIPSE Stratified Analysis on overall study period | ||||||
| (Non-eosinophilic vs. eosinophilic) | No exacerbations in the prior year (788 vs. 223) | 1 exacerbation in the prior year (373 vs. 99) | ≥2 exacerbations in the prior year (316 vs. 96) | |||
| IRR (95% CI) | p value | IRR (95% CI) | p value | IRR (95% CI) | p value | |
| Eosinophil ≥300 at screening | 1.03 (0.85–1.24) | 0.77 | 1.15(0.87–1.51) | 0.33 | 1.40(1.15–1.70) | <0.001 |
Validation in ECLIPSE
We prospectively validated the increased exacerbation risk with increased eosinophil counts in the ECLIPSE study. Among 2303 subjects with GOLD grade 2–4 COPD, 1895 with baseline CBC and complete covariate information were included in the analysis. There were 133 subjects who participated in screening visit but did not have follow up visits or had missing laboratory information. There was no difference in the proportion of eosinophilic and non-eosinophilic COPD among these subjects compared to the overall study population. Similar to COPDGene, those with eosinophil counts ≥ 300 cells/μL at the screening visit were more likely to be male. WBC counts were increased and neutrophil percentage was decreased (Table 1). There was no difference in quantitative CT measurements of emphysema and SGRQ score in ECLIPSE, in contrast to COPDGene (Supplementary Table 1). As in COPDGene, there was no association between eosinophil counts ≥ 300 cells/μL and spirometric measures or 6 minute walk distance.
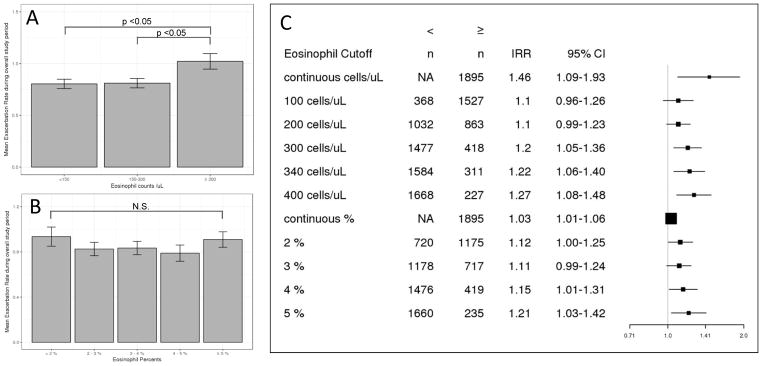
We calculated exacerbation rates by dividing the total number of moderate to severe exacerbations by observed time at two different time points: 1 year follow up and the overall study period (4981 person-years total; mean 959 days). Models were adjusted for age, sex, race, and known risk factors for COPD exacerbations (prior history of exacerbations, GERD, SGRQ total score, baseline smoking status, post bronchodilator FEV1 percent predicted and WBC count). There was no difference in risk estimates when we accounted for subjects with a change in smoking status, therefore we only included baseline smoking status in the model. An eosinophil count ≥ 300 cells/μL at screening was consistently predictive of future exacerbations (IRR 1.20, 95% CI 1.05–1.36 at 1 year, IRR 1.22, 95% CI 1.06–1.42 for overall study period) (Table 3). Comparable to COPDGene, increasing eosinophil counts were associated with higher risk of exacerbations (Figure 2). Addition of eosinophils was statistically significant based on the likelihood ratio test (p <0.001 for 1 year follow-up and overall study period). Area under the curve in ROC analyses did not differ with different eosinophil count cutoffs (Supplementary Figure 3). Similar to the COPDGene longitudinal follow up study, the exacerbation risk associated with elevated eosinophil counts was driven by the frequent exacerbators with ≥ 2 exacerbations prior to study entry in the stratified analysis(IRR 1.40, 95% CI1.15–1.70, p < 0.001) (Table 4). Subjects with frequent exacerbation history and eosinophil ≥300 cells/μL had a higher annual exacerbation rate (overall 2.41/year) than those with frequent exacerbation history and lower eosinophil counts (overall 1.61/year, Supplementary Table 2). In the longitudinal analysis, eosinophilic and non-eosinophilic COPD subjects had similar changes in FEV1, FVC, SGRQ score, and 6 minute walk distance.
Table 3.
Multivariable models of blood eosinophil count ≥ 300 cells/μL and exacerbation risk in ECLIPSE.
| ECLIPSE | ||||
|---|---|---|---|---|
| Factors associated with Exacerbation Rate | 1 year | overall study period | ||
|
| ||||
| IRR (95% CI) | p value | IRR (95% CI) | p value | |
|
| ||||
| Age | 1.01 (1.00–1.02) | 0.008 | 1.01 (1.00–1.02) | 0.01 |
| Female | 1.23 (1.10–1.38) | <0.001 | 1.31 (1.15–1.49) | <0.001 |
| Non White race | 0.82(0.56–1.18) | 0.29 | 0.71(0.45–1.08) | 0.12 |
| SGRQ total score* | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| Post-bronchodilator FEV1 % predicted† | 0.99 (0.98–0.99) | <0.001 | 0.98 (0.97–0.98) | <0.001 |
| GERD | 1.39 (1.24–1.56) | <0.001 | 1.36 (1.19–1.56) | <0.001 |
| Current smoking | 1.10(0.98–1.24) | 0.11 | 1.21(1.06–1.39) | 0.005 |
| previous exacerbations | 2.45 (2.18–2.74) | <0.001 | 2.87 (2.51–3.29) | <0.001 |
| WBC | 1.04 (1.01–1.06) | 0.001 | 1.04 (1.02–1.07) | <0.001 |
| Eosinophil ≥300 at screening | 1.20 (1.05–1.36) | 0.005 | 1.22 (1.06–1.42) | 0.006 |
Risk estimate from negative binomial regression.
per 1 point increase in score,
per percentage point increase in FEV1
Figure 2.
