Abstract
Self-regulation refers to controlling our emotions and actions in the pursuit of higher-order goals. Although research suggests commonalities in the cognitive control of emotion and action, evidence for a shared neural substrate is scant and largely circumstantial. Here we report on two large-scale meta-analyses of human neuroimaging studies on emotion or action control, yielding two fronto-parieto-insular networks. The networks’ overlap, however, was restricted to four brain regions: posteromedial prefrontal cortex, bilateral anterior insula, and right temporo-parietal junction. Conversely, meta-analytic contrasts revealed major between-network differences, which were independently corroborated by clustering domain-specific regions based on their intrinsic functional connectivity, as well as by functionally characterizing network sub-clusters using the BrainMap database for quantitative forward and reverse inference. Collectively, our analyses identified a core system for implementing self-control across emotion and action, beyond which, however, either regulation facet appears to rely on broadly similar yet distinct subnetworks. These insights into the neurocircuitry subserving affective and executive facets of self-control suggest both processing commonalities and differences between the two aspects of human self-regulation.
Keywords: Cognitive control, Emotion regulation, Executive functions, ALE meta-analysis, Resting-state fMRI, Functional decoding, Control theory
1. Introduction
Everyday life is full of situations that elicit or suggest spontaneous emotional and behavioural responses. Such impulsive responses or response tendencies, however, are often at odds with our overarching goals. Imagine, for instance, a situation where you are offered a tasty piece of cake, which elicits joy and the impulse to accept the offer, but which conflicts with your diet plan. In order for the diet plan to win, you need to exert top-down control over your stimulus-driven emotion and ensuing action tendency. Or think of loving parents being repeatedly frustrated by their child, who may need to struggle hard with themselves to stifle their anger and refrain from acting on an impulse for physical punishment. What these examples illustrate is that self-regulation, or the ability to cognitively control both our emotions and our actions, is crucial for attaining long-term or higher-level goals. Here we investigated the neural mechanisms behind this important mental faculty.
Cognitive emotion regulation (CER) refers to intentionally generating, enhancing, reducing, or stopping a given emotion. In experimental studies on CER, participants are typically confronted with emotional stimuli, such as pictures or film clips, and are then instructed to up- or down-regulate the stimulus-induced emotion using one of various strategies (Dörfel et al., 2014; Gross, 1998; Koole, 2009; Webb et al., 2012). In the above diet example, a useful down-regulation strategy might consist of reappraising the cake via a “non-consummatory transformation” (i.e., imagining odd or novel uses for the piece of cake, unrelated to consumption; cf. Hofmann et al., 2010). Other effective CER strategies include suppressing the emotional response or mentally distancing oneself from the emotional scene via taking an observer perspective (Dörfel et al., 2014; Leiberg et al., 2012). Cognitive action regulation (CAR), in turn, refers to intentionally withholding or stopping a prepotent action, often in combination with performing a competing alternative action. In our above diet example, CAR would be at work when you override the impulse to reach out for receiving the offered cake. For studying CAR in the laboratory, many experimental paradigms have been devised, ranging from simple response-inhibition tasks to tasks inducing conflicts between response alternatives to paradigms that require switching between tasks (see Section 2.1 for details).
At the heart of any such self-regulatory situation lies a conflict between a predominant but inadequate (goal-incongruent) response and a non-dominant but adequate (goal-conducive) one. Self-regulation, in turn, consists of biasing the decision toward the (initially) non-dominant, adequate option by suppressing the dominant goal-incongruent one and facilitating the goal-congruent alternative. This top-down modulation in response to the perceived discrepancy between current and goal states has been suggested as the “common core” of emotion- and action-directed self-control (Cohen et al., 2013; Heatherton and Wagner, 2011; Muraven et al., 2006; Posner and Rothbart, 1998).
The assumption of common mechanisms across CER and CAR is supported by several lines of evidence: First, CER and CAR abilities in healthy adults have been found positively correlated (McRae et al., 2012; Schmeichel et al., 2008). Second, this association is echoed by deficits across both domains in mental disorders characterized by impulsivity, such as substance abuse (Tabibnia et al., 2011) or attention-deficit/hyperactivity disorder (Walcott and Landau, 2004). Third, in children, CER and CAR abilities show common developmental trajectories and are both linked to parent-reported levels of children’s self-control (Carlson and Wang, 2007; Rothbart and Rueda, 2005; Simonds et al., 2007). Fourth, practicing mindfulness meditation, a form of self-regulation training, has been shown to improve both CER and CAR (Chambers et al., 2008; Jha et al., 2010; Teper et al., 2013). Fifth, similar (broadly defined) brain regions have been discussed as potential neural substrates of either regulatory domain. These regions include dorsolateral parts of the frontal lobes (inferior frontal sulcus/middle frontal gyrus [MFG] extending to the inferior frontal junction [IFJ]), the dorsal premotor cortex (PMd), medial premotor regions (supplementary and pre-supplementary motor area [SMA, preSMA]) extending to anterior midcingulate cortex (aMCC), regions around the intraparietal sulcus (IPS), and parts of the anterior insula/frontal operculum (aI/fO; cf. Buhle et al., 2014; Cieslik et al., 2015; Kohn et al., 2014; Niendam et al., 2012). These lines of evidence have led some researchers to suggest that a set of executive functions, typically related to CAR, also mediate CER abilities (Hofmann et al., 2012; Schmeichel and Tang, 2015; Teper et al., 2013).
Relatedly, it has been argued that both CER and CAR are implemented by a shared domain-general brain network devoted to cognitive (or executive) control (e.g., Buhle et al., 2014; Etkin et al., 2016; Rothbart et al., 2011; Schweizer et al., 2013). This assumption appears plausible not only from the arguments listed above, but also because the CAR network is recruited by many different tasks that require effortful attention and cognitive control (Cabeza and Nyberg, 2000; Fedorenko et al., 2013; Müller et al., 2015; Niendam et al., 2012). In fact, this versatility has led to these brain regions being labelled “multiple-demand network” (Duncan, 2010) – and CER might as well be just another instance of such demand for cognitive control. Others have further differentiated control-related regions into two relatively independent networks, a fronto-parietal and a cingulo-opercular one (Dosenbach et al., 2007; Power et al., 2011), with especially the latter being implicated in self-regulation across emotion and action (Petersen and Posner, 2012). This notion is in part based on observations indicating that the aMCC, a central node of this network, is preferentially involved in cognitive control, while the rostro-ventrally located anterior cingulate cortex is preferentially involved in emotion processing (Bush et al., 2000; see also Beckmann et al., 2009). Although this long-held dichotomy has been repeatedly questioned and qualified (Etkin et al., 2011), the available evidence still puts the aMCC in an optimal position for bridging cognition and emotion when it comes to self-regulation (cf. Posner et al., 2007; Shackman et al., 2011).
