Abstract
Platelet dense granules are membrane bound compartments that store polyphosphate and small molecules such as ADP, ATP, Ca2+ and serotonin. The release of dense granule contents plays a central role in platelet aggregation to form a hemostatic plug. Accordingly, congenital deficiencies in the biogenesis of platelet dense granules underlie human genetic disorders that cause storage pool disease and manifest with prolonged bleeding. Dense granules belong to a family of lysosome-related organelles, which also includes melanosomes, the compartments where the melanin pigments are synthesized. These organelles share several characteristics including an acidic lumen and, at least in part, the molecular machinery involved in their biogenesis. As a result, many genes affect both dense granule and melanosome biogenesis and the corresponding patients present not only with bleeding but also with oculocutaneous albinism. The identification and characterization of such genes has been instrumental in dissecting the pathways responsible for organelle biogenesis. Because the study of melanosome biogenesis has advanced more rapidly, this knowledge has been extrapolat ed to explain how dense granules are produced. However, some progress has recently been made in studying platelet dense granule biogenesis directly in megakaryocytes and megakaryocytoid cells. Dense granules originate from an endosomal intermediate compartment, the multivesicular body. Maturation and differentiation into a dense granule begins when newly synthesized dense granule specific proteins are delivered from early/recycling endosomal compartments. The machinery that orchestrates this vesicular trafficking is composed of a combination of both ubiquitous and cell type specific proteins. Here we review the current knowledge on dense granule biogenesis. In particular, we focus on the individual human and murine genes encoding the molecular machinery involved in this process and how their deficiencies result in disease.
Keywords: AP-3 complex, BLOC, HOPS, Rab38, protein traffic, Hermansky-Pudlak syndrome
Introduction
Circulating platelets are anucleate cells that originate from bone marrow megakaryocytes and have fundamental functions in preventing bleeding and minimizing blood vessel damage. Many of the platelets functions are mediated by secreted molecules, which in the resting platelet are stored in membrane-bound compartments known as granules. The contents of these granules are released during platelet activation as a result of granule fusion with the plasma membrane. Platelets contain three types of well-known granules: dense granules (DGs, also known as dense bodies and δ-granules), α-granules and lysosomes [1–4]. A forth type of platelet granule, the T granule, was discovered more recently [5].
Normal human platelets contain three to eight DGs typically measuring 200–300 nm in diameter although both smaller and larger DGs can also be observed [6]. Underscoring DG function in hemostasis, patients with inherited DG deficiency have bleeding problems of variable severity [7–17]. In comparison to α-granules that package hundreds of proteins, DGs contain relatively few small molecules at high concentrations (high mM range): serotonin, ADP, ATP, Ca2+, pyrophosphate (PPi) and polyphosphate of 70–75 phosphate units (polyP) as well as Mg2+ and K+ [18–21]. These small molecules are typically transported from the megakaryocyte and platelet cytosol into the DG lumen. Our understanding of the integral membrane proteins that mediate small molecule cargo transport across the DG membrane is limited [22]. Likewise, the intracellular trafficking mechanisms that deliver newly synthesized transporters and other proteins to the limiting membrane of maturing DGs are also partially understood.
Platelet dense granules belong to a family of acidic compartments known as lysosome-related organelles (LROs), which also include melanosomes in melanocytes and retinal-pigmented epithelial cells, lytic granules in cytotoxic T lymphocytes and natural killer cells, and many other cell type specific organelles [11, 23–27]. DG biogenesis has been assumed to use similar mechanisms as other LROs. Supporting this idea, several diseases manifest with prolonged bleeding due to DG deficiency together with other manifestations such as oculocutaneous albinism or immune deficiency, which are caused by defects in melanosomes and lytic granules, respectively [11, 23]. The melanosome in particular has served as the prototype LRO whose biogenesis mechanisms are best understood [25, 26]. The relative paucity in direct DG biogenesis knowledge is in part due to the difficulty of conducting cell biology studies with megakaryocytes [1, 28]. Recently, some progress has been made in studying and understanding DG biogenesis using primary megakaryocytes and appropriate models. Initial results are consistent with the concept that DG biogenesis follows analogous pathways to those described for the synthesis of melanosomes and other LROs. Here we review the current knowledge on the mechanisms of DG biogenesis and associated diseases.
Diagnosis of dense granule disorders
Dense granule disorders typically result in defective platelet aggregation of variable severity [9, 11]. Specifically, the secondary aggregation response to exogenous stimuli is absent. A significant reduction in the content and ratio of ADP to ATP or a reduced ATP release measured by lumi-aggregometry is diagnostic of a DG disorder [9]. Serotonin accumulation deficiency can also be used to diagnose DG disorders [29–31]. The fluorescent dyes mepacrine and DAPI are useful to label and observe the presence of DGs by fluorescence microscopy [20, 32]. Mepacrine uptake and anti-serotonin staining have also been applied to flow cytometric assays as a measure of DG disorder [29, 33]. Super-resolution fluorescence microscopy using anti-CD63 staining as a marker has recently been proposed as a potential approach to diagnose DG deficiency [34]. However, many of these assays do not distinguish between a defect in DG biogenesis and DG release or may not be fully specific for detection of DG deficiency. Dense granules are inherently opaque and can be identified and quantified by electron microscopy using the whole mount technique with unfixed, unstained human platelets [6]. Dense granules are also readily visualized by electron microscopy in thin sections of platelets and megakaryocytes fixed and subsequently treated with osmic acid [6]. Consequently, electron microscopy continues to be the best method to determine the presence or absence of DGs [6, 11].
Dense granule deficiency can occur in isolation (δ-storage pool disease, δ-SPD), combined with α-granule deficiency (α/δ-SPD) and as part of a syndrome [7, 10, 11, 18]. Hermansky-Pudlak Syndrome (HPS) patients exhibit bleeding diathesis caused by DG deficiency and hypopigmentation of skin, hair and eyes due to melanosome defects [11, 12, 35–37]. Some HPS patients present additional manifestations such as lung fibrosis and immune deficiency because the specific gene that is mutated also affects other LROs and cell types [11, 12]. There are 9 well-established HPS types labeled according to the gene that is mutated and numbered chronologically with their discovery (Table 1). Recently a new mutation was described in a patient likely representing HPS10 [38]. As discussed below, elucidation and study of the genes and proteins mutated in HPS has begun to illuminate the mechanisms involved in synthesizing DGs. Individuals suffering from Chediak-Higashi Syndrome (CHS) have bleeding diathesis due to DG deficiency as well as decreased pigmentation and severe immune deficiency due to malformation of additional LROs [39]. CHS patients have giant intracellular organelles that are pathognomonic of the disease [39, 40]. Chediak-Higashi Syndrome is caused by mutation of the CHS1 gene [39]. HPS and CHS are uncommon, autosomal recessive diseases.
Table 1.
Genes involved in dense granule biogenesis in humans and mice.
| Gene Symbol |
Human Disease | Rodent Mutation | Protein | Function |
|---|---|---|---|---|
| HPS1 | HPS-1 | pale ear | HPS1 | BLOC-3 subunit |
| AP3B1 | HPS-2 | pearl | AP-3 β3A | AP-3 subunit |
| HPS3 | HPS-3 | cocoa | HPS3 | BLOC-2 subunit |
| HPS4 | HPS-4 | light ear | HPS4 | BLOC-3 subunit |
| HPS5 | HPS-5 | ruby-eye 2 | HPS5 | BLOC-2 subunit |
| HPS6 | HPS-6 | ruby-eye | HPS6 | BLOC-2 subunit |
| DTNBP1 | HPS-7 | sandy | Dysbindin | BLOC-1 subunit |
| BLOC1S3 | HPS-8 | reduced pigmentation | BLOS3 | BLOC-1 subunit |
| PLDN | HPS-9 | pallid | Pallidin | BLOC-1 subunit |
| AP3D1 | HPS-10 | mocha | AP-3 δ | AP-3 subunit |
| CNO | - | cappuccino | Cappuccino | BLOC-1 subunit |
| MUTED | - | muted | Muted | BLOC-1 subunit |
| CHS1/LYST | CHS | beige | CHS1/LYST | ? |
| VPS33A | - | buff | VPS33A | HOPS/CORVET subunit |
| RAB38 | - | chocolate | Rab38 | small GTPase |
| RAB27B | - | Rab27b* | Rab27b | small GTPase |
| RABGGTA | - | gunmetal | RABGGTA | Rab geranylgeranyl transferase α subunit |
All mice described in this table, with the exception of Rab27b, were spontaneous mutants.
