Abstract
Aquaporins (AQPs), ordinarily regarded as water channels, have recently been shown to participate in other cellular functions such as cell-to-cell adhesion, cell migration, cell proliferation etc. The current investigation was undertaken to find out whether AQP5 water channel plays a role in corneal epithelial wound healing. Expression of AQP5 in mouse cornea and transfected Madin Darby canine kidney (MDCK) cells was detected using immunofluorescence or EGFP tag. Cell migration and proliferation, the two major events in wound healing, were studied in vitro using cell culture scratch-wound healing model and cell proliferation assay, in vivo by conducting wound healing experiments on corneas of wild-type and AQP5 knockout mouse model and ex vivo on corneal epithelial cells isolated from wild type and AQP5 knockout mice. MDCK cells stably expressing AQP5 showed significantly higher levels of cell migration and proliferation compared to control cells. Likewise, corneal epithelial cells of wild type mouse with innate AQP5 exhibited faster wound healing than those of AQP5 knockout in vivo and under ex vivo culture conditions. In vitro, in vivo and ex vivo studies showed that presence of AQP5 improved cell migration, proliferation and wound healing. The data collected suggest that AQP5 plays a significant role in corneal epithelial wound healing.
Keywords: AQP5, aquaporin, water channel, AQP5 knockout mouse, corneal epithelium, wound healing
1. Introduction
Aquaporin (AQP) water channels are transmembrane proteins that play a significant role in maintaining cellular water homeostasis (Preston et al., 1992). Thirteen AQPs (AQP0-AQP12) are expressed in mammals. The AQP superfamily consists of channels that allow diffusion of only water molecules (aquaporins), or water and certain solutes like glycerol and urea (aquaglyceroporins) across cell membranes (Agre and Kozono, 2003). Malfunction of AQPs is linked to several eye diseases, such as cataract (Varadaraj et al., 2008), glaucoma (Verkman, 2008), keratoconus (Garfias et al., 2008) and bullous keratopathy, and Fuchs’ dystrophy (Kenney et al., 2004).
Cornea, the outermost transparent tissue of the eye serves as a protective barrier and provides 65–75% of the refractive power needed for normal vision (Zhang et al., 2011). Corneal transparency is critical for normal refraction of the incident light which travels through the pupil and lens, and ultimately reaches the retina where visual input is transduced into neural signaling. Cornea has five layers (Wiley et al., 1991; DelMonte and Kim, 2011; Kumari et al., 2012). They are, from outer to inner: corneal epithelium, Bowman’s Layer, stroma, Descemets layer and endothelium. AQP1, AQP3 and AQP5 are present in the cornea (Patil et al., 1997; Funaki et al., 1998; Thiagarajah and Verkman, 2002). Among these, AQP5 is abundant in the epithelial layer (Funaki et al., 1998; Ren et al., 2017). AQP5 and AQP3 in epithelial cells and AQP1 in stroma and endothelial cells provide major routes for water movement across the epithelial and endothelial barriers of cornea (Thiagarajah and Verkman, 2002). Since corneal epithelium is directly exposed to the environment, it is prone to injuries. Epithelial injuries occur due to surgery, misuse and overuse of contact lenses, dry eye disease, infections, environmental and accidental exposures to chemicals, combat operations as well as aging. If corneal injuries do not heal fast, pathogenic invasion could ensue leading to corneal inflammation, opacification, ulceration and blindness.
Besides the channel function, several AQPs perform additional functions such as cell-to-cell adhesion (Hiroaki et al., 2006; Kumari and Varadaraj, 2009; Varadaraj et al., 2010, Kumari et al., 2011) cell migration, proliferation (Saadoun et al., 2005; Levin and Verkman, 2006; Hara-Chikuma and Verkman, 2008; Papadopoulos et al., 2008; Ruiz-Ederra and Verkman, 2009; Saadoun et al., 2009), and differentiation (Watanabe et al., 2009). Jiang et al. (2012) found that when aquaporin-5 expression was downregulated, there was reduction in the migration of Ishikawa cells (derived from an adenocarcinoma cell line). Recent studies showed that up-regulation of AQP5 increased cell migration and proliferation in non-cancerous cells and malignant cells (Zhang et al., 2010; Jung et al., 2011; Yan et al., 2014; Guo and Jin, 2015; Jensen et al., 2016, 2018; Direito et al., 2017; Yang et al., 2017). In cornea, AQP1 plays an important role in cell migration while AQP3 promotes both cell migration and proliferation during wound healing (Levin and Verkman, 2006; Ruiz-Ederra and Verkman, 2009; Verkman, 2011). However, research conducted on AQP5 yielded contradictory results (Shankardas et al., 2010; Chen et al., 2011). Besides, there is no in vivo experimental evidence, thus far, to show the involvement of AQP5 in corneal epithelial wound repair. We hypothesized that like AQP3, AQP5 could play an additional role in one or more phases of corneal epithelial regeneration. Using in vitro, in vivo and ex vivo experiments, we explored the possibility of a unique role for AQP5 in corneal epithelial wound healing. Our results show that AQP5, indeed, facilitates both cell migration and proliferation and hastens the process of corneal re-epithelialization.
2. Materials and methods
2.1. Animals
Wild type (WT; C57BL/6J: Jackson Laboratory) and AQP5 knockout (AQP5-KO (Krane et al., 2001) mice were reared and bred in the Animal Care Facility of Stony Brook University, NY. All procedures followed were approved by the American Association for Accreditation of Laboratory Animal Care (AAALAC); the animals were treated in accordance with ‘The Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research’.
2.2. Cloning, Cell Culture and Transfection
For experiments involving cell culture, the coding sequence of mouse AQP5 was amplified by Polymerase Chain Reaction (PCR) and cloned into a mammalian expression vector, pIRES2-EGFP in between the EcoR I and BamH I restriction sites. Cloning methods followed were as described (Varadaraj et al., 2008). A single colony positive for the cloned DNA was selected and grown in 2YT medium against Kanamycin antibiotic; plasmid DNA was isolated and purified using kits from Qiagen Inc. (Valencia, CA).
Cell culture and transfection were performed in Madin–Darby canine kidney epithelial cells (MDCK) that were purchased from American Type Culture Collection (ATCC, Manassas, VA). These cells were selected for experiments for the following reasons: MDCK cells are commonly used as an in vitro model to conduct epithelial cell studies; in situ corneal wound healing involves migration and proliferation of epithelial cells. Additionally, MDCK cells do not express AQP5 (endogenously). In a previous study (Kumari, et al., 2012), we observed that membrane localization of AQP5 in MDCK cells was similar to that in corneal epithelial cells. MDCK cells are cubical in shape and has apical and basolateral sides similar to those in corneal epithelial cells. Further, MDCK cells may lack the receptor-linked signal cascades that induce cellular carcinogenesis (Jensen et al., 2018).
