Abstract
Training chronic, cortically-blind (CB) patients on a coarse [left-right] direction discrimination and integration (CDDI) task recovers performance on this task at trained, blind field locations. However, fine direction difference (FDD) thresholds remain elevated at these locations, limiting the usefulness of recovered vision in daily life. Here, we asked if this FDD impairment can be overcome by training CB subjects with endogenous, feature-based attention (FBA) cues. Ten CB subjects were recruited and trained on CDDI and FDD with an FBA cue or FDD with a neutral cue. After completion of each training protocol, FDD thresholds were re-measured with both neutral and FBA cues at trained, blind-field locations and at corresponding, intact-field locations. In intact portions of the visual field, FDD thresholds were lower when tested with FBA than neutral cues. Training subjects in the blind field on the CDDI task improved FDD performance to the point that a threshold could be measured, but these locations remained impaired relative to the intact field. FDD training with neutral cues resulted in better blind field FDD thresholds than CDDI training, but thresholds remained impaired relative to intact field levels, regardless of testing cue condition. Importantly, training FDD in the blind field with FBA lowered FDD thresholds relative to CDDI training, and allowed the blind field to reach thresholds similar to the intact field, even when FBA trained subjects were tested with a neutral rather than FBA cue. Finally, FDD training appeared to also recover normal integration thresholds at trained, blind-field locations, providing an interesting double dissociation with respect to CDDI training. In summary, mechanisms governing FBA appear to function normally in both intact and impaired regions of the visual field following V1 damage. Our results mark the first time that FDD thresholds in CB fields have been seen to reach intact field levels of performance. Moreover, FBA can be leveraged during visual training to recover normal, fine direction discrimination and integration performance at trained, blind-field locations, potentiating visual recovery of more complex and precise aspects of motion perception in cortically-blinded fields.
Keywords: hemianopia, rehabilitation, stroke, perceptual learning, global motion
Introduction
Cortically blind (CB) subjects possess multiple residual visual abilities within their blind field (Mazzi, Savazzi, & Silvanto, 2017; Sanchez-Lopez et al., 2017; Weiskrantz, Warrington, Sanders, & Marshall, 1974). Visual perceptual training in CB fields can further improve performance on trained tasks, even if the subject initially had no residual ability to perform the task, recovering some of the vision lost at the trained locations (Bergsma, Elshout, van der Wildt, & van den Berg, 2012; Bergsma & van der Wildt, 2009; Chokron et al., 2008; Das, Tadin, & Huxlin, 2014; Huxlin et al., 2009; Raemaekers, Bergsma, van Wezel, can der Wildt, & van den Berg, 2011; Raninen, Vanni, Hyvarinen, & Nasanen, 2007; Sahraie et al., 2006; Sahraie et al., 2003; Vaina et al., 2014). Whereas performance enhancements tend to be most pronounced for trained tasks, relearning can also transfer to untrained tasks and stimuli. For instance, training on a coarse (left-right) direction discrimination and integration (CDDI) task inside CB fields improved contrast sensitivity (Das et al., 2014), fine direction discrimination (Cavanaugh et al., 2015; Das et al., 2014), the ability to discriminate untrained directional axes (Das et al., 2014) and to detect lights of different intensities, as in Humphrey perimetry (Cavanaugh & Huxlin, 2017). However, in spite of this broad transfer of learning, vision recovered post-CDDI training is not normal, remaining coarse and of poor contrast (Das et al., 2014), potentially limiting its usefulness in daily life (Cavanaugh, Lilley, Melnick, Reisner, & Huxlin, 2016). Specifically, whereas CDDI training recovers the ability to discriminate large direction differences, the ability to discriminate small direction differences remains impaired. Fine direction discrimination (FDD) is mediated by neurons with preferences near the most relevant directions of motion (Jazayeri & Movshon, 2007; Purushothaman & Bradley, 2005). Given that CB subjects have lost a large number of their direction- (and orientation-) selective neurons, they may simply lack the neural architecture required to perform fine-grained, selective tasks, while retaining enough direction (and orientation) selectivity to perform coarse discriminations.
Alternatively, our results in CB patients (Cavanaugh et al., 2015) and research in visually intact subjects with the Perceptual Template Model (PTM; (Dosher & Lu, 1999) suggest that fine discrimination performance could also be limited by a different form of impaired signal processing. Internal processing noise within the visual system can increase following visual cortex damage (Hayes & Merigan, 2007). Indeed, according to the PTM and Linear Amplifier Model (LAM), CB patients exhibit dramatically elevated internal processing noise for global direction discriminations performed in their blind field (Cavanaugh et al., 2015). After training on the CDDI task, internal noise decreased at trained blind-field locations, but they never reached intact field levels (Cavanaugh et al., 2015). Thus, a training task better able to reduce internal processing noise could potentially help recover fine discriminations closer, or even back to normal levels.
One way to decrease internal processing noise is to incorporate a visual attention cue into the training task. In visually intact subjects, spatial covert attention has been shown to modulate visual processing, improving performance on a wide-range of visual tasks and noise levels (for review, see Carrasco, 2011). These spatial attention cues increase efficacy of training and enable perceptual learning (Donovan, Szpiro, & Carrasco, 2015; Szpiro & Carrasco, 2015) as well as learning transfer to untrained locations (Donovan et al., 2015; Szpiro & Carrasco, 2015)..
Another type of attention, feature based attention (FBA), in which a subject attends to a specific feature of the stimulus rather than to a specific location in space, can also improve FDD (Ling, Liu, & Carrasco, 2009). In addition, FBA can benefit performance in various visual tasks; e.g., stimulus detection (Baldassi & Verghese, 2005), as well as discrimination of motion direction and speed (Ling et al., 2009; Treue & Martinez Trujillo, 1999), orientation (AL White & Carrasco, 2011) and saturation (A White, Rolfs, & Carrasco, 2015). FBA has been well characterized using psychophysics, neuroimaging and electrophysiology (reviewed in Carrasco, 2011). FBA cues are thought to improve performance across multiple tasks through a combination of boosted gain and sharpened tuning for population responses to the attended feature (Herrmann, Heeger, & Carrasco, 2012; Ling et al., 2009). This combination effect is able to provide performance improvements when tested at multiple external noise levels, which is a critical impairment in CB subjects (Cavanaugh et al., 2015). In addition, numerous studies have shown that unlike spatial attention, which results in retinotopically specific performance enhancement, FBA improvements occur even when relevant and irrelevant information overlaps spatially (Liu, Larsson, & Carrasco, 2007), and they generalize across the [intact] visual field (Martinez-Trujillo & Treue, 2004; Serences & Yantis, 2007; Treue & Martinez Trujillo, 1999; AL White & Carrasco, 2011). For these reasons, we hypothesized that manipulating FBA may be an optimal way of enhancing training-induced visual recovery of FDD in CB fields.
Though not extensively studied, some aspects of visual attention have been shown to remain effective in CB subjects following V1 damage. For instance, covert spatial attention was reported to improve stimulus detection (Poggel, Kasten, Muller-Oehring, & Sabel, 2006) and decrease reaction times (Kentridge, Heywood, & Weiskrantz, 2004), and temporal cueing was reported to improve detection (Kentridge, Heywood, & Weiskrantz, 1999). In addition, emotional stimuli within the blind field can act as a cue to modify performance in the intact field (Bertini, Cecere, & Làdavas, 2017), likely by utilizing visual pathways that bypass the primary visual cortex (Gerbella, Caruana, & Rizzolatti, 2017). Only one study has manipulated attention during training in CB, reporting that spatial cueing enhanced the amount of vision recovered following training in a detection task (Poggel, Kasten, & Sabel, 2004). However, this study used the NovaVision detection-training paradigm, which lacked proper fixation control, likely confounding the results attained (Horton, 2005; Reinhard et al., 2005; Schreiber et al., 2006).
