Abstract
Existing programs which aim to prevent and treat childhood obesity often do not take into account individual variation and the underlying mechanisms that impact child eating behavior. Individual differences in children’s appetitive traits have been shown to appear as early as during infancy and become more pronounced as children grow older and become more exposed to the obesogenic food environment. Differences in genetic predispositions interacting with factors in children’s early environment account in part for individual differences in appetitive traits. It is very likely that obesogenic eating phenotypes manifest themselves before the onset of childhood obesity. If so, identifying these phenotypes early is expected to move primary prevention strategies in a new direction and holds great potential to significantly enhance our ability to prevent childhood obesity. The aim of this narrative review is to discuss the role of behavioral phenotyping as an innovative approach for the development of more personalized obesity prevention and treatment interventions that are tailored to children’s individual predispositions. We describe several examples of appetitive traits which have been linked to overeating and excess weight gain in children and thus may represent modifiable risk factors for future interventions. The review concludes with a comprehensive synthesis of opportunities for future human ingestive behavior research on identifying behavioral phenotypes for childhood obesity.
1. Introduction
Despite considerable efforts to reverse the trend in childhood obesity, prevention and treatment approaches have shown limited long-term success. Systematic reviews of school-based prevention, for instance, suggest that interventions targeting both healthy eating and physical activity are only moderately successful in improving body mass index (BMI) outcomes in children (Brown and Summerbell, 2009, Brown et al., 2016). Further, many lifestyle interventions to treat pediatric obesity have proven efficacious in the short term but show mixed efficacy at follow-up time points (Wilfley et al., 2007). This poses a significant public health concern especially given that youth with obesity have a 70% higher chance of becoming adults with obesity (U.S. Department of Health and Human Services, 2007).
One of the reasons why prevention and treatment approaches for childhood obesity show limited long-term success may be that they fail to take into account individual variation and the underlying mechanisms that impact child eating behavior. Individual differences in children’s appetitive traits have been shown to appear as early as during infancy (Stunkard et al., 1999, van Jaarsveld et al., 2011) and become more pronounced as children grow older and become more exposed to the obesogenic food environment. Differences in genetic predispositions interacting with factors in children’s early environment account in part for individual differences in appetitive traits. For example, twin studies have shown high heritability for appetitive traits such as satiety responsiveness (‘My child gets full easily’; heritability estimate (h2) = 63%) or food cue responsiveness (‘My child’s always asking for food’, h2 = 75%) (Carnell et al., 2008) which can increase the risk of overeating in ‘obesogenic’ food environments. For the successful prevention and treatment of childhood obesity, it will be critical that we identify modifiable behavioral risk factors and interventions that are tailored to children’s individual predispositions.
The aim of this narrative review is to discuss the role of behavioral phenotyping in relation to ingestive behavior as a novel approach for the development of more personalized interventions for the prevention and treatment of childhood obesity. We describe several examples of appetitive traits which have been associated with overeating and excess weight gain in children and thus may represent modifiable risk factors for future interventions. The review concludes with a comprehensive synthesis of opportunities for future research on identifying behavioral phenotypes for childhood obesity.
2. Behavioral Phenotyping
According to the National Institutes of Health (NIH), precision medicine refers to the “tailoring of medical treatment to the individual characteristics of each patient” and the “ability to classify individuals into subpopulations that differ in their susceptibility to a particular disease, in the biology or prognosis of those diseases they may develop, or in their response to a specific treatment” (Committee on a Framework for Developing a New Taxonomy of Disease, 2011). Precision medicine thus seeks to maximize effectiveness by taking into account individual variability in genes, environments, and lifestyles.
Behavioral phenotyping is expected to play an important role in identifying novel targets for individual and population level approaches to weight management. Behavioral phenotypes refer to patterns of behavior that are distinct (i.e., explain individual variation), are quantifiable and measurable, and result from an interaction of a genotype with the environment. In the context of this review, we refer to individual appetitive traits that combine to make up a broader eating phenotype. Behavioral phenotypes can subsequently be used to help predict an individual’s response to an intervention and thus match individuals with targeted prevention and treatment approaches (Epstein and Wrotniak, 2010). In other words, rather than using a ‘one-size-fits all’ approach, knowing a child’s eating behavior phenotype may enable us to develop more targeted prevention and treatment approaches that are tailored to a child’s individual predisposition and focus on the individual components of the appetitive phenotype. Early childhood is an opportune time for identifying behavioral phenotypes which may put children at risk for excess weight gain.
It is very likely that obesogenic eating phenotypes manifest themselves before the onset of childhood obesity. If so, identifying these phenotypes early is expected to move primary prevention strategies in a new direction and holds great potential to significantly enhance our ability to prevent childhood obesity. One of the most important tasks will be to identify children who are at greatest risk for developing obesity before the onset of excess weight gain.
3. Prevention of Childhood Obesity
3.1. Identifying children at-risk for developing obesity
There is a pressing need to develop tools that help identify children who are at greatest risk for excess weight gain before they develop obesity. The finding that obesity tends to ‘run in families’ points to both genetic and environmental influences affecting energy balance.
It has been well established that body weight and the hormones, peptides, and neurons that play a functional role in the central and peripheral pathways that regulate energy balance are under considerable genetic control (Maes et al., 1997). Parental obesity is considered one of the strongest risk factors for childhood obesity. Data from a pivotal study by Whitaker and colleagues (1997) showed that both children with and without obesity were at greater risk of developing obesity as adults if at least one parent had obesity. Parental obesity, and maternal obesity, in particular, more than doubled the risk of adult obesity among both children with and without obesity under age 10. Once established, obesity tends to track from childhood into adulthood. Data from a systematic review and meta-analysis of 15 prospective studies showed that about 55% of children with obesity maintain their obesity status during adolescence and about 80% of adolescents with obesity will have obesity in adulthood (Simmonds et al., 2015, Simmonds et al., 2016). Importantly, along with obesity itself, a series of prospective cohort studies and controlled trials provided evidence for two key modifiable behaviors, dietary intake and physical activity patterns, to also track from childhood into adulthood and therefore represent important targets for early intervention (Craigie et al., 2011).
Besides genetic influences, a large body of evidence has also shown that aspects of the early home environment, which includes availability and quantities of foods and beverages in the home, family meals, parent feeding practices and modeling of eating behaviors, play an important role in shaping children’s appetitive behaviors and food preferences (Johnson, 2016, Dwyer et al., 2015, Draxten et al., 2014). It is possible that genetic predispositions to obesity may in part exert their effects through appetitive traits which in turn may confer a behavioral susceptibility to overeating in obesogenic environments among affected children.
3.2. Individual differences in appetite regulation
Human ingestive behavior involves complex interactions between biological and environmental factors that are integrated in the brain to control energy intake. The formation of appetitive behavior, including its underlying biological pathways, begins early in life, and, once formed, become difficult to change or override later on (Young-Hyman, 2017). Individual differences in appetitive traits can emerge as early as during infancy (e.g., sucking behavior). Other appetitive traits may emerge or become more pronounced later in life as children grow older and have more experiences in the greater obesogenic food environment. Some of these traits represent food-seeking appetitive behaviors, such as sensitivity to the reward, the reinforcing value of food, and susceptibility to eating when full in response to food cues in the environment. Children with a genetic predisposition to these traits are thought to have a behavioral susceptibility to overeating in environments where highly palatable, energy-dense foods are easily available and accessible. There are also food-avoidant appetitive traits, such as food selectivity, food neophobia, or fussy (or picky) eating (Carnell and Wardle, 2007), which can lead to refusal of (novel) foods and mealtime difficulties (Bandini et al., 2010, Carnell and Wardle, 2007, Gibson and Cooke, 2017). Other appetitive traits, such as satiation or satiety, relate more to the self-regulation of energy intake. Satiation refers to processes that bring an eating episode to an end (intra-meal satiety), whereas satiety refers to processes that inhibit further eating in the postprandial period until the next meal (inter-meal satiety) (Kral and Rolls, 2004). In the current review, we focus mostly on food-seeking appetitive and self-regulatory traits as disruptions in those traits may lead to an overconsumption of calories and excess weight gain. That is, food-seeking appetitive traits may impede or override self-regulatory traits and lead to an overconsumption of calories over time. Therefore, it is possible that a strengthening of food-seeking appetitive traits and a simultaneous weakening of self-regulatory systems such as satiety may represent a behavioral phenotype for childhood obesity.
