Abstract
Background
An Artificial Placenta (AP) utilizing extracorporeal life support (ECLS) could revolutionize care of extremely premature newborns, but its effects on gastrointestinal morphology and injury need investigation.
Methods
Lambs (116-121 days GA, term=145; n=5) were delivered by C-section, cannulated for ECLS, had total parenteral nutrition (TPN) provided, and were supported for 7 days before euthanasia. Early and Late Tissue Controls (ETC, n=5 and LTC, n=5) delivered at 115-121 days and 125-131 days, respectively, were immediately sacrificed. Standardized jejunal samples were formalin-fixed for histology. Crypt depth (CD), villus height (VH), and VH:CD ratios were measured. Measurements also included enterocyte proliferation (Ki-67), Paneth cell count (Lysozyme), and injury scores (H&E). ANOVA and Chi Square were used with p<0.05 considered significant.
Results
CD, VH, and VH:CD were similar between groups (p>0.05). AP demonstrated more enterocyte proliferation (95.7±21.8) than ETC (49.4±23.4; p=0.003) and LTC (66.1+11.8; p=0.04), and more Paneth cells (81.7±17.5) than ETC (41.6±7.0; p=0.0005) and LTC (40.7±8.2, p=0.0004). Presence of epithelial injury and congestion in the bowel of all groups were not statistically different. No villus atrophy or inflammation was present in any group.
Conclusions
This suggests preserved small bowel mucosal architecture, high cellular turnover, and minimal evidence of injury.
Keywords: Artificial Placenta, Extracorporeal Membrane Oxygenation, Gastrointestinal development, Gastrointestinal injury, Premature lambs, Premature gastrointestinal tract
Introduction
Prematurity remains a global health problem, with extremely low gestational age newborns (ELGANS, born <28 weeks) suffering the highest morbidity and mortality (1, 2). Of premature infants that survive, morbidities include respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), retinopathy of prematurity (ROP), and necrotizing enterocolitis (NEC) (1–4). NEC, in particular, is one of the most common and lethal gastrointestinal emergencies of prematurity with a mortality rate as high as 35% and up to 50% needing surgery (5, 6). Most infants who suffer from NEC are supported initially with total parenteral nutrition (TPN). Although this allows nutritional support, the lack of enteral feeds can lead to intestinal atrophy and increased risk of bacterial translocation and sepsis (7–9).
An artificial placenta (AP) could offer a paradigm shift in the management of extremely preterm infants, simulating the intrauterine environment by utilizing extracorporeal life support (ECLS) to allow for the maintenance of fetal circulation, fluid filled lungs, and no mechanical ventilation. While we have previously reported that the AP protects against lung injury and allows normal lung development (10), it is also important to evaluate the effects on other organ systems such as the gastrointestinal tract.
We aimed to evaluate the effects of AP support on bowel morphology and injury. We specifically evaluated mucosal architecture, cellular proliferation, and injury from AP-supported lambs compared to gestational age-matched controls. We hypothesized architecture would be preserved during AP support and injury would be comparable to tissue controls.
Methods
The experimental procedure was performed in an ovine model following protocol approval by the University of Michigan Institutional Animal Care and Use Committee (IACUC) (protocol 00007211). All sheep used for the experiment were treated in compliance with the Guide for Care and Use of Laboratory Animals, 8th edition.(11).
Lambs used for the experiment were divided into three groups: Artificial Placenta (AP) Group, Early Tissue Control (ETC), and Late Tissue Control (LTC). Age and weight of each lamb were recorded.
AP Group
Premature lambs at 116-121 days GA (term = 145; n =5) were delivered via C-Section. 10-14Fr cannulas (Terumo: Ann Arbor, MI) were placed in the jugular vein (drainage) and umbilical vein (reinfusion). The circuit was completed with ¼” tubing (Tygon: Lima, OH), a collapsible-tubing roller pump (MC3: Ann Arbor, MI), and oxygenator/heat exchanger (either Medos HiLite, Xenios: Heilbronn, Germany or Capiox Baby Rx, Terumo, Ann Arbor, MI; Figure 1). VV-ECLS was initiated and the lambs were monitored closely. A 5 Fr arterial line (Covidien-Medtronic: Minneapolis, MN) was placed into the umbilical artery for hemodynamic monitoring and arterial blood gas (ABG) blood draws. The second umbilical vein was cannulated with a 5 Fr triple lumen venous line (Covidien-Medtronic: Minneapolis, MN) for intravenous fluid, TPN, heparin sulfate (SAGENT, Schaumburg, IL) (100 U/hr, titrated to a goal activated clotting time (ACT) of 200-250 seconds), and Prostaglandin E1 (Pfizer, New York, NY) (0.2mcg/kg/min) infusion to maintain ductal patency. The lambs were intubated and lungs were filled with fluid (amniotic fluid, Ringer’s Lactate, or perfluorodecalin [Origen: Austin, TX]).