Here, we asked whether cueing could leverage FBA in CB subjects to improve FDD back to intact field levels. The present study is the first to: (a) manipulate FBA in CB subjects, allowing us to examine the effect of FBA in intact and blind portions of the visual field; (b) utilize FBA to enhance training and rehabilitation in CB patients, and (c) examine the effects of FBA on perceptual learning.
Methods
Subjects
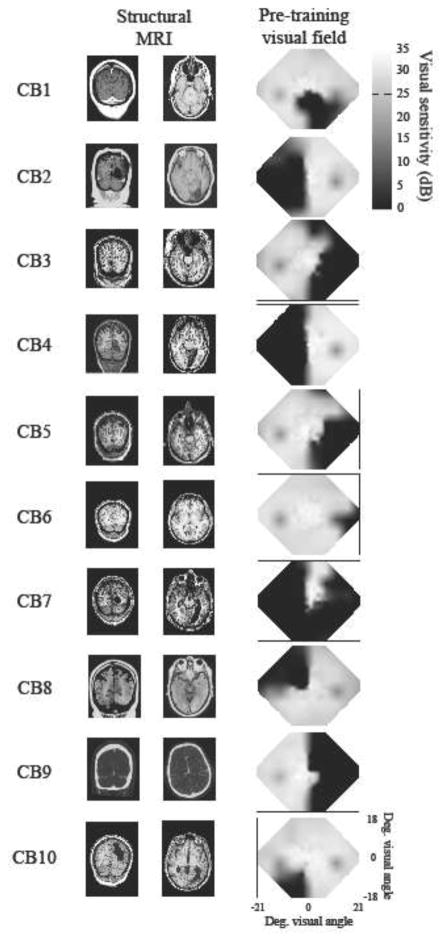
Ten CB subjects (Table 1) were recruited at least six months after a stroke resulting in damage to the primary visual cortex (V1). Injury was verified using structural MRIs or CTs (Figure 1) and homonymous visual field defects were confirmed with Humphrey visual field tests (HVF). Composite renditions of these HVFs shown in Figure 1 were created by first plotting luminance detection values obtained from 24-2 and 10-2 HVFs into a matrix (see detailed protocol in Cavanaugh and Huxlin, 2017). When the locations coincided, the values were averaged together before performing a two-dimensional interpolation between tested data points. Subjects were excluded if they were unable to fixate precisely (error greater than ± 1° relative to fixation spot) during psychophysical testing. We also excluded subjects suffering from neglect or ocular diseases, which could interfere with visual performance. None of the subjects used psychoactive drugs, such as anti-depressants, and all had their visual acuity corrected to normal (with glasses or contact lenses) during training and testing. All patient-related procedures performed in the present study were approved by the Institutional Review Board of the University of Rochester Medical Center and adhered to the tenets of the Declaration of Helsinki.
Table 1. Subject information.
Each training tasks was performed at a different blind field location
| Subjects | Gender | Age (years) | Time post-lesion (months) | Affected hemifield | Eye(s) trained & tested | First training task | Second training task | Third training task |
|---|---|---|---|---|---|---|---|---|
| CB1 | M | 49 | 24 | Bilateral | Both | CDDI | N/A | N/A |
| CB2 | F | 55 | 24 | Left | Right | CDDI | N/A | N/A |
| CB3 | M | 72 | 60 | Right | Left | CDDI | N/A | N/A |
| CB4 | F | 55 | 48 | Left | Both | CDDI | FDD+FBA | N/A |
| CB5 | M | 67 | 72 | Right | Left | CDDI | FDD+FBA | N/A |
| CB6 | F | 38 | 24 | Right | Both | CDDI | FDD+FBA | N/A |
| CB7 | M | 21 | 48 | Bilateral | Both | CDDI | FDD+Neutral | FDD+FBA |
| CB8 | M | 69 | 6 | Left | Both | FDD+Neutral | FDD+FBA | N/A |
| CB9 | M | 76 | 24 | Right | Left | FDD+Neutral | N/A | N/A |
| CB10 | F | 69 | 36 | Left | Both | FDD+Neutral | N/A | N/A |
Figure 1. Brain scans and visual fields of CB subjects.
A. T1-weighted MRI images for CB1–7 and CB9–10, and CT images for CB8 (for whom MR images were unavailable) showing lesion locations. Left is left and right is right on each picture. B. Composite baseline visual fields. Areas of relative vision are indicated by white to grey shading, while relative blindness is shown in black. Composites fields were created by plotting luminance detection values obtained from 24-2 and 10-2 Humphrey visual field tests into a matrix (as in Cavanaugh and Huxlin, 2017). If these locations coincided, the dB values were averaged together. Two-dimensional interpolation between tested data points then filled the empty spaces between values. All values are measured in dB (scale bar on far right).
Apparatus and eye tracking
Fixation accuracy could not be assessed during in-home training, but threshold performance reported here was measured during pre- and post-training in-lab testing when eye position was tracked using an Eyelink 1000 eye tracker (SR Research, Mississauga, Ontario, Canada). Tracking was binocular (CB1, CB4, CB6, CB7, CB8, CB10, or monocular using a subject’s dominant eye with the non-tracked eye patched in subjects with convergence issues (CB2, CB3, CB5, CB9). During each trial, subjects were asked to fixate a small target at the center of a CRT monitor (HP 7217A, 48.5×31.5 cm, 1024×640 pixel resolution, 120 Hz frame rate), whose luminance was calibrated with a ColorCal II automatic calibration system (Cambridge Research Systems). Stimuli were presented in a gaze-contingent manner in either intact or blind regions of the visual field. For subjects CB1 and CB7, who possessed bilateral cortical damage and vision loss, regions with spared vision were used in place of the contralateral intact hemifield, as an internal control. Viewing distance to the CRT monitor was 42 cm, enforced by a chin/forehead rest. In-lab experiments were conducted using MATLAB (The MathWorks, Natick, MA) and Psychtoolbox (Pelli, 1997). The Eyelink 1000 eye tracker used to enforce fixation is accurate to within 0.25°, with a sampling frequency of 1000 Hz (SR Research, Mississauga, Ontario, Canada). We allowed our subjects a fixation window of only ±1° around the fixation spot. If they broke fixation, the trial was aborted, reshuffled and patients received a noxious auditory tone as feedback, reminding them to improve their fixation accuracy.
Testing and training stimuli and tasks
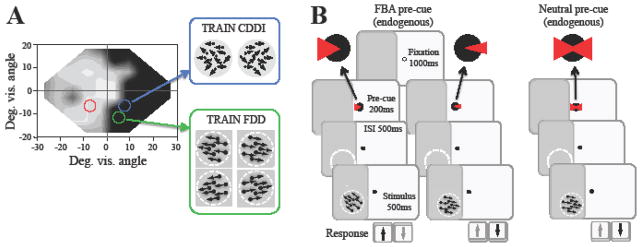
Coarse direction discrimination and integration (CDDI) task (Figure 2A)
Figure 2. Training and attention paradigms.
A. Sample visual field of a CB subject illustrating regions of relatively normal vision (white-gray) and regions of impairment (black). Blue circle indicates a CDDI training location, while the green circle marks an FDD training location. The red circle marks an eccentrically identical intact-field testing location for the blue training location. B. Subjects trained on FDD performed one of two potential versions of the task. In the FBA condition, subjects fixated for 1000ms at a central fixation point, followed by a wedge-shaped pre-cue at fixation for 200ms. The side of presentation (left or right) indicated the base direction of the upcoming trial, while the size of the cue indicated the relative difficulty of the upcoming trial (smaller cue indicating a smaller angle of discrimination and thus a harder task). In the Neutral condition, the pre-cue was presented simultaneously on both sides of fixation, providing no information about the base direction of the upcoming trial. The size of the cue also did not change to indicate relative difficulty. In both versions of the task, the cue was followed by a 500ms ISI, and then a 500ms duration stimulus to be discriminated. Subjects are asked to indicate if the stimulus was moving above or below the horizontal meridian.