Substantial individual differences exist in appetitive traits and not all children are equally susceptible to overeating when exposed to highly palatable, energy-dense foods. Individual differences in appetitive traits are in part attributed to genetic predispositions interacting with factors in children’s early home environment. The study of genetic influences on appetite traits can be carried out using either measured or unmeasured genotype approaches. The measured genotype approach makes use of direct measurement of genetic variation (e.g., frequencies of alleles at specific loci) and relates measured indicators of DNA sequence variation to the phenotype under study using methods such as linkage analysis and heritability estimation. The unmeasured genotype approach, on the other hand, utilizes phenotype data from families (e.g., full and half siblings, monozygotic and dizygotic twins, adoptees) to infer transmission of genetic variation (Malina et al., 2004).
Data from family and twin studies provided evidence that many dietary and eating behaviors are shared and heritable (Faith et al., 1999). In addition, to date more than 127 single nucleotide polymorphisms (SNPs) in candidate genes have been identified which have been shown to affect the central and peripheral regulation of energy intake and are implicated in behavioral traits such as hyperphagia, satiety responsiveness, meal size, and food reinforcement (Carr et al., 2013, Farooqi et al., 2002, de Krom et al., 2007, Wardle et al., 2008). On the other hand, child eating behaviors are also learned and shaped by upbringing and, possibly, by the early family and home environment. For example, there is evidence that parental use of restrictive and controlling feeding practices was significantly associated with greater eating in the absence of hunger (EAH) and poorer self-regulation of intake in both cross-sectional (Johnson and Birch, 1994, Fisher and Birch, 2000) and longitudinal (Birch et al., 2003) studies. It is important to note the bi-directional nature of the relationship in which children both elicit and respond to parents’ behavior. While laboratory studies provide evidence for environmental influences, such as the portion size of foods, to affect children’s energy intake (Rolls et al., 2000, Fisher and Kral, 2008, Fisher et al., 2007a, Kral et al., 2014), it currently remains unknown to what extent the home food environment may interact with children’s genetic predispositions to help shape their eating behaviors. Longitudinal studies are needed which can identify cause-and-effect relationships between home environmental influences and child eating behaviors. Furthermore, identifying children who exhibit hyperphagic eating traits before they develop obesity presents an important target for early intervention.
There is also growing evidence for individual differences in appetitive traits to be accompanied by underlying neuronal correlates. Recent advances in functional magnetic resonance imaging (fMRI), for example, suggest that individual differences in the portion size effect among children are associated with brain responses to visual portion size cues in brain regions implicated in valuation, salience, inhibitory control, and reward (Keller et al., 2018). Findings from a study using a novel neuroimaging paradigm with preadolescent boys and girls suggest that loss of control (LOC), a form of disinhibited eating, may be related to unique patterns of neural activation in response to ingestion of palatable foods, particularly in regions associated with self-regulation (Goldschmidt et al., 2018).
In the following sections of the review, we will discuss select appetitive traits, such as impaired eating self-regulation and food-seeking behaviors, in greater detail and highlight implications for energy intake regulation. We will focus on appetitive traits for which experimental paradigms have been developed and which can be assessed in the laboratory under carefully controlled conditions. We further discuss innovative ways in which the field of pediatric ingestive behavior research can be extended to generate tools which can be used in clinical settings for the prevention of childhood obesity. In this context, we discuss how parent-report instruments may serve as proxy for laboratory-based assessments of child eating phenotypes.
4. Appetitive Traits
4.1. Self-Regulation
It has been proposed that some, but not all, infants are born with the capacity to self-regulate energy intake. A study with 3,022 infants and toddlers between 4 and 24 months of age showed that infants’ amounts of foods consumed were inversely related to the energy density of the foods they consumed which points to the presence of an ability to self-regulate energy intake at a young age (Fox et al., 2006). In addition, there is evidence that suggests that infants who were breastfed as opposed to bottle fed were better able to regulate their energy intake and were at lower risk for excess weight gain during childhood (Taveras et al., 2004, Hess et al., 2015). Similarly, a study with a slightly older sample of 2–5-year-old children whose 24-hour ad libitum energy intake was studied in a day care center for six days showed that energy intake at a given meal or snack was highly variable (coefficient of variation (CV): 33.6%) while the variability in daily energy intake was much lower (CV: 10.4%). This led the authors to conclude that “intake at individual meals was not independent, and there was evidence that high energy intake at one meal was often compensated for by low energy intake at the next” (Birch et al., 1991). It still remains widely unknown, however, how successful these compensatory mechanisms are in keeping infants and toddlers in energy balance long-term. Subsequent studies on the self-regulation of intake in children also pointed to age-dependent changes (Johnson and Taylor-Holloway, 2006, Birch and Davison, 2001). To date it remains unclear why some children have the ability to regulate energy intake at a young age and others do not. We need a better understanding as to why some children’s ability to regulate energy intake weakens as they get older. We also need to examine the formation and possible changes in appetitive traits from a developmental perspective ranging from early infancy to toddlerhood to early and late childhood. It is possible that food cues in the environment may be strong enough for some children to override their ability to self-regulate. One way to experimentally assess children’s ability to self-regulate energy intake in the laboratory is to study individual differences in satiety.
4.1.1. Individual differences in satiety (caloric compensation)
As early as in the 1960s, it was proposed that individuals with obesity showed impaired sensitivity to internal satiety cues (Schachter, 1968). The satiety value of food is typically assessed by consuming it as a compulsory first course (preload) at a meal and measuring the effect on ad libitum energy intake at a subsequent main course (test meal) (Blundell et al., 2010). In the context of energy intake regulation, energy compensation refers to adjustments in intake in response to changes in the energy density (ED; kcal/g) of the compulsory preload. Energy compensation thus provides a measure of individual differences in satiety (Rolls, 2009).
The majority of previous studies provided evidence that young children have the ability to compensate for energy from a preload that varies in energy density (Carnell et al., 2017, Kral et al., 2012, Cecil et al., 2005). Birch & Fisher (Birch and Fisher, 1997), however, pointed out that “(…) this compensation is usually partial and incomplete, and (…) there are individual differences in how responsive children are to manipulations of the energy density of foods.” Poorer compensation ability in children has been associated in some, but not all, studies with higher child weight status (Kral et al., 2012, Johnson and Birch, 1994, Carnell et al., 2017), older age (Cecil et al., 2005, Johnson and Taylor-Holloway, 2006), racial/ethnic minority status (Faith et al., 2012), being male (Faith et al., 2012), and having mothers who use controlling or restrictive feeding practices (Birch and Deysher, 1985).
4.2. Food-seeking Appetitive Traits
Several appetitive traits have been identified which can lead to a behavioral susceptibility to overeating in environments where highly palatable energy-dense foods are easily accessible. Individuals who have these traits are thought to exhibit a heightened responsiveness to food cues in the environment. Following are three examples of food-seeking appetitive traits which have been studied in children.
4.2.1. Eating in the Absence of Hunger
Eating in the absence of hunger (EAH) refers to children’s susceptibility to eat beyond satiation in response to the presence of high energy-dense snack foods (see Lansigan et al., 2015 for a review). EAH in children has been typically assessed in laboratory settings by the free access procedure (Fisher and Birch, 1999a, Cutting et al., 1999). After a short delay (e.g., 5–10 minutes) after completion of a meal, children are given unrestricted access to a variety of palatable snack foods, which they consume ad libitum over a period of ~15 minutes. EAH has been most often reported as the absolute number of calories consumed from the snacks when fully satiated.
In cross-sectional studies, EAH has been identified as a behavioral eating trait that increases short-term energy intake (Fisher and Birch, 1999b, Fisher and Birch, 2000) and is increased in children with overweight and obesity in some (Kral et al., 2012, Fisher et al., 2007b, Moens and Braet, 2007, Hill et al., 2008) but not all studies (Remy et al., 2015). Some (Butte et al., 2007, Fisher and Birch, 2002, Birch et al., 2003, Francis and Birch, 2005), but not all (Kelly et al., 2015), prospective studies also support a positive association between youth’s weight status or weight gain and EAH over time.
The trait appears to be stable during childhood (Fisher and Birch, 2002, Birch et al., 2003), heritable (Fisher et al., 2007b), and is increased in children whose caregivers use parental control (Liang et al., 2016, Harris et al., 2014), restrictive feeding practices, prompts to eat (Galindo et al., 2018), and increased monitoring (Birch et al., 2003, Fisher and Birch, 1999a). However, when taking into consideration the bidirectional nature of maternal restrictive feeding practices and child EAH, a recent study by Bauer and colleagues (2017) found a significant inverse association between maternal restriction at 21 months and EAH at 27 months suggesting that maternal restriction may be protective against increased EAH during early toddlerhood. Recent data also suggest that child temperament may predispose certain children to increased EAH. In a study of 88 children, aged 7 to 9 years, greater EAH was associated with greater impulsivity (Nederkoorn et al., 2015). Another study of low income preschoolers found that children with higher surgency, which is characterized by impulsivity, intense pleasure seeking, high activity level, and low levels of shyness, were more likely to show EAH (Leung et al., 2014).
