Abstract
After spinal cord injury (SCI) in mammals, severed axons fail to regenerate, due to both extrinsic inhibitory factors, e.g., the chondroitin sulfate proteoglycans (CSPGs) and myelin-associated growth inhibitors (MAIs), and a developmental loss of intrinsic growth capacity. The latter is suggested by findings in lamprey that the 18 pairs of individually identified reticulospinal neurons vary greatly in their ability to regenerate their axons through the same spinal cord environment. Moreover, those neurons that are poor regenerators undergo very delayed apoptosis, and express common molecular markers after SCI. Thus the signaling pathways for retrograde cell death might converge with those inhibiting axon regeneration. Many extrinsic growth-inhibitory molecules activate RhoA, whereas inhibiting RhoA enhances axon growth. Whether RhoA also is involved in retrograde neuronal death after axotomy is less clear. Therefore, we cloned lamprey RhoA and correlated its mRNA expression and activation state with apoptosis signaling in identified reticulospinal neurons. RhoA mRNA was expressed widely in normal lamprey brain, and only slightly more in poorly-regenerating neurons than in good regenerators. However, within a day after spinal cord transection, RhoA mRNA was found in severed axon tips. Beginning at 5 days post-SCI RhoA mRNA was upregulated selectively in pre-apoptotic neuronal perikarya, as indicated by labelling with fluorescently labelled inhibitors of caspase activation (FLICA). After 2 weeks post-transection, RhoA expression decreased in the perikarya, and was translocated anterogradely into the axons. More striking than changes in RhoA mRNA levels, RhoA was continuously active selectively in FLICA-positive neurons through 9 weeks post-SCI. At that time, almost no neurons whose axons had regenerated were FLICA-positive. These findings are consistent with a role for RhoA activation in triggering retrograde neuronal death after SCI, and suggest that RhoA may be a point of convergence for inhibition of both axon regeneration and neuronal survival after axotomy.
Keywords: Apoptosis, RhoA, Axotomy, Caspase, FLICA, Lamprey, Spinal cord injury
Introduction
RhoA is a member of the Rho family of small GTPases, which cycle between inactive GDP-bound and active GTP-bound states and function as molecular switches in signal transduction cascades. By regulating actin cytoskeletal dynamics, RhoA plays a role in determining cell shape, attachment, motility and proliferation. In recent years, RhoA has been implicated in two important responses of neurons to spinal cord injury (SCI): 1) apoptotic neuronal death near the injury; and 2) failure of severed axons to regenerate. In mammalian models of SCI in vivo, technical limitations make interpretation of data regarding the precise nature of these two effects ambiguous. We have used the sea lamprey as a model to address these ambiguities because its large, individually identified reticulospinal neurons show great heterogeneity in the ability of their axons to regenerate through the same spinal cord environment (Davis and McClellan, 1994b, Jacobs et al., 1997) and to survive long term after axotomy (Shifman et al., 2008). These and other advantages of the lamprey have allowed us to investigate the neuron-intrinsic factors underlying axotomy-induced neuronal death as well as failure of axonal regeneration. Those neurons that are poor regenerators also are poor long-term survivors after axotomy (Shifman et al., 2008), suggesting a possible convergence of pathways for inhibition of axon regeneration and neuronal survival, possibly through RhoA.
Post-axotomy neuronal apoptosis
Rho induced apoptosis in hippocampal (Donovan et al., 1997) and cortical neurons (Zhang et al., 2007) in vitro, but in vivo studies have delivered conflicting evidence. Application of a Rho antagonist (C3-05) reduced the number of TUNEL-labeled cells in spinal cord injured mouse and rat, suggesting a role for Rho activation in cell death near a SCI (Dubreuil et al., 2003). A dominant negative form of RhoA reduced apoptosis in the developing cortex, while overexpression of RhoA in cortical neurons increased apoptosis (Sanno et al., 2010). Similarly, knockdown of RhoA increased retinal ganglion cell (RGC) survival after optic nerve injury (Koch et al., 2014). On the other hand, earlier investigators had found a decrease in developmental motor neuron survival in spinal cords of embryonic mice conditionally expressing a dominant negative form of either RhoA or Rho kinase (ROCK) that blocks activities of all Rho or ROCK isoforms, respectively (Kobayashi et al., 2004). Thus, RhoA seems to modulate neuronal death, but precisely how is not clear. In particular, we wish to know whether RhoA participates in retrograde neuronal death after axotomy. The occurrence of retrograde neuronal death in supraspinal neurons after SCI has been questioned (Kwon et al., 2002, Nielson et al., 2010, Nielson et al., 2011) (but see (Novikova et al., 2000)), while the neuronal apoptosis near a SCI and attributed to RhoA activation (Dubreuil et al., 2003) could be due to factors other than axotomy, e.g., inflammatory influences (Beattie et al., 2002), particularly because the apoptosis is not restricted to neurons. Indeed, after SCI, many of the apoptotic cells are glia (Liu et al., 1997), particularly oligodendrocytes, both near the lesion and remotely in association with axons undergoing Wallerian degeneration (Crowe et al., 1997). These other mechanisms can be ruled out in the very delayed retrograde apoptosis seen selectively among the reticulospinal neurons known to be poor regenerators (Shifman et al., 2008), since their cell bodies are located far from the site of spinal cord transection.
Inhibition of axon growth
In developing neurons, RhoA activation leads to collapse and retraction of the growth cone, thus affecting axonal projection, guidance and extension (Luo, 2000, Fujita and Yamashita, 2014). Both the myelin-associated growth-inhibitory proteins (MAIs) and the chondroitin sulfate proteoglycans (CSPGs) block axon regeneration in vitro and axon sprouting in vivo, at least in part by activating RhoA after neuronal injury (Kopp et al., 2012, Sharma et al., 2012). It is difficult to determine whether RhoA affects axon regeneration after SCI in mammals because axon regeneration does not occur spontaneously in mammalian CNS, and technical limitations require that most experiments on SCI employ partial injury models, in which true regeneration of severed axons is difficult to distinguish from collateral sprouting by spared ones. These limitations do not apply to the lamprey, in which axons of some reticulospinal neurons regenerate, even after complete transection (Rovainen, 1976, Selzer, 1978, Banerjee et al., 2016) and functional recovery is substantial (Cohen et al., 1986, 1988, Davis and McClellan, 1993). Although early on, investigators focused on the inhibitory environment to explain failure of axon regeneration in CNS, manipulation of environmental growth inhibitors has produced limited and inconsistent results (Kim et al., 2003, Simonen et al., 2003, Zheng et al., 2003), perhaps because of the great variety of these factors, and their functional redundancy (Lee et al., 2010, Sharma et al., 2012). Thus attention is shifting toward neuron-intrinsic factors (Park et al., 2010, Sun and He, 2010, Kaplan et al., 2015, He and Jin, 2016), including the ability to respond to the environmental inhibitory cues. For example, our previous studies have found negative correlations between regenerative probability and mRNA expression of the CSPG receptors, leukocyte common antigen-related phosphatase (LAR) and protein tyrosine phosphatase σ (PTPσ) (Zhang et al., 2014), members of the receptor protein tyrosine phosphatase (RPTP) family. Recently, we reported in the lamprey that knockdown of RhoA by a translation-blocking morpholino antisense oligonucleotide both reduced activation of caspases in reticulospinal neurons and increased regeneration of their axons (Hu et al., 2017). In the present study, we report the cloning of RhoA, its mRNA expression in reticulospinal neurons, and its activation after axotomy in individually identified reticulospinal neurons whose caspase activities have been determined (Hu et al., 2013). The results show that caspases and RhoA both are activated selectively in neurons with poor regenerative/poor surviving abilities, and that activation of RhoA, rather than its mRNA expression, is the better correlate with both poor regeneration and apoptotic signaling after SCI. The findings are consistent with convergence of signaling pathways for failure of neuronal survival and axonal regeneration post-SCI.
Materials and Methods
Identification and PCR cloning of the RhoA gene from a lamprey genomic database
The sea lamprey “ensemble database” and whole-genome sequencing (WGS) “trace database” maintained by the National Center for Biotechnology Information (NCBI, NIH) were used. These databases currently consist of assembled, partially assembled, and raw unassembled sequencing data from the sea lamprey genome (Smith et al., 2013). RhoA sequences from other species, including human, chicken and zebrafish, were used to query these databases, and the contigs found in the lamprey genomic library were confirmed using the basic local alignment search tool (BLAST) on NCBI servers. The contigs were further analyzed by aligning them with homologous genes of other species. Then oligonucleotide primers for polymerase chain reaction (PCR) were designed based on the lamprey RhoA gene sequence in regions highly identical with those of other species. Total RNA from lamprey CNS was isolated using Trizol reagent (Invitrogen). The first- strand cDNA synthesis reaction from total RNA was catalyzed by Superscript III Reverse Transcriptase with oligo-dT or 8 bp random primers. The synthesized total cDNA served as templates for PCR cloning using the Expand™ Long Template PCR System (Roche Applied Science) as per the manufacturer’s protocol. Following amplification, PCR fragments of expected size were purified on 1% agarose gels and ligated into the pGEM-T Easy Vector (Promega). The cloned fragments were sequenced (GENEWIZ, NJ), analyzed and compared by BLAST.
Riboprobe synthesis
The cloned lamprey RhoA cDNAs were used as templates for generating biotin-labeled antisense riboprobes. The lamprey RhoA protein contains 194 amino acids and shared >90% amino acid identity with human, mouse, chicken and zebrafish (Fig. 1). A PCR strategy for rapid generation of template was used. The PCR primers were designed to amplify cDNA sequences (513 bp) of the underlined region in Fig. 1, which included almost the full length of the RhoA gene. A T7 promoter sequence was added to the 5′ end of the antisense primer. Biotin-labeled antisense RNA probes were constructed from the amplified cDNA template, which contained the T7 promoter sequence upstream of the antisense-strand, using T7 RNA polymerase (Promega) with Biotin RNA-labeling Mix (Roche Applied Science), as recommended by the manufacturer. PTPσ probes were described previously (Zhang et al., 2014).
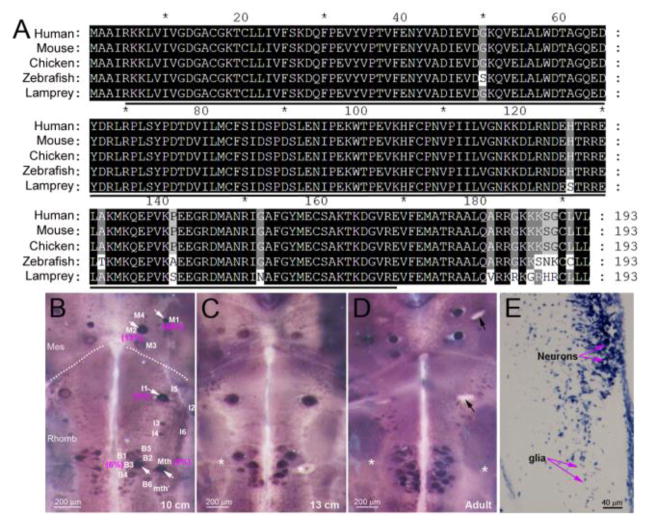
Fig. 1. Lamprey RhoA is highly homologous to other vertebrate RhoAs and widely expressed in reticulospinal neurons of lamprey.