Fig. 1.
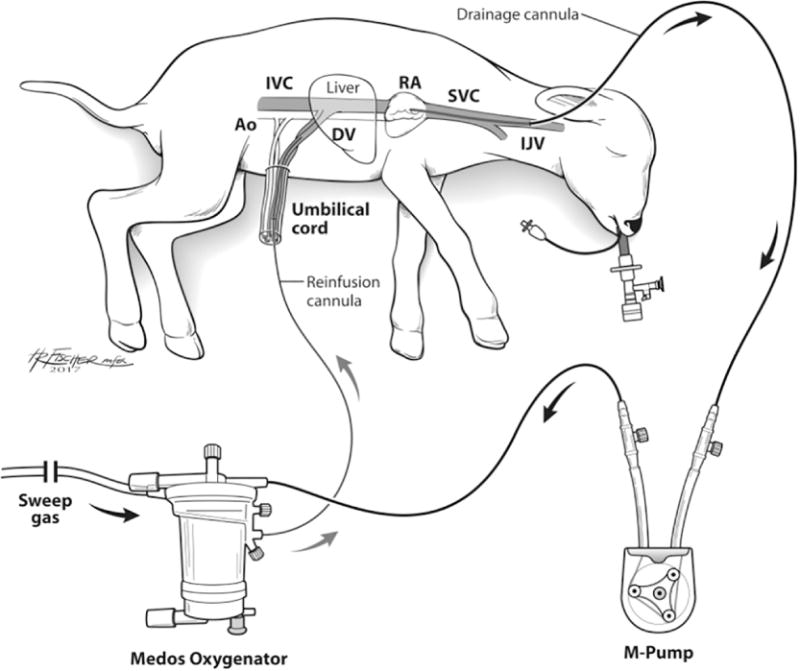
Schematic of the artificial placenta (AP) circuit in a premature lamb model demonstrating the cannulated lamb for VV ELCS, collapsible-tubing roller pump (M-Pump), and oxygenator/heat exchanger. Ao: Aorta; IVC: Inferior Vena Cava; DV: Ductus Venosus; RA: Right Atrium; SVC: Superior Vena Cava; IJV: Internal Jugular Vein
All lambs were supported on TPN infused via the umbilical vein. The TPN (ExactMix, Baxter Healthcare Corporation, Englewood, CO. Baxter International Inc. Supplied by the University of Michigan HomeMed- Home Infusion Pharmacy) was made with a standard composition including: Amino Acids (15%) (16 GM); Dextrose (45 GM); IntraLipds (80 ML); and electrolytes (Sodium Phosphate (5.4 MM); Potassium Chloride (8.1 MEQ); Magnesium Sulfate (1.6 MEQ); and Calcium Gluconate (1 MEQ)) in 400 mL volume administered at a rate of 5 mL/kg/hr. All AP lambs remained nil per os (NPO) during support. Hemodynamics, urine output, and bowel movements were monitored. All lambs were given prophylactic intravenous antibiotics (piperacillin-tazobactam [Hospira Inc., Lake Forest, IL]) and antifungals (fluconazole [SAGENT, Schaumburg, IL]) to prevent infection. Solumedrol (Pfizer, New York, NY) 0.63 mg/kg was given every 12 hours to prevent hypocortisolemia. Diazepam (Hospira Inc., Lake Forest, IL) 2.5mg and Buprenorphine (Parr Inc., Spring Valley, NJ) 0.3mg were used sparingly for pain or agitation. In cases of volume-resistant hypotension, vasopressors (norepinephrine (Claris LifeSciences Inc., North Brunswick, NJ), epinephrine (Hospira Inc., Lake Forest, IL), or dopamine (Baxter, Deerfield, IL)) were used to maintain a MAP >40 mmHg. AP support was continued for 7-10 days, then the animals were euthanized.
Tissue Control Groups
Early and Late Tissue Controls (ETC; n=5 and LTC; n=5) at 115-121 and 125-131 days, respectively. These lambs were delivered and immediately sacrificed.
