Abstract
Background
The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial established the safety of omitting axillary lymph node dissection (ALND) for early-stage breast cancer patients with limited nodal disease undergoing lumpectomy. We examined the extent of axillary surgery among women eligible for Z0011 based on patient age and tumor subtype.
Methods
Patients with cT1–2, cN0 breast cancers and one or two positive nodes diagnosed from 2009 to 2014 and treated with lumpectomy were identified in the National Cancer Data Base. Sentinel lymph node biopsy (SLNB) was defined as the removal of 1–5 nodes and ALND as the removal of 10 nodes or more. Tumor subtype was categorized as luminal, human epidermal growth factor 2-positive (HER2+), or triple-negative. Logistic regression was used to estimate the odds of receiving SLNB alone versus ALND.
Results
The inclusion criteria were met by 28,631 patients (21,029 SLNB-alone and 7602 ALND patients). Patients 70 years of age or older were more likely to undergo SLNB alone than ALND (27.0% vs 20.1%; p <0.001). The radiation therapy use rate was 89.4% after SLNB alone and 89.7% after ALND. In the multivariate analysis, the uptake of Z0011 recommendations increased over time (2014 vs 2009: odds ratio [OR] 13.02; p < 0.001). Younger patients were less likely to undergo SLNB alone than older patients (age <40 vs ≥70: OR 0.59; p <0.001). Patients with HER2+ (OR 0.89) or triple-negative disease (OR 0.79) (p < 0.001) were less likely to undergo SLNB alone than those with luminal subtypes.
Conclusions
Among women potentially eligible for ACOSOG Z0011, the use of SLNB alone increased over time in all groups, but the extent of axillary surgery differed by patient age and tumor subtype.
Axillary node involvement is an important prognostic factor in breast cancer, and axillary staging has evolved over time. In 1994, Giuliano et al.1 described sentinel lymph node biopsy (SLNB) as an alternative to axillary lymph node dissection (ALND) for clinically node-negative breast cancer patients.2,3 In 2010, the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial compared SLNB with ALND among women who had clinical T1–2, N0 breast cancers with one or two positive sentinel lymph nodes (SLNs) undergoing lumpectomy.4 At 10 years, neither locoregional recurrence nor overall survival differed between the groups, demonstrating the oncologic safety of SLNB for women with low-volume nodal disease.5 Recommendations from the American Society of Clinical Oncology, National Comprehensive Cancer Network, and American Society of Breast Surgeons reflect the Z0011 findings.6–8 We examined the association of patient age and tumor subtype with the extent of axillary surgery among women potentially eligible for Z0011.
METHODS
In this Duke Institutional Review Board-exempted study, Z0011-eligible patients, defined as women 18–90 years of age undergoing lumpectomy for cT1–2, cN0 breast cancers with one or two positive nodes diagnosed from 2009 to 2014 were identified in the American College of Surgeons National Cancer Data Base (NCDB). Patients were excluded from the study if they had undergone neoadjuvant chemotherapy; had an unknown number of positive lymph nodes, more than two positive lymph nodes, or no positive nodes; or had unknown receptor status. Because nodal procedures are not reliably coded within the NCDB, SLNB alone was defined as examination of 1–5 nodes and ALND as examination of 10 nodes or more.9 Patients with six to nine nodes were excluded from the study.
Patient characteristics were summarized as number and percentage for categorical variables and as median and interquartile range (IQR) for continuous variables. Comparison of the SLNB-alone and ALND patients was performed using the Chi square test for categorical variables and t test for continuous variables.
Multivariate logistic regression identified factors associated with the receipt of SLNB alone versus ALND. The covariates in the model included subtype, age, race, year of diagnosis, education, income, insurance, facility type, comorbidity score, cT stage, and grade. Interaction of age and subtype was included in an adjusted model to determine whether the effect of subtype on receipt of SLNB alone or ALND varied by age. Pairwise comparisons were conducted by subtype [HER2+ vs luminal, HER2+ vs triple-negative breast cancer (TNBC), and luminal vs TNBC] and age group (<40 vs 40–69, <40 vs ≥70, and 40–69 vs ≥70 years) and included a Tukey adjustment.