Stimulus dots moved globally with a variable range of directions, uniformly distributed around the left- or rightward vectors (Huxlin et al., 2009; Das et al., 2014). On each trial, subjects were asked to report the stimulus’ global direction of motion by pressing the left or right arrow keys on a keyboard. Task difficulty was adjusted using a 3:1 staircase, which increased direction range from 0° to 360° in 40° steps after each set of 3 consecutive, correct responses, and decreased it by one 40° step for every incorrect response (Huxlin et al., 2009; Das et al., 2014). Auditory feedback was provided indicating the correctness of each response. For each session, we computed performance as a function of direction range level and then fitted a Weibull function to the data. We obtained direction range thresholds from the Weibull fits by determining the direction range level at which performance reached 75%-correct. Finally, direction range thresholds were normalized to the maximum possible range of dot directions (360°), generating a normalized direction range (NDR) threshold, defined as:
Fine direction discrimination (FDD) tasks (Figure 2B): stimulus dots moved with 0° range (100% coherence) in one of two base directions (either leftward or rightward), but at an angle above or below the horizontal meridian. Whether dots moved left or right, and up or down, was chosen randomly for each trial. Task difficulty was adjusted with a 3:1 staircase, which decreased the size of the angle between the direction of motion and the horizontal meridian using the following steps: 90, 75, 60, 45, 30, 25, 15, 10, 5, 2.5, and 1°. Subjects were only asked to discriminate the upward/downward deviation from horizontal, not the rightward/leftward motion component. This was an essential aspect of task design that allowed us to use FBA cues during training and testing. Two cueing conditions were always present in the fine discrimination task. In the first condition, the basic endogenous, FBA cue consisted of a white triangle that appeared 350ms prior to the visual stimulus and lasted for 200 ms, followed by a 150 ms inter-stimulus interval (Figure 2B). This cue could point to the left or the right of fixation to indicate that the following trial would contain a leftward or a rightward base direction, respectively (Herrmann et al., 2012). Additionally, the width of the cue changed size to reflect the relative difficulty of the upcoming trial, with a smaller size indicating a smaller, upcoming angle of discrimination, and thus a harder trial.
In the neutral cueing condition, subjects performed an identical task, except that they were presented with a central cue indicating both leftward and rightward base directions simultaneously, so that the temporal certainty regarding the stimulus onset was the same in both cueing conditions. Thus, the neutral cue provided no information about the base-direction of the upcoming trial. The triangles comprising the cue also remained of a constant size, and thus did not indicate the relative difficulty of the upcoming trial.
Both coarse and fine global direction discrimination tasks required subjects to fixate on the central spot for 1000 ms before a random dot stimulus appeared in a 5° diameter circular aperture, at a pre-destined location in their peripheral visual field. Black dots moved on a mid-grey background with a 250 ms lifetime, a speed of 10 deg/s, and a density of 3 dots/deg2. Stimuli were presented for 500 ms, accompanied by a tone to indicate stimulus onset. Subjects responded using arrows on a keyboard to indicate the perceived direction of motion, either left vs. right for the CDDI tasks or up vs. down for FDD tasks. Auditory feedback was provided on every trial to indicate correct and incorrect responses. Performance for each session was fit using a Weibull function with a threshold criterion of 75%-correct used to calculate direction difference thresholds for the FDD task, and direction range thresholds for the CDDI task.
Training protocols
After in-lab instructions and pre-training testing, CB subjects trained at home using a lab-issued chin/forehead rest and software customized to their own computer and monitor’s specifications (dimensions, resolution and refresh rate). Viewing distance from the chin-rest to the monitor was 42 cm. We assessed by the accuracy of in-home set up by having patients repeat several of the in-lab pre-tests at home. Only if/when they were able to replicate in-lab performance at home were they allowed to start training. Subjects were also told that poor fixation during training could prevent recovery, and that their home-training results would be verified in-lab with eye tracking. All subjects in the present study generated home-training data that was replicated with eye tracking in the lab during post-training tests.
Initial training locations were chosen following in-lab mapping of the visual field with the training tasks. Each training session consisted of 300 trials, and subjects were expected to complete one session per day, a minimum of five days per week, at each training location. After completing a session, the program automatically closed and created a log file detailing trial-by-trial performance, which was sent weekly to the laboratory for analysis and fitting to compute thresholds. For CDDI training, once thresholds at a given blind field location reached the normal range (defined by each subject’s measured performance at equivalent locations in their intact field of vision pre-training), and stayed within the normal range for at least 5 consecutive training sessions, the training location was moved deeper into the blind field by 1° along the x-axis (in Cartesian coordinate space). For FDD training, we did not know if performance could return to intact field levels, and as such, subjects trained until thresholds stabilized for at least 20 sessions before shifting training deeper into the blind field by 1° along the x-axis. This process was repeated for at least six months, or until a subject had successfully trained a minimum of 2 blind field locations. At that point, subjects were brought back to the lab for verification of their home training performance with fixation control.
Baseline and post-training tests
All subjects were tested identically regardless of planned training category. All ten subjects were tested at intact and future blind field training locations with both CDDI and FDD with FBA cues, neutral cues and without cues (Figure 2B). For each task, mapping started at the vertical meridian and if a subject was able to perform above chance (50% correct), the stimulus was moved deeper into their blind field by a 1° lateral shift along the x-axis. This was repeated for a given axis until performance dropped to chance, at which point testing was moved to a new y-axis. For all subjects, training locations were chosen as the first location where performance on their respective training task dropped from above chance, to chance, following a one-degree lateral shift along the x-axis. As such, all training began at approximately chance performance with no measurable direction range or direction difference thresholds.
CB1–CB7 underwent CDDI training (Table 1). CB4–CB8 trained on FDD with FBA cues. CB7–CB10 trained on FDD with neutral cues. In all cases, training was performed only at previously untrained (i.e. naive), blind field locations. This was particularly important for CB4–7, who trained on CDDI before FDD training, and CB7–8, who trained with neutral cues before training with FBA cues. The ability to train two different blind field locations in the same subject was made possible by the large size of most CB visual field defects, and the fact that prior work from our group (Cavanaugh & Huxlin, 2017; Cavanaugh et al., 2015; Das et al., 2014; Huxlin et al., 2009) showed training to recover discrimination performance only at trained, blind field locations. Untrained, blind field locations never exhibited spatial transfer of learning; this was verified in the present experiments by showing that initial discrimination performance at all training locations was at chance (i.e., around 50% correct). Once participants were deemed to have improved and stabilized on their trained tasks at home (see above for criteria), they were brought back to the laboratory for a repeat of all baseline tests under conditions of gaze-contingent stimulus presentation and fixation control.
