Abstract
Purpose
We evaluated whether brain development continues and brain injury is prevented during Artificial Placenta (AP) support utilizing extracorporeal life support (ECLS).
Methods
Lambs at EGA 118 days (term=145; n=4) were placed on AP support (venovenous ECLS with jugular drainage and umbilical vein reinfusion) for 7 days and sacrificed. Early (EGA 118; n=4) and late (EGA 127; n=4) mechanical ventilation (MV) lambs underwent conventional MV for up to 48 hours and were sacrificed, and early (n=5) and late (n=5) tissue control (TC) lambs were sacrificed at delivery. Brains were harvested, formalin-fixed, rehydrated, and studied by magnetic resonance imaging (MRI). The gyrification index (GI), a measure of cerebral folding complexity, was calculated for each brain. Diffusion-weighted imaging was used to determine fractional anisotropy (FA) and apparent diffusion coefficient (ADC) in multiple structures to assess white matter (WM) integrity.
Results
No intracranial hemorrhage was observed. GI was similar between AP and TC groups. ADC and FA did not differ between AP and late TC groups in any structure. Compared to late MV brains, AP brains demonstrated significantly higher ADC (0.45±0.08 vs. 0.27±0.11, p=0.02) and FA (0.61±0.04 vs. 0.44±0.05; p=0.006) in the cerebral peduncles.
Conclusions
After 7 days of AP support, WM integrity is preserved relative to mechanical ventilation.
Keywords: Artificial Placenta, Extracorporeal life support, White matter injury, Brain development, Prematurity
Introduction
Extremely low gestational age newborns (ELGANs), defined as neonates born at ≤28 weeks estimated gestational age (EGA), face unacceptably high morbidity and mortality, with survival well below 50% for infants born before 24 weeks.[1] Morbidities in these patients arise from the immaturity of multiple organ systems.[2–5] In particular, ELGANs suffer a disproportionate amount of neurologic disability, with nearly 40% of survivors being neurologically impaired.[6, 7] Both intracranial hemorrhage (ICH) and white-matter injury (WMI) have been recognized as major problem within this population. The pathophysiology of these injuries is multifactorial, and evidence suggests hypoxia-ischemia, inflammation, mechanical ventilation, fluid shifts, and hemodynamic instability all contribute.[8, 9]
A radical solution to minimize these factors is to recreate the intrauterine environment with an Artificial Placenta (AP). This consists of veno-venous extracorporeal life support (VV-ECLS) with jugular vein drainage and umbilical vein reinfusion, avoidance of mechanical ventilation, maintenance of fluid-filled lungs, and preservation of fetal circulation. We recently reported the ability of the AP to prevent lung injury and allow continued lung development in extremely premature lambs.[10] However, neurologic complications, in addition to being common in ELGANs, are also frequently encountered in patients supported by ECLS.[11] The effects of the AP on the premature brain are unknown.
With AP-supported patients having two major risk factors for neurologic injury, it is imperative to assess the effects of the AP on the premature brain prior to clinical translation. We therefore used MRI to assess cortical folding and gray- and white-matter integrity in extremely preterm lambs supported by the AP, and compared these findings to those in gestational age-matched controls, and to age-matched lambs that were mechanically ventilated. Despite the theoretic risk conferred by ECLS, we hypothesized the AP would not cause ICH or WMI, and would be superior to mechanical ventilation in preserving brain WM integrity.
Methods
The sheep in this experiment were treated in compliance with the Guide for Care and Use of Laboratory Animals (US National Institutes of Health publication No. 85-23, National Academy Press, Washington D.C., revised 1996) and all methods were approved by the University of Michigan Institutional Animal Care and Use Committee (protocol 00007211).
