Abstract
Activation of pain (nociceptive) fibers can sensitize neural circuits within the spinal cord, inducing an increase in excitability (central sensitization) that can foster chronic pain. The development of spinally-mediated central sensitization is regulated by descending fibers and GABAergic interneurons. In adult animals, the co-transporter KCC2 maintains a low intracellular concentration of the anion Cl−. As a result, when the GABA-A receptor is engaged, Cl− flows in the neuron which has a hyperpolarizing (inhibitory) effect. Spinal cord injury (SCI) can down-regulate KCC2 and reverse the flow of Cl−. Under these conditions, engaging the GABA-A receptor can have a depolarizing (excitatory) effect that fosters the development of nociceptive sensitization. The present paper explores how SCI alters GABA function and provides evidence that the loss of descending fibers alters pain transmission to the brain. Prior work has shown that, after SCI, administration of a GABA-A antagonist blocks the development of capsaicin- induced nociceptive sensitization, implying that GABA release plays an essential role. This excitatory effect is linked to serotonergic (5HT) fibers that descend through the dorsolateral funiculus (DLF) and impact spinal function via the 5HT-1A receptor. Supporting this, blocking the 5HT-1A receptor, or lesioning the DLF, emulated the effect of SCI. Conversely, spinal application of a 5HT-1A agonist up-regulated KCC2 and reversed the effect of bicuculline treatment. Finally, lesioning the DLF reversed how a GABA-A antagonist affects a capsaicin-induced aversion in a place conditioning task; in sham operated animals, bicuculline enhanced aversion whereas in DLF-lesioned rats biciculline had an antinociceptive effect.
Keywords: GABA, GABAA receptor, KCC2, central sensitization, allodynia, pain, spinal cord injury, serotonin, 5HT-1A receptor
INTRODUCTION
It is commonly recognized that the neurotransmitter GABA regulates neural activity by modulating neural excitability, an effect that is largely attributable to its action at the ionotropic GABA-A receptor, which impacts neural function through the regulation of the anion Cl− (Ben-Ari et al., 1989; Kuffler and Edwards, 1958). How the GABA-A receptor affects cellular function depends upon the relative concentration of Cl− within the cell. In the mature nervous system, membrane-bound proteins (the co-transporters KCC2 and NKCC1) maintain a low intracellular Cl− concentration (Ben-Ari, 2002; Ben-Ari et al., 2012). Under these conditions, engaging the GABA-A receptor allows Cl− to flow into the cell, which has a hyperpolarizing (inhibitory) effect that dampens neural excitability (Ben-Ari et al., 2012). Within the dorsal horn of the spinal cord, the inhibition of neural excitability modulates afferent pain (nociceptive) signals, helping to prevent an over-excitation that can sensitize nociceptive circuits (central sensitization) and lead to chronic pain (Gwak and Hulsebosch, 2011; Latremoliere and Woolf, 2009; Sivilotti and Woolf, 1994). This general view is supported by studies demonstrating that the local [intrathecal (i.t.)] administration of a drug agonist (e.g., muscimol) that engages the GABA-A receptor inhibits nociceptive reactivity (antinociception) and the development of nociceptive sensitization (Hwang and Yaksh, 1997; Kaneko and Hammond, 1997; Roberts et al., 1986). Conversely, i.t. administration of a drug antagonist (e.g., bicuculline) that blocks the GABA-A receptor has a pronociceptive effect that enhances nociceptive reactivity and promotes the development of nociceptive sensitization (Baba et al., 2003; Dougherty and Hochman, 2008; Roberts et al., 1986; Sivilotti and Woolf, 1994; Sorkin et al., 1998; Zhang et al., 2001).
What is less widely recognized is that how GABA affects neural circuits changes with development and injury. Early in development, NKCC1 is expressed on the cellular membrane before KCC2 (Ben-Ari, 2002; Ben-Ari et al., 2012; Delpire, 2000; Rheims et al., 2009). This promotes the inward flow of Cl−, which increases its intracellular concentration. Under these conditions, administration of a GABA-A antagonist dampens, rather than promotes, neural excitability (Ben-Ari et al., 1989). Development brings an increase in membrane-bound KCC2, which promotes the transport of Cl− out of the cell and lowers its intracellular concentration (Ben-Ari et al., 2012; Delpire, 2000), establishing the ionic balance that causes GABA to have a hyperpolarizing effect. This shift in KCC2 appears linked to the maturation of ascending/descending pathways. Supporting this, if the spinal cord is transected early in development, KCC2 is not up-regulated and the developmental shift in GABA function fails to occur (Jean-Xavier et al., 2006). At a functional level, GABA-dependent neural excitation could foster the adaptive wiring of spinal circuits early in development. An increase in GABA-dependent inhibition as the organism matures would limit excitability/plasticity within the spinal cord, yielding a system that appears more immutable/hardwired in adults.
Recent work suggests that the regulation of intracellular Cl− can also alter GABA function in the mature nervous, modulating its relative inhibitory/excitatory effect, a concept known as ionic plasticity (Rivera et al., 2005). A clear example of this is found after spinal cord injury (SCI), which brings about a reduction in membrane-bound KCC2 in tissue caudal to injury, recapitulating the earlier developmental state wherein GABA has a depolarizing (excitatory) effect (Grau et al., 2017; Huang et al., 2017; Huang et al., 2016). This transformation reverses how treatments that target the GABA-A receptor impact nociceptive reactivity. For example, application of the irritant capsaicin is known to activate cellular markers of nociceptive sensitization (e.g., the phosphorylation of ERK) and enhance reactivity to mechanical stimulation (Gao and Ji, 2009). These indices of central sensitization are blocked by pretreatment with a GABA-A antagonist (bicuculline or gabazine), an outcome opposite to what is observed in uninjured animals (Huang et al., 2016). The implication is that SCI can flip how GABA affects nociceptive circuits within the dorsal horn, unleashing an excitatory effect that drives nociceptive sensitization. Under these conditions, blocking the GABA-A receptor has an antinociceptive effect.
Here we explore why SCI transforms GABAergic function, relating this effect to the loss of serotonergic (5HT) fibers within the dorsolateral funiculus (DLF). This link was suggested by prior work exploring factors that modulate spinal cord plasticity (Crown and Grau, 2005). Research has shown that afferent stimulation of nociceptive fibers can induce a form of long-term potentiation (LTP) within the lower (lumbosacral) spinal cord in rats that have undergone a rostral injury (transection) that cuts communication with the brain (Grau et al., 2006). Likewise, we have shown that nociceptive stimulation applied in an uncontrollable/unpredictable manner induces a form of maladaptive plasticity within the lumbosacral spinal cord that enhances nociceptive behavioral reactivity to mechanical stimulation and interferes with adaptive learning (Crown et al., 2002; Ferguson et al., 2006; Grau et al., 2014; Lee et al., 2015). Interestingly, in the absence of spinal cord injury, nociceptive stimulation fails to induce LTP or maladaptive plasticity. This implies that brain-dependent processes normally regulate neural excitability within the spinal cord. This regulatory effect has been linked to serotonergic (5HT) fibers that descend through the dorsolateral funiculus (DLF) (Crown and Grau, 2005). Here we show that this pathway alters spinal excitability by impacting GABA function and that this modification brings about a change in the motivational significance of pain signals relayed to the brain.
EXPERIMENTAL PROCEDURES
Subjects
Male Sprague-Dawley rats were obtained from Envigo (Houston, TX, USA). Rats were 100–120 days old and weighed 350–400g at the time of surgery. Subjects were housed in pairs and maintained on a 12 hour light-dark cycle. Food and water were available ad libitum. All experiments were carried out in accordance with NIH standards for the care and use of laboratory animals (NIH publications No. 80-23) and were approved by the University Laboratory Animal Care Committee at Texas A&M University. Every effort was made to minimize suffering and limit the number of animals used.
Surgery and intrathecal cannulization
Subjects were anesthetized with isoflurane gas, induced at 5%, and maintained at 2–3%. Each subject’s head was rendered immobile in a stereotaxic apparatus with a small (5 × 4 × 2.5 cm) gauze pillow under the subject’s chest to provide support for respiration. An anterior to posterior incision over the second thoracic vertebrae (T2) was made and the tissue just rostral to T2 was cleared using rongeurs until the cord was exposed. For spinal cord transection, a cautery device was then used to transect the cord. Dorsolateral funiculus (DLF) lesions were made under visual guidance at T2 using a scalpel blade (#11, Lance Blades) as described in Crown & Grau (2005). Sham operated rats were treated the same, except the spinal tissue was not transected/lesioned. Next, a 25-cm polyethylene cannula (PE-10, VWR International, Bristol, CT, USA) for intrathecal (i.t.) drug administration was threaded 7.5 cm down the vertebral column, into the subarachnoid space between the dura and the white matter, and placed over the lumbar enlargement of the spinal cord. The wound was closed with Michel clips (Fisher Scientific, Waltham, MA, USA) and animals were given an intraperitoneal (i.p.) injection (3 mL) of 0.9% saline solution to prevent dehydration. During the recovery period, rats were placed in a temperature-controlled environment (25.5 °C) and monitored until awake. All rats were checked every six to eight hours during the 18–24 hr post-surgical period. During this time, hydration was maintained with supplemental injections of saline, and the rats’ bladders and colons were expressed as necessary.
Drug preparation
Bicuculline (0.82 nmole, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% saline (0.3μg in 1 μL). Sixty nmole of 8-Hydroxy-DPAT hydrobromide (8-OH-DPAT; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 6 μL of 0.9% saline. One hundred nmole of WAY-100635 maleate salt (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 10 μL of 0.9% saline. All the drugs were administered i.t. and followed by a 20 μL saline (0.9%) flush that was slowly infused over a period of 2 min.
