Abstract
Objective
To assess changes in orofacial tactile sensitivity and gnawing related to capsaicin-mediated cutaneous, myogenic, and arthrogenic nociception in the rat.
Design
After recovery from anesthesia, orofacial tactile sensitivity and gnawing were assessed using operant testing methods following capsaicin application. Twenty female CD-Hairless rats were tested with bilateral capsaicin cream application to the cheek or with isoflurane anesthesia alone. Following several weeks of recovery, animals (n=20) received either 10 μL unilateral masseter injections of vehicle, or phosphate buffered saline (PBS) to assess injection sensitization. After several weeks, masseter capsaicin (1.0%) injections (10 μL) were assessed compared to vehicle and PBS (n=13). Weeks later capsaicin TMJ injections were evaluated. Animals (n=11) received either 10 μL unilateral TMJ injections of capsaicin solution (1%) or vehicle.
Results
Capsaicin cream to the skin significantly altered gnawing activity (increased puncture time by 248 seconds (p=0.0002)) and tactile sensitivity (decreased tolerated bottle distance by 0.980 cm compared to isoflurane only (p=0.0001)). Similarly, capsaicin masseter injection increased puncture time (339.6 seconds, p=0.07) and decreased tolerated bottle distance (1.04 cm, p=0.005) compared to vehicle). However, intra-articular capsaicin in the TMJ only modified gnawing (increased puncture time by 133 seconds), with no changes found in tactile sensitivity compared to vehicle.
Conclusion
Application of capsaicin to the skin and masseter had similar behavioral effects; however, intra-articular injections to the TMJ only affected gnawing. These data indicate the behavioral changes in rodent models of myogenic and cutaneous pain may be markedly different than models of arthrogenic pain originating from the TMJ.
Keywords: Capsaicin, Pain, Orofacial, Behavior, Operant
1. Introduction
Temporomandibular disorders (TMDs) are the second most commonly occurring musculoskeletal condition resulting in pain and disability, affecting 5-12% of the population (NIH, 2018). Patients with TMDs present a variety of symptoms, including irregular temporomandibular joint (TMJ) sounds, muscle and joint pain, and limited jaw movement (Peck et al., 2014; Velly et al., 2010). Providing new treatment options has proven difficult, exacerbated by the diversity of clinical causes and symptoms.
Behavioral testing is often used to investigate rodent models of pain and disability. For orofacial tactile sensitivity, current methods typically measure a withdrawal response using an investigator driven stimulus (Imamura, Kawamoto, & Nakanishi, 1997; Vos & Strassman, 1995). While semi-quantitative in nature, these particular tests can produce misrepresentative results due animal stress and experimenter bias, making comparisons across experimenters and labs challenging (Balcombe, Barnard, & Sandusky, 2004; Chesler, Wilson, Lariviere, Rodriguez-Zas, & Mogil, 2002; Imbe, Iwai-Liao, & Senba, 2006; Terman, Shavit, Lewis, Cannon, & Liebeskind, 1984). Operant-based behavioral tests allow the animal to make a participation decision, the results of which can be interpreted to assess pain and dysfunction. Moreover, allowing the animal to make decisions factoring their individual pain or capabilities removes the experimenter from the assay, reducing both animal stress and experimenter bias. As such, operant assays of orofacial pain have potential to produce more consistent results across experimenters and labs (Cha, Kohan, Zuo, Ling, & Gu, 2012; Mauderli, Acosta-Rua, & Vierck, 2000; Neubert et al., 2005; Nolan, Hester, Bokrand-Donatelli, Caudle, & Neubert, 2011).
Capsaicin can be used to elicit hypersensitivity in tissues and provide a localized pain response. Capsaicin selectively binds to TRPV1 receptors (Rosenbaum & Simon, 2007) causing a release of neuropeptides from sensitized afferent terminals. Arteriolar vasodilation, plasma extravasation, and pain including hypersensitivity and allodynia are a result of application to tissues (Gazerani, Wang, Cairns, Svensson, & Arendt-Nielsen, 2006; P Holzer, 1993; Peter Holzer, 1991; Jancsó, Jancsó-Gábor, & Szolcsányi, 1967, 1968; LaMotte, Lundberg, & Torebjörk, 1992; Lembeck & Holzer, 1979; Lin, Li, Xu, Zou, & Fang, 2007; Nilsson et al., 2014). Injected capsaicin produces thermal and mechanical hyperalgesia and edema at the injection site for over two hours in the hind paw, (Gilchrist, Allard, & Simone, 1996; Kinnman & Levine, 1995; Zhang, Wu, Fang, & Willis, 2003), masseter muscle (Ro, Lee, & Zhang, 2009; Ro, Lee, Capra, & Zhang, 2007) and TMJ of rats (Tang, Haas, & Hu, 2004). Application of capsaicin can also be used to model clinical pain related to TRPV1 activation.
In this paper, tactile hypersensitivity and gnawing are assessed in capsaicin-mediated models of cutaneous, myogenic, and arthrogenic pain using operant testing methods developed by our group (Rohrs et al., 2015). While capsaicin application does not directly mimic clinical etiology of TMD, the methods of evaluating rodent models of orofacial pain and TMJ dysfunction described here can be used to increase our understanding of clinical TMD etiology and pain. Etiologies of TMDs can have varying presentation of clinical symptoms including tactile hypersensitivity, masticatory muscle pain and joint pain. As such, future rodent models of TMD, that may more appropriately mimic clinical TMD etiology, can be appropriately evaluated using operant-based testing for orofacial hypersensitivity and TMJ dysfunction. Evaluating clinically relevant rodent models of TMD using operant-based methods will lead to more effective treatment options for TMD patients.
2. Methods
2.1. Animals
The testing procedures and general handling of animals described herein are in compliance with the ethical guidelines and standards established by the Institutional Animal Care & Use Committee (IACUC) at the University of Florida and the Guide for Care and Use of Laboratory Animals. All procedures were performed on IACUC approved research protocols (National Research Council (US), 2011).