AP Support
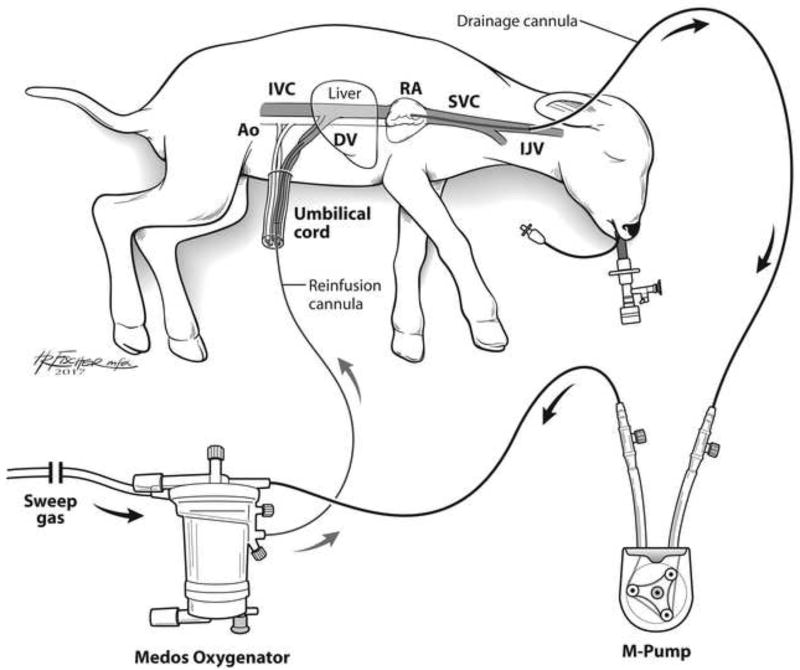
Premature lambs of EGA 118±3 days (n=4) were delivered via midline laparotomy and transverse hysterotomy. This gestational age was selected, as previous experimentation has determined fetal sheep lung development at this stage is analogous to that of a 24-week human fetus.[12] The right jugular vein was exposed and cannulated with a 10–14Fr drainage cannula (Terumo: Ann Arbor, MI). A 10–12Fr reinfusion cannula was placed in the umbilical vein, and the circuit was completed using ¼” tubing (Tygon: Lima, OH), a roller pump (MC3: Ann Arbor, MI), and oxygenator/heat exchanger (either Capiox Baby Rx, Terumo: Ann Arbor MI, or Medos HiLite, Xenios: Heilbronn, Germany; Figure 1). Venovenous (VV) ECLS was initiated. A 5Fr triple lumen venous line was placed in the second umbilical vein and used for IV fluid and medication administration, and a 5Fr umbilical arterial line (both lines from Covidien-Medtronic: Minneapolis, MN) was placed in the umbilical artery for hemodynamic monitoring and arterial blood gas (ABG) sampling.
Figure 1. Schematic of the Artificial Placenta.
Blood is drained from the right jugular vein by a collapsible-tubing roller pump (M-pump, MC3: Ann Arbor, MI) and propelled to an oxygenator/heat exchanger (Medos HiLite, Xenios: Heilbronn, Germany), then returned via an umbilical vein. The second umbilical vein is accessed for IV fluid and medication administration, and an umbilical arterial line is placed for hemodynamic monitoring and blood gas sampling. The lamb is intubated and the lungs remain filled with amniotic fluid by clamping the endotracheal tube. Ao – aorta; DV – Ductus venosus; IJV – internal jugular vein; IVC – inferior vena cava; RA – right atrium; SVC – superior vena cava
The lambs were then intubated, and the endotracheal tube filled with amniotic fluid and capped. ECLS was managed according to goal ABG parameters: pH 7.30–7.45, pCO2 35–50 mmHg, pO2 25–40 mmHg, and SpO2 65–80%. AP support was continued for 7 days. Lambs were given total parenteral nutrition (TPN), empiric piperacillin-tazobactam, and solumedrol (0.63mg/kg every 6 hours) at regular intervals. Heparin was infused IV and titrated to an ACT of 200–250.
Mechanical Ventilation (MV) Lambs
Early MV (delivered at 118±3 days EGA; n=4) or late MV (delivered at 128±2 days; n=4) lambs were delivered in the same method as AP lambs. They were immediately intubated, their lungs were suctioned of fluid, surfactant was administered, and pressure-controlled mechanical ventilation was initiated. Goal ABG values were pH 7.25–7.35, pCO2 40–60 mmHg, and SpO2 > 85. If peak airway pressures exceeded 25 cmH2O, permissive hypercapnia was practiced with administration of IV alkaline therapy. High frequency oscillatory ventilation was employed in the case of refractory hypoxia provided it did not worsen hypercarbia. Support was continued until lung failure, or until 48 hours, at which point the animal was sacrificed.
Tissue Control (TC) Lambs
Early TC (delivered at 118±3 days EGA; n=5) and late TC (delivered at 128±3 days; n=5) lambs were delivered in the same method as AP lambs and immediately sacrificed.
Brain Procurement and Preparation
After animal sacrifice, the neck was incised, jugular veins transected, and angiocatheters were placed in the bilateral carotid arteries. The carotid arteries were then each flushed with 60 mL phosphate-buffered saline (PBS) + ethylenediaminetetraacetic acid (EDTA) to clear the cerebral vasculature of blood, followed by 60 mL 10% formalin for fixation. Brains were then harvested intact via craniotomy and placed in 10% formalin.