Nociceptive stimulation
Nociceptive sensitization was induced using the irritant capsaicin. In the first three experiments, 50 μL of 3% capsaicin (dissolved in Tween 20 [7%] and saline [93%]; Sigma-Aldrich, St. Louis, MO, USA) was subcutaneously (s.c.) injected into the dorsal surface of a hindpaw while the subject was loosely restrained in a Plexiglas tube. We applied capsaicin to the dorsal surface of the hind paw. Capsaicin has both a local effect that induces a homosynaptic potentiation in the stimulated pathway and a heterosynpatic facilitation that produces an area of flare in the surrounding region. Because the latter is coupled to the induction of central sensitization, mechanical reactivity was assessed by applying the stimuli to the opposite (plantar) surface of the paw. In the last experiment, we sought a method that could be used in a place conditioning procedure. For this purpose, it was important to minimize the pain associated with the vehicle treatment. In humans, it has been shown that a capsaicin cream can induce nociceptive sensitization (Petersen and Rowbotham, 1999). We emulated this procedure by dissolving capsaicin in a mixture of ethanol (50%) and distilled water. Capsaicin (2%) or the vehicle alone was applied to the dorsal surface of one hindpaw using a cotton swab. A pilot experiment confirmed that topical capsaicin induces a robust EMR in sham operated rats and that this effect lasts at least 3 hrs (F(3, 18) = 58.76, p < 0.0005).
Mechanical testing
Mechanical reactivity was assessed using von Frey filaments (Stoelting, Wood Dale, IL, USA) that were applied while rats were loosely restrained in Plexiglas tubes. Sensitivity was determined by stimulating the mid-plantar surface of each hindpaw by up-down method until a flexion response was elicited. Stimuli were presented twice to each paw in an ABBA counterbalanced fashion (A = left, B = right), with testing on the same leg separated by a 2 min interval. Filament thickness/force was related to behavior using the transformation provided by the manufacturer: Intensity = log10 (10,000g). This transformation yields a scale that is approximately linear and amenable to parametric analyses. Data were converted to change from baseline scores for purposes of analysis. The experimenter performing the behavioral tests was unaware of the subject’s treatment condition.
Place preference conditioning
The training and testing boxes used to assess place conditionings were the same as described in Ferguson et al. (2004). Acclimation took place in gray plywood boxes [41 cm (length)×41 cm (height)×38 cm (width)] with wire grid floors. Capsaicin place conditioning training occurred in plywood boxes [41 cm (l)×41 cm (h)×38 cm (w)] that were painted either white or black. To assure that the two contexts could be easily discriminated, they also differed in odor and type of flooring. The black boxes had smooth black Plexiglas floors and were wiped clean with a mild (3%) vinegar solution. The floors of white boxes were covered with cedar wood chips and were cleaned with the disinfectant Novalsan. Preference testing was performed in another box that was 91 cm (l)×41 cm (h)×38 cm (w). The testing box had two ends that were both 41 cm (l)×41 cm (h)×38 cm (w), each with the physical characteristics of one of the training boxes. These distinct regions were separated by a narrow gray strip [9 cm (l)×41 cm (h)×38 cm (w)] with a 1-cm wire-grid floor similar to the acclimation boxes.
On days 1 and 2, rats were acclimated to being handled and placed in the gray boxes for 45 min (see Fig. 6A). On day 3, rats were placed in the test box for 15 min and the time in each region was recorded to establish whether there was a preference/bias prior to conditioning. The amount of time each rat spent in the three regions (white, black, and gray) of the testing box was recorded by assessing the location of the rat every 6 second for 15 min. This testing procedure yielded 150 evenly spaced observation points for each rat. Animals that had strong baseline preference, defined as spending more than an 80% in a single region, were eliminated from further testing. Following the pretesting phase, rats received a DLF lesion or sham surgery. Conditioning was conducted on Days 4 and 5, with all of the subjects receiving 0.3% bicuculline (i.t.) on one day and the vehicle on the other (counter-balanced across conditions). On Day 4, 15 min after the drug injection, half of the DLF lesioned and sham operated rats had capsaicin applied to one hind paw and were placed in one of the conditioning chambers (black or white). On Day 5, these subjects received the alternative drug treatment, followed by capsaicin (applied to the opposite paw), and were placed in the alternative chamber. Which chamber was presented first, and whether subjects were treated on the left or right paw on Day 4, was also counterbalanced. The remaining subjects were treated the same except that they had the vehicle solution applied to the paw prior to being placed in each training chamber. On day 6, animals were placed into the testing box and their preference/aversion for black/white sides of the apparatus was assessed. To control for baseline preferences, test scores were calculated as a percentage of time spent in each region relative to the pretesting period.
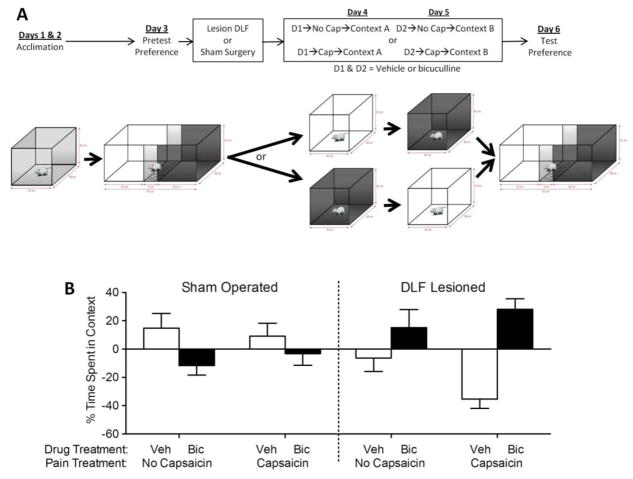
Fig. 6.
DLF lesions alter how bicuculline affects place conditioning. (A) The experimental design is illustrated at the top of the figure. After acclimation and a pretest of baseline preferences, rats received bilateral DLF lesions or a sham surgery. Over the next two days (D1 & D2), they received bicuculline (i.t.) or its vehicle prior to being placed in one of two distinctive contexts (A and B). On both days, half of the rats had topical capsaicin applied after the i.t. injection. The relative aversion to each set of contextual cues was then assessed in a preference test. (B) The left and right panel depicts sham-operated and DLF lesioned groups, respectively. Each subject had received vehicle (Veh; white bar) on one conditioning day and bicuculline (Bic; black bar) on the other. The adjoining white and black bars indicate the relative preference for each context within a group. The four groups differed in whether they had received a sham surgery (left) or DLF lesions (right) and in whether or not they were treated with capsaicin prior to context exposure. The y-axis depicts the change from baseline preferences. Error bars depict ± SEM.
Protein extraction and Western blotting
Tissue was collected for Western blotting immediately following mechanical testing. Subjects were deeply anesthetized with a lethal dosage of pentobarbital (100 mg/kg) and a 1-cm portion of the spinal cord containing the lumbosacral enlargement was removed and rapidly frozen. The spinal cord was further subdivided into dorsal and ventral portions. Total protein was extracted using the QIAzolTM lysis reagent protocol (Hummon et al., 2007) for isolation of genomic DNA and/or proteins from fatty tissue, as described in a previous report from our laboratory (Huang et al., 2016). After determining the protein concentration using a Bradford Assay (BioRad, Hercules, CA), protein samples were diluted in Laemmli sample buffer and were stored at −80°C at known concentrations (usually 2–5μg/μl). Western blotting was used for the protein quantification of ERK1/2 and pERK1/2 (~ 44/42 kDa). Equal amounts (30μg) of total protein were subjected to SDS-PAGE with 12% Tris-HEPES precast gels (Pierce, Rockford, IL). After transferring onto PVDF membranes (Millipore, Bedford, MA) by Bio-Rad Semi-dry transfer apparatus, the blots for ERK1/2 and other non-phosphorylated proteins (see below) were blocked for one hour in 5% blotting grade milk (BioRad, Hercules, CA) in Tris-Buffered Saline Tween-20 (TBST), while blots for pERK1/2 were blocked in 5% BSA in TBST. After blocking, the PVDF membranes were incubated overnight at 4° C in one of the following primary antibodies generated in rabbit: ERK1/2 (1:2000; #06-182 - Millipore, Temecula, CA; AB_310068), pERK1/2 (1:500; #07-467 - Millipore, Temecula, CA; AB_310640). β-actin (1:2500; #Ab8227 - Abcam, Cambridge, MA; AB_2305186) served as the control. All primary antibodies were diluted in blocking solution. The following day, PVDF membranes were washed in TBST (3 × 5 min) at room temperature and incubated in HRP-conjugated goat anti-rabbit or anti-mouse secondary antibodies (1:5,000; #31460 or 31430, respectively; Pierce, Rockford, IL) for 1 hour at room temperature. After another 3 × 5 min series of washes, the blots were incubated with ECL (Pierce, Rockford, IL) and were imaged with Fluorchem HD2 (ProteinSimple, Santa Clara, CA). The protein expression for each gene of interest was normalized to β-actin expression and presented as a fold change relative to the sham controls. Other targets of interest including KCC2, phospho-Ser940KCC2, and N-cadherin were assessed in the same fashion.
Fractionation
To assess KCC2 (1:500; #07-432 - Millipore, Temecula, CA; AB_310611) and phospho-Ser940KCC2 (1:1000; p1551-940 - PhosphoSolutions, Aurora, CO; AB_2492213) expression, spinal cord specimens were homogenized with dounce homogenizer (Kontes), followed by 5 passes through a 22 gauge needle in ice-cold buffer, pH 7.5, containing 10 mm Tris, 300 mm sucrose, and a complete mini protease inhibitor mixture (Roche). Crude homogenates were centrifuged at 5000 RCF for 5 min at 4° C. Supernatant was further fractionated at 13,000 RCF for 30 min. After centrifugation, supernatant was collected as cytoplasmic fraction. A membrane rich fraction was then obtained by resuspending the pellet in PBS (50 μl) containing protease inhibitor. All samples were sonicated and stored at −80° C for later processing (Western blotting). N-cadherin (1:1000; #4061 - Cell Signaling, Danvers, MA; AB_2077426) was used to confirm plasma membrane enrichment.
Statistics
All data were analyzed using repeated measures analysis of variance (ANOVA) or analysis of covariance (ANCOVA). Differences between group means were assessed using Duncan’s New Multiple Range post hoc tests when necessary.