(A) The sequence of cloned lamprey RhoA cDNA was aligned with those of other species, as indicated. They are more than 93% identical. The underlined region was used to create the template for construction of the RhoA probe. (B–D) RhoA mRNA expression in wholemounted 10 cm (B) and 13 cm (C) larval and 14 cm adult (D) lamprey brains. Labeling appeared to increase gradually with development. For unknown reasons, the Mauthner neuron was sometimes unlabeled in older animals (white asterisks). The black arrows in adult point to empty spaces where giant neurons were dislodged during tissue preparation. Mes, mesencephalon; Rhomb, rhombencephalon. Dotted lines indicate the approximate boundary between Mes and Rhomb. The 18 paired identified RS neurons are labeled in 10 cm larva brain (B). White arrows point to neurons labeled darkly by the RhoA probe, and their regeneration probabilities are indicated in magenta parentheses. Most of the densely-labeled neurons are poor regenerators. (E) transverse paraffin section through the brainstem processed by ISH, showing expression of RhoA in both neurons and glia. Mth, Mauthner neuron; mth’, auxiliary Mauthner neuron.
Animals and spinal cord transection
Larval sea lampreys (Petromyzon marinus), 6–14 cm in length (3–5 years old), were obtained from streams of Lake Michigan and maintained in freshwater tanks at 16°C until the day of use. The protocol was approved by the Temple University Institutional Animal Care and Use Committee. Lampreys were deeply anesthetized by immersion in saturated aqueous benzocaine until motionless to tail pinch, then pinned onto a Sylgard-coated dissecting dish filled with ice-cold lamprey Ringer (Lurie et al., 1994). The spinal cords were completely transected at the level of the 5th gill, and the animals allowed to recover up to 10 weeks. A total of 118 animals were investigated; 28 were used in RhoA in situ hybridization (ISH) studies on wholemounted lamprey brains, 13 in RhoA ISH on paraffin sections, and another 77 were used to study activated RhoA labeling in brain wholemounts alone or with CSPG treatment, as summarized in Table 1. To assess developmental changes in RhoA mRNA expression by wholemount ISH, 2 adults (13 and 14 cm) were included in the control group.
Table 1. Numbers of animals used for each experiment.
Times in top row are in weeks (wks) after spinal cord transection. Ctr, control; ISH, in situ hybridization; WM, wholemount; Sec, paraffin section; GST-RBD, glutathione-S-transferase-linked Rho binding domain of human Rhotekin protein (measure of activated RhoA); CSPG+, with chondroitin sulfate proteoglycans added; CSPG−, CSPG not added.
| Experiment | Ctr | 1 wk | 2 wks | 3 wks | 4 wks | 7–10 wks | Total |
|---|---|---|---|---|---|---|---|
| RhoA WM ISH | 8 | 2 | 6 | 6 | 6 | 28 | |
| RhoA Sec ISH | 3 | 4 | 4 | 2 | 13 | ||
| GST-RBD | 10 | 8 | 18 | 10 | 5 (9 wks) | 51 | |
| CSPG+ | 7 | 4 | 3 | 14 | |||
| CSPG− | 5 | 2 | 5 | 12 |
Detecting apoptotic reticulospinal neurons with FLICA
To label the cells that are undergoing apoptosis we used cell permeable FLICA. This identified an occasional apoptotic reticulospinal neuron as early as one week post-transection, reaching a peak by 4 weeks (Hu et al., 2017). Terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling (TUNEL) commonly has been used as an apoptotic marker, but it took approximately 4 weeks after SCI before any axotomized neurons were TUNEL positive, and TUNEL labeling did not peak until 12–16 weeks (Shifman et al., 2008).
The brains were removed under re-anesthesia at desired recovery times, as indicated in Table 1, then stripped of choroid plexus and processed for activated caspases using the Image-iT™ LIVE Green Poly Caspases Detection Kit, which detects most caspases, including caspase-1, -3, -4, -5, -6, -7, -8, and -9 (I35104, Invitrogen). To prevent mRNA degradation, we have modified the protocol provided by the manufacturer, so that all FLICA staining procedures were carried out at 4°C. Freshly removed brains were incubated immediately in 300 μl of phosphate buffer saline (PBS) containing 2 μl of 150x FLICA labeling solution for 1 hour and washed with wash buffer in the dark on a rotator 5 times for 5 minutes each. After washes, the cerebrotectal commissure was cut and brains were pinned flat on a small strip of Sylgard, fixed in 4% paraformaldehyde (PFA) in PBS for 2 hours, and then washed with PBS in the dark on a rotator 3 times for 15 minutes each. The brains were mounted wet on a glass slide, observed and photographed (80i, Nikon). The brains were then washed again with PBS 3 times for 5 minutes each and stored at −20 °C in 70% ethanol for ISH or activated Rho assay. The correlation between FLICA positivity was correlated with expression of RhoA mRNA and/or Rho activation in the same neurons (see below).
In situ hybridization on wholemounted brain
Wholemount ISH was carried out on the brains with or without FILCA-labeling by a modification of the chromogenic method previously described (Swain et al., 1994). Briefly, wholemounted brain preparations were washed in PTW (0.1% Tween-20 in PBS) and pre-hybridized at 50–55 °C in hybridization solution (50% deionized formamide, 5X SSC, 100 mg/ml Torula yeast RNA, 100 mg/ml wheat germ tRNA, 50 mg/ml heparin, 0.1% Tween-20) for 1 hour, followed by hybridization overnight on a nutator at 55 °C in above solution containing 1 μg/ml biotin-labeled antisense RNA probes for RhoA. Specimens were washed in hybridization solution at 55 °C, followed by room temperature washes in PTW and PBT (0.1% bovine serum albumin, 0.2% Triton X-100 in PBS). Alkaline phosphatase (AP)- conjugated Streptavidin (1:1000, Roche Applied Science) was applied to tissue overnight at 4 °C. The tissue was washed sequentially in PBT and SMT (100 mM NaCl, 50 mM MgCl2, 100 mM Tris, pH 9.5, 0.1% Tween-20), and the chromogenic reaction was carried out in a solution containing 20 μl of NBT/BCIP stock solution (Roche Applied Science) per 1 ml of SMT, as described in the manufacture’s manual, on ice in the dark for 15 minutes or until the reaction was completed (staining was monitored under a dissecting microscope). Finally, specimens were washed in PBS and mounted in 50% glycerol. Bright-field images were captured on a Nikon 80i microscope.
In order to correlate RhoA mRNA expression with actual axon regeneration, in two animals at 8 weeks post- transection, a dextran tetramethyl-rhodamine- (DTMR, 10 kDa, 5% in 0.1 M Tris buffer, pH 7.4) soaked Gelfoam plug was inserted into a second spinal cord transection 5 mm caudal to the first transection, in order to label neurons whose axons had regenerated to this level. One week later, when the fluorescent tracer had successfully backfilled regenerated reticulospinal neurons, the brain was removed and processed for FLICA and ISH for RhoA as above.
In situ hybridization on paraffin sections
To confirm the results seen in wholemounts, ISH also was performed on paraffin sections. Lampreys were re- anesthetized and the brain and spinal cord proximal to the transection site were fixed in 4% PFA for 3 to 4 hours. Tissues were rinsed in PBS, dehydrated in serial ethanols overnight in a tissue processor and embedded in paraffin. Horizontal, sagittal or transverse paraffin- sections of brain or spinal cord (10 μm, will mention in figure legends) were deparaffinized and processed for ISH as described previously (Swain et al. 1994). Briefly, after rehydration, sections were washed in PTW, and pre-hybridized as above at 50–55 °C for 1 hour. The biotin-conjugated RhoA probe was applied to slides at a concentration of 0.5 μg/ml in hybridization solution, and incubated overnight at 55 °C. To prevent evaporation, the slides were covered by HybriSlip (Sigma-Aldrich) and kept in a moist chamber. The next day, slides were washed in hybridization solution at 55 °C, then PTW and PBT at room temperature. AP-conjugated Streptavidin diluted 1:1000 in PBT was applied overnight at 4°C. The sections were washed in PBT then in SMT and the NBT/BCIP chromogenic reaction was performed as described above for 10–15 minutes. The reaction was stopped in PBS and the slides were dehydrated, cleared and mounted in Permount.
Lectin histochemistry
The distribution of microglial cells was determined, at 2 weeks post- transection. The brain and spinal cord rostral to the transection site were fixed and treated with a microglia-specific label, horseradish peroxidase-conjugated GSA isolectin B4 (Sigma), which binds to D-galactose residues in both resting and activated microglial cells (Streit, 1990). Serial sagittal paraffin-sections (10 μm) were deparaffinized, rehydrated, washed in PBS and treated in 0.3% H2O2 dH2O for 30 min. The sections were incubated with GSA I- B4-HRP (5 μg/ml in PBS) at room temperature for 2 hours, followed by 3 washes in PBS with 0.1% Triton-X 100, and once in PBS, 5 min each. The sections were colorized in Metal-Enhanced DAB Substrate (Thermo Scientific) for 10 min, then washed, dehydrated and mounted in Permount. Six sagittal sections near the midline were collected in each of five 0.3 mm lengths of spinal cord, starting from the transection site and going rostralward. Images were captured with a Nikon 80i microscope, and theB4-positive cells were counted in each length. The results were compared to those from uninjured controls.
In situ GTP-Rho binding assay to detect RhoA activation
The Rho-GTP binding domain (RBD) of human Rhotekin binds specifically to the active GTP-bound forms of Rho A/B/C proteins, but not the GDP-bound forms (Reid et al., 1996), thereby serving as an activation-specific probe. Glutathione-S-transferase-fused Rhotekin-RBD (GST-RBD) is commonly used in RhoA pulldown activation assays, and has been adapted to assay in situ Rho GTPase activity (Li et al., 2002, Dubreuil et al., 2003). To investigate activation of RhoA in lamprey reticulospinal neurons after SCI, following FLICA staining, the brains were rinsed 3 times for 1 hour each at room temperature in TPBS (0.2 % Tween-20 in PBS), blocked with 10% fetal bovine serum (FBS) in PBS for 1 hour, and incubated in GST-RBD (5 μg/ml) for 2 days at 4°C. Brains were rinsed and blocked as above and incubated overnight at 4°C in AP-conjugated GST antibody (1:200 in blocking solution, Rockland Immunochemicals, Inc). The brains then were washed 3 times for 30 minutes each in PBT 3 × 30 minutes and 3 times for 30 minutes each in SMT. The chromogenic reaction was carried out as described above for wholemount ISH. GST-RBD binds not only to RhoA but also to Rho B and C, which share more than 85% amino acid sequence identity with RhoA. Although in neurons RhoA is expressed at higher levels than RhoB and RhoC (Lehmann et al., 1999), to confirm that neurons labeled by GST-RBD also expressed RhoA, we performed triple labeling of FLICA, GST-RBD and RhoA ISH, and found colocalization of RhoA ISH and GST-RBD labeling.
RhoA immunostaining
To investigate colocalization of RhoA protein with RhoA mRNA and activated Rho in axons near the transection site, serial sagittal paraffin-sections were deparaffinized, rehydrated, and washed in PBS then processed for ISH, GST-RBD as above, and RhoA immunostaining. For RhoA immunostaining, antigen retrieval was performed - sections were immersed in sodium citrate buffer (10 mM sodium citrate, pH 6.0) and microwaved for 20 min, then allowed to cool in room temperature for 20 min. Sections were rinsed in PBS twice for 5 min/each and blocked (10% FBS in PBS with 0.2% Tween-20) for 10 min at room temperature and incubated with anti-RhoA (SC-418, Santa Cruz) in blocking buffer 1:200 at 4 °C overnight. Sections were washed 3 times with PBS, and then incubated with an ABC kit (VECTASTAIN, Vector laboratories), as per the manufacturer’s protocol. The sections were dehydrated and mounted in Permount.
