Abstract
Stroke remains one of the leading causes of death worldwide. The underlying neuropathology for stroke is ischemic brain injury. Carboxyl terminal modulator protein (CTMP), an endogenous inhibitor of the prosurvival Akt, may increase brain ischemic injury in young animals. Aging decreases brain ischemic tolerance. We hypothesize that CTMP is increased with aging and that this increase contributes to the decreased brain ischemic tolerance. To address these hypotheses, we determined the expression of CTMP and its downstream proteins in the brain of various ages of rats (Fischer 344 and Sprague-Dawley rats). The role of CTMP in ischemic brain injury was investigated by RNA interference. Here, we showed that CTMP in the brain was increased with aging in rats. The phosphorylated/activated Akt was decreased with aging. Six-month and twenty-month old rats had poorer neurological outcome than did 2-month old rats after brain ischemia. The neurological outcome of 2-month old rats was worsened by LY294002, an Akt inhibitor. The poor neurological outcome in 6-month old rats was improved by silencing CTMP. CTMP was increased in ischemic penumbral brain tissues. Silencing this increase activated Akt. These results suggest that CTMP increase with aging contributes to the aging-dependent decrease of brain ischemic tolerance.
Keywords: Aging, Akt, brain ischemic tolerance, CTMP
Introduction
Stroke is the major cause of acute brain injury in the elderly patients [1]. Studies have shown that the brain of elderly has decreased ischemic tolerance [2, 3]. Although many factors contributing to the aging process, such as mitochondrial dysfunction and increased fragility of blood vessels, have been considered to account for this phenomenon [4], the contribution of changes in the prosurvival signaling to the decreased brain ischemic tolerance in the elderly is poorly understood.
Akt plays a key role in mediating neuronal survival [5, 6]. Akt is activated by phosphorylation of Thr308 and Ser473 residues [7]. Akt can promote neuronal survival via phosphorylating the pro-apoptotic target glycogen synthase kinase 3β (GSK-3β) at Ser9 to reduce GSK-3β activity [5, 8]. Carboxyl terminal modulator protein (CTMP) is an endogenous inhibitor of Akt. CTMP binds to the carboxyl terminal regulatory domain of Akt and inhibits the activity of Akt by interfering with the phosphorylation of Thr308 and Ser473 residues [9]. Importantly, increased CTMP may be critical for ischemia-induced neuronal death after global cerebral ischemia in young rats [10]. Our previous study has shown that CTMP increase contributes to the difficulty for isoflurane to induce neuroprotection in high fat diet-fed mice [11]. Thus, we hypothesize that CTMP increase inhibits Akt signaling and that this inhibition reduces ischemic tolerance of the elderly brain. To test these hypotheses, the expression of CTMP in the brain of various ages of rats was determined. Focal brain ischemia was applied to rats with or without downregulation of CTMP.
Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 2011 and reported according to the ARRIVE guidelines.
Experiments
In the first experiment, brains of 2-month and 20-month old male Fischer 344 rats from National Institute of Aging, U.S.A., were harvested for Western blotting and immunofluorescent staining.
In the second experiment, 2-month and 20-month old male Fischer 344 rats were subjected to embolic focal brain ischemia. Neurological deficit scores, motor coordination and brain infarct volumes were evaluated 24 h after the embolic stroke.
In the third experiment, brains of 2-month, 6-month and 10-month old male Sprague-Dawley (S-D) rats from Charles River Laboratories were harvested for Western blotting. Also, 6-month old male S-D rats were randomly assigned to 3 groups: 1) control, 2) non-targeting miRNA, and 3) CTMP-miRNA. The randomization was generated by a computer program. Their brain was harvested for Western blotting 14 days after receiving the miRNA.
In the fourth experiment, 2-month old male S-D rats were randomly assigned to be subjected to: 1) middle cerebral arterial occlusion (MCAO) induced by suture method, 2) MCAO plus vehicle, and 3) MCAO plus LY294002. Six-month old male S-D rats were randomly assigned to be subjected to: 1) MCAO, 2) MCAO plus non-targeting miRNA, and 3) MCAO plus CTMP-miRNA. Left MCAO was for 30 min. Neurological deficit scores, motor coordination and brain infarct volumes were evaluated 24 h after the MCAO.
In the fifth experiment, 2-month or 6-month old male S-D rats were subjected to left MCAO and their left frontal cortex area 1 (Fr1) was harvested 24 h later for Western blotting. Six-month old male S-D rats were randomly assigned to be subjected to: 1) MCAO, 2) MCAO plus non-targeting miRNA, and 3) MCAO plus CTMP-miRNA. Their left Fr1 was harvested 24 h after the MCAO for Western blotting.
Embolic focal brain ischemia
The clot preparation and induction of embolic stroke were modified from a protocol described previously [12]. Briefly, a blood donor Fischer 344 rat was intubated and anesthetized with 3% isoflurane. The left femoral artery was cannulated with a 24-gauge catheter. Blood was withdrawn from the arterial line into a 1 ml syringe and retained in the syringe for 4 h at room temperature. The syringe was then kept in 4°C refrigerator for 3 days for clot formation. The clotted blood strip was injected into a PE-50 tube (ID: 0.58 mm). A single 10 mm3 clot (40 mm long clot in PE-50 tube) was ready for use to induce embolic stroke. No thrombin was used for the preparation of the blood clots.