Results
Effect of FBA and neutral cues in intact portions of CB visual fields
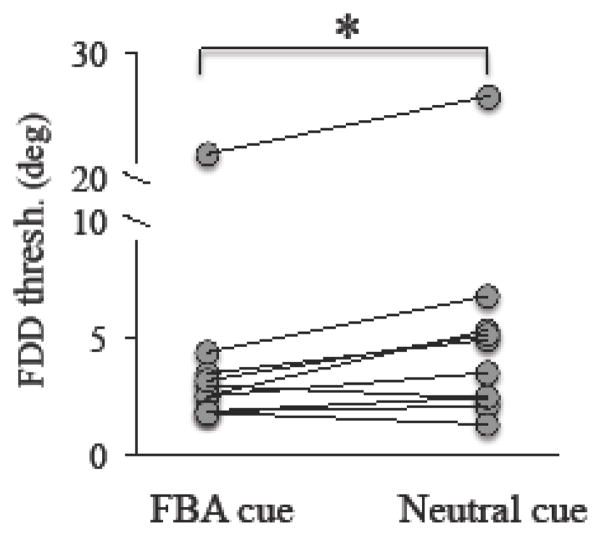
When tested at intact field locations, FBA cues resulted in significantly lower FDD thresholds than in the neutral cueing condition (paired t-test, t9 = 2.72, p = 0.024; this and all other comparisons performed in the present study are two-tailed tests). With neutral cues, CB patients achieved average FDD thresholds of 6.04 ± 7.40° (average ± SD), ranging from 1.3° to 26.5° (Figure 3). The same locations reached average thresholds of 4.64 ± 6.12° when tested with Endo-FBA cues, ranging from 1.8° to 21.9°. In one subject (CB9), thresholds under both testing conditions were more than two standard deviations from the mean. Removal of these thresholds did not significantly affect the analysis (FBA cued threshold: 2.72 ± 0.87°, Neutral cued threshold: 3.77 ± 1.85°; paired t-test, t8 = 2.54, p = 0.035).
Figure 3. Effect of FBA in the intact visual field.
In the intact field, performance on fine discrimination was significantly improved by testing with the FBA cue compared to testing with the Neutral cue. Each circle represents a single subject’s performance, with lines connecting an individual’s performance under the FBA and Neutral cue conditions. Paired student’s t-test, alpha = 0.05, *p=0.024.
Effect of CDDI training in CB fields
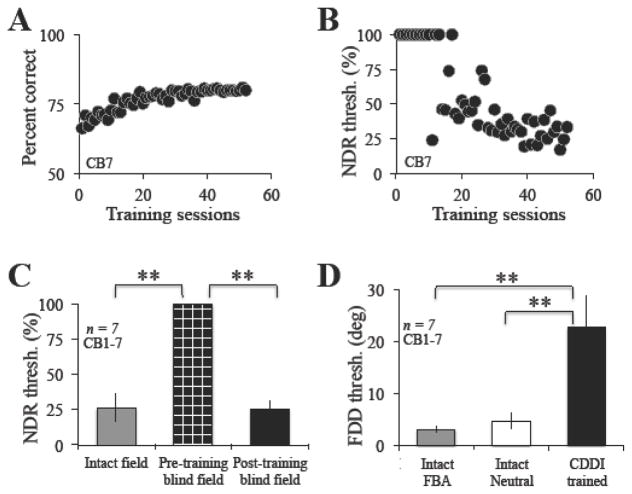
At CDDI training locations, baseline performance was at or just above chance and we were initially unable to measure an NDR threshold (Figure 4A–C). During training, performance increased until it plateaued around a ceiling of 80% correct, due to the 3:1 staircases used (Figure 4A). Once performance reached this level, subjects began to generate measurable, NDR thresholds (Figure 4B), which steadily fell until they reached levels similar to those of the intact field (Figure 4C, N = 7, Intact: 26.2 ± 9.8%; Trained Blind Field: 25.5 ± 6.1%; paired t-test, t6 = 0.26, p = 0.80).
Figure 4. Effect of CDDI training.
A. Representative example of training performance from CB7. Blind field coarse discrimination performance prior to training was around chance, but gradually rose until it plateaued at ~80%, the upper limit of percent correct performance due to the 3:1 staircase utilized in training. B. Once performance reached this upper limit, subjects began to generate a normalized direction range threshold, which steadily improved during the course of training, as in this example with CB7. C. Following training, normalized direction range (NDR) thresholds became significantly lower (black bar) than pre training (checkered black bar) and statistically similar to performance in intact portions of the visual field (grey bar). D. Following CDDI training, subjects partially recovered FDD thresholds (black bar). However, performance remained impaired relative to the intact field, regardless of how the intact field was tested (grey bar = FBA cue test, white bar = neutral cue test). Values are means ± SD. Paired student’s t-test, alpha = 0.05, **p<0.001.
Pre-training, FDD performance at blind field locations intended for CDDI training was also initially at or just above chance, and FDD thresholds could not be measured. Following CDDI training, and recovery of normal NDR thresholds, FDD thresholds averaged 24.2 ± 6.5 degrees (Figure 4D). Although this was a clear improvement relative to pre-training performance, post-training thresholds remained impaired relative to those at equivalent locations in the intact field of vision, even when measured with neutral cues, which provided temporal information about stimulus onset (Figure 4D, unpaired t-test, t14 = 4.9, p < 0.001).
Effect of FDD training with FBA cues in CB fields
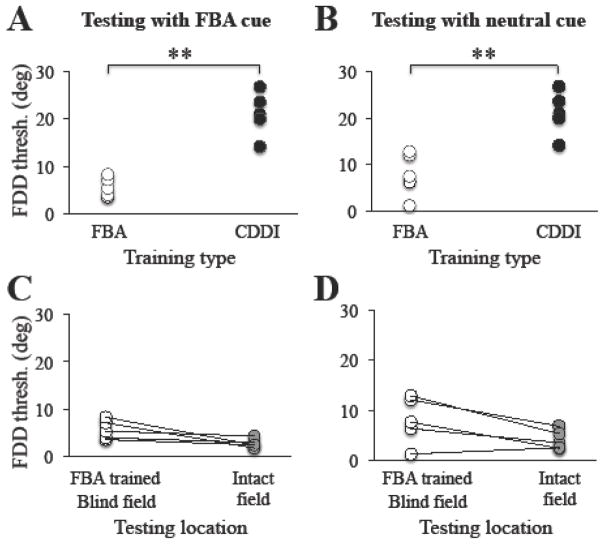
Initial performance on the FDD task was below that required to generate FDD thresholds regardless of whether subjects had previously trained with the CDDI task or not (CDDI trained subjects: N = 4, FDD threshold = 62.3 ± 5.7 deg; naïve (previously untrained) subjects: N = 3, FDD threshold = 65.7 ± 16.5 deg; unpaired t test: t5 = 0.39, p = 0.71). As subjects trained on the FDD task with FBA cues, their percent correct levels increased until they reached ~80%, at which time thresholds became measureable, then began to decrease and eventually stabilized (Figure 5A). On average this required 72 ± 13.3 training sessions. In-lab verification of performance tested with FBA cues showed that on average (± SD), trained CB subjects reached thresholds of 5.7 ± 1.8 deg at retrained, blind field locations; when tested with neutral cues at the same locations, they achieved thresholds of 8.0 ± 4.2 deg. Therefore, FDD+FBA trained subjects achieved significantly lower FDD thresholds than CDDI-trained subjects, whether post-tested with FBA cues (Figure 6A, unpaired t-test, t10 = 6.1, p < 0.001) or neutral cues (Figure 6B, unpaired t-test, t10= 4.7, p < 0.001),
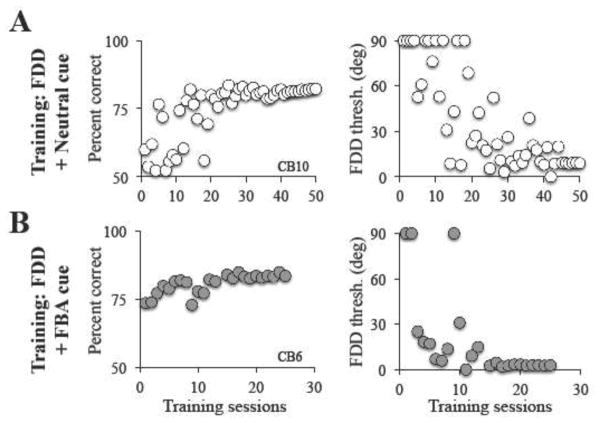
Figure 5. Sample training performance on FDD tasks with FBA and neutral pre-cues.
