Abstract
In response to new emphasis by regulatory agencies regarding socialization, behavioral management programs are allocating greater resources to maximize socialization opportunities for laboratory primates. Information regarding predictors of compatibility and risk of injury for all laboratory-housed species of macaques are needed to make social introductions and pairings as efficient and safe as possible. This study presents data on 674 pairs of pigtailed macaques (Macaca nemestrina) at the Washington National Primate Research Center over a 7-year period. During pair introduction, behavior was monitored while the degree of tactile contact was gradually increased. Based on observed behavior, pairs were assigned a behavioral introduction score (BIS), rating the quality of their interactions for each day of introduction. Animals deemed compatible, based on the BIS and technologist judgment, were allowed to progress to continuous contact with no staff present. A small proportion of animals deemed compatible at introduction was later separated for subsequent incompatibility or aggression; these proportions were higher in full contact compared to protected contact pairings. Of 674 pairs, 75% were deemed compatible at introduction in protected contact; 86 of these pairs were later transitioned to full contact with 98% compatibility. Predictors of decreased compatibility assessed during protected contact introductions included age (adult pairs were less compatible), the BIS on the last day of introduction, and aggression or injury during the introductory period. Predictors of injuries during the protected contact introduction process included: aggression on the first day of introduction, a negative BIS on the first or last day of introduction, and, surprisingly, the presence of grooming on the first day of introduction. Injuries during both introduction and subsequent pairing in protected contact were rare; however, injury rates increased significantly during full-contact pairing. These findings underscore the necessity of species-specific data to guide decision-making during the social introduction process.
Keywords: pigtailed macaques, socialization, compatibility, injury, environmental enrichment
INTRODUCTION
Regulatory agencies, as well as behavioral managers, have recognized the critical nature of social housing in non-human primates (NHPs). Both the Guide for the Care and Use of Laboratory Animals [2011] and The Association for Assessment and Accreditation of Laboratory Care International [AAALAC, 2011] endorse social housing as the norm for all facilities. The 2011 Guide specifically states, “Social animals should be housed in stable pairs or groups of compatible individuals unless they must be housed alone for experimental reasons or because of social incompatibility.” [p. 51]. However, in the most recent survey conducted on laboratory-housed NHPs, only 46% of indoor housed macaques were housed socially [Baker et al., 2007]. In order to comply with the requirement for social housing, research facilities must actively implement a social housing and monitoring program.
Social housing has a wide variety of positive behavioral and physiological effects in NHPs. A social partner provides the most effective form of enrichment [Baker et al., 2012b; Schapiro et al., 1996] since, in addition to satisfying basic social needs, a partner’s behavior is constantly changing and requires social problem solving involving complex cognitive, situation-contingent species-typical responses. In macaques, duration in single housing is a significant risk factor for developing abnormal behavior [Bellanca & Crockett, 2002; Lutz et al., 2003; Novak, 2003; Rommeck et al., 2009]. Social housing can significantly decrease the expression of both abnormal and anxiety related behaviors (self-biting, hair pulling, vocalization, self-directed behaviors) and increase the expression of species typical behaviors such as social grooming and play [Baker et al., 2012a; Eaton et al., 1994; Schapiro et al., 1996; Reinhardt & Rossell, 2001; Weed et al., 2003]. Social housing also has putatively beneficial effects on physiological measures such as heart rate [Doyle et al., 2008] and immunology [Schapiro et al., 2000] and can buffer individuals from negative behavioral and physiological outcomes in response to stressful events [Gilbert & Baker, 2011; Gust et al., 1994; Roberts & Platt, 2005]. Benefits appear to accrue to both dominant and subordinate members of a social pair [Baker et al., 2012a; Eaton et al., 1994]. Animals with surgical implants and on food or water restriction can be successfully paired [Roberts & Platt, 2005]. Even animals with behavioral problems can be socialized with success rates not differing significantly from pairs comprised of normal animals [Lee et al., 2005a]. Thus, many lines of evidence suggest that most laboratory NHPs can be socially housed and this leads to increased well-being.
Despite the positive effects of social housing, macaques can inflict serious injury and be a source of distress for one another in incompatible social pairings. Behavioral managers must be able to identify predictors of compatibility, incompatibility, and wounding that will allow social introductions to be as time efficient and safe as possible for the animals. Past studies in macaques and other NHP species have found species differences, even within the same genus, in socialization outcomes; this emphasizes the need for published data on all species of laboratory housed NHPs [Baker et al., 2012b; Jorgensen et al., 2015; Lee et al., 2012]. Although there are some reports on pair housing in long-tailed macaques [Baker et al., 2012b; Lee et al., 2012], the vast majority of published data on pair housing in macaques comes from rhesus [Baker et al., 2012a; Reinhardt, 1989, 1994, 2002; Reinhardt et al., 1988]. To date, no large-scale published reports exist describing socialization of pigtailed macaques (Macaca nemestrina) in the laboratory. Bayne et al. 1995, [p. 40] has noted that “The tendency to plan social housing strategies on methods that are successful in rhesus monkeys is an ill-conceived approach and clearly does not take into account the highly variable bonds between species.”
Information about the behavioral ecology of pigtailed macaques in the wild is scarce. The paucity of field data has been attributed to that fact that they are shy, do not habituate to observation, have high rates of dispersion and high travel rates which makes observation difficult [Caldecott, 1986; Choudhury, 2008; Crockett & Wilson, 1980]. Although pigtailed macaques’ social organization is multi-male/multi-female, the entire group is not often aggregated together but instead forms small subgroups to move and forage, separating and rejoining during the day [Caldecott, 1986; Crockett & Wilson, 1980; Oi, 1990b]. These subgroups tend to consist of one adult male and multiple adult females with attendant juveniles and infants [Caldecott, 1986]. Unlike rhesus macaques, pigtailed macaques are not seasonal breeders, facilitating adult males’ ability to monopolize estrous females [Caldecott, 1986; Oi, 1996]. Adult male to female sex ratios range from 1:5.5 to 1:8 which is the highest of any macaque species [Caldecott, 1986; Choudhury, 2008; Oi, 1990a,b]. This ratio is about twice that reported for rhesus [1:2.8; Caldecott, 1986]. Adult males not included in groups are peripheral or solitary and, unlike rhesus, do not appear to form all male groups [Caldecott, 1986; Oi, 1990a,b].
