Abstract
Background
Necrotizing enterocolitis (NEC) continues to be a devastating condition among preterm infants. Nitric oxide, which is synthesized in the intestine by endothelial nitric oxide synthase (eNOS), acts as a potent vasodilator and antioxidant within the mesentery and may play a role in prevention of NEC. We hypothesized that loss of endothelial nitric oxide would worsen both intestinal and associated lung injury and increase local and systemic inflammation during experimental NEC.
Methods
NEC was induced in five-day-old wild type (WT) and eNOS-knockout (eNOSKO) mouse pups. Experimental groups (n=10) were formula fed and subjected to intermittent hypoxic and hypothermic stress, while control groups (n=10) remained with their mother to breastfeed. Pups were monitored by daily clinical assessment. After sacrifice on day nine, intestine and lung were assessed for injury, and cytokines were measured in tissue homogenates by ELISA. Data was compared with Mann-Whitney, and p<0.05 was significant.
Results
Each NEC group was compared to its respective strain’s breastfed control to facilitate comparisons between the groups. Both NEC groups were significantly sicker than their breastfed controls. eNOSKO NEC animals had a median clinical assessment score of 3 (IQR=1–5), and the WT NEC animal’s median score was 3 (IQR=2–5). Despite similar clinical scores, intestinal injury was significantly worse in the eNOSKO NEC groups compared to WT NEC groups (median injury scores of 3.25 (IQR=2.25–3.625) and 2 (IQR=1–3), respectively (p=0.0474). Associated lung injury was significantly worse in the eNOSKO NEC group as compared to the WT NEC group (median scores of 8.5 (IQR=6.75–11.25) and 6.5 (IQR=5–7.5), respectively, p=0.0391). Interestingly, cytokines in both tissues were very different between the two groups, with varying effects noted for each cytokine (IL-6, IL-1β, VEGF, and IL-12) in both tissues.
Conclusion
Nitric oxide from eNOS plays a key role in preventing the development of NEC. Without eNOS function, both intestinal and lung injury are more severe, and the inflammatory cascade is significantly altered. Further studies are needed to determine how eNOS-derived nitric oxide facilitates these beneficial effects.
Keywords: Necrotizing enterocolitis, Animal model, Nitric oxide, Neonatal, Intestine, Ischemia
INTRODUCTION
Necrotizing enterocolitis (NEC) is one of the most common and morbid conditions in the premature neonatal population [1]. It affects about 7% of preterm infants, and unfortunately has proven difficult to eradicate over the years. Mortality continues to range from 20 to 30%, and is highest in patients with surgical disease [2]. Extensive surgical resection remains the mainstay of therapy for advanced disease, and often leads to long term morbidity secondary to short bowel syndrome [3]. New therapies for this disease are needed, and further understanding of the mechanism of disease is integral for further research in this direction.
Unfortunately, the sequelae of NEC are not limited to the intestine. Associated lung injury is a well-described phenomenon in NEC patients and animal models [4]. One of the highest risk factors for development of chronic lung disease in premature infants is the presence of necrotizing enterocolitis [5]. Additionally, lung disease in patients with NEC is commonly more severe than in matched patients without NEC [6].
The development of NEC is multifactorial, but seems to be directly related to intermittent insults secondary to hypoxia, hypothermia and enteral feeding in an already susceptible intestine [7]. The pathogenesis is complex and not completely understood. While intestinal mucosal epithelial injury is a defining characteristic of NEC, microvascular injury, and specifically, endothelial alterations, are thought to play a role as well [8].
Endothelial nitric oxide synthase (eNOS) is a constitutively-expressed enzyme that produces nitric oxide [9]. NO is a gaseous signaling molecule endogenously produced in the endothelium of all tissues by eNOS and has been identified as the most important vasodilator in the perinatal period in animal models [10]. Mice with homozygous knockout of eNOS (eNOSKO) were found to have worse experimental NEC pathology in the intestine [9, 11]. Another study demonstrated that L-arginine, an eNOS substrate, reduced injury, while nitric oxide inhibitors such as N-nitroarginine methyl ester (L-NAME) worsened injury in a neonatal piglet model of NEC [12]. The role of eNOS has not yet been thoroughly evaluated in the lungs of animals with experimental NEC.