We controlled for individual variability in mechanical reactivity in two ways: (1) By analyzing the test data using an ANCOVA, entering the baseline score as a covariate; and (2) By computing a change from baseline score and analyzing the data using an ANOVA. An advantage of the latter is that our index of variability [the standard error of the mean (SE)] is computed after we adjust for individual differences. Because this simplifies the assessment of group differences, and because an ANCOVA performed on the raw scores and an ANOVA conducted on the change from baseline values yielded the same pattern of statistical significance across experiments, we present just the change from baseline scores. In all cases, p < .05 was used to determine statistical significance.
RESULTS
Experiment 1: Blocking serotonergic transmission emulates the effect of spinal cord injury
In the absence of SCI, blocking GABAergic transmission enhances nociceptive sensitization (Reeve et al., 1998; Roberts et al., 1986; Sorkin et al., 1998). After SCI, GABA-A antagonists have the opposite effect (Boulenguez et al., 2010; Huang et al., 2016), implying that the loss of descending fibers has induced a shift in function, wherein GABA transmission potentiates the development of central sensitization. We have suggested that this shift is due to the loss of descending serotonergic fibers, which normally quell over-excitation by engaging the 5HT-1A receptor. If this is how SCI alters GABA function, then blocking 5HT-1A receptors within the spinal cord with the drug antagonist WAY-100635 should have the same effect as injury and reverse how a GABA-A antagonist (bicuculline) affects nociceptive sensitization. The 5HT-1A receptor is expressed predominantly in the dorsal horn, especially laminae I and II (Marlier et al., 1991). Some expression is also observed on primary afferent fibers within dorsal horn and initial axon segment (Otoshi et al., 2009).
Sham operated rats underwent a laminectomy, but the spinal cord was not injured (see Fig. 1A). An intrathecal (i.t.) catheter was inserted and, 24 hrs later, animals were microinjected with 10 μl of WAY-100635 (100 nmole, i.t.) or its vehicle (n=6 per group). Fifteen minutes after drug delivery, subjects received an i.t. injection of bicuculline (0.3 μg in 1 μl saline) or its vehicle. These doses were chosen on the basis of past work implicating 5HT and GABA in the regulation of maladaptive plasticity (Crown and Grau, 2005; Ferguson et al., 2003; Huang et al., 2016). Fifteen minutes later, the irritant capsaicin was applied to the left or right hind paw (counter-balanced across subjects). This yielded a 2 (WAY-100635 vs. vehicle) x 2 (bicuculline vs. vehicle) factorial design. Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), after drug treatments, and again 0, 1, 2, 3 hr following capsaicin treatment. A change from baseline score was calculated to assess the impact of the experimental manipulations. Immediately after the last behavior test, subjects were sacrificed. One centimeter of spinal cord around the lumbar enlargement (L3–L5) region was rapidly removed for cellular assays. ERK1/2 and pERK1/2 protein expression were measured using Western blotting.
Fig. 1.
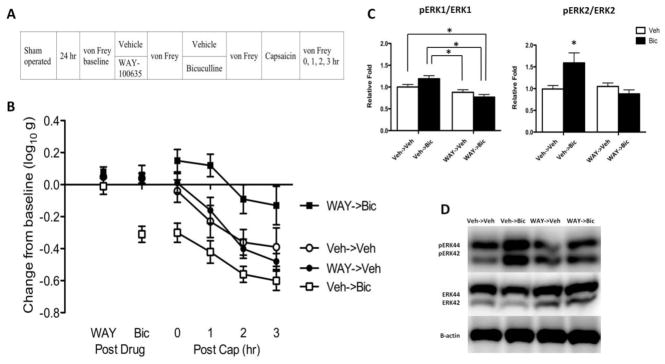
WAY-100635 switches how bicuculline affects nociceptive sensitization in intact rats. (A) The experimental design is illustrated at the top of the figure. A day after surgery, rats received WAY-100635 (WAY; black) or vehicle (Veh; white). Fifteen min later, rats received bicuculline (Bic; squares) or its vehicle (Veh; circles). Another fifteen min later, capsaicin was applied to the dorsal surface of one hind paw. (B) Mechanical reactivity after drug and capsaicin treatment. The y-axis depicts the linearized mechanical scores [log 10 (10,000g)] as a change from baseline after WAY-100635 and bicuculline treatment (Post Drug), and 0, 1, 2, 3 hr after capsaicin treatment (Post Cap). (C) Ratio of pERK1 to ERK1 (44 kDa) and pERK2 to ERK2 (42 kDa) expression in subjects that had previously received vehicle or WAY-100635. (D) Representative Western blots for ERK1/2 and pERK1/2 expression. Error bars depict ± SEM
Prior to drug treatment, mechanical reactivity scores ranged from 5.68 ± 0.04 to 5.73 ± 0.06 (mean ± standard error of the mean [SE]) across groups. These differences were not statistically significant (F(1, 20) < 1, p > 0.05). The administration of WAY-100635 alone (Post Drug, Fig. 1B) did not have a significant effect (F(1, 20) = 2.32, p > 0.05). Administration of bicuculline induced an EMR in the vehicle treated (Veh->Bic), but not WAY-100635 treated (WAY->Bic), rats. An ANOVA confirmed that the main effect of WAY-100635 and bicuculline, as well as their interaction, were statistically significant (all Fs > 13.89, p < 0.05). Post hoc comparisons confirmed that the group that received bicuculline alone (Veh->Bic) differed from the other groups (p < 0.05). No other group comparison was significant (p > 0.05).
As in prior studies (Huang et al., 2016), rats exhibited a greater EMR when tested on the treated leg (all Fs > 14.28), but the overall effect of our experimental treatments remained the same. For this reason, we collapsed the results across test leg and present the mean values (Fig. 1B). Capsaicin treatment (Post Cap) induced a lasting EMR (Veh->Veh) that was enhanced by bicuculline treatment (Veh->Bic; F(3, 60) = 39.95, p < 0.0001). Pretreatment with WAY-100635 (WAY->Bic) transformed how bicuculline affected capsaicin-induced EMR, unveiling an antinociceptive- like effect that attenuated capsaicin- induced EMR. An ANOVA showed that the main effect of WAY-100635 and its interaction with bicuculline were statistically significant (both Fs > 13.63, p < 0.005). Post hoc comparisons of the group means confirmed that the group that received WAY-100635 and bicuculline (WAT->Bic) differed from the other groups (p < 0.05). In addition, the group that received bicuculline alone (Veh->Bic) differed from the group that did not receive bicuculline (Veh->Veh, WAY->Veh). No other group comparison was significant (p > 0.05).
Seeking converging evidence that drug treatment affected the development of nociceptive sensitization, we also assessed the activation (phosphorylation) of ERK1/2 (44/42) within the lumbosacral dorsal horn (Fig. 1C & D). Protein expression for each target was normalized to β-actin expression level and the impact of drug treatment was computed relative to the vehicle treated controls. ERK activation was then derived by computing the ratio of pERK to ERK for both isoforms. In both cases, bicuculline alone (Veh->Bic) increased pERK expression and this effect was reversed by pretreatment with WAY-100635 (Way->Bic). An ANOVA confirmed that the interaction between WAY-100635 and bicuculline treatment was statistically significant (all Fs > 4.88, p < 0.05). Post hoc comparisons of the group means showed that the group that received WAY-100635 before bicuculline (WAT->Bic) differed from the group that received vehicle before bicuculline (Veh->Bic) and this was true for both ERK isoforms (p < 0.05).
In summary, we found that bicuculline alone enhanced reactivity to mechanical stimulation and augmented the development of nociceptive sensitization (at both a behavioral and cellular level). Blocking the 5HT-1A receptor with WAY-100635 had an effect analogous to SCI, eliminating the EMR induced by bicuculline and inverting the drug’s effect on capsaicin-induced EMR.
Experiment 2: Pretreatment with a 5HT-1A agonist restores the inhibitory effect of GABA after SCI
The results imply that SCI transforms GABAergic function because it disrupts descending serotonergic fibers that regulate nociceptive processing through the 5HT-1A receptor. If this is how SCI affects GABA function, then administratio n of a 5HT-1A agonist could have a restorative effect after SCI and reinstate GABA-dependent inhibition. We tested this hypothesis by pretreating spinally transected rats with a 5HT-1A agonist (8-OH-DPAT) prior to bicuculline. We predicted that engaging the 5HT-1A receptor will substitute for the loss of descending fibers and restore GABAergic inhibition. Behaviorally, this should reverse the effect of bicuculline; when 5HT-1A receptors are artificially driven, bicuculline should promote, rather than inhibit, nociceptive reactivity.
To test these predictions, rats (n=6 per group) were spinally transected and an i.t. catheter was implanted. The next day, animals were microinjected with either vehicle or 8-OH-DPAT [DPAT; 60 nmol, i.t.; see Fig. 2A]. This dose was chosen because it has previously been shown to block the development of maladaptive plasticity in spinally transected rats (Crown & Grau, 2005). Fifteen minutes after drug delivery, rats received either the vehicle (saline) or bicuculline (0.3 μg, i.t.). Fifteen minutes later, capsaicin was applied to the left or right hind paw (counterbalanced across animals). Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), after drug (DPAT and Bic) injection, and again 0, 1, 2, 3 hr following capsaicin treatment. A change from baseline score was calculated to assess the impact of the experimental manipulations.
Fig. 2.