For experiments, MDCK cells were grown in Minimum Essential Medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum, 1% non-essential amino acids, 2 mM L-glutamine, 100U/ml penicillin and 0.1 mg/ml streptomycin. Cell cultures were maintained at 37°C and 5% CO2 in a humidified atmosphere. Cells grown to 80–90% confluency on 60×15 mm dish were split and seeded in 35×10 mm plates. Transfection of the AQP5-pIRES2-EGFP construct was carried out using the Effectene reagent (Qiagen, CA), following the manufacturer’s protocol. The transfection mixture was added dropwise to 70–80 % confluent cells, mixed gently and kept in a 37°C incubator under the culture conditions mentioned above. After 24–48 hrs, cells were viewed under a microscope for AQP5 protein expression. Using G418 antibiotic, stably expressing cells were selected; only cells with high degrees of transfected DNA integration survived the G418 treatment. Stable expression of AQP5 was further confirmed by immunostaining (Kumari et al., 2017). Stable cell line expressing AQP5, and MDCK cells without AQP5 expression were investigated in vitro for the cell migration and proliferation stages of wound healing.
MDCK cell epithelial monolayers form dome-shaped structures when confluent; the cells lift up and transport solutes from the monolayer to the plastic surface below. Before conducting experiments, the untransfected and transfected clones were tested and verified for MDCK cell characteristic of ‘forming domes’ at 100% confluence on conventional cell culture plastic dishes.
2.3. AQP5 protein expression and localization in mouse cornea and in MDCK cells stably expressing AQP5
Fluorescence method
Eyeballs were dissected out from mice and placed in physiological saline solution, pH 7.0. Corneas were separated under a dissection microscope. Cornea, and MDCK cells stably expressing mouse AQP5 (plated on coverslips) were fixed in 4% paraformaldehyde for 24 hrs at 4°C. Corneas were cryosectioned at 14 μm thickness using a cryomicrotome (Leica) and stored at −20°C until use. Corneal tissue sections were thawed and incubated in 0.15% (v/v) Triton X-100 for 15 min. at room temperature to perforate the membranes, washed in PBS, blocked with normal goat serum and exposed to rabbit anti-Aquaporin 5 affinity purified antibody raised against rat AQP5 (Chemicon/Millipore, Billerica, MA), at 1:100 dilution in 5% (w/v) bovine serum albumin in 1×PBS, overnight. After washing, the corneal sections were incubated in fluorescein 5′-isothiocyanate (FITC) conjugated goat anti-rabbit IgG in PBS with 5% bovine serum albumin, washed with PBS, mounted using anti-fade Vectamount (Vector Labs, Burlingame, CA) containing the nuclear stain DAPI and viewed. Optimized Z-sectional digital images were acquired with a Zeiss Axiovert 200 inverted epifluorescent confocal microscope equipped with AxioCam and Zeiss AxioVision 4.8.2 software (Carl Zeiss Inc.), and processed using Adobe Photoshop 7.0 version. Coverslips containing AQP5-EGFP (AQP5-pIRES2-EGFP) transfected MDCK cells fixed in paraformaldehyde were observed directly under the epifluorescent confocal microscope and imaged as described previously.
2.4. Wound Healing Assays
2.4.1. In vitro Cell Migration Assay
Control MDCK cells and those stably expressing AQP5 were seeded into six well plates and incubated at 37°C until they reached 80–90% confluence as a monolayer. Cells were starved in MEM with 0.1% fetal bovine serum overnight at 37°C. A scratch wound (width ~300 μm) was made as a marked line in the cultures using a sterile 10-μl pipette tip. Wells were washed three times with culture medium to remove loose or dead cells, and photographed under the 10X objective of a Zeiss Axiovert 200 inverted microscope equipped with AxioCam and Zeiss AxioVision 4.8.2 software (0 h). Cells were incubated at 37°C and those migrated into the marked reference area to repair the wound were photographed. The images were analyzed by digitally drawing lines using SigmaScan software. Wound healing was assessed from the ratio of the difference between the original wound area at time 0 and the remaining wound area at 2, 4, 6 and 8 hr time points and compared with those of controls.
2.4.2. In vitro Cell Proliferation Assay
MDCK cells stably expressing AQP5 and control cells without endogenous expression were cultured in 35×10 mm culture dishes in the medium containing 2% bovine calf serum but without phenol red, which would otherwise interfere with the spectrophotometric measurements followed in the procedure. Cell proliferation was quantified using CytoSelect™ MTT Cell Proliferation Assay (Cell Biolabs) according to the manufacturer’s instruction. In brief, control or experimental MDCK cells were plated in 96-well culture plates at 5×103 cells (100 μl/well). Cells were incubated at 37°C in 5% CO2 in a humidified incubator for 72 hrs. Twenty microliter of 5% MTT Cell Proliferation Assay Reagent was added to each well and incubated for 3 hrs and then carefully medium and MTT reagents were aspirated. Cells were incubated with 100 μl of detergent solution (DMSO) for 2 hrs on the rotary shaker. Optical density was measured at 540 nm using a Thermo Scientific™ NanoDrop 2000.
2.4.3. In vivo wound healing studies
Wound healing was explored in vivo using the Epithelial Debridement method, as described previously (Yang et al., 2013), in age-matched (2.5 months young) wild type (AQP5+/+) and AQP5 knockout (Krane et al., 2001) mice; both heterozygous (AQP5+/−) and knockout (AQP5−/−) mice were tested. Male mice (25 g) were anesthetized with an intraperitoneal injection of ketamine/xylazine (45 mg/kg and 4.5 mg/kg, respectively); a topical anesthetic (proparacaine ophthalmic solution) was applied to the ocular surfaces until the blink sensation was lost. In the right cornea, a 2.5-mm diameter epithelial wound was made using a battery-powered mechanical device Algerbrush II (Alger Co., Inc. Lago Vista, TX), without disturbing the Bowman’s membrane, and was stained green by topical application of sodium fluorescein dye. Photographs of the cornea were taken immediately following wounding and every 12 hrs subsequently, after anesthetizing the mice. The size of the injured area remaining was measured on the images using a SigmaScan Software. Data at each time point were analyzed. Three eyes for control and AQP5 knockout mice separately were used and the experiment was repeated twice.
2.4.4. Ex-vivo wound healing studies
Eyes from wild type or AQP5 knockout mice were dissected out; the sheet of corneal epithelial cells was peeled off from each eye using Algerbrush II and dissociated into single cells using 0.25% trypsin-EDTA (Invitrogen, Life Technologies). The dissociated control and knockout cells were cultured separately in fibronectin-coated culture dishes in serum-free DMEM/F12 medium that contained growth supplements. Wound healing assay was performed as described above for ‘In Vitro Cell Migration Assay’. Mouse corneal epithelial cell migration rate was quantified from the values measured for the initial wound area and the healed area.