In addition to direct impact on the affected tissues, as a free radical, NO is intimately involved in the inflammatory signaling seen in necrotizing enterocolitis [13]. Specifically, certain cytokines are known to have a close relationship with endothelial NO as a mediator in the inflammatory cascade. For example, interleukin 6 (IL-6) has been shown to be modulated by NO in some tissues [14]. Vascular endothelial growth factor (VEGF) is an endothelial specific marker that is typically elevated during endothelial cell injury or hypoxia, but is dependent on nitric oxide for release [15]. Interleukin 1β (IL-1β) is also typically released by the endothelium in response to stress [16]. Finally interleukin 12 (IL-12) has been shown in vitro to be upregulated in the endothelium by inhibition of eNOS [17]. These are just a few of the cytokines involved in the complex systemic response to inflammation, of which endothelial NO is an integral mediator.
Observations in animal studies suggest that eNOS has a critical role in the development of NEC intestinal injury. We therefore hypothesized that eNOS would not only be a key component in limiting intestinal injury, but would also play a key role in limiting lung injury and altering the systemic inflammatory response during experimental NEC.
MATERIALS AND METHODS
Experimental NEC Model
Indiana University Institutional Animal Care and Use Committee approved the experimental protocol and animal use. Wild type (WT) mouse pups (C57BL/6J, bred in house from adult mice, Stock No: 00664, Jackson Laboratory, Bar Harbor, ME) and eNOSKO mice (B6.129P2-Nos3tm1Unc/J, bred in house from adult mice, Stock No: 002684, Jackson Laboratory, Bar Harbor, ME) were permanently separated from their mother on postnatal day (P) five. Pups were housed in a neonatal incubator with humidity 40% and temperature 32°C from P5 to P9. All groups had 10 pups per group. The control groups of both strains remained with their mother and breastfeed ad libitum. Experimental groups were gavage fed hyperosmolar formula three times daily with a 2-French silicone catheter. Formula was prepared using 4g of Esbilac canine supplement and 6g Similac in 20 mL of nanopure filtered water (Barnstead Nanopure, APS Water Services Inc., Van Nuys, CA). Formula was newly prepared every 48 hours. Animals were fed 300 kcal/kg/day and all feeds were supplemented with 8 mg/kg/day lipopolysaccharide (LPS, lipopolysaccharides from Escherichia coli O111:B4, Sigma-Aldrich Company LLC, Dorset, UK).
Before each feed, pups were stressed in a chamber with 5% O2 and 95% N2 for 10 minutes. Twice daily, after the morning and evening feeds, pups were placed in the 4°C refrigerator for 10 minutes. Because of the high rate of very low birth weight in the knock out strain specifically, litters with pups less than 1.5 grams in weight were excluded due to the technical difficulties of gavage feeding animals this small. Mice who died less than 24 hours into the protocol were excluded, as their death was more likely due to other causes than NEC. Any animal whose tissue was liquefied or unusable for histologic evaluation (i.e. died overnight and discovered hours later) was excluded completely from evaluation. Other animals that died during the study but were identified immediately were still included in analysis for all data points. This included 1 in the eNOSKO NEC group and 2 in the WT NEC group.
Clinical Assessment
While control groups were weighed and assessed daily to minimize maternal separation, experimental groups were weighed daily but re-assessed with each feed. Assessment proceeded in a systematic fashion to ensure consistency. The reported score is the pup’s last score prior to death or euthanasia. Clinical sickness score was slightly modified from Zani et. al. [18] to include specific criteria for scoring as previously reported.
Intestinal Histologic Evaluation
On P9, pups were sacrificed by decapitation and the terminal ileum and distal jejunum of each animal was then formalin fixed, paraffin embedded, and stained with hematoxylin and eosin. Two blinded authors evaluated degree of injury, and scores were averaged for each specimen. The scoring system used was published by Zani et. al. [18] with scores ranging from 0 to 4. 0 = normal intestine; 1 = disarrangement of villus enterocytes, villus-core separation; 2 = significant disarrangement of villus enterocytes, villus-core separation down sides of villi, blunting of villi; 3 = epithelial sloughing of villi, loss of villi; 4 = intestinal necrosis or perforation. A score of 2 or higher was considered consistent with development of experimental NEC. Severe NEC was defined as grade 3 or 4 intestinal injury.
Lung Histologic Evaluation
At the time of euthanasia, the right lower lobe of the lung was formalin fixed, paraffin embedded, and stained with hematoxylin and eosin. Two blinded authors evaluated degree of injury using a modified version of the scale developed by the American Thoracic Society (ATS). The scale was modified to be more appropriate for immature lungs (Table 1). Scores from 0 (normal) to 2 (acute lung injury) are given in six different parameters and added together to yield a score between 0 and 12, with 0 representing normal lung and 12 indicating severe acute injury with hemorrhage [19].