Triple labeling with FLICA, GST-RBD, and PTPσ ISH
To investigate co-localization among activated RhoA, PTPσ mRNA, and FLICA positivity in the same reticulospinal neurons, FLICA staining was first performed on fresh tissue of five lamprey brains at 9 weeks post- transection, and the results photographed. Then double chromogenic AP detection for GST-RBD and ISH were performed, each using a different AP substrate. This required that the first substrate be washed out and AP be inactivated before the second labeling can be processed. Lamprey brains were processed for GST-RBD labeling as above, except that they were first developed in the dark at room temperature for 20 minutes, or until the reaction was complete, in the alcohol-soluble AP chromogen INT/BCIP (7.5 μl stock solution per 1 ml of SMT, Roche Applied Science). This was followed by 3 washes in dH2O, 5 minutes each, and then one 30-minute wash in 0.1M Tris-HCl (pH 8.2). The samples were mounted in 20% glycerol in Tris-buffer and photographed with a bright- field microscope (80i Nikon). The AP-conjugated GST antibody was inactivated by treating the specimens with 0.1M glycine buffer (pH 2.2) in 0.1% Tween-20 for 20 minutes at 37°C. After briefly washing in Tris-buffer 3 times, the magenta color of INT/BCIP was removed by washing through serial ethanols of 70%, 80%, 90% and 95%, then back to 70%, 5 minutes each, then 3 times in PTW, 5 minutes each. The samples were rephotographed. Although often, a faint blue INT/BCIP staining remained, this did not affect co-localization observations. To verify that the procedure removed all AP activity, following the above procedure, a sample was treated with a second AP substrate (NBT/BCIP), and the absence of new staining confirmed the elimination of AP activity. The brain samples were then processed for PTPσ ISH, and colorized by NBT/BCIP as described above. Because NBT/BCIP cannot be removed easily, it had to be used as the final chromogen. As described in the company literature, the sensitivity of INT/BCIP is not as great as that of NBT/BCIP. Therefore, to insure that the results above were not affected by differential sensitivity of the AP substrates, in some experiments, the order of labeling was reversed, i.e., ISH labeling was carried out first, using INT/BCIP, followed by GST-RBD, using NBT/BCIP. No differences in labeling frequency or intensity were observed.
Semiquantitative scale for neuronal labeling
To correlate expression of RhoA mRNA or activation of caspases or RhoA with the previously-determined probabilities of regeneration for identified reticulospinal neurons, a semi-quantitative integer scale from 0 to 3 was used to estimate the staining intensity of RhoA ISH, FLICA and GST-RBD in individual identified reticulospinal neurons, as described previously (Zhang et al., 2014). For each reticulospinal neuron type, a normalized RhoA ISH, FLICA or GST-RBD labeling score (% maximum) was calculated for each group of animals as follows: The staining intensity scores of each individual neuron of that type were summed and the result was divided by the maximum possible summed staining intensity scores (i.e., the number of animals x 2 cells of each type per animal x the maximum semiquantitative score of 3 per cell) multiplied by 100. For example, if in 25 animals at a given time post- transection, the sum of the semiquantitative FLICA scores in all the Mauthner cells equaled 55, then the FLICA labeling score would be ((55/25 animals) x 2 Mauthner cells per animal x 3)) x 100 = 36.7%. The intensity scores for individual neuron types were then correlated with the previously- determined probabilities that their axon would regenerate 5mm beyond the lesion by 10 weeks post- transection (Jacobs et al., 1997). The activations of caspases and RhoA in each identified neuron type were investigated from 1–9 weeks post- transection.
Effect of CSPGs on retrograde neuronal death
It has been suggested that CSPGs secreted at the site of CNS injury inhibit axon regeneration by activating RhoA downstream of their RPTPs. To test whether exogenous CSPGs activate RhoA and accelerate neuronal death, a mixture of purified CSPGs containing neurocan, versican, phosphacan and aggrecan (5.2 μg/ml; Millipore) was applied to the transection site via a soaked Gelfoam. After survival times indicated in Table 1, the brains were removed under anesthesia and processed for FLICA followed by GST-RBD staining and PTPσ ISH as described above. The number of FLICA-positive neurons were counted and compared among the groups.
Statistical analysis
Data sets were analyzed with the InStat software (GraphPad3). The effect of SCI on expression levels of RhoA mRNA was determined for each of the 18 paired identified reticulospinal neuron types by calculating a % of maximum possible score compared to the controls (see above) for each time post- transection (ANOVA and post hoc two-tailed t-test with Bonferroni correction for multiple comparisons). The effect of exogenous CSPGs on apoptosis signaling was determined for each post- transection time by comparing total numbers of FLICA-labeled neurons in the CSPG-treated group with that in the corresponding control group, using the unpaired two-tailed t-test. Correlation analysis was by Pearson Correlation test. All values were expressed as mean ± SEM.
Results
Cloning Lamprey RhoA
The Rho family is a subfamily of the Ras superfamily, and has three primary members: RhoA, RhoB and RhoC. Their amino acid sequences share approximately 85% identity, but their functional differences are not completely clear (Causeret et al., 2004). RhoA predominates in the CNS (Erschbamer et al., 2005) and has been studied extensively with regard to axon regeneration (Fujita and Yamashita, 2014). RhoA is activated when CSPGs bind to RPTPs (Sharma et al., 2012)(Fisher et al., 2011). The lamprey genomic database was searched with sequences from human, chicken and zebrafish RhoA, and lamprey RhoA sequenced from overlapping contigs. Full length lamprey RhoA was cloned and its amino acid sequence found to be highly homologous to that of mammalian RhoAs (Fig. 1A). Lamprey RhoA shares 93.3% amino acid identity with human and zebrafish RhoAs and 93.8% with chicken RhoA. At the N- terminal, which contains most of the GTP binding region, sequence identities are almost 100%. Therefore, we predicted that reagents inhibiting or activating RhoA in mammals would work similarly in lamprey.
RhoA mRNA is expressed widely in non-injured lamprey CNS
We first determined the distribution of RhoA expression in normal lamprey brains at different stages of development. A total of 8 uninjured control lampreys, including 6 larvae from 6–13 cm long (3–5 years) and 2 recently transformed young adults (13 and 14 cm) were examined by wholemount ISH. RhoA mRNA was widely expressed in both neurons and glial cells at all stages (Fig. 1B). The level of expression was heterogeneous among the identified neurons, and, although statistically significant, the magnitude of the correlation was small, i.e., mRNA scores were only slightly higher in poorly-regenerating reticulospinal neurons (white arrows in the 10 cm larva brain of Fig. 1B) than in good regenerators. However, in the brains of larger premetamorphic larvae and young adults, the Mauthner cells often were labeled weakly or not at all (asterisks in 13 cm larva and adult brains Fig. 1B), even though they are among the poorest regenerators.
The effect of SCI on neuronal RhoA mRNA levels
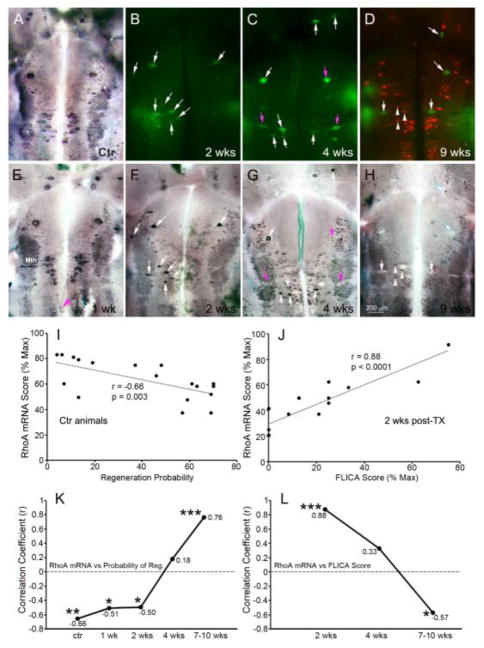
Previously we reported that caspases are activated selectively in poorly-regenerating neurons (Barreiro-Iglesias and Shifman, 2012, Hu et al., 2013), beginning by 2 weeks post- transection, and persisting for several weeks. This was confirmed in the present study (Fig. 2B–D, labeled green by FLICA). At 1 week post- transection, only a few neurons were FLICA+, and this was true also in the present study. This pattern was compared with the effect of spinal cord transection on mRNA expression. The identified reticulospinal neurons of 28 lampreys were investigated for RhoA mRNA expression in wholemounted preparations from 0–10 weeks after SCI. In 2 animals at 1 week post- transection, there was little change in mRNA labeling that was measurable with our semiquantitative scale, compared to control animals. If anything, expression was qualitatively increased (Fig. 2E vs. A). However, by 2 weeks post- transection, caspases were activated selectively in poorly-regenerating/poorly- surviving neurons (Fig. 2B–D; (Barreiro-Iglesias and Shifman, 2012)), and RhoA mRNA labeling scores declined significantly (Fig. 2F–H), though less so in poorly-regenerating/poorly-surviving neurons (Fig. 2K, L). This correlation broke down at 4 weeks (Fig. 2C, G, K, L), and was even reversed at 7–10 weeks (Fig. 2D, H, K, L). As reported previously (Zhang et al 2005), long-distance retraction of axons belonging to some poorly-regenerating neurons was observed at 1 week post-transection (Fig. 2E). The ISH label in these retracted axons suggested that they contain RhoA mRNA, consistent with our previous demonstration of mRNA and other elements of translational machinery in reticulospinal axon tips post-transection (Jin et al., 2016). To retrogradely label those reticulospinal neurons whose axons had regenerated by 8 weeks post- transection, DTMR was applied to a second transection 5 mm caudal to the original lesion, and the animals allowed to recover an additional week. Almost none of the neurons with regenerated axons were FLICA+ (Fig. 2D). RhoA mRNA labeling persisted in reticulospinal neurons with regenerated axons (white arrowheads in Fig. 2D, H), but was greatly reduced in FLICA+ neurons (white arrows). Some cells with undetectable amounts of RhoA mRNA were neither FLICA+ nor retrogradely labeled (asterisks in Fig. 2D, H), possibly because apoptosis was already too far advanced to synthesize new mRNA. Thus after axotomy, RhoA mRNA was widely distributed in neurons, was expressed preferentially in poor regenerators only early (2 weeks post-transection), and by 9 weeks was downregulated in neurons that were caspase+.
Fig. 2. RhoA mRNA expression in reticulospinal neurons is negatively associated with regenerative probability.
At 1 week post-SCI, RhoA mRNA levels (E) were similar to those in normal brain (A) and negatively correlated with regenerative probability of reticulospinal neurons (I and K) during the first two weeks. The magenta arrowhead in E points to a retracting axon tip labeled for RhoA mRNA. After 2 weeks post-SCI, apoptotic signaling was detected in reticulospinal neurons by FLICA (B–D), followed by wholemount ISH for RhoA (F–H). At 2 weeks post-transection, RhoA mRNA expression was seen selectively in caspase-positive RNs (white arrows in B & F; J). At 4 weeks post-transection (C, G), expression of RhoA mRNA in FLICA+ neurons varied, low in some (magenta arrows), high or unchanged in others (white arrows). At 8 weeks post-transection (D, H), regenerated reticulospinal neurons were labeled with DTMR applied 5 mm caudal to the original transection. One week later, RhoA mRNA expression was normal in regenerated reticulospinal neurons (H, white arrowheads) but reduced in all FLICA+ cells (white arrows). The correlation coefficients (r) between RhoA mRNA score and either regenerative probability of reticulospinal neurons or FLICA intensity score at different post-transection times are plotted in K & L. *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; wk = week; Mth, Mauthner neuron.
