Abstract
Purpose
Necrotizing enterocolitis (NEC) remains a devastating disease in premature infants. We previously showed that four stem cell (SC) types equivalently improve experimental NEC. Exosomes are intercellular nanovesicles containing RNA, miRNA, DNA, and protein. Because SC therapy faces challenges, our aim was to determine if the beneficial effects of SC are achievable with cell-free exosomes.
Methods
Exosomes from four SC types were compared: (1) amniotic fluid-derived mesenchymal SC (AF-MSC); (2) bone marrow-derived MSC (BM-MSC); (3) amniotic fluid-derived neural SC (AF-NSC); and (4) neonatal enteric NSC (E-NSC). Rat pups exposed to NEC received a varying concentration of a single type of exosome with control pups receiving PBS only. Intestinal damage was graded histologically.
Results
The incidence of NEC was 0% in unstressed pups and 60.7% in control pups subjected to NEC. Exosomes (4.0×108) reduced NEC incidence to: AF-MSC 25.0%; BM-MSC 23.1%; AF-NSC 11.1%; E-NSC 27.3%. When administered at a concentration of at least 4.0×108, all groups demonstrated a significant reduction in NEC compared to untreated pups. At this minimum concentration, there was no difference in treatment efficacy between exosomes and the SC from which they were derived.
Conclusion
Stem cell-derived exosomes reduce the incidence and severity of experimental NEC as effectively as the stem cells from which they are derived, supporting the potential for novel cell-free exosome therapy for NEC.
Keywords: Stem cells, Exosomes, Necrotizing enterocolitis
Necrotizing Enterocolitis (NEC) has been the subject of a substantial amount of research, however little progress has been made in significantly improving patient outcomes, with overall mortality for infants requiring surgery remaining greater than 30%.[1] The cost of treating premature infants affected with NEC exceeds $1 billion annually in the United States alone. Morbidity and mortality remain unacceptably high and have been relatively unchanged in several decades.[2] We have previously shown that four types of stem cells all equivalently promote improved gut barrier function and reduce the incidence and severity of experimental NEC.[3,4]
Translation of stem cell research to the clinic is challenging, with many ethical, legal, and scientific ramifications.[5] Interest has emerged in the potential diagnostic and therapeutic use of exosomes, small extracellular vesicles approximately 100 nm in size.[6] Exosomes are exocytosed by cells and contain RNA, miRNA, DNA, and proteins that are not only reflective of intracellular activity in the cells from which they were derived, but also are able to affect changes in neighboring cells and remote cells throughout the body via hematogenous spread.[7] These nanovesicles have been shown to have therapeutic potential in a number of areas, and interest has become so strong that in 2012 the NIH established a Common Fund initiative specifically to fund research into exosome origins, distribution, and potential impact.[8–10] In the current study, we sought to determine if stem cell-derived exosomes have potential as a cell-free therapy in NEC, and if so, to determine if the therapeutic effects are dose-dependent.
1. Methods
1.1 Stem Cell Culture and Verification
Using previously described methods, the following stem cell lines were cultured from Lewis rats: amniotic fluid-derived mesenchymal stem cells (AF-MSC), bone marrow-derived MSC (BM-MSC), amniotic fluid-derived neural stem cells (AF-NSC), and neonatal enteric NSC (E-NSC).[3] These specific stem cell lines were chosen because we have previously demonstrated their individual efficacy in treating experimental NEC. [3, 4] In addition, we have demonstrated that different types of stem cells target different tissues. For example, NSC target the injured enteric nervous system in our experimental NEC model, and improve intestinal motility after NEC. Because of these different effects, we wanted to investigate whether exosomes derived from these different types of stem cells might function differently from one another in protection of the intestines from NEC.
All stem cell lines were confirmed by flow cytometry for specific cell surface markers consistent with the different stem cell lines. AF-MSC cells were positive for CD29 (ThermoFisher, Waltham, MA), CD49e (ThermoFisher), CD90 (ThermoFisher), and Oct4 (Novus Biologicals, Littleton, CO), which are markers of MSCs and cells derived from AF. These cells were also negative for the hematopoietic precursor cell markers CD11 (ThermoFisher) and CD45 (ThermoFisher), as expected. BM-MSCs were positive for CD90, confirming their identity as MSC, and negative for CD11 and CD45. Both NSC cell populations were confirmed to be positive for Nestin (R&D Systems, Minneapolis, MN), a transient intermediate filament protein only expressed in NSCs and not in mature neural tissue.