There are differing accounts regarding the role that aggression plays in the formation and maintenance of pigtailed macaque social bonds. Thierry [2004] hypothesized that pigtailed macaques have a somewhat more relaxed and less despotic style of dominance compared to rhesus. However, other authors have suggested that pigtails have a hierarchical social structure and despotic style closely resembling that of rhesus [Kienast & Preuschoft, 2005]. Gust et al. [1996] reported that pigtails appear to take longer to establish dominant-subordinate relationships compared to rhesus and may do so in the absence of overt aggression. In captivity, isosexual groups of females engage in significantly more aggression than groups containing males [Dazey et al., 1977; Sackett et al., 1975]. Adult pigtailed macaque males are known to play a strong role in regulation of female aggression [Flack et al., 2005a, b]. Several studies have shown that experimental removal of the male from established harem groups resulted in dramatic increases in female–female aggression with decreased aggression when males were reinstated into the group [Erwin, 1978; Oswald & Erwin, 1976].
Considerations of the natural history of this species may be important when establishing laboratory pairings. For example, since adult males do not form bachelor groups they may more problematic to pair, isosexual female pairs may engage in more aggression due to the absence of a male, and signs of compatibility or incompatibility in social pairings may be subtle and emerge over time during the introduction period.
In this study, we describe the social introductions and outcomes of 674 pairs of pigtailed macaques over seven years at the Washington National Primate Research Center (WaNPRC). The predominant form of social housing over this period was protected contact [Crockett et al., 1997] consisting of contact through either widely spaced mesh or grooming-contact bars. With the advent of the more restrictive definition of social housing (two animals in full contact) in the Guide for the Care and Use of Laboratory Animals [National Research Council Committee, 2011] many existing protected contact pairs were transitioned into full contact, and full contact is now the predominant form of social housing at the WaNPRC. This report encompasses demographic and predictive factors for compatibility and wounding in both protected and full contact pairings of pigtailed macaques.
METHODS
Subjects
Subjects were 674 pairs of juvenile and adult pigtailed macaques that underwent social introduction to protected contact between January 2005 and December 2011 at the Washington National Primate Center (WaNPRC) in Seattle, WA. Prior to the publication of the 2011 Guide, the majority of animals were restricted to protected contact due to veterinary and research staff concerns regarding potential injury. However, in response to the new guidelines for social housing, the policy of WaNPRC changed to full-contact housing as the standard for socialization. With the advent of the new policy, animals that were currently housed in protected contact began to be transitioned into full contact. By the end date of this study’s time period, a subset (N = 86) of these pairs had been placed in full contact housing. These 86 pairs had been housed in protected contact for an average of 117 days. The data set includes all full and protected contact pairings for this species that were attempted during this period. Most of the animals (94%) participated in four or fewer pairings: 361 in one pairing; 163 in two pairings; 80 in three pairings; and 43 in four pairings.
Subjects ranged in age from 1.22 to 25.89 years at the time of initial pairing in protected contact. Age was dichotomized on the basis of approximate age at sexual maturity in this species [Hill, 1974; Napier & Napier, 1967; Sirianni & Swindler, 1985]. Individuals were categorized as adult if they were ≥4 years of age and juvenile if they were between the ages of 1 and 4 years. The majority of the animals were obtained from domestic sources or imported rather than being born at WaNPRC, and complete background information (i.e., social and rearing history) was not available; 8% of the animals were reared in the nursery at the WaNPRC. Numbers of sex and age categories for pair types are listed in Table I.
TABLE I.
Numbers of Pairs and Percent Compatible in Each Age and Sex Category in Protected Contact and in Full Contact (in Bold)
| Age classification | Pair type | N | Percent compatible |
|---|---|---|---|
| Adult–adult | Female–female | 413 | 67.3 |
| 51 | 100 | ||
| Male–male | 63 | 76.2 | |
| 3 | 66.7 | ||
| Male–female | 69 | 84.1 | |
| – | – | ||
| Total | 545 | 70.5 | |
| 54 | 98.1 | ||
| Adult–juvenile | Female–female | 9 | 100 |
| 3 | 100 | ||
| Male–male | 17 | 100 | |
| 1 | 100 | ||
| Male–female | 17 | 82.4 | |
| – | – | ||
| Total | 43 | 74.4 | |
| 4 | 100 | ||
| Juvenile–juvenile | Female–female | 11 | 81.8 |
| 2 | 100 | ||
| Male–male | 56 | 96.4 | |
| 26 | 96.2 | ||
| Male–female | 19 | 94.7 | |
| – | – | ||
| Total | 86 | 94.2 | |
| 28 | 96.4 | ||
| Grand total | 674 | 74.9 | |
| 86 | 97.7 |
Animals were considered to be adult if they were over the age of 4 and juvenile if they were between the ages of 1 and 4 years. No male–female pairs were transitioned to full contact to eliminate the chance of unwanted pregnancies.
Animals were maintained in accordance with the Guide for Care and Use of Laboratory Animals [National Research Council Committee, 2011]. They were fed a nutritionally balanced diet of monkey biscuits twice per day, participated in the WaNPRC Environmental Enhancement Plan, and were provided enrichment items on a daily basis. The WaNPRC is accredited by AAALAC (American Association for Assessment of Laboratory Care) International and all research was conducted under protocols approved by the University of Washington Institutional Animal Care and Use Committee (IACUC). The research adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates.
Housing
All animals were housed indoors on a 12-hr light cycle. Most subjects were housed in two-tiered stainless steel cages with 1.32 or 1.83 m2 floor space and 76.2 or 81.28 cm height, complying with Animal Welfare Act USDA standards for NHPs based on animal weight. Heavier subjects were housed in single-tier cages with 2.44 m2 floor space and 91.44 cm height. The back half of each cage consisted of solid panels to provide privacy from adjacent neighbors. Each cage had a metal bar perch running front to back along one side of the cage. The front half of each cage included two different sizes of mesh (fine 0.64 cm mesh on the left side [fine mesh], 2.54 × 2.54 cm mesh on the right side [wide mesh]) with the bottom portion of each side consisting of sliding mesh side-gates [Crockett et al., 1997; Lee et al., 2012]. Opening the mesh side-gates of two adjacent cages would expose either fixed vertical grooming-contact (GC) bars with 5.16–9.73 cm openings between bars, or an unobstructed opening (34.3 × 38.7 cm to 48.3 × 49.6 cm, depending on cage size) allowing partners to move freely between both cages (for a photograph of caging see Lee et al. [2012]; Fig. 1). The variation in cage openings corresponds to cage size. Protected contact consisted of contact through either wide mesh or grooming bars.
Fig. 1.

Behavioral scoring. Interaction scores based on the above behaviors were assigned for each 5-min phase of the introduction. Based on these scores an overall behavioral introductions score (BIS) was given for each 30-min introductory session. Pairs could receive a BIS of −−, −, +, ++, or ig. Pairs could also receive mixed scores for a session (e.g., +/− or ig/+) if multiple components were observed equally during the 5-min sampling periods. The overall BIS gave more weight to Interaction scores recorded in the later phases of the observation period. For regression analyses the BIS was combined into four groups: positive (+ and ++ codes), negative (−, −−), neutral (ig), and ambivalent (any mixed codes including +/−, +/ig, etc.).