Accumulation of RhoA mRNA in axons
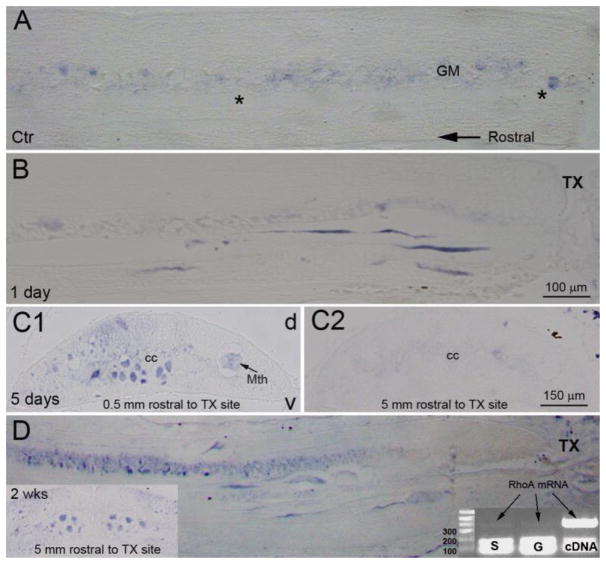
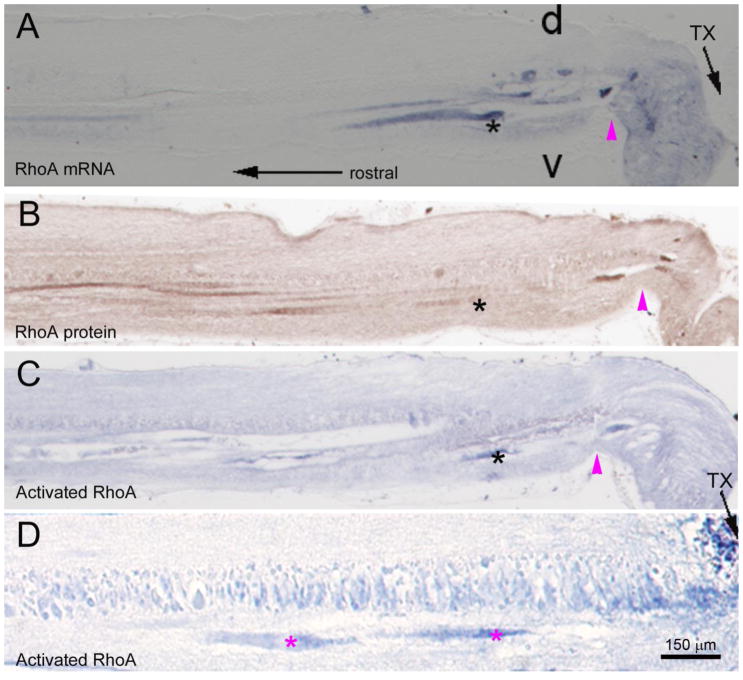
Previously we reported the accumulation of mRNA and other elements of protein synthesis machinery in lamprey reticulospinal axons after spinal cord transection (Jin et al., 2016). Since RhoA signaling by receptor-mediated action of environmental cues is assumed to be at least partly local (in the distal axon), in the present study, we determined whether RhoA mRNA also was present in the injured axon. ISH on paraffin sections at the site of injury and rostral to it showed that under normal conditions, i.e., in the uninjured spinal cord, almost no mRNA is detectable in axons (Fig. 3A). However, within 24 hours, mRNA was concentrated in the distal axon tips (Fig. 3B). Even at 5 days post- transection, RhoA mRNA remained concentrated in the distal cut axons (Fig. 3C1), but was absent from the same axons more rostrally (Fig. 3C2). By 2 weeks post- transection, the mRNA was found both distally (Fig. 3D) and at the level of the second gill, 5 mm proximal to the injury (Fig. 3D left insert). The identity of the mRNA label was confirmed by PCR of microaspirated axoplasm from the axon tips (Fig. 3D gel image).
Fig. 3. Early accumulation of axonal RhoA mRNA in severed axon tips.
Sagittal spinal cord sections at the level of the 5th gill processed for RhoA ISH, taken from an uninjured control animal (Ctr) and an animal 1 day post-transection. RhoA mRNA was barely seen in axons of control animals, but was seen in the injured axon tips post-SCI. In transverse sections at 5 days post-SCI, dense RhoA mRNA label was seen in giant reticulospinal axons within 0.5 mm rostral to the transection site (C1) but not in axons of the same spinal cord closer to the brain (C2). At 2 weeks post-SCI, RhoA mRNA was seen in axon tips near the transection site, as well as in more proximal axons near the brain (transverse section in left lower corner). An agarose gel (right lower corner) shows PCR products amplified from microaspirated individual static (S) or growing (G) giant axon tips. The heavy bands on the bottom are dimerized primers. RhoA mRNA was detected (light bands pointed to by arrows) in both. Total cDNA from lamprey CNS served as a positive control. cc, central canal; v, ventral; d, dorsal; transection, transection site; Mth, Mauthner axon.
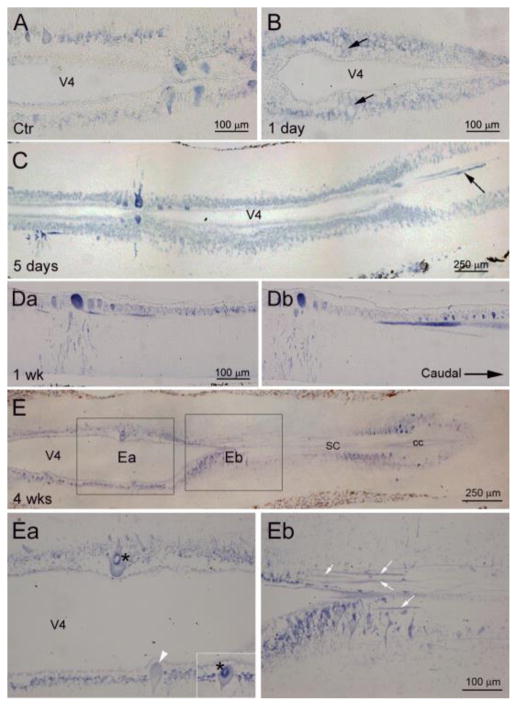
To better understand how axotomy affected the RhoA mRNA distribution, ISH was carried out on horizontal sections of lamprey brainstem. In control brains, neurons with previously-determined low probabilities of regeneration tended to show dense RhoA mRNA labeling (e.g., the I1 cells in Fig. 4A). The intensity of labeling was increased primarily during the first week post- transection (Fig. 4C). The most-affected neurons were poor regenerators, such as M2 and M3 in the mesencephalon; and I1, Mauthner, B1, and B3 in the rhombencephalon. Swollen and chromatolytic reticulospinal neurons containing multiple vesicles (arrows in Fig. 4B) were often observed as early as 1 day post- transection. In neurons that were most darkly stained, the in situ label extended into the dendrites (Fig. 4C) and proximal axons (arrow in Fig. 4C), but did not extend far beyond the obex before 1 week post- transection. RhoA mRNA label persisted in axons for at least 4 weeks (Fig. 4Eb), while beginning to decrease in most neuronal cell bodies (Fig. 4Ea). This suggests possible translocation of the mRNA from the perikaryon to the proximal axon. Thus, soon after axotomy, RhoA mRNA appeared in the distal axon tip, but was sparse more proximally. By 5 days post- transection, RhoA mRNA was upregulated in the perikaryon, and was translocated into the proximal axon. By 2 weeks, the mRNA had spread centrifugally to the level of the 2nd gill.
Fig. 4. Descending axons labeled for RhoA mRNA appear by 5 days post-SCI.
Horizontal sections of lamprey brains at indicated times post-transection. In control spinal cord and at 1 day post-transection, large RS neurons expressed relatively low levels of RhoA mRNA (A and B), and no descending axons were labeled. Some poorly-regenerating RS neurons appeared swollen and had vacuoles (arrows in B). RhoA mRNA appeared in descending axons as early as 5 days post-transection (arrow in C) and persisted at 1 wk (D), 2 wks (not show) and 4 wks (E). Adjacent sections of a brain at 1week post-transection, show a RN whose desending axon was labeled for RhoA mRNA. Ea and Eb are enlargements of boxed areas in E, showing declining RhoA mRNA label in perikarya (Ea), while the numbers of labeled axons increased (white arrows in Eb). The insert in Ea shows an adjacent section through the same neuron pointed to by the white arrowhead. Dense RhoA mRNA label was seen in the perimeter of the nucleus (*). V4, 4th ventricle; SC, spinal cord; cc, central canal.
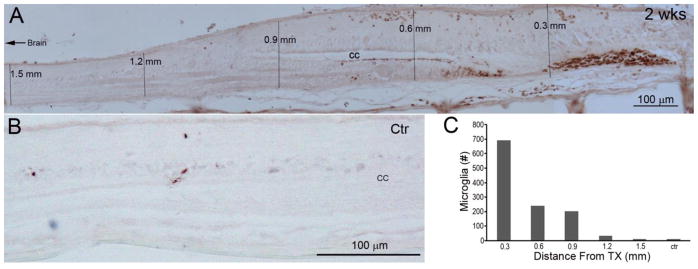
Microglial accumulation is limited to the area of the lesion
The present study examines the role of RhoA in retrograde cell death due to axotomy. Previous studies of RhoA’s role in apoptotic cell death after SCI did not distinguish death due to local factors, such as inflammation, from death due to axotomy, and described evidence for death of both neurons and glia near the lesion (Dubreuil et al., 2003). We determined how far signs of inflammation could be detected from the site of SCI at 2 weeks post-transection, when caspase activation was well underway, by labeling microglia with GSA I-B4- HRP (Fig. 5A). Microglia were heavily concentrated in the first half mm from the center of the lesion. By 1.5 mm from the transection site, their numbers were no longer significantly different from those in uninjured controls (Fig. 5B, C). We saw few microglia in the brainstem. Thus, the apoptotic signaling seen in the large identified RS neurons is likely to be due to intra-axonally transported signals remote from the site of injury, and not inflammation or other changes that are restricted to the region close to the injury. This does not preclude the possibility that such local changes participate in creating the retrograde signal.
Fig. 5. Restriction of inflammatory changes (microglia) to the transection site.
Spinal cords were sectioned sagittally at 10 μm and processed for GSA I-B4-HRP histochemistry. A, a spinal cord at 2 weeks post-transection, showing that microglia are located mostly near the transection site. B, few microglia are found in a non-injured animal. C, graph of the summed microglial counts in 6 sections from five 0.3 mm-length bins bounded by the vertical lines in A, and compared with microglial counts from 6 sections from the same level (gill region) of spinal cord in an uninjured control (ctr) animal. Note that the microglia are found almost exclusively within 0.5 mm of the transection site, and would not come near the cell bodies of reticulospinal neurons in the brainstem.