2.5. Statistical analysis
Statistical analysis was performed using SigmaPlot 2000 version 6.10. Values shown represent the mean ± standard deviation. Data at each time point were statistically analyzed using the t-test (P < 0.05).
3. Results
3.1. Expression of AQP5
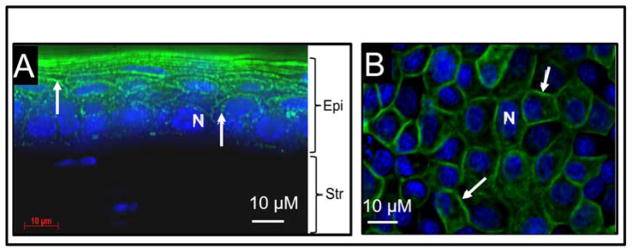
To find out whether there is noticeable difference in the expression pattern of AQP5 under in vivo, and in vitro experimental conditions, mouse corneal tissue sections were immunostained with anti-AQP5 antibody (Figure 1A), and MDCK cells were transfected with AQP5-EGFP plasmid DNA (Figure 1B). Both images showed membrane localization of AQP5 (green). After protein extraction, Western blotting was performed. Anti-AQP5 antibody bound to two bands from each sample (Fig. 1C); a ~28 kDa lower band corresponding to the unmodified AQP5, and a ~34 kDa band corresponding to post-translationally modified AQP5, possibly in the phosphorylated state. Results from both immunostaining and Western blotting showed that AQP5 expression under in vivo and in vitro conditions were comparable. There was no detectable morphological difference between control and AQP5-expressing MDCK cells. Therefore, we proceeded with in vitro, in vivo and ex vivo studies to test the efficacy of AQP5 on corneal epithelial wound healing.
Figure 1.
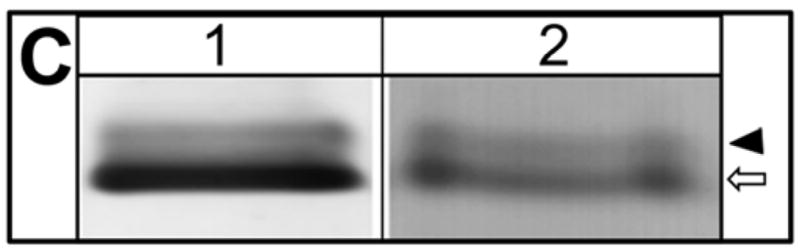
AQP5 expression (green). A. Mouse corneal tissue section immunostained with anti-AQP5 antibody. B. MDCK cells transfected with AQP5-EGFP plasmid DNA showing AQP5-EGFP fluorescence. Arrows indicate antibody binding or AQP5-EGFP fluorescence. Epi- Epithelial cells; Str- Stroma. Nucleus (N) stained with nuclear stain DAPI (Blue). C. Western blot. Membrane proteins extracted from mouse corneal tissue (lane 1) and MDCK cells transfected with AQP5-EGFP (lane 2) were subjected to Western blotting using anti-AQP5 antibody. Arrow – 28 kDa, unmodified AQP5, Arrowhead – 34 kDa, posttranslationally modified AQP5.
3.2. Wound healing in an in vitro model
To test whether AQP5 promotes wound healing in vitro, MDCK cells expressing AQP5-EGFP and untransfected (control) cells were cultured separately, scratch-wounded and monitored for wound healing. The first major step in wound healing is cell migration. Control and experimental culture dishes were observed under a microscope and photographed just after and at regular intervals of 2 hours, until completion of the wound repair process. MDCK cells transfected with AQP5 expression showed more cells migrating toward the wound area compared to the control cells. Six hours post-wounding, control dishes revealed considerable unrepaired area whereas the AQP5 transfected dishes showed ~80% restoration (Figure 2A). The average percentage of wound area remaining at different time points was calculated and plotted (Figure 2B). At 2, 4, 6 and 8 hours after wounding, MDCK cells expressing AQP5 showed 22, 44, 79 and 88% wound closure, respectively. Comparatively, control MDCK cells at the recorded time points of 2, 4, 6 and 8 hours after wounding, exhibited only 10, 24, 44, and 63% wound