Table 1.
Lung Injury Scoring System
| Parameter | Score per field (400x magnification) | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Neutrophils in the alveolar space | None | 1–5 | >5 |
| Neutrophils in the interstitial space | None | 1–5 | >5 |
| Hyaline membranes | None | 1 | >1 |
| Proteinaceous debris filling the airspaces | None | 1 | >1 |
| Alveolar septal thickening | <2x | 2x–4x | >4x |
| Red blood cells in alveolar space | Few | Half filled | Filled |
Lung injury score = sum of all scores, range from 0 to 12
Cytokine Analysis
Following euthanasia, sections of small intestine and lung were snap frozen in liquid nitrogen and stored at −80°C. Tissue was thawed and homogenized with the Bullet Blender (Next Advance, Averill Park, NY) in RIPA buffer (Sigma, St. Louis, MO) with 1:100 dilutions of both phosphatase and protease inhibitors (Sigma, St. Louis, MO). After homogenization, samples were centrifuged at 12,000 rpm and supernatants were collected for further analysis. Total protein was quantified with the Bradford Assay using a spectrophotometer (VersaMax microplate reader, Molecular Devices, Sunnyvale, CA).
Murine interleukin 6 (IL-6), vascular endothelial growth factor (VEGF), interleukin 1β (IL-1β), and interleukin 12 (IL-12) were measured by ELISA (R&D Systems, Bio-Techne Corporation, Minneapolis, MN). ELISAs were repeated twice to ensure consistency. In order to correct for variations in ELISA plates, values for both WT and eNOSKO mice were normalized to their respective breastfed controls and therefore concentrations are expressed as “fold change compared to control.” Assays were performed at 1:20 dilution and cytokine concentrations were normalized to total protein for each sample.
Statistical Analysis
Continuous variables were reported as mean ± standard error of mean (SEM) and ordinal variables were reported using median and interquartile range. All non-parametric data was compared with Mann-Whitney U-tests. GraphPad Prism 7 (GraphPad Software, La Jolla, CA) was used for all statistical analysis and figures. P values less than 0.05 were considered significant.
RESULTS
Loss of eNOS does not worsen overall clinical sickness in NEC
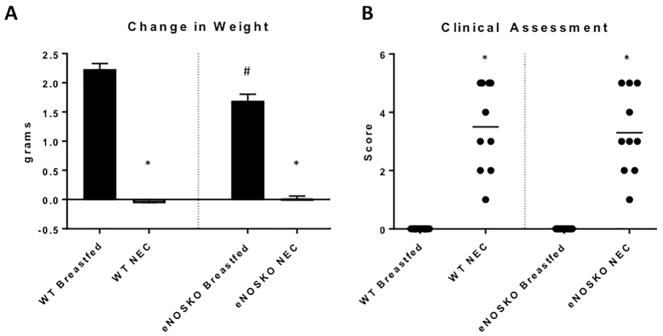
The breastfed eNOSKO pups had reduced weight gain (1.69g ± 0.11) over the four-day protocol compared to WT (2.23g ± 0.10, p=0.0015). This may indicate a reduction in baseline health compared to the WT pups. In both WT NEC and eNOSKO NEC groups, there was little to no weight gain over the course of the four-day experiment with no significant difference between the NEC groups (Figure 1A).
Figure 1. Loss of eNOS did not result in significant change in clinical severity of NEC.
A) There was a significant difference between weight gain over the four-day protocol in the breastfed groups (#: p=0.0015 vs WT Breastfed) indicating a possible baseline health deficiency in the eNOSKO pups. Each NEC group had little to no weight gain over the length of the protocol (*: p<0.0001 vs. respective breastfed control). B) Clinical assessment scores of each group were significantly worse than their respective controls (*: p<0.0001), but there was not a significant difference between WT and eNOSKO NEC groups.
Clinical assessment scores for both NEC groups were significantly higher (worse) compared to their respective controls (p<0.0001 for each), but there was no difference between clinical scores in the WT and eNOSKO NEC groups (Figure 1B).