Adjusted proportions for each subtype and age interaction group were estimated from the interaction model. A generalized estimating equations framework model with an exchangeable correlation structure accounted for correlation between patients treated at the same facility.
Only patients with data for all covariates were included in each model. Except for pairwise comparisons, no adjustments were made for multiple comparisons. All statistical analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC, USA).
RESULTS
The study enrolled 28,631 patients (21,029 with SLNB alone and 7602 with ALND) (Table 1). The median age was 61 years (IQR 53–70 years) and was similar between groups. Women 70 years of age or older were more likely to undergo SLNB alone than women younger than 40 years (78.8% vs 64%; p < 0.001). Adjuvant chemotherapy was administered to 47.8% of the patients who underwent SLNB alone compared with 66.6% of the patients who underwent ALND. Endocrine therapy was received by 81.7% of the SLNB-alone versus 77.7% of the ALND patients. 89.4% of women received radiation therapy after SLNB and 89.7% after ALND. Receipt of ALND was associated with grade 3 (32.0% vs 22.5%), cT2 (29.9% vs 24.5%), HER2+ (10.3% vs 7.0%), or triple-negative (10.2% vs 6.3%) (all p < 0.001) breast cancers. The rates of SLNB alone increased over time, from 34.3% in 2009 to 86.7% in 2014.
TABLE 1.
Baseline patient characteristics
| All patients (n = 28,631) n (%)a |
SLNB alone (1–5 LN) (n = 21,029) n (%)a |
ALND (≥10 LN) (n = 7602) n (%)a |
p value | |
|---|---|---|---|---|
| Age (years) | <0.001 | |||
| < 40 | 755 (2.6) | 483 (2.3) | 272 (3.6) | |
| 40–69 | 20,675 (72.2) | 14,874 (70.7) | 5801 (76.3) | |
| ≥70 | 7201 (25.2) | 5672 (27) | 1529 (20.1) | |
| Median (IQR) | 61 (53–70) | 62 (53–70) | 60 (51–68) | |
| Race | <0.001 | |||
| White | 24,242 (84.7) | 17,997 (85.6) | 6245 (82.1) | |
| Black | 2999 (10.5) | 2045 (9.7) | 954 (12.5) | |
| Other | 1149 (4) | 816 (3.9) | 333 (4.4) | |
| Ethnicity | 0.02 | |||
| Hispanic | 1438 (5) | 1021 (4.9) | 417 (5.5) | |
| Non-Hispanic | 26,273 (91.8) | 19,375 (92.1) | 6898 (90.7) | |
| Year of diagnosis | <0.001 | |||
| 2009 | 461 (1.6) | 158 (0.8) | 303 (4) | |
| 2010 | 4534 (15.8) | 1994 (9.5) | 2540 (33.4) | |
| 2011 | 5527 (19.3) | 3914 (18.6) | 1613 (21.2) | |
| 2012 | 5837 (20.4) | 4540 (21.6) | 1297 (17.1) | |
| 2013 | 5938 (20.7) | 4932 (23.5) | 1006 (13.2) | |
| 2014 | 6334 (22.1) | 5491 (26.1) | 843 (11.1) | |
| Median distance traveled (IQR) | 8.7 (4.2–17.5) | 8.7 (4.2–17.6) | 8.6 (4.1–17.4) | 0.57 |
| Income level ($) | 0.004 | |||
| <35,000 | 6780 (23.7) | 4887 (23.2) | 1893 (24.9) | |
| ≥35,000 | 20,922 (73.1) | 15,449 (73.5) | 5473 (72) | |
| Insurance status | <0.001 | |||
| Private | 15,160 (52.9) | 10,924 (51.9) | 4236 (55.7) | |
| Government | 12,655 (44.2) | 9530 (45.3) | 3125 (41.1) | |
| Not insured | 511 (1.8) | 356 (1.7) | 155 (2) | |
| Education level | <0.001 | |||
| ≤80% HS graduation rate | 9168 (32) | 6573 (31.3) | 2595 (34.1) | |
| >80% HS graduation rate | 18,529 (64.7) | 13,758 (65.4) | 4771 (62.8) | |
