Abstract
Brevibacillus borstelensis AK1 is a thermophile which grows between the temperatures of 45°C and 70°C. The present study is an extended genome report of B. borstelensis AK1 along with the morphological characterization. The strain is isolated from a hot spring in Saudi Arabia (southeast of the city Gazan). It is observed that the strain AK1 is rod-shaped, motile, and strictly aerobic bacterium. The whole genome sequence resulted in 29 contigs with a total length of 5,155,092 bp. In total, 3,946 protein-coding genes and 139 RNA genes were identified. Comparison with the previously submitted strains of B. borstelensis strains illustrates that strain AK1 has a small genome size but high GC content. The strain possesses putative genes for degradation of a wide range of substrates including polyethylene (plastic) and long-chain hydrocarbons. These genomic features may be useful for future environmental/biotechnological applications.
1. Introduction
Thermophiles are a group of heat-loving microorganisms which have an optimum growth temperature of at least 50°C [1]. The genus Brevibacillus (family Paenibacillaceae and class Bacilli) was initially described as Bacillus brevis in 1900 by Migula [2]. In later years, many new strains were placed in the same group (e.g., B. brevis) including the strains that were not following the sensu stricto criteria that challenged the overall classification. This included discrepancies in maximum growth temperatures and a wide range of GC values, hence confirming B. brevis as a heterogeneous group [3, 4]. To resolve these ambiguities, a new genus named Brevibacillus was proposed in 1996 that resulted in reclassification of nearly 10 Bacillus species, based on 16S rDNA analysis [5, 6]. Since then, many strains have been reclassified as novel species of the genera originally reported as members of Bacillus brevis [6–9]. The genus Brevibacillus comprised environmental bacteria that have been observed in diverse habitats including agricultural soil, wastewaters, and hot springs [10, 11].
Earlier studies have shown the importance of B. borstelensis in different spheres of industry and the environment [23–26]. For instance, Arya et al. [25] reported that B. borstelensis possess great potential to degrade the fungicide carbendazim from agricultural fields at high rates, especially when coupled with Streptomyces albogriseolus. Hadad et al. [24] showed that inert polyethylene could be degraded by B. borstelensis strain 707. Tsai et al. [26] evinced that the lipolytic B. borstelensis strain SH168 can enhance the transformation of food wastes into biofertilizer. Baek et al. [23] reported that B. borstelensis strain BCS-1 could produce D-amino acid amidases, a catalyst for the formation of optically pure D-amino acids; these are the intermediates of pharmaceuticals production, food additives, insecticides, synthetic sweeteners, and agrochemicals [27]. Furthermore, an alkaline pectin lyase applicable in fruit juice and oil extract has also been isolated from B. borstelensis P35. The reassociation analyses, cellular fatty acid profile, and isoprenoid quinone composition analysis confirmed that B. borstelensis owns these properties based on unique DNA base compositions [6, 7].
To date, 23 species of the genus Brevibacillus have been recognized including B. borstelensis. Nevertheless, only four strains of B. borstelensis are subjected to whole genome sequencing so far. These are B. borstelensis cifa_chp40 [PRJNA200540], B. borstelensis 3096-7 [NZ_JAQG01000000], B. borstelensis LChuR05 [PRJNA271204], and B. borstelensis AK1 [PRJNA191598]. In this study, we report the genomic insights of B. borstelensis strain AK1 that may help unravel the potential importance of the species in biotechnology. Additionally, since the strain has been previously reported for potential polyethylene (plastic) degradation, we identified the putative genes/enzymes based on KEGG orthology.
2. Materials and Methods
2.1. Sampling
The sampling was performed in January 2012 at a hot spring “Al-Ain Alhara”, located in the southeast of Gazan city in Saudi Arabia (16°56′N, 43°15′E). Water samples were taken in sterile thermal glass containers while maintaining the physicochemical quality parameters.
2.2. Growth Conditions and Genomic DNA Preparation
A 5 ml aliquot of the water sample was inoculated in 250 ml of tetrathionate (TT) broth (ATCC medium 697). Incubation was performed at 55°C for 24 to 48 h at shaking speed of 300 rpm. The bacterial cells were then harvested by centrifugation (14,000 g) and the pellet was spread over TT agar media. Statistically relevant numbers of distinctive colonies were picked and the procedure was repeated thrice to purify the strain for subsequent genomic analysis.