Mouse models
The availability of mouse models of HPS, CHS, α/δ-SPD and δ-SPD has been especially useful in understanding the mechanisms of DG biogenesis (Table 1). Moreover, the discovery of most of the genes known to be involved in DG biogenesis in humans was preceded and facilitated by the identification of the corresponding gene in mouse disease models [17]. Existing mouse disease models without patient counterparts yet described predict that additional human diseases will be defined at a molecular level in the future (Table 1). Conversely, the existence of patients presenting with HPS, α/δ-SPD and δ-SPD that do not have a mutation in any of the currently known disease-associated genes implies new forms of these diseases must exist [11, 16].
Multivesicular bodies and the dense granule origin
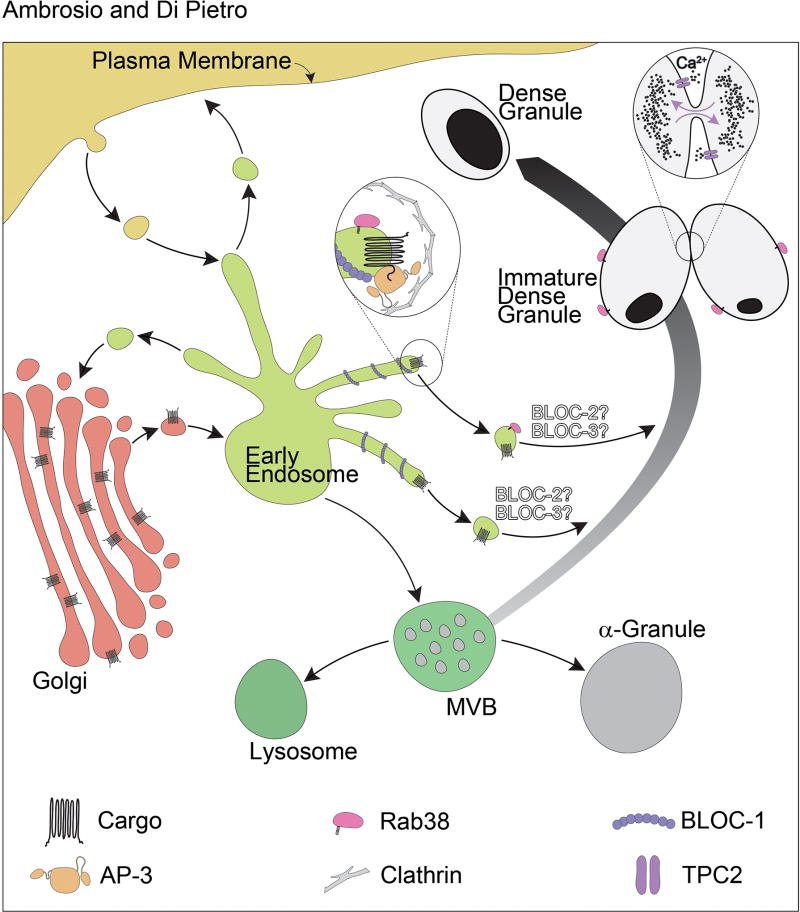
Unlike most secretory granules produced in other cell types, DGs do not originate directly from the trans-Golgi network. The biogenesis of DGs involves a specialized biosynthetic mechanism that connects the secretory and endocytic pathways (Figure 1). First, vacuolar domains of the endosomal system give rise to multivesicular bodies (MVBs) [41]. The MVB has been shown to constitute the precursor to DGs, α-granules and conventional lysosomes (Figure 1) [27, 41–43]. Consequently, all three organelles can be accessed by material internalized from the plasma membrane by endocytosis [43, 44]. The fact that MVBs are precursors to the platelet granules is also evidenced by the fact that young megakaryocytes have large numbers of MVBs but mature megakaryocytes have less MVBs and more α-granules and DGs. It is unclear whether the development from MVB into DG and differentiation from α-granules and lysosomes occurs through a continuous process of maturation and remodeling of the same compartment or the budding off of an immature DG from the MVB. Nevertheless, development into a mature DG requires delivery of newly synthesized proteins specific to DGs such as serotonin and ADP transporters (Figure 1). In the next sections we will cover the machinery and intermediate compartments that mediate this intracellular trafficking.
Figure 1. Model for the biogenesis of platelet dense granules.
In megakaryocytes, multivesicular bodies (MVBs) mature into dense granules upon receiving newly synthesized specific transmembrane proteins through multiple vesicular trafficking pathways. Dense granule cargo is sorted by adaptor protein (AP) complexes at the level of the early/recycling endosome tubules that are likely stabilized by BLOC-1. The best understood pathway to dense granules is defined by AP-3, which binds sorting signals present in the cytosolic tails of cargo proteins and recruits clathrin, facilitating the formation of the coated vesicle (see inset). Rab38 is present on to the transport vesicle that mediates targeting of the vesicle to the maturing dense granule. Very little is known regarding BLOC-2 and BLOC-3 function in DG biogenesis. Based on melanosome research they are tentatively placed downstream BLOC-1 and AP-3, and may also work independently of AP-3. TPC2 regulates dense granule pH, the pool of releasable Ca2+, and a “kiss-and-run” mechanism of dense granule membrane dynamics and content exchange (see inset).
Protein machinery
The AP-3 complex
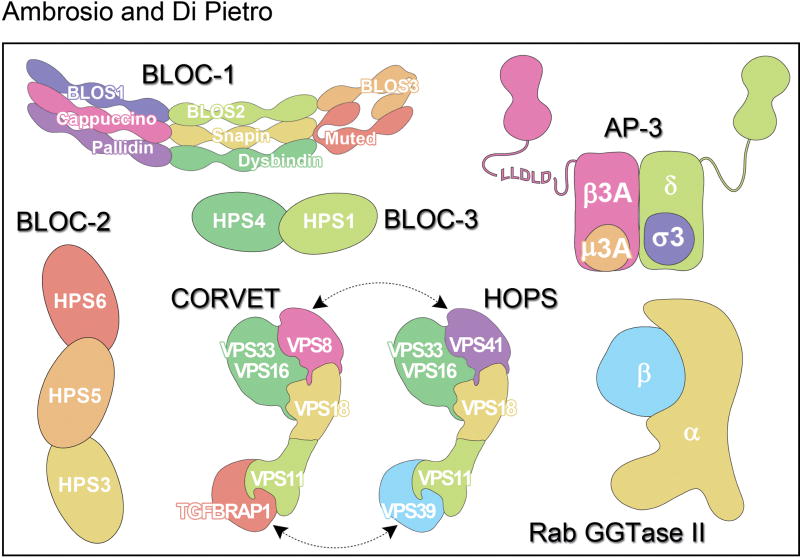
The proteins encoded by most HPS-associated genes indicated in Table 1 assemble into heteromeric complexes (Figure 2). The Adaptor Protein (AP)-3 complex is a tetramer composed of one molecule each of δ, β3, μ3, and σ3 subunits [45]. AP-3 was originally identified on the basis of its homology with the clathrin-associated adaptor protein complexes AP-1 and AP-2, which work in vesicle mediated trafficking [46, 47]. It should be noted that there are two forms of the AP-3 complex, one that is ubiquitously expressed and composed of δ, β3A, μ3A, and σ3A/σ3B subunits and a neuronal form composed of δ, β3B, μ3B, and σ3A/σ3B subunits. The δ subunit is common to both forms of AP-3 and the σ3A σ3B subunits can be in either complex. The importance of AP-3 for the biogenesis of DGs in particular and LROs in general became clear when the mutations carried by the mocha and pearl mouse models of HPS were revealed to impact the genes encoding δ and β3A, respectively [48, 49]. Mutations in β3A were also found in HPS patients, thus defining HPS type 2 (Table 1) [50]. At the time, the discovery that mutation of AP-3 causes HPS had conceptual significance because it demonstrated that Hermansky-Pudlak syndrome is a disease of protein trafficking and organelle biogenesis.
Figure 2. Schematic representation of protein complexes involved in dense granule biogenesis.
The cartoon representation of BLOC-1, AP-3, HOPS/CORVET and Rab GGTase II complexes is based on structural information while BLOC-2 and BLOC-3 simply represent their subunit composition.