Prior to imaging, the brains were removed from formalin and soaked in 0.05% sodium azide and 1 equivalent PBS at room temperature. This solution was changed daily for 3–4 weeks prior to MRI.[13, 14] For each MRI experiment, the brain was immersed in Fluorinert, a 1H proton-free, perflurocarbon that improves signal-to-noise ratio in explanted tissue MRI (FC-3283, 3M Company: Maplewood, MN).[15]
Magnetic Resonance Imaging
We used MRI to assess brain development and injury in our experimental lambs. MRI is a validated modality for detecting cerebral abnormalities, including WM injury, in preterm and stillborn neonates, and even in explanted formalin-fixed brains [16, 17], making it highly advantageous for our study.
MRI data were collected in an Agilent/Varian 4.7-T small-animal MR scanner equipped with a DirectDrive™ console (Santa Clara, CA), built around an Oxford Instruments (Oxford, United Kingdom) horizontal magnet with a 40-cm clear bore diameter. The system utilizes an actively shielded, 12-cm inner diameter Agilent/Magnex gradient-coil assembly driven by Oy International Electric Company (IEC; Helsinki, Finland) model A-240 amplifiers (300 V and 300 A), producing a maximum magnetic field gradient of 60 Gauss/cm per axis with a rise time of ~300 µs. MRI images were collected with a quadrature transmit/receive coil (outer/inner diameter, 108/63 mm).
Diffusion Tensor Imaging (DTI) measurements were made using a 2D, spin-echo, diffusion-tensor sequence, with [15]. T1-weighted images were collected using a magnetization-prepared rapid gradient echo (MPRAGE) 3D sequence. The T1-weighted MPRAGE had the same voxel plan as the DTI measurement (i.e., the data are co-registered), allowing for concomitant analysis of both imaging types.[14, 18, 19].
Measurements
The complexity of cerebral folding was assessed by calculating the gyrification index (GI). This has been supported as an excellent metric of cortical folding, and has been shown to be higher in term vs. preterm neonates.[17, 18] It is calculated as the ratio of the brain surface area divided by the surface area of the cerebral hull (the surface that would be measured if the brain were covered in “cling wrap” which would not penetrate the sulci). For a completely smooth brain, the GI is 1. As folding increases, the GI becomes larger. GI increases markedly during early brain development. Volumetric brain segmentations were manually generated using the ITK-software toolkit.[19] The segmentations were traced along the GM/CSF boundary (pial surface) and included deep gray matter/brain stem. The cerebellum was excluded and the inferior boundary of the brainstem was defined at the take-off of the cerebellum/4th ventricle. Caret software was used to generate pial and cerebral hull surfaces from this segmentation in each sheep brain’s native space.[20, 21]
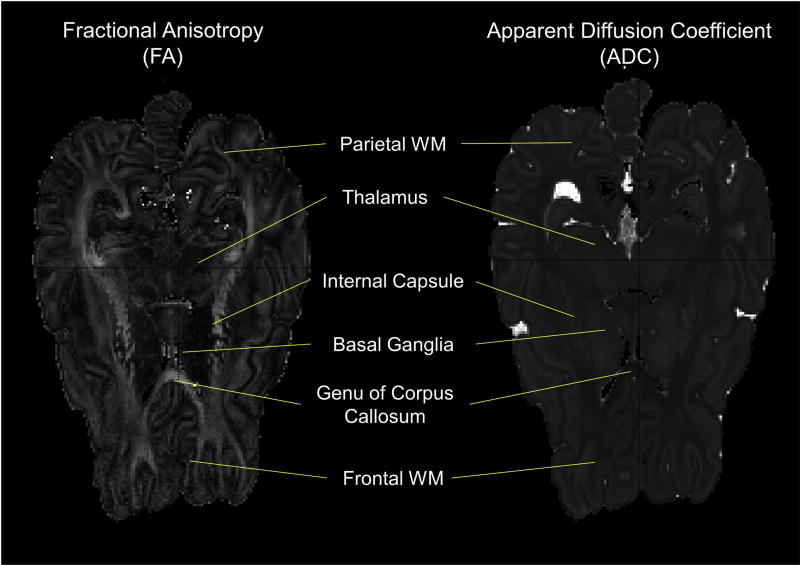
Diffusion-weighted imaging (DWI) was used to create parametric maps of apparent diffusion coefficients (ADC) and fractional anisotropy (FA; Figure 2) according to standard methods using Matlab-based software. DWI provides measures of tissue water displacements. The apparent diffusion coefficient (ADC) represents the overall magnitude of water displacements at each voxel in the brain. During development, ADC values fall as brain water content decreases. Fractional anisotropy (FA) is a measure of directional differences in water displacements. In highly ordered tissue, such as myelinated white matter, water displacements parallel to axons are greater than those perpendicular to them because of hindrance to motion caused by the need to cross myelin sheets and axons when moving perpendicular to axons. White matter FA values increase in association with normal development, and dramatically in association with myelination.