To induce embolic stroke, rats were anesthetized with 5% isoflurane for 3–5 min, and then intubated. Anesthesia was maintained with 2% - 3% isoflurane. A longitudinal incision was made in the midline of the ventral neck. The left common carotid artery (CCA), left external carotid artery (ECA) and left internal carotid artery (ICA) were isolated from the arterial sheath. The distal portion of the left ECA was double-ligated with sutures and the left ECA was cut between these two sutures. The left CCA and left ICA were clamped by microvascular clips. A small puncture was made on the cut end of the left ECA. A PE-50 tube containing a clot was introduced into the lumen of the left ICA through the puncture. A silk suture was placed around the origin of left ECA with PE-50 tube in place and tightened. The clips on the left CCA and ICA were removed. The PE-50 tube was advanced to pass the origin of the pterygopalatine artery in the left ICA. The clot in PE-50 tube was gently injected into the left middle cerebral artery (MCA) through ICA. The PE-50 tube in the left ICA was removed 5 min after injection. The left ECA was then ligated. The Rat was allowed to recover from anesthesia and returned to the original cage. During the surgery, the body temperature was monitored continuously with a rectal probe and maintained at 37.0 ± 0.5°C with a feedback-controlled heating system. The inhaled and exhaled gasses were monitored to maintain normal end-tidal CO2 concentrations.
Middle cerebral arterial occlusion
Rats were subjected to the left MCAO with intravascular suture technique as we described previously [13, 14]. Briefly, rats were anesthetized with isoflurane, maintained the rectal temperature at 37 ± 0.5°C by the feedback-controlled heating system and kept normal end-tidal CO2 concentrations. The MCAO was achieved by advancing a monofilament nylon suture (Beijing CiNontech Co. Ltd., Beijing, China) with a rounded tip to the left ICA via the ECA until slight resistance was felt. The ipsilateral CCA was occluded temporarily during the placement of the suture. Rats were re-anesthetized by isoflurane at 30 min after the onset of MCAO to remove the suture. After recovery from anesthesia, rats were placed back in their cages with ad libitum access to food and water.
Evaluation of motor coordination, neurological deficit scores, and infarct volumes
The motor coordination was evaluated by the performance on rotarod as we described before [14, 15]. All rats were trained for three continuous days before the induction of stroke. The formal test was performed 24 h before and after the induction of stroke. The rats were placed on an accelerating rotarod. The speed of the rotarod was increased from 4 rpm to 40 rpm in 5 min. The latency and the speed were recorded when a tested rat fell off the rod. Each rat was tested five times. The speed–latency index (latency in seconds × speed in rpm) of each of the five tests was calculated, and the mean index of the five trials was used to reflect the motor coordination function of each rat before or after the stroke.
The neurological deficit scores were assessed 24 h after the stroke by a blinded investigator as we described before [14, 15]. Rats were scored as follows: zero, no apparent deficits; one, failure to extend right forepaw fully; two, decreased grip of the right forelimb; three, spontaneous movement in all directions, contralateral circling only if pulled by the tail; four, circling or walking to the right; five, walking only if stimulated; six, unresponsiveness to stimulation and with depressed level of consciousness; and seven, dead.
The assessment of infarct volumes at 24 h after the stroke was performed by a blinded investigator after 2,3,5-triphenyltetrazolium chloride staining as we described before [14, 15]. Briefly, rats were euthanized by 5% isoflurane and transcardiacally perfused by saline. Brains were removed and coronal sections were taken by using a microtome at 2 mm intervals (usually total 6 slices) over the entire brain. The sum of the infarct areas in the rostral and caudal sides of each brain slice was divided by two to get the average infarct area of the brain slice. The infarct volume of the brain slice was calculated by multiplying the average infarct area of the slice by the thickness of the slice. The total infarct volume in the brain was the sum of infarct volume of each brain slice. To account for the cerebral edema and differential shrinkage resulting from brain ischemia and tissue processing and to correct for the individual difference in brain volumes, the percentage of infarct volume in the ipsilateral hemisphere volume was calculated.