As with CDDI training, blind field fine discrimination performance prior to training was initially too poor to generate a threshold, both for subjects to be trained with the FBA cue (A) and subjects to be trained with the Neutral cue (B). Over the course of training, performance gradually rose until plateauing at ~80% correct due to the use of a 3:1 staircase. Once reaching ~80% correct subjects began to generate fine direction discrimination (FDD) thresholds within their blind field (right-most graphs).
Figure 6. Effect of FDD training with FBA cues across subjects.
A. Following fine direction discrimination (FDD) training with FBA cues, and when tested with the FBA cue, CB subjects obtained FDD thresholds (z paired student’s t-test). D. FDD thresholds measured at BFA-trained, blind field locations were also not significantly different from those in the intact visual field when tested with the neutral cue (grey circles, paired student’s t-test). Each circle represents a single subject’s performance, with lines connecting an individual’s performance across different categories when a paired comparison was used, **p<0.001.
In addition, when tested with FBA cues, FDD thresholds at trained, blind field locations reached levels statistically indistinguishable from those attained in the intact field (Figure 6C, Intact: 2.8 ± 0.85 deg; paired t-test, t4 = 2.56, p = 0.06). This effect persisted when testing trained blind-field and intact field with a neutral cue (Figure 6D, Intact: 4.1 ± 1.7 deg; paired t-test, t4 = 2.6, p = 0.06).
Effect of FDD training with neutral cues in CB fields
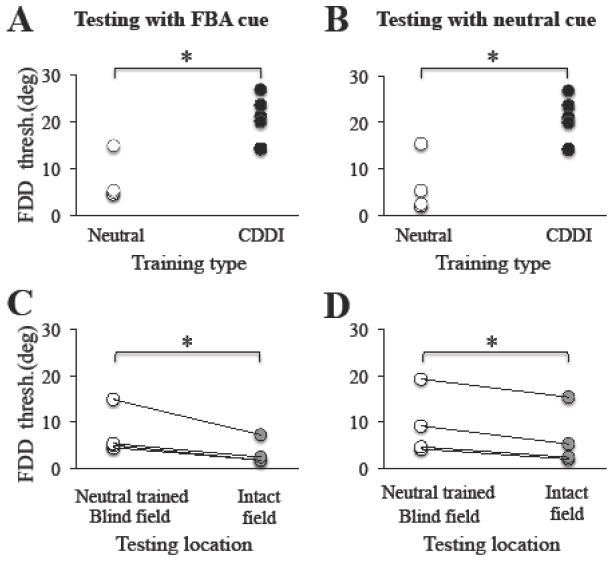
Subjects in the FDD+neutral cue training group also began training at locations where FDD thresholds initially could not be measured. They trained for a similar length of time as FBA cued subjects, requiring 82.8 ± 23.9 training sessions (Figure 5B). Following FDD training with a neutral cue, blind field locations attained thresholds averaging 9.4 ± 7 deg when tested with a neutral cue. This performance was significantly better than that of CDDI-trained subjects (Figure 7B, unpaired t test, t9=3.56, p=0.006). When tested at the same locations with FBA cues, mean FDD thresholds averaged 7.5 ± 5 deg, again significantly lower than FDD thresholds in CDDI-trained subjects (Figure 7A, unpaired t test, t9=4.43, p=0.0016). However, FDD thresholds remained higher than in the intact field, whether subjects were tested with FBA cues (Figure 7C, Intact: 3.4 ± 2.6; paired t-test, t3=3.45, p=0.04), or neutral cues (Figure 7D, Intact: 6.3 ± 6.2; paired t-test, t3=5.98, p=0.0093).
Figure 7. Effect of FDD training with neutral cues across subjects.
A. Following fine direction discrimination (FDD) training with a neutral cue, when tested with the FBA cue, CB subjects obtained FDD thresholds (white circles) significantly lower than CDDI-trained subjects (black circles, unpaired student’s t-test, p = 0.0016). All thresholds for the CDDI training group were collected using uncued testing described in the methods. B. This effect was also seen when subjects were tested with the neutral cue (white circles, unpaired student’s t-test, p = 0.006). C. However, in contrast with FDD training in the presence of FBA cues, FDD thresholds measured at blind field locations following FDD training with neutral cues remained higher than those in the intact visual field when tested with FBA cues (grey circles, paired student’s t-test, p = 0.04). D. FDD thresholds measured at FDD-trained, blind field locations in the presence of neutral cues were also higher than those in the intact visual field when tested with neutral cues (grey circles, paired student’s t-test, p = 0.0093). Each circle represents a single subject’s performance, with lines connecting an individual’s performance across different categories when a paired comparison was used, *p<0.05.
FDD training improves direction integration thresholds
As CDDI training results in partial transfer of learning regarding fine discriminations, we next tested whether FDD training with coherently moving dots improved integration of motion directions. As such, we measured direction range thresholds in 6 of the 8 subjects who trained on FDD tasks in their blind field (3 with FBA cues, 3 with neutral cues) prior to and following recovery of FDD thresholds. Testing was performed at both FDD-trained locations and at equivalent locations within intact portions of the visual field. Initially, blind field performance on the CDDI task was impaired relative to that of the intact field, averaging 63 ± 11% correct in the blind field (compared to 82 ± 4 % correct in the intact field - Figure 8, paired t-test, t6 = 4.4899, p = 0.004). Unsurprisingly, initial NDR thresholds in the blind field averaged 98.0 ± 5.4%, and were much higher than those in the intact field, 28.8 ± 10.9% (paired t-test, t6 = 13.895, p < 0.001). However, following FDD training, NDR thresholds reached an average of 33.9 ± 11.6%, significantly better than prior to training (paired t-test, t6=17.79, p < 0.001), and not significantly different from thresholds in the intact field of vision (paired t-test, t6 = 1.1243, p = 0.30).
Figure 8. Effect of FDD training on normalized direction range (NDR) thresholds.
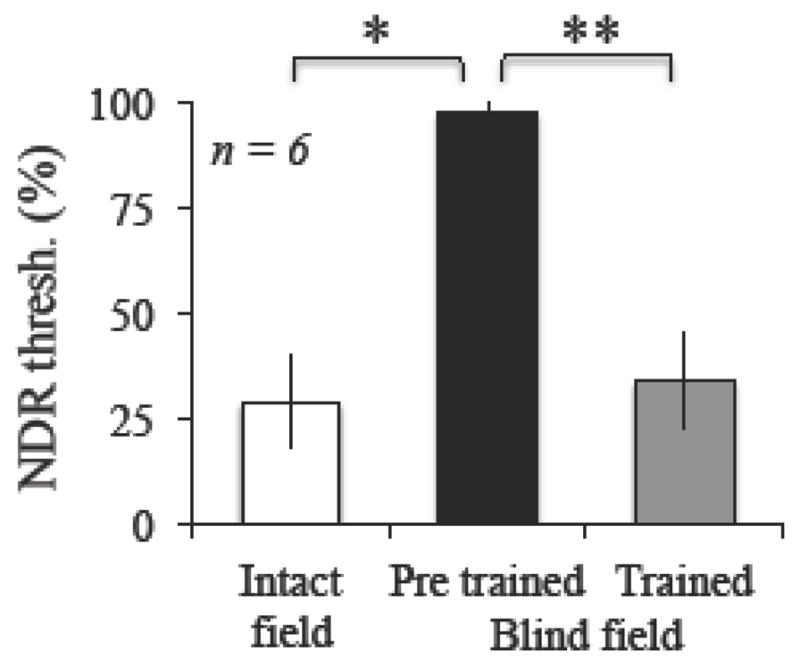
Prior to training, NDR thresholds in the blind field (black bar) were significantly impaired relative to the intact visual field (white bar) in all subjects. Following FDD training, regardless of cueing condition (grey bar), direction range thresholds improved. Performance following training became statistically similar to that of the intact visual field. No differences were observed between FBA-cued and Neutral-cued training cohorts (data not shown). Values are means ± SD. Paired student’s t-tests, alpha = 0.05, *p=0.004, **p<0.001.