Risk of prospective COPD exacerbations with increasing blood eosinophil levels in ECLIPSE. (A) Mean exacerbation rate during overall study period per blood eosinophil count range in moderate to severe COPD subjects. Error bars indicate standard errors. Difference between group means determined by one-way ANOVA and Tukey honestly significant difference test. (B) Mean exacerbation rate during overall study period per blood eosinophil percent range in moderate to severe COPD subjects. Error bars indicate standard errors. Difference between group means determined by one-way ANOVA and Tukey honestly significant difference test. (C) Adjusted incidence rate ratios for COPD exacerbations with different eosinophil cutoffs. Risk estimates are derived from negative binomial regression models adjusted for age, sex, race, previous exacerbations, gastroesophageal reflux, Saint George’s Respiratory Questionnaire score, post bronchodilator forced expiratory volume at 1 second percent predicted, and white blood cell count. IRR: incidence rate ratio. CI: confidence interval.
We also analyzed the subset of subjects (1839 COPD cases and 243 controls) with repeated eosinophil measurements over the course of the ECLIPSE study. Eosinophil counts were relatively stable over time, with an interclass correlation coefficient (ICC) of 0.57. There was no significant difference in ICC between COPD cases and controls (0.57 and 0.56 respectively). For a subset of 1345 COPD cases (71%) with four CBC measurements over the study (0, 1, 2, and 3 years), 90 (6.7%) had persistently elevated eosinophil levels above 300/μL. The majority (784, 58%) had eosinophil levels below 300/μL for the entire study. The exacerbation risk in subjects with persistently elevated eosinophil counts was higher compared to the subjects with persistently low eosinophil counts and those with fluctuating eosinophil counts (Supplementary Table 3), suggesting that while baseline eosinophil level was predictive of exacerbation risk up to 3 years, patients with persistent eosinophilia are at the greatest risk of exacerbation.
Sensitivity Analysis
To evaluate for confounding by inhaled corticosteroid (ICS) usage, we included use of ICS as covariate in the multivariable model. The association between eosinophilic COPD and exacerbations in COPDGene and ECLIPSE remained (COPDGene IRR 1.34, 95% CI 1.01–1.63, ECLIPSE 1 year follow up IRR 1.18, 95% CI 1.04–1.34, overall study period IRR 1.21, 95% CI 1.05–1.39). In addition we performed a sensitivity analysis focusing on the subgroup of subjects not taking ICS. While increasing eosinophil counts were associated with increased exacerbation risk, the cutoff of 300 cells/μL was no longer statistically significant. This is likely related to the reduced sample size (Supplementary Figure 4) as previously validated risk factors for COPD exacerbations, including GERD and WBC,14 were no longer significant in the subgroup analysis (data not shown).
Epidemiologic relationship between eosinophilic COPD and Asthma-COPD overlap
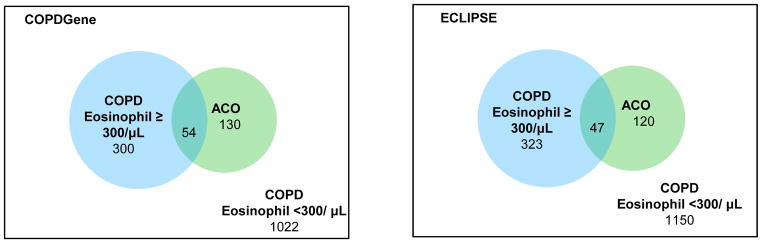
We analyzed the relationship between eosinophilic COPD, ACO and exacerbation risk. ACO has previously been shown to be associated with increased exacerbation risk in COPDGene29,30 and ECLIPSE.31 COPD subjects with eosinophil counts ≥ 300 cells/μL were more likely to have ACO, using the COPDGene definition of self-report of doctor’s diagnosis of asthma before age 4030,32 (OR 1.51 for COPDGene and OR 1.69 for ECLIPSE) (Supplementary Table 4). However, the overall concordance between eosinophilic COPD and asthma was low (N=54 in COPDGene, N=47 in ECLIPSE) (Figure 3). In negative binomial regression, ACO and eosinophilic COPD were independently associated with exacerbation risk in COPDGene and ECLIPSE (Table 5).
Figure 3.
Venn Diagrams of the number of subjects with asthma-COPD overlap (defined by asthma diagnosis before the age of 4030) and COPD subjects with blood eosinophil counts ≥ 300 cells/μL in COPDGene and ECLIPSE.
Table 5.
Asthma-COPD overlap, defined by asthma diagnosis before age 40, and elevated eosinophils (≥ 300 cells/μL) independently predict exacerbations.