Additionally, both mesenchymal stem cell lines were confirmed to be multipotent by differentiation along adipocytic and osteocytic cell lines using commercially-available differentiation kits (StemPro Adipogenesis Differentiation Kit, and StemPro Osteogenesis Differentiation Kit, ThermoFisher).
1.2 Exosome Generation and Collection
Exosomes were generated and collected based upon published methods.[9,11] To generate exosomes from MSC populations, which grow in an adherent fashion in cell culture flasks, SC growth-supporting medium was replaced with media lacking fetal bovine serum (FBS). This exosome generation media was composed of Minimum Essential Medium Alpha with GlutaMAX™ (MEM-α, ThermoFisher) and 1% penicillin/streptomycin/amphotericin B (PSA, ThermoFisher).
Exosomes were generated from NSC populations by placement of SC into media lacking SC-supportive growth factors (EGF, FGF). Generation media was composed of Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12, ThermoFisher) supplemented with 4% chicken embryo extract (Gemini Bio-Products, West Sacramento, CA), 2% PSA, and 1X N-2 supplement (ThermoFisher). Cells in culture were centrifuged at 400 × g for 10 minutes, after which supernatants were removed and pellets resuspended in exosome generation medium.
All SC were then placed into exosome generation media for 48 hours. Supernatants were then collected and exosomes purified by differential ultra-centrifugation. Five rounds of centrifugation were conducted: (1) 300 × g for 10 minutes, with supernatants retained for subsequent stages; (2) 2000 × g for 10 minutes, with supernatants retained; (3) 10,000 × g for 30 minutes, with supernatants retained; (4) 100,000 × g for 70 minutes, with the pellet retained and resuspended in 1% phosphate-buffered saline (PBS, Corning, Manassas, VA); (5) 100,000 × g for 70 minutes, with the pellet retained and resuspended in 200 μl of PBS. These final 200 μL solutions were characterized using nanotracking analysis (NTA) on a NanoSight NS300 instrument (NanoSight, Malvern Instruments Ltd, Worcestershire, UK). Particle concentrations were determined over 2–5 runs and then averaged for each sample. The exosome solutions were then diluted to reach a concentration of 4×108 exosomes/50 μL. This concentration was the initial concentration used in all experiments for all SC-derived exosome groups.
1.3 Experimental Model of NEC
All animal experiments were ethically conducted under protocol #AR15-00012 approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children’s Hospital. The model is based upon the model originally devised by Barlow et al. as previously described.[3,4,12–14] Sprague-Dawley rat pups were delivered prematurely at estimated day of gestation 21 (E21) of an average 22-day gestation period. After delivery, pups were randomized to one of the following groups: (1) breastfed, unstressed (n = 10); (2) NEC + 50 μL 1% PBS (n = 28); (3) NEC + AF-MSC exosomes (n = 38); (4) NEC + BM-MSC exosomes (n = 28); (5) NEC AF-NSC exosomes (n = 9); (6) NEC + E-NSC exosomes (n = 20). Each pup received a single intraperitoneal (IP) injection of the respective exosome preparation in 50 μL in PBS, or the same volume of PBS alone in control pups, within one hour after delivery. Over the next 96 hours, pups in the NEC groups were subjected to a series of stresses to induce experimental NEC. These stresses included hypercaloric feeds every 4 hours, and exposure to hypoxia and hypothermia every 8 hours.
Hypercaloric feeds were delivered orogastrically and composed of Esbilac milk replacer (PetAg, Hampshire, IL) fortified with Similac 60/40 powder (Ross Pediatrics, Columbus, OH). This combination delivered 836.8 kJ/kg per day. Feeds were initiated at 0.1 mL of formula at each feed on day one and increased by 0.1 mL of formula per day to a total of 0.4 mL per feed on day 4. Additionally, on day one, the second feed included 2 mg/kg of lipopolysaccharide (LPS, Sigma-Aldrich, St. Louis, MO). To induce hypoxia, pups were placed in a sealed container for 90 seconds into which nitrogen gas was introduced to achieve an ambient oxygen concentration of < 1.5%. Induction of hypothermia was through placement of pups into 4°C for 10 minutes. Breastfed, unstressed control pups were placed with a surrogate dam immediately after C-section delivery and not subjected to experimental stress.