RhoA is activated selectively in pre-apoptotic, poorly-regenerating RS neurons
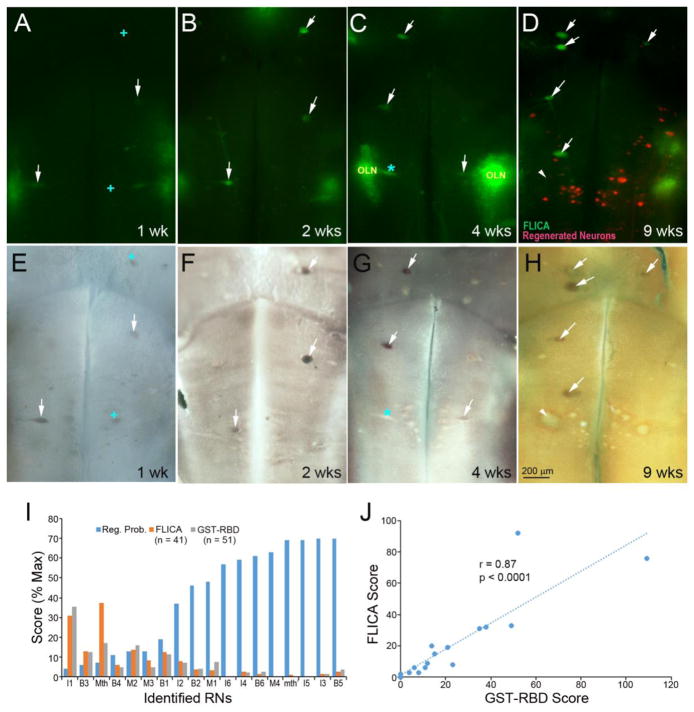
Although RhoA mRNA labeling was poorly correlated with caspase activity, the latter correlated well with RhoA activation. GST-RBD binds to the GTP-bound (active) forms of Rho subfamily members. In triple-labeling experiments, FLICA, GST-RBD and RhoA ISH frequently co-localized in poor regenerators during the first 2 weeks post-transectrion (Fig. 6). To determine whether there is a systematic relationship between RhoA activation and retrograde cell death following SCI, the brains of animals up to 9 weeks post- transection were processed for FLICA staining, followed by fixation and GST-RBD binding in situ. Both FLICA and GST-RBD labels were found primarily in the poorly-regenerating RS neurons, and neurons that were positive for FLICA were almost always positive for GST-RBD (Fig. 5, white arrows). The Mauthner neuron was an exception because it often was FLICA+ but not stained by GST-RBD (red * in Fig. 6C, G) or vice versa (+ in Fig. 6A, E). Activated caspases often were found in Mauthner neurons, whose dendrites usually were damaged during live brain dissection, suggesting that acute neural injury could have induced immediate local caspases activation. This acute response of caspase activation to injury was also seen in octavolateralis neurons (OLN) located laterally to Mauthner and extremely vulnerable to dissection. A previous study in lamprey showed caspase 8 activation in severed axon tips near the lesion at 2 hours post-transection, the earliest time studied. Whether caspase activation was triggered by RhoA or through another signaling pathway is not known. To correlate RhoA activation with axon regeneration, activated RhoA was localized by GST-RBD immunohistochemistry in the brains of animals 9 weeks post-SCI, in which neurons whose axons had regenerated were retrogradely labeled from 5 mm caudal to the original transection (Fig. 6D). Activated RhoA was found selectively in neurons undergoing apoptosis (FLICA+), but not in neurons with regenerated axons. Some swollen RS neurons were not labeled by either FLICA or GST-RBS, e.g., the Mauthner (Fig. 6H, white arrowhead), possibly because they were already in late stages of apoptosis. These results suggest a possible link between RhoA activation and retrograde neuronal death.
Fig. 6. RhoA activation is correlated with caspase activity in poorly-regenerating reticulospinal neurons.
Lamprey brains were labeled by FLICA for poly-caspases at the indicated recovery times (A–D), then by GST-RBD for activated RhoA (E–H). Red cells in D are labeled retrogradely with DTMR from a 2nd cut 5 mm caudal to the original lesion at 8 weeks post-SCI, indicating that their axons had regenerated. After 1 week to allow for retrograde labeling, brains were processed for NBT/BCIP (E–G) or INT/BCIP (H) to image active RhoA. The white arrows indicate the neurons labeled by both FLICA and GST-RBD. The + in A and E indicate neurons labeled by GST-RBD only. The blue * in C and G is a Mauthner neuron labeled by FLICA but not by GST-RBD. The Mauthner neuron lies laterally, and its dendrites usually were damaged during brain dissection, inducing almost immediate FLICA labeling. Regenerated RNs (red-labeled cells in D) were stained neither by FLICA (D) nor by GST-RBD (H). A white arrow head points to a swollen Mauthner cell that is negative for both GST-RBD and FLICA, probably because it is in a late stage of apoptosis. I, Semi-quantitative scores for caspase and RhoA activities in identified RNs were inversely correlated with their previously determined regeneration probabilities (FLICA, r = −0.769, p = 0.0002; GST-RBD, r = −0.739, p = 0.0005). The differences in labeling scores among the groups was not statistically significant. Therefore, the data for all post-transection times were pooled. Intensity of GST-RBD and FLICA labeling correlated significantly in those reticulospinal neurons (J).
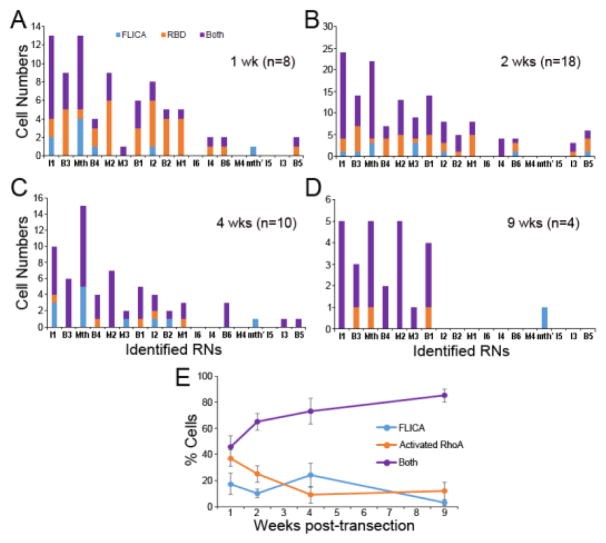
For the 18 pairs of identified RS neurons, highly significant inverse correlations were found between their probability of axon regeneration and activation of caspases (Fig. 6I). Activated RhoA often appeared in RS neurons of uninjured lampreys, whereas caspase activity was detected only after injury. The RS neurons that stained for activated RhoA in non-injured animals were mostly poor regenerators. Thus, activation of RhoA in those neurons may not represent a rapid response to injury during sample collection as described above for caspase activity. The numbers of neurons labeled by GST-RBD were not significantly different between the control and SCI groups, but most FLICA+ neurons were also positive for GST-RBD, and the intensity scores of those two labels were strongly correlated (Fig. 6J). Co-localization of activated caspases and RhoA in each neuron type is shown in Fig. 7. Since caspase activation was not observed in non-injured brains, no control group was included. There were more neurons labeled for only activated RhoA (44%) than for only activated caspases (11%) at 1 week post-SCI. Thereafter, the proportion of double-labeled cells increased (to 85% at 9 weeks post-transection), while the proportion labeled for only activated RhoA decreased, and for only activated caspases was static. Most reticulospinal neurons labeled by FLICA and GST-RBD were those whose axons regenerate poorly. The results suggest that RhoA activation contributes to a neuron-intrinsic property of poor survival and poor regeneration.
Fig. 7. The number of neurons showing dual activation of caspases and RhoA increased with time post-transection.
To correlate activities of caspases and RhoA within identified reticulospinal neurons, the number of cells that were double-labeled (purple), FLICA-labeled only (blue) and GST-RBD-labeled only (orange) were counted at 1, 2, 4 and 9 weeks post-transection (A-D). The reticulospinal neurons are arranged in order of regeneration probability from low (left) to high (right). At 1 week, less than 50% of the neurons were double-labeled, while 44% were only GST-RBD+ and 11% were only FLICA+. Thereafter, the % (means + SEM) of reticulospinal neurons that were double-labeled for activated RhoA and caspases increased to 65, 73, and 85 at 2, 4 and 9 weeks post-SCI, respectively (E).
RhoA and caspases are activated selectively in neurons expressing mRNAs for PTPσ
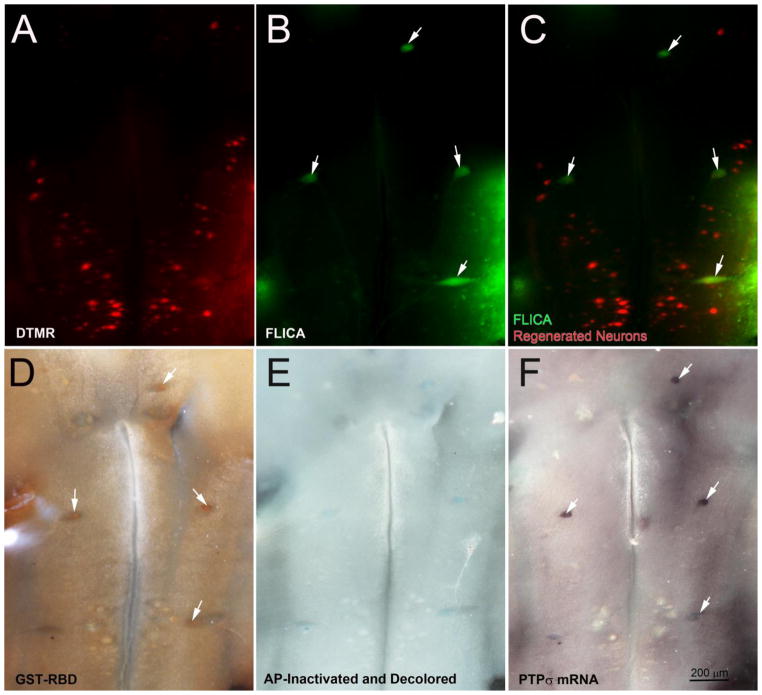
The axon growth-inhibiting effects of CSPGs partly involve binding to the RPTPs, and to downstream activation of RhoA through undetermined mechanisms (Sharma et al., 2012, Ohtake et al., 2016). RhoA also has been implicated in apoptosis near a SCI lesion (Dubreuil et al., 2003), although the relationship to axotomy is not known. Previously, we reported that PTPσ and LAR are expressed selectively in the poor-regenerator reticulospinal neurons (Zhang et al., 2015). Similarly, in the present study, activated RhoA was found selectively in neurons undergoing apoptosis. To assess the possibility that the CSPG pathway contributes to axotomy-induced reticulospinal neuronal death via activation of RhoA, we performed spinal cord transection in five lampreys and after 8 weeks recovery, retrogradely labeled regenerated reticulospinal neurons with DTMR (Fig. 8A). After one more week to allow for retrograde tracer transport, we performed triple labeling with FLICA (Fig. 8B), GST-RBD (Fig. 8D) and PTPσ ISH (Fig. 8F). There was almost no overlap between the population of neurons that were FLICA positive and those whose axons had regenerated (Fig. 8C). However, neurons that were FLICA positive were also likely to show activation of RhoA and to express PTPσ mRNA. In Fig. 8, the white arrows point to neurons that are triple-labeled for FLICA, GST-RBD and mRNA for PTPσ. None of these triple-labeled neurons were retrogradely labeled with DTMR. In two additional animals at 4 weeks post-transection, triple labeling was performed, but with ISH for LAR instead of PTPσ. As with PTPσ, LAR mRNA was detected primarily in neurons that were positive for activated caspases and activated RhoA (not shown).
Fig. 8. Co-localization of activated caspases and RhoA with PTPσ mRNA.
At 8 weeks post-SCI, reticulospinal neurons with regenerated axons were labeled by DTMR applied to a 2nd transection 5 mm caudal to the original lesion. After allowing one more week for retrodrade labeling, brains were removed and processed as indicated. Neurons whose axons had regenerated are labeled red (A). There was no overlap with FLICA-positive neurons (B, C). Lamprey brains were further processed for activated RhoA protein (D) followed by ISH for PTPσ (F), a receptor for CSPGs. White arrows point to neurons that were triply labeled by FLICA, GST-RBD and PTPσ ISH.