Western blotting
The frontal cortex was micro-dissected and placed in ice-cold RIPA buffer supplemented with a 1% cocktail of protease and phosphatase inhibitors. Tissue was homogenized in lysis buffer and centrifuged at 13,000 rpm for 10 min at 4°C. The supernatant was collected. Its protein concentration was determined by using BCA method. Aliquots of protein (30–40 μg per lane) were separated on a polyacrylamide gel and then blotted onto a polyvinylidene difluoride membrane. The membranes were blocked with Protein-Free T20 Blocking Buffer (catalog number: 37573, Thermo Scientific) and incubated with the following primary antibodies overnight at 4°C: rabbit polyclonal antibody to Akt (1:1000, catalog number: 9272; Cell Signaling), mouse monoclonal antibody to p-S473-Akt (1:1000, catalog number: 12694; Cell Signaling), mouse monoclonal antibody to p-T308-Akt (1:1000, catalog number: 5106 Cell Signaling;), rabbit polyclonal antibody to CTMP (1:1000, catalog number: 4612; Cell Signaling), rabbit monoclonal antibody to p-S9-GSK-3β (1:1000, catalog number: 9323; Cell Signaling), mouse monoclonal antibody to GSK-3β (1:1000, catalog number:9832; Cell Signaling), and rabbit polyclonal antibody to β-actin (1:1000, catalog number: 4967; Cell Signaling). Appropriate secondary antibodies were used. Band volumes of the target protein were normalized to those of β-actin for non-phosphorylated protein and to those of non-phosphorylated protein for phosphorylated protein. The band volumes of samples from experimental animals were normalized to the corresponding mean data from control animals.
Immunofluorescent labeling
Immunofluorescent labeling in the frontal cortex of Fischer 344 rats was performed to detect neuronal nuclear (NeuN), Akt phosphorylated at Ser473 (p-S473-Akt) and CTMP [16, 17]. In brief, rats were sacrificed under deep anesthesia and transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were harvested and post-fixed in 4% paraformaldehyde in 0.1 M PBS at 4°C for 24 h, dehydrated, and embedded in paraffin. Coronal 5-μm sections of the cerebral hemisphere were cut sequentially and mounted on microscope slides. Antigen retrieval with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) was performed at 95 – 100°C for 20 min. After being washed in Tris-buffered saline (TBS) containing 0.1% triton-X 100, sections were blocked in 10% donkey serum plus 1% bovine serum albumin in TBS for 2 h at room temperature and then incubated at 4°C overnight with the following primary antibodies: mouse monoclonal anti-NeuN (1:200, catalog number: MAB377, Merck Millipore), rabbit polyclonal anti-p-S473-Akt (1:200, catalog number: AF887, R&D Systems,) and rabbit polyclonal anti-THEM4 (another name for CTMP, 1:500, catalog number: ab106435, Abcam). Sections were rinsed in TBS with 0.1% triton-X 100 and then incubated with donkey anti-mouse IgG antibody conjugated with Alexa Fluor 488 (1:200, catalog number: A11055; Invitrogen) or donkey anti-rabbit IgG antibody conjugated with NL557 (1:200, catalog number: NL004; R&D Systems) for 1 h at room temperature in the dark. Specificity of immune-labeling was confirmed by incubation of sections with pre-immune rabbit IgG (1:250, catalog number: ab199376; Abcam) in place of primary antibody. No detectable labeling was observed under these conditions. Images of immunostaining were acquired with a fluorescence microscope equipped with a charge-coupled device camera.
LY294002 application
Twenty-five microgram LY294002 (catalog number: 9901; Cell Signaling) in 5 μl or the vehicle (1% dimethyl sulfoxide) was administered intracerebroventricularly (coordinates: 0.8 mm anteroposterior, 1.5 mm lateral to Bregma, and 3.5 mm in depth) [18] 2 h before the MCAO.
Lentiviral vectors carrying CTMP miRNAs
CTMP and non-targeting miRNA sequences were as described [10] and used to produce lentivirus as we did before [17]. Briefly, CTMP and non-targeting miRNA sequences were cloned into the pcDNA™6.2-GW/miR miRNA expression vector (Invitrogen). Their sequences were: CTMP miRNA-1: (5–′ GAAACAAGGAGACCGTCCTCTGTTTTGGCCACTGACTGACAGAGGACG CTCCTTGTTTC–3′); CTMP miRNA-2: (5–′ ATAACCTCCTAGCTGCTCCTGGTTTTGGCCACTGACTGACCAGGAGCAT AGGAGGTTAT–3′); and non-targeting-miRNA, a silencer-resistant miRNA sequence that does not target any known eukaryotic gene (5–′ AAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCAC GCAGTACATTT–3′). To enhance CTMP silencing, miRNA-1 and 2 were chained into the same vector. The sequences were verified by sequencing. These miRNAs in the pcDNA™ 6.2-GW/miR expression vector were sub-cloned into pLenti6/V5-DEST by standard procedure to generate CTMP miRNA and non-targeting miRNA transfer constructs. 293FT cells were transfected with the help of lipofectamine LTX (Invitrogen) to produce lentivirus as instructed by the protocol from Invitrogen. High-titer pseudotyped lentiviral solutions were harvested by PEG-it Virus Precipitation Solution (System Biosciences). All viruses were titered by qPCR lentivirus titration kit (Applied Biological Materials Inc.). Titers were 2×107 (CTMP miRNA1/2) and 5×107 (non-targeting miRNA) transducing units/ml. The viruses were then aliquoted and kept at −80°C until use.