After 96 hours or upon development of clinical signs of NEC, pups were sacrificed and intestinal tissue from duodenum through colon collected and fixed in 10% formalin (Fisher Scientific, Pittsburgh, PA). Tissue samples collected for analysis were comprised of three sections from each of duodenum, jejunum, and ileum.
1.4 Histologic Grading
Intestinal tissue collected from pups was embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E). Samples were graded independently by two blinded reviewers to assess for mucosal damage using an established scale: 0, normal mucosa; 1, epithelial cell lifting; 2, necrotic damage up to the mid-villus level; 3, necrotic damage to the entire villus; 4, transmural necrosis of the entire mucosa [15]. Any score of grade 2 or higher was considered consistent with NEC.
1.5 Exosome Dose-Response Determination
To determine whether exosome therapy was dose-dependent, varying concentrations of exosomes derived from all four SC types were delivered in the experimental NEC model. We have previously shown a concentration of ~2.5 × 109 exosomes/50 μL to be as effective as the MSC from which they were derived when injected IP in a model of experimental NEC.[9] The initial starting concentration chosen for the current study was 4×108 exosomes/50 μL. This concentration of SC-derived exosomes then underwent further serial 1:5 dilutions in PBS to produce the following additional concentrations: 8×107 exosomes/50 μL; 1.6×107 exosomes/50 μL; 3.2×106 exosomes/50 μL, 6.4×105 exosomes/50 μL, 1.3×105 exosomes/50 μL. All SC-derived exosome concentrations were tested for efficacy in the experimental NEC model.
1.6 Statistical Analysis
To determine the statistical significance of differences in NEC incidence and severity between groups, chi-square or Fisher’s exact test was used where appropriate. Analysis of NEC incidence between breastfed unstressed pups and NEC + PBS pups used Welch’s t-test. All p values < 0.05 were considered significant.
2. Results
2.1 Exosome Characterization
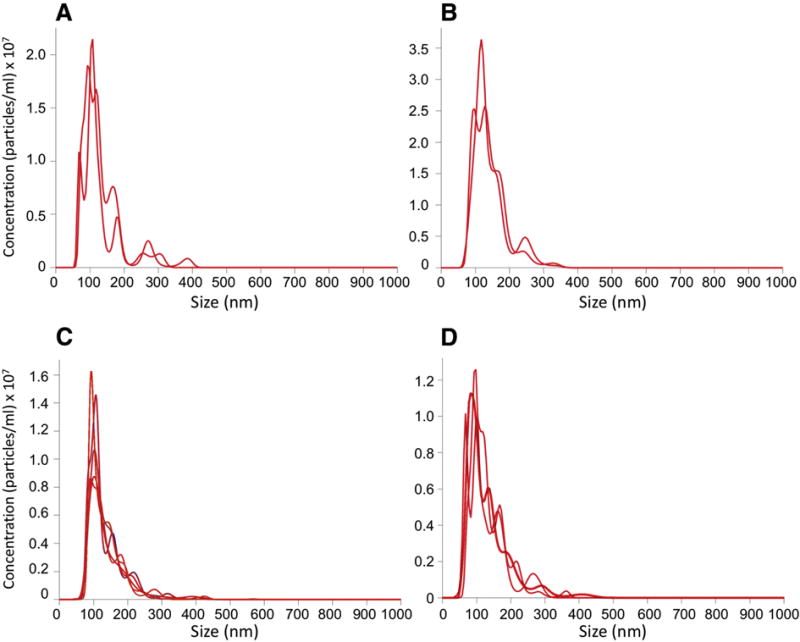
Differential ultracentrifugation led to samples that were highly enriched in nanoparticles (Figure 1). Purified particles from AF-MSC cells were enriched in the size range of 60–200 nm, with a mean diameter of ~120 nm, consistent with their identity as exosomes (Figure 2). Purified particles from BM-MSC cells were also enriched in the size range of 60–200 nm, with a mean diameter of ~120 nm. Purified particles from AF-NSC cells were enriched in the size range of 85–200 nm, with a mean diameter of ~135 nm. Purified particles from E-NSC cells were enriched in the size range of 70–250 nm, with a mean diameter of ~130 nm.
Figure 1. Representative NanoSight image of purified exosomes.
The exosomes shown were purified from AF-MSC. Similar results were seen with exosomes purified from the other three types of SC. Scale bar = 100 nm.
Figure 2. Representative plots of particle size in exosome suspensions.