Loss of eNOS increases severity of intestinal injury
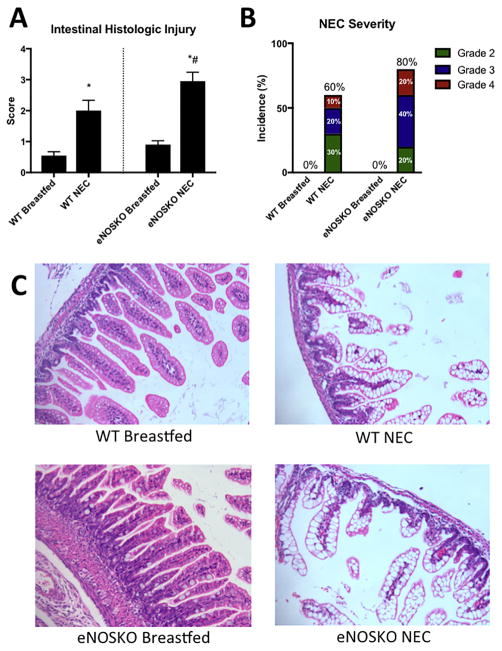
At baseline, the WT and eNOSKO breastfed groups had similar intestinal appearances, with median histologic scores of 0 (IQR=0-0) and 0 (IQR=0–1) respectively. Both NEC groups had significantly worse intestinal injury compared to their respective controls. The eNOSKO NEC group had a median histologic injury score of 3.25 (IQR=2.25–3.625) and the WT NEC group had a median score of 2 (IQR=1–3, p=0.0474, Figure 2A). The eNOSKO NEC group had a higher incidence of NEC development as well as a higher incidence of severe NEC, defined as grade 3 and 4 injury (Figure 2B).
Figure 2. Loss of eNOS results in more severe intestinal injury secondary to NEC.
A) Histologic injury scores were significantly worse in both NEC groups compared to the breastfed pups, and the eNOSKO NEC group had more severe injury than the WT NEC group. (*: p<0.05 vs. respective breastfed control, #: p<0.05 vs. WT NEC). B) The eNOSKO NEC group developed NEC at a higher rate and developed more severe NEC compared to WT. C) Representative histologic sections of each group (H&E stained, 20x magnification) demonstrate villus disarrangement and villous sloughing in the NEC groups, with significant villous core separation noted specifically in the eNOSKO NEC group.
Loss of eNOS increases severity of NEC-associated lung injury
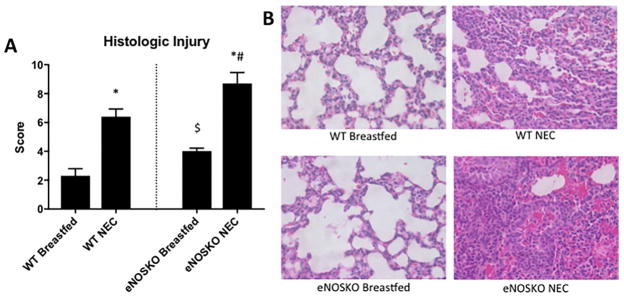
At baseline, the breastfed eNOSKO group had more significant lung injury, with a median score of 4 (IQR=3.75–4.25) compared to 2.5 (IQR=0.75–4) in the breastfed WT group (p=0.0099). After exposure to the NEC model, the eNOSKO NEC group developed more significant lung injury with a median score of 8.5 (IQR=6.75–11.25) compared to 6.5 (IQR=5–7.5) in the WT NEC group (Figure 3).
Figure 3. Loss of eNOS results in more severe lung injury secondary to NEC.
A) Lung injury scores in eNOSKO mice was significantly worse than their WT counterparts in both the breastfed and NEC groups (*: p<0.05 vs. respective breastfed control, #: p<0.05 vs. WT NEC, $: p<0.05 vs. WT breastfed). B) Representative histologic sections of each group (H&E stained, 40x magnification) demonstrate relative alveolar septal thickening, fibrinous and cellular airspace deposits, increased neutrophils, and blood in the airspaces in the NEC groups.