In contrast, others have argued for another focal common neural substrate and proposed that ventrolateral prefrontal cortex (including fO and dorsally adjacent parts of right inferior frontal gyrus [IFG]) constitutes the major convergence zone for CER and CAR in the brain, given its role in inhibition and the importance of inhibitory processing for both CER and CAR (e.g., Cohen et al., 2013; Cohen and Lieberman, 2010; Tabibnia et al., 2011). Indeed, in a pioneering study, Tabibnia and colleagues found the only correlations of gray-matter volume with both CER and CAR abilities in right opercular IFG (Tabibnia et al., 2011). More recently, using functional magnetic resonance imaging (fMRI) in abstinent heavy smokers, Tabibnia et al. (2014) found left IFG and preSMA jointly activated across three tasks taxing CER (reappraisal), CAR (stop-signal performance), and a combination thereof (resisting a smoking-related temptation). Finally, Lopez et al. (2014) recently reported that increased activity in left opercular IFG during CAR (go/no-go task) predicted resistance to food temptations in daily life.
Considering the inconsistency of earlier findings in a recent review on the neural basis of human self-regulation, Kelley et al. (2015) argued that one reason for this variability may be that “successful self-regulation involves many executive functions, each of which may have a neural signature that differs depending on specific task demands (e.g., controlling thoughts, reappraising emotion, or inhibiting prepotent responses during a Stroop task), and any one test of executive function may tap only one piece of a larger control system. Thus, a network-level approach may help delineate which systems are important for successful self-regulation.” (p. 400). We fully agree with this evaluation and contend that meta-analysis is an ideally suited tool for testing the assumptions of domain-specific subnetworks and a potential domain-general part within a brain system for self-regulation.
In fact, the neural underpinnings of CER or CAR have been separately examined in several recent meta-analyses (e.g., Buhle et al., 2014; Cieslik et al., 2015; Kohn et al., 2014; Morawetz et al., 2017; Niendam et al., 2012), but they have never been jointly analysed and directly compared on a meta-analytic scale. To close this gap and provide quantitative evidence for shared and disparate neural correlates of these two essential, seemingly related self-regulatory processes, we performed two large-scale meta-analyses of neuroimaging studies on CER and CAR, respectively, and tested for commonalities and differences. Subsequently, the connectional architecture of the ensuing meta-analytic networks was investigated in an independent data set at the individual-subject level by clustering domain-specific regions based on their functional connectivity in the resting state. Given that regions closely connected intrinsically (i.e. at rest) typically also “collaborate” during appropriate tasks (cf. Smith et al., 2009), a connectivity-based distinction of CER- and CAR-specific regions concurring with our meta-analytic results would corroborate the notion of two (partially) distinct networks. This approach also identified within-domain subclusters, possibly corresponding to functional modules. Both domain-general regions and domain-specific subclusters were functionally characterized using BrainMap database meta-data (www.brainmap.org; Laird et al., 2009), statistically testing the quantitative association between mental functions (“behavioural domains”) and regional brain activity across many studies. By combining these three lines of evidence (i.e., meta-analytic network identification and comparison, connectivity-based clustering of network nodes, and quantitative functional inference), we identified a general-purpose neural core network for self-regulation as well as preferentially emotion- versus action-related regulatory subnetworks.
2. Methods
2.1. Study selection for meta-analysis
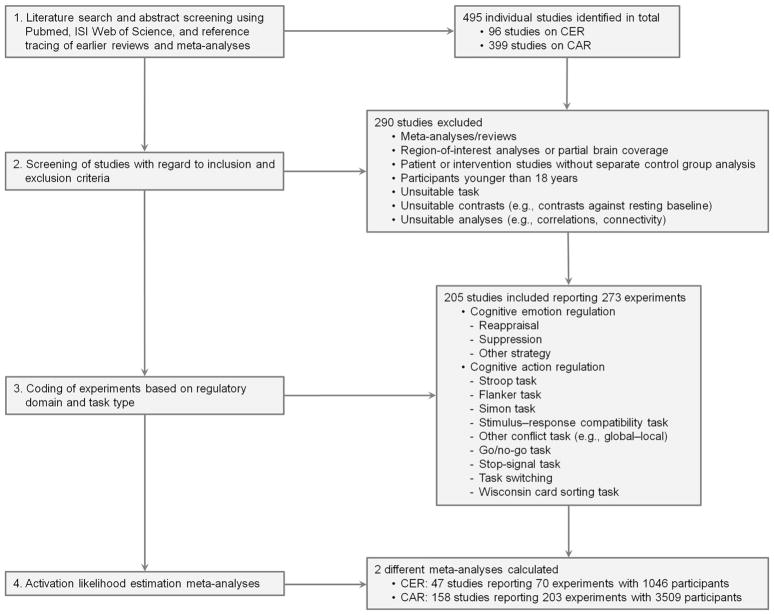
We used a step-wise procedure to identify relevant neuroimaging studies (Fig. 1; see Supplementary Methods for details). In brief, we performed a literature search using PubMed (http://www.pubmed.gov) and Web of Science® (http://apps.webofknowledge.com), supplemented by tracing references in topical papers, for experiments on CER that examined brain activity specifically associated with a task that required participants to intentionally up- or down-regulate their emotion in response to an affective stimulus (e.g., picture, film, story). Acceptable experimental strategies for CER included (1) cognitive reappraisal of the emotional situation (e.g., re-interpreting tears as resulting from joy rather than sadness); (2) distancing from, or empathizing with, the emotional scene presented (e.g., adopting a third-vs. first-person perspective or vice versa); or (3) suppressing the emotional response. For the meta-analysis on CAR, experiments were retained only if they examined brain activity associated with intentionally overcoming a predominant but inadequate action tendency. This included paradigms that required inhibiting responses (i.e., go/no-go and stop-signal tasks), solving response conflicts (i.e., Stroop, Simon, Eriksen flanker, stimulus–response compatibility, or other non-emotional interference tasks), adjusting responses to changing task rules (i.e., Wisconsin card sorting task), or switching between tasks. Finally, experiments were retained only if their results were reported as coordinates in a standard neuroanatomical reference space and if the data resulted from whole-brain group analyses comparing target and “high-level” control conditions, aiming to minimize stimulus- or response-related cognitive processing.
Fig. 1.
Flow Diagram of Study Selection for the Meta-analyses. CER, cognitive emotion regulation; CAR, cognitive action regulation.
Based on these criteria, we identified 70 experiments as eligible for inclusion into the meta-analysis on CER (see Supplementary Table S4), and 203 experiments for inclusion into the meta-analysis on CAR (see Supplementary Table S5). For supplemental analyses with comparable power between CER and CAR, we drew a subset of n = 67 CAR experiments using a stratified random sampling approach, which also ensured a commensurate number of experiments across different CAR task types (see Supplementary Methods).
2.2. Activation likelihood estimation (ALE)
Meta-analyses were performed using the current ALE algorithm for coordinate-based meta-analysis of neuroimaging results (Eickhoff et al., 2012; Eickhoff et al., 2009; Turkeltaub et al., 2012) implemented in Matlab (see Supplementary Methods for details). This algorithm seeks to identify brain areas whose activity converges across experiments more strongly than expected from a random spatial association using a permutation procedure (k = 10,000). The obtained non-parametric p-values were thresholded at p < .05 (family-wise error–corrected at cluster level; cluster inclusion threshold at voxel level: p < .001; cf. Eickhoff et al., 2016) and transformed into z-scores for display. Results were anatomically labelled by reference to probabilistic cytoarchitectonic maps of the human brain using the Anatomy Toolbox (Eickhoff et al., 2005) of SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8).