2.3. Morphological Characterization
For morphological characterization, bacterial cells were obtained from stationary phase and subjected to SEM microscopy. Briefly, fixation was performed in 2.5% glutaraldehyde, which was buffered at pH 7.2 with phosphate buffer saline (PBS). Subsequently, the mixture was placed over ice for 2 h followed by washing of bacterial cells for 20 min at room temperature. The post-fixation was performed in osmium tetraoxide (1%) leading to dehydration in a graded ethanol series up to 100% concentration. Lastly, the specimens were gold-plated (10 nm) and studied under a field emission SEM.
2.4. DNA Extraction
Total bacterial DNA extraction was performed using Genomic DNA isolation kit (Norgen Biotek) as per the manufacturer's guidelines. DNA yield was measured using Qbit Assay that was approximately 40 μg, while the quality was determined using Bioanalyzer and Agarose gel before proceeding to library preparation for sequencing.
2.5. Genome Sequencing, Assembly, and Functional Annotation
The genome sequencing was performed using a 454-genome sequencer (FLX titanium, Roche), based at Bioscience Core Laboratory of King Abdullah University of Science and Technology (KAUST) in Saudi Arabia. Briefly, 500 ng of genomic DNA was used to construct the fragment library using Rapid Library Preparation Kit. Average insert sizes of the yielded libraries were 750 bp in length. These libraries were then quantified by qPCR. The sequences were subjected to quality control to trim low-quality reads. The filtered sequences were assembled into contigs using Newbler assembler v2.5.3. The automated assembly algorithm yielded over 100 contigs. Out of these contigs, 29 were selected based on high contig length as they represented the expected whole genome length. The assembly was then annotated using Prokaryotic Genome Automatic Annotation Pipeline (PGAAP) available at NCBI. The annotation yielded a total of 5,090 coding genes and out of these 4,951 appeared to be protein-coding genes. Additionally, gene prediction analyses were completed within Integrated Microbial Genomes Expert Review (IMG-ER). The circular visualization of the assembled contigs was generated using an online web-server Circos plotting tool (ClicOFS) [22]. The project summary, as well as associated MIGS information, is shown in Table 1.
Table 1.
The summary of the project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Complete - High-quality draft |
| MIGS-28 | Libraries used | 454 shotgun libraries |
| MIGS-29 | Sequencing platforms | 454-GS-FLX |
| MIGS-31.2 | Fold coverage | 26.52x |
| MIGS-30 | Assemblers | Newbler v. 2.5.3 |
| MIGS-32 | Gene calling method | Glimmer |
| Genbank Accession | APBN00000000 | |
| Genbank Date of Release | 2013-11-05 | |
| GOLD ID | Gi0043156 | |
| MIGS-13 | Project relevance | Industrial |
2.6. Phylogenetic Analysis
For phylogenetic analysis, the sequence of the 16S rRNA gene was aligned with equivalent 16S rRNA genes of closely related strains as appeared in BLAST search. The tree was calculated with an improved neighbor-joining algorithm known as BioNJ.
2.7. Accession Numbers
The sequenced genome is deposited in GenBank with master record accession no. APBN00000000: the associated 29 contigs are assigned IDs according to this record (accession numbers APBN01000001 to APBN01000029).