Loss of eNOS leads to altered intestinal inflammatory response
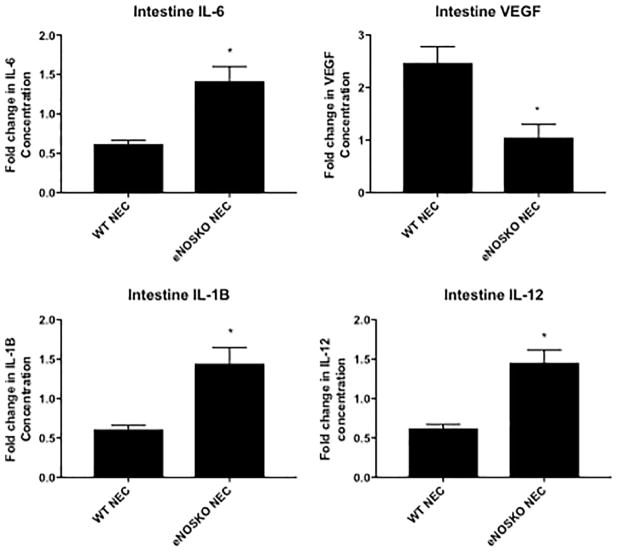
IL-6 was increased in the eNOSKO NEC group (1.414 ± 0.1872) compared to the WT NEC group (0.6187 ± 0.04663, p=0.0003). VEGF was significantly higher in the WT NEC group (2.473 ± 0.3057 compared to the eNOSKO NEC group (1.051 ± 0.2509, p=0.0008). IL-1β was higher in eNOS NEC (1.446 ± 0.2007 compared to WT NEC (0.6104 ± 0.0507, p=0.0005). Finally, intestinal IL-12 was significantly higher in eNOS NEC (1.458 ± 0.1593) compared to WT NEC (0.622 ± 0.0506, p=0.0002, Figure 3).
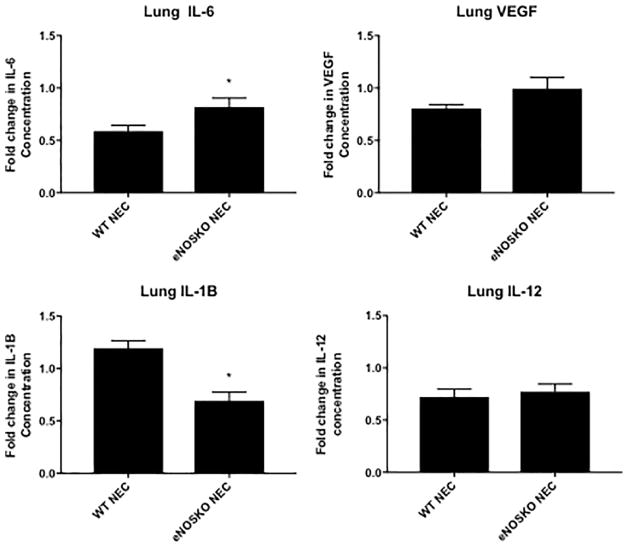
Loss of eNOS leads to altered lung inflammatory response
Lung IL-6 was significantly lower in the WT NEC group (0.5899 ± 0.05402) compared to the eNOSKO NEC group (0.8189 ± 0.0860, p=0.0320). Average lung VEGF levels were lower in WT NEC (0.8055 ± 0.0380) compared to eNOSKO NEC (0.9944 ± 0.1058), but there was no significant difference between the two groups. Lung IL-1β was significantly higher in WT NEC (1.194 ± 0.0722) compared to eNOSKO NEC (0.6934 ± 0.0809, p=0.0002, Figure 4). Lung IL-12 was not significantly different between the WT and eNOSKO NEC groups (0.724 ± 0.0737 vs. 0.773 ± 0.0733.
Figure 4. Loss of eNOS results in altered intestinal inflammatory response.
Concentrations of cytokines are expressed as folds of control (breastfed group). IL-6, VEGF, IL-1β, and IL-12 had varying responses in eNOS and WT NEC, with significant differences between the two NEC groups for all four cytokines. (*: p<0.05 vs. WT NEC)
DISCUSSION
Endothelial nitric oxide synthase (eNOS) is thought to play an integral role in intestinal microcirculatory regulation, especially in the neonatal period [10]. In the intestine, eNOS expression is upregulated by enteral feeding, which helps to modulate the microcirculatory response in mature tissues [20]. Therefore, it stands to reason that knocking out the function of this enzyme, reducing the ability of the intestine and mesentery to produce nitric oxide, would worsen intestinal injury in necrotizing enterocolitis [21]. This has been demonstrated in previous studies, but the effect of eNOS ablation on the NEC-associated lung injury has not been as well studied. In our study, we demonstrated significantly worse intestinal and lung injury in eNOSKO mice exposed to an animal model of NEC as well as dysregulation of the intestinal and pulmonary inflammatory response in experimental NEC.
Surprisingly, clinical scores were not significantly worse with loss of eNOS. While intestinal injury was certainly worse, the eNOSKO pups seemed to be able to compensate enough via other pathways to reduce overall clinical deterioration. A previous study has shown that LPS-induced systemic septic shock is less severe in eNOSKO mice as compared to wild type animals, likely because eNOS acts as both a direct producer and an indirect regulator of NO production [22]. Downregulation of NO reduces the systemic hypotension and vascular permeability that characterizes shock [15, 23]. Therefore, despite the increased injury in the intestine and lung in eNOSKO mice, they may be somewhat protected from the overall septic picture and worsening clinical status when compared to wild type mice.