Commonalities between CER and CAR were examined by a conjunction analysis, which identifies voxels where a significant effect is present in two separate analyses. To compute the conjunction between two ALE analyses, we used the conservative minimum statistic (Nichols et al., 2005), which is equivalent to identifying the intersection between the two (corrected) results.
For testing differences between CER and CAR, we first computed the voxel-wise difference between the respective ALE maps. A label-exchange permutation test (see Supplementary Methods for details) was performed to assess the significance of the observed difference in each voxel’s ALE scores by thresholding at a posterior probability of P > .95 for a true difference between the two samples (Eickhoff et al., 2011). Surviving voxels were inclusively masked by the respective main effect (i.e. the significant effect of the ALE analysis for the minuend; cf. Langner and Eickhoff, 2013).
2.3. Hierarchical clustering on resting-state functional connectivity between CER and CAR nodes
Next, we aimed to (i) validate the distinctness of the meta-analytic CER- versus CAR-specific networks at the individual subject level, and (ii) identify subclusters, or “cliques,” within either domain-specific network. To this end, we used resting-state functional connectivity (FC) as an independent measure of functional brain organization (Cole et al., 2014; Smith et al., 2009) and applied hierarchical cluster analysis to pairwise FC between all domain-specific clusters of convergence obtained from the two meta-analytic contrasts between CER and CAR (cf. Smith et al., 2013). This way, we grouped all domain-specific network nodes in an independent, data-driven manner such that regions in the same group (or cluster) were as similar as possible as to their FC profile, while that profile was maximally different between the ensuing sub-clusters (cf. Amft et al., 2015). The resulting cluster solution was then compared to the results of our meta-analytic contrast analyses and used to define sub-networks for subsequent meta-analytic functional profiling.
Interregional FC strength was computed from resting-state fMRI data of n = 192 adults (65% female, 20–75 years old, mean [ ± SD] age = 46.4 ± 16.7 years, no current psychiatric or neurologic diagnosis) of the publicly available enhanced NKI/Rockland sample (http://fcon_1000.projects.nitrc.org; Nooner et al., 2012). The analysis was approved by the local ethics committee of the Heinrich Heine University Düsseldorf. For details on data acquisition and preprocessing, please see Supplementary Methods.
For each participant, the BOLD signal time course of each domain-specific node (as obtained from the meta-analytic contrasts; cf. above) was extracted as the first eigenvariate of activity in those 50% of voxels of each node that had the highest probability of being located in gray matter. Gray-matter voxels were selected via median split of the gray-matter probabilities of all voxels in a given node as provided by the segmentation of each participant’s mean functional image performed with SPM8 (see Supplementary Methods for further details on preprocessing). Using the FSLNets toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets), partial temporal correlations between all regions’ time series data were computed to estimate pairwise FC (Marrelec et al., 2006). For each pairwise connection, Fisher’s Z–transformed FC values were submitted to one-sample t-tests. The resulting t values, reflecting connection strength as well as consistency across the sample, were z-transformed (into units of the standard normal distribution) and then fed into an agglomerative hierarchical cluster analysis. For clustering, we used Ward’s method as implemented in Matlab. This approach permits the heuristic identification of neurobiologically plausible and functionally interpretable subclusters within a given network.
2.4. Quantitative functional profiling
Brain regions involved in both CER and CAR, as well as the domain-specific subnetworks were functionally characterized by analysing the association of each region/subnetwork with descriptors for mental processes as provided by the BrainMap database (Laird et al., 2009; Laird et al., 2013). These descriptors indicate the “behavioural domain” of each experimental contrast included in the database according to a taxonomy with the following main categories: cognition, action, perception, emotion, and interoception (for subcategories, see www.brainmap.org/scribe). Our analysis was based on 8377 “normal mapping” experiments performed in healthy adults, excluding intervention studies and between-group comparisons.
The behavioural domain meta-data were analysed by way of quantitative forward and reverse inference (Langner et al., 2015; see also Poldrack, 2011). For forward inference, we tested whether the probability of finding activation in voxels of interest given a particular behavioural domain [P(Activation | Domain)] was higher than the baseline probability of finding activation there across the entire database [P(Activation)]. A binomial test assessed significance at p < .05, corrected for multiple comparisons by controlling the false-discovery rate (FDR). Reverse inference identified the most likely behavioural domains given activation in voxels of interest. This likelihood [P(Domain | Activation)] was derived from P(Activation | Domain), P(Domain) and P(Activation) using Bayes’ rule; significance was assessed by chi-square tests (p < .05, FDR-corrected for multiple comparisons).
3. Results
3.1. Meta-analysis on cognitive emotion regulation
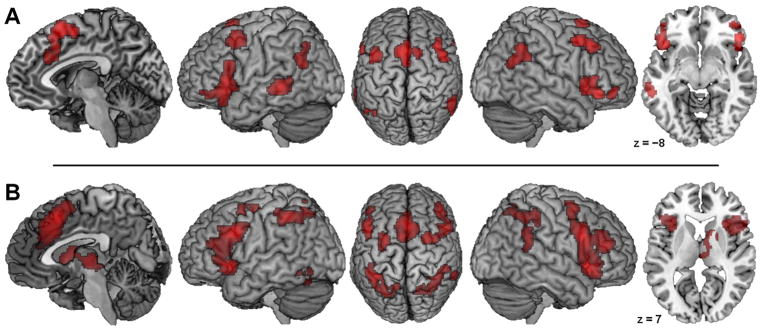
The analysis of 70 experiments on CER revealed significant convergence in a broad network (Fig. 2A; see also Supplementary Table S1) comprising a large dorsomedial frontal region, extending from SMA/preSMA to aMCC; bilateral aI/fO, inferior frontal gyrus (IFG), and lateral orbitofrontal cortex; bilateral posterior MFG and adjacent pre-central gyrus; bilateral posterior temporo-parietal junction (TPJ), extending more anteriorly in the right hemisphere; as well as left posterior middle temporal gyrus (MTG).
Fig. 2.
Meta-analytic Main Effects within Either Regulatory Domain. Foci of brain activity with significant convergence across (A) all 70 experiments on cognitive emotion regulation and (B) all 203 experiments on cognitive action regulation. Coordinates refer to Montreal Neurological Institute space. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.2. Meta-analysis on cognitive action regulation
The analysis of 203 experiments on CAR revealed significant convergence in a broad network (Fig. 2B; see also Table S2) comprising a large dorsomedial frontal region, extending from SMA/preSMA further into medial superior frontal gyrus and aMCC; large bilateral prefrontal-insular clusters (covering parts of aI/fO, IFJ, IFG, and MFG); bilateral PMd; bilateral IPS extending to superior parietal lobule and primary somatosensory cortex (Area 2; Grefkes et al., 2001); right TPJ; left fusiform gyrus; as well as right anterior/medial caudate nucleus, anterior putamen, and thalamus (predominantly parts with prefrontal projections; cf. Behrens et al., 2003). A supplemental analysis of a stratified random subsample of n = 67 CAR experiments, conducted to avoid any power bias in comparison with the CER sample, revealed essentially the same – if somewhat more focused – network (see Supplementary Figure S1).