The multiple lines represent multiple analytic runs of a single sample solution, which were then averaged. The exosomes analyzed were from: (A) AF-MSC; (B) BM-MSC; (C) AF-NSC; (D) E-NSC.
2.2 Histologic Analysis
Pups exposed to experimental NEC that were treated with exosomes had significantly less intestinal injury compared to pups that received PBS only (Figure 3).
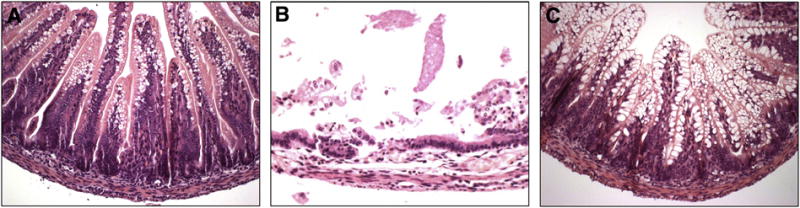
Figure 3. Representative images of histologic injury scores.
H&E stained images demonstrating: (A) uninjured breast fed control intestine (grade 0); (B) intestine from a pup exposed to experimental NEC with no therapy (grade 3 complete villus destruction); and (C) intestine from a pup exposed to experimental NEC that received 4.0×108 BM-MSC-derived exosomes (grade 0).
2.3 Dose-Response Results
In the current study, six different concentrations of exosomes were compared for each of the four types of SC-derived exosomes: 1.3×105 exosomes/50 μL, 6.4×105 exosomes/50 μL, 3.2×106 exosomes/50 μL, 1.6×107 exosomes/50 μL, 8.0×107 exosomes/50 μL, 4.0×108 exosomes/50 μL. Overall, as the concentration of exosomes administered increased, the incidence of NEC decreased, with the best results obtained at exosome concentrations of 8 × 107 or 4.0×108 exosomes/50 μL (Figure 4).
Figure 4. Exosome dose-response analysis.
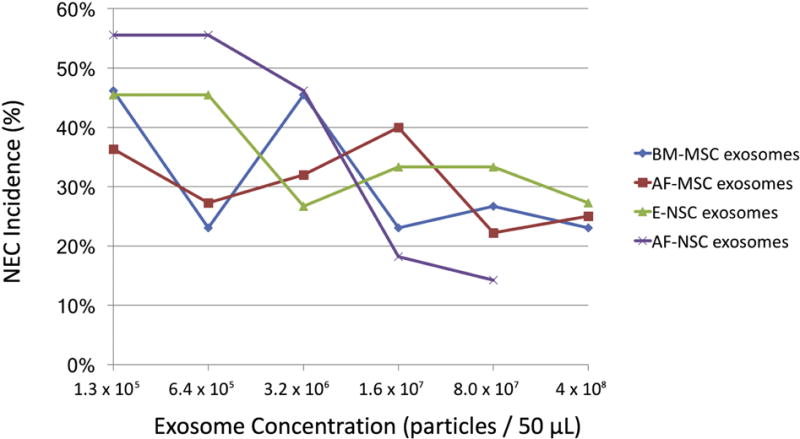
Pups received the indicated doses of SC-derived exosomes prior to exposure to experimental NEC.
2.4 Incidence and Severity Results
Breastfed control pups that were not exposed to experimental stress did not develop NEC (Figure 5). Pups that were exposed to experimental NEC that received PBS alone had an incidence of NEC of 60.7% (n = 28).
Figure 5. Incidence and severity of NEC.