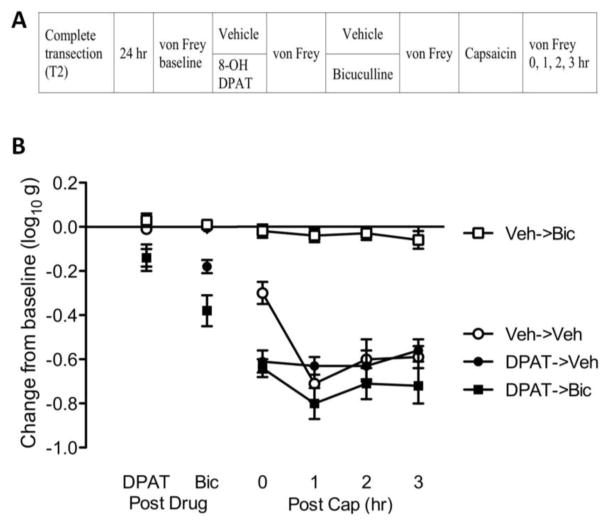
8-OH-DPAT switches how bicuculline affects nociceptive sensitization in spinally-transected rats. (A) The experimental design is illustrated at the top of the figure. A day after surgery, rats received 8-OH-DPAT (DPAT; black) or vehicle (Veh; white). Fifteen min later, rats received bicuculline (Bic; squares) or its vehicle (Veh; circles). Another fifteen min later, capsaicin was applied to the dorsal surface of one hind paw. (B) Mechanical reactivity after drug and capsaicin treatment. The y-axis depicts the linearized mechanical scores [log 10 (10,000g)] as a change from baseline after 8-OH-DPAT and bicuculline treatment (Post Drug), and 0, 1, 2, 3 hr after capsaicin treatment (Post Cap).
Average baseline mechanical reactivity scores ranged from 6.12 ± 0.04 to 6.28 ± 0.06 (mean ± standard error of the mean [SE]) across groups. These differences were not statistically significant (F(1, 20) = 1.78, p > 0.05). Prior to bicuculline treatment (DPAT, Fig. 2B), 8-OH-DPAT inhibited mechanical reactivity (F(1, 20) < 17.77, p < 0.001). Administration of bicuculline (Bic) enhanced mechanical reactivity in rats that had received 8-OH-DPAT (DPAT->Bic). An ANOVA showed that the main effects of 8-OH-DPAT and bicuculline, as well as their interaction, were statistically significant (all Fs > 5.99, p < 0.05). Post hoc comparisons confirmed that the group that received 8-OH-DPAT and bicuculline (DPAT->Bic) differed from the other groups (p < 0.05).
Capsaicin treatment (Post Cap) induced a robust EMR in vehicle treated (Veh->Veh) rats. Bicuculline blocked the development of nociceptive sensitization (Veh->Bic) and pretreatment with 8-OH-DPAT (DPAT->Bic) eliminated this effect. An ANOVA showed that the main effect of 8-OH-DPAT and bicuculline, as well as their interaction, were statistically significant (all Fs > 22.85, p < 0.0001). Also, the main effect of time, and the Time x 8-OH-DPAT x Bicuculline three-way interaction, were significant (both Fs > 7.98 p < 0.0001). Post hoc comparisons of the group means confirmed that the group that received bicuculline alone (Veh->Bic) differed from the other groups (p < 0.05).
Experiment 3: Pretreatment with a 5HT-1A agonist up-regulates KCC2 expression
We have previously shown that acute SCI down-regulates membrane-bound KCC2 in the lumbosacral spinal cord (Huang et al., 2016), which would explain why GABA has an excitatory effect after injury. The results reported above show that pretreatment with a serotonergic agonist can reverse the effect of bicuculline on nociceptive reactivity and sensitization, restoring a pattern of results that mirror the drug’s effect in uninjured animals. Administration of 8-OH-DPAT could have this effect by increasing the levels of membrane-bound KCC2 within the spinal cord, which would increase intracellular Cl− concentrations and the hyperpolarizing effect of GABA. The present experiment evaluates this possibility by separating the membrane enriched and cytoplasmic fractions and assessing KCC2 expression in each using Western blotting.
Spinally-transected and cannulized rats (n=6 per group) were microinjected with either vehicle or 8-HO-DAPT (DPAT; 60 nmole, i.t.). Thirty minutes after drug delivery, subjects were euthanized and a one-centimeter section of the spinal cord containing the lumbar enlargement (L3–L5) region was collected. Samples were hemi-dissected into dorsal and ventral halves, and went through fractionation for Western Blotting.
Both KCC2 and phospho-KCC2 (pKCC2) expression were normalized to β-actin expression level. To determine whether our experimental treatment affected the distribution of each ligand across the membrane and cytoplasmic fractions, we divided the values observed within the membrane fraction by those from the cytoplasmic component, providing a ratio (fold change) measure of each. N-cadherin was used in Western blotting to verify the fractionation procedure and confirm plasma membrane enrichment.
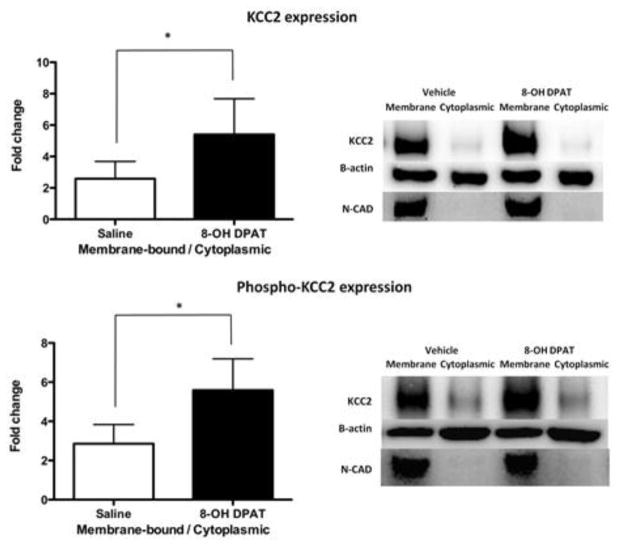
8-OH-DPAT treatment increased the ratio of membrane-bound to cytoplasmic KCC2 and pKCC2 (Fig. 3) within the dorsal region. As illustrated in the accompanying blots, this effect was evident from an increase within the membrane component. An ANOVA confirmed that the main effect of drug treatment on both forms of KCC2 was statistically significant (both Fs > 29.6, p < 0.0001). 8-OH-DPAT treatment did not have a significant effect on KCC2 or pKCC2 expression within the ventral horn (data not shown).
Fig. 3.
8-OH-DPAT upregulates KCC2 and phopho-KCC2 expression in spinally transected rats. A day after spinal transection, rats were given 8-HO-DPAT (black) or its vehicle (white) and tissue was collected 30 min later. Upper panel represents the fold change of membrane-bound/cytoplasmic KCC2 ratio; the lower panel represents the fold change of membrane-bound/cytoplasmic phospho-KCC2 ratio. Representative Western blots are provided to the right of each panel. The error bars depict ± SEM.
Experiment 4: Lesions limited to the DLF down-regulate KCC2 expression
Our results imply that the transformation in GABAergic function observed after SCI is linked to a loss of descending serotonergic fibers and the 5HT-1A receptor. Blocking the 5HT-1A receptor in intact rats with WAY-100635 had the same effect as SCI, reversing how a GABA-A antagonist affects nociceptive sensitization; in the presence of WAY-100635, bicuculline not only failed to promote nociceptive reactivity (pro-nociception), it blocked the development of central sensitization (antinociception). Conversely, in spinally transected rats, administration of a 5HT-1A receptor agonist (8-OH-DPAT) had a restorative effect, that increased membrane bound KCC2 and reversed the effect of bicuculline, causing it to enhance rather than diminish nociceptive sensitization. The majority of descending serotonergic fibers are found within the DLF (Davies et al., 1983). If these fiber pathways regulate GABA function in the lumbosacral spinal cord, lesions limited to the DLF should be suffic ient to down-regulate membrane-bound KCC2.
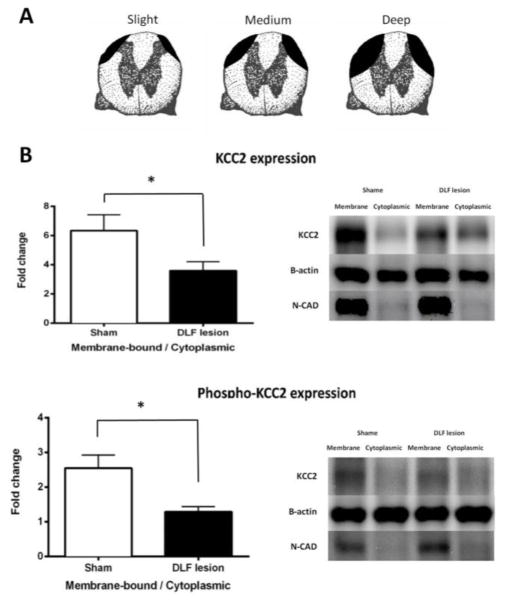
Rats were randomly assigned to receive a DLF lesion at T2 or a sham-operation (n=10 per group). Twenty-four hours later, animals were sacrificed and a one-centimeter section of the spinal cord containing the lumbar enlargement (L3–L5) region was rapidly removed. Samples were hemi-dissected into dorsal and ventral halves, and went through fractionation for Western Blotting. Both KCC2 and phospho-KCC2 (pKCC2) expression were normalized to β-actin expression level, and are presented as a fold change relative to the vehicle group. To assess how DLF lesions affected the distribution of KCC2/pKCC2, we then calculated the membrane-bound to cytoplasmic ratio. N-cadherin was used in Western Blotting to verify fractionation procedure and confirm plasma membrane enrichment. The extent of damage produced by the DLF lesions is illustrated in Fig. 4A. Bilaterally lesioning the DLF at T2 reduced KCC2 and pKCC2 protein within the membrane fraction (Fig. 4B). An ANOVA confirmed that these effects were statistically significant (both Fs > 4.83, p < 0.05).
Fig. 4.
DLF lesions down-regulate KCC2 expression. (A) Samples were selected to illustrate the range of DLF lesions, from deep through medium to slight (n=4, 6, and 3, respectively). (B) The fold change in the membrane-bound/cytoplasmic KCC2 and phosphor-KCC2 ratio in DLF lesioned (black) and sham-operated (white) rats. Representative Western blots are provided to the right of each panel. The error bars depict ± SEM.
Experiment 5: Lesioning just the DLF reverses the effect of bicuculline on nociceptive sensitization
Lesions limited to the DLF had the same effect on KCC2 as a complete spinal transection, down-regulating expression in the membrane fraction. Given this, we anticipated that DLF lesions would also reverse how bicuculline affects nociceptive reactivity.