Figure 2. Example Fractional Anisotropy (FA) and Apparent Diffusion Coefficient (ADC) maps.
The FA and ADC maps shown represent the same axial slice from a Late Tissue Control (TC) lamb. ADC values were determined from regions of interest (ROI) from bilateral gray matter (GM) structures (basal ganglia and thalamus) and white matter (WM) structures (Frontal WM, Parietal WM, Internal Capsule, Genu of Corpus Callosum). FA values were determined from ROI from the aforementioned WM structures as well. Not pictured in these slices are the WM structures Splenium of the Corpus Callosum and the Cerebral Peduncles, from both of which ADC and FA values were determined as well.
ADC was calculated in two deep-gray-matter structures (bilateral thalamus and basal ganglia) and 12 white-matter structures (bilateral internal capsule, genu and splenium of corpus callosum, cerebral peduncles, subcortical frontal WM, and subcortical parietal WM bundles); FA was calculated only for WM structures. Regions of interest (ROI) of consistent size were selected within each of the aforementioned anatomic brain regions, and average ADC and FA within each ROI were measured using ITK-SNAP, a segmentation software application for 3D medical images (www.itksnap.org).[20] For each anatomic region, average ADC and FA values were calculated from corresponding values for the right and left hemispheres.
Statistical Analysis
Statistical analysis was performed with SPSS v.22.0 (IBM Corp: Armonk, NY). Values for each anatomic region, and mean values for the collective structures, were compared using ANOVA, as these values were continuous and comparisons were made between multiple groups. A p-value < 0.05 was considered statistically significant. In cases of statistical significance, differences between specific groups were pinpointed using Hochberg’s (if homogeneity of variance using Levene’s test) or Games-Howell (if no homogeneity of variance using Levene’s test).
Results
Support and Survival
AP lambs were supported for 163±11 hours, compared to 7.3±1.0 hours for early MV and 40±18 hours for late MV lambs (p<0.001). AP lambs demonstrated normal hemodynamics, on average, during support (heart rate 195±23 bpm, mean arterial pressure 47±7mmHg). Early MV lambs were profoundly acidotic (mean pH 6.97±0.02) and hypercarbic (mean pCO2 154±35mmHg), whereas these values were normal during AP support (pH 7.36±0.04, pCO2 43±2mmHg), and nearly normal in late MV lambs (pH 7.25±0.20, pCO2 51±24mmHg). Umbilical arterial O2 saturation was similar in all 3 groups (81±8 vs. 82±15 vs. 89±17mmHg; p=0.70).
Cerebral Folding
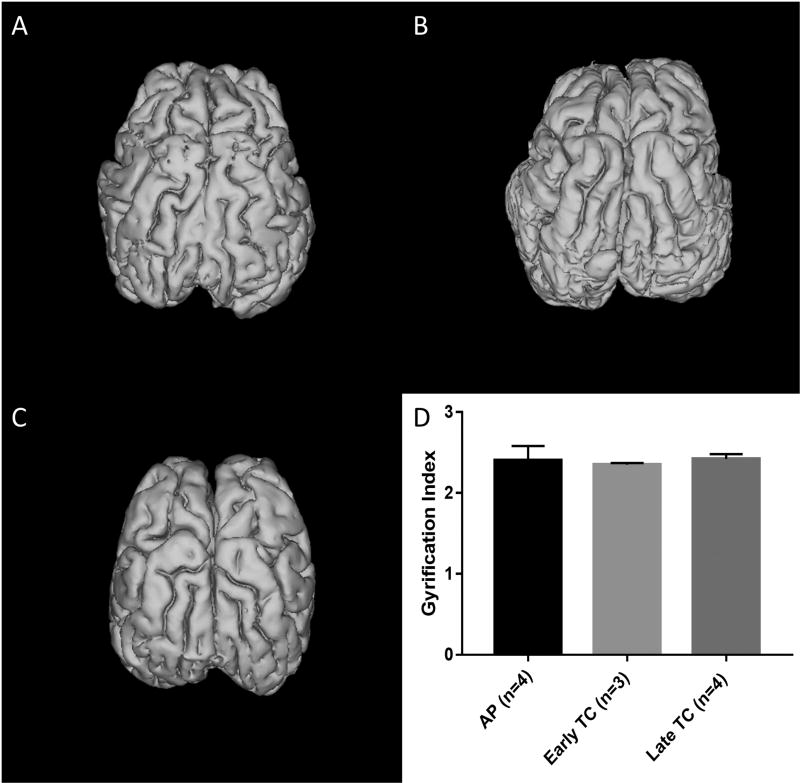
Gyrification index was calculated for AP and early and late TC brains. Two early TC and one late TC brains were excluded due to minor cortical damage resulting from the cranial extraction. No significant differences were seen in GI between AP, early TC, and late TC brains (2.40±0.18 vs. 2.35±0.02 vs. 2.42±0.06; p=0.77). Representative 3D reconstructions of brains from each of the three groups are displayed in Figure 3.