Lentiviral application
As we described before [17], 10 μl CTMP miRNA or 4 μl non-targeting miRNA viral solution was delivered into the left cerebral cortex. In brief, animals were placed in a stereotaxic frame, anesthetized with 5% isoflurane and maintained on 2.0% isoflurane anesthesia. CTMP miRNA-1/2 virus solution or non-targeting miRNA virus solution was injected into the left cortex (3.0 mm posterior to Bregma, 2.0 mm lateral to Bregma, 2.0 mm in depth) by using a 10 μl Hamilton syringe with a 34-guage needle at a flow rate of 0.2 μl/min. The needle was left in place for an additional 5 min and gently withdrawn. Fourteen days later, animals were subjected to 30 min MCAO.
Statistical analysis
Parametric results were presented in mean ± S.E.M. (n≥6). The results of speed-latency index ratio, infarct volume and Western blotting results were analyzed by one way analysis of variance followed by the Tukey test after confirmation of normal distribution of the data or by Kruskal-Wallis analysis of variance on ranks followed by the Dunn’s test when the data are not normally distributed. Neurological deficit scores were analyzed by Kruskal–Wallis analysis of variance on ranks followed by the Dunn’s test. Differences were considered to be significant at P < 0.05 based on two-tailed hypothesis testing. All statistical analyses were performed with SigmaPlot 12.5 (Systat Software, Inc., Point Richmond, CA).
Results
CTMP was increased and its downstream kinases were inactive in older Fischer 344 rats
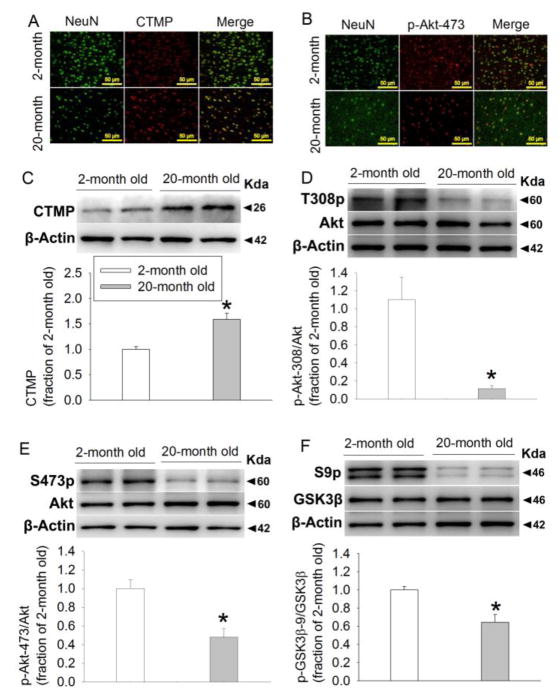
The staining of CTMP and phosphorylated Akt appeared to be co-localized with that of NeuN (Figs. 1A and 1B), suggesting that CTMP and phosphorylated Akt are expressed in the neurons. Interestingly, the immunostaining of CTMP appeared to be circular around the NeuN staining in 2-month old rats, suggesting that CTMP surrounds nuclei in the neurons of 2-month old rats. In contrast, the immunostaining of CTMP was diffuse in 20-month old mice (Fig. 1A).
FIGURE 1. Aging increases CTMP and decreases phosphorylated Akt and GSK-3β in the cerebral cortex of Fischer 344 rats.
(A) Representative images of immunolabeling of NeuN (green) and CTMP (red). Magnification x 200. Scale bar equals to 50 μm. (B) Representative images of immunolabeling of NeuN (green) and Akt phosphorylated at Ser473 (p-Ser473-Akt) (red). Magnification x 200. Scale bar equals to 50 μm. (C) Representative images and quantification of Western blot of CTMP. (D) Representative images and quantification of Western blot of Akt phosphorylated at Thr308. (E) Representative images and quantification of Western blot of Akt phosphorylated at Ser473. (F) Representative images and quantification of Western blot of GSK-3β phosphorylated at Ser9. Data are mean ± S.E.M. (n = 6–9). * P < 0.05 compared with 2-month old rats.
CTMP levels in the 20-month old Fischer 344 rats were higher than that in the 2-month old rats (Fig. 1C). Akt phosphorylated at Thr308 and Ser473 as well as GSK-3β phosphorylated at Ser9 were decreased in the 20-month old rats. The amount of total Akt and GSK-3β was not different between 2-month and 20-month old Fischer 344 rats (Figs. 1D–1F). These results suggest that the phosphorylated forms of Akt and GSK-3β are increased with aging.