Selection of Pairs and Pairing Procedures
The WaNPRC’s behavioral management policy requires socialization of as many NHPs as possible. Animals not exempted from tactile social contact on the basis of research protocol or clinical status were eligible for socialization. Potential pairings were constrained by each animal’s research project assignment and specific pathogen free (SPF) status. Additionally, pairs were not retried if they had proved to be incompatible with each other in the past. Within these constraints there were no fixed criteria for choosing potential pair-mates other than the professional judgement of behavioral technologists as to potential compatibility. All technologists conducting introductions had been trained by an experienced technologist prior to conducting introductions. Prior to pairing, prospective partners were moved into adjacent cages. To avoid possible distress due to concurrent relocation and social introduction, animals were given at least 2 days to acclimate to their new housing before pairing began. The basic socialization protocol called for three 30-min supervised introduction sessions conducted on three different days over the course of a week. However, experienced technologists were given latitude to either increase or decrease the number of actual introductions. Pairs received between 1 and 6 introductory sessions. Fifty-four percent of the pairings required fewer than three introductions, and only 7.8% required more than three introductions before a decision on compatibility was made.
On the first day of introduction, potential partners were given gradually increasing amounts of tactile contact at 5-min intervals barring any intense (lunging, chasing, striking, pushing, wrestling, hair pulling, biting) or prolonged (3–5 sec) aggression. If such aggression occurred, the pair would spend another 5-min interval at the current level of progression. If aggression exceeded 5 sec in duration, the pair was given a timeout by closing the side-gates and waiting for approximately 5 min before re-opening the side-gates to the level of contact in effect when the time-out was initiated. If two bouts of contact aggression requiring a timeout occurred, the introduction was ended for the day. Introductions on subsequent days began with the highest level of contact achieved at the previous session. However, ifcontact aggression had occurred, the pair started the subsequent session at the initial contact level, and the gradual procedure was repeated. Wounding requiring clinical intervention immediately ended the day’s session; subsequent introductions were delayed until approved by the veterinarian and then only if the pair’s compatibility seemed promising to the behavioral staff.
During introductions, the technologist recorded affiliative (grooming, nonaggressive contact, social play, lipsmacking, presenting, etc.) and agonistic (contact and noncontact aggression, grimacing, crouching, screeching, displacement/withdraw, etc.) behaviors, and behaviors indicative of tension (yawning, chomping, scratching, etc.) on a datasheet during each 5-min period using 1/0 sampling. Recorded behaviors were used to assign an interaction score for each 5-min period (Fig. 1). Based on these 5-min scores, an overall behavioral introduction score (BIS) for the 30-min introductory session was then assigned to each pair for each session. Similar to the 5min scores, pairs could receive a BIS of −−, −, +, ++, or ig. Pairs could also receive mixed scores for a session (e.g., +/− or ig/+) if multiple components were observed equally during the 5-min sampling periods. These finely detailed codes resulted in an unwieldy number of categories for data analysis and were combined into four broader categories for analysis as described below. Based on the BIS and technologist judgement, introductory sessions were ended when pairs were allowed to move to unsupervised contact (deemed compatible) or judged to be incompatible. Technologists were also tasked with identifying the dominant and subordinate member of each pair based on dominance and submissive behaviors (threat, displacement, fear grimace, etc.).
Injuries
Any injuries sustained by a pair during introductory sessions or subsequent unsupervised contact were recorded. For description of injuries, severity classifications were used: (i) injuries requiring no veterinary treatment; (ii) injuries requiring veterinary treatment but not sedation (e.g., administration of medication); and (iii) injuries requiring sedation for treatment such as wound suturing. For descriptive information presented on wounding, pairs are characterized according to the most severe injury incurred. For the purposes of regression analyses, the injury categories were combined, and injury was recorded on a yes (an injury occurred) versus no (no visible injury occurred) basis for each pair.
Data Analysis
Relevant data used in analyses were entered into an ongoing database during the period of introduction. All analyses were performed using SYSTAT® 13 (Chicago, IL). For all analyses, P-values that were ≤0.05 were considered to be statistically significant. The unit of analysis was the pair, regardless of whether individuals in a given pair had been members of other pairs. Logistic regression analyses were employed to ascertain: (i) potential variables influencing the likelihood of pairs being judged compatible and (ii) potential variables influencing the likelihood of pairs sustaining injury during the introduction process, or later, during sustained pairing. Predictor variables are listed in Tables II and III. For regression analyses, the BIS was combined into four groups: positive (+ and ++ codes), negative (−, −−), neutral (ig), and ambivalent (any mixed codes including +/−, +/ig, etc.) (See Fig. 1). Reliability, scored as percent agreement, was 80% between the two observers for these combined codes.
TABLE II.
Predictors for Compatibility and Injuries During Introductory Sessions (Protected Contact)
| Predictor | Compatibility
|
Injuries during introduction
|
||
|---|---|---|---|---|
| Odds ratio | P-value | Odds ratio | P-value | |
| Sex category | ||||
| Male–male (vs. female–female) | NS | NS | ||
| Male–female (vs. female–female) | NS | NS | ||
| Age class | ||||
| Adult–juvenile (vs. adult–adult) | 5.6 | 0.032 | NS | |
| Juvenile–juvenile (vs. adult–adult) | 9.2 | 0.002 | NS | |
| Number of intro sessions | 1.7 | <0.001 | – | |
| Age difference | NS | NS | ||
| Weight difference | NS | NS | ||
| Grooming 1st day | NS | 2.4 | 0.02 | |
| Contact aggression 1st day | 0.29 | <0.001 | 6.3 | <0.001 |
| Injury in introduction | 0.16 | <0.001 | – | |
| BIS first day | ||||
| Negative (vs. positive) | NS | 2.4 | 0.04 | |
| Neutral (vs. positive) | NS | NS | ||
| Ambivalent (vs. positive) | NS | NS | ||
| BIS last day | ||||
| Negative (vs. positive) | 0.04 | <0.001 | 3.1 | 0.003 |
| Neutral (vs. positive) | 0.23 | <0.001 | NS | |
| Ambivalent (vs. positive) | 0.21 | 0.021 | NS | |
TABLE III.