Figure 3. Cerebral Folding and Gyrification Index (GI) Determination.
3D images of (A) AP, (B) Early TC, and (C) Late TC brains are pictured. Note the folding patterns look nearly identical in all three groups. (D) This was reflected in near equal gyrification indices (mean±SD).
Gray and White Matter Integrity
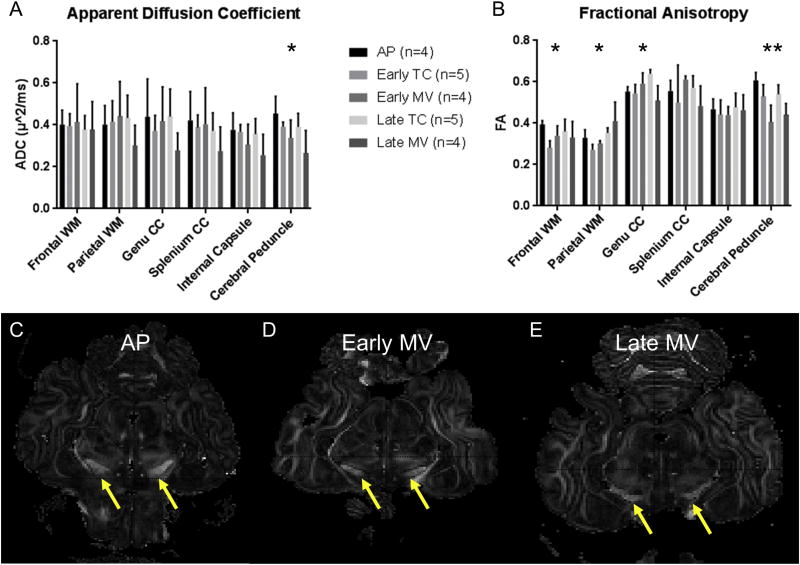
ADC and FA values for each WM anatomic region are displayed in Figure 4. ADC differed significantly between groups in the cerebral peduncles, specifically between the AP and late MV brains (0.45±0.08 vs. 0.27±0.11, p=0.02). ADC did not differ between groups in any other WM region (Figure 4A), and did not differ between groups in the GM regions of the basal ganglia (p=0.33) or thalami (p=0.48).
Figure 4. Comparisons of Apparent Diffusion Coefficient (ADC) and Fractional Anisotropy (FA) by Anatomic Region.
Mean (A) Apparent Diffusion Coefficient (ADC; µm2/ms) and (B) Fractional Anisotropy (FA) Values are displayed by Anatomic Region. *p<0.05 (ANOVA, with Hochberg or Games-Howell used for post-hoc analysis depending on homogeneity of variance). Representative axials slices of FA maps are shown at the level of the midbrain, with cerebral peduncles indicated by the yellow arrows. The AP brain (C) demonstrates greater enhancement of the cerebral peduncles than both Early (D) and Late (E) MV brains, indicating higher FA values.
FA differed between groups in several anatomic regions, including the frontal (p=0.047) and parietal (p=0.004) WM, the genu of the corpus callosum (p=0.005), and cerebral peduncles (p<0.001; Figure 4B). In frontal WM, AP brains displayed higher FA than early TC brains (0.39±0.02 vs. 0.28±0.03; p=0.003), and similar FA to late TC brains (0.36±0.06). Late TC brains demonstrated higher FA values than early TC brains in the parietal WM (0.35±0.04 vs. 0.27±0.03; p=0.005) and genu of the corpus callosum (0.64±0.02 vs. 0.54±0.04; p=0.04). In the cerebral peduncles, AP brains demonstrated higher FA values than early MV brains (0.61±0.04 vs. 0.41±0.08; p=0.001) and late MV brains (0.44±0.05; p=0.006), which is evident in the axial images in Figure 4C–E. The FA of early MV brains was also lower than that of early TC brains (0.53±0.06; p=0.04) in the cerebral peduncles. FA did not differ between AP and late TC brains in any anatomic region.