To date, EAH has only been assessed using energy-dense, palatable snack foods during the free access procedures. It remains yet to be determined if EAH is food-specific (i.e., applies only to energy-dense snacks) or if it also extends to low energy-dense healthy snacks (e.g., fruit). Data by Asta and colleagues (2016) in toddlers showed that when examined by snack type (sweet vs. salty) both total calorie intake and calories consumed from sweet snack foods, but not salty snack foods, at 27 months predicted children’s BMI z-scores at 33 months.
Further, if EAH does extend to low energy-dense snacks, such as fruit, we can use this behavioral phenotype in susceptible children strategically to promote consumption of healthy snacks while at the same time reducing children’s energy intake. If successful, this would help modify or at least mitigate the risk of what used to be thought of as an obesity-promoting eating behavior to one that promotes healthy eating in children. It is possible that due to the higher water content of fruit, fruit may be more filling than energy-dense snacks and therefore a smaller volume of food will be consumed in the absence of hunger. Given the low energy density of fruit, children likely will consume fewer calories and more nutrients when being offered fruit. It would also have important implications for caregivers when designing family meals in that they could structure snack times in a way that substitute low energy-dense snacks for high energy-dense snacks and thereby reduce energy intake in children. It is important to note that while some children have a higher propensity to EAH than others, all children will likely benefit from being given access to healthier after-meal snack/dessert options. However, the magnitude of the potential benefit (i.e., reducing caloric intake) will likely be largest for children who have a high propensity to EAH.
4.2.2. Relative Reinforcing Value of Food
The relative reinforcing value of food (RRVF) examines how hard individuals are willing to work to gain access to a preferred food rather than an appealing nonfood alternative (Epstein et al., 2007). The RRVF uses concepts of behavioral economics for understanding how individuals allocate effort and make choices among alternatives. The RRVF can be measured using a behavioral choice task in which subjects perform operant responses on a progressive ratio schedule of reinforcement. Typically the task is set up in such a way that the response requirement to obtain food increases while the response requirement for the nonfood alternative remains constant. Subjects who work more towards the food reinforcer rather than the nonfood alternative are thought to perceive food as more reinforcing and may devote more time and effort to eating compared to subjects who perceive food as less reinforcing (Temple et al., 2008).
A study by Temple et al. (2008) was among the first to assess whether the RRVF differed as a function of weight status in 8- to 12-year-old children. The findings of the study revealed a significant interaction between children’s weight status, reinforcer type, and the schedule of reinforcement such that children with overweight or obesity found food more reinforcing than nonfood alternatives and were more motivated to work for food than their leaner peers.
A study which assessed parent-child relationships in the RRVF showed similarities in their choices for food versus monetary alternatives as the behavioral cost of the two reinforcers was varied (Epstein et al., 2008). The RRVF has further shown to predict 1-year changes in age- and sex-adjusted BMI standard deviation (SD) scores and fat mass index (i.e., fat mass divided by height) in 7- to 10-year-old children (Hill et al., 2009). While children’s RRVF did not impact children’s response to portion size changes at a fast food type meal (Kral et al., 2014), recent evidence suggests that the RRV of an energy-dense snack (cookies) was significantly higher for pre-school children with overweight and obesity while normal-weight children showed a higher RRV for a healthy snack (fruit) (McCullough et al., 2017). To date it remains unknown to what extent the RRVF can be modified to promote healthier food choices among at-risk children.
4.2.3. Reward sensitivity
Reward sensitivity explains individual differences in reward motivation (Carver and White, 1994). Individuals with high reward sensitivity are believed to be more vulnerable to the rewarding properties of palatable food cues in the environment and generate behaviors to approach and consume the associated reward. Reward sensitivity in youth can be assessed using the Drive subscale of the Behavioral Inhibition/Behavioral Activation Scales (BIS/BAS) (Carver and White, 1994). This subscale reflects strong pursuit of appetitive goals and has been shown to be associated with neural responses to food cues in the brain reward circuity (Beaver et al., 2006, De Decker et al., 2016).
Youth with a high sensitivity to reward have been shown to have higher intakes of palatable, energy dense foods and sugar-sweetened beverages (De Decker et al., 2016, De Cock et al., 2016a, De Cock et al., 2016b) and a higher BMI (De Decker et al., 2016). A longitudinal study in a cohort of Flemish children furthermore indicated that girls, but not boys, with heightened reward sensitivity showed significantly higher fat mass levels at baseline and higher fat tissue accretion over a 4-year period (De Decker et al., 2017).
Data from a magnetic resonance imaging study with adolescents and young adults provided evidence for structural differences in brain regions associated with reward processing, somatosensory processing, and motivation (Moreno-Lopez et al., 2012, Yokum et al., 2012, Stice et al., 2008). Future research is needed to tease apart which of these brain differences precede the onset of obesity and which are the results of long-term overeating in youth.
4.2.4. Eating Rate
Rapid eating rate has been identified as another possible behavioral phenotype for childhood obesity. A study by Fogel and colleagues (2017) examined the relationship between eating rate, energy intake at a meal, and weight status/adiposity in 386 boys and girls, ages 4.5 years. Children consumed a buffet lunch ad libitum which was video-recorded and had their eating behaviors (bites, chews, and swallows) and eating rate (g/min) assessed. The results of the study showed a significant positive association between children’s eating rate and 1) total calories consumed at the meal (r = 0.61; P<0.001) and 2) BMI z-scores (r = 0.20; P<0.001). Children who were classified as ‘faster eaters’ consumed, on average, 75% or 132 more calories at the meal than ‘slower eaters’ (307 ± 10 kcal vs. 175 ± 6 kcal; P<0.001) and had 26% higher subcutaneous adipose tissue volume (P=0.014). Children’s eating rate was positively associated with caloric intake, independent of meal duration.
The rate of eating has been shown to be heritable (h2 = 62%) (Llewellyn et al., 2008), appears to emerge early in life, and may be a risk factor for excess weight gain in children. In a longitudinal study for growth and development, 3-month-old infants born at high-risk for obesity on the basis of maternal pre-pregnancy BMI, significantly differed in their nutritive sucking behavior when compared to infants born at low-risk for obesity (Stunkard et al., 1999). During an assessment in the laboratory, infants in both groups either received breast milk or their customary formula from a nutritive sucking apparatus. High-risk infants showed significantly greater total intakes (150g +/− 57 vs. 123g +/− 48), total number of sucks (920 +/− 559 sucks vs. 620 +/− 293 sucks), and higher sucking rates (0.75 sucks/s +/− 0.25 vs. 0.59 sucks/s +/− 0.26) than low-risk infants. The findings further showed that nutrient sucking behavior and daily energy intake at 3 months of age explained ~17% of the variability in infants’ weight, weight-for-length, body fat, fat-free mass, and skinfold thickness at 12 months of age. The same cohort of children was studied in the laboratory several years later. This time, investigators assessed the association between children’s rate of eating during a videotaped buffet test meal and their weight status. The findings indicated that a higher rate of eating at 4 years of age, expressed as mouthfuls of food per minute, predicted child overweight status at 6 years of age, as well as excess weight gain from ages 4 to 6 years (Berkowitz et al., 2010).
5. Opportunities for Future Research
Given the pronounced individual variation in eating phenotypes, there is renewed interest in studying the underlying mechanisms that impact ingestive behavior and elucidating pathways that can lead to behavioral susceptibility to overeating in some children. The vast knowledge gleaned from the past several decades of ingestive behavior research in children builds the basis for novel research approaches to help advance the development of tailored prevention and treatment programs for at-risk children.
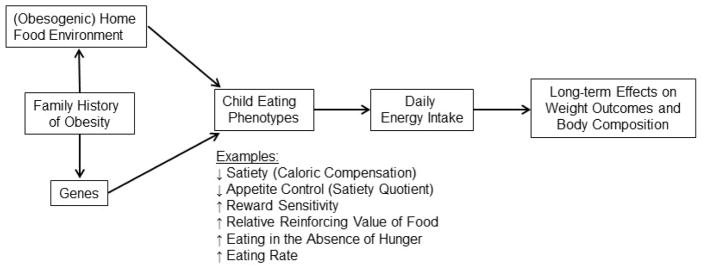
While we are making significant strides towards improving the greater food and built environment for the general public, individual differences in obesity susceptibility will likely always exist due to variations in genetic predispositions that interact with cultural, psychosocial, economic, and environmental factors. Behavioral phenotyping aims to identify individual variation in children’s differences in satiety (caloric compensation), appetite control (perceived hunger and fullness), reward sensitivity, food cue reactivity, and neural responses to food in conjunction. When combined with targeted genotypying approaches (e.g., to identify SNPs of the FTO gene), behavioral phenotyping can be a powerful tool which can help identify underlying causal pathways for excess weight gain among at-risk children. Programs that are tailored to children’s individual predispositions are likely to be more impactful than traditional approaches because they target specific aspects of problematic appetitive behaviors and can provide more focused parent training on how to address these appetite traits. We provide a proposed conceptual model in Figure 1 and a summary of novel intervention targets for future research opportunities in Table 1; both are described in the sections below.