Necropsy, Tissue Preparation, and Histological Analysis
After sacrifice, the bowel was removed en bloc and formalin-fixed. Standardized 2 cm longitudinal sections of jejunum were taken from 6 cm distal to the duodeno-jejunal junction to be used for mucosal measurements and staining. Sections from proximal, mid, and distal jejunum, terminal ileum, and cecum were also harvested for injury scoring. All samples were sectioned 3-5 μm thick. Slides were stained with Hematoxylin and Eosin (H&E), Ki-67, and Lysozyme, then digitized and reviewed via ImageScope (Leica Biosystems Imaging, Inc 2016, USA). Cell counts and measurements were summed for each sample then averaged for each group. Injury scores were marked as present or absent in the 5 anatomic locations for each animal. All measurements were done in a blinded fashion.
Histological Measurements
H&E Stain to Evaluate GI Mucosa Crypt Depth and Villus Height
Slides were stained with H&E (Fischer Scientific, Pittsburgh, PA). After review of each slide using ImageScope, Crypt depth (CD), villus height (VH), and VH:CD ratio (12/slide) were measured in micrometers and averaged. Measurements of CD and VH were only taken for those in which the plane of sectioning ran vertically from the tip of the villus to the base of the adjacent crypt. VH was measured from tip of villus to the crypt mouth, and CD was measured from crypt mouth. For each slide, 12 of the most representative villi were used for measurement and the corresponding 12 crypts.
Ki67Antibody Stain to Measure GI Epithelial Cell Proliferation
Slides stained with Ki67 Antibody (Abcam, Cambridge, MA) were used to evaluate GI enterocyte proliferation. 50 crypts from 5 different places of 10 consecutive crypts were reviewed per slide, and all the cells in the crypts stained dark brown with Ki67 were counted per slide and averaged. Measurements were taken and compared between groups.
GI Mucosal Injury Severity Scores
H&E-stained slides were reviewed and scored by a pathologist blinded to groups. Bowel injury scores were measured using presence of inflammation, epithelial injury, congestion, hemorrhage, and villus atrophy. Presence was measured as 1 and absence as 0. Since there was absence of inflammation, villus atrophy, and only minor hemorrhage among the groups, only epithelial injury and congestion were compared between groups.
Paneth Cells Stained with Lysozyme Antibody to Measure Immune Response
Proximal jejunal sections were stained with Lysozyme antibody (Invitrogen, IL, USA). Images with clearly defined Paneth cells within the crypts were included in the analysis. Paneth cell counts were averaged per slide. Those values were then averaged per animal in each group and compared between groups.
Statistical Analysis
Using the Graphpad Prism version 7.0 statistical software (GraphPad Software Inc, 2017, USA), ANOVA with post-hoc Tukey’s Test was used to compare groups with Ki-67 stain, Lysozyme stain, and CD, VH, and VH:CD Ratio. This test was chosen because the cell counts included continuous variables comparing means from multiple groups. IBM Corp. Released 2013. IBM SPSS Statistics, Version 22.0 (Armonk, NY), Fisher’s Exact Test was used to compare presence of injury score for each anatomic GI location for epithelial injury and congestion. P<0.05 defined statistical significance.
Results
GI Mucosal Development in Premature Lambs (H&E Stain)
There was no significant difference between CD in bowel of ETC (68.5±7.3) and LTC (77.2±8.2) with p=0.18. Although the CD in AP bowel (77.5±5.9) was not statistically different than ETC (p=0.16) and LTC (p=0.99), there was an increasing trend of AP CD similar to that of the LTC group. VH of the AP Group (315.6±70.4) was comparable to ETC (314.9±7.3; p=0.99) and LTC (297.9±32.6; p=0.88), without differences compared to ETC and LTC VH (p=0.89). Similarities were also found between the AP VH:CD Ratio (4.1±0.9) compared to ETC (4.7±1.4; p=0.58) and LTC (3.9±0.6; p=0.96). No differences were found between VH:CD ratio of ETC and LTC (p=0.42) (Figure 2).
Fig. 2.