Predictors for Injuries During Protected and Full Contact (Post-Introduction)
| Predictor | Injuries during protected contact
|
Injuries during full contact
|
||
|---|---|---|---|---|
| Odds ratio | P-value | Odds ratio | P-value | |
| Sex category | ||||
| Male–male (vs. female–female) | 3.1 | 0.001 | – | – |
| Male–female (vs. female–female) | 2.2 | 0.03 | – | – |
| Age class | ||||
| Adult–juvenile (vs. adult–adult) | NS | NS | – | – |
| Juvenile–juvenile (vs. adult–adult) | 0.3 | 0.01 | – | – |
| Sex/age category (vs. female–female/adult–adult vs. male–male/juvenile–juvenile) | – | – | 8.0 | .008 |
| Age difference | NS | NS | NS | NS |
| Weight difference | NS | NS | NS | NS |
| Grooming 1st day | NS | NS | NS | NS |
| Contact aggression 1st day | 2.2 | 0.006 | NS | NS |
| Injury in introduction | NS | NS | – | – |
| BIS first day | ||||
| Negative (vs. positive) | NS | NS | NS | NS |
| Neutral (vs. positive) | NS | NS | NS | NS |
| Ambivalent (vs. positive) | NS | NS | NS | NS |
| BIS last day | ||||
| Negative (vs. positive) | NS | NS | – | – |
| Neutral (vs. positive) | NS | NS | NS | NS |
| Ambivalent (vs. positive) | – | – | – | – |
All terms were initially entered into the model, and each term’s contribution to the model was assessed by the change in the model fit when that term was removed (likelihood ratio chi-square). Terms were retained if the significance of the change in the model fit was ≤0.05. Finally, we used Z-tests for equality of proportions to examine differences in injury rates for pairs in protected contact versus full contact.
RESULTS
Demographics
The majority of protected contact pairings were comprised of two females (female–female: 64%; male–male: 20%; male–female: 16%). Most pairings consisted of two adults (adult–adult: 81%; juvenilejuvenile: 13%; adult–juvenile: 6%). The mean age difference in the pairs was 2.4 years (SD = 2.6), and the mean weight difference was 1.8 kg (SD = 1.7). Of the initial 674 pairs introduced to protected contact, 505 (75%) were judged compatible after the introduction process and given unsupervised protected contact. Of these 505 compatible protected contact pairs, 86 were later given full contact introductions. These animals had been paired for a mean of 117 days prior to introduction into full contact (range 14–857 days). Only 2 of the 86 pairs introduced to full contact (one male–male/adult–adult and one male– male/juvenile–juvenile) were judged incompatible at full contact introduction (2.3%). No male–female protected contact pairs were introduced to full contact to avoid unwanted pregnancies. Proportions of compatible partners for each sex and age category are listed in Table I.
The pairing status of all compatible pairs housed in both protected (N = 505) and full contact (N = 84) paired over the preceding 7 years was assessed in November 2012. At that date, only 5% of all pairs were still together; there were no pairs still housed in protected contact and 26 pairs still housed in full contact. The mean time spent together (including time in both protected and full contact) for pairs separated before November 2012 was 220.2 days (SD = 212.9). The majority of separations in both full and protected contact were due to research project requirements with much smaller percentages owing to a shortage of appropriate caging, or to provide social housing for high-needs animals (reprioritization). Only a small percentage of the compatible protected contact pairings (4.0%) were later separated for aggression with another 4.6% separated for clinical concerns (Fig. 2a). For full contact, 8.3% were separated for aggression and 5.9% for clinical concerns (Fig. 2b). Proportions of pairs separated for aggression were not significantly different across age and sex categories.
Fig. 2.
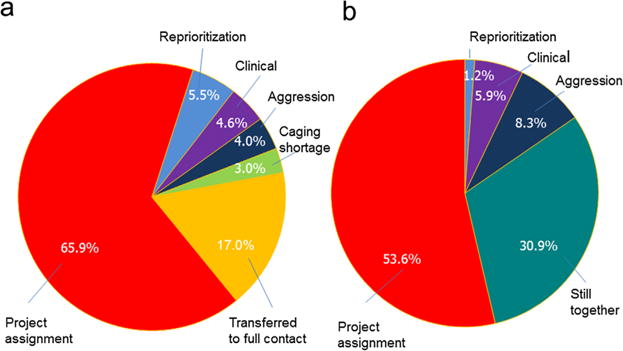
Pairing status. Socialization status or reason for pair ending as of November 2012 for all pairings attempted between 2005 and 2012 and found to be compatible after introduction in (a) protected contact (N = 505) and (b) full contact (N = 84). While the majority of pairs were no longer together, few pairs were separated due to aggression. Most separations occurred due to project assignments.
Protected Contact Pairings
Predictors of compatibility in protected contact
Protected contact consisted of either wide mesh or grooming contact bars. Preliminary analysis indicated that the type of protected contact did not substantially affect outcome measures, so both types of contact were combined for these analyses. Dominance between pairs during introduction was able to be determined for less than half (46.5%) of pairs and its determination was not significantly different across pairs that were compatible (47.5%) or incompatible (43.2%). Predictor variables for the regression analysis, odds ratios, and significance levels are listed in Table II.
Age classification was a significant predictor of compatibility. Both adult–juvenile and juvenile–juvenile pairs were significantly more likely to be compatible than adult–adult pairs. Pairs receiving more introductory sessions were more likely to be judged compatible. As might be expected, the presence of aggression on the first day of introduction, and injury at any time during introduction process, significantly decreased the odds of compatibility. The BIS given to a pair on the last introductory session was also significantly related to the probability of compatibility. Pairs rated negative, ambivalent, and neutral all had decreased odds of being judged compatible when compared with positive rated pairs. A pair’s BIS on the first day of introduction was not a significant predictor. Sex category, and age and weight differences of pairs were not significant in predicting whether pairs would be judged compatible.
Predictors of injuries during protected contact introduction
Injuries during introduction to protected contact were low with most pairings sustaining no injuries (91.6%). Minor wounds requiring no veterinary treatment occurred in 4.5% of introductions; veterinarians provided treatment without sedation in 1.9% of introductions and treatment involving sedation another 1.9% of introductions. The data were subjected to logistic regression to determine what factors contributed to the probability of injury during introduction. Predictor variables, odds ratios, and significance levels are listed in Table II. Pairs with a negative BIS on the first or last day of introduction had significantly increased odds of injury during introduction as did pairs that engaged in contact aggression on the first day of introduction. Surprisingly, pairs engaging in grooming on the first day also had increased odds of injury. Further inspection of the data revealed that receiving a negative BIS combined with grooming behavior on the first day of introduction was especially indicative of injury: 58% of pairs with grooming behavior and a negative BIS had injuries. The probability of injury during introduction was not significantly related to age classification, sex category, or a pair’s age difference or a weight difference.