Figure 1.
Conceptual model of select eating phenotypes for childhood obesity
Table 1.
Novel intervention targets for research on child eating phenotypes
| Novel intervention targets | Rational | Example | Expected Outcome |
|---|---|---|---|
| Concurrent study of multiple eating phenotypes | Develop obesity risk profiles |
Self-regulation:
|
Develop tailored interventions that target individual components of phenotype |
| Study appetitive traits across multiple meals | Determine impact of shorter-term appetitive traits on longer-term intake regulation and weight outcomes | %COMPX over 24-hr period | Identify appetitive traits that impact energy balance and weight |
| Evaluate parent-report instruments in conjunction with lab-based assessments of respective child eating phenotype | Develop low-cost, time-efficient instruments as proxy for more time- and resource-intensive lab-based assessments | EAH-P + laboratory-based EAH assessment | Use in clinical practice to identify children with high-risk eating phenotypes |
| Study eating phenotypes in context of familial predisposition to obesity and home environmental factors | Gain insights into possible gene-environment interactions affecting child eating behavior | Family history of obesity, food security status, availability of palatable foods in home, RRVF | Develop tailored interventions that take into consideration familial risk factors that may be predisposing children to food-seeking appetitive traits |
EAH = eating in the absence of hunger; EAH-P = eating in the absence of hunger questionnaire for parents; %COMPX = percentage compensation index; RRVF = relative reinforcing value of food
5.1. Concurrent study of multiple eating phenotypes
The majority of previous research has focused on studying one eating trait at a time. The advantage of this approach was that the trait could be carefully studied under a variety of experimental conditions. As more and more eating traits are carefully characterized we now have an opportunity to use a more integrated approach to study multiple eating phenotypes concurrently under both states of hunger and satiety. This new approach may enable us to identify child-specific obesity risk profiles which may contain “clusters” of food-seeking traits or impairments in self-regulatory behaviors. These obesity risk profiles can then be used to develop tailored interventions for subgroups of children who present with similar risk profiles to target the individual components of the phenotype. Targeting clusters of problematic appetitive traits will likely be more impactful for moderating energy intake and may yield better dietary and health outcomes faster than targeting one individual trait at a time. It is also possible that different aspects of an appetitive trait may be missed if not addressed together. For example, a child who is susceptible to EAH may also be a rapid eater. A tailored treatment program would then aim to modify both of these food-seeking appetitive traits simultaneously.
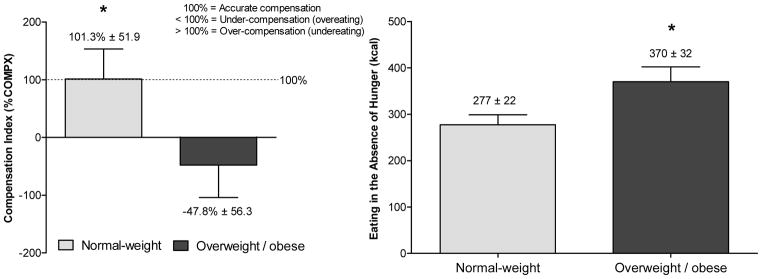
There are several examples of experimental research which have used a more integrative approach to study multiple eating traits concurrently. For example, using a behavioral genetics design, Kral et al. (2012) aimed to compare siblings who were discordant in their weight status in caloric compensation and EAH to elucidate potential differences in siblings’ ability to regulate short-term energy intake. In this study, 47 same-sex sibling pairs (53% female, 55% full siblings), ages 5 to 12 years, were served dinner once a week for three weeks. Across conditions, siblings were served the same dinner, but, 25 min before dinner, they either consumed or did not consume in full one of two preloads that varied in ED (0.57 or 0.97 kcal/g). On the day when no preload was consumed, EAH was assessed after dinner and defined as the number of calories consumed from snacks. Overweight/obese siblings under-compensated and therefore overate after the high ED preload, whereas normal-weight siblings showed accurate compensation (Figure 2). Further, overweight/obese siblings consumed 34% more calories (93 kcal) in the absence of hunger than normal-weight siblings. This study further showed that EAH and caloric compensation show important conceptual differences as indicated by a moderate-strength inverse relationship (r = −0.3; P = 0.01; unpublished), which suggests that children who rely less on internal hunger/satiety cues may be more responsive to external food cues in the environment. On the other hand, no association between %COMPX and EAH was observed in a slightly younger group of 3- to 5-year-old French children (r=0.05; P=0.46) (Remy et al., 2015). This illustrates that for some children, a behavioral phenotype for obesity may present itself as an impaired ability to regulate short-term energy intake, which includes adhering less to internal cues of hunger and fullness and eating when satiated. For these children targeted intervention approaches may focus on teaching parents about making healthy foods available after meals and providing satiety training to children. Other children may only adhere less to internal cues of hunger and fullness, but show little EAH, and thus may benefit from satiety training alone.
Figure 2.
Mean (± SEM) compensation index (%COMPX) and eating in the absence of hunger for normal-weight and overweight/obese children
The American Journal of Clinical Nutrition, 2012, 96, 574–583, American Society for Nutrition
In addition to studying caloric compensation at meals, it is also important to examine the temporal effects of food on children’s overall appetite control when studying individual differences in satiety. Appetite sensations such as perceived hunger, desire to eat, or fullness reflect both objective (unconditioned or physiological) and subjective (conditioned or learned) components of appetite control (Stubbs et al., 2000). Two constructs used to measure appetite control are the satiety quotient and the post-meal area under the curve (AUC) of appetite sensations (Drapeau et al., 2005, Drapeau et al., 2007). The satiety quotient assesses the individual-level satiating capacity of a preload and the extent to which it reduces subjective appetite sensations per unit of intake (e.g., kcal) (Green et al., 1997). In contrast to caloric compensation, which assesses actual adjustments (reduction or increase) in food intake following a preload, the satiety quotient measures the extent to which a preload reduces subjective appetite sensations (McNeil et al., 2013) and therefore provides information about children’s perceived satiety. An important area of future research will be to determine if subjective measures of appetite map onto objectively measured, actual eating behavior in children.
By contrast, the post-meal AUC is a summary indicator of appetite sensations measured over time after a meal has ended. In a study with adults, both the satiety quotient and the post-prandial AUC of fullness were significantly correlated with total energy intake over a 12-hour day (Drapeau et al., 2005), but comparable data in children are lacking. Data from two small samples of children, ages 7 to 12, indicated that a test meal’s macronutrient composition differentially affected children’s post-prandial AUC of hunger and fullness (Maffeis et al., 2010, Lomenick et al., 2009). Knowledge gleaned from studying different aspects of satiety will help develop new approaches for future obesity interventions.
A study by Rollins and colleagues (2014) aimed to examine relationships between the RRVF, reward sensitivity, and BMI in pre-school children ages 3 to 5 years. Children worked on a modified behavioral choice task to gain access to two types of food reinforcers (i.e., graham crackers). The schedule of reinforcement for both reinforcers doubled each time a reward was earned. In a separate session, children were given free access to both snack foods and their food intake was recorded. Children’s reward sensitivity was assessed using the parent-reported Behavioral Activation System (BAS) scale. This study found a significant association between children’s weight status and reward sensitivity and their response rate in the behavioral choice task. Specifically, children with higher BMI z-scores and a higher reward sensitivity worked at a faster rate (i.e., increased number of mouse clicks per minute) to access the food reinforcers. Furthermore, children’s total number of responses (i.e., number of mouse clicks) predicted children’s intake of the snack foods. This study was among the first to show that individual differences in the RRVF and its relationship to reward sensitivity can be observed in children as young as 3 years of age. When examining the RRVF and EAH in a low-income sample of 230 children, aged 7 to 10 years, Gearhardt and colleagues (2017) found that, among girls, higher RRVF was indirectly associated with overweight/obesity through EAH, which points to a possible mediating role of EAH between RRVF and obesity. Novel intervention targets gleaned from these studies may include modifying the home environment to reduce palatable food cues while maximizing children’s access to healthy foods.