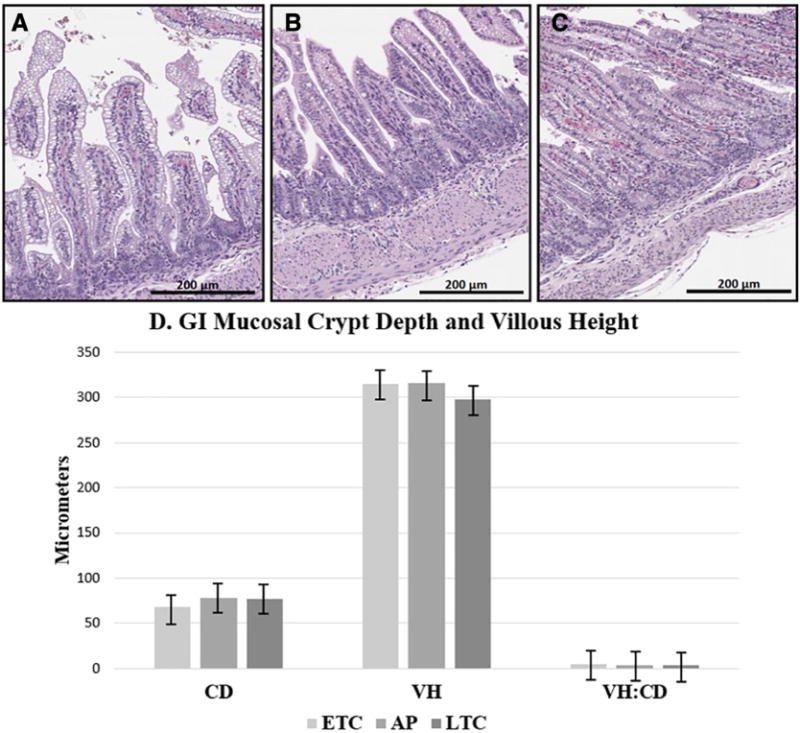
Representative histological images and graph comparing GI morphology of all groups. (A) ETC [H&E Stain]; (B) AP [H&E Stain]; (C) LTC [H&E Stain]; H&E, 200x magnification; (D) Graph demonstrating no significant difference when comparing Crypt Depth (CD), Villus Height (VH), or VH:CD Ratio of all groups. ETC: Early Tissue Control; AP: Artificial Placenta; LTC: Late Tissue Control. P-values calculated by ANOVA. * Indicates significance p<0.05.
GI Proliferation in Premature Lambs (Ki67 Stain)
Significantly more enterocyte proliferation was found in the AP group (95.7±21.8) when compared to ETC (49.4±23.4; p=0.003) and LTC (66.1+11.8; p=0.04). There were no significant differences between both tissue control groups (p=0.35) (Figure 3).
Fig. 3.
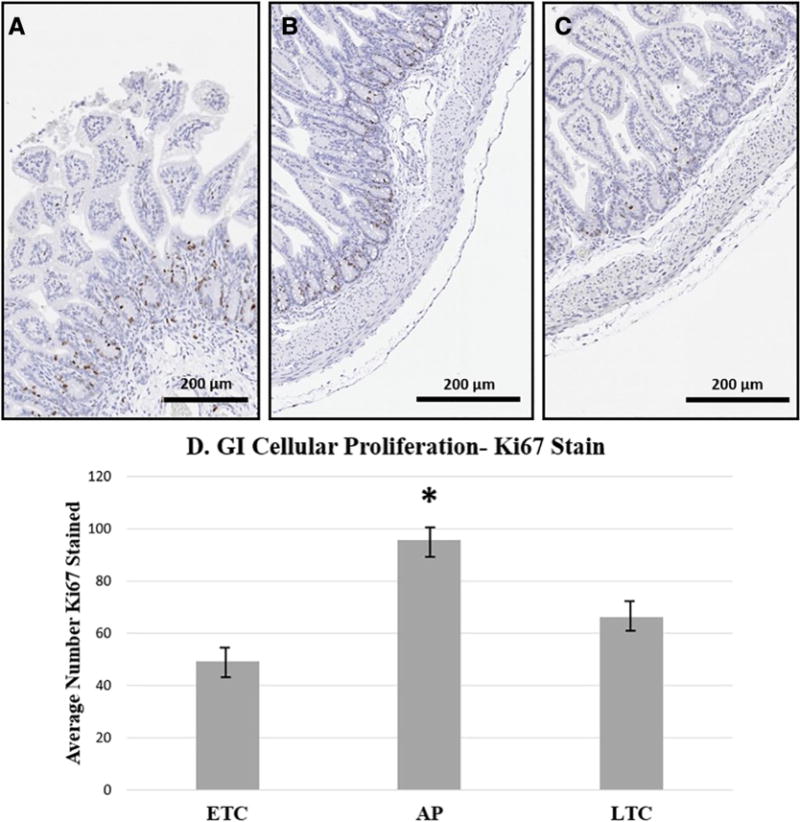
Representative histological images and graph comparing enterocyte proliferation of all groups. (A) ETC [Ki-67 Stain]; (B) AP [Ki-67 Stain]; (C) LTC [Ki-67 Stain]; Ki-67, 200x magnification; (D) Graph demonstrating significantly increased proliferation in enterocytes of the AP group compared to both ETC and LTC groups. ETC: Early Tissue Control; AP: Artificial Placenta; LTC: Late Tissue Control. P-values calculated by ANOVA. * Indicates significance p<0.05.