closure, respectively. The rate of wound closure in AQP5 expressing cells was statistically significant compared to control cells that are without AQP5 expression (P<0.001). Control and AQP5 expressing MDCK cells were tested for cell proliferation, the second major stage in wound healing using CytoSelect™ MTT Cell Proliferation Assay kit (Figure 3). The rate of cell proliferation in AQP5 expressing MDCK cells was statistically significant compared to the control cells which do not express AQP5 (P<0.001).
Figure 2.
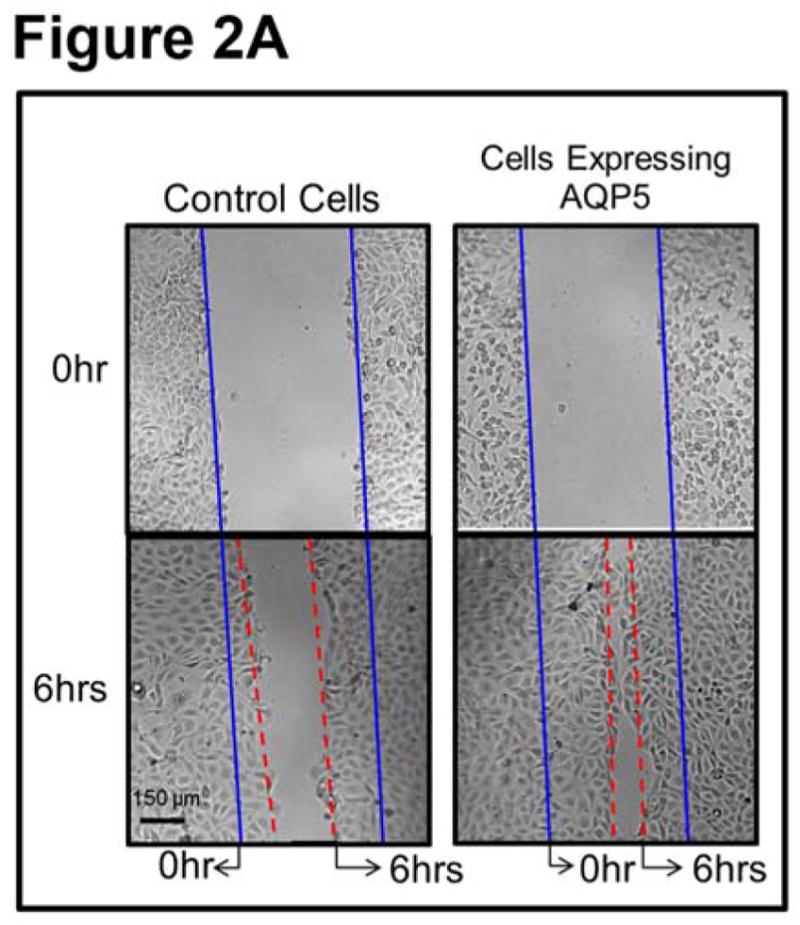
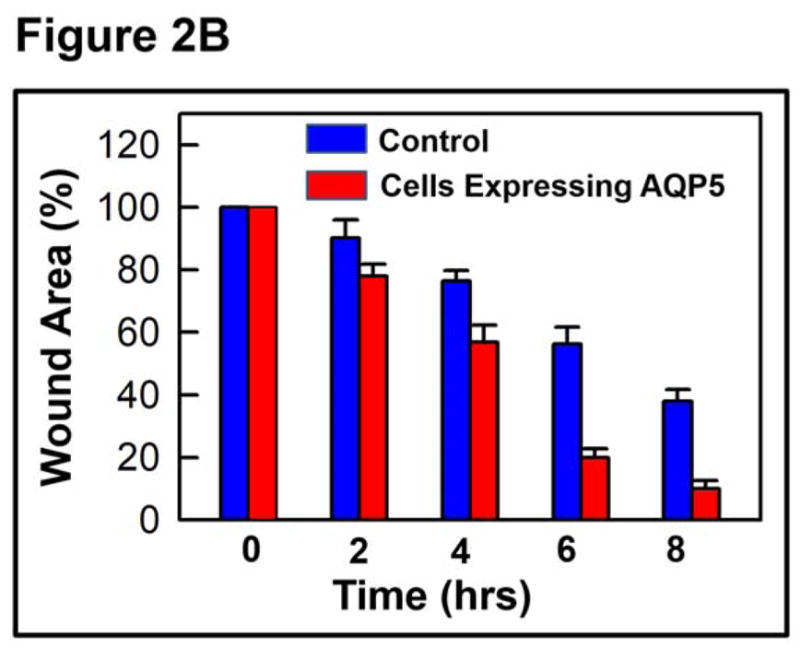
In vitro wound healing model. A. Sample micrographs of wound healing in confluent MDCK cell monolayers without (control) or with AQP5 expression. Monolayers were scratch-wounded, and repair was monitored at regular intervals, under a light microscope. Blue solid lines: wound margin immediately after making the scratch wound. Red dashed lines: cell migration after 6hrs. B. Histogram showing the rate of wound healing. MDCK cells expressing AQP5 showed significantly (P<0.001) faster cell migration than control MDCK cells.
Figure 3.
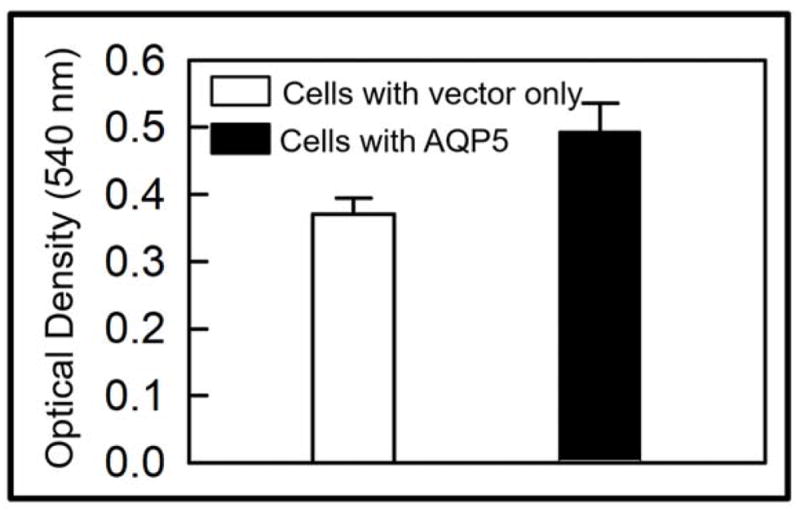
Role of AQP5 in cell proliferation. In vitro cell proliferation assay was performed to study the role of AQP5 in cell proliferation in control MDCK cells and those stably expressing transfected AQP5. Absorbance increased as a function of time due to increase in the number of cells (cell proliferation) and the resultant hike in the quantity of lactate dehydrogenase release in the culture medium. Statistically significant (P < 0.01) increase in cell proliferation was seen in cells expressing AQP5.
3.3. Wound Healing studies in an in vivo model
3.3.1. Corneal Epithelial Debridement
Results of in vitro experiments indicated involvement of AQP5 in cell migration and proliferation and prompted us to extend the studies to in vivo and ex vivo models.
Figure 4A is a schematic representation of corneal epithelial debridement method and shows a marked area from which the epithelial cells were scraped off without harming the underlying Bowman’s membrane and stroma. Using Algerbrush II, debridement of mouse corneal epithelium was performed manually; the corneal tissue was sectioned, and stained with DAPI (Figure 4B). Complete removal of the corneal epithelium without disturbing the Bowman’s membrane and stroma is demarcated in yellow. As labeled on the left, all three major layers including the unscraped epithelium are seen at the normal area of the cornea.
Figure 4.
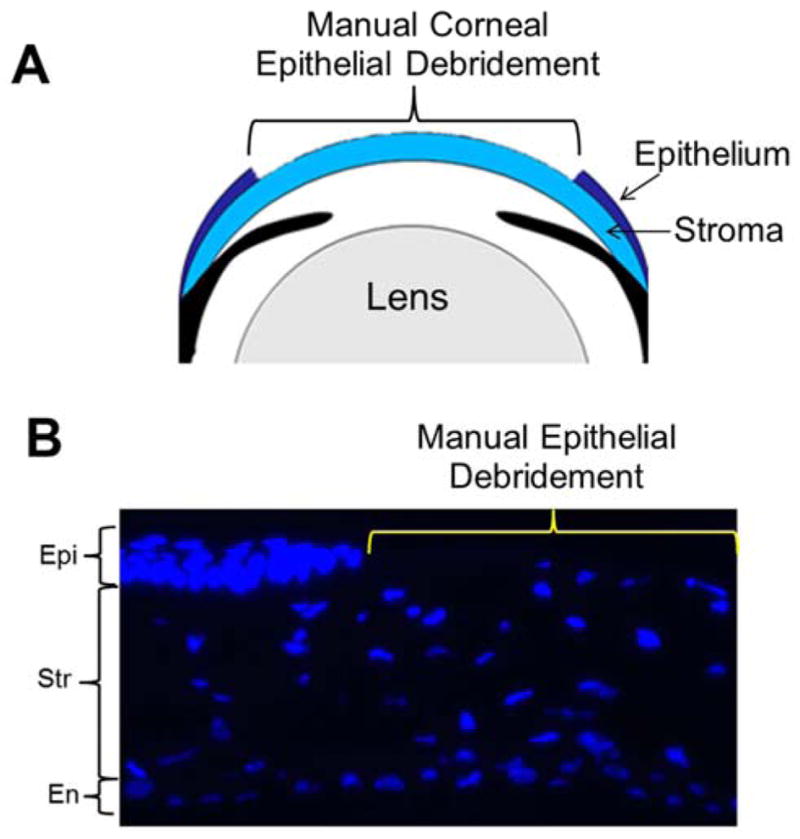
Corneal debridement. A. Schematic model of the anterior eye showing corneal epithelial debridement method. B. Manual debridement of mouse corneal epithelium. Using Algerbrush II corneal epithelium was carefully scraped off from the cornea. Tissue sections were stained with nuclear stain DAPI (blue). The area demarcated in yellow shows complete removal of the corneal epithelium. The unmarked area shows corneal epithelium and other major layers as labeled. Epi – Epithelium; Str - Stroma; En – Endothelium.
