Abstract
Objectives
New sex partners put adolescents at increased risk for sexually transmitted infections (STIs), even when these sex partners are nonoverlapping. Although the risk of partner change is well described, little is known about its antecedents. We prospectively examined associations between relationship characteristics, partner change, and subsequent STI during intervals of “serial monogamy.”
Methods
As part of a longitudinal study, 332 adolescent women were interviewed and tested for gonorrhea, chlamydia, and trichomonas every 3 months for up to just over 6 years. Interviews covered partner-specific relationship characteristics and sexual behaviors. The quarterly interval, a 3-month period bracketed by interviews and STI testing, was the unit of analysis. We examined associations among relationship factors, partner change, and subsequent STI using a series of mixed regression models, controlling for age, STI at Time 1, and condom nonuse.
Results
Age, lower relationship quality, and lower levels of partner closeness to friends and family predicted partner change from Time 1 to Time 2. In turn, partner change was associated with acquisition of a new STI at Time 2. Although relationship factors did not exert a direct effect on STI at Time 2, they improved partner change—STI model fit. Similar patterns were seen with each organism.
Conclusion
Relationship factors drive partner change, which in turn contributes to STI acquisition. STI prevention research may need to focus on the relationship antecedents to partner change, in addition to the partner change itself.
Introduction
Adolescents have the highest age-specific rates of common sexually transmitted infections (STIs) such as gonorrhea and chlamydia.1 This STI acquisition has been linked to partner change.2,3 While sex partner concurrency is a subset of partner change, “Serial monogamy,” or sequential nonoverlapping partners, is more characteristic of adolescent relationships, and these nonoverlapping partner changes are similarly associated with STIs.2 In these nonoverlapping partner changes, STI transmission presumably occurs because the gap between partners is shorter than the infectious period of the disease.4 A better understanding of why adolescents change partners, including the relationship processes leading to partner change, could inform STI prevention efforts.
Relationship characteristics have been long associated with adolescent sexual behavior. Relationship characteristics such as relationship quality, power, love, and trust have been linked to condom nonuse.5–7 Relationship quality has additionally been associated with subsequent chlamydia infection.5 Adolescent relationships occur in the broader context of adolescents’ social networks, and that sexual partner’s place in a social network has been linked to the index participants’ STI risks behaviors (such as condom nonuse),8 as well as STIs diagnoses.9,10 The behavioral mechanisms by which relationship characteristics influence STI acquisition are only partially understood. While it seems intuitive that relationship factors influence STI risk through partner selection, there may be other mechanisms by which they act, such as increased coital frequency and condom nonuse.5–7 For example, longer relationships,11,12 higher quality relationships,13 desires for intimacy,14 and partner closeness to an adolescent’s social network8 are all associated with lower levels of condom use. With the exception of work by Sayegh et al,5 less is known about partner change as an intermediary between relationship characteristics and STI. Alternatively, relationship factors might influence other mediators, such as the tendency to choose an already infected partner, or a partner’s propensity to have concurrent sex partners.
The purpose of this analysis is to prospectively examine, among adolescent women, the associations among relationship characteristics, partner change, and subsequent infection with chlamydia, gonorrhea, and trichomonas. We examined relationship characteristics first as mediators, and then as an indirect effect. We specifically target intervals of “serial monogamy,” in which participants report only one partner, or have only one partner but change from one partner to a second, temporally nonoverlapping partner.
MATERIALS AND METHODS
Participants
As part of a longitudinal study of STI, 332 adolescent women were enrolled from 3 adolescent primary care clinics, and were followed for up to just over 6 years, from 1999 to 2007. The clinics serve primarily low to middle income urban communities with high rates of early sexual onset and STIs,15 representing a population of particular interest for STI prevention. Inclusion criteria included female gender, ages 14.0 to 17.9 years at enrollment, and not pregnant. Sexual experience was not an inclusion criterion.