GI Injury Severity in Premature Lambs (H&E Stain)
There was no evidence of inflammation or villus atrophy in any of the three groups (AP, ETC, LTC). One AP animal exhibited presence of hemorrhage in the mid jejunum and colon, but otherwise there was no hemorrhage found in any other animals or anatomic locations. Epithelial injury was present in ETC and AP groups, while congestion was present in LTC and AP groups. There was an increased presence of epithelial injury and congestion in the distal jejunum of the AP group, but not statistically significant when using a Fisher’s Exact Test (p=0.05; p=0.07, respectively). In all other areas of the bowel, there were no statistical differences between groups (Table 1).
Table 1.
Numbers represent number of samples manifesting injury type within specified region (out of n=5). AP: Artificial Placenta; ETC: Early Tissue Control; LTC: Late Tissue Control; TI: Terminal ileum; Jej: jejunum; Prox: proximal; p-values calculated by Fisher’s Exact Test.
| AP (n=5) | ETC (n=5) | LTC (n=5) | p-value | ||
|---|---|---|---|---|---|
| Prox Jej | Epithelial Injury | 2 | 1 | 0 | 0.73 |
| Congestion | 2 | 0 | 1 | 0.73 | |
|
| |||||
| Mid Jej | Epithelial Injury | 3 | 1 | 0 | 0.23 |
| Congestion | 3 | 0 | 1 | 0.23 | |
|
| |||||
| Distal Jej | Epithelial Injury | 4 | 1 | 0 | 0.05 |
| Congestion | 4 | 0 | 2 | 0.07 | |
|
| |||||
| TI | Epithelial Injury | 3 | 1 | 0 | 0.23 |
| Congestion | 3 | 0 | 2 | 0.25 | |
|
| |||||
| Colon | Epithelial Injury | 1 | 0 | 0 | 1.0 |
| Congestion | 1 | 0 | 0 | 1.0 | |
Indicates significance p<0.05.
GI Paneth Cell Count in Premature Lambs (Lysozyme Stain)
There were significantly more positively stained Paneth cells in the AP Group (81.7±17.5) when compared to both ETC (41.6±7.0; p=0.0005) and LTC (40.7±8.2; p=0.0004). The number of lysozyme stained Paneth cells were similar between the ETC (41.6±7.0) and the LTC (40.7±8.2), p=0.99.
Discussion
In very premature neonates, the immature gut is highly susceptible to injury after delivery. Before clinical translation of the AP, it is critical to understand its effects on the preterm developing bowel. We therefore investigated bowel architecture, growth, injury, and response to injury in extremely premature lambs supported by the AP. The findings indicate that premature lambs supported by the AP have maintained GI morphology and integrity with minimal injury. We also found increased cellular turnover and an appropriate response to injury with increased Paneth cells.
Studies have shown that CD, VH, and VH:CD ratio are markers of bowel integrity and increase during development. Development and mucosal proliferation of the villi and crypts occur between 9-20 weeks of human gestation. The first signs of structure and fold formation of the villi is around 9-10 weeks, 12 weeks for the crypts, and 17-20 weeks for the muscularis mucosa (12–14). Near 10-12 weeks, mucosal proliferation is primarily located in the epithelial crypts and intervillus regions (9, 12). During the last trimester, the rest of the GI epithelial cells develop, absorption improves, intestinal length is doubled, and there is a dramatic increase in growth and development of the GI mucosa villi and microvilli (7). The GI mucosal growth and development can be measured by a number of histological characteristics including VH, CD, and VH:CD Ratio. (15, 16). The current study found that there was a slight increase in VH in both the AP and LTC groups, although not statistically significant. This could indicate development emulating what is found in utero. The CD and VH:CD ratio was similar to that of the tissue controls, which indicates preserved mucosal architecture in AP animals.
Despite CD and VH findings of preserved mucosal morphology, there did appear to be minor injury in the AP animals. Previous studies have used variables such as epithelial cell inflammation, necrosis, hemorrhage, and edema as markers of injury in bowel mucosa (17). The current study investigated similar variables. After comparing groups, there was no epithelial inflammation or atrophy in any of the groups (ETC, AP, LTC) and minimal hemorrhage present. This was consistent with our findings that mucosal architecture was preserved in the AP animals and observations during necropsy that there was no gross inflammation, ischemia, or bleeding. Presence of congestion and epithelial injury in the distal jejunum on AP support may indicate increased susceptibility in this area of the bowel but our sample size is too small to make any definitive conclusions. Two of the 5 AP lambs were maintained on vasopressors for 3-6 days, which may have caused vasoconstriction and hypoperfusion resulting in injury. The congestion may be related to fluid boluses received by the AP animals on ECLS support. There were otherwise no differences between the AP and the tissue controls, again supporting that bowel integrity was maintained.