| Factors | COPDGene | ECLIPSE | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cross-sectional Exacerbation Frequency | Prospective Exacerbation Rate | |||||
|
| ||||||
| 1yr | overall study period | |||||
|
| ||||||
| IRR (95% CI) | p value | IRR (95% CI) | p value | IRR (95% CI) | p value | |
|
| ||||||
| Age | 0.99 (0.97–1.00) | 0.02 | 1.01 (1.00–1.02) | 0.006 | 1.01 (1.00–1.02) | 0.009 |
| Non White race | 0.69 (0.54–0.88) | 0.003 | 0.83(0.54–1.25 | 0.37 | 0.69 (0.41–1.12) | 0.15 |
| Female | 1.39 (1.16–1.66) | <0.001 | 1.20 (1.06–1.36) | 0.004 | 1.29 (1.11–1.49) | <0.001 |
| SGRQ score* | 1.02 (1.02–1.03) | <0.001 | 1.01 (1.01–1.01) | <0.001 | 1.01(1.01–1.01) | <0.001 |
| post-bronchodilator FEV1 % predicted † | 0.98 (0.98–0.99) | <0.001 | 0.99 (0.98–0.99) | <0.001 | 0.98(0.98–0.99) | <0.001 |
| GERD | 1.37 (1.14–1.64) | <0.001 | 1.40 (1.23–1.59) | <0.001 | 1.39(1.20–1.61) | <0.001 |
| Current smoking | 0.73(0.58–0.90) | 0.004 | 1.10 (0.97–1.25) | 0.12 | 1.25(1.08–1.44) | 0.003 |
| previous exacerbations | NA | NA | 2.35 (2.08–2.65) | <0.001 | 2.77(2.40–3.21) | <0.001 |
| WBC | 1.01(0.97–1.05) | 0.70 | 1.03 (1.01–1.06) | 0.006 | 1.04(1.01–1.07) | 0.004 |
| Asthma COPD overlap | 1.33 (1.04–1.71) | 0.02 | 1.29 (1.08–1.54) | 0.005 | 1.33(1.08–1.63) | 0.006 |
| Eosinophils ≥300 | 1.26 (1.03–1.54) | 0.02 | 1.21 (1.06–1.39) | 0.005 | 1.22(1.04–1.42) | 0.01 |
Results from negative binomial regression analysis are shown.
per 1 point increase in score,
per percentage point increase in FEV1
DISCUSSION
In this study of the two multicenter, longitudinal cohorts of moderate to severe COPD subjects, we found that exacerbation risk increased linearly with higher blood eosinophil counts. We identified a threshold blood eosinophil count of ≥300 cells/μL to be associated with exacerbations and then validated this cutoff as a predictor of future exacerbations using prospective data from the ECLIPSE study. We further showed that the increased COPD exacerbation risk associated with elevated eosinophil counts was driven by subjects in both studies with a history of frequent exacerbations, defined as two or more exacerbations per year.
The most commonly used cutoff to define eosinophilic COPD is 2%, which originates from a sentinel study describing increased exacerbation risk with blood eosinophils ≥ 2 % that was predictive of sputum eosinophilia (≥3%) at the time of exacerbation.10 A previous analysis in ECLIPSE showed 88% concordance between blood eosinophil thresholds of 2% and 150 cells/μL.33 Many subsequent studies have used these lower thresholds.11,34–38 In our study, we found that higher baseline eosinophil levels were associated with future exacerbation risk up to three years and that absolute eosinophil counts were consistently associated with increased exacerbations while the eosinophil percentage did not show a linear relationship in either COPDGene or ECLIPSE. The absolute eosinophil count may be more relevant biologically, as WBC count can vary widely and single percentage value may not capture the entire range of blood eosinophils. In contrast, a percentage cutoff has been used for sputum, as sputum differential count is typically based on counting a fixed number of cells (400–600 cells) on a cytospin slide.13,39–42
Our finding is in line with the recently published Copenhagen General Population Study, where an eosinophil cutoff of 340 cells/μL was associated with COPD exacerbations.15 Two studies based on health care utilization data also demonstrated increased exacerbation risk with blood eosinophil counts ≥ 300 cells/μL17 and ≥ 450 cells/μL.16 In common with our study, these studies included large numbers of subjects with moderate to severe COPD (FEV1 <80% or GOLD C and D) and applied higher eosinophil cutoffs defined by absolute counts. Other studies reporting a lack of association between blood eosinophils and COPD exacerbations had differences compared to our study. Most of these studies were smaller.11,12,22,23 AERIS 11, BPCO 22 and a recent meta analysis used only percentage cutoffs for eosinophils.38 The importance of higher, count based eosinophil cutoff is demonstrated by the lack of association between eosinophil cutoff of 2% and exacerbation risk in a subset of the ECLIPSE study.33 No association was found in SPIROMICS, which included GOLD 0 subjects (37% of the study population), while moderate to severe COPD comprised 22% of the subjects.13 As low lung function is a risk factor for COPD exacerbations, selection of moderate to severe COPD patients may enrich for subjects prone to exacerbations. These observations suggest that patient population, disease severity and eosinophil cutoffs all matter in understanding the significance of eosinophils in COPD.
Our findings highlight the utility of measuring eosinophil counts in patients with frequent exacerbations, as eosinophilic COPD is a significant risk factor for future exacerbations in this subgroup. In the prospective follow up study of both COPDGene and ECLIPSE, frequent exacerbators with eosinophil counts ≥ 300 cells/μL had an average of one additional exacerbation episode per year compared to frequent exacerbators with lower eosinophil counts. While the most significant predictive factor for future exacerbation is a prior history of exacerbation in COPD patients,14 we showed that measurement of eosinophil counts in a high risk group may serve as a biomarker for additional risk stratification. Measurements of eosinophil counts may not have much utility in COPD in subjects with few exacerbations. Furthermore, eosinophil measurements in patients with frequent exacerbations may identify subjects who can be treated with anti-eosinophilic therapy, based on recent mepolizumab trials.24 METREX and METREO were phase 3, randomized, placebo-controlled, double blind trials that enrolled patients with two or more moderate exacerbations or one or more severe exacerbations on triple inhaled therapy.24 Eosinophilic COPD phenotype based on blood eosinophil count was part of the enrollment criteria in METREO but not for METREX.24 In line with our study, these parallel trials also showed that exacerbation risk can be further stratified based on blood eosinophil counts even in patients with significant prior exacerbation history.24
Subjects with a history of 2 or more exacerbations in the past year included 14% of moderate to severe COPD subjects in COPDGene and 22% in ECLIPSE. Based on the current guidelines, frequent exacerbators on maximal inhaled therapy would be considered for long-term azithromycin or roflumilast to prevent future exacerbations.43,44 Eosinophilic frequent exacerabators comprised 2.8% of moderate to severe COPD subjects in COPDGene and 5% of ECLIPSE, corresponding to 20% of the frequent exacerbator group. Further studies are required to determine the effects of anti-inflammatory therapy vs. anti-eosinophilic therapy, in eosinophilic and non-eosinophilic exacerbation-prone subjects.