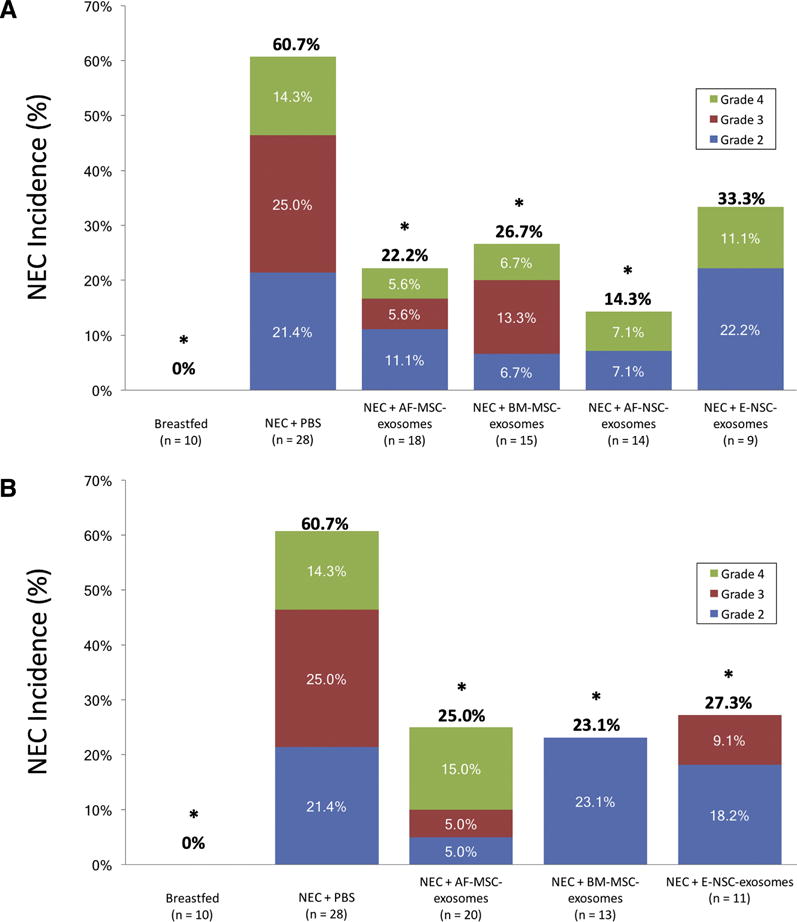
Bars represent the total incidence of NEC in each group, with the different colors depicting NEC severity. The numbers within the colored areas of the bars represent the incidence of the corresponding grade of NEC, and the numbers above the bars represent the total incidence of NEC. *p < 0.05 compared with control pups that received PBS only. (A) results for pups treated with 8.0×107 exosomes/50 μL; (B) results for pups treated with 4.0×108 exosomes/50 μL.
Pups exposed to experimental NEC that were treated with exosomes had significantly less intestinal injury compared to pups that received PBS only. Compared to pups that received PBS alone, treatment with 8.0×107 SC-derived exosomes/50 μL reduced the incidence of NEC as follows: AF-MSC-derived exosomes, 22.2% (n = 18, p = 0.005); BM-MSC-derived exosomes, 26.7% (n = 15, p = 0.012); AF-NSC-derived exosomes, 14.3% (n = 14, p = 0.002); E-NSC-derived exosomes, 33.3% (n = 9, p = 0.076). Increasing the exosome concentration to 4.0×108 SC-derived exosomes/50 μL further reduced the incidence of NEC for E-NSC-derived exosomes to 27.3%, which was significantly lower than the incidence of NEC for untreated pups (p = 0.03). There was no further significant reduction for other SC-derived exosomes. When comparing treatment with different concentrations of each of the different types of exosomes, there was a trend toward decreased severity of NEC as the concentration of exosomes increased, however these differences did not reach statistical significance for any of the groups.
In our previous studies, we showed that treatment with the same four types of SC from which the exosomes in the current study were derived resulted in comparable reductions in the incidence of experimental NEC.[3] In those experiments, treatment with SC reduced the incidence of NEC from 61% to: AF-MSC, 19.1% (n = 42, p < 0.0001); BM-MSC, 22.9% (n = 48, p < 0.0001); AF-NSC, 18.9% (n = 37, p < 0.0001); E-NSC, 22.2% (n = 36, p = 0.0002).[3] Thus, treatment with SC-derived exosomes is equivalent to treatment with the SC from which these exosomes are derived.