Discussion
Neurologic injury is very common in extremely premature neonates, and results in long-term disability in survivors.[6, 7] It is likely that mechanical ventilation with increased intrathoracic pressures, as well as the hemodynamic instability and hypoxemia that frequently are seen with the attempted transition from fetal to neonatal circulation, contributes to this pathophysiology.[8, 9] An AP would avoid potentially injurious mechanical ventilation, preserve fetal circulation, and maintain hemodynamic stability, but its effects on the preterm brain were unknown. We therefore evaluated the brains of extremely premature lambs undergoing prolonged AP support. In our study, we found no ICH in brains of any experimental group. We also observed no WMI in AP-supported lambs, as ADC and FA did not differ between AP or late TC lambs in any WM region. We remind the reader that white matter FA values increase during maturation. AP lambs did appear to manifest ongoing frontal WM myelination during support, as FA values were higher in these lambs than early TC lambs. Furthermore, WMI in the cerebral peduncles appeared to result from mechanical ventilation, with early MV brains manifesting significantly lower FA than age-matched tissue controls. This injury was not seen during AP support. With respect to cerebral folding, no differences were observed between AP brains and either TC group, with gyrification index nearly identical between AP and late TC lambs. This suggests that cortical folding during AP support is similar to that which occurs in utero, again indicating that AP support allows for continued normal brain development.
While white matter myelination, as reflected by FA, increases with increasing gestational age [21], FA has been shown to decrease in major WM tracts with as few as 2 days of mechanical ventilation in preterm neonates, and has also been shown to be reduced in infants with chronic lung disease.[22] In our lambs, FA was most reduced in the cerebral peduncles of mechanically ventilated lambs compared to age-matched controls or AP-supported lambs. The cerebral peduncles represent part of the corticospinal tract, consisting of upper motor neurons. Injury to these tracts in preterm human infants predicts neuromotor developmental abnormalities.[23] Therefore, the fact that FA was significantly higher following AP support suggests that these complications could potentially be avoided with use of the artificial placenta as opposed to mechanical ventilation.
Cerebral perfusion has been investigated in ECMO using the jugular vein for cannulation. Though jugular ligation appears to decrease cerebral blood flow (CBF) in VA-ECMO with carotid reinfusion, these changes are either temporary or not observed with jugular cannulation for VV-ECMO, despite the theoretic risk of venous hypertension.[24–26] Furthermore, we have previously studied cerebral perfusion and oxygen delivery in lambs supported with the artificial placenta, and similarly observed normal CBF and oxygenation.[27] This preserved perfusion and oxygenation is likely what preserves white-matter integrity in premature lambs supported by the AP. WM injury is a known consequence of cerebral hypoxia and hypoperfusion, also demonstrated in fetal lamb models.[28, 29] Despite our best efforts, early MV controls, which had the lowest FA in the cerebral peduncles, all eventually became profoundly hypoxic during their course of support. These lambs also required high-pressure ventilation, which increases intrathoracic pressure and impedes venous drainage from the brain. This predisposes the premature lamb, or human neonate, to venous hypertension and ICH.[30] The avoidance of such elevated intrathoracic pressure represents another advantage of AP support.
Our ovine animal model is of particular importance in this study. The gestational age of the model was selected primarily for lung development, as the lungs of a 118-day fetal sheep are developmentally analogous to a 24-week human fetus.[12] However, brain development occurs more rapidly in sheep. The ovine germinal matrix at midgestation (~70 days) already resembles of that of a 26–30 week human fetus, and has completely disappeared by term.[31] This makes premature sheep brains less prone to ICH compared to human brains, an observation that has been observed in study of hypoxia, hypo- and hypervolemia in fetal sheep.[32] However, oligodendrocyte maturation is delayed relative to the germinal matrix, and the ovine brain is indeed susceptible to WMI at the gestational ages used in our study, particularly in response to hypoxic/ischemic injury.[33] Therefore, the absence of WM injury in our AP-supported sheep is clinically relevant.