At a single time point, both the COPDGene and ECLIPSE studies had approximately 20% of moderate to severe COPD patients with eosinophil count ≥ 300 cells/μL. However, in ECLIPSE only 6.7% had persistently elevated eosinophil levels above 300 cells/μL which carried the greatest risk of exacerbations. A recent study of the UK Clinical Practice Research Datalink showed that blood eosinophil counts are more variable in COPD, particularly for patients with higher baseline eosinophil levels.45 While we did not observe differences in eosinophil stability between COPD patients and controls, we found that about 60% of COPD patients never had eosinophil counts ≥ 300 cells/μL.
In COPDGene, eosinophilic COPD was associated with an approximately 30% increased risk of exacerbations in both cross-sectional and prospective data. However, eosinophil level was not statistically significant in the prospective analysis. The data collection for the longitudinal follow up program was different than the main COPDGene study, since exacerbations were assessed every six months using a web and phone based telecommunication system.46 It is possible that the longitudinal follow up data may not represent the same type of exacerbation information collected in person at the study visits.
We have also found differences between COPDGene and ECLIPSE. For example, current smoking was negatively associated with exacerbation frequency in the COPDGene study, while it was associated with an increased exacerbation rate in ECLIPSE. This likely reflects indication bias in the COPDGene population; subjects with more severe disease may preferentially quit smoking.47 ECLIPSE subjects had a higher proportion of bronchodilator reversibility, which may be due to the withdrawal of bronchodilators prior to assessment, which was not performed in COPDGene. Individuals with COPD and blood eosinophil counts ≥ 300 cells/μL had higher SGRQ scores in COPDGene, but not in ECLIPSE.
As eosinophilic inflammation is classically regarded as a feature of allergic asthma,48 we examined the relationship between eosinophilic COPD and asthma. Both COPDGene and ECLIPSE did not exclude subjects with asthma history. In univariate analysis there was no significant associations between eosinophilic COPD and asthma-COPD overlap, defined as asthma diagnosis before the age of 40 in the COPDGene study.30 The limited concordance between eosinophilic COPD and asthma could be due to the incomplete definition of ACO, as we relied on patient report of a diagnosis of asthma, or due to the heterogeneous nature of ACO itself. ACO can include asthmatics with fixed obstruction or smokers with COPD and features of asthma or a Th2 immune response that may develop independent of asthma.49
A count-response relation between blood eosinophil counts and asthma-related outcomes has well been described in patients with asthma; blood eosinophil counts of 290–400 cells/μL have been associated with increased asthma exacerbations.50–53 However, eosinophilic COPD subjects in our study had an average of fifty pack-year smoking history, while studies of eosinophilic asthma typically exclude greater than ten pack-year smokers or subjects with COPD. Therefore, even if a subset of COPD patients had prior asthma that progressed to COPD in our cohorts, these subjects still had significant cigarette smoke exposure that is much higher than populations in asthma studies. Despite the differences in smoking exposure, eosinophilic asthma and eosinophilic COPD patients show similar relationships between blood eosinophil counts and frequent exacerbations, which points to a shared role of eosinophilic inflammation. This is in line with a recently published study demonstrating asthma-like airway remodeling and inflammation in eosinophilic COPD patients without allergies or asthma history.54
Response to therapeutics in eosinophilic COPD also provides clues to a potential shared mechanism with eosinophilic asthma. Previous studies have shown that elevated blood eosinophil counts in COPD patients predict favorable response to oral55,56 and inhaled corticosteroids,18–20,57 which have been cornerstones for asthma therapy. In a recent trial, benralizumab (anti IL5 receptor α monoclonal antibody) reduced exacerbations in COPD subjects with blood eosinophils greater than 300 cells/μL.58 As mentioned above, mepolizumab reduced exacerbations in COPD subjects with elevated blood eosinophils.24 The blood eosinophil threshold for targeting intervention requires additional investigation and is an effort in the COPD Biomarker Qualification Consortium.59
The strength of our study is the inclusion of two large cohorts of well-characterized subjects with COPD that were prospectively analyzed as discovery and validation populations to identify an eosinophil threshold increasing exacerbation risk. We are also aware of limitations of this study. We measured blood eosinophils and not sputum or lung tissue eosinophils, which may be more closely related to the disease process. We excluded patients taking oral steroids, however approximately 50% of the COPDGene and 70% of the ECLIPSE subjects were using ICS. While ICS use has minimal impact on blood eosinophil counts,60 eosinophilic COPD patients are known to be more responsive to ICS therapy and have less exacerbations on ICS, which may reduce the impact of the exacerbation risk. The analysis restricted to non-users of ICS showed increasing eosinophil counts were associated with increased risk of exacerbation but the cutoff of 300 cells/μL was no longer significant likely due to the reduced number of subjects. In the COPDGene study, CBC were only obtained at the Phase 2 visit, so we could not assess changes in eosinophil counts over time or use the full longitudinal data from Phase 1 to Phase 2. Self-report of exacerbation history may be a limitation, though this definition has been used in multiple prior studies in both COPDGene28 and ECLIPSE.14 Finally, as patients with asthma history were enrolled in the cohorts, there could be misclassification between eosinophilic COPD and asthma. We did not have skin testing results or IgE to independently assess allergic component. However as there is no gold standard definition of ACO, we aimed to assess the relationship of ACO and eosinophilic asthma utilizing this dataset.