3. Results and Discussion
3.1. Morphological Features and Classification
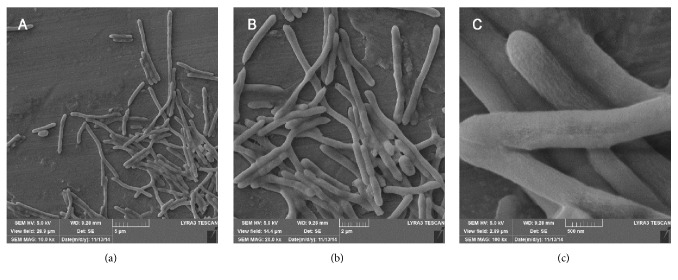
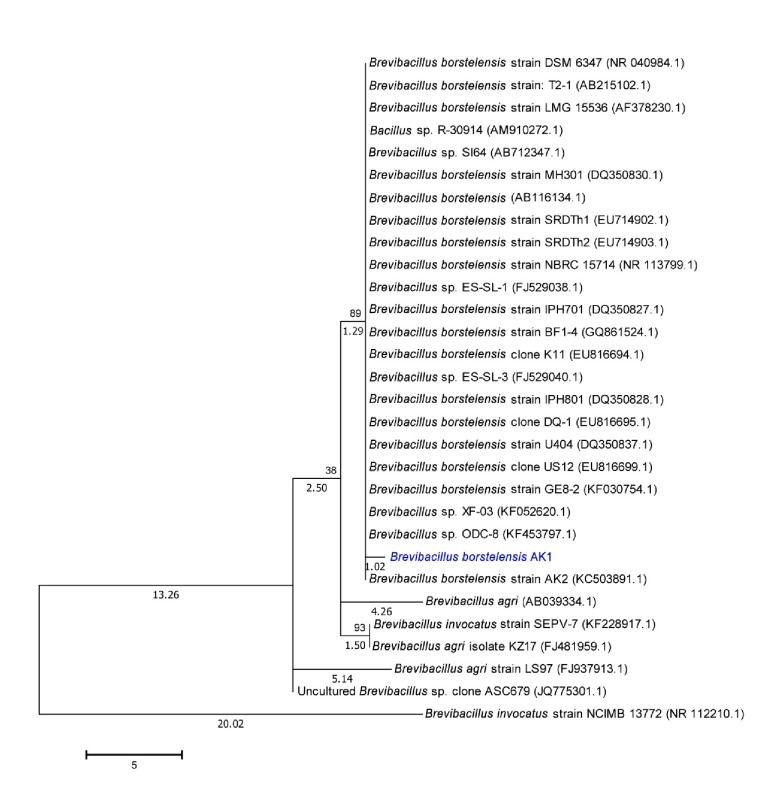
Morphological analysis revealed that, in the stationary phase, average diameter of B. borstelensis AK1 ranged from 0.2 to 0.5 μm whereas the length varied between 2.0 μm and 15.0 μm (Figure 1). The cells appeared to be fast growing, forming colonies of 3 mm diameter within a period of 24 hours. The average generation time was recorded to be 30 min at previously described conditions. The colonies were yellow-pigmented and had smooth margins while the strain was able to withstand a pH range of 5.5–8.5. The general feature information as suggested by Minimum Information about the Genome Sequence (MIGS) is presented in Table 2. Likewise, phylogenetic analysis displayed that the strain was closely related to B. borstelensis AK2 and Brevibacillus sp. ODC-8 (Figure 2).
Figure 1.
Scanning electron micrographs of Brevibacillus borstelensis strain AK1 at 10.0 kx (a), 20.0 kx (b), and 100.0 kx (c).
Table 2.
Classification and general features of B. borstelensis strain AK1 according to the MIGS recommendations.
| MIGS ID | Property | Term | Evidence Code |
|---|---|---|---|
| Current Classification | Domain Bacteria | TAS [12] | |
| Phylum Firmicutes | TAS [13–15] | ||
| Class Bacilli | TAS [16, 17] | ||
| Order Bacillales | TAS [18] | ||
| Family Paenibacillaceae | TAS [19] | ||
| Genus Brevibacillus | TAS [20, 21] | ||
| Species borstelensis | TAS [21] | ||
| Strain: AK1 | TAS [In this report] | ||
| Gram stain | Positive | TAS [In this report] | |
| Cell shape | Rod | TAS [In this report] | |
| Motility | Motile | TAS [In this report] | |
| Sporulation | Spore forming | TAS [In this report] | |
| Temperature range | 40–70°C | TAS [In this report] | |
| Optimum pH | 7.5 | TAS [In this report] | |
| Optimum temperature | 45–50°C | TAS [In this report] | |
|
| |||
| pH range | 6–8 | TAS [In this report] | |
|
| |||
| MIGS-22 | Carbon source | Maltose, cellobiose, d-fructose, d-galactose, d-glucose lactose, lactulose, d-mannose, sucrose, trehalose, d-xylose | TAS [In this report] |
|
| |||
| MIGS-6 | Habitat | Hot spring | TAS [In this report] |
|
| |||
| MIGS-6.3 | Salinity | No growth with > 1% NaCl (w/v) | TAS [In this report] |
|
| |||
| MIGS-15 | Biotic relationship | Free-living | TAS [In this report] |
|
| |||
| MIGS-14 | Pathogenicity | Non-pathogen | TAS [In this report] |
|
| |||
| MIGS-4 | Geographic location | 50 km southeast of Gazan, Saudi Arabia | TAS [In this report] |
|
| |||
| MIGS-5 | Collection date | January 2012 | NAS [In this report] |
|
| |||
| MIGS-4.1 | Latitude | 16°56′N | TAS [In this report] |
|
| |||
| MIGS-4.2 | Longitude | 43°15′E | TAS [In this report] |
Evidence codes - IDA: Inferred from Direct Assay; TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project. If the evidence is IDA, then the property was directly observed for a live isolate by one of the authors or an expert mentioned in the acknowledgements.