Ischemic brain injury in older Fischer 344 rats was severer than that in younger rats
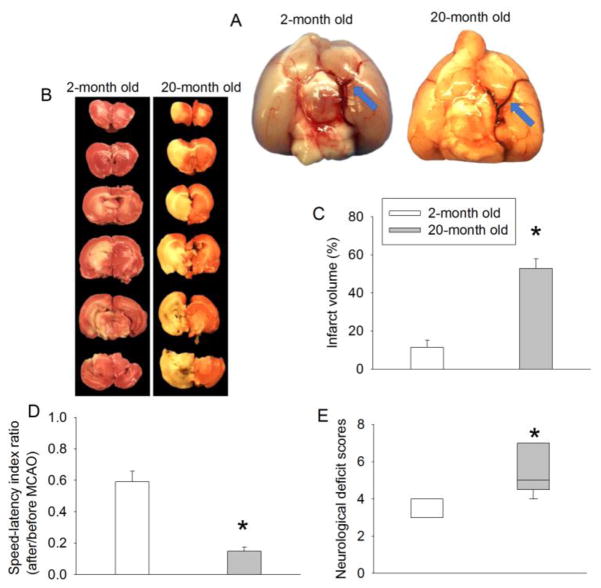
To simulate clinical situation, embolic stroke was applied to 2-month and 20-month old rats. The clots in our embolic stroke model appeared to occlude the middle cerebral artery in 2-month and 20-month old rats (Fig. 2A). Twenty-month old rats had worse neurological outcome than 2-month old rats as reflected by increased infarct volume, poor performance on rotarod and worsened neurological deficit scores (Fig. 2B–2E). These results suggest that the neurological outcome is worsened in aging rats after an embolic stroke.
FIGURE 2. Ischemic brain injury is increased with aging in Fischer 344 rats.
Rats were subjected to an embolic stroke. (A) Representative images of the occlusion of the middle cerebral artery by clots. Brains were harvested 10 min after the injection of the clot. Blue arrows indicate the clot occluding the middle cerebral artery. (B) Representative brain sections stained with 2,3,5-triphenyltetrazolium chloride. (C) Quantification of brain infarct volume. (D) Performance of rats on rotarod. Results in panels B and C are mean ± S.E.M. (n = 8). (E) Neurological deficit scores presented in box plot (n = 9 to 13). * P < 0.05 compared with 2-month old rats.
CTMP was increased and its downstream kinases were inactive with increased age in S-D rats
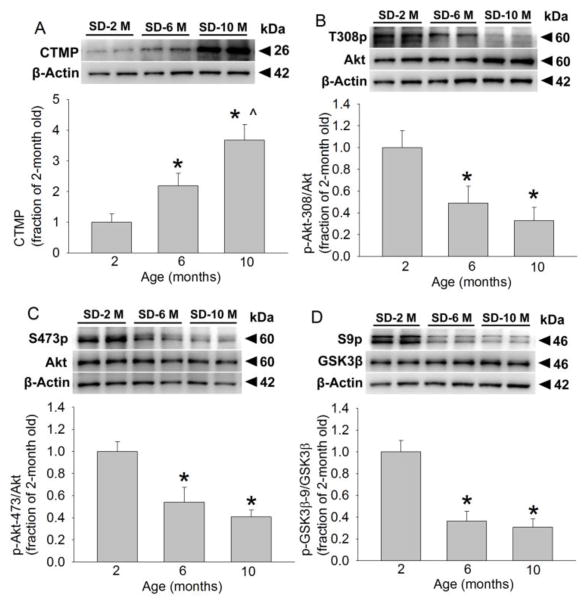
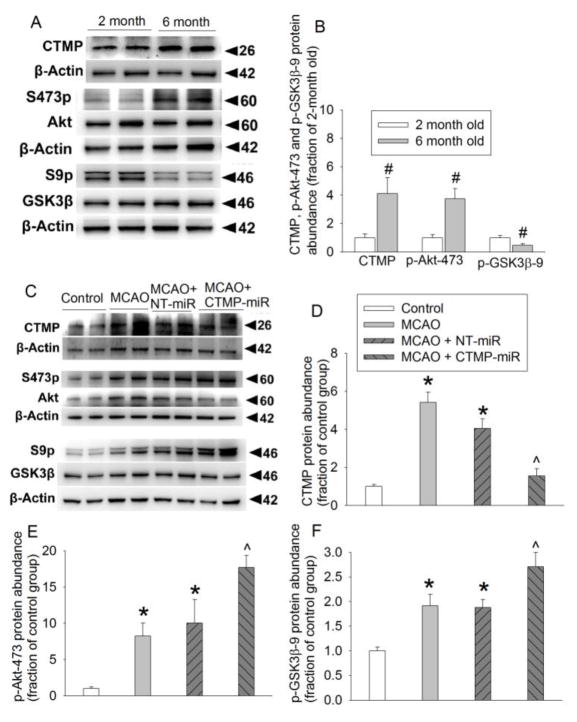
Similar to the situation in Fischer 344 rats, 6-month and 10-month old S-D rats had an increased CTMP level and decreased phosphorylated Akt and GSK-3β compared with 2-month old S-D rats. The amount of total Akt and GSK-3β levels was not different among 2-month, 6-month and 10-month old S-D rats (Fig. 3). These results suggest that the increase of CTMP and decease of phosphorylated forms of Akt and GSK-3β with aging are not strain-specific in rats.
FIGURE 3. Aging increases CTMP and decreases phosphorylated Akt and GSK-3β in the cerebral cortex of S-D rats.
(A) Representative images and quantification of Western blot of CTMP. (B) Representative images and quantification of Western blot of Akt phosphorylated at Thr308. (C) Representative images and quantification of Western blot of Akt phosphorylated at Ser473. (D) Representative images and quantification of Western blot of GSK-3β phosphorylated at Ser9. Data are mean ± S.E.M. (n = 6). * P < 0.05 compared with 2-month old rats. ^ P < 0.05 compared with 6-month old rats.