AP-3 mediates transport of integral membrane proteins from tubular domains of early/recycling endosomes to lysosomes and LROs [51–53]. AP-3 engages dileucine- and tyrosine-based sorting signals in the cytosolic tail of integral membrane protein cargos and packages them into transport vesicles destined for the LRO (Figure 1) [44, 52, 54–58]. AP-3 also has the ability to bind other components of the trafficking machinery including the terminal domain of the clathrin heavy chain to orchestrate formation of the transport vesicle [59, 60]. Several likely DG integral membrane protein components have cytosolic tails harboring sequences that conform to the dileucine- and tyrosine-based sorting signal consensus. One example is the protein SLC35D3, a member of the nucleotide sugar transporter family, whose deficiency causes δ-SPD in mice [31, 61]. SLC35D3 steady state levels in platelets from AP-3 deficient mice are decreased compared to wild type mice, consistent with a defect in transport to DGs during organelle biogenesis [62]. Moreover, SLC35D3 populates early/recycling endosomal tubules labeled with syntaxin 13 and transferrin receptor in megakaryocytes, suggesting these compartments are intermediate stations in the pathway to DGs (Figure 1) [62]. Other examples are LAMP2 and the serotonin transporter VMAT2, which are expressed in megakaryocytes/platelets and reside on the DG membrane [63–66]. The corresponding tyrosine-based and dileucine-based sorting signal mutants of LAMP2 and VMAT2 were mislocalized to the plasma membrane [44]. These observations suggest AP-3 and the sorting signals it binds function to mediate transport of newly synthesized integral membrane proteins from early/recycling endosomal tubules to DGs (Figure 1). However, AP-3 binding to the sorting signal consensus sequences in cargo such as SLC35D3 and VMAT2 has yet to be confirmed experimentally. Interestingly, the SLC35D3, LAMP2 and VMAT2 phenotypes described above were not complete, suggesting a partial defect in transport of cargo to DGs. An analogous scenario has been observed in the study of melanosomal proteins and documented to reflect the existence of multiple pathways that cargo may follow to target this LRO [26, 52, 53, 67–69]. Therefore, it is likely that a similar set of parallel pathways deliver cargo to DGs (Figure 1).
As mentioned above, there are two forms of the AP-3 complex, the ubiquitous AP-3A and the neuronal form, AP-3B. Consistently, the mocha mice – with a mutation in the δ subunit common to both AP-3 forms – present neurological phenotypes in addition to bleeding and oculocutaneous albinism that define HPS [48]. Mutation of AP-3 subunits also causes immunodeficiency in mice and patients. Recently, a patient was reported with a mutation in the δ subunit of AP-3 and showing albinism, neurological manifestations and immunodeficiency [38]. It was not confirmed, as of yet, if the patient has DG deficiency but it likely represents HPS10.
BLOC-1
The Biogenesis of Lysosome-related Organelles Complex-1 (BLOC-1) is composed of one copy each of 8 subunits: BLOS1 (BLOC1S1), BLOS2 (BLOC1S2), BLOS3 (BLOC1S3), Cappuccino (BLOS4 or BLOC1S4), Muted (BLOS5 or BLOC1S5), Pallidin (BLOS6 or BLOC1S6), Snapin (BLOS7 or BLOC1S7), and Dysbindin (BLOC1S8 or DTNBP1) (Figure 2) [70–74]. Five of them – Dysbindin, BLOS3, Pallidin, Cappuccino, and Muted – are encoded by genes mutated in HPS mouse models (Table 1). Of those, the first three were also found to be mutated in HPS patients thus defining HPS-7, HPS-8 and HPS-9, respectively (Table 1) [75–77]. It is therefore likely that uncharacterized HPS patients carry mutations in the genes encoding Cappuccino and Muted (Table 1). Interestingly, the Snapin, BLOS1 and BLOS2 subunits of BLOC-1 are also components of a separate complex known as BORC (BLOC-one-related complex) [78]. BORC functions in lysosome positioning and appears to be fundamental for life since Snapin-KO mice are perinatally inviable and BLOS1-KO mice are embryonic lethal [78]. Alternatively, BLOC-1 and BORC may have partially overlapping functions needed for viability. Consequently, mutations in the genes encoding for Snapin, BLOS1 and BLOS2 are not predicted to be found among HPS patients. Clinical data on patients with BLOC-1 deficiency is limited but the severity appears to be variable [77].
BLOC-1 function at the molecular level could not be deduced from sequence homology with previously known proteins. BLOC-1 was obtained in recombinant form allowing an initial structural characterization [72]. The complex consists of a linear chain of globular domains spanning 30 nm in length and 3 nm in diameter that can bend by as much as 45° (Figure 2). In melanocytes, BLOC-1 localizes to early/recycling endosome tubules and functions in tubule formation by coordinating the kinesin KIF13A-dependent pulling of the tubules along microtubules with the actin-dependent tubule stabilization [53, 79, 80]. BLOC-1 activity is key to deliver integral membrane proteins to maturing melanosomes through both AP-3-dependent and AP-3-independent pathways [53, 81–86]. Furthermore, BLOC-1 interacts physically with AP-3 and the endosomal SNARE syntaxin 13 [53, 87]. It is likely that BLOC-1 performs similar functions in the transport of DG proteins during organelle biogenesis (Figure 1). Supporting this idea, the phenotype of decreased SLC35D3 steady state levels in platelets from AP-3 mice described above was also observed with platelets from BLOC-1 deficient mice [62].
BLOC-2
The Biogenesis of Lysosome-related Organelles Complex-2 (BLOC-2) is composed of the HPS3, HPS5, and HPS6 proteins (Figure 2) encoded by the genes mutated in HPS-3, HPS-5 and HPS-6 disease patients (table 1) [88, 89]. While the sum of the theoretical molecular weights of the three subunits approximates the mass estimated for the native complex, the existence of additional small subunits cannot be ruled out [88]. The corresponding mouse mutant strains are cocoa, ruby-eye 2 and ruby-eye (Table 1) [89]. Patients with deficiency in the BLOC-2 subunits have similarly mild forms of the disease and do not appear to have manifestations other than bleeding and hypopigmentation [11, 12].
BLOC-2 function in DG biogenesis is very poorly characterized but it is starting to be understood in the context of melanosome biogenesis. In melanocytes, BLOC-2 localizes to early/recycling endosomal compartments and functions in the transport of melanosomal cargo, at least in part in a pathway separate from the one defined by AP-3 [53, 90]. BLOC2 interacts physically with BLOC-1 but appears to have functions downstream of BLOC-1 [53]. BLOC-2 also interacts physically with Rab38 and Rab32, which are two small GTPases that function in melanosome and DG biogenesis (see below) [44, 67, 91]. In melanocytes BLOC-2 and Rab38 regulate the membrane-associated pool of each other [67]. Furthermore, a recent study suggested BLOC-2 works in tethering of transport carriers originating from early/recycling endosomal tubules with the maturing melanosome [92]. BLOC-2 probably performs a similar function in the context of DG biogenesis (Figure 1).
BLOC-3
The Biogenesis of Lysosome-related Organelles Complex-3 (BLOC-3) is composed of the HPS1 and HPS4 proteins (Figure 2) encoded by the genes mutated in patients with HPS-1 and HPS-4 disease (table 1) [93]. Based on the characterization of the recombinant HPS1–HPS4 complex, BLOC-3 does not contain additional subunits [94]. The corresponding mouse mutant strains are pale ear and light ear, respectively (Table 1) [17]. Patients with deficiency in the BLOC-3 subunits have similarly severe forms of HPS presenting with additional manifestations besides bleeding and hypopigmentation. HPS-1 and HPS-4 patients frequently develop granulomatous colitis and suffer adult-onset fatal pulmonary fibrosis [11, 12]. Mutations in HPS1 are the most common cause of HPS [95, 96].
We are only beginning to understand BLOC-3 function in DG biogenesis. The MRP4 protein (also known as ABCC4) was initially localized to DGs in normal platelets by biochemical approaches and immunofluorescence microscopy analysis [97]. However, in platelets from an HPS-4 patient, MRP4 staining was reduced and mostly found on the plasma membrane suggesting BLOC-3 may function in transport of MRP4 to DGs [97]. Nevertheless, there is a controversy regarding normal MRP4 localization and function (as we discuss below) potentially invalidating the conclusion that BLOC-3 mediates its transport.