Local accumulation and activation of RhoA post-transection
Because of the strong correlation between caspase and RhoA activations in the neuronal perikarya, we asked whether the rapid accumulation of RhoA mRNA in severed axons was accompanied by the appearance of RhoA protein and RhoA activation after SCI. In serial sagital sections of spinal cord near the injury site, axotomized axon tips that at 24 hours post- transection contained RhoA mRNA (Fig. 9A) also stained positively for both total RhoA protein (Fig. 9B) and activated RhoA (Fig. 9C). Although it was difficult to obtain adjacent sections of over long stretches of the same axon, RhoA mRNA, protein and activation tended to be more heavily concentrated distally in the injured axon than more proximally (e.g., Fig. 9D in a different animal). Whether the RhoA protein was primarily the product of local translation (Jin et al., 2016) is not known.
Fig. 9. Early appearance of total and activated RhoA in axon tips that contain mRNA.
Serial saggital sections of spinal cord just rostral to a 1-day old transection were processed for RhoA mRNA by ISH, total RhoA protein by immunostaining and activated RhoA protein by GST-RBD labeling. RhoA mRNA appeared rapidly in severed axon stumps as in Fig. 3. RhoA mRNA-positive axons also stained positively for total and activated RhoA. d = dorsal, v = ventral. In A–C, the black asterisks indicate the same axon labeled by RhoA ISH, RhoA immunohistochemistry and GST-RBD. The magenta arrowheads point to the location where the sample is deformed by tweezer handling. D, a second spinal cord showing preferential RhoA activation in injured axon tips (magenta asterisks).
Effect of exogenous CSPGs on caspase and RhoA activations, and on expression of PTPσ mRNA
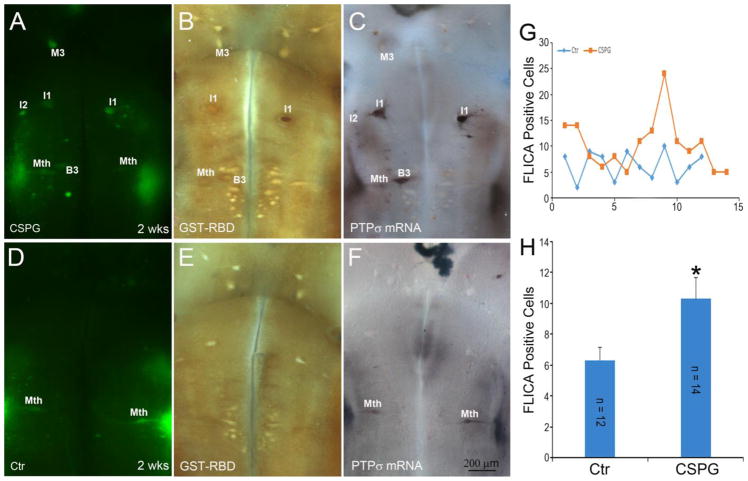
Because in cultured mouse neurons, the binding of CSPGs to PTPσ and LAR induced activation of RhoA (Lim and Joe, 2013, Ohtake et al., 2016), we determined the effect of exogenous CSPGs on activation of RhoA and caspases in identified reticulospinal neurons. At the time of transection, exogenous CSPGs were applied to the injury site. Two weeks later, compared with control lampreys, the brains of CSPG-treated animals had significantly more FLICA+ neurons (Fig. 10A vs. D, G), GST-RBD+ neurons (Fig. 10B vs. E), and PTPσ mRNA+ neurons (Fig. 10D vs. F).
Fig. 10. Exogenous CSPGs enhance activation of caspases and RhoA.
Exogenous CSPGs (A–C) or vehicle only (D–F) were applied to the transection site at the time of SCI. Two weeks later, the brains were processed for FLICA (A & D), GST-RBD (B & E). The tissue was processed by INT/BCIP-decoloration and AP-inactivation, followed by PTPσ ISH (C & F). CSPGs increased the activation of caspases (A) and RhoA (B). Most capase-positive neurons also expressed PTPσ mRNA, linking CSPGs, PTPσ and RhoA in a putative neuronal death pathway. G, counts of FLICA+ reticulospinal neurons in individual animals treated with CSPGs and in controls. The animals are numbered randomly in the abscissa. the number of FLICA-positive neurons (Means + SEM) was significantly greater in animals treated with CSPGs than in vehicle-treated controls (p = 0.025; ANOVA and post hoc two-tailed t-test).
Discussion
Recently, we reported that knockdown of RhoA by a retrogradely-delivered translation-blocking morpholino promoted axon regeneration and also reduced caspase activity in axotomized lamprey reticulospinal neurons (Hu et al., 2017), suggesting convergence onto RhoA of intraneuronal signaling for both apoptosis and inhibition of regeneration. In the present study, we examined the time course of RhoA mRNA expression and of RhoA activation after spinal cord transection, in order to gain insight into how RhoA responds to SCI and at what processing level its influence on retrograde apoptosis is regulated. Because among the identified reticulospinal neurons, those that are intrinsically poor regenerators also undergo a very delayed form of retrograde apoptosis after axotomy (Shifman et al., 2008)(see below) we anticipated that RhoA expression would be higher in the poor regenerators than in good regenerators, or that its expression would be dramatically upregulated selectively in the poor regenerators. However, this was not the case. Although in control animals, the poor regenerators did have slightly higher levels of RhoA mRNA, the difference was not dramatic. Moreover, the correlation between RhoA expression and apoptotic signaling decreased with time after transection and actually reversed after 4 weeks post-transection, a time when axons had completed their initial retraction and had not yet grown past the injury site. Instead, it was activation of RhoA that was more dramatic in the poorly-regenerating/poorly-surviving neurons, and correlated strongly with caspase activation and with expression of CSPG receptors (Zhang et al., 2014), both molecular markers of neurons that undergo delayed cell death post-axotomy. Thus it is the activation of RhoA by injury, rather than its mRNA expression, that is likely to be a dominant factor regulating post-axotomy apoptosis.
Role of RhoA in retrograde neuronal death following axotomy in vivo
RhoA activation is a signal common to many environmental cues, e.g., the myelin- associated growth inhibitors, which bind to the Nogo receptor complex (McGee and Strittmatter, 2003, Mimura et al., 2006), and CSPGs (Gallo, 2006, Zhang et al., 2014) which are secreted at the site of CNS injury (Hu et al., 2013, Hu et al., 2017) and bind to the receptor protein tyrosine phosphatases PTPσ (Gopalakrishnan et al., 2008) and LAR (Fisher et al., 2011)(Lim and Joe, 2013, Ohtake et al., 2016). RhoA inactivation fosters axon growth and functional recovery following SCI (Lehmann et al., 1999, Dergham et al., 2002, Fournier et al., 2003), but because in mammalian SCI experiments it is difficult to distinguish regeneration of severed axons from collateral sprouting by spared axons, it is not clear whether RhoA is suppressing axon regeneration or only sprouting, two processes that appear to have different mechanisms (Lee et al., 2010, Koch et al., 2014). We have used the lamprey as an experimental model to get around this difficulty because its axons regenerate after complete spinal cord injury and regeneration is easily documented by histological and electrophysiological means (Rovainen, 1976, Selzer, 1978, Yin and Selzer, 1983, 1984, Cohen et al., 1988, Davis and McClellan, 1994a, Banerjee et al., 2016).
Although lamprey reticulospinal axons can regenerate after SCI, the regeneration is not complete. The large identified reticulospinal neurons vary greatly in their regenerative abilities (Davis and McClellan, 1994b, Jacobs et al., 1997), and those that are poor regenerators often undergo a very delayed form of apoptosis (Shifman et al., 2008). Thus, after axotomy, a common pathway might mediate both failure of axon regeneration and failure of survival in the axotomized neuron. The question raised in the present study is whether RhoA activity is a common signal for both effects. Although this idea has been suggested by studies on mammalian neurons, both in vitro and in vivo, those studies do not unequivocally relate axon growth failure and cell death to axotomy of the affected neuron (see below).
Reticulospinal neuron death after spinal cord transection is due to axotomy
Apoptosis was described in unidentified cells in the region of SCI in rats and monkeys, and remotely in oligodendrocytes associated with Wallerian axonal degeneration (Crowe et al., 1997). Later in rats, apoptosis was seen in both neurons and glia, and inhibition of RhoA activity with C3 decreased apoptotic death in both (Dubreuil et al., 2003), but the observations were restricted to the region near the injury. Thus, the apoptosis described in neuro ns was not necessarily related to axotomy. Similarly, viral vector-mediated knockdown of RhoA increased survival and axonal regeneration of retinal ganglion cells in mice (Koch et al., 2014), but because the experiments involved optic nerve crush very close to the eye, an inflammatory or ischemic cause for the neuronal death, as opposed to axotomy, cannot be excluded. By contrast, in the lamprey, we have demonstrated a very delayed form of apoptotic death in axotomized reticulospinal neurons (Shifman et al., 2008) more than 1.5 cm from the site of spinal cord transection. In the present study, microglial/macrophage accumulation was concentrated much closer to the transection, and few microglia were seen in the brainsetem, so these other mechanisms are unlikely. Thus, observations relating RhoA expression and activation to death of reticulospinal neurons after SCI probably reflect a role for RhoA in axotomy-induced neuronal death.
Role of RhoA in cell death
The Rho/ROCK pathway is activated by myelin-associated growth inhibitors, CSPGs, and chemorepulsive guidance molecules (Tan et al., 2011, Liu et al., 2015)(Monnier et al., 2003, Lin et al., 2007, Gopalakrishnan et al., 2008, Koch et al., 2014, Banerjee et al., 2016, Ohtake et al., 2016, Hu and Selzer, 2017). Blocking RhoA/ROCK signaling can reverse the inhibitory effects of these molecules on axon outgrowth and promotes axonal sprouting and functional recovery in animal models of CNS injury (Dergham et al., 2002, Fournier et al., 2003, Mueller et al., 2005). The RhoA Activation was also found to play a role in neuronal survival and death (Stankiewicz and Linseman, 2014). A diabetic rat model showed that overexpression of proNGF, a precursor of nerve growth factor, resulted in retinal neuron death via activation of RhoA (Al-Gayyar et al., 2013). In vivo studies also demonstrate RhoA activation in neurons after SCI (Dubreuil et al., 2003, Madura et al., 2004)(Fu et al., 2007). In rats and mice, SCI induced Rho activation in both neurons and glial cells at lesion sites. Application of a Rho antagonist (C3-05) reversed Rho activation and reduced the number of TUNEL-labeled cells by approximately 50%, showing a role for activated Rho in cell death after CNS injury (Dubreuil et al., 2003). Furthermore, viral vector-mediated downregulation of RhoA increased survival and axonal regeneration of retinal ganglion cells (Koch et al., 2014). Our previous demonstration that expression of mRNAs for the CSPG receptors PTPσ and LAR is correlated with activation of caspases in axotomized reticulospinal neurons [Zhang, 2014 #146], links the CSPG receptors to this retrograde neuronal death. In the current study, RhoA activity was co-expressed with PTPσ mRNAs, and correlated with apoptotic signaling, suggesting that the contribution of CSPGs to retrograde cell death after SCI also may be mediated via RhoA activity.
RhoA mRNA labeling was weakly related to neuronal regenerative ability and retrograde apoptotic signaling following SCI
Since morpholino knockdown of RhoA translation increased reticulospinal axon regeneration (Zhang et al., 2015), we anticipated that neurons having a high likelihood of regeneration might also have low levels of RhoA mRNA expression. However, RhoA mRNA was expressed widely in neurons and glial cells throughout the lamprey CNS. In control animals, although RhoA expression was negatively correlated with a priori regenerative ability, the effect was small (though statistically significant). After axotomy, RhoA mRNA labeling decreased in the reticulospinal neurons from 2 to 4 weeks post- transection, but the degree of reduction was poorly correlated with regenerative ability. Similarly, reticulospinal neuronal RhoA mRNA labeling correlated with caspase activity, but only during the first 2 weeks post- transection. Thus transcriptional upregulation of RhoA probably does not account for the delayed apoptosis observed in poorly-regenerating reticulospinal neurons, and if a blunted downregulation of RhoA mRNA expression contributed to the delayed apoptosis seen in poor regenerators, the effect was small, and would have been involved only during the first 2 weeks post- transection.