CTMP and its downstream kinases affected the neurological outcome after brain ischemia
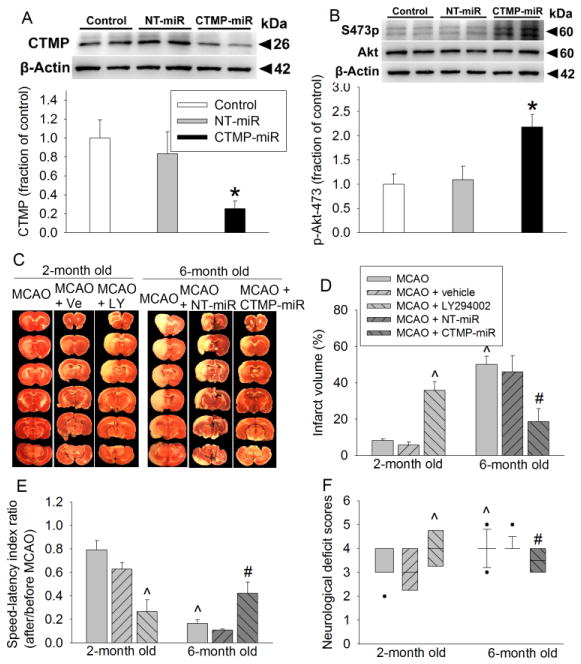
As shown in figures 4A and 4B, CTMP miRNA but not the non-targeting miRNA silenced CTMP expression and increased Akt phosphorylation at Ser473 in 6-month old S-D rats. This CTMP silencing reduced infarct brain volume and improved neurological function as reflected by better performance on rotarod and lower neurological deficit scores than those 6-month old rats without CTMP silencing. Six-month old S-D rats had increased infarct brain volume and worse neurological function than 2-month old rats after focal brain ischemia. LY294002, an Akt inhibitor, increased the infarct brain volume and worsened neurological function of 2-month old rats (Figs. 4C–4F). These results suggest that phosphorylated/activated Akt is neuroprotective\ and that increased CTMP and its associated deactivation of Akt with aging are detrimental.
FIGURE 4. CTMP increase contributes to the worse neurological outcomes in older S-D rats.
Viruses carrying code for CTMP miRNA and non-targeting (NT) miRNA were injected into cerebral cortical tissues 14 days before the brain tissues were harvested or before the rats were subjected to a 30 min MCAO. (A) Representative images and quantification of Western blot of CTMP. (B) Representative images and quantification of Western blot of Akt phosphorylated at Ser473. Results for panels A and B are mean ± S.E.M. (n = 6–10). * P < 0.05 compared with control and NT-miRNA groups. (C) Representative brain sections stained with 2,3,5-triphenyltetrazolium chloride. (D) Quantification of brain infarct volume. (E) Performance of rats on rotarod. Results in panels D and E are mean ± S.E.M. (n = 6–8). (F) Neurological deficit scores presented in box plot (n = 8 to 21). ^ P < 0.05 compared with 2-month old rats with MCAO only. # P < 0.05 compared with 6-month old rats with MCAO only. Ve: vehicle, LY: LY294002.
CTMP was increased and Akt was inactivated in the ischemic brain tissues
The expression of CTMP in the left Fr1, an ischemic penumbral region [19], of 6-month old S-D rats was higher than that in 2-month old S-D rats. The phosphorylated Akt was also increased but the phosphorylated GSK-3β was decreased in the left Fr1 of 6-month old S-D rats compared with 2-month old rats (Figs. 5A and 5B). These results suggest that the increased CTMP with aging remains in the ischemic tissues.
FIGURE 5. Aging-dependent increase of CTMP and its effects on downstream kinase phosphorylation in the ischemic brain tissues.
Rats with or without receiving viruses carrying code for CTMP miRNA and non-targeting (NT) miRNA in the cerebral cortical tissues 14 days before the brain tissues were subjected to a 30 min left MCAO. Their left Fr1 was harvested 24 h later. (A) and (B) Representative images and quantification of Western blot of CTMP, phosphorylated Akt and phosphorylated GSK-3β. (C), (D), (E) and (F) Representative images and quantification of Western blot of CTMP, phosphorylated Akt and phosphorylated GSK-3β. Results for panels B, D, E and F are mean ± S.E.M. (n = 6–8). # P < 0.05 compared with 2-month old rats. * P < 0.05 compared with control. ^ P < 0.05 compared with control and NT-miRNA groups.
Consistent with previously reported [10], the expression of CTMP, phosphorylated Akt and phosphorylated GSK-3β was increased in the left Fr1 of rats subjected to a left MCAO compared with that in control rats. Although the non-targeting miRNA did not affect the expression of CTMP, phosphorylated Akt and phosphorylated GSK-3β, CTMP miRNA decreased CTMP and increased the phosphorylated Akt and phosphorylated GSK-3β in the ischemic brain tissues (Figs. 5C to 5F). These results suggest that ischemia increases CTMP and that silencing this increase recovers Akt signaling.