Injuries sustained during protected contact housing (post-introduction)
No injury occurred in most pairings (88%). Twelve percent of pairs sustained injury during protected contact housing; 5.3% required no veterinary treatment; 1% of pairs required treatment without sedations; and 5.9% of pairs required treatment with sedation. Data were subjected to logistic regression to determine what factors contributed to the likelihood of an injury occurring during protected contact housing. Predictor variables, odds ratios and significance levels are listed in Table III. Male–male and male–female pairs had increased likelihood of injury compared with female-female pairs, and juvenile–juvenile pairs had a decreased likelihood of injury compared with adult–adult pairs. Pairs engaging in contact aggression on the first day also had an increased likelihood of injury compared with pairs not observed to engage in contact aggression on the first day.
Full Contact Pairings
Predictors of compatibility in full contact
Since only two pairs (both male–male) introduced into full contact were judged incompatible, it was not possible to assess factors impacting judged compatibility for full contact pairs (Table I).
Predictors of injury during introduction to full contact
Only 1 of the 86 pairs introduced to full contact sustained injury during the introduction (1.1%). This male–male/adult–adult pair was one of the two pairs judged incompatible in the full contact phase.
Injuries sustained during full contact (post-introduction)
Injuries during full contact housing were significantly higher (Z-test for equality of proportions = 3.6, P < 0.001) when compared to protected contact with 23 (27%) of the pairs sustaining injury (15.5% requiring no treatment; 5.9% treated without sedation; and 5.9% treated with sedation). The data were subjected to logistic regression to determine factors contributing to the likelihood of an injury in full contact. Because almost all full contact pairs were either female–female/adult–adult pairs (N = 51) or male–male/juvenile–juvenile pairs (N = 25), only one predictor was included in the model to identify these two groups; eight pairs who did not fit these two categories (one adult–juvenile/female–female, three adult–juvenile/male–male, two adult–adult/male– male, and two female–female/juvenile–juvenile) were, therefore, excluded from this analysis. Only one full contact pair incurred injury during introduction, so this variable was also not included as a potential predictor in the logistic regression. Similarly, negative and ambivalent BIS on the last day of introduction were not included due to low numbers of pairs in the sample receiving these codes (1 and 3, respectively). Predictor variables, odds ratios, and significance levels are listed in Table III.
The only significant predictor of injury in full contact was the sex and age category of the pair. Female–female/adult–adult pairs were more likely to injure than were male–male/juvenile–juvenile pairs.
The proportion of female–female/adult–adult sustaining injuries was significantly higher in full contact than protected contact. This was true when pairs were compared to themselves in protected contact (protected contact = 5.9%, full contact = 41.7%: Z = 4.5, P < 0.001) or when these pairs were compared to all female–female/adult–adult pairs housed in protected contact (protected contact = 9.7%, full contact = 41.2%, Z = 5.00, P < 0.001). This difference was not significant in male–male/ juvenile–juvenile pairs.
DISCUSSION
The optimal social management of laboratory primates requires awareness of the risks associated with pair introductions, as well as a reasonable expectation for the chances of success. Current literature suggests that, in general, outcomes will vary with age, sex, and pair composition. However, it is critical not to anticipate outcomes for a species that have been developed on the basis of experience with another species, or to use methodologies that have not been tested in the species at hand. Evidence-based assessment of risks and success are critical for the design of appropriate species-specific socialization strategies. Information contained in this manuscript can be used as a guide for designing appropriate introduction techniques for pigtailed macaques as well as making decisions about prioritizing introductions when time or resources are limited, setting appropriate balance of risks and benefits, and advising other stakeholders regarding anticipated outcomes.
In this study, overall compatibility at introduction for all protected contact pairs was 75% and compatibility for full contact pairs (after a period of compatible protected contact) was 98%. Although proportions of compatibility in our full contact pairs may be somewhat inflated because they previously had been compatibly housed in protected contact for an average of 117 days, they are comparable to levels varying from 80% to 100% compatibility reported for other macaque species [Coleman, 2012; Eaton et al., 1994; Reinhardt, 1998; Reinhardt et al., 1988, 1989; Roberts & Platt, 2005]. Compatibility in protected contact varied with age classification and was lowest in pairs containing two adults and highest in pairs containing one adult and one juvenile, with juvenile— juvenile pairs being intermediate. These data replicate earlier findings from this lab that found that juveniles of several macaque species, including pigtailed macaques, were more compatible than adults [Crockett et al., 2006]. Studies at other facilities have also reported much higher levels of compatibility in younger animals when compared to adults [Abney et al., 2011; Capitanio et al., 2015; Reinhardt, 1994].
Logistic regression identified several significant behavioral predictors of compatibility in protected contact. Aggression on the first day of introduction and injury during introduction significantly decreased the odds of compatibility. Crockett et al. [1994] reported that the presence of fighting during introduction predicted incompatibility in long-tailed macaque adult male–male pairs. In our facility, technologists were cognizant of the gravity of injury in social pairs and thus would end the introductory process when injuries requiring treatment occurred. It is possible that animals with initial injuries could have gone on to become compatible pairs as has been shown in rhesus macaques [Oettinger et al., 2008]; however, the policy in force at the time at WaNPRC was that the risk of further injury was not deemed to be worth the possible benefit, given that there were many other potentially compatible partners to choose from.
It is not surprising that pairs with a BIS of negative, ambivalent, or neutral on the last day of the introduction had decreased odds of being deemed compatible compared to those pairs scored as positive. In contrast, neither the BIS nor grooming on the first day of introduction was predictive of a pair’s success. This indicates that in pigtailed macaques, positive interactions need not be present during the initial introduction day for animals to ultimately be compatible. This has practical implications for decision making in the introductory process as it indicates that animals not seen to be overtly compatible on the initial day of pairing should be given additional introductions before being deemed incompatible.
We were not able to discern clear dominance relationships in pigtailed macaques during the introductory period for a majority of the pairs, and dominance assessment did not differ significantly between compatible and incompatible pairs. Similar to our findings, Gust et al. [1996] reported that pigtailed macaques do not appear to immediately establish dominant–subordinate relationships. In this regard, pigtailed macaques differ from rhesus macaques, who often establish a clear dominance relationship prior to pairing that predicts pairing success [DiVincenti & Wyatt, 2011; Reinhardt, 1994]. This further emphasizes the need for species-specific pairing strategies.