2.2. Experimental Design
Twenty, female CD-Hairless rats (3 month old, Charles River) underwent the testing procedures described in Figure 1. To establish baseline behavior, animals were tested every 2-4 days until a consistent behavioral baseline was established (Baseline 1). Consistent baseline behavior was classified as 3 consecutive trials without significant change in mean result (bottle distance or puncture time, procedures detailed below). If animals did exhibit consistent baseline behavior or failed to participate in the operant based behavioral assays, the animal was excluded from all subsequent testing (3 animals removed).
Figure 1.
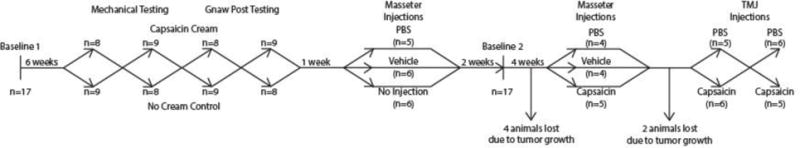
Timeline of experiments performed. After baseline behavior was established, orofacial capsaicin cream application was investigated over four separate trials. Capsaicin cream application’s effect on tactile sensitivity was tested over the first two trials, then capsaicin cream application’s effect on gnawing was assessed over two trials. Then non-noxious injections were investigated, testing tactile sensitivity and gnawing during a single trial. Baseline behavior was re-established and capsaicin injections were investigated, again, testing tactile sensitivity and gnawing during a single trial. Finally, TMJ capsaicin injections were investigated over two trials, switching vehicle and capsaicin groups between trials.
Orofacial capsaicin cream application was first utilized to investigate behavioral changes associated with cutaneous hypersensitivity (procedures detailed below). For this experiment, animals were separated into two groups (n=8, 9). Group 1 received capsaicin cream applied to the skin over the masseter under isoflurane anesthesia, while Group 2 animals received isoflurane only. The groups were then switched every 2 days and this process was repeated for three additional trials, resulting in four total capsaicin cream sensitivity trials. The first two trials were used to test orofacial tactile sensitivity, while the third and fourth trials were used to test gnawing. Using the crossed-group experimental design, all animals were tested under both treatment conditions for both behavioral tests.
Following cutaneous testing, animals were separated into three groups to examine the behavioral effects of masseter injections (non-noxious, procedures detailed below). The first set of experiments investigated the masseter injection alone, as muscle injection can incite hypersensitivity by themselves (Lund et al., 2010). Two groups received masseter injections of phosphate buffered saline (PBS, pH 7.4, n=5) or vehicle (n=6) under isoflurane anesthesia, while the third received isoflurane anesthesia only (n=6). At 20 minutes after recovery from anesthesia, gnawing was tested for 10 minutes immediately followed by 10 minutes of orofacial tactile sensitivity testing.
To ensure baseline behavior remained consistent, remaining animals (n=13) were tested at mid-point, with all animals assessed for tactile sensitivity and gnawing over three days with no treatments (Baseline).
Following the second baseline, the effects of capsaicin masseter injections were investigated. Here, animals were separated into three groups: injection of capsaicin (1%), vehicle, or PBS (n=5, 4, 4). Again, at 20 minutes after recovery from anesthesia, gnawing was tested for 10 minutes immediately followed by 10 minutes of orofacial tactile sensitivity testing.
Finally, the remaining eleven CD-Hairless rats were used to evaluate the effects of intra-articular capsaicin (1%) injection to the rat TMJ. Here, animals were separated into two groups: injection of capsaicin or vehicle. As with cutaneous sensitivity testing, animal groups were switched three days later and re-assessed, resulting in n=11 for both treatments. For behavioral testing on both testing days, animals recovered from anesthesia for 20 minutes; then, gnawing was tested for 10 minutes immediately followed by 10 minutes of orofacial tactile sensitivity testing. Following this experiment, all animals were humanely euthanized.
2.3. Capsaicin Application to the Skin
To assess the behavioral effects of capsaicin-mediated cutaneous hypersensitivity in the orofacial region of rats, animals were lightly anesthetized over 5 minutes using 3-4% isoflurane in an induction box, then transferred to 2% isoflurane via mask inhalation where capsaicin cream (0.1%, Capsazin-HP) was applied bilaterally to the skin over the masseter (Neubert, Rossi, Malphurs, Vierck, & Caudle, 2006; Rohrs et al., 2015). After 5 minutes, capsaicin cream was removed with a damp towel. For controls, animals were anesthetized for 10 minutes to match the total anesthesia time for capsaicin treatment, but did not receive any cream or solution on their skin. All animals were placed in a warm recovery box for 20 minutes prior to behavioral testing.
2.4. Masseter Injection Protocol
To assess the behavioral effects of capsaicin-mediated myogenic hypersensitivity in the orofacial region of rats, capsaicin solution was prepared by dissolving 10% capsaicin (Sigma-Aldrich) in ethanol, then mixing capsaicin in ethanol solution with Tween-80 (Sigma-Aldrich) and PBS in 1:1:8 ratio respectively. Vehicle solutions were prepared by mixing ethanol, Tween-80, and PBS (1:1:8 ratio). Animals were lightly anesthetized over 5 minutes using 3-4% isoflurane in an induction box, then transferred to 2% isoflurane via mask inhalation. The skin was prepared for injection using povidone-iodine and ethanol in triplicate, followed by a fourth application of povidone-iodine. For injections, all animals received a 10 μL injection to the right masseter using a 29 gauge insulin syringe (Ambalavanar et al., 2006; Niu, Saloman, Zhang, & Ro, 2011; Tang et al., 2004). Isoflurane only control group was anesthetized for 10 minutes to match injection group’s anesthesia time and did not receive an injection. All animals were placed in a warm recovery box for 20 minutes prior to behavioral testing.