Discussion
Our study has provided evidence to support the idea that increased CTMP with aging contributes to the decreased brain ischemic tolerance in the elderly. First, CTMP was increased with aging no matter whether Fischer 344 rats, an inbred rat strain, or S-D rats, an outbred rat strain, were examined. This change remained in ischemic brain tissues. Consistent with this change, the phosphorylation of Akt and GSK-3β was decreased with aging under control condition. Although phosphorylated Akt was increased but the phosphorylated GSK-3β was decreased in the ischemic brain tissues. Second, CTMP was expressed in neurons, cells that are very sensitive to ischemia. Third, 6- and 20-month old rats had worse neurological outcome than 2-month old rats after focal brain ischemia. Finally, the neurological outcome of 2-month old rats was worsened by inhibition of Akt, a target of CTMP, and the neurological outcome of 6-month old rats was improved by CTMP silencing that also increased the phosphorylated Akt and GSK-3β. Together, these lines of evidence strongly suggest a critical role of CTMP in determining the brain ischemic tolerance with aging.
The role of CTMP in ischemic brain injury has been shown in young animals [10, 11, 20]. CTMP was increased in the ischemic tissues after global brain ischemia in young rats. Silencing CTMP expression reduced neuronal death in these rats [10]. We showed that isoflurane application after brain ischemia induced neuroprotection. However, this protection was attenuated in mice fed high fat diet, possibly due to increased CTMP [11]. Finally, sevoflurane applied before brain ischemia induced neuroprotection possibly via inhibition of CTMP expression in young rats [20]. These results suggest a critical role of CTMP in brain ischemia-induced neuronal death in young rodents. Our current study extends this previous finding by suggesting an important role of CTMP increase in aging-dependent decrease of brain ischemic tolerance.
CTMP is an endogenous inhibitor of Akt [9]. Akt is activated by phosphorylation of the Thr308 residue in the Akt kinase domain and the Ser473 residue in the carboxy-terminal regulatory domain [10, 11]. CTMP binds the carboxyl terminal regulatory domain of Akt at the plasma membrane and inhibits the activity of Akt by interfering the phosphorylation of Akt on its Thr308 and Ser473 residues [9]. Active Akt can phosphorylate GSK-3β at Ser9, which decreases GSK-3β activity to reduce cell injury/death [10, 11]. One of the mechanisms for GSK-3β to induce cell injury is that GSK-3β can enhance the release of cytochrome c from the mitochondria to the cytosol and, therefore, induce cell apoptosis [21, 22].
Interestingly, there was a large increase in CTMP in the ischemic brain tissues of 6-month old rats in our study. Associated with this increase, the phosphorylated Akt was increased and there was a relatively small increase of phosphorylated GSK-3β in the ischemic brain tissues compared with brain tissues from control rats. This change pattern is consistent with a previous study using a global brain ischemia model in 5- to 6-week old rats [10]. The increased Akt phosphorylation in the presence of increased CTMP may be a compensatory effect. However, the increased activated/phosphorylated Akt may not translate into a proportional increase of Akt activity as shown in the previous study [10] and as reflected by a small increase in the phosphorylated GSK-3β in our study. Importantly, RNA silencing of CTMP further increased the phosphorylated Akt and GSK-3β in the ischemic brain tissues. These results, together with the findings that CTMP silencing improves neurological outcome after brain ischemia, suggest a critical role of CTMP-Akt-GSK-3β pathway in determining the severity of brain ischemic injury.
Our study clearly showed that phosphorylation of Akt was decreased with aging in cerebral cortex. We have found only one study determining changes of Akt phosphorylation with aging in the brain. That study showed that phosphorylation of Akt was increased with aging in the hippocampus, cerebellum, striatum and thalamic area but not in the cerebral cortex of rats [23]. The reasons for the different finding between this previous study and our study are not clear. However, the previous study did not specify which phosphorylation site of Akt was detected. Also, phosphorylated Akt in the cerebral cortex of the older rats trends to be lower than that in the younger rats in the previous study [23].
Our finding may have significant implications. The pathway of CTMP-Akt may be targeted to improve neurological outcome after brain ischemia. The finding that CTMP is increased with aging may advance our understanding of brain aging process at molecular level. This aging-related change may contribute to not only the decreased brain ischemic tolerance but also neurodegenerative diseases in the aged brain.
We used two focal brain ischemia models in this study. Initially, we used an intraluminal suture method to induce MCAO, a popular rodent model for focal brain ischemia [24], in both young and older rats. However, all of our 25 20-month old Fischer 344 rats died within 24 h after the MCAO. All of the 25 older rats had subarachnoid hemorrhage, assuming that this bleeding contributed to the death of the animals and was caused by sudden reperfusion to the ischemic brain region with fragile blood vessels in the older rats. The embolic focal brain ischemic model was then used. No rats in the 2-month and 20 month old groups developed subarachnoid hemorrhage in this model. Thus, two focal brain ischemia models were used in our study. The increased ischemic brain injury with aging in both models suggests that this phenomenon is not animal brain ischemia model specific.