There are several limitations of our study. Sample sizes were limited by the cost and time investment required for each experiment. However, this implies that statistical differences arose from the magnitude of these differences rather than the power behind them, which we believe makes these differences more clinically relevant and translatable. We did not obtain cerebral perfusion/oxygenation data. While we have examined this previously, we would have preferred to correlate perfusion with WM integrity and brain volume. However, nearly all the sheep in our study had hyperpigmented scalps, which precluded consistent near-infrared spectroscopy, and we did not want to confound our results with a carotid flow probe. In addition, we did not corroborate our MRI findings with histopathology. However, as described previously, MRI’s ability to detect WMI, even in ex vivo brains, is well-validated.[16, 17] Histologic correlation will be a future direction of this study as we attempt to better define the effect of the AP on fetal brain development within various anatomic regions. Finally, AP support in this study still required systemic anticoagulation with heparin. Although we did not observe ICH, heparin use in an extremely premature human neonate would presumably increase the risk of clinically-significant cerebral hemorrhage. We are therefore investigating the use of non-thrombogenic coated circuits in our lab that would obviate the need for systemic anticoagulation, thereby reducing this risk.[34]
Conclusion
During support with an Artificial Placenta, brain development appears to continue normally, as it would in utero. Furthermore, the Artificial Placenta avoids white-matter injury that is seen with mechanical ventilation in extremely premature lambs, especially in the cerebral peduncles. These findings support the use of an Artificial Placenta as a paradigm shift in the care of extremely premature neonates.
Acknowledgments
The Authors would like to thank Cindy Cooke for her editorial assistance.
Statement of Financial Support
The research presented in this manuscript was supported by the National Institutes of Health NIH grants R01 HD073475 and P30 HD062171.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (London, England) 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. Jama. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughon M, O'Shea MT, Allred EN, Bose C, Kuban K, Van Marter LJ, et al. Chronic lung disease and developmental delay at 2 years of age in children born before 28 weeks' gestation. Pediatrics. 2009;124:637–648. doi: 10.1542/peds.2008-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manktelow BN, Seaton SE, Field DJ, Draper ES. Population-based estimates of in-unit survival for very preterm infants. Pediatrics. 2013;131:e425–432. doi: 10.1542/peds.2012-2189. [DOI] [PubMed] [Google Scholar]
- 6.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. The New England journal of medicine. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 7.Msall ME. Developmental vulnerability and resilience in extremely preterm infants. Jama. 2004;292:2399–2401. doi: 10.1001/jama.292.19.2399. [DOI] [PubMed] [Google Scholar]
- 8.Barton SK, Tolcos M, Miller SL, Christoph-Roehr C, Schmolzer GM, Moss TJ, et al. Ventilation-Induced Brain Injury in Preterm Neonates: A Review of Potential Therapies. Neonatology. 2016;110:155–162. doi: 10.1159/000444918. [DOI] [PubMed] [Google Scholar]
- 9.Volpe JJ, editor. Volpe's Neurology of the Newborn. Philadelphia, PA: Saunders; 2008. Hypoxic-Ischemic Encephalopathy: Clinical Aspects. [Google Scholar]
- 10.Church JT, Coughlin MA, Perkins EM, Hoffman HR, Bartlett RH, Mychaliska GB. The Artificial Placenta: Does Lung Development Continue during Extracorporeal Support?; American Academy of Pediatrics Meeting; San Francisco, CA.. 2016. [Google Scholar]
- 11.Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO journal (American Society for Artificial Internal Organs : 1992) 2017;63:60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 12.Bryner B, Gray B, Perkins E, Davis R, Hoffman H, Barks J, et al. An extracorporeal artificial placenta supports extremely premature lambs for 1 week. Journal of pediatric surgery. 2015;50:44–49. doi: 10.1016/j.jpedsurg.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepherd TM, Thelwall PE, Stanisz GJ, Blackband SJ. Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magnetic resonance in medicine. 2009;62:26–34. doi: 10.1002/mrm.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yann Leprince BS, Élodie Chaillou, Christophe Destrieux, Laurent Barantin, Alexandre Vignaud, Denis Rivière, Cyril Poupon. Optimization of sample preparation for MRI of formaldehyde-fixed brains; 23rd Annual Meeting of ISMRM; May 2015; Toronto, Canada. 2015. [Google Scholar]