2.5. TMJ Injection Protocol
To assess the behavioral effects of capsaicin-mediated arthrogenic hypersensitivity in the orofacial region of rats, capsaicin and vehicle solution was prepared as described for the masseter injections. Animals were lightly anesthetized over 5 minutes using 3-4% isoflurane in an induction box, then transferred to 2% isoflurane via mask inhalation. The skin over the TMJ was prepared for injection using povidone-iodine and ethanol in triplicate, followed by a final application of povidone-iodine. For injections, all animals received a 10 μL injection to the right TMJ using a 29-gauge insulin syringe. Injection protocol followed previous research with entrance under the zygomatic arch (Tang et al., 2004; Wang et al., 2012). The needle was confirmed to be within the TMJ when gentle contact was made with the temporal bone. All animals were placed in a warm recovery box for 20 minutes prior to behavioral testing.
2.6. Behavioral Testing
As described above, experimental behavioral testing was performed 20 minutes after recovery from anesthesia; however, animals were not anesthetized prior to baseline trials.
For gnaw tests, an animal was placed in an acrylic chamber (19 cm × 19.7 cm × 14.6 cm) with a window (5.55 cm × 5.08 cm). A pressure-gauge instrumented silicone bite tube with barbed Leur-lock attachments was fixed across the window (0.635 cm OD, 0.318 cm ID, 7.62 cm length, Figure 2A). One end of this tube was attached to a differential pressure gauge (MPXV7002DPT1, Freescale Semiconductor, Inc), while the other end of the tube had a manual valve to open or seal the tube. Prior to testing, the tube was charged with air using a syringe to elevate the tube’s internal pressure above ambient pressure; then, the tube was sealed. When punctured, the tube’s internal pressure immediately returned from offset to ambient, allowing a puncture time to be recorded. To encourage gnawing behavior, the tubing was coated with Nutella (Ferrero).
Figure 2.
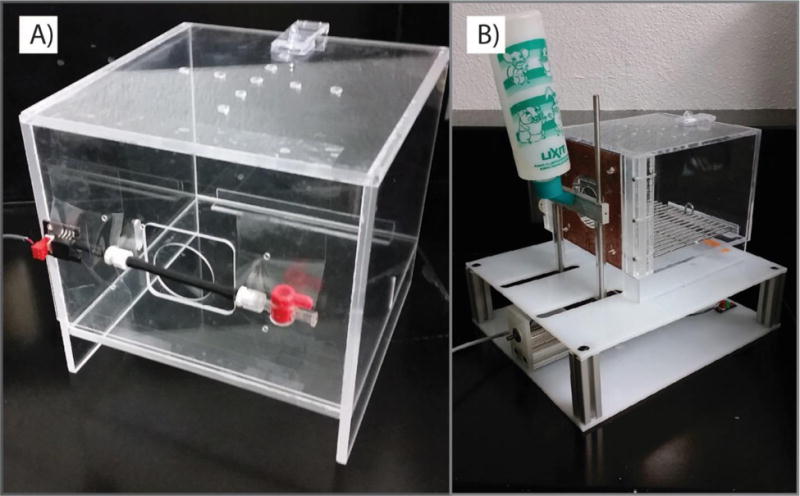
Behavioral assessment cages. (A) Operant test for gnawing behavior. (B) Operant test for tactile sensitivity.
For orofacial tactile sensitivity, an operant-based assessment was conducted as previously described (Rohrs et al., 2015). Briefly, the animal is placed in an acrylic testing chamber (19 cm × 19.7 cm × 14.6 cm). A window (5.55 cm × 5.08 cm) in the chamber allowed access to a reward bottle filled with diluted sweetened condensed milk (1:2 mix of milk and water). A tactile stimulus device consisting of looped stainless steel wires (0.0254 cm thickness, 1.78 cm diameter opening in center) was fixed to the window around the reward bottle nozzle (Figure 2B). Initially, the animal had free access to the reward bottle without contacting the stimulus. However, consistent contact (15/30 ms) with the reward bottle triggered a motor that moved the reward bottle away from the chamber. As the bottle moved, the animal was required to contact the tactile stimulus array to continue to receive the reward bottle. As the rat moved further through the device, the wires bend to accommodate the rat applying increasing pressure with bottle distance. The bottle stopped moving when there was no contact with the bottle for 15 out of 30 ms. The bottle returned to the chamber after 2 minutes. This process repeated over 10 minutes, producing 5 total displacements or maximum tolerated bottle distances.
2.7. Data Analysis and Statistics
To reduce inter-animal variability, mean animal behavior (puncture time, bottle distance) was compared to the most recent baseline data for that same animal, thereby converting the data into residual difference. This residual represents the change of individual animals from that same animal’s baseline behavior. To evaluate differences between treatment groups, 1-way analysis of variance (ANOVA) was used to compare the residuals from each treatment. Tukey’s HSD post-hoc analysis was used to assess differences between groups where indicated. In addition, non-parametric sign tests were used to evaluate whether a change from baseline had occurred.
3. Results
3.1. Tactile Hypersensitivity
Capsaicin cream application to the orofacial region was found to reduce tolerated bottle distance (Figure 3). Application of capsaicin cream reduced tolerated bottle distance by 0.577 cm compared to baseline (p=0.0001) and 0.980 cm compared to isoflurane only (p=0.0001). Isoflurane alone increased tolerated bottle distance over baseline (p=0.02).
Figure 3.