3. Discussion
Although significant advances have been made in neonatal care in recent decades, the morbidity and mortality of NEC have not improved concomitantly. We have demonstrated that multiple types of SC are able to reduce the incidence and severity of NEC [4], and others have shown similar findings in a model of intestinal ischemia/reperfusion injury [16]. Although SC engraftment with replacement of injured tissue was initially believed to be critical for SC efficacy, anti-inflammatory mechanisms may more likely account for the benefits of SC therapy.[17–19] Although the pathophysiology of NEC is not entirely clear, the heightened inflammatory response associated with the disease appears to be reduced with SC treatment, supporting a SC-associated anti-inflammatory process.[20,21]
While these studies are promising, SC treatment faces many challenges. There remain ethical concerns surrounding the use of SC in research, and in the translation of SC research to the bedside.[5,22] SC therapy also faces the daunting risk of potential immunogenicity and tumorigenicity.[23] There are potential links between genes activated in stem cells and in tumors that also remain unclear, with long-term risks of SC treatment unknown.[24,25] We have also shown that pulmonary sequestration of intravenously administered MSCs results in significant entrapment of SC in the lungs, leading to decreased MSC delivery to the target area of interest.[26]
We have shown in the current study that the benefits seen in treating experimental NEC with SC-derived exosomes can be equivalent to treatment with SC themselves. Extracellular vesicles such as exosomes are not subjected to the same immunogenic response as SC due to their size and mechanism of action. These bilipid-membrane-bound vesicles are able to fuse with recipient cell membranes and deposit their contents (proteins and nucleic acids) into the recipient cells.[27] They have been shown to play key roles not only in regulation of the immune system and its response to disease, but in many other important physiological processes. Treatment with exosomes derived from SC has been shown to be beneficial in rodent models of traumatic brain injury, where these vesicles are able to cross the blood-brain barrier (unlike the SC from which they are derived), in regeneration of cardiac muscle after scarring in animals and humans, and in improving kidney function in patients with stage III-IV chronic kidney disease.[10,28,29]
While the precise mechanisms by which exosomes deposit their contents into cells are unknown, previous rodent studies demonstrating their ability to cross the blood-brain barrier are particularly encouraging for NEC research.[10,30] A devastating complication of NEC is subsequent neurological dysfunction. Exosomes have the ability to exert their effects on neighboring cells in a paracrine fashion, and to also impact cells in remote locations throughout the body. Coupled with the observed benefits in studies on traumatic brain injury, it is exciting to consider future research into the potential long-term benefits of exosome therapy in NEC-induced brain injury.
Although the current study supports the efficacy of exosome therapy for NEC, it is unclear precisely which nucleic acids and proteins contained within the exosomes are providing the observed benefits. It is also currently unclear precisely what the half-life and activity of exosomes are in our in vivo model. Future research focused on exosome contents may help to elucidate which particular messengers are responsible for the observed benefits. Interestingly, our previous studies have shown that exosomes specifically target injured rather than non-injured intestine. In addition, exosomes can be used as delivery vehicles for intestinal cytoprotective agents such as growth factors. Current studies in our laboratory are focused on the use of exosomes as delivery vehicles to specifically target heparin-binding EGF-like growth factor (HB-EGF) to NEC-injured intestine. Although the current studies examine the ability of exosomes to prevent NEC, we also plan to investigate the ability of exosomes to protect the intestines from NEC once injury has already occurred.
Our current findings could lead to specific therapies that may more effectively treat NEC and be more readily translatable in the future. For example, amniotic fluid could be harvested at the time of amniocentesis or delivery, AF-derived stem cells harvested and expanded in vitro, and exosomes purified and frozen. If clinically indicated, these exosomes could be retrieved and administered, likely intravenously, as a prophylactic treatment to prevent NEC in the future.
4. Conclusions
The current study confirms that exosomes derived from four different types of SC (AF-MSC, BM-MSC, AF-NSC, E-NSC) not only significantly reduce the incidence of experimental NEC, but are as therapeutically effective as the SC from which they are derived. This supports the potential for development of cell-free therapies for NEC that may provide the benefits of SC therapy without the associated risks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thyoka M, de Coppi P, Eaton S, Khoo K, Hall N, Curry J, et al. Advanced Necrotizing Enterocolitis Part 1: Mortality. Eur J Pediatr Surg. 2012;22:008–12. doi: 10.1055/s-0032-1306263. [DOI] [PubMed] [Google Scholar]