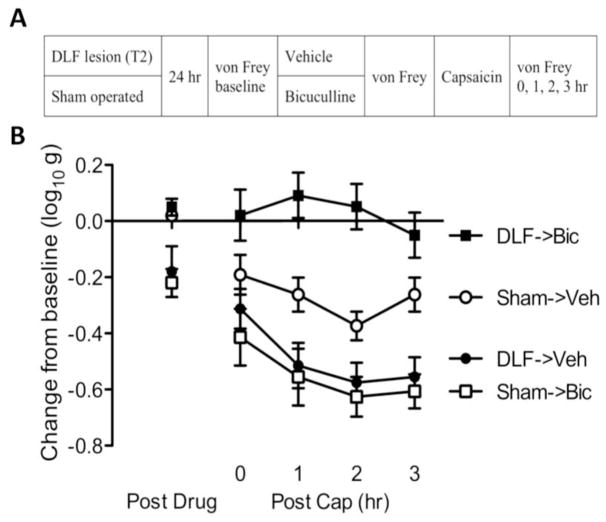
Rats received DLF lesions at T2 or underwent a sham surgery (see Fig. 5A). Baseline behavioral reactivity was tested using von Frey stimuli 24 hr later. Half of the rats from each group (n=6 per group) were then microinjected with of bicuculline (0.3 μg, i.t.) or vehicle. Fifteen minutes after drug delivery, all subjects were treated with 2% topical capsaicin applied to the dorsal surface of the left or right hind paw (counter-balanced across animals). Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), prior to topical capsaicin administration, and again 0, 1, 2, 3 hr following capsaicin treatment.
Fig. 5.
DLF lesions switch how bicuculline affects nociceptive sensitization. (A) The experimental design used to examine how DLF lesions affect GABA function. Subjects received either DLF lesion (DLF; black) or sham surgery (Sham; white). A day after surgery, rats received either bicuculline (Bic; squares) or vehicle (Veh; circles). Fifteen min later, capsaicin was applied to the dorsal surface of one hind paw. (B) The impact of DLF lesions and drug treatment on mechanical reactivity in capsaicin-treated rats. The y-axis depicts the linearized mechanical scores [log 10 (10,000g)] as a change from baseline after bicuculline treatment (Post Drug), and 0, 1, 2, 3 hr after capsaicin treatment (Post Cap). Error bars depict ± SEM.
Prior to drug treatment, mechanical reactivity scores ranged from 5.38 ± 0.08 to 5.59 ± 0.07 (mean ± standard error of the mean [SE]) across groups. Lesioning the DLF, per se, did not have a significant effect (F(1, 20) = 1.57, p > 0.05). Prior to capsaicin treatment (Post Drug), bicuculline enhanced mechanical reactivity in sham operated, but not DLF lesioned, rats (see Fig. 5B). An ANOVA confirmed that the Surgery X Bicuculline interaction was statistically significant (F(1, 20) < 17.27, p < 0.0005). Post hoc comparisons showed that sham-operated rats that received bicuculline (Sham->Bic) and DLF lesioned rats that received vehicle (DLF->Veh) differed from other groups (p < 0.05).
After capsaicin treatment (Post Cap), bicuculline inhibited capsaicin-induced EMR in DLF lesioned. In sham-operated rats, bicuculline appeared to exacerbate the capsaicin-induced EMR. An ANOVA revealed that the main effect of surgery, and its interaction with bicuculline, were statistically significant (both Fs > 7.14, p < 0.05). Also, the main effect of time, and the Time x Surgery x Bicuculline three-way interaction were significant (both Fs > 4.09, p < 0.05). Post hoc comparisons of the group means confirmed that DLF lesioned rats that received bicuculline (DLF->Bic) differed from the other groups (p < 0.05).
Experiment 6: Spinally-mediated alterations in GABA function impact place conditioning
Our results show that SCI down-regulates KCC2 expression within the lumbosacral spinal cord and that this transforms how GABA affects nociceptive processing, inducing a state wherein GABA appears to drive rather than inhibit nociceptive sensitization (Huang et al., 2016). In this state, blocking the GABA-A receptor with bicuculline attenuates nociceptive sensitization and this conclusion is supported by both behavioral and cellular data. The results reported above have extended these observations by demonstrating that the change in GABA function is linked to 5HT fibers within the DLF. What is missing is evidence that these alterations within the lumbosacral spinal cord affect pain transmission to the brain. The last experiment addresses this issue by examining the impact of DLF lesions and bicuculline on topical capsaicin-induced motivated behavior using a place conditioning procedure (King et al., 2009). In this task, rats are exposed to two distinct environments (contexts) across days. Suppose that rats are treated with topical capsaicin before they are placed in each conditioning chambers, but in one case they are pretreated with i.t. bicuculline. In intact rats, we would expect biciculline to enhance pain (pro-nociceptive) and increase the aversion to the paired context. In contrast, the current study suggests that i.t. bicuculline blocks nociceptive sensitization in DLF lesioned rats. If this transformation affects the transmission of pain signals to the brain, DLF lesioned rats given bicuculline prior to capsacin should feel less pain (antinociception), which would reduce the aversion to the paired context. To evaluate the impact of our experimental treatments, rats are then allowed to choose between the two contexts. The prediction is that sham operated rats will prefer the vehicle-paired context whereas DLF lesioned rats will choose the context where they received bicuculline prior to capsaicin treatment.
Thirty-two rats went through the place preference conditioning procedure (see Fig. 6A) and were randomly assigned to receive bilateral lesions of the DLF or a sham-surgery after Pretesting on Day 3 (baseline). Conditioning occurred over the next two days. Half of the subjects in each condition (n=8 per group) had capsaicin applied topically to one paw prior to being placed in the each training context. The remaining rats had the vehicle applied to the paw. Fifteen min before rats were placed in one context, they received an injection of bicuculline (0.3 μg, i.t.). Prior to the other context, they received an injection of the vehicle. Rats were treated on different paws across days and the paw treated, context presented first, and whether bicuculline or vehicle was given first, were counter-balanced across animals. On Day 6, animals were placed into the test chamber and their relative preference/aversion to each context was assessed. For each subject, the relative aversion/preference for the bicuculline paired context was computed by subtracting the time spent in the bicuculline paired context from the time spent in the vehicle paired context. On this scale, negative scores reflect an aversion while positive scores represent a preference.
Sham operated rats exhibited an aversion to the bicuculline paired context whereas DLF lesioned rats exhibited a preference for this context (Fig. 6B). The latter effect was evident in DLF lesioned animals that received bicuculline prior to capsaicin treatment (DLF->Cap). An ANOVA confirmed that there was a significant effect of surgery (F(1, 28) = 14.5, p < .001. Post hoc comparisons of the group means showed that DLF lesioned rats treated with capsaicin differed from both of the sham operated groups (p < 0.05).
As predicted, sham operated rats exhibited an aversion to the context where they had received i.t. bicuculline, implying that blocking the GABA-A receptor increased pain. Bicuculline had the opposite effect in DLF lesioned rats, inducing a conditioned preference, which suggests that the drug had an antinoiceptive effect.
DISCUSSION
Prior work has shown that SCI can transform how GABA affects nociceptive circuits within the spinal cord, reversing the effect of drugs that target the GABA-A receptor (Boulenguez et al., 2010; Ferguson et al., 2003; Huang et al., 2016). The experiments reported here were designed to address two key issues: 1) Is the alteration in GABA function linked to the loss of a particular fiber pathway; and 2) Does the change in nociceptive processing within the spinal cord impact pain transmission to the brain?
Serotonin Regulates GABA Function within the Spinal Cord
In the uninjured system, blocking the GABA-A receptor enhances nociceptive reactivity and the development of central sensitization (Baba et al., 2003; Dougherty and Hochman, 2008; Roberts et al., 1986; Sivilotti and Woolf, 1994; Sorkin et al., 1998; Zhang et al., 2001), an outcome consistent with the idea GABAergic interneurons normally inhibit neural excitability. But after SCI, local application of the GABA-A antagonist bicuculline has an antinociceptive effect that counters the development of nociceptive sensitization and this is evident from its effect at both a behavioral and molecular level (Huang et al., 2016). The implication is that SCI does more than remove a source of tonic inhibition—it reverses how GABA acts, so that it now drives the development of sensitization. After SCI, engaging GABA-A receptors appears essential (necessary) to central sensitization.
We proposed that SCI brings about an alteration in GABA function because it disrupts descending fibers within the DLF that regulate neural excitability within the dorsal horn through the 5HT-1A receptor (Crown and Grau, 2005; Gjerstad et al., 2001; Sandkuhler and Liu, 1998). After spinal cord injury, noxious stimulation can induce a form of long-term potentiation (LTP) (Sandkuhler, 2000). If prolonged, this can saturate NMDA-receptor dependent plasticity and impair adaptive learning (Grau et al., 2014). These effects are not observed if the same amount of stimulation is applied to awake (unanesthetized) animals prior to SCI (Washburn et al., 2007), which suggests that descending fibers normally exert a dampening force that quells the development of nociceptive sensitization. Further work linked these effects to fibers that descend through the DLF (Crown & Grau, 2005). Pharmacological studies examining the relative contribution of 5HT and noradrenergic fibers implicated 5HT and the 5HT-1A receptor (Crown and Grau, 2005; Garraway and Hochman, 2001).