Molecular level functional information on BLOC-3 can be extrapolated from other systems. Based on low level sequence homology between HPS4 and the Ccz1 subunit of the yeast Ypt7/Rab7 guanine nucleotide exchange factor (GEF), it was suspected that BLOC-3 could work as a Rab-GEF [98]. In order to function in membrane trafficking, Rab GTPases become activated by the action of GEFs, which catalyze the release of GDP turning them into active, GTP-bound Rabs. Recombinant BLOC-3 demonstrated GEF activity specifically towards Rab32 and Rab38, thus linking BLOC-3 to the trafficking machinery that transports cargo to DGs and melanosomes [98]. Additionally, BLOC-3 binds with high affinity to Rab9a, another Rab protein recently reported to function in melanosome biogenesis [94, 99]. Therefore, BLOC-3 may function in a Rab cascade to regulate biogenesis of LROs [100]. The expectation is that BLOC-3 performs similar functions along the pathways to DGs (Figure 1).
HOPS and CORVET
The buff mouse model of HPS carries a mutation in the VPS33A gene encoding the VPS33A protein (Table 1) [101]. VPS33A is a subunit of the homotypic fusion and vacuole protein sorting (HOPS) complex. HOPS is composed of one copy each of six subunits: VPS33A, VPS11, VPS16, VPS18, VPS39, and VPS41 [102, 103]. Figure 2 shows the organization of the HOPS subunits, which is based on significant available structural information [102]. Four subunits of HOPS (VPS33A, VPS11, VPS16, and VPS18) are shared with a related complex known as class C core vacuole/endosome tethering (CORVET). The VPS39 and VPS41 subunits of HOPS are replaced by TGFBRAP1 and VPS8 in CORVET (Figure 2) [103]. No HPS patient has yet been confirmed to have a deficiency in VPS33A or the other complex subunits.
HOPS and CORVET function in membrane trafficking as tethering complexes bringing compartments in close proximity and facilitating SNARE-mediated membrane fusion [102, 103]. CORVET functions at early endosomes and HOPS at late endosomes/MVBs. This specificity is based on interaction with Rab5 and Rab7, master regulators of early and late endosomes/MVBs, respectively. Importantly, the crystal structure of VPS33A confirmed it belongs to the Sec1/Munc18 family of proteins that regulate the formation of cognate SNARE complexes driving membrane fusion [104]. Even though HOPS and CORVET have not been directly studied in DG biogenesis, it is logical to envision their function in generating the precursor MVB compartment and/or mediating tethering and fusion of DG cargo-containing vesicles with MVBs.
It is worthwhile noting that VPS33A is related to VPS33B. Mutation of VPS33B causes Arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome, which shows α-granule deficiency among other manifestations [105–107]. It is tempting to speculate that two different forms of HOPS/CORVET, one containing VPS33A and another containing VPS33B, would mediate transport to DGs and α-granules, respectively. However, VPS33B may not form a complex with HOPS or CORVET and instead form a separate complex with VPS16B (also known VIPAS39 or VIPAR) [103, 108, 109].
Rab32 and Rab38
The fawn hooded rat carries a mutation in the RAB38 gene and is one of the earliest known rodent models of storage pool disease and HPS [18, 110–112]. Unlike the fawn hooded rat, which is a null, the chocolate mouse strain carries a hypomorphic mutation in the RAB38 gene and displays pigmentation dilution but does not appear to have bleeding problems [113, 114]. Rab32 is a very close homolog of Rab38 with partially overlapping functions in LRO biogenesis [67, 91]. Illustrating they perform at least some independent functions, Rab32 deficiency in melanocytes, but not Rab38 deficiency, causes a loss of the melanosomal protein Tyrp2 [67].
Rab32 and Rab38 work in transport of cargo from early/recycling endosomes to maturing DGs in megakaryocytes (Figure 1) and to maturing melanosomes in melanocytes [44, 67, 69, 91]. In melanocytes, Rab32 and Rab38 were shown to interact physically and colocalize partially with AP-3 and BLOC-2 [67]. Rab32 and Rab38 also localize in part to vesicles and melanosomes [67, 69, 91]. Deficiency of Rab32 and Rab38 causes mistrafficking of melanosomal resident proteins, melanosome malformation and lower melanin pigment synthesis [67, 91, 111, 113]. In megakaryocytes, Rab32 and Rab38 localize to AP-3 and clathrin-labeled structures as well as to transport vesicles and DGs (Figure 1) [44]. Rab32 and Rab38 were also shown to function either in tethering or fusion of cargo-containing vesicles with the maturing DG or both [44]. This function of Rab32 and Rab38 is in line with what we know about Rab proteins as key regulators of vesicular trafficking that confer target specificity to vesicle motility, tethering and fusion [100].
Most of the components of the DG biogenesis machinery such as AP-3 and the BLOCs are ubiquitously expressed. This is consistent with their function in the biogenesis of lysosomes, which are ubiquitous organelles. A long-standing question in the field was how the same machinery could mediate transport to two separate organelles, lysosomes and DGs (or lysosomes and melanosomes) that are produced and co-exist in the same cell. This question was answered with the discovery that Rab38 and to large extent Rab32 work as cell type specific factors that re-direct the ubiquitous machinery towards DGs (or melanosomes) [67, 68, 91, 100, 113, 115].
Rab27b
Deficiency of Rab27a elicits Griscelli syndrome, an autosomal recessive disorder similar to HPS but without DG deficiency and bleeding [11]. However, a mouse knock out for the closely related RAB27B gene was created and found to have prolonged bleeding and impaired platelet aggregation due to DG deficiency (Table 1) [116]. This mouse does not have hypopigmentation or any of the other manifestation observed in HPS or Griscelli syndrome. Consequently, Rab27b deficiency is a model for δ-SPD [116]. Significantly, a patient displaying Rab27b deficiency presents with absent dense granules and bleeding [14]. Additional work is needed to confirm Rab27b deficiency causes δ-SPD in humans.
RABGGTA
The gunmetal mouse (Table 1) has a mutation in the gene encoding the α-subunit of the Rab geranylgeranyl transferase (RabGGTase, Figure 2) and displays a defect in both DGs and α-granules thus serving as a model of α/δ-SPD [117]. Gunmetal mice also have hypopigmentation [117]. The RabGGTase enzyme adds two geranylgeranyl 20-carbon isoprenoid residues to each of two cysteine residues at the C-terminus of Rab proteins. This modification is needed for normal Rab association with membranes and function. The gunmetal mouse is a hypomorph with RabGGTase activity reduced four-fold but not absent [117]. As a consequence, the modification of Rab proteins and their association with membranes is compromised but not totally abolished. This defect becomes more exacerbated in cell types that express higher levels of Rabs such as megakaryocytes/platelets and melanocytes [118]. The DG and melanosome biogenesis defect can be explained by reduced function of Rab32, Rab38, and/or Rab27b. This observation suggests that another Rab or Rabs are likely involved in α-granule biogenesis.
CHS1/LYST
The CHS1 gene is mutated in patients suffering Chediak-Higashi Syndrome and the beige mouse model of the disease [39]. The function of CHS1/LYST protein remains unclear, but its ability to interact with SNARE proteins and the presence of huge organelles typical of CHS suggest a function in membrane trafficking [11, 39]. CHS1 has a BEACH domain, similar to NBEAL2 whose mutation causes α-granule deficiency (Gray Platelet syndrome) [119–121].
Cargos of the dense granule biogenesis pathways
There is a remarkable scarcity of proteins truly confirmed as components of the DG membrane. As indicated above, SLC35D3 is believed to reside on DGs and the corresponding KO mouse is considered a model of δ-SPD showing drastically reduced platelet serotonin levels [31]. In megakaryocytoid cells SLC35D3 mostly populates early/recycling endosomes, probably because its transport to DGs takes place at later stages during megakaryocyte maturation [62]. While SLC35D3 was found in platelets by immunoblotting, its localization to DGs should be confirmed.
Multidrug resistance protein 4 (MRP4) was initially reported to reside on DGs in normal platelets but mislocalized to the plasma membrane in platelets from an HPS-4 patient [97]. Furthermore, MRP4 was suggested as the ADP transporter in DGs and two patients with δ-SPD-like phenotype (reduced adenine nucleotides but normal serotonin levels) show undetectable platelet MRP4 [22, 97, 122]. Nevertheless, no mutation in the MRP4 gene was found in these patients and direct evidence that MRP4 actually transports ADP is lacking. Two recent papers using MRP4-KO mice confirmed bleeding problems but reported results that contradict the earlier studies. Both papers implicate MRP4 in cAMP rather than ADP transport [123, 124] and one of them localized MRP4 to the plasma membrane by both structured illumination microscopy and biochemical approaches [124]. The emerging picture is one in which MRP4 is needed for normal platelet function probably by transporting cAMP. However it is not clear if MRP4 functions at DGs, the plasma membrane, or in both locations.