Our study may have limitations. For example, we have not investigated how aging increases CTMP. Future studies shall investigate it so that possible targets may be identified for improving neurological outcome after stroke in the elderly.
In summary, our results suggest that CTMP increase may contribute to the decreased brain ischemic tolerance with aging. CTMP may be a therapeutic target for attenuating the neurological injury after ischemic stroke in the elderly.
Acknowledgments
Grant support: This study was supported by grants (R01 GM098308 and R21 AG047472 to Z Zuo) from the National Institutes of Health, Bethesda, MD, the Robert M. Epstein Professorship endowment, University of Virginia, Charlottesville, VA, and National Key Research and Development Program of China (2016YFC1300600).
Footnotes
Disclosure/conflict of interest: The authors declare no competing financial interests.
Author contributions: ZZ conceived the project. JL, WS and ZZ designed the study, JL and WS performed the experiments. JL did the initial data analysis and drafted the manuscript. ZZ performed the final data analysis and rewrote the manuscript.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Ay H, Koroshetz WJ, Vangel M, et al. Conversion of ischemic brain tissue into infarction increases with age. Stroke. 2005;36(12):2632–2636. doi: 10.1161/01.STR.0000189991.23918.01. [DOI] [PubMed] [Google Scholar]
- 3.Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol. 2007;113(3):277–293. doi: 10.1007/s00401-006-0164-7. [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi H, Wu Z. Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res. 2009;201(1):1–7. doi: 10.1016/j.bbr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Zuo Z. Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neurosci. 2011:19944–50. doi: 10.1016/j.neuroscience.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34(3):249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 8.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 9.Maira SM, Galetic I, Brazil DP, Kaech S, Ingley E, Thelen M, Hemmings BA. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science. 2001;294(5541):374–380. doi: 10.1126/science.1062030. [DOI] [PubMed] [Google Scholar]
- 10.Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A, Zukin RS. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12(5):618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu H, Deng J, Zuo Z. High-fat diet reduces neuroprotection of isoflurane post-treatment: Role of carboxyl-terminal modulator protein-Akt signaling. Obesity (Silver Spring) 2014;22(11):2396–2405. doi: 10.1002/oby.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CX, Yang Y, Yang T, Shuaib A. A focal embolic model of cerebral ischemia in rats: introduction and evaluation. Brain Res Brain Res Protoc. 2001;7(2):115–120. doi: 10.1016/s1385-299x(01)00049-6. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Yin J, Li L, Deng J, Feng C, Zuo Z. Isoflurane postconditioning reduces ischemia-induced nuclear factor-kappaB activation and interleukin 1beta production to provide neuroprotection in rats and mice. Neurobiol Dis. 2013;54:216–224. doi: 10.1016/j.nbd.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JJ, Li L, Jung H-H, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng J, Zhang J, Feng C, Xiong L, Zuo Z. Critical role of matrix metalloprotease-9 in chronic high fat diet-induced cerebral vascular remodelling and increase of ischaemic brain injury in mice. Cardiovasc Res. 2014;103(4):473–484. doi: 10.1093/cvr/cvu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Tan H, Jiang W, Zuo Z. Amantadine alleviates postoperative cognitive dysfunction possibly by increasing glial cell line-derived neurotrophic factor in rats. Anesthesiology. 2014;121(4):773–785. doi: 10.1097/ALN.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Feng C, Zhao H, Ren X, Peng S, Zuo Z. Autoregulation of inducible nitric oxide synthase expression by RNA interference provides neuroprotection in neonatal rats. Theranostics. 2015;5(5):504–514. doi: 10.7150/thno.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin J, Li H, Feng C, Zuo Z. Inhibition of brain ischemia-caused Notch activation in microglia may contribute to isoflurane postconditioning-induced neuroprotection. CNS NEUROL DISORD-DR. 2014;13(4):718–732. doi: 10.2174/1871527313666140618110837. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Huang W, Zuo Z. Perioperative aspirin improves neurological outcome after focal brain ischemia possibly via inhibition of Notch 1 in rat. J Neuroinflammation. 2014:1156. doi: 10.1186/1742-2094-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Nie H, Tian L, et al. Sevoflurane preconditioning-induced neuroprotection is associated with Akt activation via carboxy-terminal modulator protein inhibition. Br J Anaesth. 2015;114(2):327–335. doi: 10.1093/bja/aeu271. [DOI] [PubMed] [Google Scholar]
- 21.Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20(7):2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endo H, Nito C, Kamada H, Nishi T, Chan PH. Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006;26(12):1479–1489. doi: 10.1038/sj.jcbfm.9600303. [DOI] [PubMed] [Google Scholar]
- 23.Song GY, Kang JS, Lee SY, Myung CS. Region-specific reduction of Gbeta4 expression and induction of the phosphorylation of PKB/Akt and ERK1/2 by aging in rat brain. Pharmacol Res. 2007;56(4):295–302. doi: 10.1016/j.phrs.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27(9):1616–1622. doi: 10.1161/01.str.27.9.1616. discussion 1623. [DOI] [PubMed] [Google Scholar]