We did note more severe intestinal injury in eNOSKO pups. A previous study has demonstrated that endothelial toll-like receptor 4 (TLR4) signaling acts through downregulation of eNOS to modulate intestinal perfusion and exacerbate NEC [9]. In that study, when TLR4 was knocked out, relative eNOS activity was increased, leading to less severe NEC [9]. Lack of eNOS activity exerts the opposite effect, and alters the delicate microcirculation of the neonatal intestine, which is predominantly regulated by nitric oxide at this stage, resulting in more severe injury [21]. In this era of research, reproducibility of results is not always appreciated, but our work serves to support this proposed mechanism.
Lung injury was also more severe in the eNOSKO NEC pups compared to WT. While part of this may be partially due to the baseline differences in the lungs between the knock-out and wild-type mice, the injury secondary to the NEC model was certainly more severe in the eNOSKO NEC group. In previous studies, this injury has been shown to be secondary to intestinal NEC and its resulting systemic effects, not direct injury to the lung, as expected based on human data [4]. Additionally, as we have confirmed in this study, eNOSKO mice suffer more severe intestinal injury secondary to NEC [9, 21], which likely explains the more severe lung injury in these animals as well.
Loss of eNOS activity was found to significantly alter the inflammatory response in both intestine and lung. In non-stressed endothelium, NO is constitutively produced by eNOS at steady states, which maintains hemostasis and keeps vascular structure intact [24]. Stimulation by any sort of stress induces NO production, which contributes to the vascular permeability associated with inflammation [24]. Because of this, loss of endothelial nitric oxide significantly impacts the inflammatory response. We chose to look at IL-6, IL-1β, IL-12, and VEGF based on findings in previous studies which have demonstrated alterations in these cytokines in experimental NEC [13, 25–28], as well as studies showing intimate involvement of endothelial NO with production of these cytokines [14–17], and these were the only cytokines evaluated.
IL-6 was elevated in eNOSKO NEC group’s intestine compared to WT NEC group. IL-6, while typically thought of as a pro-inflammatory cytokine, has been shown to play a more protective role in the intestine and may act as an anti-inflammatory mediator in NEC [29]. Additionally, previous studies have shown multiple interactions between IL-6 and NO. In macrophages stimulated by LPS, a NO inhibitor has been shown to increase IL-6 release [30], indicating that a loss of eNOS may result in increased IL-6 during inflammation. In the lung, IL-6 was higher in the eNOSKO NEC group than the WT NEC group. Because IL-6 release is at least partially modulated by nitric oxide, it is reasonable that the eNOSKO NEC pups would have higher IL-6 in the lung compared to WT NEC after prolonged and repeated systemic insult.
A significant difference between WT and eNOSKO pups was noted in VEGF production. The WT group had significantly elevated intestinal VEGF and decreased VEGF in the lung, while the eNOSKO NEC group exhibited no change from baseline in either tissue. VEGF is a growth factor that is typically released by endothelial cells in response to hypoxic stress [16]. Therefore, VEGF was expected to increase during NEC in intestinal tissue, however it’s response in the lung is not well understood. VEGF also plays an important role in inflammation as a inducer of vascular permeability and stimulator of angiogenesis [24]. Previous studies have shown that VEGF is predominantly dependent on eNOS-derived NO to provide these effects [15].
Significant differences were noted in the production of IL-1β in both intestine and lung. In the intestine, IL-1β was decreased in the WT NEC group compared to the eNOSKO NEC group, while the opposite effect was noted in the lung. One function of IL-1β is to activate endothelial cells and stimulate NO production [31]. Theoretically, loss of endothelial NO would decrease negative feedback of IL-1β and increase its production in response to injury. Systemically, IL-1β acts a neutrophil chemoattractant, and therefore it is expected that it would be increased in the WT NEC lungs. The result in the eNOSKO pups is more surprising. A study in organoids of human pulmonary artery endothelial cells demonstrated that expression of eNOS was intimately linked to hypoxia and LPS-induced stress as well as IL-1β concentration over time [32]. In stressed conditions, IL-1β contributes to downregulation of eNOS expression [32]. In the case of eNOSKO pups, the lungs may decrease IL-1β production due to absence of eNOS.