While past studies indicated that a loss of 5HT fibers and/or damage to the DLF can set the stage for nociceptive sensitization, it was not known how this transformation occurred. The present study fills this gap by demonstrating that descending 5HT fibers impact GABA function, inducing an increase in neural excitability through a biological switch linked to the regulation of GABA. To show that descending 5HT fibers play an essential role, we tested whether blocking the 5HT-1A receptor with WAY-100635 alters GABA function. As expected, in the absence of WAY-100635, i.t. bicuculline had a pronociceptive effect that enhanced reactivity to mechanical stimulation and the development of capsaicin-induced central sensitization (Baba et al., 2003; Dougherty and Hochman, 2008; Sivilotti and Woolf, 1994; Sorkin et al., 1998; Zhang et al., 2001). Blocking just the 5HT-1A receptors reversed how bicuculline affected nociceptive reactivity, unveiling an antinociceptive effect that attenuated the development of nociceptive sensitization at both the behavioral and molecular level. We then attempted a form of substitution, by driving the 5HT-1A receptors after spinal cord injury with 8-OH-DPAT. After spinal cord injury, application of 8-OH-DPAT increased the membrane expression of KCC2 and reversed the effect of bicuculline; now, instead of blocking capsaicin- induced nociceptive sensitization (antinociception), bicuculline had a pronociceptive effect. This outcome implies that engaging the 5HT-1A receptor was sufficient to reinstate GABA-dependent inhibition.
Fibers within the DLF Impact GABA and Pain Transmission to the Brain
We posited that an essential fiber pathway descended through the DLF. If this is true, then bilaterally lesioning the DLF should have the same effect as a complete spinal cord transection. We first showed that lesions limited to the DLF were sufficient to induce a down-regulation in membrane-bound KCC2. DLF lesions also reversed how i.t. bicuculline affected nociceptive sensitization—in DLF lesioned animals, bicuculline blocked, rather than facilitated, the development of central sensitization.
Because DLF lesions preserve ascending pathways, this manipulation provided a means to address a key issue: Does the alteration in GABA function within the spinal cord affect pain transmission to the brain? We addressed this issue using a place conditioning procedure (King et al., 2009), wherein subjects received topical capsaicin before they were placed in one of two distinctive contexts. Prior to one context, rats received an i.t. injection of bicuculline, which we predicted would enhance pain and conditioned aversion in uninjured rats (Sivilotti and Woolf, 1994; Sorkin et al., 1998). As expected, when subsequently allowed to choose between the two capsaicin-paired chambers, uninjured rats showed an aversion to the context where they received bicuculline. If i.t. bicuculline has an antinociceptive effect in DLF lesioned rats, the drug should reduce pain and the development of a conditioned aversion. The unique prediction is that DLF lesioned rats given bicuculline should prefer the bicuculline-paired context, and this is what we found. The implication is that an injury- induced alteration in spinal GABA function affects both local nociceptive processing within the spinal cord and pain transmission to the brain.
Regulation of Neural Excitability Through KCC2
The present results are consistent with past work demonstrating that SCI can cause a reduction in membrane-bound KCC2 at and caudal to the site of injury that reduces GABA-dependent inhibition (Boulenguez et al., 2010; Cramer et al., 2008; Huang et al., 2016; Lavertu et al., 2014). This modification has been linked to both the development of chronic pain and spasticity. The hypothesis is supported by studies demonstrating that blocking KCC2 at the level of the spinal cord in uninjured rats (with the drug DIOA) has the same effect as SCI, inducing physiological signs of spasticity and reversing how bicuculline affects nociceptive sensitization (Boulenguez et al., 2010; Huang et al., 2016). Conversely, blocking the inward flow of Cl− through NKCC1 with the drug bumetanide, which should lower intracellular Cl− concentrations and restore GABAergic inhibiton, blocks the effect of SCI on nociceptive processing and spasticity (Cramer et al., 2008; Hasbargen et al., 2010; Huang et al., 2016).
The regulation of KCC2 and intracellular Cl− provides a cellular process (ionic plasticity) that can modulate neural excitability. Interestingly, other signal pathways known to regulate plastic potential (metaplasticity) appear to act, in part, by affecting KCC2. Of particular interest to injury, pain, and recovery is brain derived neurotrophic factor (BDNF). In uninjured animals, local application of BDNF down-regulates KCC2 within the spinal cord, inducing an increase in neural excitability that fosters the development of nociceptive sensitization (Coull et al., 2005; Huang et al., 2017; Merighi et al., 2008). This maladaptive plasticity has been linked to the release of BDNF from activated microglia (Coull et al., 2005). In contrast, after SCI, BDNF inhibits the development of nociceptive sensitization and promotes adaptive plasticity (Huang et al., 2017; Huie et al., 2012; Khaing et al. 2016). BDNF appears to do so by restoring GABA-dependent inhibition through an increase in membrane-bound KCC2. Thus, BDNF can affect KCC2 in opposite ways, depending upon the overall level of neural excitability (Shulga et al., 2008). This fits with the more general view that BDNF has a homeostatic function that helps to maintain an optimal level of neural excitability (Wenner, 2014).
Regulation of Spinal Nociceptive Circuits by Serotonin
The present results are consistent with prior work implicating 5HT, and the 5HT-1A receptor, in the regulation of nociceptive circuits within the dorsal horn (Crown and Grau, 2005; Garraway and Hochman, 2001; Gjerstad et al., 2001; Sandkuhler and Liu, 1998). Here we showed that drugs targeting this receptor impact GABA function and the expression of KCC2. Other work suggests that 5HT can also regulate KCC2 within the ventral region (Bos et al., 2013). However, this effect appears to depend on the 5HT-2A receptor (Bos et al., 2013).
Other work suggests that dysfunction in descending pain modulatory circuits contribute to pain “chronification” (Ossipov et al., 2014). This may be due to a disruption in the balance of descending modulatory circuits that favors facilitation over inhibition, and thereby impacts nociceptive sensitization. Supporting this, as in models of inflammation, descending inhibition generally predominates over descending facilitation in the primary pain circuits with input from the inflamed tissue; while in the secondary pain circuit, descending facilitation predominates over descending inhibition with input from neighboring tissues (Millan, 2002). Also, the inhibitory descending control from the PAG-RVM system preferentially suppresses nociceptive inputs mediated by C-fibers, preserving sensory-discriminative information conveyed by more rapidly conducting A-fibers (Heinricher et al., 2009; Suzuki and Dickenson, 2005; Suzuki et al., 2004; Vanegas and Schaible, 2004). Combined with the present results, it appears that descending serotoninergic fibers normally inhibit neural excitability within the spinal cord. SCI can disrupt this source of inhibition and contribute to hyperexcitation by changing how GABA affects spinal cord circuits, resulting in the facilitation of nociceptive reflexes.
Our findings are also consistent with past studies indicating that increasing serotonergic input through drug application or cell engraftment can counter the development of central sensitization and neuropathic pain after SCI (Bardin et al., 2000; Bruce et al., 2002; Hains et al., 2002; Horiuchi et al., 2003). Here too, research has implicated the 5HT1A receptor (Gjerstad et al., 1996; Hains et al., 2003; Otoshi et al., 2009). BDNF has also been shown to play a role in this process. For example, Hains et al. (2001) showed that engraftment of serotonergic neurons following SCI restores spinal serotonin, increases BDNF tissue content, and reduces central pain in rat. Further, work suggests that BDNF release from serotonergic precursors transplanted into the spinal cord contributes to the amelioration of chronic pain (Eaton et al., 1998), and this effect too has been related to a change in GABA function (Eaton et al., 1998; Kato et al., 2006; Stubley et al., 2001). Likewise, the antinociceptive effect of serotonin has been tied to GABA and the 5HT1A receptor (Bonnefont et al., 2005; Huo et al., 2008). Taken together, these data suggest that descending serotonergic input, partly through 5HT1A receptor, determines how BDNF affect KCC2 expression and GABA-dependent ionic plasticity.
Serotonergic input has also been implicated in other neuropathies. For example, research has shown that serotonergic transmission plays a pivotal role in spasticity post SCI (Dentel et al., 2013; Elbasiouny et al., 2010; Nardone et al., 2015). Other work has shown that increasing serotonergic transmission can enhance locomotor recovery post SCI (Ghosh and Pearse, 2014; Hains et al., 2001). Research has also shown that serotonergic transmission and KCC2 play a role in spasticity associated with Amyotrophic lateral sclerosis (Modol et al., 2014) and neuropathic pain post SCI (Sanchez-Brualla et al., 2017). Here too, changes in spinal function have been related to alterations in KCC2 expression and GABA-dependent inhibition (Boulenguez et al., 2010; Chopek et al., 2015; Gackiere and Vinay, 2014; Tashiro et al., 2015). Other work has shown that serotonergic processes and alterations in GABA function contribute to brain-dependent processes, including stress, epilepsy, and seizures (Miller & Maguire, 2014; Buchanan et al., 2014; Kahle et al., 2016; Maguire, 2014; Silayeva et al., 2015). These findings support a more nuanced view of how GABA affects neural excitability in adult animals, a view that assumes a bidirectional effect and allows for both the inhib ition and facilitation of neural excitability. While these effects are especially evident after trauma, ionic plasticity may play a more general role, to dynamically shift neural systems from a hard-wired state to one that is malleable (Grau & Huang, 2018).
Ionic Plasticity Enables Nociceptive Sensitization
Early pain theorist viewed the spinal cord as a more-or-less hard-wired system and assumed that variation in pain was largely attributable to the top-down regulation of incoming pain signals through descending fibers (Basbaum and Fields, 1984). Work over the last two decades has challenged this view, providing evidence that noxious input can sensitize nociceptive circuitry within the dorsal horn to enhance behavioral reactivity and pain transmission to the brain (Latremoliere and Woolf, 2009; Sandkuhler, 2009; Willis, 2001). This, and other work, suggests a very different view of spinal function—that it is a dynamic system that regulates nociceptive signals on the basis of environmental relations and past experience (Grau et al., 2012; Ji et al., 2003; Sandkuhler, 2000).