While there was minor injury seen in the AP bowel, an increase in proliferation and Paneth cell count was also observed. Cellular turnover and growth can be seen with development as infants get closer to term (15) and in response to injury (18). An increase in Paneth cells is also seen with postnatal development (19) and in response to injury (19–22) (23, 24). Paneth cells play an important role in fighting off infection as they release several factors into the bowel lumen, such as microbicidal peptides, growth factors, and digestive enzymes (lysozyme) from their apical secretory granules following exposure to bacteria and fungi (19–22). Studies have shown that during acute active infection such as NEC, Crohn’s disease, or decreased immune response in graft vs host disease (GVHD), there is a decrease in the number of Paneth cells present (23, 24). However, after recovery from acute inflammatory conditions such as NEC, there is an upregulation of Paneth cell proliferation and hyperplasia (6, 25). It has been suggested that this increase in abundance of Paneth cells allows for enhanced immune response, secretion of active antimicrobial peptides, and increased eradication of ingested pathogens (6). The increase in both proliferation and Paneth cells in the current study may indicate enhanced growth and response to mild injury. These changes suggest that increased cellular turnover and upregulation of Paneth cell proliferation may play a protective role during AP support.
In lambs supported by the AP, there are a few potential causes of injury and cellular turnover. Previous studies have shown that a loss of integrity can be seen with the “starved” bowel related to splanchnic ischemia. A study done by Piena et al. investigated this by looking at changes in the GI mucosa in critically ill infants on ECLS with TPN vs enteral nutrition. It was concluded that enteral feeds did not compromise the gut integrity and should therefore be continued during ECLS (26). The AP utilizes ECLS and could therefore affect blood flow to the gut. Decreased gut perfusion is also seen with intermittent hypotension and this was observed in some of the AP animals. Most of the lambs were hemodynamically stable, however early experiments had very low thresholds for starting vasopressors. As a result, 2/5 AP lambs were supported on vasopressors for 3-6 days, which may have decreased perfusion to the bowel. Near-infrared spectroscopy (NIRS) would be a potential modality to monitor splanchnic blood flow. Several studies have suggested that NIRS can be used to measure splanchnic tissue oxygenation and detect early onset NEC and other causes of acute abdomen (27, 28). Other studies have even used NIRS to evaluate altered oxygenation to the mesenteric vasculature and development of NEC during blood transfusions (29). While these studies seem promising in humans, they may not be as useful in the sheep model. Monitoring of splanchnic perfusion via NIRS was attempted in several AP lambs; however, due to the dark pigmentation and difficulty with placement of the skin probes in the majority of the lambs, there were no meaningful data obtained from these measurements.
Another potential cause of mucosal injury could be related to the NPO status of the AP lambs. In utero, the human fetus ingests about 700-1,000 mL of amniotic fluid daily and about 100-1,000 mL daily in the sheep fetus (15, 30). Studies have shown that this ingestion of amniotic fluid may enhance bowel mucosa development, while the lack of fluid ingestion leads to leads to decreased birth weight and crown-rump length in humans and decreased bowel mucosal thickening, villus height, and villus cell density in the small intestine in fetal sheep (30).
Another study found piglets to have significantly shorter villi and deeper crypts when they were given a dry diet as opposed to a milk diet, suggesting that liquid enteral nutrition in the developing gut plays a role in structure and function (16). The lambs in the current study had no fluid ingestion and received all nutrition via TPN, Future studies will investigate the effects of trophic feeds with either amniotic fluid or enteral feeds during AP support.
There are several potential limitations to our study. Small sample size was a limitation in this study due to time investment required for each experiment, cost, and the goal to sacrifice the least number of animals as possible. Lack of statistical difference in the mucosal architecture could be related to limited power as this depends on both the magnitude of the difference and the sample size. This could be improved with a larger sample size for future studies. Another potential limitation is that significant changes to the bowel mucosa CD and VH might require more than 7 days of support. This could be evaluated in lambs that are supported longer than 7 days on the AP. Further studies will also include GI tract analysis of lambs that are supported by the AP until closer to term (about 14-17 days of AP support) and are able to be weaned off to room air. Also, earlier protocols had a lower threshold for starting vasopressors for the lambs. Current guidelines in AP management avoid vasopressors unless absolutely necessary. We have found that lambs do better with this protocol, anditcould therefore lead to decreased bowel injury in the future. Previous studies have also indicated the importance of trophic enteral nutrition on the developing gut (15, 16, 30), which will be assessed in subsequent experiments. Lastly, another area of investigation is the premature bowel microbiome and how support on the AP affects the colonization of the gut. Future studies will examine bowel mucosa in lambs supported by the AP with TPN vs ingestion of amniotic fluid or enteral feeds, as well as the changes this might have on the microbiome.