Discussion
Training CB subjects on visual discrimination tasks within their blind field improves performance on the trained tasks and reduces the size of the perimetrically-measured visual deficit (Cavanaugh & Huxlin, 2017; Chokron et al., 2008; Das et al., 2014; Huxlin et al., 2009; Raninen et al., 2007; Sahraie et al., 2006; Vaina et al., 2014). However, the recovered vision is not perfect. Several key visual abilities, such as contrast sensitivity and fine discrimination performance (Das et al., 2014) remain impaired, reducing the quality and therefore likely usefulness of recovered vision in daily life (Cavanaugh et al., 2016). Here, we show that providing FBA cues during FDD training in the blind field can restore FDD performance back to normal. Our results mark the first time that FDD thresholds in CB fields have been seen to reach intact field levels of performance.
Feature-based attention in the intact field of CB patients
Although some visual deficits have been reported in the intact fields of CB subjects (Bola, Gall, & Sabel, 2013), the intact field is often used as an internal control as it possesses many visual thresholds similar to those of healthy individuals (Huxlin et al., 2009; ex. Raninen et al., 2007; Vaina et al., 2014). Here we show, for the first time, that FBA functions normally in the intact field of CB subjects: on average, FDD thresholds improved by ~2 deg when tested with FBA cues relative to testing with neutral cues. The magnitude of this benefit was similar to that seen when testing visually-intact subjects (Ling et al., 2009), who improved from a threshold of ~5 deg with the neutral cue to ~3 deg when tested with the FBA cue. Thus, at least with respect to FBA in FDD tasks, the intact fields of CB subjects appear to have similar visual processing abilities as those of visually-intact subjects, justifying the use of the intact field as an internal control for the purposes of the present study.
Effect of FDD training with FBA cues in CB fields
FDD training coupled with endogenous FBA cues resulted in significantly lower FDD thresholds than training on a left-right, global CDDI task. FDD thresholds at trained, blind field locations improved so much, that for the first time, we observed FDD thresholds that were not significantly different from to those of the intact field. This finding contrasted with CB subjects who trained on FDD coupled with a neutral cue, whose thresholds decreased but never reached intact levels of performance, even when tested with FBA cues.
Importantly, the benefit of FBA training persisted when patients were tested with a neutral cue, indicating that subjects did not just learn to attend better to the trained, blind field location. Had the benefit of training with the FBA cue disappeared when tested without the cue, it would have suggested that subjects had only learned how to extract information from the cue to improve task performance. Instead, manipulating FBA during training seemed to improve stimulus representation, making such recovery potentially more useful in day-to-day life.
FDD training with neutral cues was performed as a control for training with FBA cues. The neutral cue provided stimulation at fixation and the same temporal warning as the FBA cue, allowing us to rule out the possibility of increased arousal from the central cue, or changes/improvements in fixation due to the presence of additional central stimulation. We found that subjects who trained with neutral cues achieved better FDD thresholds than CDDI-trained subjects. This result shows that FDD training alone is able to improve fine difference thresholds over those attained following CDDI training. However, no matter what testing condition was used (FDD with FBA cue or FDD with neutral cue), subjects trained on FDD with a neutral cue did not perform as well as in their own intact fields. Therefore, the substantial improvement seen in the FBA training cohort appears not to be the result of changes in fixation, increased arousal, or difference in CDDI versus FDD training, but rather due to the use of an FBA manipulation during training.
An additional point of interest here is that the five patients who underwent FDD training automatically recovered normal direction integration thresholds (NDR thresholds) at the trained locations. The fact that both CDDI and FDD training can recover normal direction integration performance does in fact suggest that at least with respect to global motion, FDD training – especially with FBA cues – may be “more efficient”, as it automatically and fully recovers both fine direction discrimination and direction integration. In contrast, CDDI training only fully recovers direction integration performance. Nonetheless, before hastily concluding that FDD training is simply “better” than CDDI training as a therapeutic tool, it will be necessary to measure its efficacy at improving several other functions which normally improve following CDDI training: contrast sensitivity for direction and static orientation discriminations, transfer to other directional axes, transfer to other trained, blind field locations and luminance detection sensitivity (Huxlin et al., 2009; Das et al., 2014; Cavanaugh et al., 2015; Cavanaugh and Huxlin, 2017).
Finally, we should note that although the present findings are persuasive, the relatively small number of participants that underwent each type of training, as well as large inter-individual variability of FDD thresholds and of FBA effects, prevented us from conducting between-subjects analyses. However, we could compare performance prior to and following training within each subject, by contrasting the subject’s trained, blind field performance to his/her own intact field performance. Our results demonstrate that FDD+FBA training can improve blind field FDD thresholds back to intact field levels – a result that has never been reported and which was not obtained through CDDI training or FDD training with neutral pre-cues. We hope that these findings inspire future studies with a larger patient group.
Mechanistic implications
The results showing that FBA directed to motion direction improves visual performance in the absence of an intact V1 represent a novel finding. FBA has been shown to modulate activity within numerous visual areas (reviewed in Carrasco, 2011), enhancing neural activity in regions specialized in processing the attended feature (Liu et al., 2007; Liu, Slotnick, Serences, & Yantis, 2003; O’Craven, Rosen, Kwong, Treisman, & Savoy, 1997; Schoenfeld et al., 2007; Serences & Boynton, 2007). As the FDD task is probably mediated by neurons in MT (Jazayeri & Movshon, 2007; Purushothaman & Bradley, 2005), it is likely that FBA modulated neural processing in this area (Martinez-Trujillo & Treue, 2004; Treue & Martinez Trujillo, 1999) to improve subjects’ performance. Further supporting evidence for a potential role of MT in the perceptual improvements observed following FDD training came from the observation that subjects trained on FDD tasks automatically recovered normal NDR thresholds at trained, blind field locations. Direction integration is thought to be mediated by area MT in primates (Newsome & Paré, 1988; Rudolph & Pasternak, 1999); critical to our case, its homologue in humans–the human MT+ complex–appears to be intact and functional in most CB patients (Cowey & Stoerig, 1991; Kaycic, Triplett, Das, Martin, & Huxlin, 2015; Martin, Das, & Huxlin, 2012; reviewed in MD Melnick, Tadin, & Huxlin, 2016; Sincich, Park, Wohlgemuth, & Horton, 2004). Interestingly, subjects who acquire CB at a young age present with enhanced LGN-MT connectivity (Mikellidou et al., 2017; Mundinano et al., 2017), suggesting that MT has an enhanced role in processing visual information for CB subjects (Tamietto & Morrone, 2016).