Figure 2.
Molecular Phylogenetic analysis by Maximum Likelihood method highlighting the position of Brevibacillus borstelensis AK1 relative to other similar bacteria. Bootstrap values based on 1000 replicates show the robustness of the branching. Scale bar represents 0.1 substitutions per nucleotide position. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site.
3.2. Genome Properties
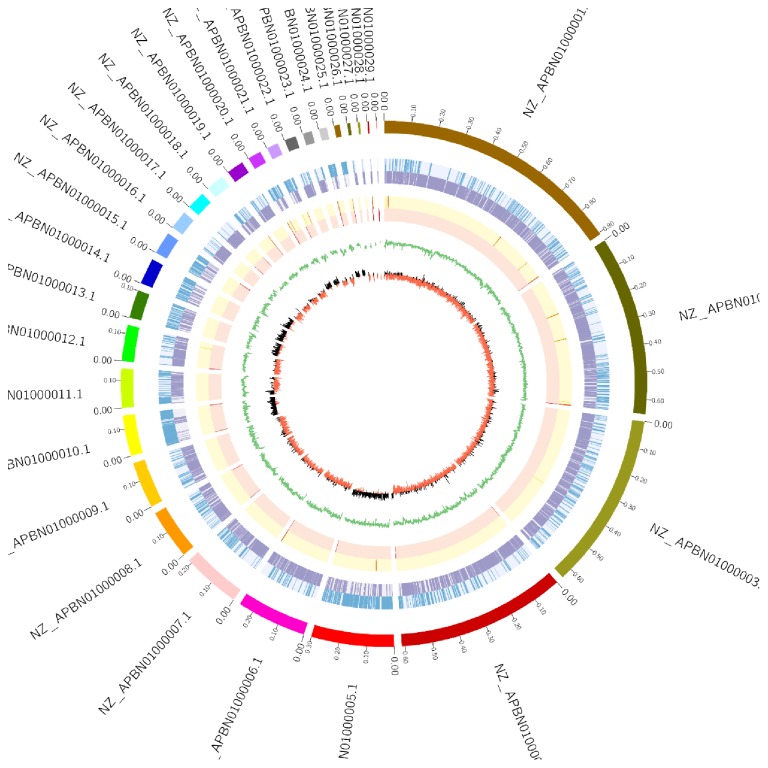
A genome sequence of 5,155,092 bp with 52% of GC content was produced out of 29 contigs of B. borstelensis AK1 (Figure 3; Table 3). More precisely, this comprised 5,090 predicted genes, of which 4,951 (97.27%) were protein-coding genes while 139 were RNA genes (2.73%). Among these RNA genes, 22 belonged to rRNA that contained eleven 16S, ten 23S, and one 5S gene; on the other hand, 117 genes appeared to be tRNA genes. The putative function was assigned to 3,921 genes (77.03%) whereas the remaining genes were annotated as hypothetical proteins. Protein-coding genes connected to KEGG pathways were 1,348 (26.48%) while genes associated with COG categories appeared to be 3,231 (63.48%). The genes distribution into COGs functional categories is displayed in Table 4.
Figure 3.
Graphical circular map drawn using ClicO FS [22]. The outer circle represents contigs information, whereas the next two inner circles present ORFs oriented in the forward (blue) and reverse (purple) direction. The 4th circle marks tRNA gene operon (orange) while 5th circle represents rRNA genes (red). The sixth circle reflects GC content plot (%age). The innermost circle shows GC skew; red indicates negative values, whereas black indicates positive values. The scale bar is in Mb.
Table 3.
Nucleotide and gene content of the genome.
| Attribute | Genome (total) | |
|---|---|---|
| Value | % of totala | |
| Size (bp) | 5155092 | 100.00 |
| G+C content (bp) | 2680425 | 52.00 |
| Coding region (bp) | 4471011 | 86.73 |
| Total genesb | 5090 | 100.00 |
| RNA genes | 139 | 2.73 |
| Protein-coding genes | 4951 | 97.27 |
| Genes with function predictions | 3946 | 77.52 |
| Protein coding genes with enzymes | 1186 | 23.30 |
| Genes assigned to COGs | 3231 | 63.48 |
| COG clusters | 1677 | 51.90 |
| Genes with signal peptides | 303 | 5.95 |
| Genes with transmembrane helices | 1366 | 26.84 |
| Fused protein coding genes | 88 | 1.73 |
The total is based on either the size of the genome in base pairs or the total number of protein coding genes in the annotated genome.