In our study, demographic variables other than age classification appeared to have little or no predictive value on compatibility. Weight differences between pairs were not significant predictors of compatibility. Some studies in other macaque species indicated that pairs more disparate in weight were more likely to be compatible [Baker, 2010; Doyle et al., 2008; Steward et al., 2013]; others found contrary or little to no effect of weight differences on compatibility [Abney et al., 2014; Lee et al., 2005b; Maguire-Herring et al., 2013; West et al., 2009]. Age differences between pairs did not affect compatibility in our study. An earlier study at this facility found that age differences accounted for only a very small amount of variance in compatibility in three species of macaques and baboons [Lee et al., 2005b]. It is possible that the relatively small age and weight difference in this study contributed to a lack of statistical significance.
Unexpectedly, sex classification did not contribute uniquely to the regression. Crockett et al. [1994, 1997] reported greater compatibility in long-tailed macaque females in both protected and full contact pairings. Greater compatibility in full contact for female rhesus has also been reported [Steward et al., 2013] although this finding is not universal [DiVincenti & Wyatt, 2011]. The absence of a significant sex effect in our regression could be a statistical anomaly due to the strong correlation of sex classification with the other significant variables of contact aggression and the BIS on the last day of introduction.
Injuries during the introductory process were relatively minor and occurred at low rates for both protected and full contact pairings. Injury rates during protected contact introductions were somewhat lower than those reported for rhesus [Oettinger et al., 2008]. Injury rates during full contact introductions in our study were also lower than rates reported in other macaque species which range from a high of 33% to a low of 2% [Abney et al., 2011; Oettinger et al., 2008; Reinhardt, 1989; Watson, 2002]. Across these studies, longer supervised introduction durations appear to be associated with fewer injuries [Abney et al., 2011 (6–30 min); Reinhardt, 1989 (>6hr); Watson, 2002 (>4 hr)]. Watson [2002] attributed low levels of injuries to close observation during introduction sessions. In our study, low injury rates can be attributed partially to close observation during the pairing process and providing “timeouts” (separation of animals) during prolonged aggressive interactions allowing for deescalation of aggression prior to injury.
Several behavioral measures predicted an injury during protected contact introductions (the occurrence of only one injury during full contact introduction precluded a similar analysis). Aggression on the first day of introduction was significantly associated with injury during the introductory period. Although this is not surprising, it does indicate that pairs with no observed aggression on the first day are less likely to later sustain injuries during introduction. The BIS on the first day of introduction was also predictive, with animals rated as negative being more likely to injure than those rated as positive. This is also unsurprising since the BIS is partially based on agonistic behaviors observed during the introduction. What does seem anomalous is the finding that pairs in which at least one animal groomed on the first day were also more likely to sustain an injury. Further inspection of the data revealed that this finding mostly reflected animals who were rated as negative on their first day of introduction (i.e., most of the interactions in the pair were negative). Therefore, this may indicate that one member of the pair was reacting inappropriately to either agonistic or affiliative cues from the other partner. As with predictions of compatibility, behavioral measures were more useful in predicting injury than demographic factors (age category, sex category, differences in age, and weight) which were not predictive of injury during protected contact introductions. Few studies have been able to fully assess the impact of these demographic factors as most have involved only one sex or one age class.
Although our basic introduction protocol called for three 30-min introductions, technologists were given latitude in deciding when a pair could proceed to unsupervised contact. In practice, most pairs required fewer than three introductions resulting in a mean introduction time of just over an hour across pairings. Some facilities have reported considerably longer introduction times [Reinhardt, 1989 (>6 hr); Watson, 2002 (>4 hr)], and other facilities have reported less time expended in introductions [Abney et al., 2011]. Different lengths of introductions across facilities could be due to either facility or species differences. In our colony, an hour of supervised introduction for pigtailed macaques appears to be optimal as injury rates during introduction were low, and only a small proportion of pairs deemed compatible at introduction in both protected and full contact required later separation due to aggression or clinical concerns.
The average length of pairing duration in our colony was relatively short, with most pair separations due to scientific project assignment. In our sample, the mean duration of pairing was approximately 7 months. This rather sobering statistic underscores the fact that maintaining laboratory macaques in social configurations compliant with regulatory demands is a continuous task that requires ongoing personnel time investment. Time invested in the actual introduction process does not encompass the additional time necessary to identify appropriate pairs, arrange for animals to be moved if necessary, and to set up appropriate caging, and so on. Socialization efforts involve not only time spent by behavioral management personnel, but also husbandry staff. It is crucial to identify factors that can predict which pairings are likely to succeed or fail as well as an optimal introduction duration so as to maximize efficiency in the pairing process while maintaining animal safety.
The ability to compare injury rates among studies is constrained by two factors. First, what is defined as an injury may vary between studies (i.e., minor wounds not requiring any veterinary attention may not be reported). Second, and perhaps more problematic, is that veterinary practices may differ between facilities or individual veterinarians, confounding the attempt to assign severity according to the extent or type of treatment provided. Therefore, conclusions drawn from species differences in wounding must be tempered by these methodological inconsistencies. Despite the fact that solutions for addressing these confounds have not yet been found, injury rates are nonetheless of great interest to behavioral managers and can be broadly useful for comparing outcomes within or among institutions. In our study, injuries post-introduction during either protected or full contact housing were relatively low. Injury rates in full contact housing were somewhat higher than comparable injuries reported in some studies of rhesus macaques [Reinhardt, 1998 (1.3–2.5%); Reinhardt, 2002 (0.6%)]. In another study, however, involving retrospective analysis of colony records for rhesus and long-tailed macaques, Bayne et al. [1995] reported that 21 of the 84 (25%) pair-housed individuals incurred injuries over a three year period. However, it appears that only eight of these (10%) required significant veterinary attention. Nearly all of the pairs transitioned into full contact in our study were female–female/adult–adult and male–male/juvenile–juvenile. Full contact female–female/adult–adult pairs were much more likely to injure than were male–male/juvenile–juvenile pairs, which had a very low injury rate. This could be partly due to the immature dentition of juvenile males as well as the fact that younger animals, in general are more compatible. Injuries sustained in full contact occurred at a significantly higher rate than in protected contact in female–female/adult–adult pairs when compared either to themselves while housed in protected contact (within subjects) or to all female–female/adult–adult pairs (between subjects) in protected contact. We speculate that this may relate to the natural history of this species, wherein male pigtailed macaques play an important role in controlling intra-group aggression among females [Oswald & Erwin, 1976]. Regardless, it is of use for behavioral managers to be aware that more frequent injuries may occur when adult females are housed in full contact in this species.