In conclusion, we showed that blood eosinophil counts greater than or equal to 300 cells/μL were associated with increased exacerbation frequency in two large COPD studies. This threshold identified an eosinophilic subgroup of 20% of subjects with moderate to severe COPD, and 20% of subjects with frequent exacerbations in whom blood eosinophils could further stratify exacerbation risk.
Supplementary Material
Key Messages.
Patients with moderate to severe COPD and elevated blood eosinophil counts have increased exacerbation risk compared to those with blood eosinophil count of less than 300 cells per μL.
Our study supports measurement of eosinophils in patients with frequent COPD exacerbations which may have utility in identifying a population for treatments targeting eosinophilic inflammation.
Acknowledgments
Funding
Supported by National Institute of Health (NIH) grants R01HL125583, R01HL130512, R01HL124233, R01HL126596, R01HL089897, R01HL089856, T32HL007427, P01HL105339, P01HL132825.
J. Vestbo was supported by NIHR Manchester Biomedical Research Centre.
COPDGene® project is also supported by the COPD foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens and Sunovion.
ECLIPSE was funded by GlaxoSmithKline.
COPDGene® Investigators – Core Units
Administrative Center: James D. Crapo, MD (PI); Edwin K. Silverman, MD, PhD (PI); Barry J. Make, MD; Elizabeth A. Regan, MD, PhD
Genetic Analysis Center: Terri Beaty, PhD; Ferdouse Begum, PhD; Robert Busch, MD; Peter J. Castaldi, MD, MSc; Michael Cho, MD; Dawn L. DeMeo, MD, MPH; Adel R. Boueiz, MD; Marilyn G. Foreman, MD, MS; Eitan Halper-Stromberg; Nadia N. Hansel, MD, MPH; Megan E. Hardin, MD; Lystra P. Hayden, MD, MMSc; Craig P. Hersh, MD, MPH; Jacqueline Hetmanski, MS, MPH; Brian D. Hobbs, MD; John E. Hokanson, MPH, PhD; Nan Laird, PhD; Christoph Lange, PhD; Sharon M. Lutz, PhD; Merry-Lynn McDonald, PhD; Margaret M. Parker, PhD; Dandi Qiao, PhD; Elizabeth A. Regan, MD, PhD; Stephanie Santorico, PhD; Edwin K. Silverman, MD, PhD; Emily S. Wan, MD; Sungho Won
Imaging Center: Mustafa Al Qaisi, MD; Harvey O. Coxson, PhD; Teresa Gray; MeiLan K. Han, MD, MS; Eric A. Hoffman, PhD; Stephen Humphries, PhD; Francine L. Jacobson, MD, MPH; Philip F. Judy, PhD; Ella A. Kazerooni, MD; Alex Kluiber; David A. Lynch, MB; John D. Newell, Jr., MD; Elizabeth A. Regan, MD, PhD; James C. Ross, PhD; Raul San Jose Estepar, PhD; Joyce Schroeder, MD; Jered Sieren; Douglas Stinson; Berend C. Stoel, PhD; Juerg Tschirren, PhD; Edwin Van Beek, MD, PhD; Bram van Ginneken, PhD; Eva van Rikxoort, PhD; George Washko, MD; Carla G. Wilson, MS;
PFT QA Center, Salt Lake City, UT: Robert Jensen, PhD
Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Douglas Everett, PhD; Jim Crooks, PhD; Camille Moore, PhD; Matt Strand, PhD; Carla G. Wilson, MS
Epidemiology Core, University of Colorado Anschutz Medical Campus, Aurora, CO: John E. Hokanson, MPH, PhD; John Hughes, PhD; Gregory Kinney, MPH, PhD; Sharon M. Lutz, PhD; Katherine Pratte, MSPH; Kendra A. Young, PhD
COPDGene® Investigators – Clinical Centers
Ann Arbor VA: Jeffrey L. Curtis, MD; Carlos H. Martinez, MD, MPH; Perry G. Pernicano, MD
Baylor College of Medicine, Houston, TX: Nicola Hanania, MD, MS; Philip Alapat, MD; Mustafa Atik, MD; Venkata Bandi, MD; Aladin Boriek, PhD; Kalpatha Guntupalli, MD; Elizabeth Guy, MD; Arun Nachiappan, MD; Amit Parulekar, MD;
Brigham and Women’s Hospital, Boston, MA: Dawn L. DeMeo, MD, MPH; Craig Hersh, MD, MPH; Francine L. Jacobson, MD, MPH; George Washko, MD
Columbia University, New York, NY: R. Graham Barr, MD, DrPH; John Austin, MD; Belinda D’Souza, MD; Gregory D.N. Pearson, MD; Anna Rozenshtein, MD, MPH, FACR; Byron Thomashow, MD
Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., MD; H. Page McAdams, MD; Lacey Washington, MD
HealthPartners Research Institute, Minneapolis, MN: Charlene McEvoy, MD, MPH; Joseph Tashjian, MD