Intra-axonal appearance of RhoA mRNA
RhoA mRNA was observed in the large reticulospinal axons only after transection. It appeared in injured axon tips within the first day post- transection, and the label spread centripetally within 5 days. In brain wholemounts and in paraffin sections, within the first week post- transection, in situ mRNA label spread from the perikaryon to the proximal reticulospinal axons in the brainstem. A similar pattern of axonal mRNA spread into axons was described previously for total mRNA (Jin et al., 2016). Whether the subsequent reduction in RhoA mRNA seen in reticulospinal neurons reflects centrifugal translocation of the mRNA, or whether there was also a reduction in transcription is not known, but the appearance of RhoA mRNA in the axon suggests the possibility that RhoA might be synthesized in the injured axon tip, as occurs in mammalian peripheral axons (Willis and Twiss, 2010). We previously detected several transcripts by ISH in both non-injured axons and transected axon tips, e.g., PTPσ (Zhang et al., 2014), L- NFL and beta-tubulin (Jin et al., 2016). Single-cell PCR of cytosolic microaspirates from the tips of regenerating lamprey axons also contained mRNA for several proteins in these axon tips, in which immunohistochemistry and electron microscopy showed ribosomes/polyribosomes (Jin et al., 2016). Intra-axonal translation of RhoA promoted axon growth inhibition by CSPGs in postnatal dorsal root ganglion (DRG) cultures, and we have found in lamprey, that RhoA mRNA co-localized with Nogo receptor (NgR), PTPσ and Rho-associated protein kinase (Rock) in axons after SCI (not published). Thus, it is possible that after SCI in the lamprey, some of the growth- and survival-inhibitory effects triggered by CSPGs are mediated through signaling molecules synthesized locally in the injured axon tips.
Role of RhoA activation in retrograde neuronal death
In rats, RhoA was activated near the site of SCI as early as 1.5 hours after injury, the earliest time investigated (Dubreuil et al., 2003). We also found caspase 8 activation in axotomized axons within 2 hours post-transection. In situ Rho GTPase analysis with GST-RBD in control lamprey brains showed RhoA activation in the neurons with poor regenerating probability as well. Moreover, activation of caspases was strongly correlated with RhoA activation in the neurons that usually regenerate poorly after SCI. The number of neurons doubly-labeled for activated caspases and activated RhoA were observed less frequently at one week post-transection (45% of total labeled cells), than at later times, increasing gradually to 85% at 9 weeks after SCI. The earlier activation of RhoA than of caspases is consistent with the possibility that activation of caspases is downstream of Rho/ROCK signaling. RhoA/ROCK up-regulated Bax, leading to apoptosis via the intrinsic (mitochondrial) death pathway, including downstream activation of caspases 9 and 3 (Del Re et al., 2007). We also observed that activated caspases appeared in some neurons without labeling for RhoA activation. This suggests either that caspases were activated through a pathway other than RhoA/ROCK, or that the amount of activated RhoA required to trigger caspase activation was too small to detect by the in situ GTPase assay. Several studies have suggested that RhoA is downstream of CSPG binding to RPTPs (Walker et al., 2012, Lim and Joe, 2013, Koch et al., 2014, Ohtake et al., 2016). Inhibitors of RPTPs can block apoptosis in non-neuronal cell lines (Huang et al., 1996, Lund-Johansen et al., 1996, Yang et al., 1996). Overexpression of LAR in mammalian cell lines activated the caspase cell-death pathway by a p53-independent mechanism (Weng et al., 1998). Whether the LAR-induced activation of caspases was mediated by RhoA has not been determined. In the current study, RhoA was activated in neurons that also expressed mRNA for the CSPG receptors PTPσ and LAR (not shown), and application of exogenous CSPGs increased activation of both RhoA and caspases. We previously showed that knockdown of RhoA in lamprey reticulospinal neurons by retrograde delivery of MOs significantly reduced the number of apoptotic neurons labeled by FLICA (Zhang et al., 2015). Furthermore, activated RhoA was not found in those neurons whose axons had regenerated at 9 weeks post-SCI, but selectively in neurons undergoing apoptosis (i.e., labeled by FLICA). Our results are consistent with the hypothesis that CSPGs induce apoptosis as well as inhibit axon regeneration in lamprey reticulospinal neurons by binding to RPTPs and activating RhoA.
Specificity of FLICA for apoptosis in lamprey reticulospinal neurons
Regenerative ability in lamprey reticulospinal neurons is stochastic, i.e., even poor regenerators sometimes regenerate. This is true also of retrograde neuronal death, and caspases may be involved in normal cellular processes, including dendritic pruning (Kuo et al., 2006, Williams et al., 2006), synaptic plasticity (Lu et al., 2006, Li et al., 2010) and inflammation (Boatright and Salvesen, 2003, Martinon and Tschopp, 2007, Pop and Salvesen, 2009). And although FLICA reagents have been used to detect activated caspases in apoptotic cells (Bedner et al., 2000, Grabarek et al., 2002, Pozarowski et al., 2003), it might be argued that neurons could be labeled by FLICA even if they are not undergoing apoptosis. However, in lamprey identified reticulospinal neurons, FLICA labeling colocalized with TUNEL (Hu et al., 2013), and is generally limited to those that have high probabilities of undergoing very delayed apoptosis and a low probability of regenerating their axons after axotomy (Barreiro-Iglesias and Shifman, 2012, Zhang et al., 2014, Barreiro-Iglesias and Shifman, 2015). In the present study, FLICA-positivity at 9 weeks post-transection was almost completely restricted to neurons whose axons had not regenerated. We used poly-caspase FLICA rather than individual caspase-specific FLICA to increase labeling intensity and to capture neurons undergoing apoptosis by any caspase pathway.
Conclusions
The present results suggest that RhoA activity is strongly increased after spinal cord transection and that this is strongly correlated with apoptotic signaling. How the increase in activated RhoA occurs is yet to be determined. The ISH observations on identified reticulospinal neurons make it unlikely that the response is regulated at the level of transcription, but the translocation of RhoA mRNA into the injured axon tip leaves open the intriguing possibility that local translation of RhoA in some way contributes to the response. Together with our previously-published demonstration that morpholino knock-down of RhoA protects neurons from apoptotic signaling after transection, the present results strongly suggest an important role for RhoA in signaling retrograde neuronal death after axotomy.
Highlights.
RhoA activity is strongly increased after spinal cord transection and is strongly correlated with apoptotic signaling.
In situ hybridization observations on identified reticulospinal neurons suggest that the response is not regulated at the level of transcription.
RhoA mRNA is translocated into the injured axon tip, suggesting the possibility that local translation of RhoA contributes to the response.
Together with our previously-published demonstration that morpholino knock-down of RhoA protects neurons from apoptotic signaling after transection, the present results strongly suggest an important role for RhoA in signaling retrograde neuronal death after axotomy.
Acknowledgments
This work was supported by grants R01-NS092876 and R01-NS097846 (NIH, M.E. Selzer PI), SHC-85400, SHC-85220, and SHC-85101 (Shriners Hospitals for Children, M.E. Selzer, PI), SHC-84293, (Shriners Hospitals for Children, J. Hu, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Gayyar MM, Mysona BA, Matragoon S, Abdelsaid MA, El-Azab MF, Shanab AY, Ha Y, Smith SB, Bollinger KE, El-Remessy AB. Diabetes and overexpression of proNGF cause retinal neurodegeneration via activation of RhoA pathway. PLoS One. 2013;8:e54692. doi: 10.1371/journal.pone.0054692. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Banerjee P, Harada H, Tassew NG, Charish J, Goldschneider D, Wallace VA, Sugita S, Mehlen P, Monnier PP. Upsilon-secretase and LARG mediate distinct RGMa activities to control appropriate layer targeting within the optic tectum. Cell Death Differ. 2016;23:442–453. doi: 10.1038/cdd.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Shifman MI. Use of fluorochrome-labeled inhibitors of caspases to detect neuronal apoptosis in the whole-mounted lamprey brain after spinal cord injury. Enzyme research. 2012;2012:835731. doi: 10.1155/2012/835731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Shifman MI. Detection of activated caspase-8 in injured spinal axons by using fluorochrome-labeled inhibitors of caspases (FLICA) Methods in molecular biology. 2015;1254:329–339. doi: 10.1007/978-1-4939-2152-2_23. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Exp Cell Res. 2000;259:308–313. doi: 10.1006/excr.2000.4955. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Causeret F, Hidalgo-Sanchez M, Fort P, Backer S, Popoff MR, Gauthier-Rouviere C, Bloch-Gallego E. Distinct roles of Rac1/Cdc42 and Rho/Rock for axon outgrowth and nucleokinesis of precerebellar neurons toward netrin 1. Development. 2004;131:2841–2852. doi: 10.1242/dev.01162. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Mackler SA, Selzer ME. Functional regeneration following spinal transection demonstrated in the isolated spinal cord of the larval sea lamprey. Proc Natl Acad Sci U S A. 1986;83:2763–2766. doi: 10.1073/pnas.83.8.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AH, Mackler SA, Selzer ME. Behavioral recovery following spinal transection: functional regeneration in the lamprey CNS. Trends in neurosciences. 1988;11:227–231. doi: 10.1016/0166-2236(88)90131-2. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Davis GR, Jr, McClellan AD. Time course of anatomical regeneration of descending brainstem neurons and behavioral recovery in spinal-transected lamprey. Brain Res. 1993;602:131–137. doi: 10.1016/0006-8993(93)90252-i. [DOI] [PubMed] [Google Scholar]
- Davis GR, Jr, McClellan AD. Extent and time course of restoration of descending brainstem projections in spinal cord-transected lamprey. The Journal of comparative neurology. 1994a;344:65–82. doi: 10.1002/cne.903440106. [DOI] [PubMed] [Google Scholar]
- Davis GR, Jr, McClellan AD. Long distance axonal regeneration of identified lamprey reticulospinal neurons. Exp Neurol. 1994b;127:94–105. doi: 10.1006/exnr.1994.1083. [DOI] [PubMed] [Google Scholar]
- Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci. 1997;17:5316–5326. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil CI, Winton MJ, McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol. 2003;162:233–243. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erschbamer MK, Hofstetter CP, Olson L. RhoA, RhoB, RhoC, Rac1, Cdc42, and Tc10 mRNA levels in spinal cord, sensory ganglia, and corticospinal tract neurons and long-lasting specific changes following spinal cord injury. The Journal of comparative neurology. 2005;484:224–233. doi: 10.1002/cne.20471. [DOI] [PubMed] [Google Scholar]
- Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27:4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Yamashita T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Frontiers in neuroscience. 2014;8:338. doi: 10.3389/fnins.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin- 3A-induced axon retraction. J Cell Sci. 2006;119:3413–3423. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan SM, Teusch N, Imhof C, Bakker MH, Schurdak M, Burns DJ, Warrior U. Role of Rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells. Journal of neuroscience research. 2008;86:2214–2226. doi: 10.1002/jnr.21671. [DOI] [PubMed] [Google Scholar]