| Facility type | <0.001 | |||
| Academic | 8885 (31) | 6722 (32) | 2163 (28.5) | |
| Integrated network | 3050 (10.7) | 2252 (10.7) | 798 (10.5) | |
| Comprehensive | 13,849 (48.4) | 10,145 (48.2) | 3704 (48.7) | |
| Community | 2847 (9.9) | 1910 (9.1) | 937 (12.3) | |
| Facility location | <0.001 | |||
| Midwest | 7751 (27.1) | 5458 (26) | 2293 (30.2) | |
| Northeast | 6244 (21.8) | 4721 (22.4) | 1523 (20) | |
| South | 9234 (32.3) | 6791 (32.3) | 2443 (32.1) | |
| West | 5402 (18.9) | 4059 (19.3) | 1343 (17.7) | |
| Charlson/Deyo comorbidity score | 0.27 | |||
| 0 | 23,721 (82.9) | 17,378 (82.6) | 6343 (83.4) | |
| 1 | 4043 (14.1) | 3010 (14.3) | 1033 (13.6) | |
| ≥2 | 867 (3) | 641 (3) | 226 (3) | |
| Laterality | 0.45 | |||
| Bilateral | 2 (0) | 1 (0) | 1 (0) | |
| Unilateral | 28,627 (100) | 21,026 (100) | 7601 (100) | |
| Grade | <0.001 | |||
| 1 | 6296 (22) | 4897 (23.3) | 1399 (18.4) | |
| 2 | 13,924 (48.6) | 10,437 (49.6) | 3487 (45.9) | |
| 3 | 7153 (25) | 4722 (22.5) | 2431 (32) | |
| ER status | <0.001 | |||
| ER+ | 25,788 (90.1) | 19,230 (91.4) | 6558 (86.3) | |
| ER− | 2843 (9.9) | 1799 (8.6) | 1044 (13.7) | |
| PR status | <0.001 | |||
| PR+ | 23,642 (82.6) | 17,669 (84) | 5973 (78.6) | |
| PR− | 4989 (17.4) | 3360 (16) | 1629 (21.4) | |
| HER2 status | <0.001 | |||
| HER2+ | 2256 (7.9) | 1474 (7) | 782 (10.3) | |
| HER2− | 26,375 (92.1) | 19,555 (93) | 6820 (89.7) | |
| Molecular subtype | <0.001 | |||
| HER2+ | 2256 (7.9) | 1474 (7) | 782 (10.3) | |
| Luminal | 24,282 (84.8) | 18,237 (86.7) | 6045 (79.5) | |
| TNBC | 2093 (7.3) | 1318 (6.3) | 775 (10.2) | |
| Clinical T stage | <0.001 | |||
| 1 | 21,207 (74.1) | 15,878 (75.5) | 5329 (70.1) | |
| 2 | 7424 (25.9) | 5151 (24.5) | 2273 (29.9) | |
| Median tumor size (cm) (IQR) | 1.7 (1.2–2.3) | 1.7 (1.2–2.2) | 1.8 (1.3–2.5) | <0.001 |
| Median no. of positive nodes (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–2) | <0.001 |
| Median no. of nodes examined (IQR) | 3 (2–10) | 2 (2–3) | 15 (12–19) | <0.001 |
| Treated with chemotherapy | 15,119 (52.8) | 10,053 (47.8) | 5066 (66.6) | <0.001 |
| Treated with endocrine therapy | ||||
| Out of all patients | 23,080 (80.6) | 17,175 (81.7) | 5905 (77.7) | <0.001 |
| Out of ER+ or PR+ patients | 23,010 (88.8) | 17,136 (88.7) | 5874 (89) | 0.60 |
| Treated with radiation | 25,617 (89.5) | 18,798 (89.4) | 6819 (89.7) | 0.46 |
SLNB sentinel lymph node biopsy, LN lymph node, ALND axillary lymph node dissection, IQR interquartile range, HS high school, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor 2, TNBC triple-negative breast cancer
Percentages may not add up to 100 due to rounding or missing values