Platelets take up serotonin by the action of the plasma membrane transporter SERT and then package it in DGs by the work of vesicular monoamine transporter 2 (VMAT2) [64–66]. Serotonin transport into DGs by VMAT2 relies on the proton electrochemical gradient generated by a vacuolar H+-ATPase [125].
Two-pore channel 2 (TPC2) is a component of the DG membrane that regulates the organelle luminal pH and the pool of releasable Ca2+ [57]. Release of Ca2+ regulated by TPC2 marks DG “kiss-and-run” events where two DGs make transient physical contact and then move away from each other (Figure 1) [57]. During kiss-and-run events DGs exchange contents, therefore this mechanism may play a role in DG maturation. TPC2 also regulates the formation of membrane tubules connected to DGs [57]. These tubules may be involved in DG biogenesis as they exchange both soluble and membrane material with the DG. Tubular extensions of DGs may also correspond to mature DGs with tail-like extensions occasionally observed in platelets by electron microscopy [6]. Consequently, TPC2 has dual properties as both cargo and machinery component of the DG biogenesis pathways. The discovery that TPC2 also regulates melanosome biogenesis and pigmentation suggests TPC2 may define an HPS gene [126–128].
LAMP2 is another integral membrane protein which presence is confirmed in the DG membrane [63, 129]. SNARE proteins needed for DG fusion with the platelet plasma membrane during activation must also be present [130]. A number of glycoproteins and 14-3-3ζ have also been reported in DGs [129]. Establishing bona fide DG markers is important to better understand DG function and the pathways and machinery involved in biogenesis.
Perspective
Although some progress has been made in understanding DG biogenesis, many aspects remain poorly defined. A major issue is the paucity of membrane proteins properly verified as DG components, particularly those specific to DGs. Given the importance of ADP for dense granule function, unequivocal identification and characterization of the corresponding transporter would be helpful. The same concept applies to transporters for most of the other molecules stored in the DG lumen. In turn, study of DG membrane protein transport will refine our understanding of the pathways, mechanisms, and molecular machinery involved in biogenesis. Are these pathways as complex and analogous to the melanosome biogenesis pathways? Do different cargos use separate pathways to DGs? How does a seemingly uniform MVB give rise to three distinct organelles, DGs, α-granules and lysosomes?
The cross talk between storage pool disease and basic biology and between animal models and patients will likely continue to be fruitful. On the one hand, elucidation of the normal function of the genes and proteins mutated in patients with storage pool disease will be needed to achieve a detailed understanding of DG biogenesis. On the other hand, the characterization of genes mutated in animal models with dense granule deficiency predicts the corresponding human disease must exist. Finally, from a clinical point of view, the well-established function of DGs in hemostasis and thrombosis raises the question of whether their biogenesis could be targeted for therapeutic purposes. Therefore, understanding DG biogenesis is a prerequisite to rationally design such approaches.
Acknowledgments
The authors acknowledge NIH grant R01HL106186.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Thon JN, Italiano JE. Platelets: production, morphology and ultrastructure. Handb Exp Pharmacol. 2012:3–22. doi: 10.1007/978-3-642-29423-5_1. [DOI] [PubMed] [Google Scholar]
- 2.Machlus KR, Italiano JE., Jr The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koseoglu S, Flaumenhaft R. Advances in platelet granule biology. Curr Opin Hematol. 2013;20:464–471. doi: 10.1097/MOH.0b013e3283632e6b. [DOI] [PubMed] [Google Scholar]
- 4.King SM, Reed GL. Development of platelet secretory granules. Semin Cell Dev Biol. 2002;13:293–302. doi: 10.1016/s1084952102000599. [DOI] [PubMed] [Google Scholar]
- 5.Thon JN, Peters CG, Machlus KR, Aslam R, Rowley J, Macleod H, Devine MT, Fuchs TA, Weyrich AS, Semple JW, et al. T granules in human platelets function in TLR9 organization and signaling. J Cell Biol. 2012;198:561–574. doi: 10.1083/jcb.201111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White JG. Electron opaque structures in human platelets: which are or are not dense bodies? Platelets. 2008;19:455–466. doi: 10.1080/09537100802132671. [DOI] [PubMed] [Google Scholar]
- 7.Nurden AT, Nurden P. Congenital platelet disorders and understanding of platelet function. Br J Haematol. 2014;165:165–178. doi: 10.1111/bjh.12662. [DOI] [PubMed] [Google Scholar]
- 8.Nurden P, Nurden AT. Congenital disorders associated with platelet dysfunctions. Thromb Haemost. 2008;99:253–263. doi: 10.1160/TH07-09-0568. [DOI] [PubMed] [Google Scholar]
- 9.Bolton-Maggs PH, Chalmers EA, Collins PW, Harrison P, Kitchen S, Liesner RJ, Minford A, Mumford AD, Parapia LA, Perry DJ, et al. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. Br J Haematol. 2006;135:603–633. doi: 10.1111/j.1365-2141.2006.06343.x. [DOI] [PubMed] [Google Scholar]
- 10.Gunay-Aygun M, Huizing M, Gahl WA. Molecular defects that affect platelet dense granules. Semin Thromb Hemost. 2004;30:537–547. doi: 10.1055/s-2004-835674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet. 2008;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 13.McNicol A, Israels SJ. Platelet dense granules: structure, function and implications for haemostasis. Thromb Res. 1999;95:1–18. doi: 10.1016/s0049-3848(99)00015-8. [DOI] [PubMed] [Google Scholar]
- 14.Salles II, Feys HB, Iserbyt BF, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Inherited traits affecting platelet function. Blood Rev. 2008;22:155–172. doi: 10.1016/j.blre.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Masliah-Planchon J, Darnige L, Bellucci S. Molecular determinants of platelet delta storage pool deficiencies: an update. Br J Haematol. 2013;160:5–11. doi: 10.1111/bjh.12064. [DOI] [PubMed] [Google Scholar]
- 16.Israels SJ, El-Ekiaby M, Quiroga T, Mezzano D. Inherited disorders of platelet function and challenges to diagnosis of mucocutaneous bleeding. Haemophilia. 2010;16(Suppl 5):152–159. doi: 10.1111/j.1365-2516.2010.02314.x. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Rusiniak ME, Chintala S, Gautam R, Novak EK, Swank RT. Murine Hermansky-Pudlak syndrome genes: regulators of lysosome-related organelles. Bioessays. 2004;26:616–628. doi: 10.1002/bies.20042. [DOI] [PubMed] [Google Scholar]
- 18.Holmsen H, Weiss HJ. Secretable storage pools in platelets. Annu Rev Med. 1979;30:119–134. doi: 10.1146/annurev.me.30.020179.001003. [DOI] [PubMed] [Google Scholar]
- 19.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 21.Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jedlitschky G, Greinacher A, Kroemer HK. Transporters in human platelets: physiologic function and impact for pharmacotherapy. Blood. 2012;119:3394–3402. doi: 10.1182/blood-2011-09-336933. [DOI] [PubMed] [Google Scholar]
- 23.Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14:1265–1278. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- 24.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks MS, Heijnen HF, Raposo G. Lysosome-related organelles: unusual compartments become mainstream. Curr Opin Cell Biol. 2013;25:495–505. doi: 10.1016/j.ceb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raposo G, Marks MS. Melanosomes--dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luzio JP, Hackmann Y, Dieckmann NM, Griffiths GM. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb Perspect Biol. 2014;6:a016840. doi: 10.1101/cshperspect.a016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leven RM. Isolation of primary megakaryocytes and studies of proplatelet formation. Methods Mol Biol. 2004;272:281–291. doi: 10.1385/1-59259-782-3:281. [DOI] [PubMed] [Google Scholar]