Curiously however, CDDI training, which restores normal direction integration thresholds and is also thought to be mediated by an intact area MT in CB patients (Martin, Das, & Huxlin, 2010; M Melnick, Merriam, Heeger, & Huxlin, 2017), does not restore normal FDD thresholds in CB fields (Cavanaugh et al., 2015; Das et al., 2014). This finding suggests that even if they both occur in MT, the computations necessary for the two tasks are different. As previously reported by our group, CDDI training improves performance in CB fields through a reduction of internal processing noise, as opposed to changes in external noise exclusion (Cavanaugh et al., 2015). It is thus possible that FDD training, which involves stimuli with no external direction noise, was more efficient at reducing internal noise compared to CDDI training. Alternatively, FDD training may have resulted in more efficient external noise exclusion in addition to decreasing internal processing noise, allowing for better overall discrimination of the “noisier” CDDI stimuli. Another important question in this context is to determine exactly how FBA acts in a visual system lacking an intact V1 to alter processing mechanisms and induce the observed fine discrimination and integration improvements. In visually intact subjects, FBA produces better discrimination performance by boosting gain and sharpening tuning of the relevant neuronal populations (Baldassi & Verghese, 2005; Ling et al., 2009; Paltoglou & Neri, 2012). However, as this is the first study to use FBA during perceptual training, and to do so in V1-damaged individuals, it remains to be determined if FBA operates through the same mechanisms in CB patients as in visually intact subjects.
Conclusion and future directions
The present study showed that FBA is effective and appears to be intact and functioning normally in CB subjects. We also show that it is possible to leverage FBA during blind field training to induce better recovery of visual performance. FBA coupled with FDD training reduced fine direction discrimination thresholds at trained, blind field locations, restoring performance to levels similar to those of the intact visual field. Importantly, this effect persisted when FBA cues were removed, indicating that the recovered fine discrimination ability reflected an actual improvement in perceptual processing, not just an increased ability to deploy attention to the blind field. Finally, we report an interesting dissociation of learning transfer between fine direction discrimination and direction integration in cortically blind fields, with specific implications for processing mechanisms likely engaged by these two tasks. Our results mark the first time that FBA has been manipulated in perceptual learning or rehabilitation. They should lead to further interesting questions, such as what effect FBA might have on performance of more V1-dependent tasks, including fine orientation discrimination of simple Gabor patches (Hubel, 1982) in CB subjects.
By showing that FBA is effective in CB subjects, this study provides converging evidence that covert attention can improve perception in individuals with visual deficits (e.g. amblyopia, Roberts, Cymerman, Smith, Kiorpes, & Carrasco, 2016). The present study also underscores the potential benefit of systematically leveraging different forms of attention, including spatial (Donovan et al., 2015; Szpiro & Carrasco, 2015) and feature-based, to improve perception and overcome perceptual deficits in both neurotypical and special populations.
Highlights.
Visual feature-based attention is intact and functional in humans with V1 damage
Feature-based attention enhances training-induced visual recovery in the blind field
Training on fine direction discrimination recovers direction integration
Acknowledgments
The present study was funded by NIH (EY021209 to KRH, Core Center Grant P30 EY001319 to the Center for Visual Science (CVS), training grant T32 EY007125 to CVS, a pre-doctoral NRSA EY025918 to MRC), and by an unrestricted grant from the Research to Prevent Blindness (RPB) Foundation to the Flaum Eye Institute. They authors wish to thank Terrance Schaeffer, who performed Humphrey visual field tests on all patients presented here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldassi S, Verghese P. Attention to locations and features: Different top-down modulation of detector weights. Journal of Vision. 2005;5(6):556–570. doi: 10.1167/5.6.7. [DOI] [PubMed] [Google Scholar]
- Bergsma DP, Elshout JA, van der Wildt GJ, van den Berg AV. Transfer effects of training-induced visual field recovery in patients with chronic stroke. Top Stroke Rehabil. 2012;19(3):212–225. doi: 10.1310/tsr1903-212. [DOI] [PubMed] [Google Scholar]
- Bergsma DP, van der Wildt GJ. Visual training of cerebral blindness patients gradually enlarges the visual field. Br J Ophthalmol. 2009;94:88–96. doi: 10.1136/bjo.2008.154336. [DOI] [PubMed] [Google Scholar]
- Bertini C, Cecere R, Làdavas E. Unseen fearful faces facilitate visual discrimination in the intact field. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.07.029. [DOI] [PubMed] [Google Scholar]
- Bola M, Gall C, Sabel B. “Sightblind”: perceptual deficits in the “intact” visual field. Front Neurol. 2013;4(80):1–5. doi: 10.3389/fneur.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: The past 25 years. Vision Research. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh MR, Huxlin K. Visual discrimination training improves Humphrey perimetry in chronic cortically induced blindness. Neurology. 2017;88:1856–1864. doi: 10.1212/WNL.0000000000003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh MR, Lilley S, Melnick M, Reisner A, Huxlin K. Visual discrimination training shrinks cortically blind fields and improves quality of life in chronic stroke patients. Journal of Vision. 2016;16(12):31. [Google Scholar]
- Cavanaugh MR, Zhang R, Melnick M, Das A, Roberts M, Tadin D, … Huxlin K. Visual recovery in cortical blindness is limited by high internal noise. Journal of Vision. 2015;15(10):1–18. doi: 10.1167/15.10.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokron S, Perez C, Obadia M, Gaudry I, Laloum L, Gout O. From blindsight to sight: Cognitive rehabiliation of visual field defects. Restorative Neurology and Neuroscience. 2008;26:305–320. [PubMed] [Google Scholar]
- Cowey A, Stoerig P. The neurobiology of blindsight. Trends Neurosci. 1991;14(4):140–145. doi: 10.1016/0166-2236(91)90085-9. [DOI] [PubMed] [Google Scholar]
- Das A, Tadin D, Huxlin K. Beyond blindsight: Properties of visual relearning in cortically blind fields. The Journal of Neuroscience. 2014;34(35):11652–11664. doi: 10.1523/JNEUROSCI.1076-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan I, Szpiro S, Carrasco M. Exogenous attention facilitates location transfer of perceptual learning. Journal of Vision. 2015;15(10):11. doi: 10.1167/15.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher B, Lu ZL. Mechanisms of perceptual learning. Vision Research. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Caruana F, Rizzolatti G. Pathways for smiling, disgust and fear recognition in blindsight patients. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.08.028. [DOI] [PubMed] [Google Scholar]
- Hayes RD, Merigan WH. Mechanisms of Sensitivity Loss due to Visual Cortex Lesions in Humand and Macaques. Cerebral Cortex. 2007;17:1117–1128. doi: 10.1093/cercor/bhl021. [DOI] [PubMed] [Google Scholar]
- Herrmann KD, Heeger D, Carrasco M. Feature-based attention enhances performance by increasing response gain. Vision Research. 2012;74:10–20. doi: 10.1016/j.visres.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. Disappointing results from Nova Vision’s visual restortation therapy. Br J Ophthalmol. 2005;89:1–2. doi: 10.1136/bjo.2004.058214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. Evolution of ideas on the primary visual cortex, 1955–1978: a biased historical account. Biosci Rep. 1982;2(7):435–469. doi: 10.1007/BF01115245. [DOI] [PubMed] [Google Scholar]