3.3. Brain activity shared between CER and CAR
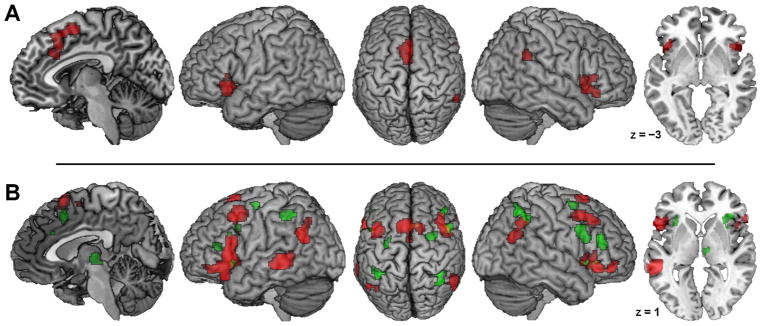
A conjunction analysis using the strict minimum statistic revealed that significant overlap across both domain-specific networks was restricted to four clusters (Fig. 3A): left aI/fO (cluster size: 236 voxels; peak voxel MNI coordinates: −42, 20, −4), right aI/fO extending into IFG (373 voxels; peak: 46, 18, −4), frontomedial cortex (778 voxels; preSMA peak: −2, 16, 54; aMCC peak: −4, 24, 30), as well as right TPJ (73 voxels; peak: 60, −46, 30; almost entirely located in cytoarchitectonically defined area PFm of the inferior parietal lobule, cf. Caspers et al., 2006).
Fig. 3.
Meta-analytic Overlap and Differences between Regulatory Domains. (A) Conjunction across meta-analytic main effects for cognitive emotion regulation (CER) and cognitive action regulation (CAR). (B) Foci of brain activity with significantly stronger convergence across experiments on CER (red) or CAR (green). Coordinates refer to Montreal Neurological Institute space. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.4. Meta-analytic differences between CER and CAR
Contrast analysis revealed significantly stronger convergence related to CER than CAR in the following brain regions (Fig. 3B, denoted in red; see also Table S3): dorsal SMA/preSMA; bilateral IFG extending into lateral orbitofrontal cortex; bilateral posterior MFG and adjacent precentral gyrus; bilateral posterior TPJ (inferior parietal lobule); and left posterior MTG.
The reverse contrast yielded significantly stronger convergence related to CAR than CER in the following brain regions (Fig. 3B, denoted in green; see also Table S3): ventral preSMA extending into right aMCC; bilateral aI/fO; bilateral IFJ and adjacent inferior frontal sulcus; bilateral dorsal IFG (mid-ventrolateral prefrontal cortex); bilateral PMd; bilateral IPS and adjacent primary somatosensory area 2 (in the left hemisphere); as well as right thalamus (prefrontally projecting parts; cf. Behrens et al., 2003). Supplemental analyses using the above-mentioned subsample of n = 67 CAR experiments yielded essentially the same results for either contrast (see Figure S1).
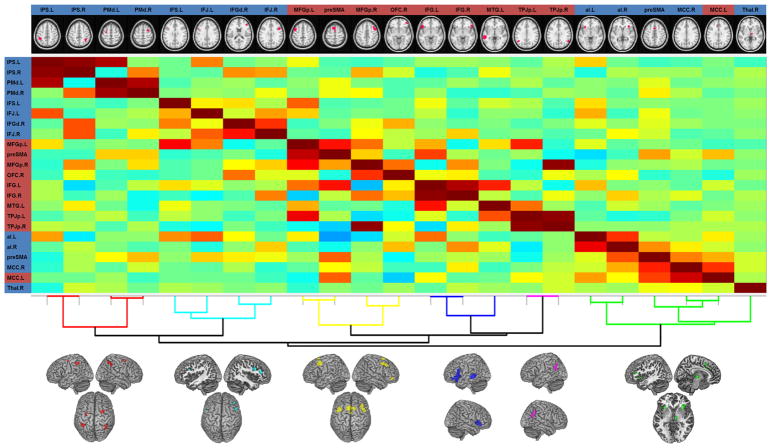
3.5. Clustering on functional connectivity between CER- and CAR-specific network nodes
The hierarchical clustering based on resting-state FC between all domain-specific regions identified in the above contrast analyses grouped these regions into subclusters with strong within-cluster similarity (Fig. 4, subclusters denoted in same colours). This data-driven grouping yielded three CER-specific and two CAR-specific subnetworks of 2–4 regions each. In addition, there was a somewhat larger third CAR-related subcluster comprising bilateral aI/fO, preSMA, right thalamus, as well as right and left (CER-related) aMCC.
Fig. 4.
Clustering on functional connectivity between domain-specific network nodes. Full (shown below the diagonal) and partial (above the diagonal) correlation matrices. Warmer colours (from yellow to dark-red) denote increasingly strong positive correlations; cooler colours (from yellow-green to dark-blue) denote increasingly strong negative correlations. Small images at the top of each column show each node’s location; the background colour of node labels indicates each node’s domain (blue = cognitive action regulation; red = cognitive emotion regulation). The nodes were reordered according to a hierarchical clustering of the partial correlation matrix, as visualized below the matrix. At the bottom, the spatial map of each subcluster is depicted. Abbreviations:. L, left;. R, right; IPS, intraparietal sulcus; PMd, dorsal premotor cortex; IFS, inferior frontal sulcus; IFJ, inferior frontal junction; IFGd, dorsal inferior frontal gyrus; MFGp, posterior middle frontal gyrus; OFC, orbitofrontal cortex; MTG, middle temporal gyrus; TPJp, posterior temporo-parietal junction; aI, anterior insula; preSMA, pre-supplementary motor area; MCC, midcingulate cortex; Thal, Thalamus (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Further agglomeration showed that all subclusters merged with each other first within their respective domain, in line with the meta-analytic results. Notably, the largest CAR-related subcluster joined the remaining ones only at the last junction of the hierarchical cluster tree (i.e., at its highest level). Thus, it was more dissimilar (in terms of internodal FC) from the other CER- and CAR-related subclusters formed up to that level than those subclusters were from each other. This may be due to the fact that all regions of that large subcluster, except for the thalamus, were directly adjacent to regions jointly involved in both CER and CAR (cf. above). Thus, even if the meta-analytic contrast analysis indicated a preferential association with CAR, these regions would still be linked to CER to some lesser degree. In summary, the data-driven FC-based clustering yielded a split into CER- versus CAR-specific regions, mirroring the meta-analytic differentiation (see Fig. 4).