Table 4.
Number of genes associated with the 25 general COG functional categories.
| Code | Value | % of total | Description |
|---|---|---|---|
| J | 245 | 3.97 | Translation |
| A | 25 | 0.41 | RNA processing and modification |
| K | 231 | 3.75 | Transcription |
| L | 238 | 3.86 | Replication, recombination and repair |
| B | 19 | 0.31 | Chromatin structure and dynamics |
| D | 72 | 1.17 | Cell cycle control, mitosis and meiosis |
| Y | 2 | 0.03 | Nuclear structure |
| V | 46 | 0.75 | Defense mechanisms |
| T | 152 | 2.46 | Signal transduction mechanisms |
| M | 188 | 3.05 | Cell wall/membrane biogenesis |
| N | 96 | 1.56 | Cell motility |
| Z | 12 | 0.19 | Cytoskeleton |
| W | 1 | 0.02 | Extracellular structures |
| U | 158 | 2.56 | Intracellular trafficking and secretion |
| O | 203 | 3.29 | Posttranslational modification, protein turnover, chaperones |
| C | 258 | 4.18 | Energy production and conversion |
| G | 230 | 3.73 | Carbohydrate transport and metabolism |
| E | 270 | 4.38 | Amino acid transport and metabolism |
| F | 95 | 1.54 | Nucleotide transport and metabolism |
| H | 179 | 2.9 | Coenzyme transport and metabolism |
| I | 94 | 1.52 | Lipid transport and metabolism |
| P | 212 | 3.44 | Inorganic ion transport and metabolism |
| Q | 88 | 1.43 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 702 | 11.38 | General function prediction only |
| S | 1347 | 21.84 | Function unknown |
| - | 1005 | 16.29 | Not in COGs |
3.3. Genomic Insights and Comparative Analysis
To date, four strains of B. borstelensis have been sequenced whose genome information is available in the IMG-JGI database. This includes B. borstelensis strain 3096-7, B. borstelensis strain cifa_chp40, B. borstelensis strain LChuR05, and B. borstelensis strain AK1. The strain LChuR05, however, has been found to be contaminated and the record has been removed from the GenBank. Hereby, we compare the genome sequence of B. borstelensis strain AK1 with the other three strains as described above. The physical comparison shows that the genome sequence of strain AK1 (5.155 Mb) is nearly equal to the strain cifa_chp40, i.e., 5.19 Mb; nevertheless, it is the smallest as compared to the other strains. By contrast, the strain 3096-7 displayed the biggest genome size, i.e., 5.46 Mb. The GC content of the strain AK1 genome is the highest (52.0%) when compared to the other strains of B. borstelensis, i.e., strain 3096-7 of 51.4% and strain cifa_chp40 of 51.9%. The gene content of the strain AK1 is the smallest; and likewise, it has the lowest number of protein-coding genes (4,951) followed by the strains cifa_chp40 and 3096-7 (5,042 and 5,352, respectively). Among these genes, AK1 had 3,921 protein-coding genes with function prediction, which is similar to the strain cifa_chp40 that had 3,922 genes. The strain 3096-7, however, had 4,073 genes which is still consistent with these numbers. Further investigations on protein-coding genes and enzymes illustrated the presence of 1,208 genes for the strain AK1, 1,221 genes for strain 3096-7, and 1,215 genes for the strain cifa_chp40. The detailed description of these parameters along with additional features is presented in Table 5. The pairwise average nucleotide identity (ANI) values of all the sequenced strains of B. borstelensis are presented in Table 6. It is found that all of the strains have similar ANI values ranging from 99.51% to 99.58%.
Table 5.