- 2.Neu J, Walker WA. Necrotizing Enterocolitis. N Engl J Med. 2011;364:255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCulloh CJ, Olson JK, Zhou Y, Wang Y, Besner GE. Stem cells and necrotizing enterocolitis: A direct comparison of the efficacy of multiple types of stem cells. J Pediatr Surg. 2017;52:999–1005. doi: 10.1016/j.jpedsurg.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCulloh CJ, Olson JK, Wang Y, Vu J, Gartner S, Besner GE. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J Surg Res. 2017;214:278–85. doi: 10.1016/j.jss.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato K, Kimmelman J, Robert J, Sipp D, Sugarman J. Ethical and Policy Issues in the Clinical Translation of Stem Cells: Report of a Focus Session at the ISSCR Tenth Annual Meeting. Cell Stem Cell. 2012;11:765–7. doi: 10.1016/j.stem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Edgar JR, Harding C, Heuser J, Stahl P, Pan B, Teng K, et al. Q&A: What are exosomes, exactly? BMC Biol. 2016;14:46. doi: 10.1186/s12915-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lässer C. Exosomal RNA as biomarkers and the therapeutic potential of exosome vectors. Expert Opin Biol Ther. 2012;12(Suppl 1):S189–97. doi: 10.1517/14712598.2012.680018. [DOI] [PubMed] [Google Scholar]
- 8.United States Department of Health and Human Services. RFA-RM-12-012: Extracellular RNA Biogenesis, Biodistribution, Uptake, and Effector Function (U19) 2012 https://grants.nih.gov/grants/guide/rfa-files/RFA-RM-12-012.html (accessed July 30, 2017)
- 9.Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. 2016;51:942–7. doi: 10.1016/j.jpedsurg.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res. 2017;12:19. doi: 10.4103/1673-5374.198966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 12.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery. 1975;77:687–90. [PubMed] [Google Scholar]
- 13.Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, Schullinger JN. An experimental study of acute neonatal enterocolitis–the importance of breast milk. J Pediatr Surg. 1974;9:587–95. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Watkins D, Chen C-L, Bhushan B, Zhou Y, Besner GE. Heparin-Binding Epidermal Growth Factor-Like Growth Factor and Mesenchymal Stem Cells Act Synergistically to Prevent Experimental Necrotizing Enterocolitis. J Am Coll Surg. 2012;215:534–45. doi: 10.1016/j.jamcollsurg.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of Asphyxia and Feeding in a Neonatal Rat Model of Necrotizing Enterocolitis. Pediatr Pathol. 1994;14:1017–28. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 16.Jensen AR, Manning MM, Khaneki S, Drucker NA, Markel TA. Harvest tissue source does not alter the protective power of stromal cell therapy after intestinal ischemia and reperfusion injury. J Surg Res. 2016;204:361–70. doi: 10.1016/j.jss.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galindo LT, Filippo TRM, Semedo P, Ariza CB, Moreira CM, Camara NOS, et al. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol Res Int. 2011;2011:564089. doi: 10.1155/2011/564089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaco N, Diamandis Z, Borlongan C. Amniotic fluid-derived stem cells as an effective cell source for transplantation therapy in stroke. Brain Circ. 2015;1:119. doi: 10.4103/2394-8108.172881. [DOI] [Google Scholar]
- 19.Rowart P, Erpicum P, Detry O, Weekers L, Grégoire C, Lechanteur C, et al. Mesenchymal Stromal Cell Therapy in Ischemia/Reperfusion Injury. J Immunol Res. 2015;2015:1–8. doi: 10.1155/2015/602597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zani A, Cananzi M, Fascetti-Leon F, Lauriti G, Smith VV, Bollini S, et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut. 2014;63:300–9. doi: 10.1136/gutjnl-2012-303735. [DOI] [PubMed] [Google Scholar]
- 21.Sharma R, Tepas JJ, Hudak ML, Mollitt DL, Wludyka PS, Teng R-J, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42:454–61. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 22.Lo B, Parham L. Ethical Issues in Stem Cell Research. Endocr Rev. 2009;30:204–13. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Orkin SH. Embryonic stem cell-specific signatures in cancer: insights into genomic regulatory networks and implications for medicine. Genome Med. 2011;3:75. doi: 10.1186/gm291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karaczyn A, Bani-Yaghoub M, Tremblay R, Kubu C, Cowling R, Adams TL, et al. Two novel human NUMB isoforms provide a potential link between development and cancer. Neural Dev. 2010;5:31. doi: 10.1186/1749-8104-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Watkins D, Chen C-L, Zhang H-Y, Zhou Y, Velten M, et al. A Technique for Systemic Mesenchymal Stem Cell Transplantation in Newborn Rat Pups. J Investig Surg. 2012;25:405–14. doi: 10.3109/08941939.2012.661519. [DOI] [PubMed] [Google Scholar]
- 27.Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim AG-E, Cheng K, Marbán E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Reports. 2014;2:606–19. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derkus B, Emregul KC, Emregul E. A new approach in stem cell research-Exosomes: Their mechanism of action via cellular pathways. Cell Biol Int. 2017;41:466–75. doi: 10.1002/cbin.10742. [DOI] [PubMed] [Google Scholar]