IL-12 was depressed in WT NEC intestine, compared to eNOSKO NEC. Previous studies have demonstrated decreased IL-12 in intestinal epithelium in experimental NEC [26], so this was anticipated in our WT group. Previous studies in human endothelial cells have demonstrated upregulation of IL-12 expression by inhibition of eNOS activity, and downregulation in the presence of endogenously produced NO [17], which is supported by our observations in this study. In the lung, there was no difference in IL-12 between WT NEC and eNOSKO NEC, which is not fully understood. In previous experimental NEC studies, IL-12 has mainly been evaluated in the intestine [26], and further work to elucidate its role in the lung would be useful.
In addition to more severe intestinal and lung injury secondary to the NEC model, the inflammatory responses of the intestine and lung vary widely between the two strains. It is clear that nitric oxide plays an integral role in inflammation, sepsis, and systemic injury, and additional research is certainly needed to further elucidate these interactions.
LIMITATIONS
Because the pathophysiology of NEC is not completely understood, experimental models of NEC are limited. The diagnosis can only be truly established at postmortem examination of the intestine, which makes it difficult to assess development of NEC during progression of the protocol. Additionally, blinding the clinical assessments of the animals is difficult, but this was standardized as much as possible by a regimented scoring system and inclusion of other more objective measures. Specific challenges with the eNOSKO mice include difficult and unpredictable breeding and high cannibalism rate of pups. Additionally, a high rate of low birth weight was noted, with some pups weighing under 1 gram on P5. Litters with pups under 1.5 grams on P5 were excluded after multiple deaths related to technical difficulties of gavage feeding pups this small.
CONCLUSION
Endothelial nitric oxide synthase plays an integral role in protection of the intestine and lung in experimental NEC. The inflammatory response both locally in the intestine and systemically is significantly altered in the absence of eNOS in knock-out mice.
Figure 5. Loss of eNOS results in altered pulmonary inflammatory response.
Concentrations of cytokines are expressed as folds of control (breastfed group). There is a significant difference in IL-6 and IL-1β between the two NEC groups, but no difference in VEGF or IL-12. (*: p<0.05 vs. WT NEC)
Acknowledgments
This work was made possible with support from:
KL2TR001106 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award
Indiana University Health, Indianapolis, IN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neu J, Walker WA. Necrotizing enterocolitis. The New England journal of medicine. 2011;364(3):255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. Journal of pediatric surgery. 2009;44(6):1072–5. doi: 10.1016/j.jpedsurg.2009.02.013. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 3.Spencer AU, Kovacevich D, McKinney-Barnett M, Hair D, Canham J, Maksym C, et al. Pediatric short-bowel syndrome: the cost of comprehensive care. The American journal of clinical nutrition. 2008;88(6):1552–9. doi: 10.3945/ajcn.2008.26007. [DOI] [PubMed] [Google Scholar]
- 4.Jia H, Sodhi CP, Yamaguchi Y, Lu P, Martin LY, Good M, et al. Pulmonary Epithelial TLR4 Activation Leads to Lung Injury in Neonatal Necrotizing Enterocolitis. Journal of immunology. 2016;197(3):859–71. doi: 10.4049/jimmunol.1600618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughon M, O’Shea MT, Allred EN, Bose C, Kuban K, Van Marter LJ, et al. Chronic lung disease and developmental delay at 2 years of age in children born before 28 weeks’ gestation. Pediatrics. 2009;124(2):637–48. doi: 10.1542/peds.2008-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganapathy V, Hay JW, Kim JH, Lee ML, Rechtman DJ. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC pediatrics. 2013;13:127. doi: 10.1186/1471-2431-13-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–23. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 8.van Haver ER, Oste M, Thymann T, Sys SU, Lamers WH, Weyns AL, et al. Enteral feeding reduces endothelial nitric oxide synthase in the caudal intestinal microvasculature of preterm piglets. Pediatr Res. 2008;63(2):137–42. doi: 10.1203/PDR.0b013e31815f00f9. [DOI] [PubMed] [Google Scholar]
- 9.Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(23):9451–6. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan WQ, Smolich JJ, Wild J, Yu VY, Walker AM. Nitric oxide modulates regional blood flow differences in the fetal gastrointestinal tract. The American journal of physiology. 1996;271(4 Pt 1):G598–604. doi: 10.1152/ajpgi.1996.271.4.G598. [DOI] [PubMed] [Google Scholar]