Full contact must currently be considered the default means for providing social contact [National Research Council Committee, 2011]. However, since there are a number of reasons that protected contact housing may be implemented, including research protocol requirements, it is important to continue to evaluate whether the behavioral and welfare benefits for full contact housing outweigh the potential costs of greater risk of injuries. It appears that the answer may vary by species. Adult female rhesus macaques clearly benefit from full contact compared to protected contact with full contact pairs engaging in significantly more grooming and significantly less abnormal behavior than when housed in protected contact [Baker et al., 2012b]. However, for long-tailed macaque females, the cost/benefit ratio is not as clear, since no differences were found in expression of abnormal or social behaviors with full contact housing [Baker et al., 2012b; Lee et al., 2012]. It has not been ascertained whether full contact housing provides greater behavioral benefits to pigtailed macaques. This study does show, however, that full contact housing was associated with greater costs in the form of injuries and pair separations due to aggression. Although the question regarding costs in relation to benefits in this species cannot be fully assessed at present, these data suggest that if there are instances where full contact housing is contraindicated due to research or behavioral considerations, protected contact could be considered as a viable option.
Acknowledgments
Contract grant sponsor: NIH; contract grant numbers: P51 OD010425, R24OD01180.
Footnotes
Conflicts of interest: None.
References
- Abney DM, Poor LL, Reuther KJ. Socialization of adult male cynomolgus macaques: benefits vs. costs. American Journal of Primatology. 2011;73:41. [Google Scholar]
- Abney DM, Toscano JE, Poor LL, Moomaw HA. Successful social housing of adult male cynomolgus macaques with similar body weights. American Journal of Primatology. 2014;76:86. [Google Scholar]
- Association for Accreditation of Laboratory Animal Care International. Position statements. 2011 Available from: http://www.aaalac.org/accreditation/positionstatements.cfm.
- Baker KC. Retrospective assessment of pair formation in laboratory rhesus macaques: refining partner selection. American Journal of Primatology. 2010;72:26–27. [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, et al. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Applied Animal Behaviour Science. 2012a;137:148–156. doi: 10.1016/j.applanim.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Crockett CM, Lee GH, et al. Pair housing for female longtailed and rhesus macaques in the laboratory: behavior in protected contact versus full contact. Journal of Applied Animal Welfare Science. 2012b;15:126–143. doi: 10.1080/10888705.2012.658330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Weed JL, Crockett CM, Bloomsmith MA. Survey of environmental enhancement programs for laboratory primates. American Journal of Primatology. 2007;69:377–394. doi: 10.1002/ajp.20347. [DOI] [PubMed] [Google Scholar]
- Bayne K, Haines M, Dexter S, Woodman D. Nonhuman primate wounding prevalence: a retrospective analysis. Laboratory Animal. 1995;24:40–44. [Google Scholar]
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. American Journal of Primatology. 2002;58:57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- Caldecott JO. In: An ecological and behavioural study of the pig-tailed macaque. Szalay FS, editor. Basel, New York: Karger; 1986. pp. 1–259. [Google Scholar]
- Capitanio JP, Blozis SA, Snarr J, Steward A, McCowan BJ. Do “birds of a feather flock together” or do “opposites attract”? Behavioral responses and temperament predict success in pairings of rhesus monkeys in a laboratory setting: temperament and pairing success. American Journal of Primatology. 2015 doi: 10.1002/ajp.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A. Ecology and behaviour of the pig-tailed macaque Macaca nemestrina leonina in some forests of Assam in North-East India. Journal of the Bombay Natural Historical Society. 2008;105:279–291. [Google Scholar]
- Coleman K. Individual differences in temperament and behavioral management practices for nonhuman primates. Applied Animal Behaviour Science. 2012;137:106–113. doi: 10.1016/j.applanim.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett CM, Bellanca RU, Bowers CL, Bowden DM. Grooming-contact bars provide social contact for individually caged laboratory macaques. Contemporary Topics in Laboratory Animal Science. 1997;36:53–60. [PubMed] [Google Scholar]
- Crockett CM, Bowers CL, Bowden DM, Sackett GP. Sex differences in compatibility of pair-housed adult longtailed macaques. American Journal of Primatology. 1994;32:73–94. doi: 10.1002/ajp.1350320202. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Lee GH, Thom JP. Sex and age predictors of compatibility in grooming-contact caging vary by species of laboratory monkey. International Journal of Primatology. 2006;27:417. [Google Scholar]
- Crockett CM, Wilson WL. The macaques: studies in ecology, behavior and evolution. New York: Van Nostrand Reinhold; 1980. The ecological separation of Macaca nemestrina and M. fascicularis in Sumatra; pp. 148–181. [Google Scholar]
- Dazey J, Kuyk K, Oswald M, Martenson J, Erwin J. Effects of group composition on agonistic behavior of captive pigtail macaques, Macaca nemestrina. American Journal of Physical Anthropology. 1977;46:73–76. [Google Scholar]
- DiVincenti JL, Wyatt JD. Pair housing of macaques in research facilities: a science-based review of benefits and risks. Journal of the American Association for Laboratory Animal Science. 2011;50:856–863. [PMC free article] [PubMed] [Google Scholar]
- Doyle LA, Baker KC, Cox LD. Physiological and behavioral effects of social introduction on adult male rhesus macaques. American Journal of Primatology. 2008;70:542–550. doi: 10.1002/ajp.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton GG, Kelley ST, Axthelm MK, Iliff-Sizemore SA, Shiigi SM. Psychological well-being in paired adult female rhesus (Macaca mulatta) American Journal of Primatology. 1994;33:89–99. doi: 10.1002/ajp.1350330204. [DOI] [PubMed] [Google Scholar]
- Erwin J. Factors contributing to intragroup aggression in captive pigtail monkey groups. In: Chivers DJ, Herbert J, editors. Recent advances in primatology, vol 1: behaviour. New York: Academic Press; 1978. pp. 581–584. [Google Scholar]
- Flack JC, Krakauer DC, de Waal FBM. Robustness mechanisms in primate societies: a perturbation study. Proceedings of the Royal Society Biological Sciences Series B. 2005a;272:1091–1099. doi: 10.1098/rspb.2004.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack J, de Waal FBM, Krakauer DC. Social structure, robustness, and policing cost in a cognitively sophisticated species. American Naturalist. 2005b;165:E126–E139. doi: 10.1086/429277. [DOI] [PubMed] [Google Scholar]