- Grabarek J, Amstad P, Darzynkiewicz Z. Use of fluorescently labeled caspase inhibitors as affinity labels to detect activated caspases. Human cell. 2002;15:1–12. doi: 10.1111/j.1749-0774.2002.tb00094.x. [DOI] [PubMed] [Google Scholar]
- He Z, Jin Y. Intrinsic Control of Axon Regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Hu J, Selzer ME. RhoA as a target to promote neuronal survival and axon regeneration. Neural Regeneration Research. 2017;12:525–528. doi: 10.4103/1673-5374.205080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhang G, Rodemer W, Jin LQ, Shifman M, Selzer ME. The role of RhoA in retrograde neuronal death and axon regeneration after spinal cord injury. Neurobiol Dis. 2017;98:25–35. doi: 10.1016/j.nbd.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhang G, Selzer ME. Activated caspase detection in living tissue combined with subsequent retrograde labeling, immunohistochemistry or in situ hybridization in whole-mounted lamprey brains. Journal of neuroscience methods. 2013;220:92–98. doi: 10.1016/j.jneumeth.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TS, Shu CH, Shih YL, Huang HC, Su YC, Chao Y, Yang WK, Whang-Peng J. Protein tyrosine phosphatase activities are involved in apoptotic cancer cell death induced by GL331, a new homolog of etoposide. Cancer Lett. 1996;110:77–85. doi: 10.1016/s0304-3835(96)04464-3. [DOI] [PubMed] [Google Scholar]
- Jacobs AJ, Swain GP, Snedeker JA, Pijak DS, Gladstone LJ, Selzer ME. Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. J Neurosci. 1997;17:5206–5220. doi: 10.1523/JNEUROSCI.17-13-05206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LQ, Pennise C, Rodemer W, Jahn K, Selzer M. Protein Synthetic Machinery and mRNA in Regenerating Tips of Spinal Cord Axons in Lamprey. The Journal of comparative neurology. 2016 doi: 10.1002/cne.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Ong Tone S, Fournier AE. Extrinsic and intrinsic regulation of axon regeneration at a crossroads. Frontiers in molecular neuroscience. 2015;8:27. doi: 10.3389/fnmol.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi M, Matsushita N, Miyazaki J, Koike M, Yaginuma H, Osumi N, Kaibuchi K, Kobayashi K. Survival of developing motor neurons mediated by Rho GTPase signaling pathway through Rho-kinase. J Neurosci. 2004;24:3480–3488. doi: 10.1523/JNEUROSCI.0295-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JC, Tonges L, Michel U, Bahr M, Lingor P. Viral vector-mediated downregulation of RhoA increases survival and axonal regeneration of retinal ganglion cells. Frontiers in cellular neuroscience. 2014;8:273. doi: 10.3389/fncel.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp MA, Liebscher T, Niedeggen A, Laufer S, Brommer B, Jungehulsing GJ, Strittmatter SM, Dirnagl U, Schwab JM. Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury. Cell Tissue Res. 2012;349:119–132. doi: 10.1007/s00441-012-1334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A. 2002;99:3246–3251. doi: 10.1073/pnas.052308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, Cho K, Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HS, Joe YA. A ROCK Inhibitor Blocks the Inhibitory Effect of Chondroitin Sulfate Proteoglycan on Morphological Changes of Mesenchymal Stromal/Stem Cells into Neuron-Like Cells. Biomolecules & therapeutics. 2013;21:447–453. doi: 10.4062/biomolther.2013.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gao HY, Wang XF. The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural regeneration research. 2015;10:1892–1896. doi: 10.4103/1673-5374.170325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Ogiya M, Takahara M, Yamaguchi W, Furuyama T, Tanaka H, Tohyama M, Inagaki S. Sema4D-plexin-B1 implicated in regulation of dendritic spine density through RhoA/ROCK pathway. Neuroscience letters. 2007;428:1–6. doi: 10.1016/j.neulet.2007.09.045. [DOI] [PubMed] [Google Scholar]
- Lu C, Wang Y, Furukawa K, Fu W, Ouyang X, Mattson MP. Evidence that caspase-1 is a negative regulator of AMPA receptor-mediated long-term potentiation at hippocampal synapses. J Neurochem. 2006;97:1104–1110. doi: 10.1111/j.1471-4159.2006.03800.x. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen F, Frey T, Ledbetter JA, Thompson PA. Apoptosis in hematopoietic cells is associated with an extensive decrease in cellular phosphotyrosine content that can be inhibited by the tyrosine phosphatase antagonist pervanadate. Cytometry. 1996;25:182–190. doi: 10.1002/(SICI)1097-0320(19961001)25:2<182::AID-CYTO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nature reviews Neuroscience. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Pijak DS, Selzer ME. Structure of reticulospinal axon growth cones and their cellular environment during regeneration in the lamprey spinal cord. The Journal of comparative neurology. 1994;344:559–580. doi: 10.1002/cne.903440406. [DOI] [PubMed] [Google Scholar]
- Madura T, Yamashita T, Kubo T, Fujitani M, Hosokawa K, Tohyama M. Activation of Rho in the injured axons following spinal cord injury. EMBO reports. 2004;5:412–417. doi: 10.1038/sj.embor.7400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends in neurosciences. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Mimura F, Yamagishi S, Arimura N, Fujitani M, Kubo T, Kaibuchi K, Yamashita T. Myelin-associated glycoprotein inhibits microtubule assembly by a Rho-kinase-dependent mechanism. J Biol Chem. 2006;281:15970–15979. doi: 10.1074/jbc.M510934200. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nature reviews Drug discovery. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Nielson JL, Sears-Kraxberger I, Strong MK, Wong JK, Willenberg R, Steward O. Unexpected survival of neurons of origin of the pyramidal tract after spinal cord injury. J Neurosci. 2010;30:11516–11528. doi: 10.1523/JNEUROSCI.1433-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson JL, Strong MK, Steward O. A reassessment of whether cortical motor neurons die following spinal cord injury. The Journal of comparative neurology. 2011;519:2852–2869. doi: 10.1002/cne.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova LN, Novikov LN, Kellerth JO. Survival effects of BDNF and NT-3 on axotomized rubrospinal neurons depend on the temporal pattern of neurotrophin administration. Eur J Neurosci. 2000;12:776–780. doi: 10.1046/j.1460-9568.2000.00978.x. [DOI] [PubMed] [Google Scholar]
- Ohtake Y, Wong D, Abdul-Muneer PM, Selzer ME, Li S. Two PTP receptors mediate CSPG inhibition by convergent and divergent signaling pathways in neurons. Scientific reports. 2016;6:37152. doi: 10.1038/srep37152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozarowski P, Huang X, Halicka DH, Lee B, Johnson G, Darzynkiewicz Z. Interactions of fluorochrome-labeled caspase inhibitors with apoptotic cells: a caution in data interpretation. Cytometry Part A: the journal of the International Society for Analytical Cytology. 2003;55:50–60. doi: 10.1002/cyto.a.10074. [DOI] [PubMed] [Google Scholar]
- Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Regeneration of Muller and Mauthner axons after spinal transection in larval lampreys. The Journal of comparative neurology. 1976;168:545–554. doi: 10.1002/cne.901680407. [DOI] [PubMed] [Google Scholar]
- Sanno H, Shen X, Kuru N, Bormuth I, Bobsin K, Gardner HA, Komljenovic D, Tarabykin V, Erzurumlu RS, Tucker KL. Control of postnatal apoptosis in the neocortex by RhoA-subfamily GTPases determines neuronal density. J Neurosci. 2010;30:4221–4231. doi: 10.1523/JNEUROSCI.3318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ME. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. The Journal of physiology. 1978;277:395–408. doi: 10.1113/jphysiol.1978.sp012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Selzer ME, Li S. Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol. 2012;237:370–378. doi: 10.1016/j.expneurol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman MI, Zhang G, Selzer ME. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. The Journal of comparative neurology. 2008;510:269–282. doi: 10.1002/cne.21789. [DOI] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, Campbell MS, Yandell MD, Manousaki T, Meyer A, Bloom OE, Morgan JR, Buxbaum JD, Sachidanandam R, Sims C, Garruss AS, Cook M, Krumlauf R, Wiedemann LM, Sower SA, Decatur WA, Hall JA, Amemiya CT, Saha NR, Buckley KM, Rast JP, Das S, Hirano M, McCurley N, Guo P, Rohner N, Tabin CJ, Piccinelli P, Elgar G, Ruffier M, Aken BL, Searle SM, Muffato M, Pignatelli M, Herrero J, Jones M, Brown CT, Chung-Davidson YW, Nanlohy KG, Libants SV, Yeh CY, McCauley DW, Langeland JA, Pancer Z, Fritzsch B, de Jong PJ, Zhu B, Fulton LL, Theising B, Flicek P, Bronner ME, Warren WC, Clifton SW, Wilson RK, Li W. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nature genetics. 2013;45:415–421. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz TR, Linseman DA. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Frontiers in cellular neuroscience. 2014;8:314. doi: 10.3389/fncel.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4) The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1990;38:1683–1686. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- Sun F, He Z. Neuronal intrinsic barriers for axon regeneration in the adult CNS. Current opinion in neurobiology. 2010;20:510–518. doi: 10.1016/j.conb.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain GP, Jacobs AJ, Frei E, Selzer ME. A method for in situ hybridization in wholemounted lamprey brain: neurofilament expression in larvae and adults. Experimental neurology. 1994;126:256–269. doi: 10.1006/exnr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Tan HB, Zhong YS, Cheng Y, Shen X. Rho/ROCK pathway and neural regeneration: a potential therapeutic target for central nervous system and optic nerve damage. International journal of ophthalmology. 2011;4:652–657. doi: 10.3980/j.issn.2222-3959.2011.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BA, Ji SJ, Jaffrey SR. Intra-axonal translation of RhoA promotes axon growth inhibition by CSPG. J Neurosci. 2012;32:14442–14447. doi: 10.1523/JNEUROSCI.0176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng LP, Yuan J, Yu Q. Overexpression of the transmembrane tyrosine phosphatase LAR activates the caspase pathway and induces apoptosis. Current biology: CB. 1998;8:247–256. doi: 10.1016/s0960-9822(98)70106-x. [DOI] [PubMed] [Google Scholar]
- Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9:1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- Willis DE, Twiss JL. Regulation of protein levels in subcellular domains through mRNA transport and localized translation. Molecular & cellular proteomics: MCP. 2010;9:952–962. doi: 10.1074/mcp.R900005-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Chang J, Gorospe M, Passaniti A. Protein tyrosine phosphatase regulation of endothelial cell apoptosis and differentiation. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1996;7:161–171. [PubMed] [Google Scholar]
- Yin HS, Selzer ME. Axonal regeneration in lamprey spinal cord. J Neurosci. 1983;3:1135–1144. doi: 10.1523/JNEUROSCI.03-06-01135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HS, Selzer ME. Electrophysiologic evidence of regeneration of lamprey spinal neurons. Exp Neurol. 1984;83:618–628. doi: 10.1016/0014-4886(84)90128-6. [DOI] [PubMed] [Google Scholar]
- Zhang G, Hu J, Li S, Huang L, Selzer ME. Selective expression of CSPG receptors PTPsigma and LAR in poorly regenerating reticulospinal neurons of lamprey. The Journal of comparative neurology. 2014;522:2209–2229. doi: 10.1002/cne.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Jin LQ, Hu J, Rodemer W, Selzer ME. Antisense Morpholino Oligonucleotides Reduce Neurofilament Synthesis and Inhibit Axon Regeneration in Lamprey Reticulospinal Neurons. PLoS One. 2015;10:e0137670. doi: 10.1371/journal.pone.0137670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gu X, Yuan X. Phenylalanine activates the mitochondria-mediated apoptosis through the RhoA/Rho-associated kinase pathway in cortical neurons. Eur J Neurosci. 2007;25:1341–1348. doi: 10.1111/j.1460-9568.2007.05404.x. [DOI] [PubMed] [Google Scholar]