Genome report for four strains of Brevibacillus borstelensis submitted to GenBank.
| Organism Name | Size (Mb) | GC% | Scaffolds | Total Genes | Proteins | Protein coding genes | RNA genes | Protein coding genes with function prediction | Protein coding genes with enzymes | Protein coding genes with COGs3 | Chromosomal cassette | COG Clusters | Symmetric Identity of AK1 with other strains (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brevibacillus borstelensis AK1 | 5.1550 | 52 | 29 | 5037 | 4817 | 4951 | 139 | 3946 | 1208 | 3231 | 493 | 1677 | - |
|
| |||||||||||||
| Brevibacillus borstelensis 3096-7 | 5.4642 | 51.4 | 192 | 5302 | 5112 | 5352 | 129 | 4073 | 1221 | 3322 | 593 | 1709 | 91.56 |
|
| |||||||||||||
| Brevibacillus borstelensis cifa_chp40 | 5.1965 | 51.9 | 38 | 5086 | 4918 | 5042 | 135 | 3922 | 1215 | 3270 | 487 | 1693 | 94.72 |
Table 6.
Genomic comparisons of different strains of B. borstelensis using ANI (in %age).
| B. borstelensis AK1 | B. borstelensis 3096-7 | B. borstelensis cifa_chp40 | |
|---|---|---|---|
| B. borstelensis AK1 | 100.00 | 99.51 | 99.58 |
| B. borstelensis 3096-7 | 99.51 | 100.00 | 99.52 |
| B. borstelensis cifa_chp40 | 99.58 | 99.52 | 100.00 |
3.4. Putative Genes Involved in Degradation Services: Metagenomic Assessment
We further attempted to identify putative genes/enzymes of the strain AK1 based on KEGG orthology, KO (i.e., enzyme commission classification), that may possess a potential in degradation services including polyethylene and hydrocarbons. We found presence of 1 cutinase, 67 lipases, 99 hydroxylases, 2 laccases, 1 polyphenol oxidase, and 116 proteases KO database (Supplementary Data) (available here). All of them have been previously reported as potential plastic degrading genes in different bacteria [28–34]. Similarly, 159 monooxygenases, 136 dioxygenases, and 118 lyases are identified (Supplementary Data). These enzymes appeared to have a potential role in a number of bioremediation studies including catabolic expression of CYP family [35–37]. In any case, the results strengthen the significance of strain AK1 for future biotechnological services that come with advantage of extremophile properties (i.e., high GC content).
4. Conclusions
Thermophilic organisms are not only of industrial importance, but they can also be exploited in pollutant degradation services. B. borstelensis AK1 was selected based on such a potential and the whole genome sequencing was performed to unravel genomic insights. A general comparison with previously sequenced strains of the same species revealed that the strain AK1 has the smallest genome size but highest GC content. Nevertheless, the presence of putative biodegradation related genes supports the idea of exploiting the species in future environmental/biotechnological services.
Acknowledgments
The authors acknowledge the financial support by KFUPM and KAUST, Saudi Arabia. Moreover, they extend their acknowledgment to PoliReddy, D., Rajan, I., Rathinam, K., and Sugumar, T., for their help and support. They are further thankful to Dr. Jochen A. Müller (UFZ, Leipzig, Germany) for providing critical remarks during the preparation of the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
A list of putative genes (entries) of the strain AK1 based on KEGG orthology, KO (i.e., enzyme commission classification), that may possess a potential in degradation of polyethylene, hydrocarbons, and so forth.
References
- 1.Seckbach J. Journey to Diverse Microbial Worlds: Adaptation to Exotic Environments. Springer Science & Business Media; 2000. [Google Scholar]
- 2.Migula W. System der Bakterien. Vol. 2. Jena, Germany: Gustav Fisher; 1900. [Google Scholar]