Procedures
Each adolescent provided written consent and parents provided written permission. The study was approved by the institutional review board of Indiana University Purdue University at Indianapolis–Clarian Health. At baseline and then at quarterly intervals, participants completed face-to-face interviews and provided a self- or provider-collected vaginal swab for deoxyribonucleic acid (DNA)-based STI testing. Participants had a 6-week window to complete their quarterly interview. Interview topics included sex partners, partner-specific sexual behaviors, and relationship characteristics. Participants diagnosed with STIs were treated according to Centers for Disease Control and Promotion STD treatment guidelines.16 Partners of participants were either contacted by the participant themselves, or contacted by a county health department Disease Information Specialist employed by the study, and referred for treatment to the county STD clinic.
Measures
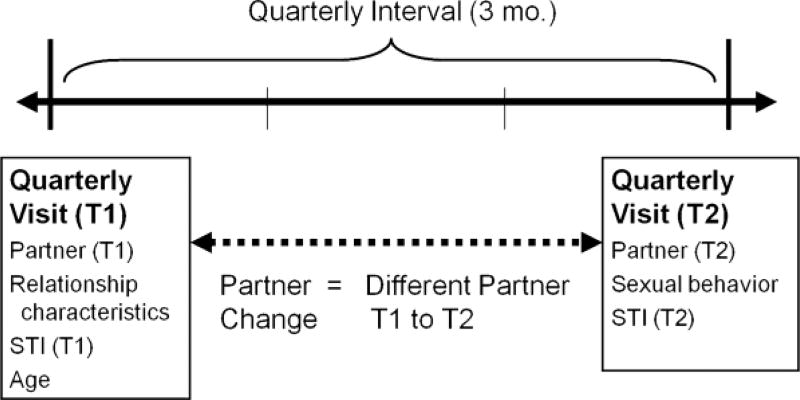
These analyses focus on changes over a quarterly interval, from 1 interview to the following quarterly interview (Fig. 1). Relationship characteristics and demographic information were measured at Time 1. STI was measured at both Time 1 and Time 2. Condom nonuse during the interval was measured at Time 2. Partner change across the interval was measured by comparing partners at Time 1 to partners at Time 2.
Figure 1.
Data structure and the quarterly interval.
Time 1 relationship characteristics were partner-specific, meaning that young women referenced a specific individual when providing the data. Relationship quality-consisted of 6 items (a = 0.83; range, 6–24), which assessed positive emotional and affiliational aspects of a relationship. Participants were asked to respond on a 4-point Likert-type scale (strongly disagree to strongly agree) to items such as, “We have a strong emotional relationship,” “We enjoy spending time together,” and “He is a very important person in my life.” Length of relationship was defined as time since first sex with that specific partner. Family-partner closeness consisted of 4 items measuring how well their family and sex partner knew each other (a = 0.92). Friend-partner closeness also consisted of 4 items, measuring how well their friends and sex partner knew each other (a = 0.88). The number of condom-unprotected vaginal sex events was measured to give an estimate of STI exposure in the quarterly interval. At each interview, participants were asked the number of vaginal sex events for each specific partner, and the number of those vaginal sex events protected with a condom. Partner change was measured across quarterly intervals (Fig. 1). At each quarterly interview, participants listed sex partners over the past 3 months by first name or nickname. We defined partner change as having a different individual listed at Time 1 and Time 2.
STIs were measured by self- or provider-collected vaginal samples at the time of each quarterly interview. Infections with chlamydia, gonorrhea, and trichomonas were assessed using deoxyribonucleic acid-based tests. Infections were treated with antibiotic regimens recommended by the Centers for Disease Control and Prevention.16 Assistance in partner treatment was offered to each young woman.
Analysis
Our unit of analysis was the quarterly interval (Fig. 1), and each participant contributed multiple quarterly intervals. The outcome variables were infection with gonorrhea, chlamydia and trichomonas at Time 2. The analysis included all intervals consistent with “serial monogamy,” where participants reported only 1 sexual partner in the interview at the start and only 1 partner at the end of the quarterly interval. Of nearly 4500 quarterly intervals, 53% included 1 partner at each interview (n = 2339, included in the analysis), 24% had no partners at either the start or the end interview, and 23% had 2 or more partners at either the start or the end interview.