3.6. Quantitative functional profiling of domain-general regions
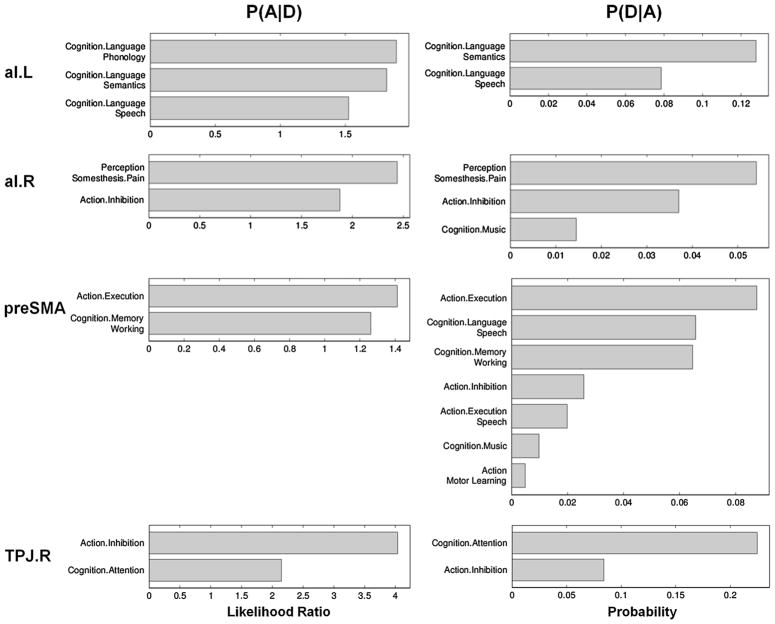
We functionally characterized the four regions consistently active during CER and CAR alike (i.e., preSMA, bilateral aI/fO, and right TPJ) by performing forward and reverse inference on these regions’ associations with behavioural domains as provided by the BrainMap database. The results are shown in Fig. 5. In brief, left aI was activated by 567 out of 8377 eligible BrainMap experiments and significantly associated with different aspects of language-related cognition (semantics, speech, and – based on forward inference only – phonology). Right aI, activated by 630 experiments, was significantly linked to pain perception, action inhibition, and music-related cognition (the latter indicated only by reverse inference). The shared frontomedial cluster (preSMA/aMCC), activated by 1509 experiments, was associated with action execution and working memory. Reverse inference indicated additional associations with speech-related cognition and speech execution, action inhibition, motor learning, as well as music-related cognition. Finally, the domain-general part of the right TPJ (activated by 92 experiments in BrainMap) was significantly linked to action inhibition and attention.
Fig. 5.
Functional Characterization of the Four Domain-general Core Regions. BrainMap behavioural domain meta-data were used for quantitative forward (left panel) and reverse (right panel) inference on significant functional associations of each core region. For abbreviations, see Fig. 4.
3.7. Quantitative functional profiling of domain-specific subclusters
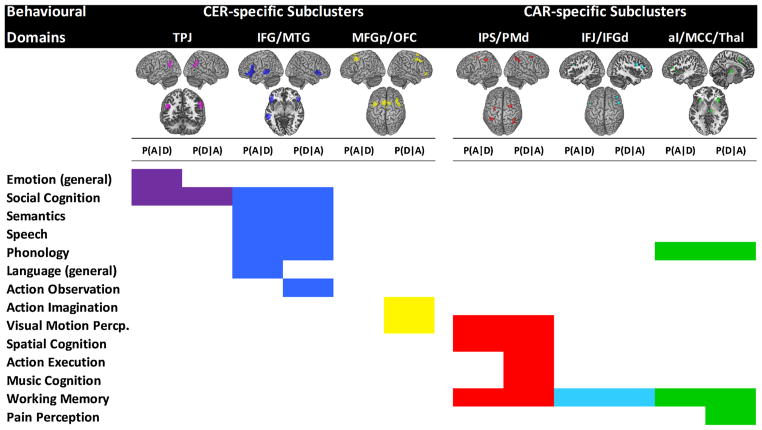
For functionally characterizing the domain-specific subclusters we only considered experiments from BrainMap that activated at least two regions of a given subcluster (with the exception of the smaller two-element TPJ subcluster). Of these two (or more) activated regions, at least one had to be non-homotopic with respect to other lateralized regions in that cluster. This constraint was applied because left and right homotopic regions often are highly similar regarding their functional involvement. Our approach avoided this potential redundancy and enforced some more dissimilarity among the two (or more) regions co-activated by any eligible experiment.
The results of these analyses are depicted in Fig. 6. At the time of analysis, 446 BrainMap experiments activated the CER-related TPJ subcluster (i.e., left OR right posterior TPJ). Forward and reverse inference alike indicated a significant association of this subcluster with social cognition, while forward inference additionally yielded an association with emotion (i.e., the label given to all studies on emotion processing that do not deal exclusively with one of the basic emotions fear/anxiety, happiness, sadness, disgust, or anger). The CER-related IFG/MTG subcluster [(left OR right IFG) AND left posterior MTG] was activated by 231 BrainMap experiments and significantly associated with language-related cognition (especially phonology, semantics, and speech) and social cognition; only reverse inference indicated an association with action observation. The third CER-related subcluster [(left OR right posterior MFG) AND (dorsal preSMA OR right lateral orbitofrontal cortex)] was activated by 226 BrainMap experiments. While forward inference yielded no significant results, reverse inference indicated significant associations with visual motion perception and action imagination.
Fig. 6.
Summary of the Functional Characterization of Domain-specific Subclusters. BrainMap behavioural domain meta-data were used for quantitative forward [P (A|D)] and reverse [P(D|A)] inference on significant functional associations of each domain-specific subcluster. Small images at the top of each column show each subcluster’s spatial map. CER, cognitive emotion regulation; CAR, cognitive action regulation. For further abbreviations, see Fig. 4. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
The CAR-related IPS/PMd subcluster [(left OR right IPS) AND (left OR right PMd)] was activated by 160 BrainMap experiments and significantly linked to spatial cognition, working memory, and visual motion perception. Reverse inference yielded additional associations with action execution and music-related cognition. The CAR-related IFJ/IFG subcluster [(left OR right IFJ OR left inferior frontal sulcus) AND right dorsal IFG] was activated by 122 experiments and significantly associated with working memory. Finally, 226 eligible BrainMap experiments activated the third CAR-related subcluster [(left OR right aI) AND (preSMA OR right aMCC OR right thalamus)]. Both kinds of inference indicated significant associations with language-related cognition (phonology) and working memory, while reverse inference yielded an additional association with pain perception.
4. Discussion
4.1. Networks subserving CER or CAR are largely distinct
This study examined to what extent cognitively controlling emotions or actions is subserved by a shared brain network. We provide evidence from three independent sources of data that this is the case only to a rather limited degree. First, we delineated and compared the sets of brain regions activated during CER and CAR by conducting two large-scale neuroimaging meta-analyses. The resulting two fronto-parieto-insular CER- and CAR-related networks agree well with previous meta-analyses on either topic (CER: e.g. Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014; CAR: e.g. Cieslik et al., 2015; Levy and Wagner, 2011; Niendam et al., 2012). And indeed, the two CER- and CAR-related networks may seem rather similar at first sight. A formal conjunction across them, however, revealed that their overlap was restricted to four regions: left and right aI/fO, preSMA/aMCC, and right TPJ. Conversely, meta-analytic contrast analyses demonstrated a broad range of significant differences between the two networks. We conclude that the majority of regions in either network appear to be preferentially associated with either CER or CAR, rather than forming a largely shared domain-general network as previously suggested (e.g., Buhle et al., 2014; Etkin et al., 2016; Rothbart et al., 2011; Schweizer et al., 2013).