5.2. Impact of short-term appetite control on longer-term intake regulation and weight outcomes
Many previous studies assessed children’s eating phenotypes at a single meal without putting them into the context of children’s overall energy needs. Children’s intake at individual meals can be highly variable (Birch et al., 1991) and it therefore remains unclear if eating traits that are observed at single meals are predictive of longer-term intake and weight gain.
Taking caloric compensation as an example, to date it remains unknown if children who overeat after a preload during a single meal compensate for the additional calories by reducing their intake at a later meal. Similarly, while percentage compensation is a useful index for short-term (single meal) intake regulation, it has yet to be determined to what extent it may serve as a marker for longer-term (daily) energy intake and weight regulation. Longitudinal studies are needed that examine if poor caloric compensation is a risk factor for excess weight gain over time and the development of obesity in children.
5.3. Evaluation of parent-report instruments as proxy for laboratory-based assessment of child eating phenotypes
Laboratory-based experiments to study child eating behaviors are often costly and time-consuming and require appropriate facilities and equipment. Further, only relative small sample sizes can be studied under controlled laboratory conditions at a time which limits generalizability and identification of behavioral eating phenotypes among the pediatric population at large. Therefore, it will be crucial to develop parent-report questionnaires of child appetitive traits which can serve as proxy for laboratory-assessed appetitive traits. To date, there exist a small number of questionnaires which have been shown to adequately predict child appetitive traits measured in the laboratory. For example, the parent-report Eating in the Absence of Hunger Questionnaire (EAH-P) (Tanofsky-Kraff et al., 2008) assesses the impact of negative affect, fatigue/boredom, and external eating on eating past satiation in youth. In a study with 90 adolescents, who ranged in ages between 13 to 17 years, parental report of their children’s EAH to external cues was positively related to youth’s observed EAH after a standardized lunch meal (r = 0.32, P < 0.01) and a multi-item buffet-style lunch meal (r = 0.33, P < 0.01) (Shomaker et al., 2013).
Another questionnaire, the Child Eating Behavior Questionnaire (CEBQ) (Wardle et al., 2001) assesses children’s responsiveness to food, enjoyment of food, satiety responsiveness, slowness in eating, food fussiness, emotional over- and undereating, and desire for drinks. A study with 149 children between 4 and 5 years of age examined associations between three CEBQ subscales (i.e., satiety responsiveness, food responsiveness, and enjoyment of food) and four aspects of child eating behavior (i.e., eating without hunger, caloric compensation, eating rate, energy intake at a meal) in the laboratory (Carnell and Wardle, 2007). The results of the study showed that higher satiety responsiveness was associated with lower EAH, improved caloric compensation, a slower eating rate and lower energy intake. Higher scores on the food responsiveness subscale were associated with a faster eating rate and higher energy intake while higher scores on the enjoyment of food subscale were associated with greater EAH, a faster eating rate and greater energy intake, respectively.
The development and validation of additional parent-report questionnaires for the assessment of child eating behaviors is an important area of future research. The use of these less expensive and time-consuming measures may subsequently serve as screening tools in clinical settings, which clinical care providers may use to identify children who are at greatest risk for obesity and intervene early. These screening tools, in conjunction with select genotyping tools, can be used across all four stages of prevention and treatment approaches in clinical care settings (e.g., at well visits) or tertiary care centers for obesity treatment. Screening for individual components of behavioral phenotypes may assist a multidisciplinary team of health care professionals make informed decisions on the type of treatments they prescribe. For example, if a child screens high for EAH, the treatment approach may include implementation of a fixed meal and snack time structure to reduce snacking throughout the day and focus on healthy snacks to reduce overconsumption of calories.
5.4. Study of eating phenotypes in context of familial predisposition to obesity and home food environment
Another important goal of future research should be identifying children who are at greatest risk for excess weight gain before they develop obesity. To date little is known about the extent to which obesogenic eating traits develop to promote overeating and excess weight gain in (still) normal weight, but at-risk children (e.g., those with a family history of obesity). Specifically, it is important to determine if high-risk, normal-weight children are phenotypically similar in their eating traits to low-risk, normal-weight children or if they may have already adopted eating traits that resemble more closely those of high-risk children with obesity, which, if so, may put them on a path to excess weight gain. Identifying children who show hyperphagic eating behaviors before they develop obesity will present powerful targets for early intervention.
When examining familial predispositions to obesity, the majority of previous studies focused on maternal influences on child feeding practices and weight development. Modern day fathers, however, play an important role in caregiving and child feeding. Recent data suggest that 62% of fathers reported sharing food parenting responsibilities with the child’s mother (Khandpur et al., 2016) and also showed that fathers’ feeding practices were independently associated with children’s BMI z-score (Penilla et al., 2017). Thus, when studying family influences on the development of child eating phenotypes it will be critically important to examine both mothers’ and fathers’ role and possibly conflicting views on child feeding and structuring of the home food environment.
It will also be important to study individual differences of eating phenotypes of children with obesity to be better able to predict long-term treatment outcomes. While some children may respond well to tailored interventions and reduce weight successfully, others may be resistant to treatment and develop severe obesity. Gaining a better understanding of how appetitive traits and eating behaviors vary among children with obesity may help care providers better predict treatment outcomes and thus prescribe right away more comprehensive multidisciplinary treatment approaches for children who are at risk of not responding to more traditional, less intensive interventions.
Furthermore, it will be important to study behavioral phenotypes in the context of children’s early home environment. Home food environments that provide easy access to highly palatable, energy-dense foods can affect children’s eating behaviors in two possible ways. One, obesogenic home food environments may interact with genetic predispositions to hyperphagic eating behaviors in children who are susceptible to overeating and excess weight gain. Two, regular exposure to highly palatable foods in the home may change children’s eating behaviors and impede their ability to regulate their energy intake.
If found that obesogenic home food environments contribute to the development of hyperphagic eating behaviors and excess weight gain, helping parents structure home environments that promote healthy eating will offer novel intervention targets for childhood obesity prevention. An example of a successful home-based behavioral intervention is the innovative skills-based behavioral clinic and home-based intervention (LAUNCH) which aimed at reducing BMI z-scores in 2- to 5-year-old preschoolers with obesity (Stark et al., 2018). Intervention components included limiting portion sizes and consumption of energy-dense foods and setting dietary and physical activity goals. The study found that LAUNCH participants demonstrated a significantly greater decrease in BMIz compared with a motivational interviewing a standard care group (P < 0.004).
6. Summary
In summary, the vast knowledge that we have gleaned over the last four decades from ingestive behavior research in children is expected to build the basis for novel integrated research paradigms that aim to identify behavioral phenotypes for childhood obesity. The development of tools that help us identify children who are at greatest risk for weight gain and the identification of modifiable behavioral risk factors should be a primary focus of future research. While laboratory-based research protocols remain of utmost importance for carefully characterizing child eating behaviors and thereby identifying possible behavioral phenotypes for childhood obesity, new and exciting opportunities exist which have the potential of moving the field of (pediatric) human ingestive behavior research forward in important ways. A comprehensive characterization of a child’s behavioral phenotypes, which takes into consideration multiple appetitive traits studied concurrently under states of hunger and fullness over multiple meals/days, as well as genetic and/or (home) environmental factors which may be contributing to the child’s risk for developing obesity, is expected to provide crucial information for the development of more targeted intervention approaches and screening tools which can be used in clinical practice to identify children who are at greatest risk for developing obesity.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01DK101480).