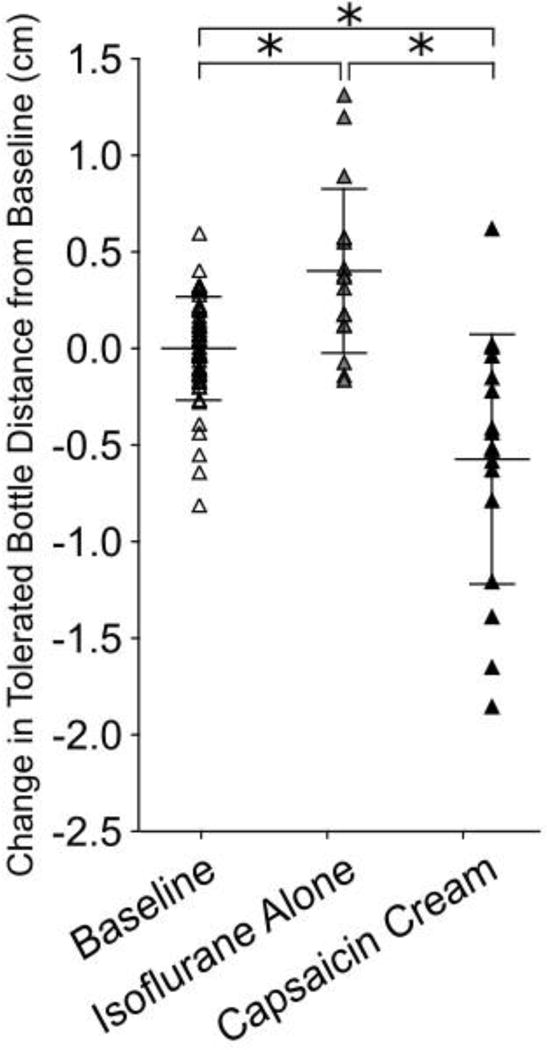
Cutaneous application of capsaicin cream decreases tolerated bottle distance compared to baseline and isoflurane alone (p=0.00003, p=0.00001 respectively). Data was normalized to individual animal baseline mean to adjust for variation among animal distance preference. * indicates p<0.05.
Intra-muscular capsaicin injections reduced tolerated bottle distance by 0.914 cm compared to baseline (p=0.0002, Figure 4B). Additionally, capsaicin injections reduced tolerated bottle distance compared to PBS injections and vehicle injections (p=0.003, p=0.005 respectively). PBS and vehicle injections were found to have no effect on tolerated bottle distance compared to baseline (p=1.0, p=0.9 respectively) and isoflurane only (p=0.5, p=0.9 respectively, Figure 4A).
Figure 4.
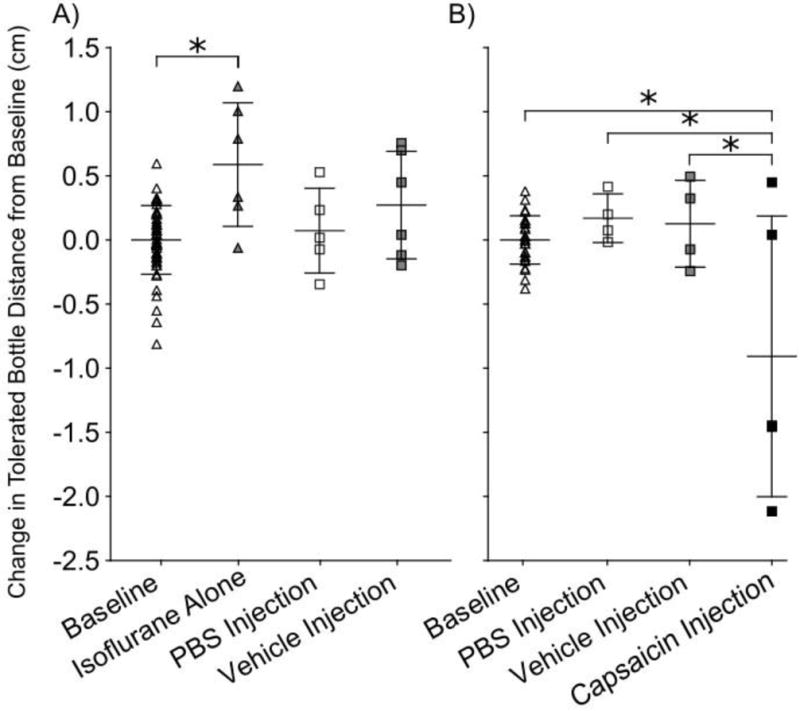
Capsaicin masseter injection decreases tolerated bottle distance. Data for non-noxious and capsaicin masseter injections trials were normalized to individual animal baseline mean to adjust for variation among animal distance preference. A) No change in tolerated bottle distance between baseline and PBS injections, or baseline and vehicle injections, (p=0.9, p=0.9 respectively), however isoflurane only group increased tolerated bottle distance compared to baseline (p=0.03). B) Capsaicin injection reduced tolerated bottle distance compared to baseline, PBS injections, and vehicle injections (p=0.0002, p=0.003, p=0.005). * indicates p<0.05.
Intra-articular capsaicin injections did not significantly reduce tolerated bottle distance compared to baseline (p=0.8, Figure 5). Intra-articular vehicle injections were also found to have no significant effect on tolerated bottle distance compared to baseline (p=0.8). Finally, no difference was found between intra-articular vehicle and capsaicin injected groups (p=0.8).
Figure 5.