Multivariate analysis was used to model the odds of receiving SLNB alone as a proxy for adoption of Z0011 guidelines (Table 2). Younger patients were less likely to receive SLNB alone than older patients [age <40 years: odds ratio (OR) 0.59, 95% confidence interval (CI) 0.49–0.71; age 40–69 years: OR 0.69, 95% CI 0.64–0.75; p<0.001]. Treatment with SLNB alone was less likely for grade 2 or 3 vs grade 1 disease (grade 2: OR 0.86, 95% CI 0.80–0.92 vs grade 3: OR 0.65, 95% CI 0.59–0.71), cT2 vs cT1 (OR 0.81, 95% CI 0.76–0.87), HER2+ (OR 0.89, 95% CI 0.80–0.98), and triple-negative vs luminal disease (OR 0.79, 95% CI 0.71–0.88) (all p <0.001). Patients treated at community centers were less likely to receive SLNB alone than those treated at academic centers (OR 0.67, 95% CI 0.57–0.78; p < 0.001). Application of Z0011 recommendations increased over time (2014 vs 2009: OR 13.02, 95% CI 9.82–17.26; p <0.001).
TABLE 2.
Adjusted logistic regression predicting receipt of sentinel lymph node biopsy (SLNB) versus axillary lymph node dissection (ALND) (n = 25,993)
| OR (95% CI) | p value | Overall p value | |
|---|---|---|---|
| Molecular subtype | <0.001 | ||
| Luminal | Reference | ||
| HER2+ | 0.888 (0.802–0.982) | 0.02 | |
| TNBC | 0.793 (0.712–0.884) | <0.001 | |
| Age (years) | <0.001 | ||
| ≥70 | Reference | ||
| 40–69 | 0.694 (0.643–0.749) | <0.001 | |
| <40 | 0.591 (0.494–0.705) | <0.001 | |
| Race | <0.001 | ||
| White | Reference | ||
| Black | 0.825 (0.74–0.919) | <0.001 | |
| Other | 0.836 (0.722–0.967) | 0.02 | |
| Year of diagnosis | <0.001 | ||
| 2009 | Reference | ||
| 2010 | 1.563 (1.199–2.038) | <0.001 | |
| 2011 | 4.935 (3.761–6.475) | <0.001 | |
| 2012 | 7.211 (5.489–9.472) | <0.001 | |
| 2013 | 9.601 (7.278–12.664) | <0.001 | |
| 2014 | 13.018 (9.819–17.259) | <0.001 | |
| Education level | 0.19 | ||
| ≤80% HS graduation rate | Reference | ||
| >80% HS graduation rate | 1.052 (0.977–1.132) | 0.18 | |
| Income level ($) | 0.86 | ||
| <35,000 | Reference | ||
| ≥35,000 | 0.993 (0.913–1.079) | 0.86 | |
| Insurance | 0.99 | ||
| Private | Reference | ||
| Government | 1.004 (0.939–1.073) | 0.91 | |
| Not insured | 1.016 (0.814–1.268) | 0.89 | |
| Facility type | <0.001 | ||
| Academic | Reference | ||
| Integrated network | 1 (0.832–1.203) | 1.00 | |
| Comprehensive | 0.888 (0.786–1.005) | 0.06 | |
| Community | 0.665 (0.57–0.777) | <0.001 | |
| Facility location | <0.001 | ||
| South | Reference | ||
| Midwest | 0.755 (0.661–0.864) | <0.001 | |
| Northeast | 0.983 (0.857–1.126) | 0.80 | |
| West | 1.049 (0.903–1.219) | 0.53 | |
| Charlson/Deyo comorbidity score | 0.72 | ||
| 0 | Reference | ||
| 1 | 1.006 (0.922–1.098) | 0.89 | |
| ≥2 | 0.935 (0.795–1.1) | 0.42 | |
| Clinical T stage | <0.001 | ||
| 1 | Reference | ||
| 2 | 0.813 (0.763–0.866) | <0.001 | |
| Grade | <0.001 | ||
| 1 | Reference | ||
| 2 | 0.857 (0.795–0.923) | <0.001 | |
| 3 | 0.648 (0.592–0.71) | <0.001 |
OR odds ratio, CI confidence interval, HER2 human epidermal growth factor 2, TNBC triple-negative breast cancer, HS high school
The interaction model demonstrated that the effect of tumor subtype on receipt of SLNB alone did not differ by age (interaction: p = 0.90) (Table 3). However, individual pairwise interaction comparisons demonstrated that the adjusted proportion of patients treated with SLNB alone was higher among older patients with HER2+ and luminal disease (multiple comparisons adjusted p < 0.05 for all comparisons).