To test whether AQP5 promotes wound healing in vivo, as observed in in vitro cell culture models, rate of wound healing was tested in the wild type (AQP5+/+) and AQP5 knockout (AQP5−/−) mice using the corneal debridement method. AQP5 heterozygous (AQP5+/−) and knockout mice were tested. Photographs of the wound areas stained with sodium fluorescein (green) clearly show the appearance of the wounds at 0 hr (just after wounding) and 12 and 24 hrs thereafter (Figure 5A). Re-epithelialization and wound healing were the fastest in wild type which expresses both copies of AQP5 (AQP5+/+). AQP5−/− showed impaired re-epithelialization with very slow wound closure. AQP5+/− showed a better rate of wound healing compared to the knockout but a slower rate compared to the wild type. These data support the notion that presence of AQP5 influences wound healing in a positive manner. Rate of wound healing was quantified after tracing the initial fluorescein stained wound area vs. the stained area remaining at each time point (on the images taken at different time points), expressed as percentage, and plotted (Figure 5B). Compared to the cornea of AQP5−/− mice (28%), that of AQP5+/+ (wild type, 60%) showed about 2-fold increase in re-epithelialization at 12 hours, and 1.5-fold increase at 24 hours. AQP5+/− mice with one copy of AQP5 showed a decrease in re-epithelialization compared to the wild type, and an increase in re-epithelialization compared to the knockout (AQP5−/−), indicating the dose-dependent effect of AQP5 in wound healing.
Figure 5.
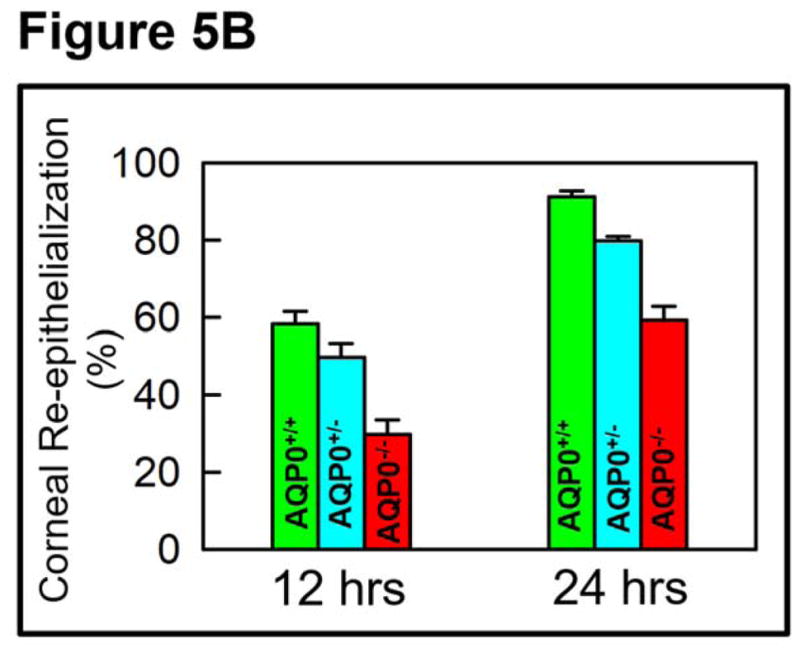
AQP5-dependent in vivo re-epithelialization after manual corneal epithelial debridement. A. At the center of the cornea, an epithelial debridement of about 2.5 mm diameter was created using Algerbrush II, stained with sodium fluorescein and imaged. Corneal resurfacing was monitored by imaging at regular intervals. Percent of remaining corneal epithelial defects in the wound area was determined by the sodium fluorescein stain remaining at 12 and 24 hrs after wounding. The images are representative of 3 corneas per condition from two independent experiments. Scale bar, 6 mm. AQP5+/+ (wild type); AQP5+/− (heterozygous); AQP5−/− (knockout). B. Rate of wound healing due to corneal re-epithelialization. Wild type (AQP5+/+) expressing AQP5 protein showed higher rate of wound healing (P<0.01) than heterozygous (AQP5+/−) or AQP5 knockout (AQP5−/−).
Tissue sections of the cornea immediately after corneal epithelial debridement and 10 hours into the repair process were immunostained with anti-AQP5 antibody to observe the expression level and pattern of AQP5 (Figure 6A,B). In the central cornea, compared to the epithelial cells at 0 hr (Figure 6A), those after 10 hrs (Figure 6B) of epithelial debridement showed several fold increase in the expression of AQP5 (green). Increased level of AQP5 expression in the corneal epithelial cells suggests involvement of AQP5 in epithelial wound repair.
Figure 6.
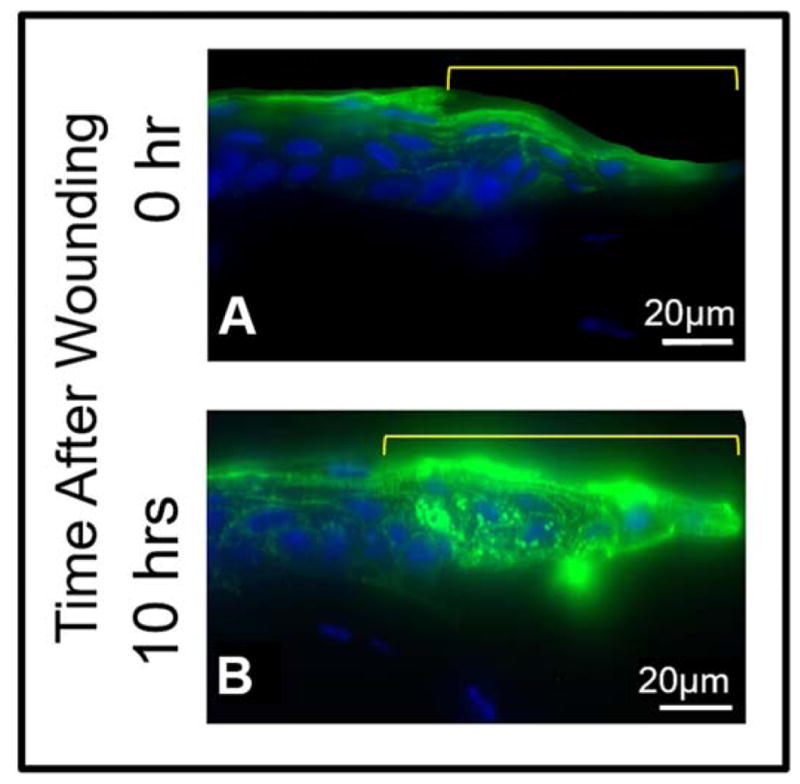
Immunostaining to show AQP5 expression after corneal epithelial debridement in the epithelial cells and stromal keratocytes from different areas of the cornea of wild type mouse. A. AQP5 expression and localization 0 hr after corneal epithelial debridement, B. AQP5 expression and localization 10 hrs after corneal epithelial debridement. The area marked in yellow on A and B indicates the wound area of the central cornea showing regenerating epithelial cells.