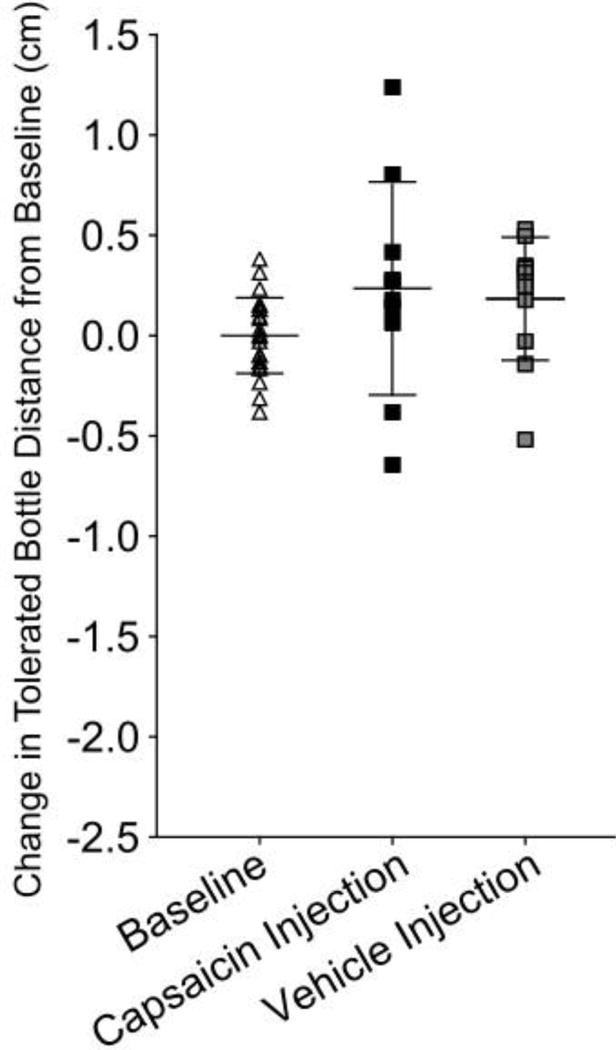
Capsaicin TMJ injection did not affect tolerated bottle distance compared with baseline or vehicle injected groups (p=0.4, p=0.8 respectively). Additionally, no difference was noted between baseline and vehicle injected groups (p=0.8). Data was normalized to individual animal baseline mean to adjust for variation among animal distance preference.
3.2. Gnaw Post Assessment
Capsaicin cream application to the orofacial region was found to increase puncture time (Figure 6). Application of capsaicin cream increased puncture time by 338 seconds compared to baseline (p=0.0002) and 248 seconds compared to isoflurane only (p=0.0002).
Figure 6.
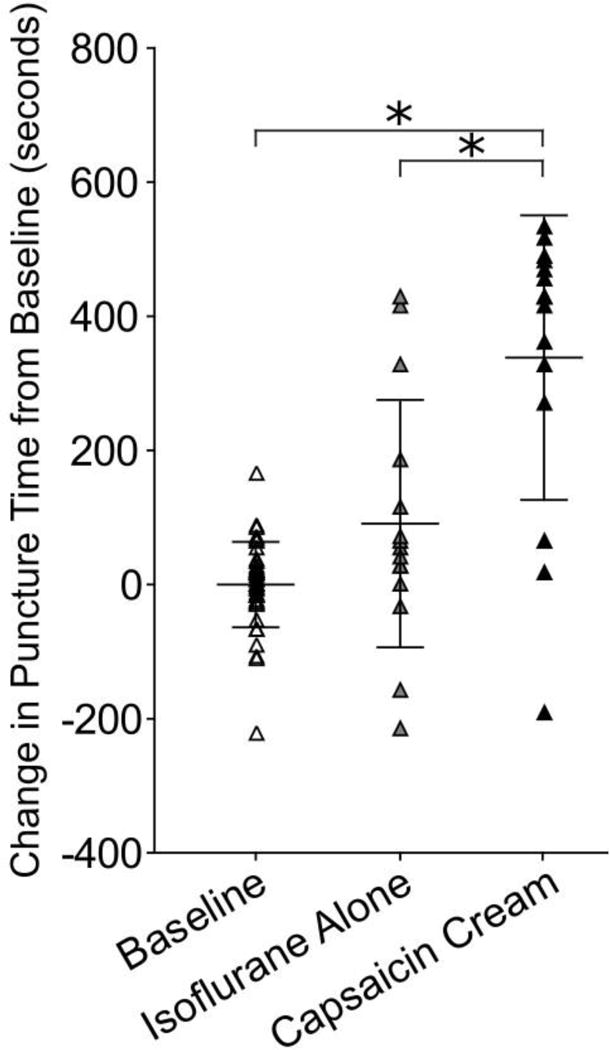
Cutaneous capsaicin cream application increases puncture time compared to baseline and isoflurane alone groups (p=0.0002, p=0.0002). Data was normalized to individual animal baseline mean to adjust for variation among animal distance preference. * indicates p<0.05.
Intra-muscular capsaicin injections increased puncture by 425 seconds compared to baseline (p=0.0002, Figure 7). Additionally, capsaicin injections increased puncture time compared to PBS injections and vehicle injections (p=0.01, p=0.07 respectively). PBS and vehicle injections were found to have no effect on tolerated bottle distance compared to baseline (p=1.0, p=0.9 respectively) and isoflurane only (p=0.5, p=0.9 respectively).
Figure 7.
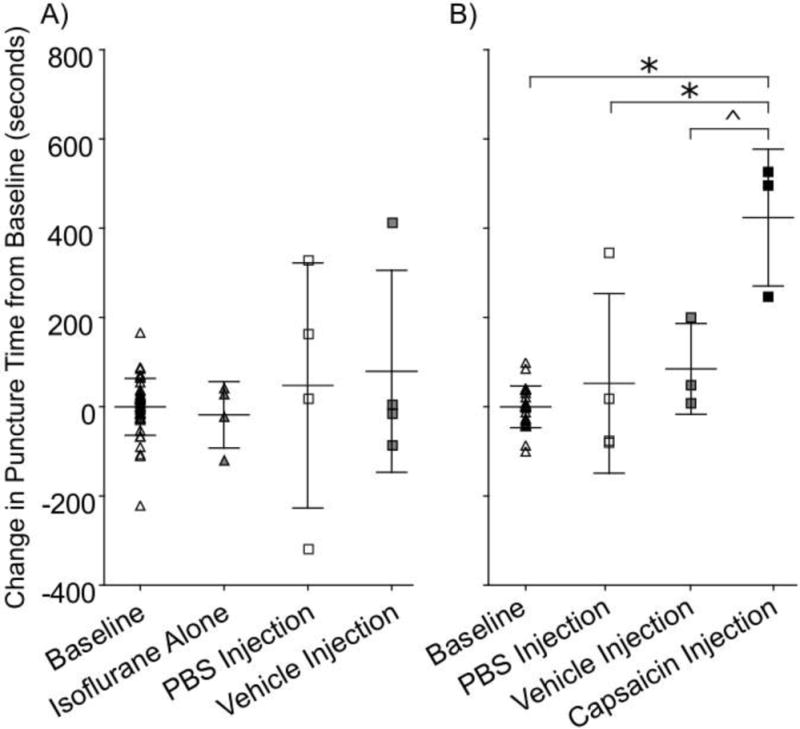
Capsaicin masseter injection increases time to puncture compared to baseline. Data for non-noxious and capsaicin masseter injections trials were normalized to individual animal baseline mean to adjust for variation among animal distance preference. A) No difference was found between baseline, isoflurane only, PBS injections, or vehicle injections (p>0.05). B) Capsaicin injection increased puncture time compared to baseline, PBS injections, and vehicle injections (p=0.0002, p=0.014, p=0.068). * indicates p<0.05. ^ indicates p<0.10.