Abbreviations
- BMI
body mass index
- EAH
eating in the absence of hunger
- %COMPX
percentage compensation index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ASTA K, MILLER AL, RETZLOFF L, ROSENBLUM K, KACIROTI NA, LUMENG JC. Eating in the Absence of Hunger and Weight Gain in Low-income Toddlers. Pediatrics. 2016:137. doi: 10.1542/peds.2015-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANDINI LG, ANDERSON SE, CURTIN C, CERMAK S, EVANS EW, SCAMPINI R, MASLIN M, MUST A. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr. 2010;157:259–64. doi: 10.1016/j.jpeds.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER KW, HAINES J, MILLER AL, ROSENBLUM K, APPUGLIESE DP, LUMENG JC, KACIROTI NA. Maternal restrictive feeding and eating in the absence of hunger among toddlers: a cohort study. Int J Behav Nutr Phys Act. 2017;14:172. doi: 10.1186/s12966-017-0630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVER JD, LAWRENCE AD, VAN DITZHUIJZEN J, DAVIS MH, WOODS A, CALDER AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–6. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERKOWITZ RI, MOORE RH, FAITH MS, STALLINGS VA, KRAL TV, STUNKARD AJ. Identification of an obese eating style in 4-year-old children born at high and low risk for obesity. Obesity (Silver Spring) 2010;18:505–12. doi: 10.1038/oby.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRCH LL, DAVISON KK. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr Clin North Am. 2001;48:893–907. doi: 10.1016/s0031-3955(05)70347-3. [DOI] [PubMed] [Google Scholar]
- BIRCH LL, DEYSHER M. Conditioned and unconditioned caloric compensation: evidence for self-regulation of food intake by young children. Learning and motivation. 1985;16:341–355. [Google Scholar]
- BIRCH LL, FISHER JO. Food intake regulation in children. Fat and sugar substitutes and intake. Ann N Y Acad Sci. 1997;819:194–220. doi: 10.1111/j.1749-6632.1997.tb51809.x. [DOI] [PubMed] [Google Scholar]
- BIRCH LL, FISHER JO, DAVISON KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr. 2003;78:215–20. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRCH LL, JOHNSON SL, ANDRESEN G, PETERS JC, SCHULTE MC. The variability of young children’s energy intake. N Engl J Med. 1991;324:232–5. doi: 10.1056/NEJM199101243240405. [DOI] [PubMed] [Google Scholar]
- BLUNDELL J, DE GRAAF C, HULSHOF T, JEBB S, LIVINGSTONE B, LLUCH A, MELA D, SALAH S, SCHURING E, VAN DER KNAAP H, WESTERTERP M. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11:251–70. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN EC, BUCHAN DS, BAKER JS, WYATT FB, BOCALINI DS, KILGORE L. A Systematised Review of Primary School Whole Class Child Obesity Interventions: Effectiveness, Characteristics, and Strategies. Biomed Res Int. 2016;2016:4902714. doi: 10.1155/2016/4902714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN T, SUMMERBELL C. Systematic review of school-based interventions that focus on changing dietary intake and physical activity levels to prevent childhood obesity: an update to the obesity guidance produced by the National Institute for Health and Clinical Excellence. Obes Rev. 2009;10:110–41. doi: 10.1111/j.1467-789X.2008.00515.x. [DOI] [PubMed] [Google Scholar]
- BUTTE NF, CAI G, COLE SA, WILSON TA, FISHER JO, ZAKERI IF, ELLIS KJ, COMUZZIE AG. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. Am J Clin Nutr. 2007;85:1478–85. doi: 10.1093/ajcn/85.6.1478. [DOI] [PubMed] [Google Scholar]
- CARNELL S, BENSON L, GIBSON EL, MAIS LA, WARKENTIN S. Caloric compensation in preschool children: Relationships with body mass and differences by food category. Appetite. 2017;116:82–89. doi: 10.1016/j.appet.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARNELL S, HAWORTH CM, PLOMIN R, WARDLE J. Genetic influence on appetite in children. Int J Obes (Lond) 2008;32:1468–73. doi: 10.1038/ijo.2008.127. [DOI] [PubMed] [Google Scholar]
- CARNELL S, WARDLE J. Measuring behavioural susceptibility to obesity: validation of the child eating behaviour questionnaire. Appetite. 2007;48:104–13. doi: 10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- CARR KA, LIN H, FLETCHER KD, SUCHESTON L, SINGH PK, SALIS RJ, ERBE RW, FAITH MS, ALLISON DB, STICE E, EPSTEIN LH. Two functional serotonin polymorphisms moderate the effect of food reinforcement on BMI. Behav Neurosci. 2013;127:387–99. doi: 10.1037/a0032026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARVER CS, WHITE TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- CECIL JE, PALMER CN, WRIEDEN W, MURRIE I, BOLTON-SMITH C, WATT P, WALLIS DJ, HETHERINGTON MM. Energy intakes of children after preloads: adjustment, not compensation. Am J Clin Nutr. 2005;82:302–8. doi: 10.1093/ajcn.82.2.302. [DOI] [PubMed] [Google Scholar]
- COMMITTEE ON A FRAMEWORK FOR DEVELOPING A NEW TAXONOMY OF DISEASE. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. 2011 Available: https://www.ncbi.nlm.nih.gov/books/NBK91503/pdf/Bookshelf_NBK91503.pdf. [PubMed]
- CRAIGIE AM, LAKE AA, KELLY SA, ADAMSON AJ, MATHERS JC. Tracking of obesity-related behaviours from childhood to adulthood: A systematic review. Maturitas. 2011;70:266–84. doi: 10.1016/j.maturitas.2011.08.005. [DOI] [PubMed] [Google Scholar]
- CUTTING TM, FISHER JO, GRIMM-THOMAS K, BIRCH LL. Like mother, like daughter: familial patterns of overweight are mediated by mothers’ dietary disinhibition. Am J Clin Nutr. 1999;69:608–13. doi: 10.1093/ajcn/69.4.608. [DOI] [PubMed] [Google Scholar]
- DE COCK N, VAN LIPPEVELDE W, GOOSSENS L, DE CLERCQ B, VANGEEL J, LACHAT C, BEULLENS K, HUYBREGTS L, VERVOORT L, EGGERMONT S, MAES L, BRAET C, DEFORCHE B, KOLSTEREN P, VAN CAMP J. Sensitivity to reward and adolescents’ unhealthy snacking and drinking behavior: the role of hedonic eating styles and availability. Int J Behav Nutr Phys Act. 2016a;13:17. doi: 10.1186/s12966-016-0341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE COCK N, VAN LIPPEVELDE W, VERVOORT L, VANGEEL J, MAES L, EGGERMONT S, BRAET C, LACHAT C, HUYBREGTS L, GOOSSENS L, BEULLENS K, KOLSTEREN P, VAN CAMP J. Sensitivity to reward is associated with snack and sugar-sweetened beverage consumption in adolescents. Eur J Nutr. 2016b;55:1623–32. doi: 10.1007/s00394-015-0981-3. [DOI] [PubMed] [Google Scholar]
- DE DECKER A, DE CLERCQ B, VERBEKEN S, WELLS JC, BRAET C, MICHELS N, DE HENAUW S, SIOEN I. Fat and lean tissue accretion in relation to reward motivation in children. Appetite. 2017;108:317–325. doi: 10.1016/j.appet.2016.10.017. [DOI] [PubMed] [Google Scholar]
- DE DECKER A, SIOEN I, VERBEKEN S, BRAET C, MICHELS N, DE HENAUW S. Associations of reward sensitivity with food consumption, activity pattern, and BMI in children. Appetite. 2016;100:189–96. doi: 10.1016/j.appet.2016.02.028. [DOI] [PubMed] [Google Scholar]
- DE KROM M, VAN DER SCHOUW YT, HENDRIKS J, OPHOFF RA, VAN GILS CH, STOLK RP, GROBBEE DE, ADAN R. Common genetic variations in CCK, leptin, and leptin receptor genes are associated with specific human eating patterns. Diabetes. 2007;56:276–80. doi: 10.2337/db06-0473. [DOI] [PubMed] [Google Scholar]
- DRAPEAU V, BLUNDELL J, THERRIEN F, LAWTON C, RICHARD D, TREMBLAY A. Appetite sensations as a marker of overall intake. Br J Nutr. 2005;93:273–80. doi: 10.1079/bjn20041312. [DOI] [PubMed] [Google Scholar]
- DRAPEAU V, KING N, HETHERINGTON M, DOUCET E, BLUNDELL J, TREMBLAY A. Appetite sensations and satiety quotient: predictors of energy intake and weight loss. Appetite. 2007;48:159–66. doi: 10.1016/j.appet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- DRAXTEN M, FULKERSON JA, FRIEND S, FLATTUM CF, SCHOW R. Parental role modeling of fruits and vegetables at meals and snacks is associated with children’s adequate consumption. Appetite. 2014;78:1–7. doi: 10.1016/j.appet.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWYER L, OH A, PATRICK H, HENNESSY E. Promoting family meals: a review of existing interventions and opportunities for future research. Adolesc Health Med Ther. 2015;6:115–31. doi: 10.2147/AHMT.S37316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN LH, DEARING KK, TEMPLE JL, CAVANAUGH MD. Food reinforcement and impulsivity in overweight children and their parents. Eat Behav. 2008;9:319–27. doi: 10.1016/j.eatbeh.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN LH, LEDDY JJ, TEMPLE JL, FAITH MS. Food reinforcement and eating: a multilevel analysis. Psychol Bull. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN LH, WROTNIAK BH. Future directions for pediatric obesity treatment. Obesity (Silver Spring) 2010;18(Suppl 1):S8–12. doi: 10.1038/oby.2009.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAITH MS, PIETROBELLI A, HEO M, JOHNSON SL, KELLER KL, HEYMSFIELD SB, ALLISON DB. A twin study of self-regulatory eating in early childhood: estimates of genetic and environmental influence, and measurement considerations. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAITH MS, RHA SS, NEALE MC, ALLISON DB. Evidence for genetic influences on human energy intake: results from a twin study using measured observations. Behav Genet. 1999;29:145–54. doi: 10.1023/a:1021683716700. [DOI] [PubMed] [Google Scholar]