To demonstrate plasticity within the spinal cord, researchers have typically used procedures that inhibit brain function (e.g., anesthesia, decerebration) or curtail communication with rostral systems (spinal cord transection) (e.g., Grau et al., 1998; Patterson, 1976; Sandkuhler and Liu, 1998; Woolf, 1983). While these procedures have yielded definitive evidence that neurons within the spinal cord can adapt in the absence of brain input (Grau et al., 2014), the procedures themselves alter the neural context in which plasticity develops, releasing caudal systems from potential inhibitory forces. As we have seen, interfering with just 5HT transmission can flip how GABA affects spinal circuits, to recapitulate an earlier developmental state that fosters neural plasticity. Likewise, inflammation and the activation of microglia can lead to the release of BDNF, which in the uninjured system down-regulates KCC2 and promotes nociceptive sensitization (Coull et al., 2005; Merighi et al., 2008). In both cases, ionic plasticity appears to set the stage for nociceptive sensitization and chronic pain. From this view, a shift in GABA function shifts the central gray from a state that seems hard-wired to one that is malleable. The corollary to this analysis is that, in the presence of GABAergic inhibition, spinally- mediated nociceptive sensitization cannot develop. This supports the more general view that promoting GABAergic inhibition can counter the development of nociceptive sensitization (Gwak and Hulsebosch, 2011; Latremoliere and Woolf, 2009). The caution is that simply enhancing GABA release may not achieve this end, because its action is regulated by ionic plasticity. A more reliable approach may involve targeting the processes that control how GABA acts, by focusing upon the co-transporters that regulate Cl− flow or the neurochemical systems (e.g., 5HT) that regulate their trafficking and activation.
Highlights.
After SCI, blocking GABA-A receptor prevents, rather than enhances, the development of nociceptive sensitization.
Transformation in GABA function is linked to the loss of serotonergic fibers descend through DLF.
Serotonin alters GABA function via the 5HT-1A receptor.
GABA-A antagonist treatment has an antinociceptive effect in rats (place conditioning) with DLF lesion.
Acknowledgments
The authors wish to thank Misty Malamakal, Joel Turtle, Josh Reynolds, Melissa Brumley, Julia Forsberg and Jason Lu for comments on an earlier version of this article.
Funding
This research was supported by the Mary Tucker Currier Professorship and grants from the Craig H. Neilsen Foundation and NIH (NINDS NS091723) to JWG.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- Bic
Bicuculline
- BSA
Bovine serum albumin
- Cap
Capsaicin
- CNS
Central nervous system
- DPAT
8-OH-DPAT
- DLF
Dorsolateral funiculus
- EMR
Enhanced mechanical reactivity
- ERK
Extracellular signal-regulated kinases
- GABA
Gamma-aminobutyric acid
- 8-OH-DPAT
8-Hydroxy-2-dipropylaminotetralin hydrobromide
- i.t
Intrathecal
- KCC2
K+-Cl− cotransporter 2
- LTP
Long-term potentiation
- N-CAD
N-cadherin
- NKCC1
Na+-K+- Cl− cotransporter 1
- pERK
Phosphorylated ERK
- pKCC2
Phospho-KCC2
- SCI
Spinal cord injury
- 5HT
Serotonin
- T2
Second thoracic
- Veh
Vehicle
- WAY
WAY100635
Footnotes
Conflict of Interest
The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Molecular and cellular neurosciences. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Bardin L, Schmidt J, Alloui A, Eschalier A. Effect of intrathecal administration of serotonin in chronic pain models in rats. European journal of pharmacology. 2000;409:37–43. doi: 10.1016/s0014-2999(00)00796-2. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annual review of neuroscience. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature reviews Neuroscience. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. The Journal of physiology. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- Bonnefont J, Chapuy E, Clottes E, Alloui A, Eschalier A. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain. 2005;114:482–490. doi: 10.1016/j.pain.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Bos R, Sadlaoud K, Boulenguez P, Buttigieg D, Liabeuf S, Brocard C, Haase G, Bras H, Vinay L. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:348–353. doi: 10.1073/pnas.1213680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nature medicine. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- Bruce JC, Oatway MA, Weaver LC. Chronic pain after clip-compression injury of the rat spinal cord. Experimental neurology. 2002;178:33–48. doi: 10.1006/exnr.2002.8026. [DOI] [PubMed] [Google Scholar]
- Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. The Journal of physiology. 2014;592:4395–4410. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopek JW, Sheppard PC, Gardiner K, Gardiner PF. Serotonin receptor and KCC2 gene expression in lumbar flexor and extensor motoneurons posttransection with and without passive cycling. Journal of neurophysiology. 2015;113:1369–1376. doi: 10.1152/jn.00550.2014. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cramer SW, Baggott C, Cain J, Tilghman J, Allcock B, Miranpuri G, Rajpal S, Sun D, Resnick D. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Molecular pain. 2008;4:36. doi: 10.1186/1744-8069-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord. II. Evidence for central mediation. Physiology & behavior. 2002;77:259–267. doi: 10.1016/s0031-9384(02)00859-4. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Evidence that descending serotonergic systems protect spinal cord plasticity against the disruptive effect of uncontrollable stimulation. Experimental neurology. 2005;196:164–176. doi: 10.1016/j.expneurol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Davies JE, Marsden CA, Roberts MH. Hyperalgesia and the reduction of monoamines resulting from lesions of the dorsolateral funiculus. Brain research. 1983;261:59–68. doi: 10.1016/0006-8993(83)91283-0. [DOI] [PubMed] [Google Scholar]
- Delpire E. Cation-Chloride Cotransporters in Neuronal Communication. News in physiological sciences: an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- Dentel C, Palamiuc L, Henriques A, Lannes B, Spreux-Varoquaux O, Gutknecht L, Rene F, Echaniz- Laguna A, Gonzalez de Aguilar JL, Lesch KP, Meininger V, Loeffler JP, Dupuis L. Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: a link to spasticity. Brain: a journal of neurology. 2013;136:483–493. doi: 10.1093/brain/aws274. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Hochman S. Spinal cord injury causes plasticity in a subpopulation of lamina I GABAergic interneurons. Journal of neurophysiolo gy. 2008;100:212–223. doi: 10.1152/jn.01104.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. Journal of chemical neuroanatomy. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Moroz D, Bakr MM, Mushahwar VK. Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabilitation and neural repair. 2010;24:23–33. doi: 10.1177/1545968309343213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Patton BC, Bopp AC, Meagher MW, Grau JW. Brief exposure to a mild stressor enhances morphine-conditioned place preference in male rats. Psychopharmacology. 2004;175:47–52. doi: 10.1007/s00213-004-1780-3. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Washburn SN, Crown ED, Grau JW. GABA(A) receptor activation is involved in noncontingent shock inhibition of instrumental conditioning in spinal rats. Behavioral neuroscience. 2003;117:799–812. doi: 10.1037/0735-7044.117.4.799. [DOI] [PubMed] [Google Scholar]
- Gackiere F, Vinay L. Serotonergic modulation of post-synaptic inhibition and locomotor alternating pattern in the spinal cord. Frontiers in neural circuits. 2014;8:102. doi: 10.3389/fncir.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? The open pain journal. 2009;2:11–17. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway SM, Hochman S. Modulatory actions of serotonin, norepinephrine, dopamine, and acetylcholine in spinal cord deep dorsal horn neurons. Journal of neurophysiology. 2001;86:2183–2194. doi: 10.1152/jn.2001.86.5.2183. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Pearse DD. The role of the serotonergic system in locomotor recovery after spinal cord injury. Frontiers in neural circuits. 2014;8:151. doi: 10.3389/fncir.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstad J, Tjolsen A, Hole K. The effect of 5-HT1A receptor stimulation on nociceptive dorsal horn neurones in rats. European journal of pharmacology. 1996;318:315–321. doi: 10.1016/s0014-2999(96)00819-9. [DOI] [PubMed] [Google Scholar]
- Gjerstad J, Tjolsen A, Hole K. Induction of long-term potentiation of single wide dynamic range neurones in the dorsal horn is inhibited by descending pathways. Pain. 2001;91:263–268. doi: 10.1016/S0304-3959(00)00448-6. [DOI] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behavioral neuroscience. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behavioral and cognitive neuroscience reviews. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grau JW, Huang Y-J. Metaplasticity through ionic plasticity: How a shift in GABA function can alter pain and the capacity to learn. Neurobiology of Learning and Memory. 2018 doi: 10.1016/j.nlm.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Huang YJ, Turtle JD, Strain MM, Miranda RC, Garraway SM, Hook MA. When Pain Hurts: Nociceptive Stimulation Induces a State of Maladaptive Plasticity and Impairs Recovery after Spinal Cord Injury. Journal of neurotrauma. 2017;34:1873–1890. doi: 10.1089/neu.2016.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Huie JR, Garraway SM, Hook MA, Crown ED, Baumbauer KM, Lee KH, Hoy KC, Ferguson AR. Impact of behavioral control on the processing of nociceptive stimulation. Frontiers in physiology. 2012;3:262. doi: 10.3389/fphys.2012.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Huie JR, Lee KH, Hoy KC, Huang YJ, Turtle JD, Strain MM, Baumbauer KM, Miranda RM, Hook MA, Ferguson AR, Garraway SM. Metaplasticity and behavior: how training and inflammation affect plastic potential within the spinal cord and recovery after injury. Frontiers in neural circuits. 2014;8:100. doi: 10.3389/fncir.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60:799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Everhart AW, Fullwood SD, Hulsebosch CE. Changes in serotonin, serotonin transporter expression and serotonin denervation supersensitivity: involvement in chronic central pain after spinal hemisection in the rat. Experimental neurology. 2002;175:347–362. doi: 10.1006/exnr.2002.7892. [DOI] [PubMed] [Google Scholar]
- Hains BC, Fullwood SD, Eaton MJ, Hulsebosch CE. Subdural engraftment of serotonergic neurons following spinal hemisection restores spinal serotonin, downregulates serotonin transporter, and increases BDNF tissue content in rat. Brain research. 2001;913:35–46. doi: 10.1016/s0006-8993(01)02749-4. [DOI] [PubMed] [Google Scholar]
- Hains BC, Willis WD, Hulsebosch CE. Serotonin receptors 5-HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Experimental brain research. 2003;149:174–186. doi: 10.1007/s00221-002-1352-x. [DOI] [PubMed] [Google Scholar]