Intra-articular capsaicin injections increased puncture time by 208 seconds compared to baseline (p=0.0002, Figure 8). Intra-articular vehicle injection did not significantly change puncture time compared to baseline (p=0.7).
Figure 8.
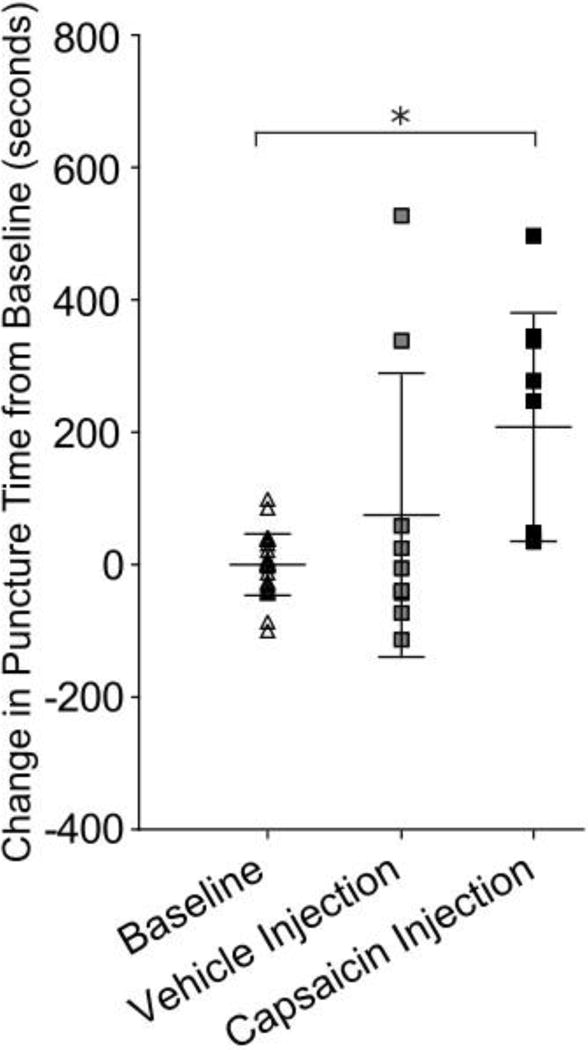
Capsaicin TMJ injection increased time to punctured compared to baseline (p=0.0001). Data was normalized to individual animal baseline mean to adjust for variation among animal distance preference. * indicates p<0.05.
4. Discussion
Quantitative methods of measuring pain related behaviors are necessary to evaluate models of orofacial pain and TMDs. Here, cutaneous, myogenic, and arthrogenic capsaicin mediated hypersensitivity assessed using operant-based tests. Cutaneous and myogenic application of capsaicin both increased puncture time compared to baseline behavior and non-noxious control groups. Intra-articular capsaicin TMJ injections increased time to puncture, but did not decrease tolerated bottle distance. As such behavioral changes were identified in all models indicated by decreased tolerated bottle distance and/or by increased time to puncture. Some animals were removed from the study prior masseter and TMJ injections models due to tumor growth unrelated to the experiment (Figure 1). Larger sample size may have increased the significance between some groups. Both devices used here can be used in parallel to assess behavioral changes associated with rodent models of cutaneous, myogenic, and arthrogenic hyper-nociception.
Using the methods described herein, tactile hypersensitivity produced by surface nociception and deep muscle intervention produce similar behavioral characteristics. Maximum tolerated bottle distance reduced by 0.577 cm and 0.914 cm from cream and injection models respectively (Figure 3), indicating operant testing for tactile sensitivity can produce similar results in deep muscle tissue as in cutaneous tissue. However, it is interesting that intra-articular TMJ capsaicin injections did not decrease tolerated bottle distance. It is possible that short-acting capsaicin does not result in changes to tactile nociception at the time scales evaluated in this study and chronic inflammation in the TMJ may ultimately result in referred hypersensitivity. These hypotheses can be evaluated in future investigations.
Clinically, tactile sensitivity and masticatory muscle pain are related. Clinicians will often use similar tactile assessments when evaluating muscle pain including pressure pain thresholds. The severity of TMD pain intensity is correlated to the pressure pain threshold over the TMJ and masticatory muscles (Herpich et al., 2018). Evaluating severity of tactile sensitivity in the masseter and TMJ region, as described here, can aid in describing overall severity of pain associated with models of TMD.
To investigate gnawing, a device was created to interpret length of time to voluntarily chew through a silicone tube. Cutaneous, myogenic, and arthrogenic application of capsaicin produced similar results as puncture time increased from baseline by 338 seconds, 425 seconds, and 208 seconds respectively (Figure 6). Increased puncture time due to either capsaicin masseter injection or capsaicin TMJ injection was expected and may be attributed to difficulty gnawing the bite post; no animals in the capsaicin masseter injected group were able to chew through the device within the 10 minute trial period (0 of 3), and only 4 of 9 animals in the capsaicin TMJ injected group were not able to chew through the device within the 10 minute trial period (Figure 7, 8). While a significant increase in puncture time was found in capsaicin injection groups, longer trial periods could provide a larger effect size between groups.