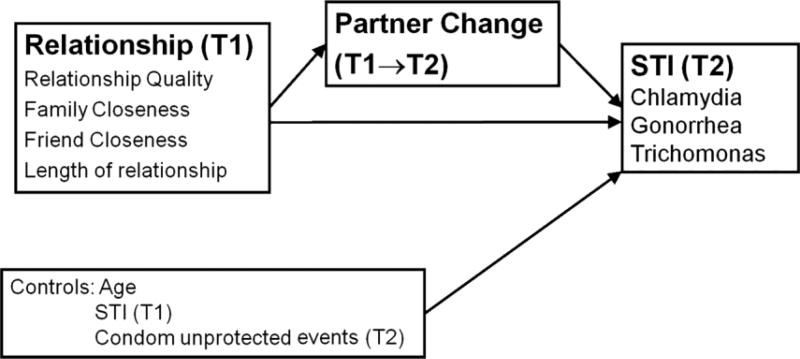
To examine how relationship characteristics and partner change together influence STI risk, we conducted a series of 4 mixed effects regression models, examining both direct and indirect effects of relationship characteristics on STI acquisition (Fig. 2). Model 1 examined the direct effects of relationship characteristics on STI at Time 2. Models 2 through 4 examined indirect effects of relationship characteristics on STI at Time 2. Model 2 examined associations between partner change and acquisition of STI at Time 2, verifying that our data are consistent with other epidemiologic data. Model 3 examined the associations between Time 1 relationship characteristics and subsequent partner change. Model 4 examined the combined effect of relationship characteristics and partner change on STI at Time 2. This last set of analyses examined the independent contributions of relationship characteristics and partner change, and whether relationship characteristics improve our ability to explain STI at Time 2. All models controlled for age, Time 1 STI, and the number of condom-unprotected sexual events reported at Time 2. The number of condom-unprotected sexual events provides a measure of potential STI exposure. Subject-specific random intercepts were used in the mixed effect models to accommodate the dependent structure introduced from multiple quarterly intervals contributed by each participant. Estimates provided are equivalent to unstandardized f3 coefficients. Analyses were conducted in SAS Version 9.1. (SAS Institute, Inc., Cary, NC).
Figure 2.
Conceptual model of partner change.
RESULTS
Participants, Sexual Behaviors, and STIs
A total of 332 participants contributed 2339 quarterly intervals of “serial monogamy” over a period of just over 6 years (Table 1). The median number of quarterly intervals contributed was 6. In all, 88% of participants were black, 9.7% white, 1.2% Latino, and 1 individual self-identified as Pacific Islander. The mean age at the start of these intervals was 18.1 years. Additional information on Time 1 STI diagnoses and behaviors is given in Table 1. STI diagnoses were common, with 8.5% of Time 2 samples associated with chlamydia infection, 3.3% associated with gonorrhea, and 4.5% associated with trichomonas.
Table 1.
Interval Characteristics
| Interval Characteristics | Time 1 | Time 2 | ||
|---|---|---|---|---|
| Mean (SD)/ Percent |
Range | Mean (SD)/ Percent |
Range | |
| Age at start of Interval (years) | 18.2 (+/−2.1) | 14 – 24 | ||
| Relationship Quality | 19.90 (+/−3.68) | 6–24 | ||
| Relationship Length | 33.06(29.2) | 0–123.5 | ||
| Friend-Partner Closeness | 0.55 (+/−0.2) | 0–1 | ||
| Family-Partner Closeness | 0.42 (+/−0.19) | 0–1 | ||
| Partner Change | 24.2% | |||
| Condom Unprotected Events | 13.1 (+/−12.5) | 0 – 50 | ||
| Any STI | 16.2% | 15.5% | ||
| CT | 8.9% | 8.5% | ||
| GC | 3.7% | 3.3% | ||
| TV | 4.8% | 4.5% | ||
Model 1: Lack of a Direct Effect of Relationship Characteristics on STI
Model 1 (not shown) demonstrated a lack of a direct effect of relationship characteristics on STI at Time 2. In bivariate analyses between individual relationship characteristics and specific STIs at Time 2, the only significant association was between relationship quality and trichomonas infection at Time 2 (P < 0.003). Borderline associations (0.05 < P < 0.10) were observed between relationship quality and any STI, relationship quality and gonorrhea, relationship length and any STI, and relationship length and chlamydia. In models controlling for age, condom unprotected events, and baseline infection with that specific organism(s), the significant association between relationship quality and trichomonas disappeared.