Johns Hopkins University, Baltimore, MD: Robert Wise, MD; Robert Brown, MD; Nadia N. Hansel, MD, MPH; Karen Horton, MD; Allison Lambert, MD, MHS; Nirupama Putcha, MD, MHS
Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Torrance, CA: Richard Casaburi, PhD, MD; Alessandra Adami, PhD; Matthew Budoff, MD; Hans Fischer, MD; Janos Porszasz, MD, PhD; Harry Rossiter, PhD; William Stringer, MD
Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, MD, PhD; Charlie Lan, DO
Minneapolis VA: Christine Wendt, MD; Brian Bell, MD
Morehouse School of Medicine, Atlanta, GA: Marilyn G. Foreman, MD, MS; Eugene Berkowitz, MD, PhD; Gloria Westney, MD, MS
National Jewish Health, Denver, CO: Russell Bowler, MD, PhD; David A. Lynch, MB
Reliant Medical Group, Worcester, MA: Richard Rosiello, MD; David Pace, MD
Temple University, Philadelphia, PA: Gerard Criner, MD; David Ciccolella, MD; Francis Cordova, MD; Chandra Dass, MD; Gilbert D’Alonzo, DO; Parag Desai, MD; Michael Jacobs, PharmD; Steven Kelsen, MD, PhD; Victor Kim, MD; A. James Mamary, MD; Nathaniel Marchetti, DO; Aditi Satti, MD; Kartik Shenoy, MD; Robert M. Steiner, MD; Alex Swift, MD; Irene Swift, MD; Maria Elena Vega-Sanchez, MD
University of Alabama, Birmingham, AL: Mark Dransfield, MD; William Bailey, MD; Surya Bhatt, MD; Anand Iyer, MD; Hrudaya Nath, MD; J. Michael Wells, MD
University of California, San Diego, CA: Joe Ramsdell, MD; Paul Friedman, MD; Xavier Soler, MD, PhD; Andrew Yen, MD
University of Iowa, Iowa City, IA: Alejandro P. Comellas, MD; John Newell, Jr., MD; Brad Thompson, MD
University of Michigan, Ann Arbor, MI: MeiLan K. Han, MD, MS; Ella Kazerooni, MD; Carlos H. Martinez, MD, MPH
University of Minnesota, Minneapolis, MN: Joanne Billings, MD; Abbie Begnaud, MD; Tadashi Allen, MD
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD; Jessica Bon, MD; Divay Chandra, MD, MSc; Carl Fuhrman, MD; Joel Weissfeld, MD, MPH
University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, MD; Sandra Adams, MD; Diego Maselli-Caceres, MD; Mario E. Ruiz, MD
ECLIPSE Investigators — Bulgaria: Y. Ivanov, Pleven; K. Kostov, Sofia. Canada: J. Bourbeau, Montreal; M. Fitzgerald, Vancouver, BC; P. Hernandez, Halifax, NS; K. Killian, Hamilton, ON; R. Levy, Vancouver, BC; F. Maltais, Montreal; D. O’Donnell, Kingston, ON. Czech Republic: J. Krepelka, Prague. Denmark: J. Vestbo, Hvidovre. The Netherlands: E. Wouters, Horn-Maastricht. New Zealand: D. Quinn, Wellington. Norway: P. Bakke, Bergen. Slovenia: M. Kosnik, Golnik. Spain: A. Agusti, J. Sauleda, P. de Mallorca. Ukraine: Y. Feschenko, V. Gavrisyuk, L. Yashina, Kiev; N. Monogarova, Donetsk. United Kingdom: P. Calverley, Liverpool; D. Lomas, Cambridge; W. MacNee, Edinburgh; D. Singh, Manchester; J. Wedzicha, London. United States: A. Anzueto, San Antonio, TX; S. Braman, Providence, RI; R. Casaburi, Torrance CA; B. Celli, Boston; G. Giessel, Richmond, VA; M. Gotfried, Phoenix, AZ; G. Greenwald, Rancho Mirage, CA; N. Hanania, Houston; D. Mahler, Lebanon, NH; B. Make, Denver; S. Rennard, Omaha, NE; C. Rochester, New Haven, CT; P. Scanlon, Rochester, MN; D. Schuller, Omaha, NE; F. Sciurba, Pittsburgh; A. Sharafkhaneh, Houston; T. Siler, St. Charles, MO; E. Silverman, Boston; A. Wanner, Miami; R. Wise, Baltimore; R. ZuWallack, Hartford, CT.
ECLIPSE Steering Committee: H. Coxson (Canada), C. Crim (GlaxoSmithKline, USA), L. Edwards (GlaxoSmithKline, USA), D. Lomas (UK), W. MacNee (UK), E. Silverman (USA), R. Tal Singer (Co-chair, GlaxoSmithKline, USA), J. Vestbo (Co-chair, Denmark), J. Yates (GlaxoSmithKline, USA).
ECLIPSE Scientific Committee: A. Agusti (Spain), P. Calverley (UK), B. Celli (USA), C. Crim (GlaxoSmithKline, USA), B. Miller (GlaxoSmithKline, USA), W. MacNee (Chair, UK), S. Rennard (USA), R. Tal-Singer (GlaxoSmithKline, USA), E. Wouters (The Netherlands), J. Yates (GlaxoSmithKline, USA).