Next, we examined the within-subject distinctness of the evidently domain-specific parts of both CER and CAR networks by assessing resting-state FC between these nodes, given that intrinsic brain connectivity provides an independent means to characterize functional networks (Cole et al., 2014; Langner et al., 2015; Power et al., 2011). Clustering all domain-specific nodes based on their internodal FC clearly separated CER- from CAR-specific regions (cf. Fig. 4). The only minor exception was the inclusion of the CER-specific left aMCC in the largest CAR-related subcluster (comprising parts of bilateral aI, preSMA/aMCC, and right thalamus), which was most likely due to the spatial proximity of the right and left aMCC regions. High FC values between such neighbouring regions may be driven by the spatial smoothness of the hemodynamic response, further adding to the generally strong intrinsic FC between homotopic areas (Stark et al., 2008; Zuo et al., 2010). Overall, the data-driven hierarchical clustering, obtained from an independent data set, corroborated our meta-analytic contrast results.
Finally, using behavioural domain meta-data of the BrainMap database, we functionally characterized the four domain-general regions as well as the six domain-specific subclusters in an objective, quantitative way. The domain-general regions were associated with “higher level” cognitive categories such as working memory, attention, language, action inhibition and execution, or bodily awareness (pain). Thus, each of these four regions is linked to processes/functions that represent facets of cognitive control, which fits with our meta-analytic results indicating an involvement of these regions in top-down control across domains. The domain-specific clusters, in turn, featured behavioural profiles with only little overlap between CER- versus CAR-related subclusters (cf. Fig. 6). In particular, the CER-related subclusters were linked to emotion, social cognition, language, action observation and imagination, while CAR-related subclusters were linked to working memory, spatial cognition, and action execution. This association of CER-related regions with socio-affective processing and of CAR-related regions with primarily non-affective cognitive control, respectively, provides independent support for our meta-analytic and FC-based network differentiation.
Taken together, our data provide conclusive evidence for substantial neurofunctional distinctions between the two regulatory domains. This suggests that CER and CAR differ in many subprocesses, with the BrainMap meta-data providing some strong clues as to the nature of these. Yet, our results also indicate that CER and CAR converge on a significant “common ground,” suggesting a shared set of core processes. Since the domain-specific functions of CER- or CAR-related brain regions have been discussed in detail elsewhere (e.g., Buhle et al., 2014; Duncan, 2010; Kohn et al., 2014; Niendam et al., 2012), we will focus on the domain-independent activity in bilateral aI/fO, preSMA/MCC, and right TPJ, which we propose as the neural substrate of exerting self-control.
4.2. A core network for self-regulation
Given the evidence for psychological and neurobiological commonalities among CER and CAR (see Introduction), the limited spatial overlap between the neurofunctional correlates of either domain may come to some as a surprise. Others, however, may be surprised that overlap actually was found in more than one brain region, since initial studies identified the opercular part of right IFG (i.e., ventrolateral prefrontal cortex) as the convergence zone for CER and CAR in the brain (Tabibnia et al., 2011). Indeed, the meta-analytic overlap in aI/fO observed in our data included part of the (bilateral) opercular IFG, with overlap in the dorsal operculum being largely restricted to the right side. However, a wide variety of research supports a central role in self-regulation for all four shared network parts. First of all, it is hardly surprising that it is these four regions that were found jointly involved across domains, since they form a tightly coupled network (Bzdok et al., 2013), distinct from other subnetworks involved in cognitive control (Dosenbach et al., 2007; Power et al., 2011; Seeley et al., 2007). More to the point of self-regulation, meta-analytic evidence shows that our four domain-general regions are consistently involved in keeping up attention to intellectually unchallenging, boring tasks (Langner and Eickhoff, 2013). Maintaining attention under such conditions, which are perceived as increasingly aversive, is another domain where self-control is seriously taxed in order to prevent mind-wandering and to stay on the “job.” The set of domain-general regions (except for right TPJ) also is consistently linked to another high-level mental function involving effortful cognitive control: working memory (Rottschy et al., 2012), the training of which has been found to result in improved impulse control in addiction patients (Bickel et al., 2011; Houben et al., 2011; see also Wiers et al., 2013).
Intriguingly, a recent meta-analysis (Goodkind et al., 2015) found the same three regions (i.e., bilateral aI/fO and preSMA/MCC) as the only ones showing consistent differences in gray-matter volume related to mental disorders across a broad range of psychiatric diagnoses (including mood and anxiety disorders, substance use disorders, and schizophrenia). That study also demonstrated that in healthy participants volumetric variance in these regions is linked to executive functioning. Those findings indicate a general association between the integrity of this circumscribed network and highly diverse psychiatric syndromes. Such a few-to-many mapping, in turn, corroborates the idea of a network that is involved in a wide variety of mental (dys)functions as it forms the domain-general core of the top-down regulatory neurocircuitry. These transnosologic aberrations in network integrity echo a recent study demonstrating that age-dependent deterioration of FC between CAR-related regions is largely restricted to the same three regions (Langner et al., 2015). Conversely, it has been suggested that this network’s maturation in children, including the proliferation of Von Economo neurons that are unique to aI and cingulate cortex, may relate to the improvement in self-regulation (or “effortful control”) between infancy and later childhood (Posner and Rothbart, 2009). We conjecture that individual differences in this network’s functional integrity, already present in middle childhood, might underlie the association observed between the tolerated delay of gratification (a marker for self-control) in pre-schoolers and their parent-reported control over negative affect (CER) and ability to concentrate (CAR) during adolescence (Shoda et al., 1990).
The paramount importance of this set of regions for self-regulation is further supported by studies on brain changes resulting from mindfulness meditation practice, which has been shown to be beneficial for both CER and CAR (cf. Teper et al., 2013; see also Tang et al., 2015): Again, these changes are predominantly found in aI, preSMA/MCC, and TPJ, beside structures of the fronto-limbic and default-mode networks (Hölzel et al., 2011). Based on the above evidence, we propose that it is the shared core network that mediates across-domain transfer effects of training procedures that (initially) target only CER or CAR abilities, respectively.
4.3. Limitations and functional considerations
In spite of the converging evidence obtained by the different analyses, some limitations are worth noting. First, our analysis did not differentiate between the various subtypes of CER or CAR, although it has been shown that different strategies may entail somewhat different brain activation patterns (Cieslik et al., 2015; Dörfel et al., 2014; see also Egner, 2008). Rather, we sampled data from a broad range of paradigms taxing either CER or CAR in order to distil brain activation that is consistent across various conceptual subcategories and task types in either domain. This way we aimed to obtain activity estimations that are less affected by strategy/task idiosyncrasies and thus correspond more closely to the high-level constructs of CER and CAR. And while reappraisal and Stroop-type conflicts constitute the most frequent paradigms in our sample, possibly introducing a bias toward these, our main effects agree well with previous meta-analyses using different selection criteria, indicating robustness against such biases. This conclusion is also supported by the high comparability of results obtained in the full versus stratified CAR sample, as the stratification removed any frequency bias for a given task.