Model 2: Partner Change and STI
Model 2 verified the association between partner change and STI acquisition during the interval (Table 2). Results were consistent across all 3 organisms. During intervals where participants changed partners, participants were also more likely to acquire chlamydia, even when controlling for age, chlamydia at Time 1, and number of condom-unprotected events. Age was inversely related to chlamydia acquisition, meaning that during intervals where participants were younger, they were more likely to acquire chlamydia. As with chlamydia, participants were more likely to acquire gonorrhea and trichomonas during intervals where they changed partners. In contrast to chlamydia, participants were more likely to acquire trichomonas during intervals in which they were older.
Table 2.
Model 2, Partner Change and STI Acquisition at Time 2 and Model 3, Relationship Characteristics and Partner Change
| Model 2, Partner Change and STI Acquisition | ||||
| Chlamydia Acquisition | Estimate | Std.Error | t-value | |
| Age | −0.158 | 0.037 | −4.266 | *** |
| CT at T1 | 0.813 | 0.139 | 5.857 | *** |
| Condom Unprotected Events | 0.000 | 0.005 | −0.087 | |
| Partner Change | 0.562 | 0.136 | 4.125 | *** |
| Gonorrhea Acquisition | Estimate | Std.Error | t-value | |
| Age | −0.076 | 0.043 | −1.764 | § |
| GC at T1 | −0.063 | 0.208 | −0.304 | |
| Condom Unprotected Events | −0.017 | 0.006 | 2.929 | ** |
| New Partner | 0.352 | 0.168 | 2.095 | * |
| Trichomonas Acquisition | Estimate | Std.Error | t-value | |
| Age | 0.105 | 0.048 | 2.199 | * |
| TV at T1 | 1.950 | 0.234 | 8.333 | *** |
| Condom Unprotected Events | −0.002 | 0.008 | −0.255 | |
| New Partner | 0.521 | 0.232 | 2.248 | * |
| Model 3, Relationship Characteristics and Partner Change | ||||
| Partner Change | Estimate | Std.Error | t-value | |
| Relationship Quality | −0.175 | 0.017 | −10.382 | *** |
| Family/Partner Closeness | −1.518 | 0.359 | −4.231 | *** |
| Friend/Partner Closeness | −0.678 | 0.291 | −2.327 | ** |
| Relationship Length | −0.004 | 0.002 | −2.058 | * |
| STI at T1 | 0.392 | 0.142 | 2.760 | ** |
| Age | −0.222 | 0.032 | −6.875 | *** |
| Relationship Quality | −0.175 | 0.017 | −10.382 | *** |
| Family/Partner Closeness | −1.518 | 0.359 | −4.231 | *** |
p<0.001;
p< 0.01;
p< 0.05,
p<.10
Model 3: Relationship Characteristics and Partner Change
Model 3 examined the effect of relationship characteristics on partner change, controlling for age and any STI at Time 1 (Table 2). We found that all relationship characteristics were significantly and negatively associated with changing partners. During intervals in which the participant reported lower relationship quality, shorter relationships, and less closeness between their partner and their family or their friends, participants were more likely to change partners.
Model 4: Independent Effects of Relationship Characteristics and Partner Change on STI
Model 4 examined the independent contributions of relationship characteristics and partner change on subsequent STI at Time 2, again controlling for Age, STI at Time 1, and condom unprotected events (Table 3). When both relationship characteristics and partner change are included in the models, relationship factors were not significant predictors of STI, suggesting that relationship characteristics do not directly contribute to STI, but instead act through partner change. However, adding relationship factors does significantly improve model fit for all 3 organisms (bottom of Table 3).
Table 3.