TABLE 3.
Odds of receiving axillary lymph node dissection (ALND) as pairwise interactions of age and molecular subtype (n = 31,026)
| Interaction | Age (years) | Molecular subtype | Adjusted OR (95% CI) | Pairwise differences | Overall interaction p value | |
|---|---|---|---|---|---|---|
| 1 | Age and subtype | <40 | HER2+ | 0.519 (0.416–0.621) | 7 8 | 0.90 |
| 2 | Luminal | 0.578 (0.523–0.631) | 7 8 | |||
| 3 | TNBC | 0.522 (0.418–0.624) | 7 8 | |||
| 4 | 40–69 | HER2+ | 0.583 (0.54–0.624) | 7 8 | ||
| 5 | Luminal | 0.612 (0.581–0.641) | 6 8 | |||
| 6 | TNBC | 0.561 (0.521–0.6) | 5 7 8 | |||
| 7 | ≥70 | HER2+ | 0.676 (0.62–0.728) | 1 2 3 4 6 | ||
| 8 | Luminal | 0.695 (0.664–0.725) | 1 2 3 4 5 6 9 | |||
| 9 | TNBC | 0.625 (0.566–0.681) | 8 |
OR odds ratio, CI confidence interval, HER2 human epidermal growth factor 2, TNBC triple-negative breast cancer
Although adoption of Z0011 guidelines increased overall, this was inconsistent across patient age and tumor subtype. Younger women, and those with HER2+ and triple-negative disease, were more likely to receive ALND than older patients with luminal-type cancers.
DISCUSSION
Our study examined the surgical management of the axilla in a nationwide cohort of women potentially eligible for the Z0011 trial. We found that use of SLNB alone among women with low-volume nodal disease has increased over time, but that women younger than 40 years with HER2+ or triple-negative disease were less likely to undergo SLNB alone than their older counterparts with luminal cancers. Physician risk-stratification by tumor biology may drive higher rates of ALND for these patients.
Other authors have reported that axillary management is inconsistent across the breast cancer population. Bilimoria et al.9 found that 20.8% of patients with positive SLN treated from 1998 to 2005 did not undergo ALND and did not experience worse overall survival. These patients were more likely to be older, to have smaller tumors, and to receive treatment at centers other than National Cancer Institute-designated centers. Caudle et al.10 found that surgeons were more likely to perform ALND for patients with larger tumors, lobular histology, fewer SLNs, larger SLN metastases, or extranodal extension. Conversely, Mamtani et al.11 demonstrated that Memorial Sloan Kettering surgeons performed SLNB alone for 85% of patients with high-risk features (TNBC, HER2+, or age<50 years) compared with 82% of patients at average risk. Importantly, the rates for additional positive nodes found at ALND were similar between the two groups (62% vs 65%; p = 0.8). Because most of the women in Z0011 were older than 50 years (63%) or had hormone-receptor-positive disease (76%), concerns arose regarding its broader applicability to patients at higher risk for locoregional recurrence or distant metastasis, including younger women or those with HER2 overexpression or triple-negative disease. Our study demonstrated that nationally, patients with high-risk features were more likely to undergo ALND despite potential eligibility for SLNB alone.