3.3.2. Ex-vivo Studies on Corneal Epithelial Wound Healing
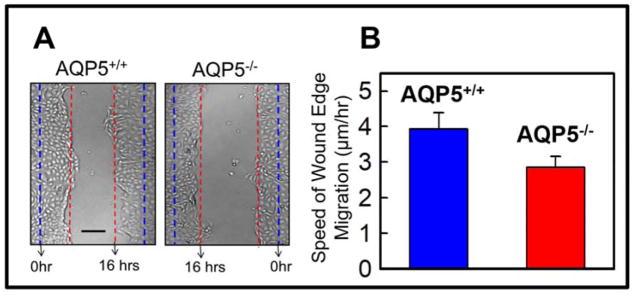
Epithelial cells isolated from the corneas of AQP5+/+ and AQP5−/− mice were cultured separately and scratch-wounded. Monitoring of wound healing in the two groups revealed that more cells in wild type (AQP5+/+) were migrating toward the wound site (Figure 7A). Rate of cell migration was calculated and plotted. AQP5 expressing wild type mouse corneal epithelial cells showed significantly (P<0.01) higher rate of cell migration to the wound area (4.0 ± 0.5 μm/hr) compared to AQP5 knockout (AQP5−/−) corneal epithelial cells (2.8 ±0.2 μm/hr; Figure 7B), suggesting the involvement of AQP5 in wound healing by enhancing cell migration.
Figure 7.
Ex vivo wound healing model to study mouse corneal epithelial cell migration. A. Corneal epithelial cells were isolated, cultured as monolayers on fibronectin-coated cell culture dishes, scratch-wounded, and repair monitored at regular intervals under a light microscope. Wild type (AQP5+/+) showed faster wound closure than AQP5 knockout (AQP5−/−) 16 hours after wounding. Blue dashed lines: wound margin immediately after wounding. Red dashed lines: cell migration after 16 hours. B. Speed of cell migration of the wound edges of isolated corneal epithelial cells of wild type (AQP5+/+) and AQP5 knockout (AQP5−/−). Wild type (AQP5+/+) showed significantly (P<0.01) faster cell migration than AQP5 knockout (AQP5−/−). Five random sample areas were selected for quantification. Scale bar = 75μm.
4. Discussion
AQP5 is an efficient water channel expressed in a wide range of cells (Raina et al., 1995). Water permeability is generally higher in secretory cells than in non-secretory cells. Knockout of AQP5 resulted in reduced transcorneal water permeability in mice (Levin and Verkman, 2004). However, other possible role(s) of AQP5 in cornea remains to be identified. Through the current investigation, we provide evidence that apart from being an efficient water transporter (Raina et al., 1995), AQP5 is a promoter of cell migration and cell proliferation during the process of corneal epithelial wound repair.
The corneal epithelium in mouse is stratified with five to six layers of cells and has a single layer of mitotically active columnar basal cells seated on a basement membrane. The basal cells are covered by one to three layers of wing cells which in turn are covered by two to three layers of flattened squamous cells. Maintenance of the epithelial layer as well as its continual regeneration is crucial for microbial barrier function, for replacing any damaged corneal surface and for corneal transparency. In the event of an injury and corneal epithelial damage, the damaged cells are replaced in three continuous stages; latent phase, cell migration and adhesion, and cell proliferation. Our experiments show that corneal epithelial cell migration and proliferation increased significantly during wound healing in the presence of AQP5.
Reports indicate the involvement of several AQPs in wound healing, in different tissues. AQP1, AQP3 and AQP5 play important roles in the maintenance of corneal transparency and homeostasis (Verkman, 2011). Under pathophysiological conditions, AQP1 (Kenney et al., 2004) and AQP5 (Rabinowitz et al., 2005) showed alterations in expression and localization. AQP1 is involved in the migration of different cell types, such as renal (Hara-Chikuma and Verkman, 2006) and gastric epithelial cells (Hayashi et al., 2009), keratocytes (Ruiz-Ederra and Verkman, 2009), endothelial and melanoma cells (Monzani et al., 2009), glioma cells (McCoy and Sontheimer, 2007), and corneal endothelial cells (Shankardas et al., 2010). Involvement of AQP5 in promoting cell migration in lung adenocarcinoma cells (SPC A-1 (Chen et al., 2011) and non small cell lung cancer cells (Chae et al., 2008) or cell proliferation in colon (Kang et al., 2008), lung (Zhang et al., 2010) and ovarian cancer cells (Yan et al., 2011) has been reported. However, Simian Virus-40 transformed corneal epithelial cell line (CEPI17) showed an increase in cell migration and proliferation, when AQP5 expression was down-regulated using short interfering RNA (Shankardas et al., 2010).
In the present study, we used cell culture and animal models to test whether AQP5 promotes wound healing. The results obtained using the MDCK cell culture model demonstrating faster wound closure, which was also reinforced by the corneal debridement studies on live mice, and the wound healing studies on ex-vivo mouse corneal epithelial cell models, strongly suggest the involvement of AQP5 in corneal re-epithelialization and wound healing. While the exact mechanism by which AQP5 promotes wound healing remains unknown, it is possible that the effect observed was exerted through its water conducting ability. In in vitro cell culture studies, control MDCK cells showed delayed healing of the scrape-wound compared to the cells transfected with AQP5 (Figure 2A). We noticed that in AQP5 transfected culture dishes, the marginal cells at the leading edge of the wound formed more lamellipodial and filipodial extensions as part of the cell migration process. Presence of similar extensions during corneal wound repair and a reduction of these structures in aquaporin-deficient cells had been reported (Hara-Chikuma and Verkman, 2006; Saadoun et al., 2005). The impaired wound healing in AQP5 knockout mice could be due to defective cell migration and proliferation, as reported for AQP3 knockout mice by Levin and Verkman (2006).