Model 4: Influences of Relationship Characteristics and Partner Change on Acquisition of STI at Time 2. [a]
| Chlamydia Acquisition | Estimate | Std.Error | t-value | |
| Age | −0.182 | 0.043 | −4.266 | *** |
| CT at T1 | 1.011 | 0.169 | 5.982 | *** |
| Condom Unprotected Events | 0.001 | 0.006 | −0.129 | |
| Partner Change | 0.669 | 0.160 | 4.192 | *** |
| Relationship Quality | 0.003 | 0.022 | 0.141 | |
| Family-Partner Closeness | 0.163 | 0.459 | 0.355 | |
| Friend-Partner Closeness | 0.456 | 0.402 | 1.133 | |
| Length of Relationship | −0.002 | 0.002 | −0.848 | |
| Gonorrhea Acquisition | Estimate | Std.Error | t-value | |
| Age | −0.084 | 0.048 | −1.756 | § |
| GC at T1 | −0.063 | 0.208 | −0.304 | |
| Condom Unprotected Events | −0.019 | 0.006 | 2.916 | ** |
| Partner Change | 0.413 | 0.189 | 2.181 | * |
| Relationship Quality | −0.034 | 0.025 | −1.373 | |
| Family-Partner Closeness | 0.825 | 0.546 | 1.510 | |
| Friend-Partner Closeness | 0.362 | 0.485 | 0.746 | |
| Length of Relationship | 0.006 | 0.003 | 1.970 | * |
| Trichomonas Acquisition | Estimate | Std.Error | t-value | |
| Age | 0.284 | 0.047 | 5.997 | *** |
| TV at T1 | 0.470 | 0.189 | 2.484 | * |
| Condom Unprotected Events | −0.008 | 0.006 | −1.333 | |
| Partner Change | 0.619 | 0.175 | 3.543 | *** |
| Relationship Quality | −0.015 | 0.021 | −0.726 | |
| Family-Partner Closeness | 0.411 | 0.551 | 0.746 | |
| Friend-Partner Closeness | 0.053 | 0.453 | 0.118 | |
| Length of Relationship | −0.002 | 0.003 | −0.641 | |
| Comparison of Model Fit with relationship characteristics (Model 4) and Without relationship characteristics (Model 2, Table 2) | Log-Likelihood | Chi-Square | ||
| Chlamydia T2 | Model 2 | −614.6 | 95.5 | *** |
| Model 4 | −566.8 | |||
| Gonorrhea T2 | Model 2 | −323.8 | 62.0 | *** |
| Model 4 | −292.8 | |||
| Trichomonas T2 | Model 2 | −370.1 | 72.5 | *** |
| Model 4 | −333.8 |
Note that associations between relationship characteristics and STI acquisition disappear after partner change is added to the model, but that model fit is improved.
p<.001,
p<.01,
p<.05,
p<.10
DISCUSSION
For the average participant, relationship characteristics predicted the decision to change partners, which in turn predicted STI acquisition, even in our constrained model of serial monogamy. These longitudinal associations were seen across 3 different sexually transmitted organisms. Our series of analyses demonstrate that these acted indirectly, with relationship characteristics influencing STI acquisition through partner change. The findings extend our existing epidemiologic understanding of partner change and adolescent STI,2,3,5 in several dimensions. First, the statistical approach, use of multiple intervals from each individual and a random subject intercept, allows a focus on within-individual differences. During intervals where the average individual reported lower levels of relationship indicators at the start of the interval, that individual was more likely to subsequently change partners and acquire an STI.