Before publication of the ACOSOG Z0011 trial, the rates of ALND had decreased with increasing patient age,12,13 and this trend continued during our study period. Women younger than 40 years commonly receive a diagnosis of higher-risk breast cancer, and young age is an independent risk factor for recurrence and death.14–16 These outcomes must be weighed against the risk for lymphedema and functional impairment in this population.17 Our pairwise comparison analysis addressed this concern (Table 3). Although the overall interaction was not significant, the individual interactions of age and tumor subtype yielded a significantly lower rate of ALND among elderly patients with luminal and HER2+ disease. However, the rates for ALND did not differ significantly between high-risk young women with triple-negative or HER2+ disease and average-risk middle-aged women with hormone receptor-positive disease.
The aforementioned findings corroborate those of previous studies demonstrating that high-risk characteristics may portend distant metastasis but not axillary tumor burden.18 Thus, although younger patients and those with HER2+ or triple-negative disease may have more aggressive biology, aggressive axillary clearance may not be warranted. We found that ALND was performed more frequently for women with TNBC than for those with HER2+ or luminal disease. This may be due to concern for higher risk of local recurrence secondary to lack of targeted therapies. The ongoing Alliance 11202 trial will address the extent of axillary surgery needed for women undergoing preoperative chemotherapy.19
We additionally found that women treated at academic centers were more likely to receive SLNB alone than those treated at community cancer centers. Prior literature has demonstrated that although many individual practitioners may be early adopters of clinical trial results, as a whole, academic centers may precede community hospitals with regard to evidence-based practice change.11,20
The limitations of this study were those inherent to retrospective analyses. Coding of HER2 status before 2009 limited analysis of pre-Z0011 practices. Because SNLB and ALND were only recently designated, they are incompletely coded during our study period. Thus, we used previously described cutoffs of 1–5 nodes removed as a proxy for SLNB and 10 or more nodes removed as a proxy for ALND.4,9 Furthermore, because NCDB captures total positive nodes and does not differentiate between those found on SLNB or ALND, our study excluded approximately 5000 patients who had two or fewer positive nodes but subsequently more positive nodes on ALND. However, this did not affect the results or conclusions of our study (data not shown). Finally, we lacked detailed pathologic features that may have contributed to treatment decisions.
Our findings suggest that incorporation of the Z0011 results into clinical practice was inconsistent across the breast cancer population. Although the perception of aggressive disease biology, risk of recurrence, or lack of targeted therapy may have influenced these patterns, further research is needed to understand better the incorporation of evidence into clinical practices and influences on surgical management of the axilla.
CONCLUSION
This study demonstrated that younger patients and those with HER2+ and TNBC were less likely to undergo SLNB alone despite meeting the eligibility criteria for the ACOSOG Z0011 trial. Future studies are needed to explore the influence of clinical trial data and patient characteristics on practice patterns.
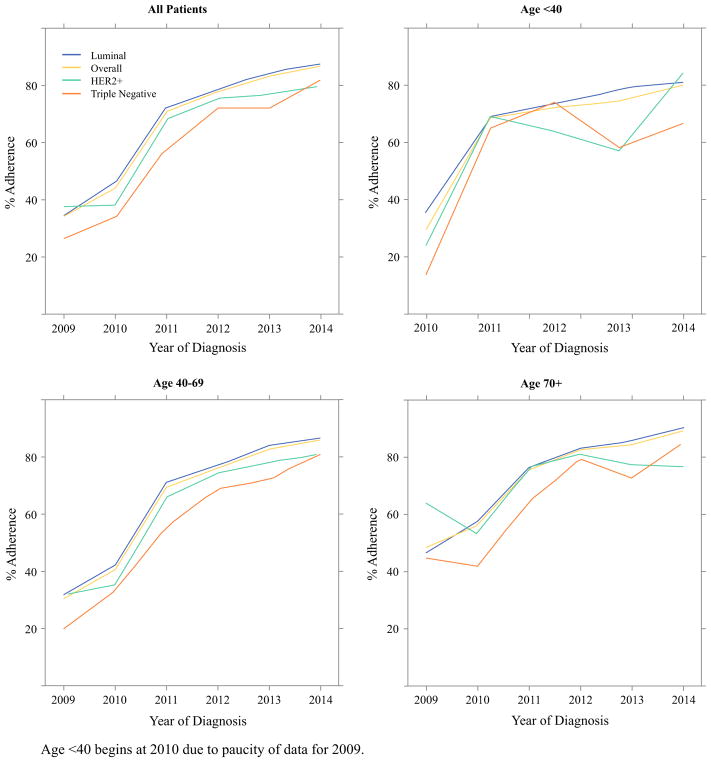
FIG. 1.