- 29.Maurer-Spurej E, Pittendreigh C, Wu JK. Diagnosing platelet delta-storage pool disease in children by flow cytometry. Am J Clin Pathol. 2007;127:626–632. doi: 10.1309/3KRYCPNAPDTVFWGY. [DOI] [PubMed] [Google Scholar]
- 30.White JG. The dense bodies of human platelets: inherent electron opacity of the serotonin storage particles. Blood. 1969;33:598–606. [PubMed] [Google Scholar]
- 31.Chintala S, Tan J, Gautam R, Rusiniak ME, Guo X, Li W, Gahl WA, Huizing M, Spritz RA, Hutton S, et al. The Slc35d3 gene, encoding an orphan nucleotide sugar transporter, regulates platelet-dense granules. Blood. 2007;109:1533–1540. doi: 10.1182/blood-2006-08-040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddington M, Novak EK, Hurley E, Medda C, McGarry MP, Swank RT. Immature dense granules in platelets from mice with platelet storage pool disease. Blood. 1987;69:1300–1306. [PubMed] [Google Scholar]
- 33.Billio A, Moeseneder C, Donazzan G, Triani A, Pescosta N, Coser P. Hermansky-Pudlak syndrome: clinical presentation and confirmation of the value of the mepacrine-based cytofluorimetry test in the diagnosis of delta granule deficiency. Haematologica. 2001;86:220. [PubMed] [Google Scholar]
- 34.Westmoreland D, Shaw M, Grimes W, Metcalf DJ, Burden JJ, Gomez K, Knight AE, Cutler DF. Super-resolution microscopy as a potential approach to diagnosis of platelet granule disorders. J Thromb Haemost. 2016;14:839–849. doi: 10.1111/jth.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermansky F, Pudlak P. Albinism associated with hemorrhagic diathesis and unusual pigmented reticular cells in the bone marrow: report of two cases with histochemical studies. Blood. 1959;14:162–169. [PubMed] [Google Scholar]
- 36.Di Pietro SM, Dell'Angelica EC. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 37.Spritz RA, Chiang PW, Oiso N, Alkhateeb A. Human and mouse disorders of pigmentation. Curr Opin Genet Dev. 2003;13:284–289. doi: 10.1016/s0959-437x(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 38.Ammann S, Schulz A, Krageloh-Mann I, Dieckmann NM, Niethammer K, Fuchs S, Eckl KM, Plank R, Werner R, Altmuller J, et al. Mutations in AP3D1 associated with immunodeficiency and seizures define a new type of Hermansky-Pudlak syndrome. Blood. 2016;127:997–1006. doi: 10.1182/blood-2015-09-671636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68:283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- 40.Higashi O. Congenital gigantism of peroxidase granules; the first case ever reported of qualitative abnormity of peroxidase. Tohoku J Exp Med. 1954;59:315–332. doi: 10.1620/tjem.59.315. [DOI] [PubMed] [Google Scholar]
- 41.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 42.Youssefian T, Cramer EM. Megakaryocyte dense granule components are sorted in multivesicular bodies. Blood. 2000;95:4004–4007. [PubMed] [Google Scholar]
- 43.Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91:2313–2325. [PubMed] [Google Scholar]
- 44.Ambrosio AL, Boyle JA, Di Pietro SM. Mechanism of platelet dense granule biogenesis: study of cargo transport and function of Rab32 and Rab38 in a model system. Blood. 2012;120:4072–4081. doi: 10.1182/blood-2012-04-420745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dell'Angelica EC. AP-3-dependent trafficking and disease: the first decade. Curr Opin Cell Biol. 2009;21:552–559. doi: 10.1016/j.ceb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 47.Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Kantheti P, Qiao X, Diaz ME, Peden AA, Meyer GE, Carskadon SL, Kapfhamer D, Sufalko D, Robinson MS, Noebels JL, et al. Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- 49.Feng L, Seymour AB, Jiang S, To A, Peden AA, Novak EK, Zhen L, Rusiniak ME, Eicher EM, Robinson MS, et al. The beta3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. Hum Mol Genet. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- 50.Dell'Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 51.Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol. 2004;164:1065–1076. doi: 10.1083/jcb.200311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SR, Marks MS, Raposo G, Dell'Angelica EC. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Honing S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huizing M, Sarangarajan R, Strovel E, Zhao Y, Gahl WA, Boissy RE. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol Biol Cell. 2001;12:2075–2085. doi: 10.1091/mbc.12.7.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sitaram A, Piccirillo R, Palmisano I, Harper DC, Dell'Angelica EC, Schiaffino MV, Marks MS. Localization to mature melanosomes by virtue of cytoplasmic dileucine motifs is required for human OCA2 function. Mol Biol Cell. 2009;20:1464–1477. doi: 10.1091/mbc.E08-07-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ambrosio AL, Boyle JA, Di Pietro SM. TPC2 mediates new mechanisms of platelet dense granule membrane dynamics through regulation of Ca2+ release. Mol Biol Cell. 2015 doi: 10.1091/mbc.E15-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Craige B, Salazar G, Faundez V. Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol Biol Cell. 2008;19:1415–1426. doi: 10.1091/mbc.E07-12-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- 60.Lefrancois S, Janvier K, Boehm M, Ooi CE, Bonifacino JS. An ear-core interaction regulates the recruitment of the AP-3 complex to membranes. Dev Cell. 2004;7:619–625. doi: 10.1016/j.devcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Novak EK, Gautam R, Reddington M, Collinson LM, Copeland NG, Jenkins NA, McGarry MP, Swank RT. The regulation of platelet-dense granules by Rab27a in the ashen mouse, a model of Hermansky-Pudlak and Griscelli syndromes, is granule-specific and dependent on genetic background. Blood. 2002;100:128–135. doi: 10.1182/blood.v100.1.128. [DOI] [PubMed] [Google Scholar]
- 62.Meng R, Wang Y, Yao Y, Zhang Z, Harper DC, Heijnen HF, Sitaram A, Li W, Raposo G, Weiss MJ, et al. SLC35D3 delivery from megakaryocyte early endosomes is required for platelet dense granule biogenesis and is differentially defective in Hermansky-Pudlak syndrome models. Blood. 2012;120:404–414. doi: 10.1182/blood-2011-11-389551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niessen J, Jedlitschky G, Grube M, Bien S, Strobel U, Ritter CA, Greinacher A, Kroemer HK. Subfractionation and purification of intracellular granule-structures of human platelets: an improved method based on magnetic sorting. J Immunol Methods. 2007;328:89–96. doi: 10.1016/j.jim.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Holtje M, Winter S, Walther D, Pahner I, Hortnagl H, Ottersen OP, Bader M, Ahnert-Hilger G. The vesicular monoamine content regulates VMAT2 activity through Galphaq in mouse platelets. Evidence for autoregulation of vesicular transmitter uptake. J Biol Chem. 2003;278:15850–15858. doi: 10.1074/jbc.M212816200. [DOI] [PubMed] [Google Scholar]
- 65.Zucker M, Weizman A, Rehavi M. Characterization of high-affinity [3H]TBZOH binding to the human platelet vesicular monoamine transporter. Life Sci. 2001;69:2311–2317. doi: 10.1016/s0024-3205(01)01301-7. [DOI] [PubMed] [Google Scholar]
- 66.Cesura AM, Bertocci B, Da Prada M. Binding of [3H]dihydrotetrabenazine and [125I]azidoiodoketanserin photoaffinity labeling of the monoamine transporter of platelet 5-HT organelles. Eur J Pharmacol. 1990;186:95–104. doi: 10.1016/0014-2999(90)94064-5. [DOI] [PubMed] [Google Scholar]