- Huxlin K, Martin T, Kelly K, Riley M, Friedman D, Burgin W, Hayhoe M. Perceptual relearning of complex visual motion after V1 damage in humans. Journal of Neuroscience. 2009;29(13):3981–3991. doi: 10.1523/JNEUROSCI.4882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, Movshon JA. A new perceptual illusion reveals mechanisms of sensory decoding. Nature. 2007;446:912–915. doi: 10.1038/nature05739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaycic V, Triplett R, Das A, Martin T, Huxlin K. Role of inter-hemispheric transfer in generating visual evoked potentials in V1-damaged brain hemispheres. Neuropsychologia. 2015;68:82–93. doi: 10.1016/j.neuropsychologia.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentridge R, Heywood C, Weiskrantz L. Effects of temporal cueing on residual visual discrimination in blindsight. Neuropsychologia. 1999;37:479–483. doi: 10.1016/s0028-3932(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Kentridge R, Heywood C, Weiskrantz L. Spatial attention speeds discrimination without awareness in blindsight. Neuropsychologia. 2004;42:831–835. doi: 10.1016/j.neuropsychologia.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Ling S, Liu T, Carrasco M. How spatial and feature-based attention affect the gain and tuning of population responses. Vision Research. 2009;49:1194–1204. doi: 10.1016/j.visres.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Larsson J, Carrasco M. Feature-based attention modulates orientation-selective responses in human visual cortex. Neuron. 2007;55(2):313–323. doi: 10.1016/j.neuron.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Slotnick S, Serences J, Yantis S. Cortical mechanisms of feature-based attentional control. Cerebral Cortex. 2003;13(12):1334–1343. doi: 10.1093/cercor/bhg080. [DOI] [PubMed] [Google Scholar]
- Martin T, Das A, Huxlin K. Visual motion retraining of a cortically-blind field increases BOLD responses in peri-lesional cortex and MT+-a case study. Journal of Vision. 2010;9(8):666. [Google Scholar]
- Martin T, Das A, Huxlin K. Visual cortical activity reflects faster accumulation of information from cortically blind fields. Brain. 2012;135(11):3440–3452. doi: 10.1093/brain/aws272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr Biol. 2004;14(9):744–751. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Mazzi C, Savazzi S, Silvanto J. On the “blindness” of blindsight: What is the evidence for phenomonal awareness in the absence of primary visual cortex (V1)? Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Melnick M, Merriam E, Heeger D, Huxlin K. Training-induced recovery of fMRI-based motion adaptation signals in V1 damaged humans. Journal of Vision. 2017;17(7):16. [Google Scholar]
- Melnick M, Tadin D, Huxlin K. Relearning to see in cortical blindness. The Neuroscientist. 2016;22(2):199–212. doi: 10.1177/1073858415621035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikellidou K, Arrighi R, Aghakhanyan G, Tinelli F, Frijia F, Crespi S, … Morrone MC. Plasticity of the human visual brain after an early cortical lesion. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.10.033. [DOI] [PubMed] [Google Scholar]
- Mundinano I, Chen J, de Souza M, Sarossy MG, Joanisse MF, Goodale MA, Bourne JA. More than blindsight: Case report of a child with extraordinary visual capacity following perinatal bilateral occipital lobe injury. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.11.017. [DOI] [PubMed] [Google Scholar]
- Newsome W, Paré E. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Craven K, Rosen B, Kwong K, Treisman A, Savoy R. Voluntary attention modulates fMRI activity in human MT-MST. Neuron. 1997;18(4):591–598. doi: 10.1016/s0896-6273(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Paltoglou A, Neri P. Attentional control of sensory tuning in human visual perception. J Neurophysiol. 2012;107(5):1260–1274. doi: 10.1152/jn.00776.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Poggel D, Kasten E, Muller-Oehring E, Sabel B. Improving residual vision by attentional cueing in patients with brain lesions. Brain Research. 2006;1097:142–148. doi: 10.1016/j.brainres.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Poggel D, Kasten E, Sabel B. Attentional cueing improves vision restoration therapy in patients with visual field defects. Neurology. 2004;63:2069–2076. doi: 10.1212/01.wnl.0000145773.26378.e5. [DOI] [PubMed] [Google Scholar]
- Purushothaman G, Bradley DC. Neural population code for fine perceptual decisions in area MT. Nat Neurosci. 2005;8(1):99–106. doi: 10.1038/nn1373. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Bergsma D, van Wezel R, can der Wildt G, van den Berg A. Effects of vision restoration training on early visual cortex in patients with cerebral blindness investigated with functional magnetic resonance imaging. J Neurophysiol. 2011;105(2):872–882. doi: 10.1152/jn.00308.2010. [DOI] [PubMed] [Google Scholar]
- Raninen A, Vanni S, Hyvarinen L, Nasanen R. Temporal sensitivity in a hemianopic visual field can be improved by long-term training using flicker stimulation. J Neurol Neurosurg Psychiatry. 2007;78:66–73. doi: 10.1136/jnnp.2006.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, Schreiber A, Schiefer U, Sabel B, Kenkel S, VonTheim R, Trauzettel-Klosinski S. Does visual restitution training change absolute homonymous visual field defects? A fundus controlled study. Br J Ophthalmol. 2005;89(1):30–35. doi: 10.1136/bjo.2003.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Cymerman R, Smith R, Kiorpes L, Carrasco M. Covert spatial attention is functionally intact in amblyopic human adults. Journal of Vision. 2016;16(15):1–19. doi: 10.1167/16.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K, Pasternak T. Transient and permanent deficits in motion perception after lesions of cortical areas MT and MST in the macaque monkey. Cerebral Cortex. 1999;9(1):90–100. doi: 10.1093/cercor/9.1.90. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, MacLeod MJ, Murray AD, Olson JA, Weiskrantz L. Increased sensitivity after repeated stimulation of residual spatial channels in blindsight. PNAS. 2006;103(40):14971–14976. doi: 10.1073/pnas.0607073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, Weiskrantz L, Olson JA, MacLeod MJ, Murray AD, … Coleman R. Spatial channels of visual processing in cortical blindness. Eur J Neurosci. 2003;18(5):1189–1196. doi: 10.1046/j.1460-9568.2003.02853.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez J, Pedersini CA, Di Russo F, Cardobi N, Fonte C, Varalta V, … Marzi CA. Visually evoked responses from the blind field of hemianopic patients. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld M, Hopf J, Martinez A, Mai H, Sattler C, Gasde A, … Hillyard S. Spatio-temporal analysis of feature-based attention. Cerebral Cortex. 2007;17(10):2468–2477. doi: 10.1093/cercor/bhl154. [DOI] [PubMed] [Google Scholar]
- Schreiber A, Vonthein R, Reinhard J, Trauzettel-Klosinski S, Connert C, Scheifer U. Effect of visual restitution training on absolute homonymous scotomas. Neurology. 2006;67:143–145. doi: 10.1212/01.wnl.0000223338.26040.fb. [DOI] [PubMed] [Google Scholar]
- Serences J, Boynton G. Feature-based attentional modulations in the absence of direct visual stimulation. Neuron. 2007;55(2):301–312. doi: 10.1016/j.neuron.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Serences J, Yantis S. Spatially selective representations of voluntary and stimulus-driven attention priority in human occipital, parietal, and frontal cortex. Cerebral Cortex. 2007;17(2):284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Sincich L, Park K, Wohlgemuth M, Horton J. Bypassing V1: a direct geniculate input to area MT. Nature Neuroscience. 2004;7(10):1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Szpiro S, Carrasco M. Exogenous Attention Enables Perceptual Learning. Psychol Sci. 2015;26(12):1854–1862. doi: 10.1177/0956797615598976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M, Morrone MC. Visual Plasticity: Blindsight Bridges Anatomy and Function in the Visual System. Curr Biol. 2016;26(2):R70–73. doi: 10.1016/j.cub.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Martinez Trujillo J. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399(6736):575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Vaina LM, Soloviev S, Calabro FJ, Buonanno F, Passingham R, Cowey A. Reorganization of retinotopic maps after occipital lobe infarction. Journal of Cognitive Neuroscience. 2014;26(6):1266–1282. doi: 10.1162/jocn_a_00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington E, Sanders M, Marshall J. Visual capacity in the hemianopic field following restricted occipital ablation. Brain. 1974;97:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- White A, Carrasco M. Feature-based attention involuntarily and simultaneously improves visual performance across locations. Journal of Vision. 2011;11(6) doi: 10.1167/11.6.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Rolfs M, Carrasco M. Stimulus competition mediates the joint effects of spatial and feature-based attention. Journal of Vision. 2015;15(14):1–21. doi: 10.1167/15.14.7. [DOI] [PMC free article] [PubMed] [Google Scholar]