Papadopoulos et al., (2008) stated that AQPs could facilitate cell migration by increasing the membrane water flux at the leading edge of migrating cells causing accelerated formation of lamellipodial projections in response to actin depolymerization and intake of ions. This mechanism of cell migration has been observed in different AQP expressing cells. The same events could be responsible for the faster cell migration in AQP5 expressing cells during wound healing in the experiments conducted in our laboratory. Water-selective AQPs, namely, AQP1 and AQP4 facilitate cell migration (Saadoun et al., 2005a, 2005b). However, AQP3, an aquaglyceroporin, promotes wound healing by exerting its water as well as glycerol permeability properties in a distinct manner; while cell migration is facilitated by the water permeability function, cell proliferation is facilitated by the glycerol permeability (Levin and Verkman, 2006; Verkman, 2011). It is interesting to note that AQP5, a water-selective AQP, facilitates both cell migration and cell proliferation. Sidhaye et al. (2012) reported the involvement of AQP5 in microtubule assembly and stability. The increased cell proliferation observed in our in vitro studies could be due to the interaction of AQP5 with microtubules, which might have enhanced microtubule stability and hastened the cell cycle progression. Changes in morphology have been reported in cancer cells due to AQP5 overexpression (Chae et al., 2008; Watanabe et al., 2009). However, there was no noticeable morphological changes in MDCK cells or cultured corneal epithelial cells due to the expression or upregulation of AQP5, indicating the non-carcinogenic status of these cells.
Overexpression of AQP5 in non-cancerous MDCK cells did not induce epithelial-to-mesenchymal transition (Jensen et al., 2018) which is a common characteristic found in epithelial cell cancer (Chae et al., 2008; Watanabe et al., 2009; Zhang et al., 2010). However, overexpression of AQP5 in non-cancerous human bronchial cells (BEAS-2B) showed one clone maintaining epithelial morphology while another expressing low levels of epithelial markers and increased levels of mesenchymal markers indicating epithelial-to-mesenchymal transition suggesting that cellular environment and signaling pathways are critical for AQP5-induced cell behavior (Jensen et al. (2018). These studies and the present investigation suggest that AQP5 expression in both non-cancerous and malignant cellular environments can induce increased cell migration and proliferation. In the current study, upregulation of AQP5 found in wild type mouse corneal epithelial wound site (Fig. 6B) did not result in cancerous growth in the cornea. It caused normal but faster wound healing by promoting enhanced cell migration and proliferation, suggesting that these immune-privileged corneal cells may not have cancerous environment and associated oncogenic signaling pathways to become invasive.
The difference between our study and cancerous cell studies is, in the former, cell migration is towards a wound to repair the damaged area. In this non-cancerous environment, the cells could be controlled by signaling pathways that limit further cell migration and proliferation once the wounded area is repaired. Events such as contact inhibition could be responsible to stop further progression of these events. In cancerous cells, the signal is to multiply, become invasive and metastasize. Therefore, the molecular mechanisms underlying the two processes, one of controlled cell migration and proliferation and the other of uncontrolled cell migration and proliferation, though considered a consequence of AQP5 upregulation, must be quite distinct depending upon the cellular environment. The molecular mechanism behind AQP5-induced cell migration and proliferation in the wound healing process will be investigated in future.
5. Conclusions
The in vitro, in vivo and ex vivo studies conducted demonstrate, to our knowledge, for the first time, that AQP5 takes part in corneal wound healing. Besides having the role of being a water channel, AQP5 performs a unique function of facilitating corneal wound healing by enhancing cell migration and proliferation, which are very complex processes. The wound healing property of AQP5 could be exploited for designing therapeutic strategies.
Highlights.
AQP5 promotes wound healing in the cornea
AQP5 facilitates cell migration
AQP5 promotes cell proliferation
Acknowledgments
This work was supported by NIH-NEI grant R01: EY20506, EY026155 (to K.V.), and NIH grant R01: DE13825 (to A.G.M.).
Abbreviations
- AQP5
Aquaporin 5
- MDCK
Madin–Darby canine kidney
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–78. doi: 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- Chae YK, Woo J, Kim MJ, et al. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLoS One. 2008;3:e2162. doi: 10.1371/journal.pone.0002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhan Z, Gu Y, et al. Impaired migration and cell volume regulation in aquaporin 5-deficient SPC-A1 cells. Respir Physiol Neurobiol. 2011;176:110–117. doi: 10.1016/j.resp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Direito I, Paulino J, Vigia E, et al. Differential expression of aquaporin-3 and aquaporin-5 in pancreatic ductal adenocarcinoma. J Surg Oncol. 2017;115:980–996. doi: 10.1002/jso.24605. [DOI] [PubMed] [Google Scholar]
- Funaki H, Yamamoto T, Koyama Y, et al. Localization and expression of AQP5 in cornea, serous salivary glands, and pulmonary epithelial cells. Am J Physiol. 1998;275:C1151–1157. doi: 10.1152/ajpcell.1998.275.4.C1151. [DOI] [PubMed] [Google Scholar]
- Garfias Y, Navas A, Perez-Cano HJ, et al. Comparative expression analysis of aquaporin-5 (AQP5) in keratoconic and healthy corneas. Mol Vis. 2008;14:756–761. [PMC free article] [PubMed] [Google Scholar]
- Guo K, Jin F. NFAT5 promotes proliferation and migration of lung adenocarcinoma cells in part through regulating AQP5 expression. Biochem Biophys Res Commun. 2015;465:644–649. doi: 10.1016/j.bbrc.2015.08.078. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J Am Soc Nephrol. 2006;17:39–45. doi: 10.1681/ASN.2005080846. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28:326–332. doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Takahashi N, Kurata N, et al. Involvement of aquaporin-1 in gastric epithelial cell migration during wound repair. Biochem Biophys Res Commun. 2009;386:483–487. doi: 10.1016/j.bbrc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- Hiroaki Y, Tani K, Kamegawa A, Gyobu N, et al. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol. 2006;355:628–639. doi: 10.1016/j.jmb.2005.10.081. [DOI] [PubMed] [Google Scholar]