- 67.Bultema JJ, Ambrosio AL, Burek CL, Di Pietro SM. BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles. J Biol Chem. 2012;287:19550–19563. doi: 10.1074/jbc.M112.351908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bultema JJ, Di Pietro SM. Cell type-specific Rab32 and Rab38 cooperate with the ubiquitous lysosome biogenesis machinery to synthesize specialized lysosome-related organelles. Small GTPases. 2013;4:16–21. doi: 10.4161/sgtp.22349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bultema JJ, Boyle JA, Malenke PB, Martin FE, Dell'Angelica EC, Cheney RE, Di Pietro SM. Myosin vc interacts with Rab32 and Rab38 proteins and works in the biogenesis and secretion of melanosomes. J Biol Chem. 2014;289:33513–33528. doi: 10.1074/jbc.M114.578948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Falcon-Perez JM, Starcevic M, Gautam R, Dell'Angelica EC. BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J Biol Chem. 2002;277:28191–28199. doi: 10.1074/jbc.M204011200. [DOI] [PubMed] [Google Scholar]
- 71.Starcevic M, Dell'Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J Biol Chem. 2004;279:28393–28401. doi: 10.1074/jbc.M402513200. [DOI] [PubMed] [Google Scholar]
- 72.Lee HH, Nemecek D, Schindler C, Smith WJ, Ghirlando R, Steven AC, Bonifacino JS, Hurley JH. Assembly and architecture of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J Biol Chem. 2012;287:5882–5890. doi: 10.1074/jbc.M111.325746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dell'Angelica EC. The building BLOC(k)s of lysosomes and related organelles. Curr Opin Cell Biol. 2004;16:458–464. doi: 10.1016/j.ceb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Moriyama K, Bonifacino JS. Pallidin is a component of a multi-protein complex involved in the biogenesis of lysosome-related organelles. Traffic. 2002;3:666–677. doi: 10.1034/j.1600-0854.2002.30908.x. [DOI] [PubMed] [Google Scholar]
- 75.Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP, Tinsley CL, Blake DJ, Spritz RA, Copeland NG, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgan NV, Pasha S, Johnson CA, Ainsworth JR, Eady RA, Dawood B, McKeown C, Trembath RC, Wilde J, Watson SP, et al. A germline mutation in BLOC1S3/reduced pigmentation causes a novel variant of Hermansky-Pudlak syndrome (HPS8) Am J Hum Genet. 2006;78:160–166. doi: 10.1086/499338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cullinane AR, Curry JA, Carmona-Rivera C, Summers CG, Ciccone C, Cardillo ND, Dorward H, Hess RA, White JG, Adams D, et al. A BLOC-1 mutation screen reveals that PLDN is mutated in Hermansky-Pudlak Syndrome type 9. Am J Hum Genet. 2011;88:778–787. doi: 10.1016/j.ajhg.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Pu J, Schindler C, Jia R, Jarnik M, Backlund P, Bonifacino JS. BORC, a multisubunit complex that regulates lysosome positioning. Dev Cell. 2015;33:176–188. doi: 10.1016/j.devcel.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delevoye C, Heiligenstein X, Ripoll L, Gilles-Marsens F, Dennis MK, Linares RA, Derman L, Gokhale A, Morel E, Faundez V, et al. BLOC-1 Brings Together the Actin and Microtubule Cytoskeletons to Generate Recycling Endosomes. Curr Biol. 2016;26:1–13. doi: 10.1016/j.cub.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Monfregola J, Napolitano G, D'Urso M, Lappalainen P, Ursini MV. Functional characterization of Wiskott-Aldrich syndrome protein and scar homolog (WASH), a bi-modular nucleation-promoting factor able to interact with biogenesis of lysosome-related organelle subunit 2 (BLOS2) and gamma-tubulin. J Biol Chem. 2010;285:16951–16957. doi: 10.1074/jbc.M109.078501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Setty SR, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salazar G, Craige B, Styers ML, Newell-Litwa KA, Doucette MM, Wainer BH, Falcon-Perez JM, Dell'Angelica EC, Peden AA, Werner E, et al. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol Biol Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newell-Litwa K, Salazar G, Smith Y, Faundez V. Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell. 2009;20:1441–1453. doi: 10.1091/mbc.E08-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larimore J, Tornieri K, Ryder PV, Gokhale A, Zlatic SA, Craige B, Lee JD, Talbot K, Pare JF, Smith Y, et al. The schizophrenia susceptibility factor dysbindin and its associated complex sort cargoes from cell bodies to the synapse. Mol Biol Cell. 2011;22:4854–4867. doi: 10.1091/mbc.E11-07-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sitaram A, Dennis MK, Chaudhuri R, De Jesus-Rojas W, Tenza D, Setty SR, Wood CS, Sviderskaya EV, Bennett DC, Raposo G, et al. Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes. Mol Biol Cell. 2012;23:3178–3192. doi: 10.1091/mbc.E11-06-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hermann GJ, Scavarda E, Weis AM, Saxton DS, Thomas LL, Salesky R, Somhegyi H, Curtin TP, Barrett A, Foster OK, et al. C. elegans BLOC-1 functions in trafficking to lysosome-related gut granules. PLoS One. 2012;7:e43043. doi: 10.1371/journal.pone.0043043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell'Angelica EC. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry. 2010;15:115, 204–115. doi: 10.1038/mp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Pietro SM, Falcon-Perez JM, Dell'Angelica EC. Characterization of BLOC-2, a complex containing the Hermansky-Pudlak syndrome proteins HPS3, HPS5 and HPS6. Traffic. 2004;5:276–283. doi: 10.1111/j.1600-0854.2004.0171.x. [DOI] [PubMed] [Google Scholar]
- 89.Gautam R, Chintala S, Li W, Zhang Q, Tan J, Novak EK, Di Pietro SM, Dell'Angelica EC, Swank RT. The Hermansky-Pudlak syndrome 3 (cocoa) protein is a component of the biogenesis of lysosome-related organelles complex-2 (BLOC-2) J Biol Chem. 2004;279:12935–12942. doi: 10.1074/jbc.M311311200. [DOI] [PubMed] [Google Scholar]
- 90.Helip-Wooley A, Westbroek W, Dorward HM, Koshoffer A, Huizing M, Boissy RE, Gahl WA. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5. J Invest Dermatol. 2007;127:1471–1478. doi: 10.1038/sj.jid.5700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dennis MK, Mantegazza AR, Snir OL, Tenza D, Acosta-Ruiz A, Delevoye C, Zorger R, Sitaram A, de Jesus-Rojas W, Ravichandran K, et al. BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery. J Cell Biol. 2015;209:563–577. doi: 10.1083/jcb.201410026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nazarian R, Falcon-Perez JM, Dell'Angelica EC. Biogenesis of lysosome-related organelles complex 3 (BLOC-3): a complex containing the Hermansky-Pudlak syndrome (HPS) proteins HPS1 and HPS4. Proc Natl Acad Sci U S A. 2003;100:8770–8775. doi: 10.1073/pnas.1532040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kloer DP, Rojas R, Ivan V, Moriyama K, van Vlijmen T, Murthy N, Ghirlando R, van der Sluijs P, Hurley JH, Bonifacino JS. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J Biol Chem. 2010;285:7794–7804. doi: 10.1074/jbc.M109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oh J, Bailin T, Fukai K, Feng GH, Ho L, Mao JI, Frenk E, Tamura N, Spritz RA. Positional cloning of a gene for Hermansky-Pudlak syndrome, a disorder of cytoplasmic organelles. Nat Genet. 1996;14:300–306. doi: 10.1038/ng1196-300. [DOI] [PubMed] [Google Scholar]
- 96.Witkop CJ, Nunez Babcock M, Rao GH, Gaudier F, Summers CG, Shanahan F, Harmon KR, Townsend D, Sedano HO, King RA, et al. Albinism and Hermansky-Pudlak syndrome in Puerto Rico. Bol Asoc Med P R. 1990;82:333–339. [PubMed] [Google Scholar]
- 97.Jedlitschky G, Tirschmann K, Lubenow LE, Nieuwenhuis HK, Akkerman JW, Greinacher A, Kroemer HK. The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood. 2004;104:3603–3610. doi: 10.1182/blood-2003-12-4330. [DOI] [PubMed] [Google Scholar]