Capsaicin cream application also significantly increased puncture time. It is unlikely capsaicin cream directly interferes with muscle activity or gnawing. Rather, increased puncture time may be produced by increased grooming time or distraction caused by capsaicin cream application. Similar pain related behaviors as grooming have been shown to increase in pain models (Vos & Strassman, 1994). As such, excessive grooming may also by why capsaicin injected animals did not puncture the tube. As such, puncture time may not be directly related to jaw function or bite strength, but rather is another characteristic pain related behavior.
Future iterations of the device will attempt to more closely evaluate gnawing frequency, duration, and intervals to more closely evaluate what behaviors are actually changing in the animal. Pressure readings were attempted throughout this study in an attempt to investigate bite strength, however, a lack of sensitivity in these pressure readings made the results inconclusive. Future studies will improve upon the bite behavior device used here.
5. Conclusion
Quantifying tactile sensitivity and gnawing behavior are essential to understanding pain characteristics in the development and treatment of orofacial pain and TMD. Both capsaicin cream application and capsaicin masseter injection cause pain behavior relating to tactile hypersensitivity. Our data also show capsaicin cream, capsaicin masseter injections, and capsaicin TMJ injections increased puncture time in an operant gnawing behavior test. As a result, it is our understanding that pain originating in the TMJ will present dramatically different behavioral characteristics than pain originating in the masticatory muscles or skin. Using these assays, further investigation of pain related behavior in other rodent models of TMDs can help us understand the development of pain related behavior as a consequence of biological changes, hopefully leading to valuable evaluations of treatments and therapies for orofacial pain and TMD in the future.
Highlights.
Behavior was assessed in rats following capsaicin application.
Tactile allodynia and gnawing behavior were investigated.
Cutaneous, myogenic and arthrogenic nociception were investigated.
Cutaneous and myogenic nociception models displayed tactile allodynia behavior.
All investigated nociception models displayed decreased gnawing behavior.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health [grant numbers K99,R00AR057426].
Abbreviations
- TMJ
Temporomandibular Joint
- TMD
Temporomandibular Disorder
- IACUC
The Institutional Animal Care and Use Committee
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Robert M. Caudle, John K. Neubert and Eric L. Rohrs are employees of Velocity Laboratories, a company that provides fee-for-service behavioral testing using operant pain assays. The other author does not have a conflict of interest to report.
References
- Ambalavanar R, Dessem D, Moutanni A, Yallampalli C, Yallampalli U, Gangula P, Bai G. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience. 2006;143(3):875–884. doi: 10.1016/j.neuroscience.2006.08.015. https://doi.org/10.1016/J.NEUROSCIENCE.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemporary Topics in Laboratory Animal Science. 2004;43(6):42–51. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15669134. [PubMed] [Google Scholar]
- Cha M, Kohan KJ, Zuo X, Ling JX, Gu JG. Assessment of chronic trigeminal neuropathic pain by the orofacial operant test in rats. Behavioural Brain Research. 2012;234(1):82–90. doi: 10.1016/j.bbr.2012.06.020. https://doi.org/10.1016/j.bbr.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neuroscience and Biobehavioral Reviews. 2002;26(8):907–23. doi: 10.1016/s0149-7634(02)00103-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12667496. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Wang K, Cairns BE, Svensson P, Arendt-Nielsen L. Effects of subcutaneous administration of glutamate on pain, sensitization and vasomotor responses in healthy men and women. Pain. 2006;124(3):338–48. doi: 10.1016/j.pain.2006.06.015. https://doi.org/10.1016/j.pain.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone Da. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67(1):179–88. doi: 10.1016/0304-3959(96)03104-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8895246. [DOI] [PubMed] [Google Scholar]
- Herpich CM, André C, De F, Gomes P, Vieira Dibai-Filho A, Politti F, Aparecida Biasotto-Gonzalez D. Correlation Between Severity of Temporomandibular Disorder, Pain Intensity, and Pressure Pain Threshold. 2018 doi: 10.1016/j.jmpt.2017.08.001. https://doi.org/10.1016/j.jmpt.2017.08.001. [DOI] [PubMed]
- Holzer P. Capsaicin : Cellular Selectivity Targets, for Thin Mechanisms of Action, Sensory Neurons *. Pharmacological Review. 1991;43(2):143–201. [PubMed] [Google Scholar]
- Holzer P. Capsaicin-sensitive nerves in the control of vascular effector mechanisms. In: Wood JN, editor. Capsaicin in the Study of Pain. London: Academic Press; 1993. pp. 191–218. [Google Scholar]
- Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale. 1997;116(1):97–103. doi: 10.1007/pl00005748. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9305818. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Frontiers in Bioscience : A Journal and Virtual Library. 2006;11:2179–92. doi: 10.2741/1960. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16720304. [DOI] [PubMed] [Google Scholar]
- Jancsó N, Jancsó-Gábor A, Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. British Journal of Pharmacology and Chemotherapy. 1967;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. https://doi.org/10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó N, Jancsó-Gábor A, Szolcsányi J. The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. British Journal of Pharmacology and Chemotherapy. 1968;33(1):32–41. doi: 10.1111/j.1476-5381.1968.tb00471.x. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1570273&tool=pmcentre z&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnman E, Levine JD. Involvement of the sympathetic postganglionic neuron in capsaicin-induced secondary hyperalgesia in the rat. Neuroscience. 1995;65(1):283–91. doi: 10.1016/0306-4522(94)00474-j. https://doi.org/10.1016/0306-4522(94)00474-J. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjörk HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. The Journal of Physiology. 1992;448:749–64. doi: 10.1113/jphysiol.1992.sp019068. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1176226&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Arch Pharmacol. 1979;310:175–183. doi: 10.1007/BF00500282. https://doi.org/PMID:93706. [DOI] [PubMed] [Google Scholar]
- Lin Q, Li D, Xu X, Zou X, Fang L. Roles of TRPV1 and neuropeptidergic receptors in dorsal root reflex-mediated neurogenic inflammation induced by intradermal injection of capsaicin. Molecular Pain. 2007;3:30. doi: 10.1186/1744-8069-3-30. https://doi.org/10.1186/1744-8069-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JP, Sadeghi S, Athanassiadis T, Caram Salas N, Auclair F, Thivierge B, Kolta A. Assessment of the Potential Role of Muscle Spindle Mechanoreceptor Afferents in Chronic Muscle Pain in the Rat Masseter Muscle. PLoS ONE. 2010;5(6):e11131. doi: 10.1371/journal.pone.0011131. https://doi.org/10.1371/journal.pone.0011131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauderli aP, Acosta-Rua a, Vierck CJ. An operant assay of thermal pain in conscious, unrestrained rats. Journal of Neuroscience Methods. 2000;97(1):19–29. doi: 10.1016/s0165-0270(00)00160-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10771071. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- Neubert JK, Rossi HL, Malphurs W, Vierck CJ, Caudle RM. Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behavioural Brain Research. 2006;170(2):308–15. doi: 10.1016/j.bbr.2006.03.008. https://doi.org/10.1016/j.bbr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116(3):386–95. doi: 10.1016/j.pain.2005.05.011. https://doi.org/10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- NIH. TMJ Disorders. 2018 Retrieved February 9, 2018, from http://www.nidcr.nih.gov/OralHealth/Topics/TMJ/TMJDisorders.htm.