Furthermore, the exact nature of the presumably highly abstract processing subserved by the domain-general network cannot be as well derived from the BrainMap meta-data as can more concrete domain-specific subprocesses. Ascribing particular processing steps involved in self-regulation to each of the four domain-general regions would therefore remain rather speculative. Psychological research, however, has successfully applied the cybernetic control-theory framework to obtain a more detailed picture of how self-regulation might be implemented at the computational level (Baumeister and Heatherton, 1996; Carver and Scheier, 1981, 1982; Zelazo and Cunningham, 2007). The core idea of this framework is that control is achieved via iterative negative feedback loops that keep emotional and behavioural impulses in check (i.e., in line with a given “higher” goal). In particular, the control-theory approach poses that successful self-regulation entails the following subprocesses (Baumeister and Heatherton, 1996; Hofmann et al., 2012): (i) monitoring the current state against the representation of an intended “standard” (i.e., the goal) to become aware of any discrepancy between goal state (adequate response) and actual state (inadequate response tendency), (ii) expending the necessary effort for top-down adjustments to remove or reduce any perceived discrepancy, and (iii) updating the current state representation and evaluating it against the goal state and the effort invested.
These processing steps generally agree with our functional decoding results as well as with functions previously ascribed to one or several of the four domain-general brain regions. Such functions, usually defined rather broadly, with relevance to self-control include task-set activation and maintenance (Dosenbach et al., 2006, 2007), schema energization and monitoring (Langner and Eickhoff, 2013; Stuss et al., 1995), salience detection (Seeley et al., 2007), bodily awareness (Craig, 2002; Critchley et al., 2004), alertness/arousal regulation (Critchley et al., 2002; Langner et al., 2012; Sadaghiani and DöEsposito, 2015), action outcome prediction and evaluation (Alexander and Brown, 2011; Silvetti et al., 2011), contextual updating (Geng and Vossel, 2013), and effort investment (Engström et al., 2014; Fischer et al., 2008; Prévost et al., 2010). We consider these attempts to verbally summarize complex, highly abstract cognitive processes as useful global approximations. Control theory, in turn, provides a computational framework that may allow integrating these perspectives and enable future research to test more specific hypotheses about the neural implementation of these interacting subprocesses, which, in their entirety, make up the core of successful self-control.
4.4. Conclusions
Our results indicate limited commonalities and important differences between CER and CAR, suggesting that both regulatory facets are akin to each other but not alike: they appear to share a common core, yet they also involve several distinct subprocesses. We propose that the four regions jointly involved in both CER and CAR (i.e., bilateral aI/fO, preSMA/aMCC and right TPJ) represent the neural substrate of a general control feedback loop essential to self-regulation across emotion and action domains. This feedback loop is thought to realize the effortful implementation of non-dominant but goal-conducive mental schemata to override spontaneous emotional/behavioural responses to external stimuli, likely mediating previously shown associations between emotion and action regulation abilities in health and disease. The observed brain activity differences between the two aspects of self-regulation, in turn, reflect how those goal-oriented schemata are put into effect: via driving semantic, evaluative and imagery-based processing to modulate affective responses versus driving spatial-attentional, working-memory and action-execution-related processing to modulate behavioural responses.
Supplementary Material
Acknowledgments
We thank all contacted authors who contributed results of relevant contrasts not explicitly reported in the original publications.
This work was supported by the Deutsche Forschungsgemeinschaft (LA 3071/3-1, EI 816/4-1); the National Institute of Mental Health (R01-MH074457); the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain”; and the European Union Seventh Framework Programme (grant agreement no. 604102).
References
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB. Definition and characterization of an extended social-affective default network. Brain Struct Funct. 2015;220:1031–1049. doi: 10.1007/s00429-013-0698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: an overview. Psychol Inq. 1996;7:1–15. doi: 10.1207/s15327965pli0701_1. [DOI] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Laird AR, Fox PT, Zilles K, Eickhoff SB. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. NeuroImage. 2013;81:381–392. doi: 10.1016/j.neuroimage.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Wang TS. Inhibitory control and emotion regulation in preschool children. Cogn Dev. 2007;22:489–510. doi: 10.1016/j.cogdev.2007.08.002. [DOI] [Google Scholar]
- Carver CS, Scheier MF. Attention and Self-Regulation: A Control-Theory Approach to Human Behavior. Springer-Verlag; New York: 1981. [Google Scholar]
- Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality-social, clinical, and health psychology. Psychol Bull. 1982;92:111–135. doi: 10.1037//0033-2909.92.1.111. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. NeuroImage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Chambers R, Lo BCY, Allen NB. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cognitive Ther Res. 2008;32:303–322. doi: 10.1007/s10608-007-9119-0. [DOI] [Google Scholar]
- Cieslik EC, Müller VI, Eickhoff CR, Langner R, Eickhoff SB. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci Biobehav Rev. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Berkman ET, Lieberman MD. Intentional and incidental self-control in ventrolateral PFC. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; New York, USA: 2013. pp. 417–440. [Google Scholar]
- Cohen JR, Lieberman MD. The common neural basis of exerting self-control in multiple domains. In: Hassin RR, Ochsner KN, Trope Y, editors. Self Control in Society, Mind, and Brain. Oxford University Press; Oxford, UK: 2010. pp. 141–162. [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional control of autonomic arousal: a functional magnetic resonance study. NeuroImage. 2002;16:909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dörfel D, Lamke JP, Hummel F, Wagner U, Erk S, Walter H. Common and differential neural networks of emotion regulation by detachment, reinterpretation, distraction, and expressive suppression: a comparative fMRI investigation. NeuroImage. 2014;101:298–309. doi: 10.1016/j.neuroimage.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends Cogn Sci. 2008;12:374–380. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, Bzdok D, Eickhoff CR. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage. 2016;137:70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Engström M, Karlsson T, Landtblom AM, Craig AD. Evidence of conjoint activation of the anterior insular and cingulate cortices during effortful tasks. Front Hum Neurosci. 2014;8:1071. doi: 10.3389/fnhum.2014.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ. Emotion regulation involves both model-based and model-free processes. Nat Rev Neurosci. 2016;17:532. doi: 10.1038/nrn.2016.79. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci USA. 2013;110:16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T, Langner R, Birbaumer N, Brocke B. Arousal and attention: self-chosen stimulation optimizes cortical excitability and minimizes compensatory effort. J Cogn Neurosci. 2008;20:1443–1453. doi: 10.1162/jocn.2008.20101. [DOI] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Geng JJ, Vossel S. Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci Biobehav Rev. 2013;37:2608–2620. doi: 10.1016/j.neubiorev.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. NeuroImage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. 1998;2:271–299. [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Deutsch R, Lancaster K, Banaji MR. Cooling the heat of temptation: mental self-control and the automatic evaluation of tempting stimuli. Eur J Soc Psychol. 2010;40:17–25. doi: 10.1002/ejsp.708. [DOI] [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. 2011;6:537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW, Jansen A. Getting a grip on drinking behavior: training working memory to reduce alcohol abuse. Psychol Sci. 2011;22:968–975. doi: 10.1177/0956797611412392. [DOI] [PubMed] [Google Scholar]