Use of SLNB alone over time among women eligible for American College of Surgeons Oncology Group (ACOSOG) Z0011 (2009–2014). Age younger than 40 years begins in 2010 due to paucity of data for 2009
Footnotes
DISCLOSURE: There are no conflicts of interest.
References
- 1.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–8. doi: 10.1097/00000658-199409000-00015. (Discussion 398–401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krag DN, Anderson SJ, Julian TB, et al. Sentinel lymph node resection compared with conventional axillary lymph node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23:4312–21. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–32. doi: 10.1097/SLA.0b013e3181f08f32. (Discussion 432–3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg. 2016;264:413–20. doi: 10.1097/SLA.0000000000001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCCN. [Accessed May 2017];NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Version 2. 2016 http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 7.Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365–83. doi: 10.1200/JCO.2013.54.1177. [DOI] [PubMed] [Google Scholar]
- 8.Surgeons ASoB. [Accessed May 2017];Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients. https://www.breastsurgeons.org/statements/guidelines/PerformancePracticeGuidelines_ALND.pdf.
- 9.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–53. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 10.Caudle AS, Hunt KK, Tucker SL, et al. American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol. 2012;19:3144–51. doi: 10.1245/s10434-012-2531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamtani A, Patil S, Van Zee KJ, et al. Age and receptor status do not indicate the need for axillary dissection in patients with sentinel lymph node metastases. Ann Surg Oncol. 2016;23:3481–6. doi: 10.1245/s10434-016-5259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bland KI, Scott-Conner CE, Menck H, et al. Axillary dissection in breast-conserving surgery for stage I and II breast cancer: a National Cancer Data Base study of patterns of omission and implications for survival. J Am Coll Surg. 1999;188:586–95. doi: 10.1016/s1072-7515(99)00056-3. (Discussion 595–6) [DOI] [PubMed] [Google Scholar]
- 13.Enger SM, Thwin SS, Buist DS, et al. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24:4377–83. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman RA, Partridge AH. Management of breast cancer in very young women. Breast. 2013;22(Suppl 2):S176–9. doi: 10.1016/j.breast.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Lee HB, Han W. Unique features of young age breast cancer and its management. J Breast Cancer. 2014;17:301–7. doi: 10.4048/jbc.2014.17.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chollet-Hinton L, Anders CK, Tse CK, et al. Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: a case-control study. Breast Cancer Res. 2016;18:79. doi: 10.1186/s13058-016-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gartner R, Jensen MB, Kronborg L, et al. Self-reported arm-lymphedema and functional impairment after breast cancer treatment: a nationwide study of prevalence and associated factors. Breast. 2010;19:506–15. doi: 10.1016/j.breast.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Chung A, Gangi A, Mirocha J, et al. Applicability of the ACOSOG Z0011 criteria in women with high-risk node-positive breast cancer undergoing breast conserving surgery. Ann Surg Oncol. 2015;22:1128–32. doi: 10.1245/s10434-014-4090-y. [DOI] [PubMed] [Google Scholar]
- 19.King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12:335–43. doi: 10.1038/nrclinonc.2015.63. [DOI] [PubMed] [Google Scholar]
- 20.Mohiuddin JJ, Deal AM, Carey LA, et al. Neoadjuvant systemic therapy use for younger patients with breast cancer treated in different types of cancer centers across the United States. J Am Coll Surg. 2016;223:717–728 e4. doi: 10.1016/j.jamcollsurg.2016.08.541. [DOI] [PMC free article] [PubMed] [Google Scholar]