- 98.Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22:2135–2139. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahanty S, Ravichandran K, Chitirala P, Prabha J, Jani RA, Setty SR. Rab9A is required for delivery of cargo from recycling endosomes to melanosomes. Pigment Cell Melanoma Res. 2016;29:43–59. doi: 10.1111/pcmr.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuki T, Oiso N, Gautam R, Novak EK, Panthier JJ, Suprabha PG, Vida T, Swank RT, Spritz RA. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc Natl Acad Sci U S A. 2003;100:1146–1150. doi: 10.1073/pnas.0237292100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuhlee A, Raunser S, Ungermann C. Functional homologies in vesicle tethering. FEBS Lett. 2015;589:2487–2497. doi: 10.1016/j.febslet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 103.van der Kant R, Jonker CT, Wijdeven RH, Bakker J, Janssen L, Klumperman J, Neefjes J. Characterization of the Mammalian CORVET and HOPS Complexes and Their Modular Restructuring for Endosome Specificity. J Biol Chem. 2015;290:30280–30290. doi: 10.1074/jbc.M115.688440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Graham SC, Wartosch L, Gray SR, Scourfield EJ, Deane JE, Luzio JP, Owen DJ. Structural basis of Vps33A recruitment to the human HOPS complex by Vps16. Proc Natl Acad Sci U S A. 2013;110:13345–13350. doi: 10.1073/pnas.1307074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp LW, Morris AA, Wraith JE, et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat Genet. 2004;36:400–404. doi: 10.1038/ng1325. [DOI] [PubMed] [Google Scholar]
- 106.Lo B, Li L, Gissen P, Christensen H, McKiernan PJ, Ye C, Abdelhaleem M, Hayes JA, Williams MD, Chitayat D, et al. Requirement of VPS33B, a member of the Sec1/Munc18 protein family, in megakaryocyte and platelet alpha-granule biogenesis. Blood. 2005;106:4159–4166. doi: 10.1182/blood-2005-04-1356. [DOI] [PubMed] [Google Scholar]
- 107.Bem D, Smith H, Banushi B, Burden JJ, White IJ, Hanley J, Jeremiah N, Rieux-Laucat F, Bettels R, Ariceta G, et al. VPS33B regulates protein sorting into and maturation of alpha-granule progenitor organelles in mouse megakaryocytes. Blood. 2015;126:133–143. doi: 10.1182/blood-2014-12-614677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu GD, Salazar G, Zlatic SA, Fiza B, Doucette MM, Heilman CJ, Levey AI, Faundez V, L'Hernault SW. SPE-39 family proteins interact with the HOPS complex and function in lysosomal delivery. Mol Biol Cell. 2009;20:1223–1240. doi: 10.1091/mbc.E08-07-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tornieri K, Zlatic SA, Mullin AP, Werner E, Harrison R, L'Hernault SW, Faundez V. Vps33b pathogenic mutations preferentially affect VIPAS39/SPE-39-positive endosomes. Hum Mol Genet. 2013;22:5215–5228. doi: 10.1093/hmg/ddt378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tschopp B, Weiss HJ. Decreased ATP, ADP and serotonin in young platelets of fawn-hooded rats with storage pool disease. Thromb Diath Haemorrh. 1974;32:670–677. [PubMed] [Google Scholar]
- 111.Oiso N, Riddle SR, Serikawa T, Kuramoto T, Spritz RA. The rat Ruby (R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm Genome. 2004;15:307–314. doi: 10.1007/s00335-004-2337-9. [DOI] [PubMed] [Google Scholar]
- 112.Ninkovic I, White JG, Rangel-Filho A, Datta YH. The role of Rab38 in platelet dense granule defects. J Thromb Haemost. 2008;6:2143–2151. doi: 10.1111/j.1538-7836.2008.03178.x. [DOI] [PubMed] [Google Scholar]
- 113.Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen Y, Wu X, Jiang Y, Bittner M, Hammer JA, 3rd, Pavan WJ. Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci U S A. 2002;99:4471–4476. doi: 10.1073/pnas.072087599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Osanai K, Oikawa R, Higuchi J, Kobayashi M, Tsuchihara K, Iguchi M, Jongsu H, Toga H, Voelker DR. A mutation in Rab38 small GTPase causes abnormal lung surfactant homeostasis and aberrant alveolar structure in mice. Am J Pathol. 2008;173:1265–1274. doi: 10.2353/ajpath.2008.080056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cohen-Solal KA, Sood R, Marin Y, Crespo-Carbone SM, Sinsimer D, Martino JJ, Robbins C, Makalowska I, Trent J, Chen S. Identification and characterization of mouse Rab32 by mRNA and protein expression analysis. Biochim Biophys Acta. 2003;1651:68–75. doi: 10.1016/s1570-9639(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 116.Tolmachova T, Abrink M, Futter CE, Authi KS, Seabra MC. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci U S A. 2007;104:5872–5877. doi: 10.1073/pnas.0609879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Detter JC, Zhang Q, Mules EH, Novak EK, Mishra VS, Li W, McMurtrie EB, Tchernev VT, Wallace MR, Seabra MC, et al. Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proc Natl Acad Sci U S A. 2000;97:4144–4149. doi: 10.1073/pnas.080517697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Q, Zhen L, Li W, Novak EK, Collinson LM, Jang EK, Haslam RJ, Elliott RW, Swank RT. Cell-specific abnormal prenylation of Rab proteins in platelets and melanocytes of the gunmetal mouse. Br J Haematol. 2002;117:414–423. doi: 10.1046/j.1365-2141.2002.03444.x. [DOI] [PubMed] [Google Scholar]
- 119.Kahr WH, Hinckley J, Li L, Schwertz H, Christensen H, Rowley JW, Pluthero FG, Urban D, Fabbro S, Nixon B, et al. Mutations in NBEAL2, encoding a BEACH protein, cause gray platelet syndrome. Nat Genet. 2011;43:738–740. doi: 10.1038/ng.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gunay-Aygun M, Falik-Zaccai TC, Vilboux T, Zivony-Elboum Y, Gumruk F, Cetin M, Khayat M, Boerkoel CF, Kfir N, Huang Y, et al. NBEAL2 is mutated in gray platelet syndrome and is required for biogenesis of platelet alpha-granules. Nat Genet. 2011;43:732–734. doi: 10.1038/ng.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Albers CA, Cvejic A, Favier R, Bouwmans EE, Alessi MC, Bertone P, Jordan G, Kettleborough RN, Kiddle G, Kostadima M, et al. Exome sequencing identifies NBEAL2 as the causative gene for gray platelet syndrome. Nat Genet. 2011;43:735–737. doi: 10.1038/ng.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jedlitschky G, Cattaneo M, Lubenow LE, Rosskopf D, Lecchi A, Artoni A, Motta G, Niessen J, Kroemer HK, Greinacher A. Role of MRP4 (ABCC4) in platelet adenine nucleotide-storage: evidence from patients with delta-storage pool deficiencies. Am J Pathol. 2010;176:1097–1103. doi: 10.2353/ajpath.2010.090425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Decouture B, Dreano E, Belleville-Rolland T, Kuci O, Dizier B, Bazaa A, Coqueran B, Lompre AM, Denis CV, Hulot JS, et al. Impaired platelet activation and cAMP homeostasis in MRP4-deficient mice. Blood. 2015;126:1823–1830. doi: 10.1182/blood-2015-02-631044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheepala SB, Pitre A, Fukuda Y, Takenaka K, Zhang Y, Wang Y, Frase S, Pestina T, Gartner TK, Jackson C, et al. The ABCC4 membrane transporter modulates platelet aggregation. Blood. 2015;126:2307–2319. doi: 10.1182/blood-2014-08-595942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dean GE, Fishkes H, Nelson PJ, Rudnick G. The hydrogen ion-pumping adenosine triphosphatase of platelet dense granule membrane. Differences from F1F0- and phosphoenzyme-type ATPases. J Biol Chem. 1984;259:9569–9574. [PubMed] [Google Scholar]
- 126.Ambrosio AL, Boyle JA, Aradi AE, Christian KA, Di Pietro SM. TPC2 controls pigmentation by regulating melanosome pH and size. Proc Natl Acad Sci U S A. 2016;113:5622–5627. doi: 10.1073/pnas.1600108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bellono NW, Escobar IE, Oancea E. A melanosomal two-pore sodium channel regulates pigmentation. Sci Rep. 2016;6:26570. doi: 10.1038/srep26570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Jakobsdottir M, Steinberg S, Gudjonsson SA, Palsson A, Thorleifsson G, et al. Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008;40:835–837. doi: 10.1038/ng.160. [DOI] [PubMed] [Google Scholar]
- 129.Hernandez-Ruiz L, Valverde F, Jimenez-Nunez MD, Ocana E, Saez-Benito A, Rodriguez-Martorell J, Bohorquez JC, Serrano A, Ruiz FA. Organellar proteomics of human platelet dense granules reveals that 14-3-3zeta is a granule protein related to atherosclerosis. J Proteome Res. 2007;6:4449–4457. doi: 10.1021/pr070380o. [DOI] [PubMed] [Google Scholar]
- 130.Graham GJ, Ren Q, Dilks JR, Blair P, Whiteheart SW, Flaumenhaft R. Endobrevin/VAMP-8-dependent dense granule release mediates thrombus formation in vivo. Blood. 2009;114:1083–1090. doi: 10.1182/blood-2009-03-210211. [DOI] [PMC free article] [PubMed] [Google Scholar]