Conclusion
The AP offers a potentially revolutionary solution to many of the problems facing prematurity. During AP support, small bowel mucosal architecture appears preserved, mucosal injury is minimal and cellular turnover is increased. Further studies will focus on decreasing the intestinal injury during AP support, including the effects of enteral nutrition on the gut during AP support.
Acknowledgments
The authors would like to thank: Cindy Cook and Marie Cornell for assistance with experiment management, ECLS laboratory students for the chronic care of the animals, and Unit for Laboratory Animal Medicine (ULAM) at University of Michigan - Wendy Rosebury-Smith and Kathy Toy for preparation of the slides. We would also like to thank the Coller Surgical Society for the Frederick A. Coller Surgical Research Fellowship Grant and National Institute of Health (NIH) Grant for funding our project. We would also like to thank Niki Matusko, BS, senior statistician for the University of Michigan Surgery Department, and Elizabeth Freiheit, PhD, University of Michigan Department of Biostatistics, for assistance with statistical review and feedback.
Funding: This work was supported by the National Institutes of Health NIH 1R01HD073475-01A1 and the Collar Grant #326501 supported by the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Level of Evidence: N/A
Conflicts of Interest: None
References
- 1.Althabe F, Bhutta Z, Blencowe H, et al. Born Too Soon: The Global Action Report on Preterm Birth. World Health Organization; 2012. pp. 1–112. [Google Scholar]
- 2.Mychaliska GB. The artificial placenta: Is clinical translation next? Pediatr Pulmonol. 2016;51(6):557–9. doi: 10.1002/ppul.23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert WM. The cost of preterm birth: the low cost versus high value of tocolysis. BJOG. 2006;113(Suppl 3):4–9. doi: 10.1111/j.1471-0528.2006.01117.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis RP, Bryner B, Mychaliska GB. A paradigm shift in the treatment of extreme prematurity: the artificial placenta. Curr Opin Pediatr. 2014;26(3):370–6. doi: 10.1097/MOP.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnoni A, Amendola CL. Necrotizing Enterocolitis. Journal of the American Academy of Physician Assistants. 2017;30(8):16–21. doi: 10.1097/01.JAA.0000521131.85173.f9. [DOI] [PubMed] [Google Scholar]
- 6.Puiman PJ, Burger-Van Paassen N, Schaart MW, et al. Paneth Cell Hyperplasia and Metaplasia in Necrotizing Enterocolitis. Pediatric Research. 2011;69(3) doi: 10.1203/PDR.0b013e3182092a9a. [DOI] [PubMed] [Google Scholar]
- 7.Neu J. Gastrointestinal Development and Meeting the Nutritional Needs of Premature Infants. Am J Clin Nutr. 2007;85:629–34. doi: 10.1093/ajcn/85.2.629S. [DOI] [PubMed] [Google Scholar]
- 8.Neu J. Gastrointestinal Maturation and Implications for Infant Feeding. Early Hum Dev. 2007;83(12):767–75. doi: 10.1016/j.earlhumdev.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Commare CE, Tappenden KA. Development of the Infant Intestine: Implications for Nutrition Support. Nutrition in Clinical Practice. 2007;22:159–73. doi: 10.1177/0115426507022002159. [DOI] [PubMed] [Google Scholar]
- 10.Coughlin MA, Werner NL, Church J, et al. Podium Presentation by J Church at American Society for Artificial Internal Organs (ASAIO) Chicago, IL: Jun 22, 2017. An Artificial Placenta Protects against Lung Injury and Promotes Continued Lung Development in Extremely Premature Lambs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 12.Montgomery RK, Mulberg AE, Grand RJ. Development of the Human Gastrointestinal Tract: Twenty Years of Progress. Gastroenterology. 1999;116:702–31. doi: 10.1016/s0016-5085(99)70193-9. [DOI] [PubMed] [Google Scholar]
- 13.Nunes T, Bernardazzi C, de Souza HS. Cell Death and Inflammatory Bowel Diseases: Apoptosis, Necrosis, and Autophagy in the Intestinal Epithelium. Biomed Res Int. 2014;2014:1–12. doi: 10.1155/2014/218493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drozdowski LA, Cloandinin T, Thomson ABR. Ontogeny, Growth, and Development of the Small Intestine: Understanding Pediatric Gastroenterology. World Journal of Gastroenterology. 2010;16(7):787–99. doi: 10.3748/wjg.v16.i7.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trahair JF, Sangild PT. Systemic and Luminal Influences on the Perinatal Development of the Gut. Equine Veterinary Journal. 1997;24:40–50. doi: 10.1111/j.2042-3306.1997.tb05077.x. [DOI] [PubMed] [Google Scholar]