- 11.Good M, Sodhi CP, Yamaguchi Y, Jia H, Lu P, Fulton WB, et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr. 2016;116(7):1175–87. doi: 10.1017/S0007114516002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Lorenzo M, Bass J, Krantis A. Use of L-arginine in the treatment of experimental necrotizing enterocolitis. Journal of pediatric surgery. 1995;30(2):235–40. doi: 10.1016/0022-3468(95)90567-7. discussion 40–1. [DOI] [PubMed] [Google Scholar]
- 13.De Plaen IG. Inflammatory signaling in necrotizing enterocolitis. Clinics in perinatology. 2013;40(1):109–24. doi: 10.1016/j.clp.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siednienko J, Nowak J, Moynagh PN, Gorczyca WA. Nitric oxide affects IL-6 expression in human peripheral blood mononuclear cells involving cGMP-dependent modulation of NF-kappaB activity. Cytokine. 2011;54(3):282–8. doi: 10.1016/j.cyto.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2604–9. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochemical pharmacology. 2009;78(6):539–52. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattaruzza M, Slodowski W, Stojakovic M, Krzesz R, Hecker M. Interleukin-10 induction of nitric-oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. The Journal of biological chemistry. 2003;278(39):37874–80. doi: 10.1074/jbc.M301670200. [DOI] [PubMed] [Google Scholar]
- 18.Zani A, Cordischi L, Cananzi M, De Coppi P, Smith VV, Eaton S, et al. Assessment of a neonatal rat model of necrotizing enterocolitis. European journal of pediatric surgery: official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 2008;18(6):423–6. doi: 10.1055/s-2008-1038951. [DOI] [PubMed] [Google Scholar]
- 19.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. American journal of respiratory cell and molecular biology. 2011;44(5):725–38. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forstermann U, Boissel JP, Kleinert H. Expressional control of the ‘constitutive’ isoforms of nitric oxide synthase (NOS I and NOS III) FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1998;12(10):773–90. [PubMed] [Google Scholar]
- 21.Watkins DJ, Besner GE. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg. 2013;22(2):83–7. doi: 10.1053/j.sempedsurg.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connelly L, Madhani M, Hobbs AJ. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: a pro-inflammatory role for eNOS-derived no in vivo. The Journal of biological chemistry. 2005;280(11):10040–6. doi: 10.1074/jbc.M411991200. [DOI] [PubMed] [Google Scholar]
- 23.Connelly L, Jacobs AT, Palacios-Callender M, Moncada S, Hobbs AJ. Macrophage endothelial nitric-oxide synthase autoregulates cellular activation and pro-inflammatory protein expression. The Journal of biological chemistry. 2003;278(29):26480–7. doi: 10.1074/jbc.M302238200. [DOI] [PubMed] [Google Scholar]
- 24.Cirino G, Fiorucci S, Sessa WC. Endothelial nitric oxide synthase: the Cinderella of inflammation? Trends in pharmacological sciences. 2003;24(2):91–5. doi: 10.1016/S0165-6147(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 25.Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nature reviews Gastroenterology & hepatology. 2016;13(10):590–600. doi: 10.1038/nrgastro.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. The Journal of surgical research. 2000;92(1):71–7. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 27.Sabnis A, Carrasco R, Liu SX, Yan X, Managlia E, Chou PM, et al. Intestinal vascular endothelial growth factor is decreased in necrotizing enterocolitis. Neonatology. 2015;107(3):191–8. doi: 10.1159/000368879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan X, Managlia E, Liu SX, Tan XD, Wang X, Marek C, et al. Lack of VEGFR2 signaling causes maldevelopment of the intestinal microvasculature and facilitates necrotizing enterocolitis in neonatal mice. Am J Physiol Gastrointest Liver Physiol. 2016;310(9):G716–25. doi: 10.1152/ajpgi.00273.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock. 2006;25(4):329–37. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- 30.Deakin AM, Payne AN, Whittle BJR. The Modulation of Il-6 and Tnf-Alpha Release by Nitric-Oxide Following Stimulation of J774 Cells with Lps and Ifn-Gamma. Cytokine. 1995;7(5):408–16. doi: 10.1006/cyto.1995.0056. [DOI] [PubMed] [Google Scholar]
- 31.Sahni A, Sahni SK, Francis CW. Endothelial cell activation by IL-1beta in the presence of fibrinogen requires alphavbeta3. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(10):2222–7. doi: 10.1161/01.ATV.0000183605.27125.6f. [DOI] [PubMed] [Google Scholar]
- 32.Ziesche R, Petkov V, Williams J, Zakeri SM, Mosgoller W, Knofler M, et al. Lipopolysaccharide and interleukin 1 augment the effects of hypoxia and inflammation in human pulmonary arterial tissue. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(22):12478–83. doi: 10.1073/pnas.93.22.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]