- 3.Nakamura L. K., Swezey J. Taxonomy of Bacillus circulans Jordan 1890: Base composition and reassociation of deoxyribonucleic acid. International Journal of Systematic Bacteriology. 1983;33(1):46–52. doi: 10.1099/00207713-33-1-46. [DOI] [Google Scholar]
- 4.Nakamura L. K., Swezey J. Deoxyribonucleic acid relatedness of Bacillus circulans Jordan 1890 strains. International Journal of Systematic Bacteriology. 1983;33(4):703–708. doi: 10.1099/00207713-33-4-703. [DOI] [Google Scholar]
- 5.Nakamura L. K. Bacillus brevis Migula 1900 taxonomy: Reassociation and base composition of DNA. International Journal of Systematic Bacteriology. 1991;41(4):510–515. doi: 10.1099/00207713-41-4-510. [DOI] [PubMed] [Google Scholar]
- 6.Shida O., Takagi H., Kadowaki K., Yano H., Komagata K. Differentiation of species in the Bacillus brevis group and the Bacillus aneurinolyticus group based on the electrophoretic whole-cell protein pattern. Antonie van Leeuwenhoek-Journal of Microbiology. 1996;70(1):31–39. doi: 10.1007/BF00393567. [DOI] [PubMed] [Google Scholar]
- 7.Shida O., Takagi H., Kadowaki K., et al. Bacillus aneurinolyticus sp. nov., nom. rev. International Journal of Systematic Bacteriology. 1994;44(1):143–150. doi: 10.1099/00207713-44-1-143. [DOI] [Google Scholar]
- 8.Nakamura L. K. DNA relatedness of Bacillus brevis migula 1900 strains and proposal of Bacillus agri sp. nov., nom. rev., and Bacillus centrosporus sp. nov., nom. rev. International Journal of Systematic Bacteriology. 1993;43(1):20–25. doi: 10.1099/00207713-43-1-20. [DOI] [Google Scholar]
- 9.Takagi H., Shida O., Kadowaki K., Komagata K., Udaka S. Characterization of Bacillus brevis with descriptions of Bacillus migulanus sp. nov., Bacillus choshinensis sp. nov., Bacillus parabrevis sp. nov., and Bacillus galactophilus sp. nov. International Journal of Systematic Bacteriology. 1993;43(2):221–231. doi: 10.1099/00207713-43-2-221. [DOI] [PubMed] [Google Scholar]
- 10.Kim M. K., Sathiyaraj S., Pulla R. K., Yang D.-C. Brevibacillus panacihumi sp. nov., a β-glucosidase-producing bacterium. International Journal of Systematic and Evolutionary Microbiology. 2009;59(5):1227–1231. doi: 10.1099/ijs.0.001248-0. [DOI] [PubMed] [Google Scholar]
- 11.Sharma V., Singh P. K., Midha S., Ranjan M., Korpole S., Patil P. B. Genome sequence of Brevibacillus laterosporus strain GI-9. Journal of Bacteriology. 2012;194(5):p. 1279. doi: 10.1128/JB.06659-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proceedings of the National Acadamy of Sciences of the United States of America. 1990;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrity G., Holt J. The Road Map to the Manual. In: Boone D., Castenholz R., Garrity G., editors. Bergey’s Manual® of Systematic Bacteriology. New York, NY, USA: Springer; 2001. pp. 119–166. [Google Scholar]
- 14.Gibbons N. E., Murray R. G. E. Proposals concerning the higher taxa of bacteria. International Journal of Systematic Bacteriology. 1978;28(1):1–6. doi: 10.1099/00207713-28-1-1. [DOI] [Google Scholar]
- 15.Murray R. Bergey's Manual of Systematic Bacteriology. Vol. 1. 1; 1984. The higher taxa, or, a place for everything; pp. 31–34. [Google Scholar]
- 16.Ludwig W., Schleifer K.-H., Whitman W. B. Class I. Bacilli class nov. Bergey's Manual of Systematic Bacteriology. 2009;3:19–20. [Google Scholar]
- 17.Oren A., Garrity G. M. List of new names and new combinations previously effectively, but not validly, published. International Journal of Systematic and Evolutionary Microbiology. 2015;65:1105–1111. doi: 10.1099/ijs.0.000178. [DOI] [PubMed] [Google Scholar]
- 18.Skerman V. B. D., McGowan V., Sneath P. H. A. Approved lists of bacterial names. International Journal of Systematic Bacteriology. 1980;30(1):225–420. doi: 10.1099/00207713-30-1-225. [DOI] [Google Scholar]
- 19.Ludwig W., Schleifer K. H., Whitman W. B. Eubacteriaceae, fam. nov. Bergey's Manual of Systematics of Archaea and Bacteria. 2010.