- Hasbargen T, Ahmed MM, Miranpuri G, Li L, Kahle KT, Resnick D, Sun D. Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Annals of the New York Academy of Sciences. 2010;1198:168–172. doi: 10.1111/j.1749-6632.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain research reviews. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, Ogata T, Morino T, Takeba J, Yamamoto H. Serotonergic signaling inhibits hyperalgesia induced by spinal cord damage. Brain research. 2003;963:312–320. doi: 10.1016/s0006-8993(02)04055-6. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Lee KH, Grau JW. Complete spinal cord injury (SCI) transforms how brain derived neurotrophic factor (BDNF) affects nociceptive sensitization. Experimental neurology. 2017;288:38–50. doi: 10.1016/j.expneurol.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Lee KH, Murphy L, Garraway SM, Grau JW. Acute spinal cord injury (SCI) transforms how GABA affects nociceptive sensitization. Experimental neurology. 2016;285:82–95. doi: 10.1016/j.expneurol.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie JR, Garraway SM, Baumbauer KM, Hoy KC, Jr, Beas BS, Montgomery KS, Bizon JL, Grau JW. Brain-derived neurotrophic factor promotes adaptive plasticity within the spinal cord and mediates the beneficial effects of controllable stimulation. Neuroscience. 2012;200:74–90. doi: 10.1016/j.neuroscience.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummon AB, Lim SR, Difilippantonio MJ, Ried T. Isolation and solubilization of proteins after TRIzol extraction of RNA and DNA from patient material following prolonged storage. BioTechniques. 2007;42:467–470. 472. doi: 10.2144/000112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo FQ, Qu CL, Li YQ, Tang JS, Jia H. GABAergic modulation is involved in the ventrolateral orbital cortex 5-HT 1A receptor activation- induced antinociception in the rat. Pain. 2008;139:398–405. doi: 10.1016/j.pain.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically- induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- Jean-Xavier C, Pflieger JF, Liabeuf S, Vinay L. Inhibitory postsynaptic potentials in lumbar motoneurons remain depolarizing after neonatal spinal cord transection in the rat. Journal of neurophysiology. 2006;96:2274–2281. doi: 10.1152/jn.00328.2006. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends in neurosciences. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Khanna AR, Duan J, Staley KJ, Delpire E, Poduri A. The KCC2 Cotransporter and Human Epilepsy: Getting Excited About Inhibition. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2016;22:555–562. doi: 10.1177/1073858416645087. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hammond DL. Role of spinal gamma-aminobutyric acidA receptors in formalin- induced nociception in the rat. The Journal of pharmacology and experimental therapeutics. 1997;282:928–938. [PubMed] [Google Scholar]
- Kato G, Yasaka T, Katafuchi T, Furue H, Mizuno M, Iwamoto Y, Yoshimura M. Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:1787–1794. doi: 10.1523/JNEUROSCI.4856-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaing ZZ, Agrawal NK, Park JH, Xin S, Plumton GC, Lee KH, Huang YJ, Niemerski AL, Schmidt CE, Grau JW. Localized and sustained release of brain-derived neurotrophic factor from injectable hydrogel/microparticle composites fosters spinal learning after spinal cord injury. Journal of Materials Chemistry B. 2016;4:7560–7571. doi: 10.1039/c6tb01602b. [DOI] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nature neuroscience. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Edwards C. Mechanism of gamma aminobutyric acid (GABA) action and its relation to synaptic inhibition. Journal of neurophysiology. 1958;21:589–610. doi: 10.1152/jn.1958.21.6.589. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The journal of pain: official journal of the American Pain Society. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavertu G, Cote SL, De Koninck Y. Enhancing K-Cl co-transport restores normal spinothalamic sensory coding in a neuropathic pain model. Brain: a journal of neurology. 2014;137:724–738. doi: 10.1093/brain/awt334. [DOI] [PubMed] [Google Scholar]
- Lee KH, Turtle JD, Huang YJ, Strain MM, Baumbauer KM, Grau JW. Learning about time within the spinal cord: evidence that spinal neurons can abstract and store an index of regularity. Frontiers in behavioral neuroscience. 2015;9:274. doi: 10.3389/fnbeh.2015.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J. Stress-induced plasticity of GABAergic inhibition. Frontiers in cellular neuroscience. 2014;8:157. doi: 10.3389/fncel.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Research. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Progress in neurobiology. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Progress in neurobiology. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller S, Maguire J. Deficits in KCC2 and activation of the HPA axis lead to depression-like behavior following social defeat. Hormonal Studies. 2014;2:2. [Google Scholar]
- Mòdol L, Mancuso R, Alé A, Francos-Quijorna I, Navarro X. Differential effects on KCC2 expression and spasticity of ALS and traumatic injuries to motoneurons. Frontiers in Cellular Neuroscience. 2014;8:7. doi: 10.3389/fncel.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Holler Y, Thomschewski A, Holler P, Lochner P, Golaszewski S, Brigo F, Trinka E. Serotonergic transmission after spinal cord injury. Journal of neural transmission. 2015;122:279–295. doi: 10.1007/s00702-014-1241-z. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Current opinion in supportive and palliative care. 2014;8:143–151. doi: 10.1097/SPC.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otoshi CK, Walwyn WM, Tillakaratne NJ, Zhong H, Roy RR, Edgerton VR. Distribution and localization of 5-HT(1A) receptors in the rat lumbar spinal cord after transection and deafferentation. Journal of neurotrauma. 2009;26:575–584. doi: 10.1089/neu.2008.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MM. Mechanisms of classical conditioning and fixation in spinal mammals. Advances in psychobiology. 1976;3:381–436. [PubMed] [Google Scholar]
- Petersen KL, Rowbotham MC. A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport. 1999;10:1511–1516. doi: 10.1097/00001756-199905140-00022. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Dickenson AH, Kerr NC. Spinal effects of bicuculline: modulation of an allodynia-like state by an A1-receptor agonist, morphine, and an NMDA-receptor antagonist. Journal of neurophysiology. 1998;79:1494–1507. doi: 10.1152/jn.1998.79.3.1494. [DOI] [PubMed] [Google Scholar]
- Rheims S, Holmgren CD, Chazal G, Mulder J, Harkany T, Zilberter T, Zilberter Y. GABA action in immature neocortical neurons directly depends on the availability of ketone bodies. Journal of neurochemistry. 2009;110:1330–1338. doi: 10.1111/j.1471-4159.2009.06230.x. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. The Journal of physiology. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LA, Beyer C, Komisaruk BR. Nociceptive responses to altered GABAergic activity at the spinal cord. Life sciences. 1986;39:1667–1674. doi: 10.1016/0024-3205(86)90164-5. [DOI] [PubMed] [Google Scholar]
- Sánchez-Brualla I, Boulenguez P, Brocard C, Liabeuf S, Viallat-Lieutaud A, Navarro X, Udina E, Brocard F. Activation of 5-HT2A receptors restores KCC2 function and reduces neuropathic pain after spinal cord injury. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.08.033. In Press. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Learning and memory in pain pathways. Pain. 2000;88:113–118. doi: 10.1016/S0304-3959(00)00424-3. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiological reviews. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. The European journal of neuroscience. 1998;10:2476–2480. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- Shulga A, Thomas-Crusells J, Sigl T, Blaesse A, Mestres P, Meyer M, Yan Q, Kaila K, Saarma M, Rivera C, Giehl KM. Posttraumatic GABA(A)-mediated [Ca2+]i increase is essential for the induction of brain-derived neurotrophic factor-dependent survival of mature central neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:6996–7005. doi: 10.1523/JNEUROSCI.5268-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silayeva L, Deeb TZ, Hines RM, Kelley MR, Munoz MB, Lee HH, Brandon NJ, Dunlop J, Maguire J, Davies PA, Moss SJ. KCC2 activity is critical in limiting the onset and severity of status epilepticus. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3523–3528. doi: 10.1073/pnas.1415126112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. Journal of neurophysiology. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Puig S, Jones DL. Spinal bicuculline produces hypersensitivity of dorsal horn neurons: effects of excitatory amino acid antagonists. Pain. 1998;77:181–190. doi: 10.1016/S0304-3959(98)00094-3. [DOI] [PubMed] [Google Scholar]
- Stubley LA, Martinez MA, Karmally S, Lopez T, Cejas P, Eaton MJ. Only early intervention with gamma-aminobutyric acid cell therapy is able to reverse neuropathic pain after partial nerve injury. Journal of neurotrauma. 2001;18:471–477. doi: 10.1089/089771501750171092. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Dickenson A. Spinal and supraspinal contributions to central sensitization in peripheral neuropathy. Neuro-Signals. 2005;14:175–181. doi: 10.1159/000087656. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends in pharmacological sciences. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Tashiro S, Shinozaki M, Mukaino M, Renault-Mihara F, Toyama Y, Liu M, Nakamura M, Okano H. BDNF Induced by Treadmill Training Contributes to the Suppression of Spasticity and Allodynia After Spinal Cord Injury via Upregulation of KCC2. Neurorehabilitation and neural repair. 2015;29:677–689. doi: 10.1177/1545968314562110. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain research. Brain research reviews. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Washburn SN, Patton BC, Ferguson AR, Hudson KL, Grau JW. Exposure to intermittent nociceptive stimulation under pentobarbital anesthesia disrupts spinal cord function in rats. Psychopharmacology. 2007;192:243–252. doi: 10.1007/s00213-007-0707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner P. Homeostatic synaptic plasticity in developing spinal networks driven by excitatory GABAergic currents. Neuropharmacology. 2014;78:55–62. doi: 10.1016/j.neuropharm.2013.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD. Role of neurotransmitters in sensitization of pain responses. Annals of the New York Academy of Sciences. 2001;933:142–156. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. C-primary afferent fibre mediated inhibitions in the dorsal horn of the decerebrate-spinal rat. Experimental brain research. 1983;51:283–290. doi: 10.1007/BF00237204. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hefferan MP, Loomis CW. Topical bicuculline to the rat spinal cord induces highly localized allodynia that is mediated by spinal prostaglandins. Pain. 2001;92:351–361. doi: 10.1016/S0304-3959(01)00276-7. [DOI] [PubMed] [Google Scholar]