- FAROOQI IS, MATARESE G, LORD GM, KEOGH JM, LAWRENCE E, AGWU C, SANNA V, JEBB SA, PERNA F, FONTANA S, LECHLER RI, DEPAOLI AM, O’RAHILLY S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER JO, ARREOLA A, BIRCH LL, ROLLS BJ. Portion size effects on daily energy intake in low-income Hispanic and African American children and their mothers. Am J Clin Nutr. 2007a;86:1709–16. doi: 10.1093/ajcn/86.5.1709. [DOI] [PubMed] [Google Scholar]
- FISHER JO, BIRCH LL. Restricting access to foods and children’s eating. Appetite. 1999a;32:405–19. doi: 10.1006/appe.1999.0231. [DOI] [PubMed] [Google Scholar]
- FISHER JO, BIRCH LL. Restricting access to palatable foods affects children’s behavioral response, food selection, and intake. Am J Clin Nutr. 1999b;69:1264–72. doi: 10.1093/ajcn/69.6.1264. [DOI] [PubMed] [Google Scholar]
- FISHER JO, BIRCH LL. Parents’ restrictive feeding practices are associated with young girls’ negative self-evaluation of eating. J Am Diet Assoc. 2000;100:1341–6. doi: 10.1016/S0002-8223(00)00378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER JO, BIRCH LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr. 2002;76:226–31. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER JO, CAI G, JARAMILLO SJ, COLE SA, COMUZZIE AG, BUTTE NF. Heritability of Hyperphagic Eating Behavior and Appetite-Related Hormones among Hispanic Children. Obesity (Silver Spring) 2007b;15:1484–1495. doi: 10.1038/oby.2007.177. [DOI] [PubMed] [Google Scholar]
- FISHER JO, KRAL TV. Super-size me: Portion size effects on young children’s eating. Physiol Behav. 2008;94:39–47. doi: 10.1016/j.physbeh.2007.11.015. [DOI] [PubMed] [Google Scholar]
- FOGEL A, GOH AT, FRIES LR, SADANANTHAN SA, VELAN SS, MICHAEL N, TINT MT, FORTIER MV, CHAN MJ, TOH JY, CHONG YS, TAN KH, YAP F, SHEK LP, MEANEY MJ, BROEKMAN BFP, LEE YS, GODFREY KM, CHONG MFF, FORDE CG. Faster eating rates are associated with higher energy intakes during an ad libitum meal, higher BMI and greater adiposity among 4.5-year-old children: results from the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) cohort. Br J Nutr. 2017;117:1042–1051. doi: 10.1017/S0007114517000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX MK, DEVANEY B, REIDY K, RAZAFINDRAKOTO C, ZIEGLER P. Relationship between portion size and energy intake among infants and toddlers: evidence of self-regulation. J Am Diet Assoc. 2006;106:S77–83. doi: 10.1016/j.jada.2005.09.039. [DOI] [PubMed] [Google Scholar]
- FRANCIS LA, BIRCH LL. Maternal weight status modulates the effects of restriction on daughters’ eating and weight. Int J Obes (Lond) 2005;29:942–9. doi: 10.1038/sj.ijo.0802935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALINDO L, POWER TG, BECK AD, FISHER JO, O’CONNOR TM, HUGHES SO. Predicting preschool children’s eating in the absence of hunger from maternal pressure to eat: A longitudinal study of low-income, Latina mothers. Appetite. 2018;120:281–286. doi: 10.1016/j.appet.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEARHARDT AN, MILLER AL, STURZA J, EPSTEIN LH, KACIROTI N, LUMENG JC. Behavioral Associations with Overweight in Low-Income Children. Obesity (Silver Spring) 2017 doi: 10.1002/oby.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON EL, COOKE L. Understanding Food Fussiness and Its Implications for Food Choice, Health, Weight and Interventions in Young Children: The Impact of Professor Jane Wardle. Curr Obes Rep. 2017;6:46–56. doi: 10.1007/s13679-017-0248-9. [DOI] [PubMed] [Google Scholar]
- GOLDSCHMIDT AB, DICKSTEIN DP, MACNAMARA AE, PHAN KL, O’BRIEN S, LE GRANGE D, FISHER JO, KEEDY S. A Pilot Study of Neural Correlates of Loss of Control Eating in Children With Overweight/Obesity: Probing Intermittent Access to Food as a Means of Eliciting Disinhibited Eating. J Pediatr Psychol. 2018 doi: 10.1093/jpepsy/jsy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN SM, DELARGY HJ, JOANES D, BLUNDELL JE. A satiety quotient: a formulation to assess the satiating effect of food. Appetite. 1997;29:291–304. doi: 10.1006/appe.1997.0096. [DOI] [PubMed] [Google Scholar]
- HARRIS H, MALLAN KM, NAMBIAR S, DANIELS LA. The relationship between controlling feeding practices and boys’ and girls’ eating in the absence of hunger. Eat Behav. 2014;15:519–22. doi: 10.1016/j.eatbeh.2014.07.003. [DOI] [PubMed] [Google Scholar]
- HESS C, OFEI A, MINCHER A. Breastfeeding and Childhood Obesity Among African Americans: A Systematic Review. MCN Am J Matern Child Nurs. 2015;40:313–9. doi: 10.1097/NMC.0000000000000170. [DOI] [PubMed] [Google Scholar]
- HILL C, LLEWELLYN CH, SAXTON J, WEBBER L, SEMMLER C, CARNELL S, VAN JAARSVELD CH, BONIFACE D, WARDLE J. Adiposity and ‘eating in the absence of hunger’ in children. Int J Obes (Lond) 2008;32:1499–505. doi: 10.1038/ijo.2008.113. [DOI] [PubMed] [Google Scholar]
- HILL C, SAXTON J, WEBBER L, BLUNDELL J, WARDLE J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7–10-y-old children. Am J Clin Nutr. 2009;90:276–81. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- JOHNSON SL. Developmental and Environmental Influences on Young Children’s Vegetable Preferences and Consumption. Adv Nutr. 2016;7:220S–231S. doi: 10.3945/an.115.008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON SL, BIRCH LL. Parents’ and children’s adiposity and eating style. Pediatrics. 1994;94:653–61. [PubMed] [Google Scholar]
- JOHNSON SL, TAYLOR-HOLLOWAY LA. Non-Hispanic white and Hispanic elementary school children’s self-regulation of energy intake. Am J Clin Nutr. 2006;83:1276–82. doi: 10.1093/ajcn/83.6.1276. [DOI] [PubMed] [Google Scholar]
- KELLER KL, ENGLISH LK, FEARNBACH SN, LASSCHUIJT M, ANDERSON K, BERMUDEZ M, FISHER JO, ROLLS BJ, WILSON SJ. Brain response to food cues varying in portion size is associated with individual differences in the portion size effect in children. Appetite. 2018;125:139–151. doi: 10.1016/j.appet.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY NR, SHOMAKER LB, PICKWORTH CK, BRADY SM, COURVILLE AB, BERNSTEIN S, SCHVEY NA, DEMIDOWICH AP, GALESCU O, YANOVSKI SZ, TANOFSKY-KRAFF M, YANOVSKI JA. A prospective study of adolescent eating in the absence of hunger and body mass and fat mass outcomes. Obesity (Silver Spring) 2015;23:1472–1478. doi: 10.1002/oby.21110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHANDPUR N, CHARLES J, DAVISON KK. Fathers’ perspectives on coparenting in the context of child feeding. Child Obes. 2016;12:455–462. doi: 10.1089/chi.2016.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAL TV, ALLISON DB, BIRCH LL, STALLINGS VA, MOORE RH, FAITH MS. Caloric compensation and eating in the absence of hunger in 5- to 12-y-old weight-discordant siblings. Am J Clin Nutr. 2012;96:574–83. doi: 10.3945/ajcn.112.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAL TV, REMIKER AM, STRUTZ EM, MOORE RH. Role of child weight status and the relative reinforcing value of food in children’s response to portion size increases. Obesity (Silver Spring) 2014 doi: 10.1002/oby.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAL TV, ROLLS BJ. Energy density and portion size: their independent and combined effects on energy intake. Physiol Behav. 2004;82:131–8. doi: 10.1016/j.physbeh.2004.04.063. [DOI] [PubMed] [Google Scholar]