- 20.Shida O., Takagi H., Kadowaki K., Komagata K. Proposal for two new genera, Brevibacillus gen. nov. and Aneurinibacillus gen. nov. International Journal of Systematic Bacteriology. 1996;46(4):939–946. doi: 10.1099/00207713-46-4-939. [DOI] [PubMed] [Google Scholar]
- 21.Tatusova T., Ciufo S., Fedorov B., O'Neill K., Tolstoy I. RefSeq microbial genomes database: new representation and annotation strategy. Nucleic Acids Research. 2015;43(7):p. 3872. doi: 10.1093/nar/gkv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheong W.-H., Tan Y.-C., Yap S.-J., Ng K.-P. ClicO FS: An interactive web-based service of Circos. Bioinformatics. 2015;31(22):3685–3687. doi: 10.1093/bioinformatics/btv433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek D. H., Song J. J., Lee S.-G., Kwon S. J., Asano Y., Sung M.-H. New thermostable D-methionine amidase from Brevibacillus borstelensis BCS-1 and its application for D-phenylalanine production. Enzyme and Microbial Technology. 2003;32(1):131–139. doi: 10.1016/S0141-0229(02)00268-5. [DOI] [Google Scholar]
- 24.Hadad D., Geresh S., Sivan A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. Journal of Applied Microbiology. 2005;98(5):1093–1100. doi: 10.1111/j.1365-2672.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- 25.Arya R., Sharma A. K. Bioremediation of carbendazim, a benzimidazole fungicide using brevibacillus borstelensis and Streptomyces albogriseolus together. Current Pharmaceutical Biotechnology. 2016;17(2):185–189. doi: 10.2174/1389201016666150930115737. [DOI] [PubMed] [Google Scholar]
- 26.Tsai S.-H., Liu C.-P., Yang S.-S. Microbial conversion of food wastes for biofertilizer production with thermophilic lipolytic microbes. Journal of Renewable Energy. 2007;32(6):904–915. doi: 10.1016/j.renene.2006.04.019. [DOI] [Google Scholar]
- 27.Yagasaki M., Ozaki A. Industrial biotransformations for the production of D-amino acids. Journal of Molecular Catalysis B: Enzymatic. 1998;4(1-2):1–11. doi: 10.1016/S1381-1177(97)00011-8. [DOI] [Google Scholar]
- 28.Rojo F. Degradation of alkanes by bacteria. Environmental Microbiology. 2009;11(10):2477–2490. doi: 10.1111/j.1462-2920.2009.01948.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Wang W., Wu Y., Zhou Z., Lai Q., Shao Z. Multiple alkane hydroxylase systems in a marine alkane degrader, Alcanivorax dieselolei B-5. Environmental Microbiology. 2011;13(5):1168–1178. doi: 10.1111/j.1462-2920.2010.02416.x. [DOI] [PubMed] [Google Scholar]
- 30.Uchida H., Nakajima-Kambe T., Shigeno-Akutsu Y., Nomura N., Tokiwa Y., Nakahara T. Properties of a bacterium which degrades solid poly(tetramethylene succinate)-co-adipate, a biodegradable plastic. FEMS Microbiology Letters. 2000;189(1):25–29. doi: 10.1016/S0378-1097(00)00246-9. [DOI] [PubMed] [Google Scholar]
- 31.Teeraphatpornchai T., Nakajima-Kambe T., Shigeno-Akutsu Y., et al. Isolation and characterization of a bacterium that degrades various polyester-based biodegradable plastics. Biotechnology Letters. 2003;25(1):23–28. doi: 10.1023/A:1021713711160. [DOI] [PubMed] [Google Scholar]
- 32.Sivan A. New perspectives in plastic biodegradation. Current Opinion in Biotechnology. 2011;22(3):422–426. doi: 10.1016/j.copbio.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Santo M., Weitsman R., Sivan A. The role of the copper-binding enzyme—laccase—in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. International Biodeterioration & Biodegradation. 2013;84:204–210. doi: 10.1016/j.ibiod.2012.03.001. [DOI] [Google Scholar]
- 34.Bhardwaj H., Gupta R., Tiwari A. Communities of Microbial Enzymes Associated with Biodegradation of Plastics. Journal of Polymers and the Environment. 2013;21(2):575–579. doi: 10.1007/s10924-012-0456-z. [DOI] [Google Scholar]
- 35.Cerniglia C. E. Microorganisms to Combat Pollution. Dordrecht, Netherlands: Springer; 1992. Biodegradation of polycyclic aromatic hydrocarbons; pp. 227–244. [Google Scholar]
- 36.Gibson D. T., Parales R. E. Aromatic hydrocarbon dioxygenases in environmental biotechnology. Current Opinion in Biotechnology. 2000;11(3):236–243. doi: 10.1016/S0958-1669(00)00090-2. [DOI] [PubMed] [Google Scholar]
- 37.Arslan M., Afzal M., Amin I., Iqbal S., Khan Q. M. Nutrients can enhance the abundance and expression of alkane hydroxylase CYP153 gene in the rhizosphere of ryegrass planted in hydrocarbon-polluted soil. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0111208.e111208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A list of putative genes (entries) of the strain AK1 based on KEGG orthology, KO (i.e., enzyme commission classification), that may possess a potential in degradation of polyethylene, hydrocarbons, and so forth.