Second, the findings demonstrate that an improved understanding of the “upstream” relationship dynamics preceding partner change allows us to know more about STI acquisition. Elaborating relationship dynamics improved our understanding of the association between partner change and STI. By controlling for infection at the start of the interval, we control for the relationship turmoil inevitably associated with an STI diagnosis. The lack of direct effects of relationship characteristics on STI acquisition suggests that alternative hypotheses relying on unmeasured variables (e.g., lower relationship quality as a marker for having baseline partners who are more likely to be infected or more likely to have other partners, bringing STI into an existing relationship) are not valid. The issue of reinfection is important, and is described for chlamydia in a separate subanalysis of the same dataset (Batteiger et al).17 Of 478 episodes of infection, 278 were repeated infections. Using ompA genotyping and behavioral data, 84% of those were categorized as definite, probably, or possible reinfections; 14% were probable or possible treatment failures, and 2% persisted with no documentation of treatment. In our data, rates of infection at the start and end of the interval are similar. Batteiger et al’s work suggests that these are most likely infections from untreated partners who may have other untreated partners.17
Our focus on the relationship dynamics preceding partner change calls into question the mechanism by which partner change influences STI. The conventional assumption is that the new partner represents a new exposure (and this assumption is supported in part by sexual network studies18). However, our findings that negative relationship dynamics (lower quality, shorter relationships, less closeness to one’s social network) precede the partner change and STI raise the possibility that at least part of the STI risk associated with partner change may come from a preceding partnership “going bad.” If the old relationship is the source of the STI, the young woman may be the person introducing it into the new partnership.
Third, important organism-specific differences are seen in our final model. Consistent with other data,19,20 lower age predicted Chlamydia acquisition, whereas older age predicted trichomonas acquisition. Baseline infection is highly predictive of subsequent infection for Chlamydia and trichomonas, and not predictive for gonorrhea. Of interest, when age, Time 1 STI, and partner change are entered into the model, condom unprotected events, a measure of STI exposure, drops out of the model for Chlamydia and Trichomonas, and is correlated only with gonorrhea at Time 2. These data are consistent with adult studies demonstrating an association between gonorrhea and number of condom unprotected sex acts in known exposures,21 and likely reflect different infectious properties of the organisms.
These analyses have limitations. First, participants are primarily black drawn from a single Midwestern city. However, the consistency of our findings with national data acts as a validity check on the sample. For example, the negative association between age and chlamydia, and the positive association with trichomonas are consistent with other published reports.20,22 Second, we did not have STI or interview data on all of the partners. This limits our ability to examine the source of the STI (Time 1 partner or Time 2 partner) or the behaviors (having side partners, etc.) of that partner. Finally, we constrained our model to serial monogamy, so that we could examine a single partner change. It is unclear how these findings would apply to multiple concurrent partners.
CONCLUSIONS
These findings have both research and clinical relevance. From a research perspective, more efforts need to be focused upstream, contextual,23 and relational antecedents to STI. From a clinical perspective, clinicians may be able to better target STI prevention efforts by inquiring about relationships and targeting those most likely to change.
References
- 1.Gavin L, MacKay AP, Brown K, et al. Sexual and reproductive health of persons aged 10–24 years—United States, 2002–2007. MMWR Surveill Summ. 2009;58:1–58. [PubMed] [Google Scholar]
- 2.Kelley SS, Borawski EA, Flocke SA, et al. The role of sequential and concurrent sexual relationships in the risk of sexually transmitted diseases among adolescents. J Adolesc Health. 2003;32:296–305. doi: 10.1016/s1054-139x(02)00710-3. [DOI] [PubMed] [Google Scholar]
- 3.Niccolai LM, Ethier KA, Kershaw TS, et al. New sex partner acquisition and sexually transmitted disease risk among adolescent females. J Adolesc Health. 2004;34:216–223. doi: 10.1016/S1054-139X(03)00250-7. [DOI] [PubMed] [Google Scholar]