- Jiang XX, Xu KH, Ma JY, et al. Reduced migration of Ishikawa cells associated with downregulation of aquaporin-5. Oncol Lett. 2012;4:257–261. doi: 10.3892/ol.2012.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen HH, Login FH, Koffman JS, et al. The role of aquaporin-5 in cancer cell migration: A potential active participant. Int J Biochem Cell Biol. 2016;79:271–276. doi: 10.1016/j.biocel.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Jensen HH, Holst MR, Login FH, et al. Ectopic expression of AQP5 in non-cancerous epithelial MDCK cells changes morphology without inducing EMT. Am J Physiol Cell Physiol. 2018 doi: 10.1152/ajpcell.00186.2017. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Park JY, Jeon HS, et al. Aquaporin-5: a marker protein for proliferation and migration of human breast cancer cells. PLoS One. 2011;6:e28492. doi: 10.1371/journal.pone.0028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SK, Chae YK, Woo J, et al. Role of human aquaporin 5 in colorectal carcinogenesis. Am J Pathol. 2008;173:518–525. doi: 10.2353/ajpath.2008.071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MC, Atilano SR, Zorapapel N, et al. Altered expression of aquaporins in bullous keratopathy and Fuchs’ dystrophy corneas. J Histochem Cytochem. 2004;52:1341–1350. doi: 10.1177/002215540405201010. [DOI] [PubMed] [Google Scholar]
- Krane CM, Melvin JE, Nguyen HV. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;276:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- Kumari S, Gao J, Mathias RT, et al. Aquaporin 0 Modulates Lens Gap Junctions in the Presence of Lens-Specific Beaded Filament Proteins. Invest Ophthalmol Vis Sci. 2017;58:6006–6019. doi: 10.1167/iovs.17-22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Eswaramoorthy S, Mathias RT, et al. Unique and analogous functions of aquaporin 0 for fiber cell architecture and ocular lens transparency. Biochim Biophys Acta. 2011;1812:1089–1097. doi: 10.1016/j.bbadis.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K. Intact AQP0 performs cell-to-cell adhesion. Biochem Biophys Res Commun. 2009;390:1034–1039. doi: 10.1016/j.bbrc.2009.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj M, Yerramilli VS, et al. Spatial expression of Aquaporin 5 in mammalian cornea and lens and regulation of its localization by Phosphokinase A. Mol Vis. 2012;18:957–967. [PMC free article] [PubMed] [Google Scholar]
- Levin MH, Verkman AS. Aquaporin-dependent water permeation at the mouse ocular surface: in vivo microfluorimetric measurements in cornea and conjunctiva. Invest Ophthalmol Vis Sci. 2004;45:4423–4432. doi: 10.1167/iovs.04-0816. [DOI] [PubMed] [Google Scholar]
- Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- McCoy E, Sontheimer H. Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia. 2007;55:1034–1043. doi: 10.1002/glia.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzani E, Bazzotti R, Perego C, et al. AQP1 is not only a water channel: it contributes to cell migration through Lin7/beta-catenin. PLoS One. 2009;4:e6167. doi: 10.1371/journal.pone.0006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil RV, Saito I, Yang X, et al. Expression of aquaporins in the rat ocular tissue. Exp Eye Res. 1997;64:203–209. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, et al. Appearance of water channels in Xenopus oocytes expressing red-cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Rabinowitz YS, Dong L, Wistow G. Gene expression profile studies of human keratoconus cornea for NEI Bank: a novel cornea-expressed gene and the absence of transcripts for aquaporin 5. Invest Ophthalmol Vis Sci. 2005;46:1239–1246. doi: 10.1167/iovs.04-1148. [DOI] [PubMed] [Google Scholar]
- Raina S, Preston GM, Guggino WB, et al. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- Ren Y, Lu H, Reinach PS, et al. Hyperosmolarity-induced AQP5 upregulation promotes inflammation and cell death via JNK1/2 Activation in human corneal epithelial cells. Sci Rep. 2017;7:4727. doi: 10.1038/s41598-017-05145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Verkman AS. Aquaporin-1-facilitated keratocyte migration in cell culture and in vivo corneal wound healing models. Exp Eye Res. 2009;89:159–165. doi: 10.1016/j.exer.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, et al. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005a;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Watanabe H, et al. Involvement of Aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005b;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Tait MJ, Reza A, et al. AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience. 2009;161:764–772. doi: 10.1016/j.neuroscience.2009.03.069. [DOI] [PubMed] [Google Scholar]
- Shankardas J, Patil RV, Vishwanatha JK. Effect of down-regulation of aquaporins in human corneal endothelial and epithelial cell lines. Mol Vis. 2010;16:1538–1548. [PMC free article] [PubMed] [Google Scholar]
- Sidhaye VK, Chau E, Srivastava V. A novel role for aquaporin-5 in enhancing microtubule organization and stability. PLoS One. 2012;7:e38717. doi: 10.1371/journal.pone.0038717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajah JR, Verkman AS. Aquaporin deletion in mice reduces corneal water permeability and delays restoration of transparency after swelling. J Biol Chem. 2002;277:19139–19144. doi: 10.1074/jbc.M202071200. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, Mathias RT. Transgenic expression of AQP1 in the fiber cells of AQP0 knockout mouse: effects on lens transparency. Exp Eye Res. 2010;91:393–404. doi: 10.1016/j.exer.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Patil R, et al. Functional characterization of a human aquaporin 0 mutation that leads to a congenital dominant lens cataract. Exp Eye Res. 2008;87:9–21. doi: 10.1016/j.exer.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS. Mammalian aquaporins: diverse physiological roles and potential clinical significance. Expert Rev Mol Med. 2008;10:e13. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS. Aquaporins at a glance. J Cell Sci. 2011;124:2107–2112. doi: 10.1242/jcs.079467. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Fujii T, Oya T, et al. Involvement of aquaporin-5 in differentiation of human gastric cancer cells. J Physiol Sci. 2009;59:113–122. doi: 10.1007/s12576-008-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley L, SundarRaj N, Sun TT. Regional heterogeneity in human corneal and limbal epithelia: an immunohistochemical evaluation. Invest Ophthalmol Vis Sci. 1991;32:594–602. [PubMed] [Google Scholar]
- Yan C, Yang J, Shen L, et al. Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch Gynecol Obstet. 2011;285:459–467. doi: 10.1007/s00404-011-1942-6. [DOI] [PubMed] [Google Scholar]
- Yan C, Zhu Y, Zhang X, et al. Down-regulated aquaporin 5 inhibits proliferation and migration of human epithelial ovarian cancer 3AO cells. J Ovarian Res. 2014;7:78. doi: 10.1186/s13048-014-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang H, Wang Z, et al. Cannabinoid receptor 1 suppresses transient receptor potential vanilloid 1-induced inflammatory responses to corneal injury. Cell Signal. 2013;25:501–511. doi: 10.1016/j.cellsig.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang JN, Chen WL, et al. Effects of AQP5 gene silencing on proliferation, migration and apoptosis of human glioma cells through regulating EGFR/ERK/p38 MAPK signaling pathway. Oncotarget. 2017;8:38444–38455. doi: 10.18632/oncotarget.16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen Z, Song Y, et al. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J Pathol. 2010;221:210–220. doi: 10.1002/path.2702. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Conrad AH, Conrad GW. Effects of ultraviolet-A and riboflavin on the interaction of collagen and proteoglycans during corneal cross-linking. J Biol Chem. 2011;286:13011–13022. doi: 10.1074/jbc.M110.169813. [DOI] [PMC free article] [PubMed] [Google Scholar]