- 15.D'Arceuil HE, Westmoreland S, de Crespigny AJ. An approach to high resolution diffusion tensor imaging in fixed primate brain. NeuroImage. 2007;35:553–565. doi: 10.1016/j.neuroimage.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Griffith JL, Shimony JS, Cousins SA, Rees SE, McCurnin DC, Inder TE, et al. MR imaging correlates of white-matter pathology in a preterm baboon model. Pediatr Res. 2012;71:185–191. doi: 10.1038/pr.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths PD, Variend D, Evans M, Jones A, Wilkinson ID, Paley MN, et al. Postmortem MR imaging of the fetal and stillborn central nervous system. AJNR American journal of neuroradiology. 2003;24:22–27. [PMC free article] [PubMed] [Google Scholar]
- 18.Brant-Zawadzki M, Gillan GD, Nitz WR. MP RAGE: a three-dimensional, T1-weighted, gradient-echo sequence--initial experience in the brain. Radiology. 1992;182:769–775. doi: 10.1148/radiology.182.3.1535892. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, He L, Zheng H, Lu ZL. Optimizing the magnetization-prepared rapid gradient-echo (MP-RAGE) sequence. PloS one. 2014;9:e96899. doi: 10.1371/journal.pone.0096899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Rogers CE, Smyser T, Smyser CD, Shimony J, Inder TE, Neil JJ. Regional white matter development in very preterm infants: perinatal predictors and early developmental outcomes. Pediatric research. 2016;79:87–95. doi: 10.1038/pr.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anjari M, Counsell SJ, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, et al. The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics. 2009;124:268–276. doi: 10.1542/peds.2008-1294. [DOI] [PubMed] [Google Scholar]
- 23.de Kieviet JF, Pouwels PJ, Lafeber HN, Vermeulen RJ, van Elburg RM, Oosterlaan J. A crucial role of altered fractional anisotropy in motor problems of very preterm children. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2014;18:126–133. doi: 10.1016/j.ejpn.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda S, Aoyama M, Yamada Y, Saitoh N, Honjoh T, Hasegawa T, et al. Comparison of venoarterial versus venovenous access in the cerebral circulation of newborns undergoing extracorporeal membrane oxygenation. Pediatric surgery international. 1999;15:78–84. doi: 10.1007/s003830050521. [DOI] [PubMed] [Google Scholar]
- 25.Weber TR, Kountzman B. The effects of venous occlusion on cerebral blood flow characteristics during ECMO. Journal of pediatric surgery. 1996;31:1124–1127. doi: 10.1016/s0022-3468(96)90100-1. [DOI] [PubMed] [Google Scholar]
- 26.Hunter CJ, Blood AB, Bishai JM, Hickerson AD, Wall DD, Peverini RL, et al. Cerebral blood flow and oxygenation during venoarterial and venovenous extracorporeal membrane oxygenation in the newborn lamb. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2004;5:475–481. doi: 10.1097/01.pcc.0000130992.73123.bc. [DOI] [PubMed] [Google Scholar]
- 27.El-Sabbagh AM, Gray BW, Shaffer AW, Bryner BS, Church JT, McLeod JS, et al. Cerebral Oxygenation of Premature Lambs Supported by an Artificial Placenta. ASAIO journal (American Society for Artificial Internal Organs : 1992) 2017 doi: 10.1097/MAT.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy K, Mallard C, Guan J, Marks K, Bennet L, Gunning M, et al. Maturational change in the cortical response to hypoperfusion injury in the fetal sheep. Pediatric research. 1998;43:674–682. doi: 10.1203/00006450-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Riddle A, Luo NL, Manese M, Beardsley DJ, Green L, Rorvik DA, et al. Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3045–3055. doi: 10.1523/JNEUROSCI.5200-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aly H, Hammad TA, Essers J, Wung JT. Is mechanical ventilation associated with intraventricular hemorrhage in preterm infants? Brain & development. 2012;34:201–205. doi: 10.1016/j.braindev.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Balasubramaniam J, Del Bigio MR. Animal models of germinal matrix hemorrhage. Journal of child neurology. 2006;21:365–371. doi: 10.1177/08830738060210050201. [DOI] [PubMed] [Google Scholar]
- 32.Ting P, Yamaguchi S, Bacher JD, Killens RH, Myers RE. Failure to produce germinal matrix or intraventricular hemorrhage by hypoxia, hypo-, or hypervolemia. Experimental neurology. 1984;83:449–460. doi: 10.1016/0014-4886(84)90114-6. [DOI] [PubMed] [Google Scholar]
- 33.Back SA, Riddle A, Dean J, Hohimer AR. The instrumented fetal sheep as a model of cerebral white matter injury in the premature infant. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9:359–370. doi: 10.1007/s13311-012-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annich GM, Meinhardt JP, Mowery KA, Ashton BA, Merz SI, Hirschl RB, et al. Reduced platelet activation and thrombosis in extracorporeal circuits coated with nitric oxide release polymers. Crit Care Med. 2000;28:915–920. doi: 10.1097/00003246-200004000-00001. [DOI] [PubMed] [Google Scholar]