- Jha AP, Stanley EA, Kiyonaga A, Wong L, Gelfand L. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010;10:54–64. doi: 10.1037/a0018438. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Wagner DD, Heatherton TF. In search of a human self-regulation system. Annu Rev Neurosci. 2015;38:389–411. doi: 10.1146/annurev-neuro-071013-014243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation: an ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole S. The psychology of emotion regulation: an integrative review. Cogn Emot. 2009;23:4–41. doi: 10.1080/02699930802619031. [DOI] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the BrainMap database: progress towards a probabilistic functional brain atlas. Front Neuroinform. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Rottschy C, Bzdok D, Ray KL, Fox PT. Networks of task co-activations. NeuroImage. 2013;80:505–514. doi: 10.1016/j.neuroimage.2013.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Cieslik EC, Behrwind SD, Roski C, Caspers S, Amunts K, Eickhoff SB. Aging and response conflict solution: behavioural and functional connectivity changes. Brain Struct Funct. 2015;220:1739–1757. doi: 10.1007/s00429-014-0758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Eickhoff SB, Boers F, Chatterjee A, Willmes K, Sturm W. Staying responsive to the world: modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Hum Brain Mapp. 2012;33:398–418. doi: 10.1002/hbm.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiberg S, Eippert F, Veit R, Anders S. Intentional social distance regulation alters affective responses towards victims of violence: an fMRI study. Hum Brain Mapp. 2012;33:2464–2476. doi: 10.1002/hbm.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RB, Hofmann W, Wagner DD, Kelley WM, Heatherton TF. Neural predictors of giving in to temptation in daily life. Psychol Sci. 2014;25:1337–1344. doi: 10.1177/0956797614531492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelec G, Krainik A, Duffau H, Pelegrini-Issac M, Lehericy S, Doyon J, Benali H. Partial correlation for functional brain interactivity investigation in functional MRI. NeuroImage. 2006;32:228–237. doi: 10.1016/j.neuroimage.2005.12.057. [DOI] [PubMed] [Google Scholar]
- McRae K, Jacobs SE, Ray RD, John OP, Gross JJ. Individual differences in reappraisal ability: Links to reappraisal frequency, well-being, and cognitive control. J Res Pers. 2012;46:2–7. doi: 10.1016/j.jrp.2011.10.003. [DOI] [Google Scholar]
- Morawetz C, Bode S, Derntl B, Heekeren HR. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2017;72:111–128. doi: 10.1016/j.neubiorev.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Müller VI, Langner R, Cieslik EC, Rottschy C, Eickhoff SB. Interindividual differences in cognitive flexibility: influence of gray matter volume, functional connectivity and trait impulsivity. Brain Struct Funct. 2015;220:2401–2414. doi: 10.1007/s00429-014-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Shmueli D, Burkley E. Conserving self-control strength. J Pers Soc Psychol. 2006;91:524–537. doi: 10.1037/0022-3514.91.3.524. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Brett M, Andersson J, Wager TD, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q, Sikka S, Gutman D, Bangaru S, Schlachter RT, Kamiel SM, Anwar AR, Hinz CM, Kaplan MS, Rachlin AB, Adelsberg S, Cheung B, Khanuja R, Yan C, Craddock CC, Calhoun V, Courtney W, King M, Wood D, Cox CL, Kelly AM, Di Martino A, Petkova E, Reiss PT, Duan N, Thomsen D, Biswal B, Coffey B, Hoptman MJ, Javitt DC, Pomara N, Sidtis JJ, Koplewicz HS, Castellanos FX, Leventhal BL, Milham MP. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Frontiers Neuroscience. 2012;6:152. doi: 10.3389/fnins.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Toward a physical basis of attention and self-regulation. Phys Life Rev. 2009;6:103–120. doi: 10.1016/j.plrev.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/CABN.7.4.391. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost C, Pessiglione M, Météreau E, Cléry-Melin ML, Dreher JC. Separate valuation subsystems for delay and effort decision costs. J Neurosci. 2010;30:14080–14090. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Rueda MR. The development of effortful control. In: Mayr U, Awh E, Keele SW, editors. Developing Individuality in the Human Brain: A Tribute to Michael I. Posner. American Psychological Association; Washington, DC: 2005. pp. 167–188. [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, Posner MI. Developing mechanisms of self-regulation in early life. Emot Rev. 2011;3:207–213. doi: 10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. NeuroImage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, D’Esposito M. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cereb Cortex. 2015;25:2763–2773. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BJ, Tang D. Individual differences in executive functioning and their relationship to emotional processes and responses. Curr Dir Psychol Sci. 2015;24:93–98. doi: 10.1177/0963721414555178. [DOI] [Google Scholar]
- Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. J Pers Soc Psychol. 2008;95:1526–1540. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- Schweizer S, Grahn J, Hampshire A, Mobbs D, Dalgleish T. Training the emotional brain: improving affective control through emotional working memory training. J Neurosci. 2013;33:5301–5311. doi: 10.1523/JNEUROSCI.2593-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoda Y, Mischel W, Peake PK. Predicting adolescent cognitive and self-regulatory competences from preschool delay of gratification: identifying diagnostic conditions. Dev Psychol. 1990;26:978–986. doi: 10.1037/0012-1649.26.6.978. [DOI] [Google Scholar]
- Silvetti M, Seurinck R, Verguts T. Value and prediction error in medial frontal cortex: integrating the single-unit and systems levels of analysis. Front Hum Neurosci. 2011;5:75. doi: 10.3389/fnhum.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds J, Kieras JE, Rueda MR, Rothbart MK. Effortful control, executive attention, and emotional regulation in 7–10-year-old children. Cogn Dev. 2007;22:474–488. doi: 10.1016/j.cogdev.2007.08.009. [DOI] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, Nichols TE, Robinson EC, Salimi-Khorshidi G, Woolrich MW, Barch DM, Ugurbil K, Van Essen DC. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17:666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, Gee DG, Roy AK, Banich MT, Castellanos FX, Milham MP. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28:13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, Picton TW. A multidisciplinary approach to anterior attentional functions. Ann N Y Acad Sci. 1995;769:191–211. doi: 10.1111/j.1749-6632.1995.tb38140.x. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Creswell JD, Kraynak T, Westbrook C, Julson E, Tindle HA. Common prefrontal regions activate during self-control of craving, emotion, and motor impulses in smokers. Clin Psychol Sci. 2014;2:611–619. doi: 10.1177/2167702614522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31:4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Posner MI, Rothbart MK, Volkow ND. Circuitry of self-control and its role in reducing addiction. Trends Cogn Sci. 2015;19:439–444. doi: 10.1016/j.tics.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Teper R, Segal ZV, Inzlicht M. Inside the mindful mind: how mindfulness enhances emotion regulation through improvements in executive control. Curr Dir Psychol Sci. 2013;22:449–454. doi: 10.1177/0963721413495869. [DOI] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott CM, Landau S. The relation between disinhibition and emotion regulation in boys with attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol. 2004;33:772–782. doi: 10.1207/s15374424jccp3304_12. [DOI] [PubMed] [Google Scholar]
- Webb TL, Miles E, Sheeran P. Dealing with feeling: a meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol Bull. 2012;138:775–808. doi: 10.1037/a0027600. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Gladwin TE, Hofmann W, Salemink E, Ridderinkhof KR. Cognitive bias modification and cognitive control training in addiction and related psychopathology: mechanisms, clinical perspectives, and ways forward. Clin Psychol Sci. 2013;1:192–212. doi: 10.1177/2167702612466547. [DOI] [Google Scholar]
- Zelazo PD, Cunningham WA. Executive function: mechanisms underlying emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford Press; New York: 2007. pp. 135–158. [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, Milham MP. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.