- Nilsson M, Lassen D, Andresen T, Nielsen AK, Arendt-Nielsen L, Drewes AM. Intradermal glutamate and capsaicin injections: intra- and interindividual variability of provoked hyperalgesia and allodynia. Clinical and Experimental Pharmacology & Physiology. 2014;41(6):423–9. doi: 10.1111/1440-1681.12229. https://doi.org/10.1111/1440-1681.12229. [DOI] [PubMed] [Google Scholar]
- Niu K, Saloman JL, Zhang Y, Ro JY. Sex differences in the contribution of ATP-sensitive K+ channels in trigeminal ganglia under an acute muscle pain condition. Neuroscience. 2011;180:344–52. doi: 10.1016/j.neuroscience.2011.01.045. https://doi.org/10.1016/j.neuroscience.2011.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan Ta, Hester J, Bokrand-Donatelli Y, Caudle RM, Neubert JK. Adaptation of a novel operant orofacial testing system to characterize both mechanical and thermal pain. Behavioural Brain Research. 2011;217(2):477–80. doi: 10.1016/j.bbr.2010.10.022. https://doi.org/10.1016/j.bbr.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck CC, Goulet JP, Lobbezoo F, Schiffman EL, Alstergren P, Anderson GC, List T. Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. Journal of Oral Rehabilitation. 2014;41(1):2–23. doi: 10.1111/joor.12132. https://doi.org/10.1111/joor.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144(3):270–7. doi: 10.1016/j.pain.2009.04.021. https://doi.org/10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro JY, Lee J, Capra NF, Zhang Y. Role of soluble guanylate cyclase in the trigeminal subnucleus caudalis in capsaicin-induced muscle hypersensitivity. Brain Research. 2007;1184:141–8. doi: 10.1016/j.brainres.2007.09.085. https://doi.org/10.1016/j.brainres.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Rohrs EL, Kloefkorn HE, Lakes EH, Jacobs BY, Neubert JK, Caudle RM, Allen KD. A novel operant-based behavioral assay of mechanical allodynia in the orofacial region of rats. Journal of Neuroscience Methods. 2015;248(248):1–6. doi: 10.1016/j.jneumeth.2015.03.022. https://doi.org/10.1016/j.jneumeth.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum T, Simon S. TRPV1 Receptors and Signal Transduction. In: Liedtke W, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton, FL: CRC Press/Taylor & Francis; 2007. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK5260/ [PubMed] [Google Scholar]
- Tang MLY, Haas Da, Hu JW. Capsaicin-induced joint inflammation is not blocked by local anesthesia. Anesthesia Progress. 2004;51(1):2–9. [PMC free article] [PubMed] [Google Scholar]
- Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: activation by stress. Science (New York, NY) 1984;226(4680):1270–7. doi: 10.1126/science.6505691. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6505691. [DOI] [PubMed] [Google Scholar]
- Velly AM, Look JO, Schiffman E, Lenton PA, Kang W, Messner RP, Fricton JR. The effect of fibromyalgia and widespread pain on the clinically significant temporomandibular muscle and joint pain disorders–a prospective 18-month cohort study. The Journal of Pain : Official Journal of the American Pain Society. 2010;11(11):1155–64. doi: 10.1016/j.jpain.2010.02.009. https://doi.org/10.1016/j.jpain.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos BP, Strassman AM. Behavioral Evidence Chronic Constriction of Trigeminal Neuropathic Pain Following Injury to the Rat’s lnfraorbital Nerve. The Journal of Neuroscience. 1994;74:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos BP, Strassman AM. Fos expression in the medullary dorsal horn of the rat after chronic constriction injury to the infraorbital nerve. The Journal of Comparative Neurology. 1995;357(3):362–75. doi: 10.1002/cne.903570304. https://doi.org/10.1002/cne.903570304. [DOI] [PubMed] [Google Scholar]
- Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Zhou YH. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PloS One. 2012;7(9):e45036. doi: 10.1371/journal.pone.0045036. https://doi.org/10.1371/journal.pone.0045036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu J, Fang L, Willis WD. The effects of protein phosphatase inhibitors on nociceptive behavioral responses of rats following intradermal injection of capsaicin. Pain. 2003;106(3):443–451. doi: 10.1016/j.pain.2003.09.002. https://doi.org/10.1016/j.pain.2003.09.002. [DOI] [PubMed] [Google Scholar]