Abbreviations
- ACO
asthma-COPD overlap
- BDR
bronchodilator reversibility
- BMI
body mass index
- CBC
complete blood count
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GERD
gastroesophageal reflux
- HU
Hounsfield units
- ICC
interclass correlation coefficient
- ICS
inhaled corticosteroid
- IRR
incidence rate ratio
- LAA950
percent of lung with attenuation less than −950 Hounsfield units
- Perc15
15th percentile of the lung density histogram
- ROC
receiver operating characteristics
- SGRQ
Saint George’s Respiratory Questionnaire
- Th2
T helper type 2
- WBC
white blood cell
Footnotes
Disclosure of potential conflict of interest
D. Singh has received grants from AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, GlaxoSmithKline, Gelnmark, Menarini, Merck, Mundipharma, Novartis, Pfizer, Pulmatrix, Teva, Therevance, Verona and has served as consultant for Apellis, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genetech, GlaxoSmithKline, Glenmark, Menarini, Merck, Mundipharam, Novartis, Peptinnovate, Pfizer, Pulmatrix, Skyepharma, Teva, Tehrevance and Verona. J. Vestbo has served as consultant for GlaxoSmithKline, Chiesi Pharmaceuticals, Boehringer Ingelheim, Novartis and AstraZeneca. R. Tal-Singer is a employee and shareholder of GlaxoSmithKline. P. Castaldi has received personal fees and grant support from GlaxoSmithKline. E. Silverman has received grants and travel expenses from COPD Foundation and GlaxoSmithKline. C. Hersh has served as a consultant for AstraZeneca, Concert Pharmaceuticals, Mylan, and 23andMe, and has received grants from Boehringer-Ingelheim and Novartis. The other authors report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61:448–54. doi: 10.1136/thx.2004.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. NatRevImmunol. 2008;8:183–92. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 3.Aleman F, Lim HF, Nair P, Fang H, Mbbs L, Uk M, et al. Eosinophilic Endotype of Asthma. Immunol Allergy Clin North Am. 2016;36:559–68. doi: 10.1016/j.iac.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:39–47. doi: 10.2147/copd.2006.1.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, et al. Eosinophilic airway inflammation and exacerbations of COPD: A randomised controlled trial. Eur Respir J. 2007;29:906–13. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 6.Liesker JJW, Bathoorn E, Postma DS, Vonk JM, Timens W, Kerstjens HAM. Sputum inflammation predicts exacerbations after cessation of inhaled corticosteroids in COPD. Respir Med. 2011;105:1853–60. doi: 10.1016/j.rmed.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–5. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 8.Pizzichini E, Pizzichini MMM, Gibsn P, Parameswaran K, Gleich GJ, Berman L, et al. Sputum Eosinophilia Predicts Benefit from Prednisone in Smokers with Chronic Obstructive Bronchitis. Am J Respir Crit Care Med. 1998;158:1511–7. doi: 10.1164/ajrccm.158.5.9804028. [DOI] [PubMed] [Google Scholar]
- 9.Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60:193–8. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–71. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 11.Kim VL, Coombs NA, Staples KJ, Ostridge KK, Williams NP, Wootton SA, et al. Impact and associations of eosinophilic inflammation in COPD: Analysis of the AERIS cohort. Eur Respir J. 2017:50. doi: 10.1183/13993003.00853-2017. [DOI] [PubMed] [Google Scholar]
- 12.Casanova C, Celli BR, de-Torres JP, Martinez-Gonzalez C, Cosio BG, Pinto-Plata V, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50:1701162. doi: 10.1183/13993003.01162-2017. [DOI] [PubMed] [Google Scholar]
- 13.Hastie AT, Martinez FJ, Curtis JL, Doerschuk CM, Hansel NN, Christenson S, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017:5. doi: 10.1016/S2213-2600(17)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to Exacerbation in Chronic Obstructive Pulmonary Disease._Supp. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 15.Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood Eosinophils and Exacerbations in Chronic Obstructive. Am J Respir Crit Care Med. 2016;193:965–74. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 16.Kerkhof M, Sonnappa S, Postma DS, Brusselle G, Agusti A, Anzueto A, et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur Respir J. 2017;50:10–3. doi: 10.1183/13993003.00761-2017. [DOI] [PubMed] [Google Scholar]
- 17.Zeiger RS, Tran TN, Butler RK, Schatz M, Li Q, Khatry DB, et al. Relationship of Blood Eosinophil Count to Exacerbations in Chronic Obstructive Pulmonary Disease. J allergy Clin Immunol Pract. 2017 doi: 10.1016/j.jaip.2017.10.004. S2213–2198:30754–7. [DOI] [PubMed] [Google Scholar]
- 18.Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–42. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, et al. Blood Eosinophils: A Biomarker of Response to Extrafine Beclomethasone/Formoterol in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192:523–5. doi: 10.1164/rccm.201502-0235LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watz H, Tetzlaff K, Wouters EFM, Kirsten A, Magnussen H, Rodriguez-Roisin R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: A post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4:390–8. doi: 10.1016/S2213-2600(16)00100-4. [DOI] [PubMed] [Google Scholar]
- 21.Barnes NC, Sharma R, Lettis S, Calverley PMA. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016:47. doi: 10.1183/13993003.01370-2015. [DOI] [PubMed] [Google Scholar]
- 22.Zysman M, Deslee G, Caillaud D, Chanez P, Escamilla R, Court-fortune I, et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1819–24. doi: 10.2147/COPD.S129787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turato G, Semenzato U, Bazzan E, Biondini D, Tine M, Torrecilla N, et al. Blood Eosinophilia Does Not Reflect Tissue Eosinophils nor Worsen Clinical Outcomes in COPD. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201708-1684LE. rccm.201708-1684LE. [DOI] [PubMed] [Google Scholar]
- 24.Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S, Lugogo N, et al. Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease. N Engl J Med. 2017;377:1613–29. doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
- 25.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic Epidemiology of COPD (COPDGene) Study Design. COPD J Chronic Obstr Pulm Dis. 2011;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31:869–73. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 27.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195:557–82. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 28.Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary aJ, et al. Pulmonary Arterial Enlargement and Acute Exacerbations of COPD. N Engl J Med. 2012;367:913–21. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardin M, Cho M, McDonald M-L, Beaty T, Ramsdell J, Bhatt S, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44:341–50. doi: 10.1183/09031936.00216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wurst KE, Rheault TR, Edwards L, Tal-Singer R, Agusti A, Vestbo J. A comparison of COPD patients with and without ACOS in the ECLIPSE study. Eur Respir J. 2016;47:1559–62. doi: 10.1183/13993003.02045-2015. [DOI] [PubMed] [Google Scholar]
- 32.Sin DD, Miravitlles M, Mannino DM, Soriano JB, Price D, Celli BR, et al. What is asthma−COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016:48. doi: 10.1183/13993003.00436-2016. [DOI] [PubMed] [Google Scholar]
- 33.Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur Respir J. 2014;44:1697–700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 34.Bafadhel M, Greening NJ, Harvey-Dunstan TC, Williams JEA, Morgan MD, Brightling CE, et al. Blood eosinophils and outcomes in severe hospitalised exacerbations of COPD. Chest. 2016;150:320–8. doi: 10.1016/j.chest.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Pavord ID, Lettis S, Anzueto A, Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med. 2016;4:731–41. doi: 10.1016/S2213-2600(16)30148-5. [DOI] [PubMed] [Google Scholar]
- 36.Brightling CE, George L. Is the eosinophil a leading villain in lung function decline? Chest. 2015;148:844–6. doi: 10.1378/chest.15-0915. [DOI] [PubMed] [Google Scholar]
- 37.Couillard S, Larivee P, Courteau J, Vanasse A. Eosinophils in chronic obstructive pulmonary disease exacerbations are associated with increased readmissions. Chest. 2016;151:366–73. doi: 10.1016/j.chest.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Ho J, He W, Chan MTV, Tse G, Liu T, Wong SH, et al. Eosinophilia and clinical outcome of chronic obstructive pulmonary disease: a meta-analysis. Sci Rep. 2017;7:13451. doi: 10.1038/s41598-017-13745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid669 phase measurements. Am J Respir Crit Care Med. 1996;154:308–17. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 40.Brightling CE, Monterio W, Green RH, Parker D, Morgan MD, Wardlaw AJ, et al. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: safety and repeatability. Respir Med. 2001;95:999–1002. doi: 10.1053/rmed.2001.1195. [DOI] [PubMed] [Google Scholar]
- 41.Singh D, Edwards L, Tal-Singer R, Rennard S. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res. 2010;11:77. doi: 10.1186/1465-9921-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52:498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.GOLD. Global Initiative for Chronic Obstructive. Glob Obstr Lung Dis. 2018 http://www.goldcopd.org.