- 16.Pluske JR, Williams IH, Aherne FX. Maintenance of Villous Height and Crypt Depth in Piglets by Providing Continuous Nutrition After Weaning. Animal Science. 1996;62(01):131–44. doi: 10.1017/s1357729800014417. [DOI] [Google Scholar]
- 17.Quaedackers J, Beuk R, Bennet L, et al. An Evaluation of Methods for Grading Histologic Injury Following Ischemia/Reprofusion of the Small Bowel. Transplantation Proceedings. 2000;32:1307–10. doi: 10.1016/s0041-1345(00)01238-0. [DOI] [PubMed] [Google Scholar]
- 18.Williams JM, Duckworth CA, Burkitt MD, et al. Epithelial Cell Shedding and Barrier Fuction: A Matter of LIfe and Death at the Small Intestinal Villus Tip. Veterinary Pathology. 2015;52(3):445–55. doi: 10.1177/0300985814559404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bry L, Falk P, Huttner K, et al. Paneth Cell Differentiation in the Developing Intestine of Normal and Transgenic Mice. Proc Natl Acad Sci. 1994;91:10335–9. doi: 10.1073/pnas.91.22.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elphick DA, Mahida YR. Paneth Cells: Their Role in Innate Immunity and Inflammatory Disease. Gut. 2005;54(12):1802–9. doi: 10.1136/gut.2005.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garabedian EM, Roberts LJJ, McNevin S, et al. Examining the Role of Paneth Cells in the Small Intestine by Lineage Ablation in Transgenic Mice. The Journal of Biological Chemistry. 1997;272(38):23729–40. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 22.Ergun E, Ergun L, Asti RN, et al. Light and Electron Microscopic Morphology of Paneth Cells in the Sheep Small Intestine. Revenue Med Vet. 2003;154(5):351–5. [Google Scholar]
- 23.Coutinho HB, Carmona da Mota H, Coutinho VB, et al. Absence of Lysozyme (Muramidase) in the Intestinal Paneth Cells of Newborn Infants with Necrotising Enterocolitis. Journal of Clinical Pathology. 1998;51:512–4. doi: 10.1136/jcp.51.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine JE, Huber E, Hammer ST, et al. Low Paneth Cell Numbers at Onset of Gastrointestinal Graft-Versus-Host Disease Identify Patients at High Risk for Nonrelapse Mortality. Blood. 2013;122(8):1505–9. doi: 10.1182/blood-2013-02-485813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewin K. The Paneth Cell in Disease. Gut. 1969;10:804–11. doi: 10.1136/gut.10.10.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piena M, Albers MJ, Van Haard PM, et al. Introduction of Enteral Feeding in Neonates on Extracorporeal Membrane Oxygenation after Evaluation of Intestinal Permeability Changes. Journal of Pediatric Surgery. 1998;33(1):30–4. doi: 10.1016/s0022-3468(98)90355-4. [DOI] [PubMed] [Google Scholar]
- 27.Zamora IJ, Stoll B, Ethun CG, Sheikh F, Yu L, Burrin DG, Brandt ML, Olutoye OO. Low Abdominal NIRS Values and Elevated Plasma Intestinal Fatty Acid-Binding Protein in a Premature Piglet Model of Necrotizing Enterocolitis. PloS one. 2015;10(6):e0125437. doi: 10.1371/journal.pone.0125437. Epub 2015/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive care medicine. 2001;27(8):1401–7. doi: 10.1007/s001340100994. Epub 2001/08/21. [DOI] [PubMed] [Google Scholar]
- 29.Marin T, Moore J, Kosmetatos N, Roback JD, Weiss P, Higgins M, McCauley L, Strickland OL, Josephson CD. Red blood cell transfusion-related necrotizing enterocolitis in very-low-birthweight infants: a near-infrared spectroscopy investigation. Transfusion. 2013;53(11):2650–8. doi: 10.1111/trf.12158. Epub 2013/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avila CG, Harding R. The Development of the Gastrointestinal System in Fetal Sheep in the Absence of Ingested Fluid. Journal of Pediatric Gastroenterology and Nutrition. 1991;12:96–104. doi: 10.1097/00005176-199101000-00019. [DOI] [PubMed] [Google Scholar]