- Gilbert MH, Baker KC. Social buffering in adult male rhesus macaques (Macaca mulatta): effects of stressful events in single vs. pair housing. Journal of Medical Primatology. 2011;40:71–78. doi: 10.1111/j.1600-0684.2010.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Brodie AR, McClure HM. Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiology and Behavior. 1994;55:681–684. doi: 10.1016/0031-9384(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, et al. Group formation of female pigtail macaques (Macaca nemestrina) American Journal of Primatology. 1996;39:263–273. doi: 10.1002/(SICI)1098-2345(1996)39:4<263::AID-AJP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hill O. Primates, comparative anatomy and taxonomy. Vol. 7. New York: John Wiley; 1974. [Google Scholar]
- Jorgensen MJ, Lambert KR, Breaux SD, et al. Pair housing of vervets/African green monkeys for biomedical research. American Journal of Primatology. 2015 doi: 10.1002/ajp.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast K, Preuschoft S. Dominance style and facial expressions of pigtail macaques (Macaca nemestrina) Primate Report. 2005;72:52–53. [Google Scholar]
- Lee GH, Bellanca RU, Crockett CM. Do behavior disorders affect the probability of a laboratory monkey’s socialization success? American Journal of Primatology. 2005a;66:149–150. [Google Scholar]
- Lee GH, Thom JP, Chu KL, Crockett CM. Comparing the relative benefits of grooming-contact and full-contact pairing for laboratory-housed adult female Macaca fascicularis. Applied Animal Behaviour Science. 2012;137:157–165. doi: 10.1016/j.applanim.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Thom JP, Crockett CM. Factors predicting compatible grooming-contact pairings in four species of laboratory monkey. American Journal of Primatology. 2005b;66:83–84. [Google Scholar]
- Lutz CK, Well A, Novak MA. Stereotypic and selfinjurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. American Journal of Primatology. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Maguire-Herring V, Stonemetz KM, Lynch LJ, Fahey MA. The effect of weight on the compatibility of isosexual pairs of captive rhesus macaques (Macaca mulatta) American Journal of Primatology. 2013;75:77. [Google Scholar]
- Napier JR, Napier PH. Handbook of living primates. London & New York: Academic Press; 1967. [Google Scholar]
- National Research Council Committee. Guide for the Care and Use of Laboratory Animals. 8th. Washington DC: National Academies Press (US); 2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK54050/ [Google Scholar]
- Novak MA. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. American Journal of Primatology. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Oettinger BC, Baker KC, Neu K, et al. Wounding incidence in isosexual pairs of adult rhesus macaques (Macaca mulatta) during introduction and in varying pair housing conditions. American Journal of Primatology. 2008;70:44. [Google Scholar]
- Oi T. Patterns of dominance and affiliation in wild pig-tailed macaques (Macaca nemestrina nemestrina) in West Sumatra. International Journal of Primatology. 1990a;11:339–356. [Google Scholar]
- Oi T. Population organization of wild pig-tailed macaques (Macaca nemestrina nemestrina) in West Sumatra. Primates. 1990b;31:15–31. [Google Scholar]
- Oi T. Sexual behaviour and mating system of the wild pig-tailed macaque in West Sumatra. In: Fa JE, Lindburg DG, editors. Evolution and ecology of macaque societies. New York: Cambridge University Press; 1996. pp. 342–368. [Google Scholar]
- Oswald M, Erwin J. Control of intragroup aggression by male pigtail monkeys (Macaca nemestrina) Nature. 1976;262:686–688. [Google Scholar]
- Reinhardt V. Behavioral responses of unrelated adult male rhesus monkeys familiarized and paired for the purpose of environmental enrichment. American Journal of Primatology. 1989;17:243–248. doi: 10.1002/ajp.1350170305. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Pair-housing rather than single-housing for laboratory rhesus macaques. Journal of Medical Primatology. 1994;23:426–431. doi: 10.1111/j.1600-0684.1994.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Pairing Macaca mulatta and Macaca arctoides of both sexes. Laboratory Primate Newsletter. 1998;37:2. [Google Scholar]
- Reinhardt V. Addressing the social needs of macaques used for research. Laboratory Primate Newsletter. 2002;41:7–11. [Google Scholar]
- Reinhardt V, Houser D, Cowley D, Eisele S, Vertein R. Alternatives to single caging of rhesus monkeys (Macaca mulatta) used in research. Zeitschrift Fur Versuchstierkunde. 1989;32:275–279. [PubMed] [Google Scholar]
- Reinhardt V, Houser D, Eisele S, Cowley D, Vertein R. Behavioral responses of unrelated rhesus monkey females paired for the purpose of environmental enrichment. American Journal of Primatology. 1988;14:135–140. doi: 10.1002/ajp.1350140204. [DOI] [PubMed] [Google Scholar]
- Reinhardt V, Rossell M. Self-biting in caged macaques: cause, effect, and treatment. Journal of Applied Animal Welfare Science. 2001;4:285–294. [Google Scholar]
- Roberts SJ, Platt ML. Effects of isosexual pair-housing on biomedical implants and study participation in male macaques. Contemporary Topics in Laboratory Animal Science. 2005;44:13–18. [PubMed] [Google Scholar]
- Rommeck I, Anderson K, Heagerty A, Cameron A, McCowan B. Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. Journal of Applied Animal Welfare Science. 2009;12:61–72. doi: 10.1080/10888700802536798. [DOI] [PubMed] [Google Scholar]
- Sackett DP, Oswald M, Erwin J. Aggression among captive female pigtail monkeys in all-female and harem group. Journal of Biological Psychology. 1975;17:17–20. [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Porter LM, Suarez SA. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Applied Animal Behaviour Science. 1996;48:159–172. [Google Scholar]
- Schapiro SJ, Nehete P, Perlman JE, Sastry KJ. A comparison of cell-mediated immune responses in rhesus macaques housed singly, in pairs, or in groups. Applied Animal Behaviour Science. 2000;68:67–84. doi: 10.1016/s0168-1591(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Sirianni JE, Swindler DR. Growth and development of the pigtailed macaque. Boca Raton (FL): CRC Press; 1985. p. 168. [Google Scholar]
- Steward AL, Sorenson A, Elliot K, et al. Variables affecting indoor pairing success in adult rhesus macaques (Macaca mulatta) American Journal of Primatology. 2013;75:48. [Google Scholar]
- Thierry B. In: Social epigenesis. Thierry B, Singh M, Kaumanns W, editors. New York: Cambridge University Press; 2004. pp. 267–290. [Google Scholar]
- Watson LM. A successful program for same- and cross-age pair-housing adult and subadult male Macaca fascicularis. Laboratory Primate Newsletter. 2002;41:6–9. [Google Scholar]
- Weed JL, Wagner PO, Byrum R, et al. Treatment of persistent self-injurious behavior in rhesus monkeys through socialization: a preliminary report. Contemporary Topics in Laboratory Animal Science. 2003;42:21–23. [PubMed] [Google Scholar]
- West AM, Leland SP, Collins MW, et al. Pair-formation in laboratory rhesus macaques (Macaca mulatta): a retrospective assessment in a compatibility testing procedure. American Journal of Primatology. 2009;71:41. [Google Scholar]