- 44.Wedzicha JA, Miravitlles M, Hurst JR, Calverley PMA, Albert RK, Anzueto A, et al. Management of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017:50. doi: 10.1183/13993003.02265-2016. [DOI] [PubMed] [Google Scholar]
- 45.Oshagbemi OA, Burden AM, Braeken DCW, Henskens Y, Wouters EFM, Driessen JHM, et al. Stability of Blood Eosinophils in Patients with Chronic Obstructive Pulmonary Disease and in Control Subjects, and the Impact of Sex, Age, Smoking, and Baseline Counts. Am J Respir Crit Care Med. 2017;195:1402–4. doi: 10.1164/rccm.201701-0009LE. [DOI] [PubMed] [Google Scholar]
- 46.Stewart JI, Moyle S, Criner GJ, Wilson C, Tanner R, Bowler RP, et al. Automated Telecommunication to Obtain Longitudinal Follow-up in a Multicenter Cross-sectional COPD Study. COPD J Chronic Obstr Pulm Dis. 2012;9:466–72. doi: 10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Au DH, Bryson CL, Chien JW, Sun H, Udris EM, Evans LE, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457–63. doi: 10.1007/s11606-009-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.George L, Brightling CE. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis. 2016;7:34–51. doi: 10.1177/2040622315609251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodruff PG, van den Berge M, Boucher RC, Brightling C, Burchard EG, Christenson SA, et al. American Thoracic Society/National Heart, Lung, and Blood Institute Asthma–Chronic Obstructive Pulmonary Disease Overlap Workshop Report. Am J Respir Crit Care Med. 2017;196:375–81. doi: 10.1164/rccm.201705-0973WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Gossage D, et al. High Blood Eosinophil Count Is a Risk Factor for Future Asthma Exacerbations in Adult Persistent Asthma. J Allergy Clin Immunol Pract. 2014;2:741–750. e4. doi: 10.1016/j.jaip.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Tran TN, Khatry DB, Ke X, Ward CK, Gossage D. High blood eosinophil count is associated with more frequent asthma attacks in asthma patients. Ann Allergy Asthma Immunol. 2014;113:19–24. doi: 10.1016/j.anai.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Price DB, Rigazio A, Campbell JD, Bleecker ER, Corrigan CJ, Thomas M, et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir Med. 2015;3:849–58. doi: 10.1016/S2213-2600(15)00367-7. [DOI] [PubMed] [Google Scholar]
- 53.Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Association of blood eosinophil and blood neutrophil counts with asthma exacerbations in the copenhagen general population study. Clin Chem. 2017;63:823–32. doi: 10.1373/clinchem.2016.267450. [DOI] [PubMed] [Google Scholar]
- 54.Kolsum U, Damera G, Pham T-H, Southworth T, Mason S, Karur P, et al. Pulmonary Inflammation in COPD Patients with Higher Blood Eosinophil Counts. J Allergy Clin Immunol. 2017;140:1181–1184. e7. doi: 10.1016/j.jaci.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 55.Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: A randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bafadhel M, Davies L, Calverley PMA, Aaron SD, Brightling CE, Pavord ID. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J. 2014;44:789–91. doi: 10.1183/09031936.00062614. [DOI] [PubMed] [Google Scholar]
- 57.Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, et al. Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD. Thorax. 2016;71:118–25. doi: 10.1136/thoraxjnl-2015-207021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brightling CE, Bleecker ER, Panettieri RA, Bafadhel M, She D, Ward CK, et al. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: A randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med. 2014;2:891–901. doi: 10.1016/S2213-2600(14)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casaburi R, Celli B, Crapo J, Criner G, Croxton T, Gaw A, et al. The COPD Biomarker Qualification Consortium (CBQC) COPD J Chronic Obstr Pulm Dis. 2013;10:367–77. doi: 10.3109/15412555.2012.752807. [DOI] [PubMed] [Google Scholar]
- 60.Kreindler JL, Watkins ML, Lettis S, Tal-Singer R, Locantore N. Effect of inhaled corticosteroids on blood eosinophil count in steroid-naive patients with COPD. BMJ Open Respir Res. 2016;3:e000151. doi: 10.1136/bmjresp-2016-000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.