- LANSIGAN RK, EMOND JA, GILBERT-DIAMOND D. Understanding eating in the absence of hunger among young children: a systematic review of existing studies. Appetite. 2015;85:36–47. doi: 10.1016/j.appet.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG CY, LUMENG JC, KACIROTI NA, CHEN YP, ROSENBLUM K, MILLER AL. Surgency and negative affectivity, but not effortful control, are uniquely associated with obesogenic eating behaviors among low-income preschoolers. Appetite. 2014;78:139–46. doi: 10.1016/j.appet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIANG J, MATHESON BE, RHEE KE, PETERSON CB, RYDELL S, BOUTELLE KN. Parental control and overconsumption of snack foods in overweight and obese children. Appetite. 2016;100:181–8. doi: 10.1016/j.appet.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLEWELLYN CH, VAN JAARSVELD CH, BONIFACE D, CARNELL S, WARDLE J. Eating rate is a heritable phenotype related to weight in children. Am J Clin Nutr. 2008;88:1560–6. doi: 10.3945/ajcn.2008.26175. [DOI] [PubMed] [Google Scholar]
- LOMENICK JP, MELGUIZO MS, MITCHELL SL, SUMMAR ML, ANDERSON JW. Effects of meals high in carbohydrate, protein, and fat on ghrelin and peptide YY secretion in prepubertal children. J Clin Endocrinol Metab. 2009;94:4463–71. doi: 10.1210/jc.2009-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAES HH, NEALE MC, EAVES LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–51. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- MAFFEIS C, SURANO MG, CORDIOLI S, GASPEROTTI S, CORRADI M, PINELLI L. A high-fat vs. a moderate-fat meal in obese boys: nutrient balance, appetite, and gastrointestinal hormone changes. Obesity (Silver Spring) 2010;18:449–55. doi: 10.1038/oby.2009.271. [DOI] [PubMed] [Google Scholar]
- MALINA R, BOUCHARD C, BAR-OR O. In: Growth, maturation, and physical activity. Malina R, Bouchard C, Bar-Or O, editors. 2004. [Google Scholar]
- MCCULLOUGH MB, GUILKEY H, STARK L. Cookie or fruit? Relative reinforcing value of snack foods among preschoolers with overweight/obesity compared to healthy weight. Appetite. 2017;111:187–194. doi: 10.1016/j.appet.2017.01.006. [DOI] [PubMed] [Google Scholar]
- MCNEIL J, DRAPEAU V, GALLANT AR, TREMBLAY A, DOUCET E, CHAPUT JP. Short sleep duration is associated with a lower mean satiety quotient in overweight and obese men. Eur J Clin Nutr. 2013 doi: 10.1038/ejcn.2013.204. [DOI] [PubMed] [Google Scholar]
- MOENS E, BRAET C. Predictors of disinhibited eating in children with and without overweight. Behav Res Ther. 2007;45:1357–68. doi: 10.1016/j.brat.2006.10.001. [DOI] [PubMed] [Google Scholar]
- MORENO-LOPEZ L, SORIANO-MAS C, DELGADO-RICO E, RIO-VALLE JS, VERDEJO-GARCIA A. Brain structural correlates of reward sensitivity and impulsivity in adolescents with normal and excess weight. PLoS One. 2012;7:e49185. doi: 10.1371/journal.pone.0049185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEDERKOORN C, DASSEN FC, FRANKEN L, RESCH C, HOUBEN K. Impulsivity and overeating in children in the absence and presence of hunger. Appetite. 2015;93:57–61. doi: 10.1016/j.appet.2015.03.032. [DOI] [PubMed] [Google Scholar]
- PENILLA C, TSCHANN JM, DEARDORFF J, FLORES E, PASCH LA, BUTTE NF, GREGORICH SE, GREENSPAN LC, MARTINEZ SM, OZER E. Fathers’ feeding practices and children’s weight status in Mexican American families. Appetite. 2017;117:109–116. doi: 10.1016/j.appet.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMY E, ISSANCHOU S, CHABANET C, BOGGIO V, NICKLAUS S. Impact of adiposity, age, sex and maternal feeding practices on eating in the absence of hunger and caloric compensation in preschool children. Int J Obes (Lond) 2015;39:925–30. doi: 10.1038/ijo.2015.30. [DOI] [PubMed] [Google Scholar]
- ROLLINS BY, LOKEN E, SAVAGE JS, BIRCH LL. Measurement of food reinforcement in preschool children. Associations with food intake, BMI, and reward sensitivity. Appetite. 2014;72:21–7. doi: 10.1016/j.appet.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROLLS BJ. The relationship between dietary energy density and energy intake. Physiol Behav. 2009;97:609–15. doi: 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROLLS BJ, ENGELL D, BIRCH LL. Serving portion size influences 5-year-old but not 3-year-old children’s food intakes. J Am Diet Assoc. 2000;100:232–4. doi: 10.1016/S0002-8223(00)00070-5. [DOI] [PubMed] [Google Scholar]
- SCHACHTER S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science. 1968;161:751–6. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. The Surgeon General’s call to action to prevent and decrease overweight and obesity. Overweight in children and adolescents. 2007 [Online]. Available: http://www.surgeongeneral.gov/topics/obesity/calltoaction/fact_adolescents.htm.
- SHOMAKER LB, TANOFSKY-KRAFF M, MOOREVILLE M, REINA SA, COURVILLE AB, FIELD SE, MATHESON BE, BRADY SM, YANOVSKI SZ, YANOVSKI JA. Links of adolescent- and parent-reported eating in the absence of hunger with observed eating in the absence of hunger. Obesity (Silver Spring) 2013;21:1243–50. doi: 10.1002/oby.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMMONDS M, BURCH J, LLEWELLYN A, GRIFFITHS C, YANG H, OWEN C, DUFFY S, WOOLACOTT N. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess. 2015;19:1–336. doi: 10.3310/hta19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMMONDS M, LLEWELLYN A, OWEN CG, WOOLACOTT N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17:95–107. doi: 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- STARK LJ, SPEAR FILIGNO S, BOLLING C, RATCLIFF MB, KICHLER JC, ROBSON SM, SIMON SL, MCCULLOUGH MB, CLIFFORD LM, ODAR STOUGH C, ZION C, ITTENBACH RF. Clinic and Home-Based Behavioral Intervention for Obesity in Preschoolers: A Randomized Trial. J Pediatr. 2018;192:115–121. e1. doi: 10.1016/j.jpeds.2017.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICE E, SPOOR S, BOHON C, VELDHUIZEN MG, SMALL DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUBBS RJ, HUGHES DA, JOHNSTONE AM, ROWLEY E, REID C, ELIA M, STRATTON R, DELARGY H, KING N, BLUNDELL JE. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84:405–15. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- STUNKARD AJ, BERKOWITZ RI, STALLINGS VA, SCHOELLER DA. Energy intake, not energy output, is a determinant of body size in infants. Am J Clin Nutr. 1999;69:524–30. doi: 10.1093/ajcn/69.3.524. [DOI] [PubMed] [Google Scholar]
- TANOFSKY-KRAFF M, RANZENHOFER LM, YANOVSKI SZ, SCHVEY NA, FAITH M, GUSTAFSON J, YANOVSKI JA. Psychometric properties of a new questionnaire to assess eating in the absence of hunger in children and adolescents. Appetite. 2008;51:148–55. doi: 10.1016/j.appet.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAVERAS EM, SCANLON KS, BIRCH L, RIFAS-SHIMAN SL, RICH-EDWARDS JW, GILLMAN MW. Association of breastfeeding with maternal control of infant feeding at age 1 year. Pediatrics. 2004;114:e577–83. doi: 10.1542/peds.2004-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMPLE JL, LEGIERSKI CM, GIACOMELLI AM, SALVY SJ, EPSTEIN LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr. 2008;87:1121–7. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN JAARSVELD CH, LLEWELLYN CH, JOHNSON L, WARDLE J. Prospective associations between appetitive traits and weight gain in infancy. Am J Clin Nutr. 2011;94:1562–7. doi: 10.3945/ajcn.111.015818. [DOI] [PubMed] [Google Scholar]
- WARDLE J, CARNELL S, HAWORTH CM, FAROOQI IS, O’RAHILLY S, PLOMIN R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- WARDLE J, GUTHRIE CA, SANDERSON S, RAPOPORT L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42:963–70. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- WHITAKER RC, WRIGHT JA, PEPE MS, SEIDEL KD, DIETZ WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- WILFLEY DE, TIBBS TL, VAN BUREN DJ, REACH KP, WALKER MS, EPSTEIN LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26:521–32. doi: 10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKUM S, NG J, STICE E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes (Lond) 2012;36:656–64. doi: 10.1038/ijo.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG-HYMAN D. Introduction to special issue: Self-regulation of appetite-it’s complicated. Obesity (Silver Spring) 2017;25(Suppl 1):S5–S7. doi: 10.1002/oby.21781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.