- 4.Foxman B, Newman M, Percha B, et al. Measures of sexual partnerships: Lengths, gaps, overlaps, and sexually transmitted infection. Sex Transm Dis. 2006;33:209–214. doi: 10.1097/01.olq.0000191318.95873.8a. [DOI] [PubMed] [Google Scholar]
- 5.Sayegh MA, Fortenberry JD, Anderson J, et al. Relationship quality, coital frequency, and condom use as predictors of incident genital Chlamydia trachomatis infection among adolescent women. J Adolesc Health. 2005;37:163. doi: 10.1016/j.jadohealth.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Tschann JM, Adler NE, Millstein SG, et al. Relative power between sexual partners and condom use among adolescents. J Adolesc Health. 2002;31:17–25. doi: 10.1016/s1054-139x(01)00418-9. [DOI] [PubMed] [Google Scholar]
- 7.Manning WD, Flanigan CM, Giordano PC, et al. Relationship dynamics and consistency of condom use among adolescents. Perspect Sex Reprod Health. 2009;41:181–190. doi: 10.1363/4118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aalsma MC, Fortenberry JD, Sayegh MA, et al. Family and friend closeness to adolescent sexual partners in relationship to condom use. J Adolesc Health. 2006;38:173–178. doi: 10.1016/j.jadohealth.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Ellen JM, Gaydos C, Chung SE, et al. Sex partner selection, social networks, and repeat sexually transmitted infections in young men: A preliminary report. Sex Transm Dis. 2006;33:18–21. doi: 10.1097/01.olq.0000187213.07551.a6. [DOI] [PubMed] [Google Scholar]
- 10.Youm Y, Laumann EO. Social network effects on the transmission of sexually transmitted diseases. Sex Transm Dis. 2002;29:689–697. doi: 10.1097/00007435-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Fortenberry JD, Tu W, Harezlak J, et al. Condom use as a function of time in new and established adolescent sexual relationships. Am J Public Health. 2002;92:211–213. doi: 10.2105/ajph.92.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ku L, Sonenstein FL, Pleck JH. The dynamics of young men’s condom use during and across relationships. Fam Plann Perspect. 1994;26:246–251. [PubMed] [Google Scholar]
- 13.Sayegh MA, Fortenberry JD, Shew M, et al. The developmental association of relationship quality, hormonal contraceptive choice and condom non-use among adolescent women. J Adolesc Health. 2006;39:388–395. doi: 10.1016/j.jadohealth.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Gebhardt WA, Kuyper L, Greunsven G, et al. Need for intimacy in relationships and motives for sex as determinants of adolescent condom use. J Adolesc Health. 2003;33:154–164. doi: 10.1016/s1054-139x(03)00137-x. [DOI] [PubMed] [Google Scholar]
- 15.Katz BP, Fortenberry JD, Tu W, et al. Sexual behavior among adolescent women at high risk for sexually transmitted infections. Sex Transm Dis. 2001;28:247–251. doi: 10.1097/00007435-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Sexually Transmitted Disease Treatment Guidelines, 2006. Atlanta, GA: Department of Health and Human Services; 2006. [Google Scholar]
- 17.Batteiger BE, Tu W, Ofner S, et al. Repeated Chlamydia trachomatis genital infections in adolescent women. J Infect Dis. 2010;201:42–51. doi: 10.1086/648734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fichtenberg CM, Muth SQ, Brown B, et al. Sexual network structure among a household sample of urban African American adolescents in an endemic sexually transmitted infection setting. Sex Transm Dis. 2009;36:41–48. doi: 10.1097/OLQ.0b013e3181860711. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2008. Atlanta, GA: U.S. Department of Health and Human Services; 2009. [Google Scholar]
- 20.Sutton M, Sternberg M, Koumans EH, et al. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis. 2007;45:1319–1326. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 21.Warner L, Newman DR, Austin HD, et al. Condom effectiveness for reducing transmission of gonorrhea and Chlamydia: The importance of assessing partner infection status. Am J Epidemiol. 2004;159:242–251. doi: 10.1093/aje/kwh044. [DOI] [PubMed] [Google Scholar]
- 22.Burstein GR, Gaydos CA, Diener-West M, et al. Incident Chlamydia trachomatis infections among inner-city adolescent females. JAMA. 1998;280:521–526. doi: 10.1001/jama.280.6.521. [DOI] [PubMed] [Google Scholar]
- 23.Buffardi AL, Thomas KK, Holmes KK, et al. Moving upstream: Ecosocial and psychosocial correlates of sexually transmitted infections among young adults in the United States. Am J Public Health. 2008;98:1128–1136. doi: 10.2105/AJPH.2007.120451. [DOI] [PMC free article] [PubMed] [Google Scholar]